Abstract
The COVID-19 pandemic has posed and is continuously posing enormous societal and health challenges worldwide. The research community has mobilized to develop novel projects to find a cure or a vaccine, as well as to contribute to mass testing, which has been a critical measure to contain the infection in several countries. Through this article, we share our experiences and learnings as a group of volunteers at the Centre for Genomic Regulation (CRG) in Barcelona, Spain. As members of the ORFEU project, an initiative by the Government of Catalonia to achieve mass testing of people at risk and contain the epidemic in Spain, we share our motivations, challenges and the key lessons learnt, which we feel will help better prepare the global society to address similar situations in the future.
Keywords: COVID-19 testing, volunteers, health, collaboration
Introduction: CRG in Orpheus’ shoes
The recent turn of events worldwide, brought on by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that uprooted the world of all normality, presented a tremendous challenge. In tragic unison, COVID-19 became the viper to humanity’s Eurydice, and the world of researchers and medics, her Orpheus, in our attempt to revive and reset. With a name inspired by this mythological allegory, the Catalan government launched the ‘ORFEU’ project – a mass testing initiative to identify infected individuals – in an attempt to reduce infection rates and facilitate an easier transition into a post-confinement era. At the time that the government decided to launch the ORFEU project, the epidemic in Catalonia was at a critical high. In April 2020, the infection rates and mortality were peaking, and the health system was rapidly overwhelmed. As a result, no systematic testing initiatives were in place in various critical settings such as nursing homes, prisons or clinical institutions due to the vast demand for tests for symptomatic people in hospitals.
With the aim of performing 170,000 PCR-based COVID-19 tests, the ORFEU project united some of the major research institutes across Catalonia. There were two main sample-processing nodes with close to 200 volunteers collectively – one at the Barcelona Biomedical Research Park (PRBB) orchestrated by the Center for Genomic Regulation (CRG) and the other at Barcelona Science Park (PCB), jointly coordinated by IRB Barcelona, IBEC and the CNAG-CRG ( Figure 1). Both nodes were supported by the CNAG-CRG informatics team and the whole process coordinated by the CRG director. The team at PRBB comprised a total of 106 volunteers including principal investigators, post-doctoral researchers, technicians, and administration personnel ( Figure 2). Here, in this short article, we, as volunteers at the PRBB node, share our motivations, and briefly outline the processes and challenges that were important to adapt from a basic research centre to an analytical and diagnostic centre for COVID-19 so that other global institutes may benefit from our experience.
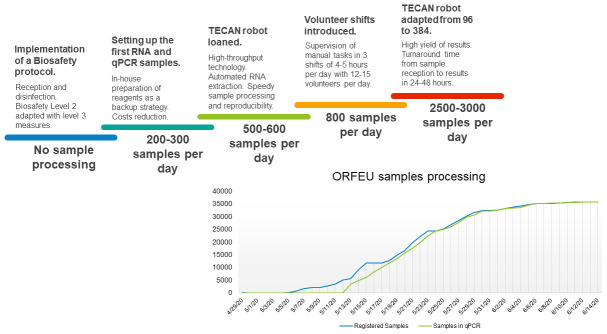
Figure 1. Key milestones towards increasing sampling processing.
(Top) Modifications to the workflow, which greatly influenced our ability to handle a larger number of samples. (Bottom) Cumulative number of samples processed from the start to the end of the ORFEU project.
Figure 2. Some of the volunteers across the Barcelona Biomedical Research Park and Barcelona Science Park nodes who took part in the ORFEU project.
We confirm that we have obtained written informed consent to use images of the individuals included in this presentation.
The motivation
“ Extraordinary times call for extraordinary measures. We saw a need that needed to be filled and we stepped in to help.” - Benet Wilson
The motivations of all the volunteers were diverse. While for some it was curiosity to better understand the on-ground scene, others hoped to get a much-desired alternative to in-house confinement. For others less fortunate, the motivation was fuelled by personal experiences with loved ones having suffered through the infection. But most of all, and unanimously, the underlying motivation was to help society fight against the pandemic by offering our specific skill sets and spreading the knowledge needed to battle the crisis. Together, obstacles such as reduced public transport, commuting distances and the inherent human fear factor, were overcome by our will to curb the pandemic through our contributions in the ORFEU project.
Organisation and pipeline
At the outset, once called into the ORFEU project, the CRG health and safety department achieved the mammoth task of adapting the research centre into an analytical and diagnostic centre. There were two major phases to this. First, a biosafety protocol outlining key information such as SARS-CoV-2 sample collection and handling, based on the procedures from the World Health Organisation 1 and Centre for Disease Control and Prevention 2 were established. In addition, a Biosafety Level 2 facility was modified by introducing level 3 measures, such as exclusive changing rooms and protective equipment disposal protocols, which was pivotal in our ability to deal with SARS-CoV-2 samples due to the need for adequate disinfection and correct storage. In the second phase, given our inexperience in handling such an emergency, adequate training of volunteers was critical. To start, a core team of volunteers of around 10 members were instructed on biosafety measures through an online training session given by an external expert in Biosafety Level 3 followed by a hands-on in situ training in smaller groups. All members were trained equally in sample collection and disinfection, sample registration, RNA extraction, PCR and validation, allowing fail-safes and contingencies in cases when members were not able to carry on for any reason. Eventually more volunteers were recruited based on sample load and divided into specialised groups led by a member from the core team. The entire workflow was monitored using a laboratory information management system (LIMS) developed ad hoc by the Bioinformatics Unit at CNAG-CRG and a user interface program for sample registration developed by the CRG bioinformatics unit. Together, the LIMS allowed for the smooth and rapid management of samples in an efficient and errorless process between various teams. Expert clinical microbiologists, from various collaborating hospitals, interpreted the PCR results online in a user-friendly module of the LIMS, and carried out the final task of diagnosis.
The ultimate aim was to automate the process for speedy sample processing as well as reproducibility using high-throughput TECAN robots (one of which was kindly loaned to the CRG by the company) and multiple RT-PCR machines (one kindly lent by the University Pompeu Fabra). Nevertheless, the importance of manual tasks and supervision was paramount, and required approximately three shifts of 4–5 hours every other day, with approximately 4–5 volunteers per shift. Having started at a few hundred samples processed per day, by the end of the project, the CRG was in a position to process 2500–3000 samples per day, with a turnaround time from sample reception to results in about 24–48 hours ( Figure 1). The workflow can be followed in an infographic created by CRG 3 .
Key challenges
To ensure the systematic running of the entire unit, it was important to establish a general workflow based on the process mentioned above. Through our implementation of the setup 4 , we identified key steps to assure process standardization and optimisation. Here we highlight some of the major points.
From the point of view of the organising committee
A project of this scale requires navigating through a large amount of administrative and legal procedures, as well as coordinating many different organisations and teams. Resolving each of these issues caused an initial delay between the proposed and actual start of the project, resulting in a lower overall turnaround of test results than intended. Moreover, the sporadic receipt of samples from various parts of Catalonia presented a challenge in terms of organising shifts, adequate storage and handling, and quick turnaround time (ideally 24–48h). The CRG now has a go-to model ready for use in future situations to share with other organizations 4 .
From the point of view of informaticians
One of the main challenges during the project was the simultaneous development of an online informatics platform, whilst the laboratory workflow was being designed. Our aim was to develop a platform that enabled tracking of all samples throughout the process internally and also provided an interactive and user-friendly analysis environment for third-party use by collaborating microbiologists to interpret the results. To achieve this, the CNAG-CRG, together with CRG bioinformaticians, developed a LIMS that included a diagnosis validation tool.
From the point of view of the analytical team
The PCR testing protocol had two major aspects – RNA extraction and real-time quantitative PCR. Identifying the optimal combination of reagents with sample processing equipment was arguably the most challenging part and is a must to be tackled at the earliest. The initial phase was subject to continual switching between different RNA extraction kits and equipment, which called for exhaustively long working hours and time-consuming daily training.
Communication and transfer of knowledge posed major challenges as well. Using dedicated social media channels and live cloud-based services helped in sharing status updates between shifts and quick responses to unexpected technical issues.
From the point of view of the protein production team
A continuous logistical challenge throughout the course of the project was the limited availability of reagents and materials. In order to circumvent this issue, reduce the pressure on manufacturing units and optimise overall project costs, the CRG organised a protein production team that performed expression and purification of all the necessary proteins as a backup in the event that the supply chain broke completely. Protocols were initially tested on a small scale by the CRG’s Protein Technologies Unit and further expanded by volunteers. Moreover, extensive further validation and certification of these proteins in PCR mixes or as other reagents are limiting steps prior to them being available for analytical use.
From a personal perspective
Often taken for granted, one of the main challenges during the coordination of a crisis initiative such as the ORFEU project was taking into account personal and professional lives. On one hand, at a personal level, the stress of the lockdown and having children at home or living with high-risk individuals made it difficult to volunteer. Accommodating for lockdown-induced anxiety and mental health needs was very important. On the other hand, from a professional point of view, the delays and late start of the project, which coincided with the reopening of many labs, made it difficult to balance between the two. In addition, and very importantly, as basic researchers, we needed to accept a new responsibility, that results from this project would directly impact people’s lives.
Success stories
The outcomes we achieved in the ORFEU project would not have been possible without some key aspects that contributed to its success.
Above all, the motivation, commitment and effort of everyone involved, working towards a common objective, was a very strong driving force that kept us on track, despite the obstacles. The trust of the administration in the volunteers allowed us to take the lead on various aspects of the project, enabling productivity and creating an atmosphere of trust and motivation.
Teamwork and cooperation were, as always, critical. Working together in a friendly, supportive and cooperative environment made it easier to cope with stress and difficult situations without unnecessary conflict. The level of mutual respect was very high and communication was transparent, preventing any tension within the team despite varying opinions, changing protocols and long working hours.
While the entire team’s effort was very important, it is necessary to highlight that without the dedication and perseverance of the initial organising team, and the various principal investigators, the project would not have been possible. Their tireless commitment for the optimisation of protocols and round-the-clock availability were indispensable stepping-stones for the rest of the team to be able to deliver on the project targets.
A major turning point in our ability to handle large volumes of samples came through the tireless efforts of highly skilled personnel at the CRG core facilities who worked to overcome technical hurdles and managed, for instance, to convert a robot that was processing 96 samples every 90 min to processing 384 samples in the same time.
Delegation of tasks and specialised teams became a critical factor in speeding up not only the sample-processing rate but also the training and induction of different groups of volunteers. Given the clear communication and coordination between specialised teams, typically overseen by rotating team leaders, the whole process adopted a very smooth and high-throughput functioning capacity.
Lessons learned
Converting a research centre into an analytical platform is not easy: Despite the resources and technical expertise, setting-up an analytical platform from scratch and under a state of emergency was a complex process. Added to the challenges intrinsic to the novel social responsibilities, and the professional responsibility of delivering accurate results, the preparation of this platform required various obstacles to be negotiated.
Create a collaborative work environment: A strong team built through collaboration between researchers, the government and health professionals, with a varied skill set and good morale proved to be essential. Stratified decision-making, accountability, and distribution of tasks can promote the efficient functioning of the unit. Moreover, cross-institute collaborations, which arise from such projects, are long-term connections that can present opportunities for collaboration on various projects across disciplines and sectors, for instance between basic researchers, clinicians, health authorities and policymakers.
Technical assurance is critical: The transition from the creation of LIMS to its usability was an important process requiring optimization and often limiting sample processing rates. During the user interface design, it was critical to avoid making assumptions. The entire development process benefited from monitoring usage of the tool after an initial training session, as well as through the course of the project. Documenting workflows and database schemas was fundamental. Additionally, prioritization and differentiation between critical and non-critical features allowed the optimal use of limited software development personnel.
Importance of good communication: Communication is one of the cornerstones of an efficient system. Establishing communication channels quickly through social media, live cloud-based services or internal institute-based communication systems available to everyone involved was very important to guarantee an effective workflow, and to ensure transparency, robustness and stability.
Protocol standardization: The initial stages of the ORFEU project witnessed a lot of changing protocols and volunteers were required to adapt very quickly. Multiple tests were needed to optimize both processes, and as mentioned above technical developments were needed to increase the capacity of the RNA extraction robots. Although not discussed here, we found numerous artefacts in the PCR reactions. For instance, something as banal as minor traces of ethanol in the reaction could induce artifactual amplification, or the contamination of neighbouring wells in plates where a patient had a very large viral load. See 4 for our final protocol.
Open access and availability of organizational workflow and protocols: All protocols, as well as optimization trials (including negative results), from situations such as the COVID-19 pandemic, would gain from being shared openly by enabling other research centres and institutions to make better use of their resources 5 . Moreover, these are important opportunities to initiate dialogue and collaboration between local and global research institutions, alongside local governments and health authorities. This would allow them to put in place emergency plans and workflows that can prevent loss of precious time and scarce resources, as was exemplified in the initial phases of the ORFEU project.
Researchers and research are important to society : Beyond knowledge creation, researchers can mobilize quickly to produce tangible outcomes for the benefit of society. Proven across Catalonia, and globally, researchers can respond and adapt rapidly and effectively to emergencies, collaborating with local healthcare systems. As a result, a greater consideration for scientific research from governments, health authorities and society would better leverage resources during a health crisis.
Our top 10 recommendations to be prepared for the next pandemic
-
1.
A general but adaptable workflow should initially be established by a core team of administrators, researchers and informaticians. Additional teams should closely follow to prevent exhaustion of the leading team and sustain productivity. To ensure readiness, an emergency response team should revisit the entire workflow annually and adapt it to address any potential issues based on current infrastructure, availability of personnel, and other possible complications.
-
2.
Define clear tasks and responsibilities as soon as possible. The combination of specialized teams and a small group of people with broader knowledge ensures efficiency and robustness.
-
3.
An initial database schema should be agreed upon ahead of time followed by suitable data transfer formats and protocols between systems (e.g. LIMS and Electronic Health Records). To ensure the systematic transfer of samples and eventually results, set up a common platform with the health authorities to assure transparent communication of all necessary logistic information.
-
4.
Set up dedicated communication channels that allow fast information transfer and updates.
-
5.
Volunteers should be recurrently tested, and not exclusively at the start of the programme. This will ensure mental peace and health safety of all directly or indirectly involved, as a result, ensuring sustainability of the project.
-
6.
Set up a pipeline to produce your own reagents (RT-PCR master mix and RNA extraction kits), so as not to solely rely on external sources to acquire them.
-
7.
Open access protocols should be shared across research centres and health institutions as soon as they are standardised. Problems arising during their development should also be communicated to avoid needless repetition.
-
8.
Equipment and pipelines should not be dismantled until normality has been reinstated globally to prepare for future outbreaks and the possible need for testing protocols.
-
9.
In emergencies, requirements are dynamic and processes can change very quickly. Hence, the volunteer recruitment process should continue as a backup for as long as possible.
-
10.
Despite being volunteer work, all participants worked at their own projects’ expense. Understanding from supervisors and recognition from funding agencies and government bodies, for instance by extending fellowships, would attract more support.
Data availability
No data are associated with this article.
Acknowledgements
We thank all volunteers from both nodes – PRBB and PCB for their participation and hard work in the ORFEU project. A list of all participants can be found below. In addition, we specifically extend our thanks to Jochen Hecht (CRG Genomics Unit Head) and Carlo Carolis (CRG Biomolecular Screening & Protein Technologies Unit Head) for their tireless work throughout the ORFEU project. We also thank the collaborating microbiologists who were instrumental in the final diagnosis process.
The testing phase would not have been possible without the efforts of the biosafety team at CRG to adapt the facilities, to establish protocols and to maintain the safety conditions.
Our thanks to Luis Serrano Pubul (Director, CRG), Juan Valcárcel (Group Leader, CRG), Guillaume Filion (previously Group Leader at CRG) and Mònica Morales Ballús (Core Facilities Programme Coordinator, CRG) for coordinating and organizing the ORFEU project. Finally, we thank Michela Bertero (Head of International & Scientific Affairs, CRG), who was a major driving force towards the creation of this manuscript.
List of volunteers from CRG: Marta Agostinho, Álvaro Aranguren Ibáñez, Niccolò Arecco, Carme Arnan, Leonor Avila, Borja Balbastre, Diego Balboa, Magalí Bartomeus, Marc Bataller, Sergi Beltran, Hannah Benisty, Marta Benito, Aina Bernal Martínez, Edgar Bernardo, Elvan Boke, Elisabetta Broglio, Sílvia Carbonell Sala, Carlo Carolis, Eloi Casals, Ludovica Ciampi, Olga Coll, Livia Condemi, Marta Cosín-Tomás, Pia Cosma, Mirabai Cuenca-Ardura, Natalia Dave, Montse Diaz, Juan Manuel Duran Serrano, María Isabel Espejo Díaz, Imma Falero, Rute Fernandes, Marcos Fernandez Callejo, Ana Belen Fernandez Llorente, Guillaume Filion, Antoni Gañez-Zapater, Ximena Garate, Raquel Garcia-Castellanos, Romina Garrido, Fátima Gebauer, Ritobrata Ghose, Juan Carlos Gomez Escobar, Julia Grawenhoff, Thomas Guegan, Jochen Hecht, Gil Henkin, Toni Hermoso Pulido, Xavier Hernandez-Alias, Jorge Herrero Vicente, Matthew Ingham, Jonas Juan Mateu, Isabel Jurado, Damjana Kastelic, Jonas Krebs, Alejandra Laguillo Diego, Claire Lastrucci, Wei Ming Lim, Sílvia Llonch, Andrew Macrae, Elena Marmesat Bertoli, Elena Martín Rodríguez, Rocco Mazzolini, Neus Mestre Farràs, Christel Michel, Irene Miguel-Escalada, Belén Miñana, Ivano Mocavini, Magda Monfort, Ariadna Montero-Blay, Cristina Navarrete Hernández, Maria Victoria Neguembor, Róisín-Ana Ní Chárthaigh, Natalia Pardo-Lorente, Laura Pascual-Reguant, Sílvia Pérez-Lluch, Reyes Perza, Martina Pesaresi, Daniel Pico Amador, Paula Pifarre, Davide Piscia, Marcos Plana Carmona, Julia Ponomarenko, Matja Popovic, Anna Puig, Leandro Radusky, Anna Ribó Rubio, Ezequiel Rivero, Natalia Rodrigo, Malgorzata Rogalska, Jacopo Scrofani, Sandrine Schwartz, Anna Sillero, Silvia Speroni, Nicholas Stroustrup, Chelsea Szu Tu, Guillem Torcal Garcia, Eduard Valera Zorita, José Wojnacki, Ivan Zadra, Roser Zaurin Quer
List of volunteers at the PCB node: CNAG – Paola Pisacane, Laetitia Casano, Lidia Sevilla Fortes, Patricia Lorden Rodriguez, Ana Gonzalez, Nuria Aventin, Domenica Marchese, Maite Rico, Sara Ruiz Gil, Maria Gallo, Angèle Bénard, Marta López Parra, Ginevra Caratu, Javier Gutiérrez Cuesta, Julie Blanc, Marta Gut, Katia Kahlem, Matthew Ingham*, Eloi Casals*, Elena Marmesat*, Davide Piscia*, Daniel Pico Amador*, Alberto Corvó*, Marcos Fernández Callejo*, Sergi Beltran* (*participation at the PRBB node as well); IRB – Victor Gonzalez, Anna Pijuan, Núria Villegas, Uxue Urdiroz, Victor Alcalde, Valentina Ramponi, Saska Ivanova, Vanessa Lopez, Berta Duran, Clara Morral, Marta Lovera, Clara Borras, Isabel Calvo, Laura Novellasdemunt, Ester Saus, Eduardo Pauls, Paloma Solá, Adrian Gabriel Torres, Cian Lynch, Joana Fort i Baixeras, Pasquale Pellegrini, Thomas Mortimer, Noelia Alcazar, Adria Nicolas, Blazej Baginski, Juan Felipe Slebe, Marc Furriols, Monica Torras, Nicolas Martin, Panagiotis Giannios, Lorena Gonzalez, Laura Gonzalez, Maria Sanchiz, Raquel Bernad, Eduard Puig, Alba Millanes, Erika Lopez, Isabelle Heath, Mariona Nadal, Israel Ramos, Claudia Arnedo, Patrick Aloy, Jesús Sánchez; IBEC – Ignasi Casanellas, Mireia Seuma, Albert Manzano, Anabel-Lise Le Roux, Sefora Conti, Adrian Lopez Canosa, Marina Pavlova, Isabela Santos Fortunato, Andrea García Lizarribar, Juanma Fernandez, Julia Rodriguez Comas, Swapnil Sanmukh, Francesco de Chiara, Nuria Camarero Palao, Gerard Rubi, Ferran Velasco, Ignasi Granero, Alba Rubio, Andrés Marco Giménez, Rafael Mestre, Marta Badia, Marc Molina, Elisabet Urrea.
Funding Statement
The ORFEU program was created by the Catalan Enterprise and Knowledge Department with the Department of Health and funded by the Government of Catalonia, who trusted the expertise of research institutes to add value to the health system during the pandemic. We also extend our thanks to the Spanish Ministry of Science and Innovation to the EMBL partnership, the Centro de Excelencia Severo Ochoa, the CERCA Programme / Generalitat de Catalunya, the Spanish Ministry of Science and Innovation through the Instituto de Salud Carlos III, the Generalitat de Catalunya through Departament de Salut and Departament d’Empresa i Coneixement, and the co-financing by the Spanish Ministry of Economy, Industry and Competitiveness (MEIC) with funds from the European Regional Development Fund (ERDF) corresponding to the 2014-2020 Smart Growth Operating Program. We acknowledge support of the Spanish Ministry of Science and Innovation through the Instituto de Salud Carlos III, to the EMBL partnership and to the Co-financing with funds from the European Regional Development Fund corresponding to the Programa Operativo FEDER Plurirregional de España (POPE) 2014-2020. We acknowledge also support of the Centro de Excelencia Severo Ochoa and the Generalitat de Catalunya through the CERCA Programme, through Departament de Salut and Departament d’Empresa i Coneixement and the Co-financing with funds from the European Regional Development Fund by the Secretaria d’Universitats i Recerca corresponding to the Programa Operatiu FEDER de Catalunya 2014-2020.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 2 approved]
References
- 1. World Health Organization: Laboratory biosafety guidance related to coronavirus disease (COVID-19). Reference Source [Google Scholar]
- 2. Centers for Disease Control and Prevention: Guidance for General Laboratory Safety Practices during the COVID-19 Pandemic. Reference Source [Google Scholar]
- 3. Centre for Genomic Regulation: ORFEU PROGRAMME. Reference Source [Google Scholar]
- 4. Centre for Genomic Regulation: ORFEU PROGRAMME, SARS-CoV-2, Testing Protocol. Reference Source [Google Scholar]
- 5. Centre for Genomic Regulation: Guidelines for Open Science on COVID-19 Research. Reference Source [Google Scholar]