Abstract
Inflammatory bowel diseases (IBD) are systemic disease that manifest not only in the gut/GI tract but also in many patients in extraintestinal organs. The quality of life of IBD patients may be significantly affected by those extraintestinal manifestations (EIMs). It is important to have knowledge of the prevalence, pathophysiology and clinical presentation of EIMs in order to adapt therapeutic options to cover all aspects of IBD. EIMs may occur in up to 24% of IBD patients before the onset of intestinal symptoms and need to be recognized to initiate appropriate diagnostic procedures. EIMs most frequently affect joints, skin, or eyes, but may also affect other organs such as the liver, lung and pancreas. It is a frequent misconception that a successful therapy of the intestinal inflammation will be sufficient to treat EIMs satisfactorily in the majority of IBD patients. In general, peripheral arthritis, oral aphthous ulcers, episcleritis, or erythema nodosum may be associated with active intestinal inflammation and may improve upon standard treatment of the intestinal inflammation. On the other hand, anterior uveitis, ankylosing spondylitis (AS) and primary sclerosing cholangitis (PSC) usually occur independent of disease flares. This review provides a comprehensive overview of epidemiology, pathophysiology, clinical presentation and treatment of EIMs in IBD.
Keywords: inflammatory bowel disease, Crohn’s disease, ulcerative colitis, extra-intestinal manifestations, arthralgias, arthritis, psoriasis, spondyloarthropathy, erythema nodosum, pyoderma gangrenosum, uveitis, primary sclerosing cholangitis
Introduction
Inflammatory bowel diseases (IBDs) not only affects the gastrointestinal (GI) tract but also may involve many other organs of the body. Involvement of organs outside the GI tract are usually termed extraintestinal manifestations (EIMs) of IBD 1–3. EIMs occur with varying frequency depending on the affected organ. EIMs may occur before or after the diagnosis of IBD. They can significantly impact the quality of life (QoL) of IBD patients, sometimes more so than the intestinal disease. Frequently, EIMs require specific treatments or at least need to be considered when deciding on the treatment of the intestinal inflammation 1–3. EIMs may occur together with flares of the underlying IBD and respond to the treatment of the intestinal inflammation or they may be independent of the IBD activity.
EIMs should be differentiated from extraintestinal complications of IBD 4. Extraintestinal complications are direct or indirect sequela of intestinal inflammation. EIMs may be defined as “an inflammatory pathology in a patient with IBD that is located outside the gut and for which the pathogenesis is either dependent on extension/translocation of immune responses from the intestine, or is an independent inflammatory event perpetuated by IBD or that shares a common environmental or genetic predisposition with IBD” 5. EIMs are common in both ulcerative colitis (UC)6 and Crohn’s disease (CD).
In both CD and UC, EIMs most commonly involve the musculoskeletal system (e.g. peripheral and axial arthritis, enthesitis), skin (e.g. pyoderma gangrenosum, erythema nodosum, Sweet syndrome, and aphthous stomatitis), hepatobiliary tract (primary sclerosing cholangitis) and eyes (episcleritis, anterior uveitis, and iritis) (Table 1). However, almost any organ can be affected. These organ manifestations may not be clinically obvious nor easy to detect. For example, an acute or chronic pancreatitis associated with IBD (and not with IBD medication such as azathioprine) is rare 7, 8. However, asymptomatic exocrine insufficiency, pancreatic duct abnormalities and hyperamylasaemia are seen in up to 18% of IBD patients 8 and antibodies against exocrine pancreatic tissue (PAbs) can be found in up to 29% of patients with CD but not in UC 9. Other conditions like pneumonitis or PSC may persist in patients with UC, even after proctocolectomy.
Table 1.
Extra-Intestinal Manifestations of Inflammatory Bowel Diseases
| Organ System | Manifestations | Prevalence |
|---|---|---|
| Gastrointestinal | Primary sclerosing cholangitis Autoimmune pancreatitis Autoimmune hepatitis |
UC: up to 5%; CD: rare rare rare (< 1%) |
| Mucocutaneous | Erythema nodosum Pyoderma gangrenosum Oral aphthous ulcers Sweet’s syndrome Orofacial granulomatosis |
5–15% in CD; 2–10% in UC 0.4 – 2.6% in IBD 5–50% in CD rare rare |
| Musculoskeletal | IBD-related arthritis peripheral arthritis axial arthritis enthesitis |
CD: 10–20% ; UC: 4–14% Up to 50% in CD (asymptomatic) |
| Ocular | Episcleritis and scleritis Anterior Uveitis |
Scleritis: up to 1%; CD 5–12%; UC 3.5–4.1% |
| Pulmonary | Pneumonitis | rare |
| Vascular | Cardiovascular disease Thromboembolism Portal vein thrombosis |
n.a. 3–4 fold increase rare |
EIMs in IBD represent a challenge for the treating healthcare providers. Multidisciplinary integrated management plans in IBD practices can improve outcomes as well as quality of life 6.
Epidemiology, frequency and chronology of EIMs in IBD
The prevalence and incidence of EIMs is dependent on the types of EIMs included in definitions as outlined above. More stringent definitions of EIMs, as suggested by the European Crohn’s and Colitis Organization’s (ECCO) working group on EIM will result in lower estimates of prevalence: “An inflammatory pathology in a patient with IBD that is located outside the gut and for which the pathogenesis is either dependent on extension/translocation of immune responses from the intestine, or is an independent inflammatory event perpetuated by IBD or that shares a common environmental or genetic predisposition with IBD” 5. Given this potential for variability it is perhaps not surprising that EIMs in IBD have been reported with frequencies ranging from 6% up to 47% 10 (Figure 1a). Further complicating assessment of prevalence is the fact that patients may be affected by more than one EIM. The Swiss IBD Cohort study (SIBDCS), a large Swiss based IBD cohort study with a focus on extraintestinal manifestations reported that up to 25% of EIM-affected IBD patients suffer from several EIMs (up to five) 11. In this cohort 29% of IBD patients were diagnosed with EIM, of those 63% presented with 1 EIM, 26% with 2, 5% with 3, 2% with 4, and 3% with 5 EIMs during the observation period 11 (Figure 1b).
Figure 1:

Epidemiology of extraintestinal manifestations in IBD patients
A) Dependent on the definition the prevalence of EIMs is reported to be between 6% and 47% of all patients. B) Patients may be affected by more than one EIM. More than 20% of all IBD patients report 2 EIMs. More than 10% of patients report 3 or more different EIMs. C) EIMs may occur before or after the diagnosis of IBD:; 26% of all patients with EIMs report occurrence of EIMs up to 25 months (median 5 months) before IBD diagnosis.
EIMs may be more frequent in early onset IBD and in younger patients12, however, this has not been found in all studies. Grossman and DeBenedetti reported EIMs in up to 68% of pediatric patients with IBD 13 and Stawarski et al. reported that 50% of patients with UC and 80% with CD had EIM14., In contrast, the SIBDCS group reported a 16.7% (55/329) prevalence of EIMs in pediatric patients with IBD 15. With a stringent definition of EIMs and analysis of 481 pediatric onset CD patients and 386 pediatric onset UC patients, only a trend towards higher rates of stomatitis in CD and of PSC and AS in UC was reported16. In addition, orofacial granulomatosis is mainly seen in male children or teens (male:female ratio at least 2:1) suffering from CD 17–20.
EIMs may present clinically either before or after the onset (or diagnosis) of IBD. Up to 26% of cases may suffer from their first EIM before IBD is diagnosed (median time 5 mo before IBD diagnosis) and in 74% of cases, the first EIM manifested after IBD diagnosis (median: 92 mo) 11 (Figure 1c). Before IBD diagnosis was made, peripheral arthritis was diagnosed in 19.7% of patients with EIMs, axial arthropathy/ankylosing spondylitis in 39.1%, aphthous stomatitis in 27.8% of patients, uveitis in 52.2%, erythema nodosum in 14.3% pyoderma gangrenosum in 14.3% and PSC in 23.8% of patients.11
Pathophysiology of EIMs in IBD
It has been assumed that the factors relevant for the pathogenesis of EIMs are similar or the same as for the intestinal inflammation 5. Genetic risk factors seem to play a role, as several are shared between IBD and various EIMs. Furthermore, environmental factors appear to play a role. The innate and adaptive immune system certainly plays an important role in the initiation and perpetuation of the organ inflammation. In addition, the interaction with components of the microbiota may be of importance.
Genetic risk factors
The contribution of the genetic risk on the pathogenesis of EIMs is illustrated by association studies that describe a concordance for EIMs in 70% of parent–child pairs and 84% of sibling pairs 21. In addition, there is a significant overlap between genetic risk loci for EIMs and IBD 5, 22. The first risk variant identified in CD patients, NOD2/CARD15, has also been associated with sacroiliitis and uveitis 23, 24 (Figure 2). Weizman and coworkers investigated skin EIMs and found associations between pyoderma gangrenosum and known IBD loci such as IL8RA, PRDM1, USP15 and TIMP3 25 (Figure 2). For erythema nodosum they found significant genetic associations with other IBD susceptibility variants including PTGER4, ITGAL, SOCS5, CD207, ITGB3 as well as rs6828740 (4q26)25 (Figure 2). In PSC patients, IBD risk variants also have been identified, including UBASH3A, BCL2L11, FOXO1, IRF8 as well as SOCS1, JAK2, STAT3, and TYK2 23.
Figure 2:
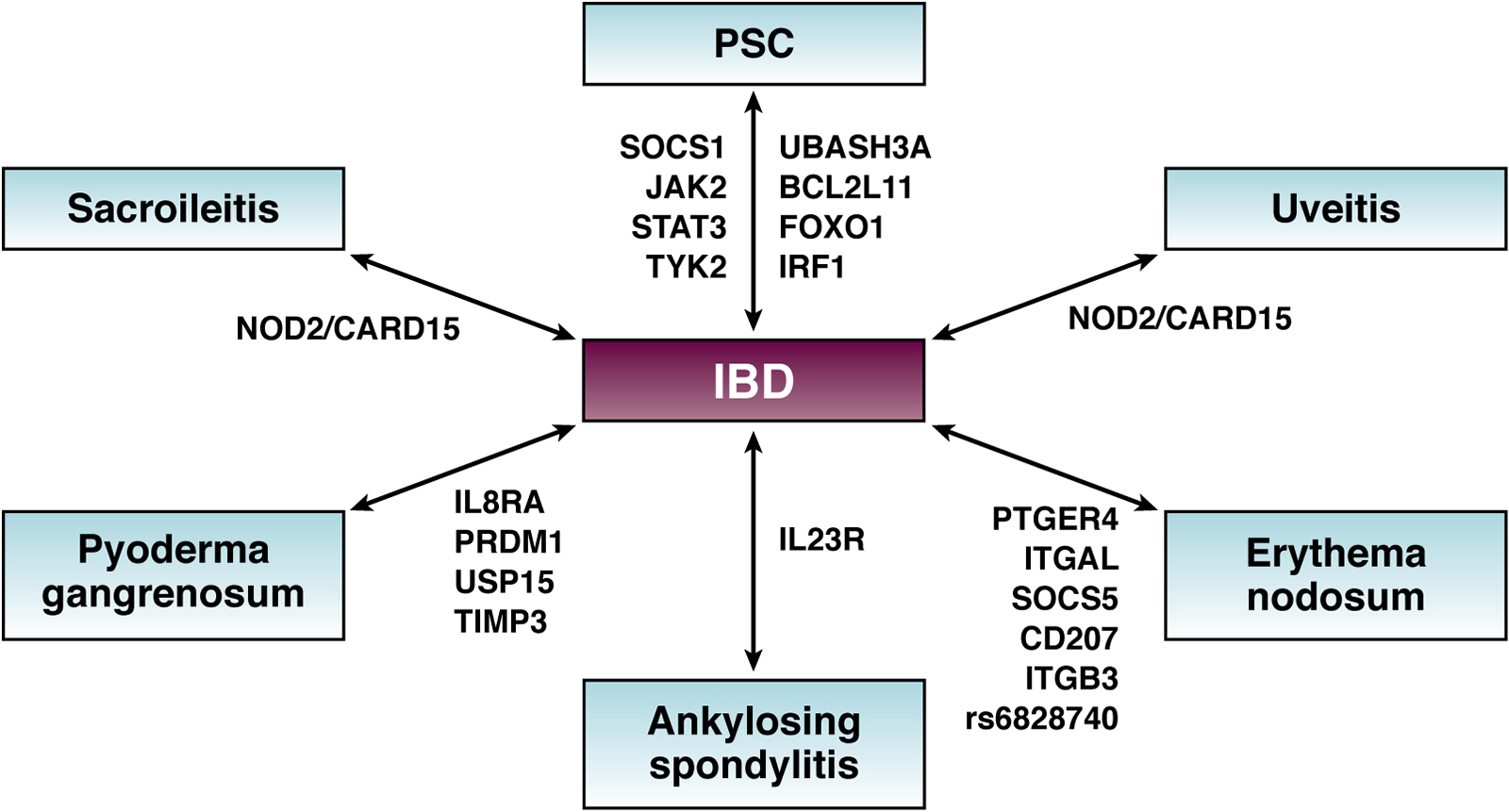
Shared genetic risk factors between IBD and extraintestinal manifestations
The Arg381Gln variant of IL23R is protective against development of CD, with reduced risk by 3-fold. Interestingly, the same polymorphism has also been shown to be protective against AS26. In a recent study applying full genome sequencing to detect monogenic IBD in a pediatric cohort, the odds ratio (OR) for EIMs in monogenic IBD patients was 15.36 (p < .0001), corresponding to a prevalence of 76% in CD patients and 42% in UC patients27. This association further supports the pathogenetic role of genetic factors for the incidence and prevalence of EIMs while suggesting that patients with very early onset of IBD and co-existing EIMs should be screened by full genome sequencing. The results of such genome screening may identify monogenic disease and the possibility of cure is suggested to be achieved by stem cell transplantation 27.
Musculoskeletal EIMs are associated with HLA-A2, HLA-DR1, and HLA-DQw5 alleles in CD patients and to DRB1*0103, B27, and B58 alleles in UC patients 28, 29. Twenty-five percent to 78% of patients with IBD and AS are HLA-B27 positive. Up to 60% of patients with AS have asymptomatic gut inflammation, 25% of them may develop overt IBD over time. Germ-free HLA-B27 transgenic rats do not develop gut or joint inflammation, suggesting that bacterial exposure is a prerequisite for the development of SpA in genetically predisposed IBD patients 30.
Environmental factors
Patients with CD who smoke are more likely to present with EIMs as compared to non-smokers 31. Whether this is mediated by a cigarette smoke induced modification of the intestinal microbiota (see below) remains to be answered.32, Biedermann, 2014 #2991,Biedermann, 2014 #2991, 33, 33a Smoking is associated with 10% higher incidence of skin and joint EIMs34. EIMs may be more prevalent with higher exposure to smoking34. Smoking cessation appears to have a positive effect on the prevalence of EIMs34. It is important to note that smoking also has been identified as an important environmental factor in the pathogenesis and for the severity of luminal and perianal CD. Interestingly, smokers are protected from the onset of UC and ex-smoking is a known risk factor for the development of UC 35, 36.
Activation of the immune system
It has been hypothesized that EIMs may arise from cross-reactivity of antigen-specific immune responses against intestinal antigens at non-intestinal sites5. Shared peptide sequences between enteric bacteria and host MHC molecules have been reported37. Whether this truly can contribute to EIMs has not been demonstrated unequivocally and the antigen specificity of potential T-cell clones mediating or causing EIMs in humans has not been defined5. Inflammatory T-cells are recruited into the intestinal wall via the interaction of α4β7 integrin (the target of respective therapeutic antibodies such as vedolizumab) with mucosal addressin cell adhesion molecule 1 (MAdCAM-1). Ectopic expression of MAdCAM-1 has been reported in the liver 38, 39, however, this is not the case in other organs affected by EIMs.
Role of the microbiota
Several pathways by which the microbiota could contribute to EIMs have been discussed in the past5. A molecular similarity between gut microbiota antigens and non-microbial epitopes present on cells in the organs affected by EIMs is seen as a potential reason for a cross reactivity of T-cell clones and immune cross-reactivity. This has never been clearly supported by evidence. Due to the leaky intestinal barrier, microbiota components such as lipopolysaccharides, bacterial antigens, or metabolites could be translocated from the gut to the extraintestinal site or may cause systemic inflammatory responses5. A dysbiosis could lead to an activation of intestinal immune cell populations that finally migrate to other organs. Preliminary evidence indicates increased abundance of Clostridiaceae in patients with IBD and arthritis40, however, this association was relatively weak. Further evidence mainly comes from studies on “dysbiosis” and “microbiota diversity”: Patients with spondyloarthritis (SpA) were reported to have decreased faecal gut microbial diversity (with increased abundance of Ruminococcus gnavus and the genus Dialister)41, 42, however, these patients did not suffer from IBD.
Musculoskeletal EIMs
Clinical characteristics and epidemiology
Musculoskeletal EIMs represent the most common EIMs in IBD, affecting up to 46% of IBD patients. The prevalence of these EIMs has been reported to range from 6% to 46% of patients, depending on the clinical and/or skeletal radiological criteria utilized. Geographical area may also contribute to the large heterogeneity in prevalence and descriptions, further complicated by a lack of specificity in clinical trial indices of joint pain (arthralgias) and joint inflammation (arthritis). The prevalence of arthritis in IBD may decrease with increasing age; it has been reported that the prevalence of musculoskeletal EIMs in the 20–30 year age group was nearly 25%, whereas in the age group of 50–60 years it was 2% 43.
From a rheumatologic standpoint, musculoskeletal EIMs of IBD are classified within the spondyloarthritis (SpA) family of conditions: in addition to IBD-related arthritis, this includes psoriatic arthritis (PsA), AS (also called axial spondyloarthritis or axSpA), enthesitis related arthritis (ERA; a type of juvenile idiopathic arthritis), reactive arthritis (sometimes referred to in the past as Reiter’s syndrome) and idiopathic acute anterior uveitis (iritis) (Table 2a). IBD arthritis may affect both the peripheral skeleton and the axial skeleton. For example, synovitis of the hands and feet may occur, with features similar to many patients with PsA. Axial involvement, including sacroiliitis, can occur with features typical of AS. Enthesitis (inflammation of the insertion of tendons, ligaments and joint capsule into bone), an important domain of SpA conditions, can affect the peripheral and axial skeleton. In addition to pain related to inflammation of synovium and entheses, pain among SpA patients can also derive from other conditions, such as fibromyalgia and osteoporosis with related fractures. Peripheral arthritis is sometimes further categorized as oligoarticular (4 or fewer joints involved) or polyarticular (more than 4 joints). Although these were sometimes considered distinct entities in the past, it appears that this relates more to duration and progression, with most polyarticular SpA patients (which is more often associated with poorer outcomes than oligoarticular) beginning their clinical course with oligoarticular involvement. Conditions within the SpA family are typically seronegative for rheumatoid factor (RF); hence the older appellation of “seronegative spondyloarthropathy”. It is worth noting however, that the actual prevalence of a positive RF test is higher among SpA patients (~ 15% or more) than the general population (5% positive, by definition). Also, tests for anti-citrullinated peptide antibodies (ACPA), are mostly negative but are positive more often than in the general population (8–12% vs 5%). In addition, distinct from rheumatoid arthritis, SpA arthritis is less commonly deforming, and less often associated with erosive changes on radiographs. However, erosive disease affecting the hips, elbows, metacarpophalangeal joints, and metatarsophalangeal joints have all been described.
Table 2:
Musculoskeletal Conditions Associated with Inflammatory Bowel Disease
Classification of arthritis associated with IBD
| Type of Arthritis | Characteristics | Prevalence (%) |
|---|---|---|
| Arthralgia without arthritis | Joint pain without synovitis | 5–16 |
| Axial Arthropathy | ||
| Ankylosing spondylitis | Inflammatory back pain with imaging evidence of sacroiliitis (SI) and/or spinal inflammation | 1–12 |
| Isolated sacroiliitis | Sacroiliac joint erosions or sclerosis on imaging | 16–46 |
| Inflammatory back pain | Back stiffness without radiologic findings | 17–22 |
| Dactylitis | Swelling of entire digit | 2–5 |
| Enthesitis | Tendon/ligament/joint capsule insertion pain | 6–54 |
Peripheral arthritis (pSpA)
Peripheral joint involvement occurs in 5–14% of UC patients, and 10–20% of CD patients (Table 2b). The diagnosis of peripheral SpA is mainly clinical, based on the evidence of objective inflammation in peripheral joints/entheses. Musculoskeletal ultrasound and MRI may support the diagnosis, showing typical signs of arthritis, enthesitis, tenosynovitis and bursitis. There is no reliable laboratory test that can be used as a diagnostic or activity index of IBD-related arthritis. A normal sedimentation rate does not exclude active disease, nor does a high level confirm. Serological diagnostic tests (rheumatoid factor, anti-cyclic citrullinated peptide) are generally negative, but a positive result by no means excludes these diagnoses.
A classic study published in 1998 that excluded patients with axial involvement, described two main patterns of peripheral arthritis. Type 1 arthropathy is the classic form, characterized by oligoarticular asymmetric arthritis affecting less than five joints, involving preferentially large joints (ankles, knees, hips, wrists, elbows and shoulders). This arthropathy usually involves acute self-limiting attacks of less than 10 weeks duration, is strongly associated with extraintestinal manifestations of IBD such as erythema nodosum and uveitis and it is often associated with active IBD. Type 2 arthropathy is characterized by polyarticular involvement, 5 or more joints, is symmetric mainly affecting small joints of both hands with pain, swelling or effusion that usually persist for months or years. This type of arthritis is largely independent of IBD activity. It is commonly associated with uveitis but not with other extraintestinal manifestations of IBD. However, more currently, these different types may be considered more of a continuum. Early on, patients tend to have less joint involvement; while some remain oligoarticular, some develop into the polyarticular pattern. This was seen in a more recent study that examined the prevalence of musculoskeletal EIMs among patients in an IBD clinic. In one study of 350 IBD patients (206 Crohn’s, 138 UC), 129 (37%) patients had one or more musculoskeletal EIM. Interestingly, it was relatively evenly split with 23% of patients having axial involvement, and 24% having peripheral involvement. There was a similar prevalence of these EIM among CD and UC 44.
As noted, enthesitis, tenosynovitis and dactylitis commonly occur. Several studies report a prevalence of enthesitis in adult IBD patients ranging from 7% to 50%. Chronic enthesitis can lead to functional disability and structural changes, including osteopenia, bone cortex irregularities and erosions, soft tissue calcifications, and abnormal new bone formation 45. Enthesitis is often missed on clinical exam, can be detectable at an earlier stage with ultrasound of the affected area.
Axial arthritis/Spondyloarthropathy (axSpA)
IBD-related spondyloarthropathy may cause a variety of symptoms due to axial involvement seen in active sacroiliitis or spondylitis. axSpA can occur concomitantly with peripheral involvement including synovitis, dactylitis, and enthesopathy such as Achilles tendinitis, plantar fasciitis, and chest wall pain 43, 46.) Whereas idiopathic AS is associated with HLA-B27 in over 90% of cases, the strength of the HLA-B27 association in spondylitis complicating IBDs is less (~50–70%); this is true for spondylitis associated with other SpA conditions, such as PsA and reactive arthritis. AS is characterized by persistent inflammatory low back pain and its clinical diagnosis is supported by MRI. In advanced cases, marginal vertebral bodies, syndesmophytes and bony proliferation aspects with axial ankylosis are observed on standard radiograph. In many patients with AS it may take years from the onset of inflammatory back pain to the development of radiographic sacroiliitis. An anteroposterior radiograph of the pelvis should be considered in IBD patients with back pain to evaluate for sacroiliitis. MRI using the short-tau inversion recovery (STIR) technique is an excellent tool to demonstrate sacroiliitis and enthesitis, it may show inflammation, bone marrow edema and bony erosions that are still not detectable by conventional radiographs. Interestingly, similar information can often be gleaned from MRE examinations of such patients; it would be worthwhile to alert the radiologist interpreting the MRE to review the bones and joints for such complications.
IBD-associated sacroiliitis is usually bilateral. It can be asymptomatic or symptomatic. Asymptomatic sacroiliitis is seen in up to 50% of CD patients on imaging. Symptomatic sacroiliitis is characterized by low back/buttock pain after rest, improves with activity. The prevalence of clinical SI was estimated to be 8%. Concomitant axial and peripheral joints disease can occur in 3–6% of patients.
Treatment of IBD Related Arthritis / Enteropathic Arthritis associated with IBD
Much of the higher quality data relevant to treating musculoskeletal EIMs of IBD come from studies in other SpA: thus, treatment of peripheral arthritis and peripheral enthesitis has been best studied in PsA, while the treatment of axial arthritis has been best studied in AS. Extrapolation of data from such studies to patients with involvement in those same domains is reasonable, in the absence of strong controlled data specifically in IBD arthritis.
Of note, with the testing and introduction of diverse immunomodulatory targeted therapies across various systemic inflammatory autoimmune and autoinflammatory diseases, much has been learned about the immunopathophysiology of the diseases themselves. For example, while inhibitors of IL-17 have been highly effective for skin psoriasis and for peripheral arthritis and enthesitis in PsA and also for axial arthritis in AS, they have been ineffective in IBD. Optimal treatment of IBD related arthritis therefore requires consideration of activity of disease across the different domains.
Management of bowel inflammation is an important therapeutic target because this may also induce remission or reduction of activity for musculoskeletal manifestations. However, in a sizable proportion of patients, more often those with polyarticular diseases, despite the amelioration or disappearance of gut inflammation, the joint disease persists. In these cases, the preferred therapies are those that are potentially effective for both diseases
Most patients with SpA will respond clinically to nonsteroidal anti-inflammatory drugs (NSAIDs), with reduced pain and improved function. The use of NSAIDs however, is controversial in IBD as they have been suggested to be associated with the development of ulcerations in the small and large intestine and flares of IBD 47. COX-2 inhibitors have been shown capable of being used safely in UC patients with quiescent disease for up to 2 weeks 48, 49. Therefore, short-term use may be acceptable for relief of symptoms or clarification of diagnoses, provided that care is taken to monitor the bowel inflammation simultaneously (Table 2b).
Corticosteroids may be helpful for peripheral arthritis but are often ineffective in controlling axial pain and enthesitis. Long-term use of steroids for arthritis should be limited due to risk of steroid related side effects, particularly osteoporosis and bone fractures. Local steroid injections may be effective and usually well tolerated in mono/oligoarthritis.
Sulfasalazine has generally demonstrated efficacy at improving peripheral arthritis in SpA patients, but not axial arthritis/back pain (Table 2b). Sulfasalazine can be considered as a low-cost treatment option for patients with UC and peripheral arthritis. Some other DMARDs including thiopurines are not effective for the treatment of articular symptoms. There are no data to support the use of hydroxychloroquine for this condition. Leflunomide has been used with some benefit for peripheral arthritis but is ineffective for axial disease 50.
Methotrexate has been used as an effective treatment in CD with concomitant peripheral arthritis, although definite data are lacking. Its use requires close monitoring for potential hepatic toxicity and risk of teratogenicity. Methotrexate is not effective in the treatment of axial SpA 51. If not effective despite 12 weeks of continuing treatment, adjustment of the treatment plan, for example adding anti-TNF therapy should be considered. Both infliximab and adalimumab have proven effective in the management of IBD-arthropathy, including axial disease. Administration of etanercept in IBD patients should be avoided, given the poor efficacy for bowel inflammation 52.
Additional Therapeutic Considerations
Ustekinumab is a monoclonal antibody against the p40 subunit of IL-12 and IL-23 approved for the treatment of moderately to severely active CD and UC. In a recent systematic review, evidence was provided that Ustekinumab may be effective for IBD associated peripheral arthritis (as well as skin EIMs) but not in axial spondyloarthritis53. Tofacitinib is an oral small molecule which inhibits mainly JAK1 and JAK3 and is available and approved for the treatment of moderately to severely active UC as well as RA and PsA. Phase 3 trials are under way to establish its safety and efficacy in AS. Filgotinib, a selective JAK1 inhibitor, has demonstrated efficacy in patients with CD54; however, there are not yet data on its use for the treatment of rheumatologic manifestations. Upadacitinib is also a selective JAK1 inhibitor which is already approved in the US for the treatment of moderate to severe RA and has demonstrated efficacy in both CD and UC 55, 56. Secukinumab, a monoclonal antibody against the IL-17, demonstrated to be effective for the treatment of AS, but not in patients with CD57, in which there have been cases of de novo IBD reported, or worsening of existing IBD58. Therefore, its use in IBD is not recommended at the current time.
Surgical removal of the diseased part of the colon or total proctocolectomy for UC usually induces remission of peripheral arthritis but has no influence on axial involvement. In CD, although colonic disease increases the likelihood of peripheral arthritis, surgical removal of the diseased part does not appear to affect the course of the arthritis59. Vedolizumab which specifically block leukocyte trafficking into the inflamed mucosa seems not to be effective for most EIMs (which was expected from due to the mechanism of action)60.
Skin EIMs in IBD
Cutaneous EIMs have been reported in 5 – 15% of IBD patients 61. Erythema nodosum (EN) and pyoderma gangrenosum (PG) are the most frequent skin EIMs in IBD patients. In a large cohort of 2402 patients 5.8% had at least 1 skin manifestation 61, 4% with EN and 0.75% had PG.
Erythema nodosum
EN is clinically characterized by tender, red (or violet), raised, subcutaneous nodules of 1–5 cm in diameter. EN typically appears on the extensor surfaces of the lower extremities, most often in the anterior tibial area, however, it may also be localized on the thighs and the forearm (in up to 15% of female patients for both localizations) 2. Skin biopsies are not necessary. The prevalence of EN in IBD patients is reported to range from 5 to 15% of patients with CD and 2 to 10% of patients with UC 2. In the SIBDCS, EN was reported in 6.8% of patients with inactive CD and 2.4% of patients with active CD 62, which is in contrast to the frequently described statement that EN is associated with active CD. In UC patients, EN was found in 2% of patients with inactive disease and 4.7% of patients with active inflammation 62. This suggests that the common recommendation to treat the intestinal inflammation may not be sufficient. A female preponderance has been reported, which is also seen in non-IBD associated EN 63. In pediatric patients it is seen less frequently. EN usually heals without scars. If treatment of the intestinal inflammation is not sufficient, corticosteroids (initially 40–60mg/daily and subsequent tapering) or anti-TNF antibodies have excellent efficacy.
Pyoderma gangrenosum
Pyoderma Gangrenosum (PG) frequently begins as an erythematous pustule or nodule rapidly developing in sometimes deep ulcers irregular violaceous edges and purulent material in the ulcer ground which is sterile on culture 63. In a systematic review of 14 studies, prevalence of PG in IBD patients ranged from 0.4 to 2.6%64. PG is found mainly on the legs but may also occur in 4–8% on the head and neck and in 4–5% on the trunk 2 (Figure 4). The ulcers can be solitary or multiple, unilateral, or bilateral, and can range in size from several centimeters to an entire limb 2. PG is less frequently seen but often more severe and even debilitating, affecting women more frequently than men 63 (Figure 4). In the SIBDCS, PG was observed in 1.4% of patients with inactive CD and 2.4% of patients with active CD. In UC patients, PG was reported in 1.5% patients with inactive disease and 3% of patients with active disease 62. This lack of commonly reported association with intestinal disease activity was also reported by others 61. Fifty percent of patients with PG have underlying IBD. Patients with severe disease and colonic involvement are most likely to develop PG 2. Peristomal PG is seen occasionally. The diagnosis is made clinically, skin biopsy should be avoided as it usually worsens the situation. Treatment includes oral steroids (40–60 mg/day and tapering, cyclosporine (initial target blood levels of 150–300 ng/ml), tacrolimus (initial target blood levels between 10 and 15 ng/ml) or anti-TNF antibodies (infliximab and adalimumab). Topical tacrolimus is successful in the treatment of early lesion (e.g. peri-stomal pyoderma: 0.1% ointment 2 times daily)
Figure 4:
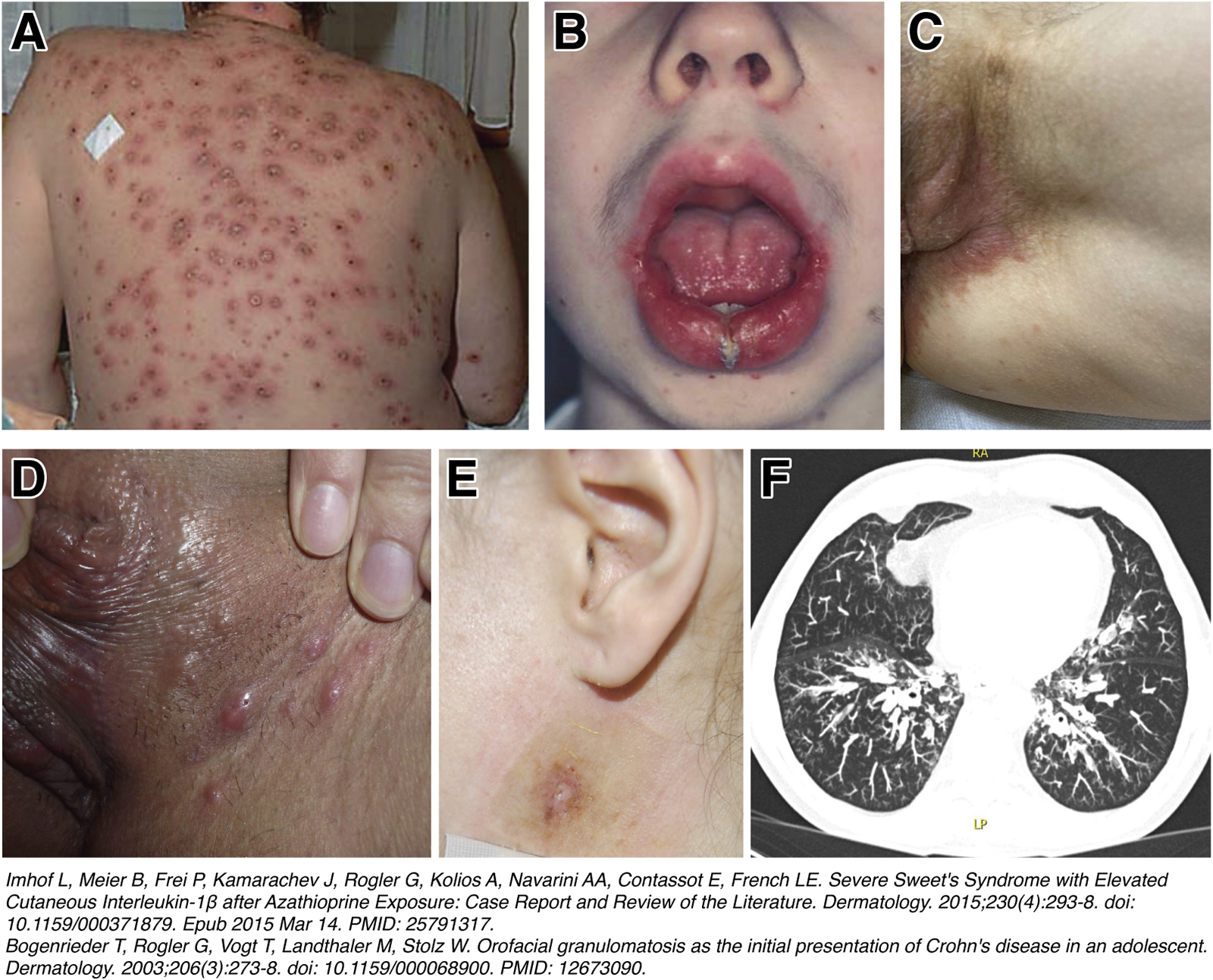
Rare extraintestinal manifestations of IBD that should be recognized: A) 50-year-old patient with indeterminate colitis, presenting with a febrile diffuse papulo-pustular and necrotizing skin eruption that healed with significant scarring and appeared 14 days after onset of treatment with azathioprine (1). B) 17-year-old male patients with CD presented with a symmetrical nontender swelling of the lower lip. The swelling had been present for the previous 5 years. The clinical and histological changes were consistent with the diagnosis of orofacial granulomatosis. Further diagnostic tests confirmed the presence of intestinal CD. (2) C) 48 year old female patient with CD and ileocecal resection presenting with “metastatic CD” of the genital skin with histologically visible granuloma. D) 34 year old female CD patient presenting with a nodular form of skin manifestation in the genital area E) Retroauricular atypical pyoderma in a 35 years of female patient with ulcerative colitis. The patient later on developed a colitis associated rectum carcinoma. F) Pulmonary involvement in a 62 year old patient with ulcerative colitis. The pulmonary changes (bronchiectasis and relapsing infection) developed rapidly after colectomy.18, 65
Sweet’s Syndrome
Sweet’s syndrome, also termed “acute febrile neutrophilic dermatosis”, is a rare cutaneous EIM in IBD patients 2, 63. The typical skin lesions (tender or papulosquamous exanthema or nodules located on the arms, legs, trunk, hands, or face) of varying size (Figure 4) are associated with malignancies, infections and less frequently, IBD. Usually Sweet’s Syndrome occurs in female patients (>80%) associated with other EIMs such as arthritis, fever, or ocular symptoms. Leukocytosis is frequently observed. Sweet’s syndrome usually parallels intestinal disease activity, so it may not be clear whether leukocytosis is caused by the skin EIM or by the underlying IBD. Sweet’s Syndrome has also been reported as a side effect of medications such as azathioprine 65, making a careful evaluation of the patient’s history mandatory (Figure 4). Sweet’s syndrome has been reported to appear before (20%), concurrently (28%), or after (52%) an IBD diagnosis is established 63, 66. Treatment recommendations include topical or systemic corticosteroids (40–60 mg/day and tapering) and immunomodulators as well as sufficient and adequate treatment of the intestinal inflammation as it frequently parallels intestinal disease activity.
“Metastatic Crohn’s”
“Metastatic” CD lesions of the skin show a histology with granulomas and may manifest anywhere on the integumentum. Genital or vulvovaginal lesions may be the most disabling severely impacting the quality of life (Figure 4). “Metastatic” CD does usually not parallel intestinal disease activity 63. Treatment include topical or systemic corticosteroids, immunomodulators, and anti-TNF agents, however, the evidence is mainly based on case reports 67–71.
Oral pathologies
It may be disputed whether oral lesions represent an EIM of IBD or just a manifestation of the disease in the first portion of the GI tract. IBD patients not only suffer from aphthous stomatitis (in CD patients) but also from periodontitis. The prevalence of oral lesions is reported in a range form 5–50% 72–74. In the pediatric patient population a prevalence of 7% to 23% has been reported74. Oral lesions are reported to be more common in CD patients, and more prevalent in children 75. In a large cohort study with evaluation by a dentist a prevalence of 10% among CD, but only 4% among UC patients was seen 62. Aphthous stomatitis presents with typical aphthous lesions similar to aphthous lesions in the ileum or colon: round or oval painful ulcers with a yellow pseudomembranous base and erythematous borders. Frequently the aphthae are in the buccal or labial mucosa. They may be treated with topical steroids and anesthetics. Most systemic treatments (e.g. corticosteroids and anti-TNF antibodies) are also successful.
Periodontitis is a chronic inflammatory condition leading to destruction of the anchoring bone and soft tissue, presenting with gingival inflammation, swelling and bleeding ultimately leading to loose teeth. With respect to gingivitis and periodontitis higher frequencies are seen in IBD patients as compared to healthy controls 76. Interestingly, this seems to be associated with differences in the oral microbiota 77. Smokers are at higher risk for periodontitis 76, 78,76, 78. Annual dentist checkups have been recommended. Aphthous stomatitis and periodontitis usually parallel intestinal disease activity and are associated with perianal disease.
A rare oral EIM of CD is the Orofacial Granulomatosis or Melkersson-Rosenthal syndrome also known as cheilitis granulomatosa (Miescher’s cheilitis) (Figure 4) 18, 19. Orofacial Granulomatosis often presents with chronic diffuse swelling of the lips or lower half of the face, oral ulceration, hyperplastic gingivitis and mucosal tags due to granulomatous inflammation of unknown causation mainly in young males in the age of 14 – 20 years. Histopathologically, a lymphedema and corium and granulomata as well as aggregates of epithelioid histiocytes are found 18, 19. Treatment includes systemic steroids (40–60 mg/day and tapering) and immunosuppression.
As mentioned, the microbiota seems to play an important pathophysiological role for oral EIMs. Besides treatment of intestinal inflammation and perianal disease topical treatments with antiseptic mouthwashes and local steroids are recommended. Anti-TNF antibodies have been reported to improve the condition 79.
Ocular EIMs in IBD
Beside joints and skin, the eye is the third major tissue type predisposed to immune-mediated EIMs. Nearly 2–7% of patients with IBD experience ocular manifestations. Episcleritis, scleritis, and anterior uveitis are the most common ocular EIMs in IBD 80. Less common ocular EIMs are retinal vasculitis, papillitis, corneal infiltrates, myositis, scleromalacia perforans, and optic neuritis 80. In pediatric patients where EIMs are more frequent in general, ocular EIMs show a higher prevalence as compared to the adult IBD population with uveitis being the most common 81. Patients with CD have a higher risk to suffer from ocular EIMs as compared to UC (OR = 2.70) 81,80. Ocular EIMs are often associated with skin and joint EIMs80.
Episcleritis and Scleritis
Episcleritis is an inflammation of the episclera, the tissue that covers the sclera. It is the most common ocular manifestation and causes moderate discomfort 82. It is associated with active IBD and flares and can be improved by treatment of the underlying disease. Scleritis is rarer than episcleritis occurring in less than 1% of cases82. Scleritis may ultimately progress to permanent visual loss and should not be missed. It can be classified as anterior (diffuse, nodular, or necrotizing, with or without inflammation) and posterior 83. Sufficient treatment of intestinal inflammation is key. Topical NSAIDs appear to be ineffective whereas topical corticosteroids lead to rapid improvement but may have side effects such as elevated intraocular pressure and cataract formation, especially with prolonged use. Scleritis requires a more aggressive treatment. As NSAIDS are relatively contra-indicted in IBD patients, COX2 inhibitors are preferred. Addition of corticoisteroids (1 to 1.5 mg/kg/day and tapering) may be necessary early. Immunosuppressive therapy will be necessary in patients with severe scleritis who do not respond sufficiently to steroids. Infliximab at the standard dose of 5 mg/kg BW has proven efficacy in those patients.
Uveitis
Uveitis is an inflammation of the uveal tract, the middle layer of the eye, which includes the iris, ciliary body, and choroid 82. In IBD patients mainly anterior uveitis is described. In contrast to episcleritis and scleritis it is less associated with intestinal inflammation. Vavricka et al found an association between uveitis and CD activity, but not with UC activity. In the SIBDCS uveitis was reported in 5.2 % of CD patients with inactive disease and in 12.2% of CD patients with active intestinal inflammation 62. In contrast, in UC patients, uveitis was found in 3.5% of patients with inactive disease and 4.1% of patients with active disease62. Uveitis in IBD patients is initially treated with corticosteroid eye drops. If not successful, systemic steroids, immunosuppression or anti-TNF agents may be used.
Hepatobiliary EIMs in IBD
Primary sclerosing cholangitis (PSC)
PSC is the most important hepatobiliary EIM seen in IBD patients. In 60–80% of PSC patients an underlying IBD can be diagnosed 84. PSC is found in a prevalence of up to 5% in patients with UC and less frequently in patients with (mainly colonic) CD 85. PSC is more often found in adult IBD patients as compared to the pediatric population 85. Risk factors for the development of PSC in patients with UC are male gender, pancolitis in UC patients, nonsmoker at diagnosis and a history of appendicectomy 86. Clinical elevated alkaline phosphatase or gamma-glutamyl transferase (GGT) serum levels should trigger further evaluation. Histologically PSC is characterized by infiltration of lymphocytes in the intrahepatic and extrahepatic biliary tree followed by an inflammatory process that triggers fibrosis, which ultimately may lead to strictures of the small or large bile ducts. This may be followed in the long run by liver cirrhosis, end-stage liver disease and cholangiocarcinoma 87. Importantly, PSC is associated with a ten-fold increased risk for the development of colorectal carcinoma in IBD patients 88, 89
The pathophysiology of PSC is not well understood. The median survival time without liver transplantation for PSC patients is reported to be 10 to 12 years 90.
Patients with PSC and dominant strictures of the bile duct benefit from scheduled ECRPs and dilatation 91. No benefit for small duct PSC without dominant strictures can be expected, however, small duct PSC is associated with better outcomes and longer median survival 92. Besides endoscopic dilatation of bile duct strictures treatment options for IBD patients with PSC are limited 93. Whereas ursodeoxycholic acid is frequently prescribed in PSC patients 94 meta-analyses do not show a significant benefit for survival or other hard endpoints such as liver cirrhosis or malignancy 95, 96. Treatment of intestinal inflammation in IBD patients also will not change the course of PSC: a worsening of PSC has been described in cases of patients undergoing colectomy for UC 97 and colonic inflammation frequently gets more severe in patients undergoing liver transplantation due to IBD associated PSC, despite installed immunosuppression to prevent graft rejection 98, 99. In patients with PSC gallbladder polyps have a high malignant potential and should be treated by cholecystectomy.
Hepatitis
Besides PSC, EIMs of the liver include autoimmune hepatitis (AIH), IgG4-related cholangitis and granulomatous hepatitis. In addition, there are multiple IBD treatments that may affect the liver and cause hepatitis (e.g. thiopurines, methotrexate, anti-TNF antibodies and JAK-inhibitors). Furthermore, immunosuppression may lead to a reactivation of hepatitis B100 or cause hepatitis mediated by other viruses such as CMV, EBV and others 101–104.
The prevalence of true hepatic EIMs is reported to be low (< 1%). The discrimination from treatment side effects usually is difficult 105. Consequently, reliably data on incidence and prevalence are lacking.
Vascular EIM in IBD
Arterial EIM
Vascular EIM of IBD are discussed as a result of systemic inflammation together with endothelial dysfunction. Aortic stiffness, seen as an independent risk factor for cardiovascular (CV) disease has been reported to be increased in adults with IBD compared with matched controls in 13 single-center studies and 2 multicenter longitudinal studies, even after adjustment for known risk factors 106. In line with this, patients with IBD have an increased risk of acute myocardial infarction and heart failure 107, 108 as well as cerebrovascular insults 109, 110. Presently no specific preventive recommendations have been published; however, there is agreement that risk reduction is very important in the IBD population.
Thromboembolic events
IBD patients are at increased risk for venous thromboembolic events (VTE), including deep vein thrombosis (DVT), splanchnic VTE and lung embolism 111. The risk for VTEs in general is about threefold increased. This has led to recent discussions with respect to medications that also increase the risk for VTEs such as certain JAK inhibitors. The pathophysiology behind the increased risk for VTEs in IBD patients is not clear. Endothelial dysfunction, platelet activation and impaired fibrinolysis may be contributing factors 112. The risk of VTE complications increases with the severity of inflammation and is highest in hospitalized IBD patients with acute severe colitis 113.
Rare EIM: Pancreatitis, pneumonitis
Pancreatitis
Acute idiopathic pancreatitis 15 is a rare EIM mainly seen in CD patients 114. Whereas a prevalence of 2.2% has been reported in pediatric patients only 0.06% of adult patients presented with AP in an Israeli cohort before the diagnosis of IBD 115. AP as an EIM has to be discriminated from pancreatitis caused by IBD specific medication such as azathioprine or in rare cases 5-ASA 116. Duodenal involvement of CD may be seen in patients presenting with AP 117. Furthermore, autoimmune pancreatitis (AIP) is seen in the context of IBD. Type2 AIP is found more frequently in IBD patients than in the general population 118. Interestingly, antibodies against exocrine pancreatic tissue (PAbs) are present in up to 15–40% of CD but not in patients with UC 9). PAbs are not associated with CD disease activity, or drug therapy. 9 PAbs have not been clearly associated with an increased risk of pancreatitis 119, however with an impaired exocrine pancreatic function 120. Elevated blood amylase levels may be found in up to 17% of CD patients and 9% of UC patients. Increased lipase values are found in up to 9% CD and 7% US patients 121. High levels of serum pancreatic enzymes may be associated with extensive and severely active colonic disease 121.
Bronchopulmonary manifestations/Pneumonitis
Bronchopulmonary manifestations are rare but increasingly recognized122. There is high variability and all segments of the bronchopulmonary tract may be affected. Besides airway affections, interstitial lung disease and granulomatous lung disease have been described as EIMs of IBD. Whereas interstitial lung disease (ILD) seems to be mainly associated with UC, granulomatous lung disease has been associated to CD 122 Bronchopulmonary EIMs may occur even after colectomy in some UC patients. (Figure 4).
Other rare EIMs (that also may represent rare drug side effects) include glomerulonephritis; amyloidosis; nephrolithiasis; pericarditis/myocarditis. Discrimination of drug side effects (i.e. pancreatitis due to azathioprine of 5-ASA therapy; skin affections due to anti-TNF therapy or liung disease due to MTX therapy) and EIMs may be particularly difficult in some patients, however, needs to be attempted to optimize treatment.
Systemic EIM: Fatigue and Pain
Fatigue and pain are very frequently reported by IBD patients 123. During disease flares 50–70% of IBD patients report episodes of pain. This may be related to EIMs such as arthritis or EN or can be interpreted as an EIM by itself 124 The prevalence of pain in IBD patients is 71–89%. 123, 125. There is no obvious difference between CD and UC patients with respect to the occurrence of pain 123. The presence of other EIMs is not significantly associated with the occurrence of pain 123. For the majority of patients, pain is a longstanding problem, more than 50% of IBD patients experience pain with a duration of >5 years. The majority of patients (up to 60%) report abdominal pain followed by back pain (38%), knee pain (29%) and hip pain (26%). In the majority of patients (59%) these pain attacks have an impact on ADLs 123. For the treatment it is important to differentiate between pain as a symptom of the intestinal disease (inflammation, stricture, abscesses, fistulae), pain as a symptom of EIMs or pain as an independent EIM not related to the first two conditions. Before interpreting pain as an EIM other pain-causes such as strictures, fistulae, abscesses or joint inflammation need to be excluded.
Fatigue is reported by the majority of IBD patients especially during flares and active disease 126 but also during the course of remission 127, 128. It is reported to affect 50% of patients in clinical remission and > 80% of those with active disease 129. The pathophysiology of fatigue is unclear 130. Interleukin-6 (IL-6) appears to be involved as a decrease in circulating Il-6 levels is usually associated with an improvement in fatigue scores 131. In addition, alterations of the fecal microbiome in IBD patients suffering from fatigue have been described 132. In general, fatigue is very difficult to treat. No firm conclusion regarding the efficacy of interventions (e.g. electroacupuncture, Cognitive behavioral therapy (CBT), solution-focused therapy, adalimumab 40 mg e.o.w, Ferric maltol) could be drawn from the data analyzed 133. Treatment in a multidisciplinary team may be beneficial, including. physical therapy, pain medicine and psychiatry, cognitive behavioral therapy, however only few good studies investigated the efficacy.
Summary
EIMs in IBD patients significantly contribute to the burden of disease. Specific anti-inflammatory and symptomatic treatments and therapies in a multidisciplinary team approach are necessary to address EIMs adequately and improve the QoL of our patients. In the absence of specific therapeutic biomarkers for EIMs, considerations of co-existing extra-intestinal manifestations in patients with IBD may inform treatment selection and decisions.
Figure 3:
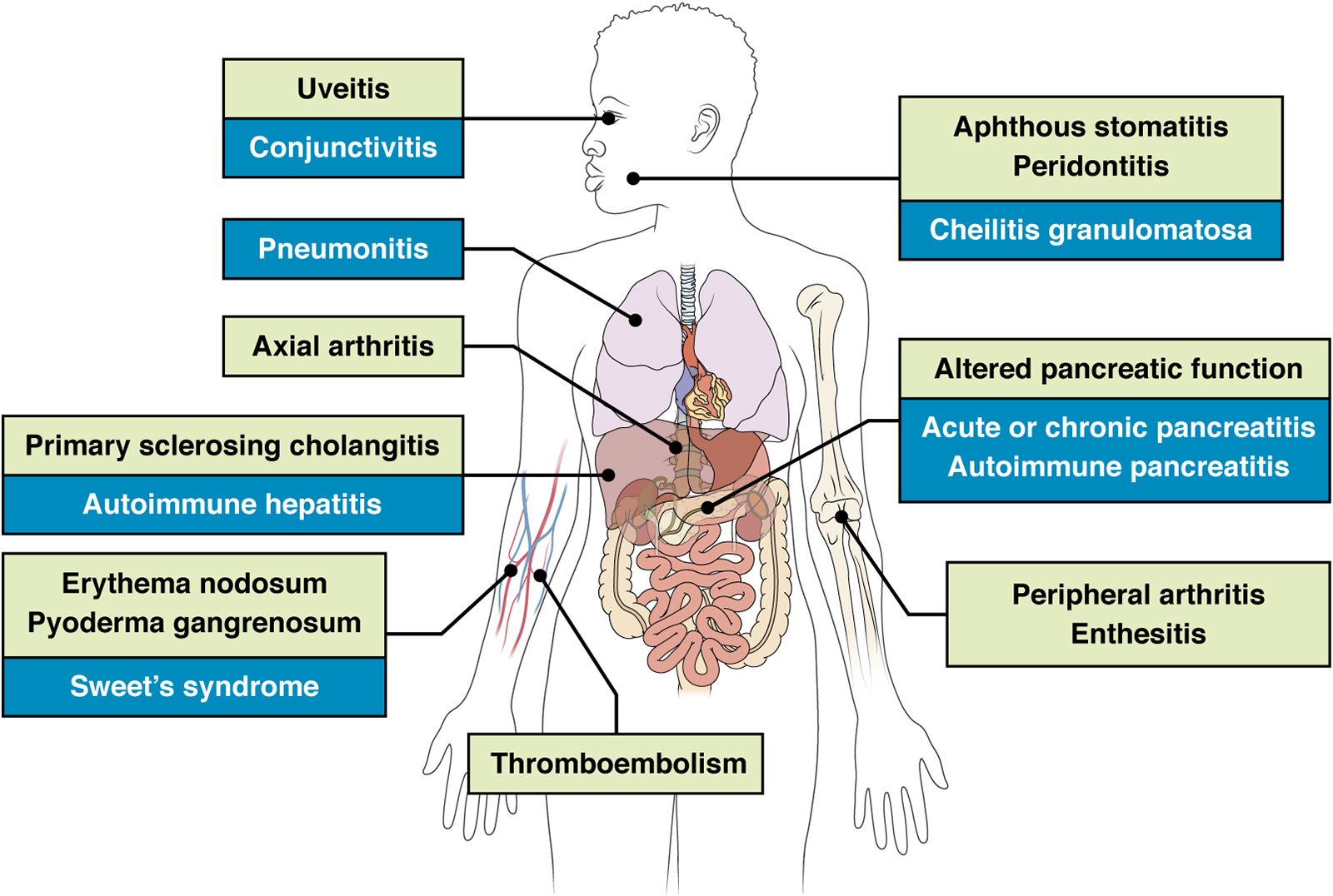
EIMS of IBD affect many organs; green = frequent EIMs; blue = rare EIMs
Table 3:
Musculoskeletal Conditions Associated With Inflammatory Bowel Disease: Therapy of Peripheral and Axial Arthritis in Inflammatory Bowel Disease
|
|
Prevalence | Diagnosis | Therapy |
|---|---|---|---|
| Peripheral arthritis (pSpA) | 5–14%i n UC 10–20% in CD |
Clinical (and US or MRI) | Treatment of intestinal inflammation COX-2 inhibitors Corticosteroids (short term) Sulfasalazine (esp. in UC) Methotrexate Anti-TNF |
| Axial arthritis/Spondyl-arthopathy (axSpA) | Up p to 50 %in CD symptomatic in up to 8% |
Clinical and MRI | anti-TNF |
References
- 1.Garber A, Regueiro M. Extraintestinal Manifestations of Inflammatory Bowel Disease: Epidemiology, Etiopathogenesis, and Management. Curr Gastroenterol Rep 2019;21:31. [DOI] [PubMed] [Google Scholar]
- 2.Vavricka SR, Schoepfer A, Scharl M, et al. Extraintestinal Manifestations of Inflammatory Bowel Disease. Inflamm Bowel Dis 2015;21:1982–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Su CG, Judge TA, Lichtenstein GR. Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol Clin North Am 2002;31:307–27. [DOI] [PubMed] [Google Scholar]
- 4.Orchard T Extraintestinal complications of inflammatory bowel disease. Curr Gastroenterol Rep 2003;5:512–7. [DOI] [PubMed] [Google Scholar]
- 5.Hedin CRH, Vavricka SR, Stagg AJ, et al. The Pathogenesis of Extraintestinal Manifestations: Implications for IBD Research, Diagnosis, and Therapy. J Crohns Colitis 2019;13:541–554. [DOI] [PubMed] [Google Scholar]
- 6.Luchetti MM, Benfaremo D, Bendia E, et al. Clinical and patient reported outcomes of the multidisciplinary management in patients with inflammatory bowel disease-associated spondyloarthritis. Eur J Intern Med 2019;64:76–84. [DOI] [PubMed] [Google Scholar]
- 7.Garcia Garcia de Paredes A, Rodriguez de Santiago E, Rodriguez-Escaja C, et al. Idiopathic acute pancreatitis in patients with inflammatory bowel disease: A multicenter cohort study. Pancreatology 2020;20:331–337. [DOI] [PubMed] [Google Scholar]
- 8.Ramos LR, Sachar DB, DiMaio CJ, et al. Inflammatory Bowel Disease and Pancreatitis: A Review. J Crohns Colitis 2016;10:95–104. [DOI] [PubMed] [Google Scholar]
- 9.Klebl FH, Bataille F, Huy C, et al. Association of antibodies to exocrine pancreas with subtypes of Crohn’s disease. Eur J Gastroenterol Hepatol 2005;17:73–7. [DOI] [PubMed] [Google Scholar]
- 10.Bernstein CN, Blanchard JF, Rawsthorne P, et al. The prevalence of extraintestinal diseases in inflammatory bowel disease: a population-based study. Am J Gastroenterol 2001;96:1116–22. [DOI] [PubMed] [Google Scholar]
- 11.Vavricka SR, Rogler G, Gantenbein C, et al. Chronological Order of Appearance of Extraintestinal Manifestations Relative to the Time of IBD Diagnosis in the Swiss Inflammatory Bowel Disease Cohort. Inflamm Bowel Dis 2015;21:1794–800. [DOI] [PubMed] [Google Scholar]
- 12.Jang HJ, Kang B, Choe BH. The difference in extraintestinal manifestations of inflammatory bowel disease for children and adults. Transl Pediatr 2019;8:4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grossman BJ, DeBenedetti CD. Extraintestinal manifestations of chronic inflammatory bowel disease in children. Proc Inst Med Chic 1970;28:119. [PubMed] [Google Scholar]
- 14.Stawarski A, Iwanczak B, Krzesiek E, et al. [Intestinal complications and extraintestinal manifestations in children with inflammatory bowel disease]. Pol Merkur Lekarski 2006;20:22–5. [PubMed] [Google Scholar]
- 15.Greuter T, Bertoldo F, Rechner R, et al. Extraintestinal Manifestations of Pediatric Inflammatory Bowel Disease: Prevalence, Presentation, and Anti-TNF Treatment. J Pediatr Gastroenterol Nutr 2017;65:200–206. [DOI] [PubMed] [Google Scholar]
- 16.Herzog D, Fournier N, Buehr P, et al. Age at disease onset of inflammatory bowel disease is associated with later extraintestinal manifestations and complications. Eur J Gastroenterol Hepatol 2018;30:598–607. [DOI] [PubMed] [Google Scholar]
- 17.Lazzerini M, Bramuzzo M, Ventura A. Association between orofacial granulomatosis and Crohn’s disease in children: systematic review. World J Gastroenterol 2014;20:7497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bogenrieder T, Rogler G, Vogt T, et al. Orofacial granulomatosis as the initial presentation of Crohn’s disease in an adolescent. Dermatology 2003;206:273–8. [DOI] [PubMed] [Google Scholar]
- 19.Girlich C, Bogenrieder T, Palitzsch KD, et al. Orofacial granulomatosis as initial manifestation of Crohn’s disease: a report of two cases. Eur J Gastroenterol Hepatol 2002;14:873–6. [DOI] [PubMed] [Google Scholar]
- 20.Khouri JM, Bohane TD, Day AS. Is orofacial granulomatosis in children a feature of Crohn’s disease? Acta Paediatr 2005;94:501–4. [DOI] [PubMed] [Google Scholar]
- 21.Satsangi J, Grootscholten C, Holt H, et al. Clinical patterns of familial inflammatory bowel disease. Gut 1996;38:738–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lees CW, Barrett JC, Parkes M, et al. New IBD genetics: common pathways with other diseases. Gut 2011;60:1739–53. [DOI] [PubMed] [Google Scholar]
- 23.Kim JM, Cheon JH. Pathogenesis and clinical perspectives of extraintestinal manifestations in inflammatory bowel diseases. Intest Res 2020;18:249–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peeters H, Vander Cruyssen B, Laukens D, et al. Radiological sacroiliitis, a hallmark of spondylitis, is linked with CARD15 gene polymorphisms in patients with Crohn’s disease. Ann Rheum Dis 2004;63:1131–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weizman A, Huang B, Berel D, et al. Clinical, serologic, and genetic factors associated with pyoderma gangrenosum and erythema nodosum in inflammatory bowel disease patients. Inflamm Bowel Dis 2014;20:525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho JH, Brant SR. Recent insights into the genetics of inflammatory bowel disease. Gastroenterology 2011;140:1704–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crowley E, Warner N, Pan J, et al. Prevalence and Clinical Features of Inflammatory Bowel Diseases Associated With Monogenic Variants, Identified by Whole-Exome Sequencing in 1000 Children at a Single Center. Gastroenterology 2020;158:2208–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheth T, Pitchumoni CS, Das KM. Musculoskeletal manifestations in inflammatory bowel disease: a revisit in search of immunopathophysiological mechanisms. J Clin Gastroenterol 2014;48:308–17. [DOI] [PubMed] [Google Scholar]
- 29.Trikudanathan G, Venkatesh PG, Navaneethan U. Diagnosis and therapeutic management of extra-intestinal manifestations of inflammatory bowel disease. Drugs 2012;72:2333–49. [DOI] [PubMed] [Google Scholar]
- 30.Arvikar SL, Fisher MC. Inflammatory bowel disease associated arthropathy. Curr Rev Musculoskelet Med 2011;4:123–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cosnes J, Carbonnel F, Carrat F, et al. Effects of current and former cigarette smoking on the clinical course of Crohn’s disease. Aliment Pharmacol Ther 1999;13:1403–11. [DOI] [PubMed] [Google Scholar]
- 32.Savin Z, Kivity S, Yonath H, et al. Smoking and the intestinal microbiome. Arch Microbiol 2018;200:677–684. [DOI] [PubMed] [Google Scholar]
- 33.Biedermann L, Zeitz J, Mwinyi J, et al. Smoking cessation induces profound changes in the composition of the intestinal microbiota in humans. PLoS One 2013;8:e59260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Severs M, van Erp SJ, van der Valk ME, et al. Smoking is Associated With Extra-intestinal Manifestations in Inflammatory Bowel Disease. J Crohns Colitis 2016;10:455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sugiyama D, Nishimura K, Tamaki K, et al. Impact of smoking as a risk factor for developing rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis 2010;69:70–81. [DOI] [PubMed] [Google Scholar]
- 36.Rubin DT, Hanauer SB. Smoking and inflammatory bowel disease. Eur J Gastroenterol Hepatol 2000;12:855–62. [DOI] [PubMed] [Google Scholar]
- 37.Scofield RH, Kurien B, Gross T, et al. HLA-B27 binding of peptide from its own sequence and similar peptides from bacteria: implications for spondyloarthropathies. Lancet 1995;345:1542–4. [DOI] [PubMed] [Google Scholar]
- 38.Grant AJ, Lalor PF, Salmi M, et al. Homing of mucosal lymphocytes to the liver in the pathogenesis of hepatic complications of inflammatory bowel disease. Lancet 2002;359:150–7. [DOI] [PubMed] [Google Scholar]
- 39.Grant AJ, Lalor PF, Hubscher SG, et al. MAdCAM-1 expressed in chronic inflammatory liver disease supports mucosal lymphocyte adhesion to hepatic endothelium (MAdCAM-1 in chronic inflammatory liver disease). Hepatology 2001;33:1065–72. [DOI] [PubMed] [Google Scholar]
- 40.Muniz Pedrogo DA, Chen J, Hillmann B, et al. An Increased Abundance of Clostridiaceae Characterizes Arthritis in Inflammatory Bowel Disease and Rheumatoid Arthritis: A Cross-sectional Study. Inflamm Bowel Dis 2019;25:902–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Breban M, Tap J, Leboime A, et al. Faecal microbiota study reveals specific dysbiosis in spondyloarthritis. Ann Rheum Dis 2017;76:1614–1622. [DOI] [PubMed] [Google Scholar]
- 42.Tito RY, Cypers H, Joossens M, et al. Brief Report: Dialister as a Microbial Marker of Disease Activity in Spondyloarthritis. Arthritis Rheumatol 2017;69:114–121. [DOI] [PubMed] [Google Scholar]
- 43.Karreman MC, Luime JJ, Hazes JMW, et al. The Prevalence and Incidence of Axial and Peripheral Spondyloarthritis in Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. J Crohns Colitis 2017;11:631–642. [DOI] [PubMed] [Google Scholar]
- 44.Stolwijk C, Pierik M, Landewe R, et al. Prevalence of self-reported spondyloarthritis features in a cohort of patients with inflammatory bowel disease. Can J Gastroenterol 2013;27:199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Horton DB, Sherry DD, Baldassano RN, et al. Enthesitis is an Extraintestinal Manifestation of Pediatric Inflammatory Bowel Disease. Ann Paediatr Rheumatol 2012;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bourikas LA, Papadakis KA. Musculoskeletal manifestations of inflammatory bowel disease. Inflamm Bowel Dis 2009;15:1915–24. [DOI] [PubMed] [Google Scholar]
- 47.Takeuchi K, Smale S, Premchand P, et al. Prevalence and mechanism of nonsteroidal anti-inflammatory drug-induced clinical relapse in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol 2006;4:196–202. [DOI] [PubMed] [Google Scholar]
- 48.Sandborn WJ, Stenson WF, Brynskov J, et al. Safety of celecoxib in patients with ulcerative colitis in remission: a randomized, placebo-controlled, pilot study. Clin Gastroenterol Hepatol 2006;4:203–11. [DOI] [PubMed] [Google Scholar]
- 49.El Miedany Y, Youssef S, Ahmed I, et al. The gastrointestinal safety and effect on disease activity of etoricoxib, a selective cox-2 inhibitor in inflammatory bowel diseases. Am J Gastroenterol 2006;101:311–7. [DOI] [PubMed] [Google Scholar]
- 50.Zochling J, van der Heijde D, Dougados M, et al. Current evidence for the management of ankylosing spondylitis: a systematic literature review for the ASAS/EULAR management recommendations in ankylosing spondylitis. Ann Rheum Dis 2006;65:423–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van der Heijde D, Ramiro S, Landewe R, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis 2017;76:978–991. [DOI] [PubMed] [Google Scholar]
- 52.Bieber A, Fawaz A, Novofastovski I, et al. Antitumor Necrosis Factor-alpha Therapy Associated with Inflammatory Bowel Disease: Three Cases and a Systematic Literature Review. J Rheumatol 2017;44:1088–1095. [DOI] [PubMed] [Google Scholar]
- 53.Guillo L, D’Amico F, Danese S, et al. Ustekinumab for Extra-intestinal Manifestations of Inflammatory Bowel Disease: A Systematic Literature Review. J Crohns Colitis 2021;15:1236–1243. [DOI] [PubMed] [Google Scholar]
- 54.Vermeire S, Schreiber S, Petryka R, et al. Clinical remission in patients with moderate-to-severe Crohn’s disease treated with filgotinib (the FITZROY study): results from a phase 2, double-blind, randomised, placebo-controlled trial. Lancet 2017;389:266–275. [DOI] [PubMed] [Google Scholar]
- 55.Sandborn WJ, Ghosh S, Panes J, et al. Efficacy of Upadacitinib in a Randomized Trial of Patients With Active Ulcerative Colitis. Gastroenterology 2020;158:2139–2149 e14. [DOI] [PubMed] [Google Scholar]
- 56.Sandborn WJ, Feagan BG, Loftus EV Jr., et al. Efficacy and Safety of Upadacitinib in a Randomized Trial of Patients With Crohn’s Disease. Gastroenterology 2020;158:2123–2138 e8. [DOI] [PubMed] [Google Scholar]
- 57.Baeten D, Sieper J, Braun J, et al. Secukinumab, an Interleukin-17A Inhibitor, in Ankylosing Spondylitis. N Engl J Med 2015;373:2534–48. [DOI] [PubMed] [Google Scholar]
- 58.Hohenberger M, Cardwell LA, Oussedik E, et al. Interleukin-17 inhibition: role in psoriasis and inflammatory bowel disease. J Dermatolog Treat 2018;29:13–18. [DOI] [PubMed] [Google Scholar]
- 59.Nascimbeni R, Di Fabio F, Lanzini A, et al. [Extraintestinal manifestations in Crohn’s disease: risk factors and influence of intestinal surgery]. Ann Ital Chir 2009;80:293–8. [PubMed] [Google Scholar]
- 60.Chateau T, Bonovas S, Le Berre C, et al. Vedolizumab Treatment in Extra-Intestinal Manifestations in Inflammatory Bowel Disease: A Systematic Review. J Crohns Colitis 2019;13:1569–1577. [DOI] [PubMed] [Google Scholar]
- 61.Farhi D, Cosnes J, Zizi N, et al. Significance of erythema nodosum and pyoderma gangrenosum in inflammatory bowel diseases: a cohort study of 2402 patients. Medicine (Baltimore) 2008;87:281–93. [DOI] [PubMed] [Google Scholar]
- 62.Vavricka SR, Brun L, Ballabeni P, et al. Frequency and risk factors for extraintestinal manifestations in the Swiss inflammatory bowel disease cohort. Am J Gastroenterol 2011;106:110–9. [DOI] [PubMed] [Google Scholar]
- 63.Greuter T, Navarini A, Vavricka SR. Skin Manifestations of Inflammatory Bowel Disease. Clin Rev Allergy Immunol 2017;53:413–427. [DOI] [PubMed] [Google Scholar]
- 64.States V, O’Brien S, Rai JP, et al. Pyoderma Gangrenosum in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Dig Dis Sci 2020;65:2675–2685. [DOI] [PubMed] [Google Scholar]
- 65.Imhof L, Meier B, Frei P, et al. Severe Sweet’s Syndrome with Elevated Cutaneous Interleukin-1beta after Azathioprine Exposure: Case Report and Review of the Literature. Dermatology 2015;230:293–8. [DOI] [PubMed] [Google Scholar]
- 66.Travis S, Innes N, Davies MG, et al. Sweet’s syndrome: an unusual cutaneous feature of Crohn’s disease or ulcerative colitis. The South West Gastroenterology Group. Eur J Gastroenterol Hepatol 1997;9:715–20. [DOI] [PubMed] [Google Scholar]
- 67.Amarapurkar DN, Sonavane A, Amarapurkar AD. Metastatic Crohn’s Disease. J Assoc Physicians India 2017;65:86–88. [PubMed] [Google Scholar]
- 68.Aberumand B, Howard J, Howard J. Metastatic Crohn’s Disease: An Approach to an Uncommon but Important Cutaneous Disorder. Biomed Res Int 2017;2017:8192150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rani U, Russell A, Tanaka S, et al. Urogenital Manifestations of Metastatic Crohn’s Disease in Children: Case Series and Review of the Literature. Urology 2016;92:117–21. [DOI] [PubMed] [Google Scholar]
- 70.Patel AV, Jones DM, Hill JC, et al. Development of metastatic Crohn’s disease of the skin while on anti-TNF biologics. Inflamm Bowel Dis 2012;18:1188–90. [DOI] [PubMed] [Google Scholar]
- 71.Cury DB, Moss AC, Elias G, et al. Adalimumab for cutaneous metastatic Crohn’s disease. Inflamm Bowel Dis 2010;16:723–4. [DOI] [PubMed] [Google Scholar]
- 72.Ribaldone DG, Brigo S, Mangia M, et al. Oral Manifestations of Inflammatory Bowel Disease and the Role of Non-Invasive Surrogate Markers of Disease Activity. Medicines (Basel) 2020;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Muhvic-Urek M, Tomac-Stojmenovic M, Mijandrusic-Sincic B. Oral pathology in inflammatory bowel disease. World J Gastroenterol 2016;22:5655–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lauritano D, Boccalari E, Di Stasio D, et al. Prevalence of Oral Lesions and Correlation with Intestinal Symptoms of Inflammatory Bowel Disease: A Systematic Review. Diagnostics (Basel) 2019;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Katsanos KH, Torres J, Roda G, et al. Review article: non-malignant oral manifestations in inflammatory bowel diseases. Aliment Pharmacol Ther 2015;42:40–60. [DOI] [PubMed] [Google Scholar]
- 76.Vavricka SR, Manser CN, Hediger S, et al. Periodontitis and gingivitis in inflammatory bowel disease: a case-control study. Inflamm Bowel Dis 2013;19:2768–77. [DOI] [PubMed] [Google Scholar]
- 77.Brito F, Zaltman C, Carvalho AT, et al. Subgingival microflora in inflammatory bowel disease patients with untreated periodontitis. Eur J Gastroenterol Hepatol 2013;25:239–45. [DOI] [PubMed] [Google Scholar]
- 78.Brito F, de Barros FC, Zaltman C, et al. Prevalence of periodontitis and DMFT index in patients with Crohn’s disease and ulcerative colitis. J Clin Periodontol 2008;35:555–60. [DOI] [PubMed] [Google Scholar]
- 79.Lofberg R, Louis EV, Reinisch W, et al. Adalimumab produces clinical remission and reduces extraintestinal manifestations in Crohn’s disease: results from CARE. Inflamm Bowel Dis 2012;18:1–9. [DOI] [PubMed] [Google Scholar]
- 80.Taleban S, Li D, Targan SR, et al. Ocular Manifestations in Inflammatory Bowel Disease Are Associated with Other Extra-intestinal Manifestations, Gender, and Genes Implicated in Other Immune-related Traits. J Crohns Colitis 2016;10:43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ottaviano G, Salvatore S, Salvatoni A, et al. Ocular Manifestations of Paediatric Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. J Crohns Colitis 2018;12:870–879. [DOI] [PubMed] [Google Scholar]
- 82.Troncoso LL, Biancardi AL, de Moraes HV Jr., et al. Ophthalmic manifestations in patients with inflammatory bowel disease: A review. World J Gastroenterol 2017;23:5836–5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Watson PG, Hayreh SS. Scleritis and episcleritis. Br J Ophthalmol 1976;60:163–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rossi RE, Conte D, Massironi S. Primary sclerosing cholangitis associated with inflammatory bowel disease: an update. Eur J Gastroenterol Hepatol 2016;28:123–31. [DOI] [PubMed] [Google Scholar]
- 85.Kaplan GG, Laupland KB, Butzner D, et al. The burden of large and small duct primary sclerosing cholangitis in adults and children: a population-based analysis. Am J Gastroenterol 2007;102:1042–9. [DOI] [PubMed] [Google Scholar]
- 86.Fraga M, Fournier N, Safroneeva E, et al. Primary sclerosing cholangitis in the Swiss Inflammatory Bowel Disease Cohort Study: prevalence, risk factors, and long-term follow-up. Eur J Gastroenterol Hepatol 2017;29:91–97. [DOI] [PubMed] [Google Scholar]
- 87.Dean G, Hanauer S, Levitsky J. The Role of the Intestine in the Pathogenesis of Primary Sclerosing Cholangitis: Evidence and Therapeutic Implications. Hepatology 2020. [DOI] [PubMed] [Google Scholar]
- 88.Bergquist A, Ekbom A, Olsson R, et al. Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. J Hepatol 2002;36:321–7. [DOI] [PubMed] [Google Scholar]
- 89.Fevery J, Verslype C, Lai G, et al. Incidence, diagnosis, and therapy of cholangiocarcinoma in patients with primary sclerosing cholangitis. Dig Dis Sci 2007;52:3123–35. [DOI] [PubMed] [Google Scholar]
- 90.Tischendorf JJ, Hecker H, Kruger M, et al. Characterization, outcome, and prognosis in 273 patients with primary sclerosing cholangitis: A single center study. Am J Gastroenterol 2007;102:107–14. [DOI] [PubMed] [Google Scholar]
- 91.Rupp C, Hippchen T, Bruckner T, et al. Effect of scheduled endoscopic dilatation of dominant strictures on outcome in patients with primary sclerosing cholangitis. Gut 2019;68:2170–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bjornsson E, Boberg KM, Cullen S, et al. Patients with small duct primary sclerosing cholangitis have a favourable long term prognosis. Gut 2002;51:731–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Goode EC, Rushbrook SM. A review of the medical treatment of primary sclerosing cholangitis in the 21st century. Ther Adv Chronic Dis 2016;7:68–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lindor KD, Kowdley KV, Harrison ME, et al. ACG Clinical Guideline: Primary Sclerosing Cholangitis. Am J Gastroenterol 2015;110:646–59; quiz 660. [DOI] [PubMed] [Google Scholar]
- 95.Triantos CK, Koukias NM, Nikolopoulou VN, et al. Meta-analysis: ursodeoxycholic acid for primary sclerosing cholangitis. Aliment Pharmacol Ther 2011;34:901–10. [DOI] [PubMed] [Google Scholar]
- 96.Poropat G, Giljaca V, Stimac D, et al. Bile acids for primary sclerosing cholangitis. Cochrane Database Syst Rev 2011:CD003626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Treeprasertsuk S, Bjornsson E, Sinakos E, et al. Outcome of patients with primary sclerosing cholangitis and ulcerative colitis undergoing colectomy. World J Gastrointest Pharmacol Ther 2013;4:61–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gelley F, Zadori G, Gorog D, et al. Recurrence of primary sclerosing cholangitis after liver transplantation - The Hungarian experience. Interv Med Appl Sci 2014;6:16–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gelley F, Miheller P, Peter A, et al. Activity of ulcerative colitis before and after liver transplantation in primary sclerosing cholangitis: the Hungarian experience. Transplant Proc 2012;44:2164–5. [DOI] [PubMed] [Google Scholar]
- 100.Zeitz J, Mullhaupt B, Fruehauf H, et al. Hepatic failure due to hepatitis B reactivation in a patient with ulcerative colitis treated with prednisone. Hepatology 2009;50:653–4. [DOI] [PubMed] [Google Scholar]
- 101.Navaneethan U, Shen B. Hepatopancreatobiliary manifestations and complications associated with inflammatory bowel disease. Inflamm Bowel Dis 2010;16:1598–619. [DOI] [PubMed] [Google Scholar]
- 102.Saubermann LJ, Deneau M, Falcone RA, et al. Hepatic Issues and Complications Associated With Inflammatory Bowel Disease: A Clinical Report From the NASPGHAN Inflammatory Bowel Disease and Hepatology Committees. J Pediatr Gastroenterol Nutr 2017;64:639–652. [DOI] [PubMed] [Google Scholar]
- 103.Restellini S, Chazouilleres O, Frossard JL. Hepatic manifestations of inflammatory bowel diseases. Liver Int 2017;37:475–489. [DOI] [PubMed] [Google Scholar]
- 104.Gizard E, Ford AC, Bronowicki JP, et al. Systematic review: The epidemiology of the hepatobiliary manifestations in patients with inflammatory bowel disease. Aliment Pharmacol Ther 2014;40:3–15. [DOI] [PubMed] [Google Scholar]
- 105.Rogler G Gastrointestinal and liver adverse effects of drugs used for treating IBD. Best Pract Res Clin Gastroenterol 2010;24:157–65. [DOI] [PubMed] [Google Scholar]
- 106.Zanoli L, Mikhailidis DP, Bruno RM, et al. Aortic Stiffening Is an Extraintestinal Manifestation of Inflammatory Bowel Disease: Review of the Literature and Expert Panel Statement. Angiology 2020:3319720918509. [DOI] [PubMed] [Google Scholar]
- 107.Panhwar MS, Mansoor E, Al-Kindi SG, et al. Risk of Myocardial Infarction in Inflammatory Bowel Disease: A Population-based National Study. Inflamm Bowel Dis 2019;25:1080–1087. [DOI] [PubMed] [Google Scholar]
- 108.Aniwan S, Pardi DS, Tremaine WJ, et al. Increased Risk of Acute Myocardial Infarction and Heart Failure in Patients With Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol 2018;16:1607–1615 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Singh AG, Crowson CS, Singh S, et al. Risk of Cerebrovascular Accidents and Ischemic Heart Disease in Cutaneous Lupus Erythematosus: A Population-Based Cohort Study. Arthritis Care Res (Hoboken) 2016;68:1664–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kirchgesner J, Beaugerie L, Carrat F, et al. Increased risk of acute arterial events in young patients and severely active IBD: a nationwide French cohort study. Gut 2018;67:1261–1268. [DOI] [PubMed] [Google Scholar]
- 111.Murthy SK, Robertson McCurdy AB, Carrier M, et al. Venous thromboembolic events in inflammatory bowel diseases: A review of current evidence and guidance on risk in the post-hospitalization setting. Thromb Res 2020;194:26–32. [DOI] [PubMed] [Google Scholar]
- 112.Danese S, Papa A, Saibeni S, et al. Inflammation and coagulation in inflammatory bowel disease: The clot thickens. Am J Gastroenterol 2007;102:174–86. [DOI] [PubMed] [Google Scholar]
- 113.Shen J, Ran ZH, Zhang Y, et al. Biomarkers of altered coagulation and fibrinolysis as measures of disease activity in active inflammatory bowel disease: a gender-stratified, cohort analysis. Thromb Res 2009;123:604–11. [DOI] [PubMed] [Google Scholar]
- 114.Jasdanwala S, Babyatsky M. Crohn’s disease and acute pancreatitis. A review of literature. JOP 2015;16:136–42. [DOI] [PubMed] [Google Scholar]
- 115.Broide E, Dotan I, Weiss B, et al. Idiopathic pancreatitis preceding the diagnosis of inflammatory bowel disease is more frequent in pediatric patients. J Pediatr Gastroenterol Nutr 2011;52:714–7. [DOI] [PubMed] [Google Scholar]
- 116.Pitchumoni CS, Rubin A, Das K. Pancreatitis in inflammatory bowel diseases. J Clin Gastroenterol 2010;44:246–53. [DOI] [PubMed] [Google Scholar]
- 117.Pimentel AM, Rocha R, Santana GO. Crohn’s disease of esophagus, stomach and duodenum. World J Gastrointest Pharmacol Ther 2019;10:35–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Suk Lee Y, Kim NH, Hyuk Son J, et al. Type 2 Autoimmune Pancreatitis with Crohn’s Disease. Intern Med 2018;57:2957–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Seibold F, Weber P, Jenss H, et al. Antibodies to a trypsin sensitive pancreatic antigen in chronic inflammatory bowel disease: specific markers for a subgroup of patients with Crohn’s disease. Gut 1991;32:1192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Seibold F, Scheurlen M, Muller A, et al. Impaired pancreatic function in patients with Crohn’s disease with and without pancreatic autoantibodies. J Clin Gastroenterol 1996;22:202–6. [DOI] [PubMed] [Google Scholar]
- 121.Heikius B, Niemela S, Lehtola J, et al. Elevated pancreatic enzymes in inflammatory bowel disease are associated with extensive disease. Am J Gastroenterol 1999;94:1062–9. [DOI] [PubMed] [Google Scholar]
- 122.Eliadou E, Moleiro J, Ribaldone DG, et al. Interstitial and Granulomatous Lung Disease in Inflammatory Bowel Disease Patients. J Crohns Colitis 2020;14:480–489. [DOI] [PubMed] [Google Scholar]
- 123.Zeitz J, Ak M, Muller-Mottet S, et al. Pain in IBD Patients: Very Frequent and Frequently Insufficiently Taken into Account. PLoS One 2016;11:e0156666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Veloso FT, Carvalho J, Magro F. Immune-related systemic manifestations of inflammatory bowel disease. A prospective study of 792 patients. J Clin Gastroenterol 1996;23:29–34. [DOI] [PubMed] [Google Scholar]
- 125.Schirbel A, Reichert A, Roll S, et al. Impact of pain on health-related quality of life in patients with inflammatory bowel disease. World J Gastroenterol 2010;16:3168–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Borren NZ, Tan W, Colizzo FP, et al. Longitudinal Trajectory of Fatigue With Initiation of Biologic Therapy in Inflammatory Bowel Diseases: A Prospective Cohort Study. J Crohns Colitis 2020;14:309–315. [DOI] [PubMed] [Google Scholar]
- 127.Qazi T Fatigue in inflammatory bowel disease: a problematic ailment. Curr Opin Gastroenterol 2020;36:284–294. [DOI] [PubMed] [Google Scholar]
- 128.Canakis A, Qazi T. Sleep and Fatigue in IBD: an Unrecognized but Important Extra-intestinal Manifestation. Curr Gastroenterol Rep 2020;22:8. [DOI] [PubMed] [Google Scholar]
- 129.Borren NZ, van der Woude CJ, Ananthakrishnan AN. Fatigue in IBD: epidemiology, pathophysiology and management. Nat Rev Gastroenterol Hepatol 2019;16:247–259. [DOI] [PubMed] [Google Scholar]
- 130.Nocerino A, Nguyen A, Agrawal M, et al. Fatigue in Inflammatory Bowel Diseases: Etiologies and Management. Adv Ther 2020;37:97–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Grygiel-Gorniak B, Puszczewicz M. Fatigue and interleukin-6 - a multi-faceted relationship. Reumatologia 2015;53:207–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Borren NZ, Plichta D, Joshi AD, et al. Alterations in Fecal Microbiomes and Serum Metabolomes of Fatigued Patients With Quiescent Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol 2020. [DOI] [PubMed] [Google Scholar]
- 133.Farrell D, Artom M, Czuber-Dochan W, et al. Interventions for fatigue in inflammatory bowel disease. Cochrane Database Syst Rev 2020;4:CD012005. [DOI] [PMC free article] [PubMed] [Google Scholar]


