Key words: Coinfections, Metschnikowia, Ordospora, priority effects, zooplankton
Over the course of seasonal epidemics, populations of susceptible hosts may encounter a wide variety of parasites. Parasite phenology affects the order in which these species encounter their hosts, leading to sequential infections, with potentially strong effects on within-host growth and host population dynamics. Here, the cladoceran Daphnia magna was exposed sequentially to a haemolymph-infecting yeast (Metschnikowia bicuspidata) and a gut microsporidium (Ordospora colligata), with experimental treatments reflecting two possible scenarios of parasite succession. The effects of single and co-exposure were compared on parasite infectivity, spore production and the overall virulence experienced by the host. We show that neither parasite benefited from coinfection; instead, when hosts encountered Ordospora, followed by Metschnikowia, higher levels of host mortality contributed to an overall decrease in the transmission of both parasites. These results showcase an example of sequential infections generating unilateral priority effects, in which antagonistic interactions between parasites can alleviate the intensity of infection and coincide with maladaptive levels of damage inflicted on the host.
Introduction
Over the course of their lifetime, most free-living organisms are bound to encounter parasites (Poulin and Morand, 2000). Realistically, individual hosts rarely encounter a single parasite, but rather progress through a series of events (exposure, infection and recovery) from a multitude of pathogens, some of which may coexist within the course of an infection. While some parasites may encounter their hosts simultaneously, such as several virus species being inoculated by a shared vector (Swanson et al., 2006), the majority of multiple infections are thought to occur sequentially (Karvonen et al., 2019). In a within-host framework, ‘priority effects’ occur when this sequence of infection alters the outcome of interactions among parasites (Halliday et al., 2020). For instance, as different strains compete for a pool of susceptible hosts, faster replicating strains are generally favoured (Levin and Pimentel, 1981; Nowak and May, 1994). However, prior residency may allow ‘weaker’ strains to prevail in coinfection, by conferring protection against more competitive genotypes (Ben-Ami et al., 2008; Seifi et al., 2012). The biological mechanisms underlying such observations are likely the product of complex interactions between the defending host and coinfecting parasites (Alizon et al., 2013), although common hypotheses have been proposed, which generally involve host immunity and competition for resources (Read and Taylor, 2001; de Roode et al., 2005). For instance, prior exposure may weaken host immunity in such a way that secondary infections are facilitated (Graham, 2008) or trigger priming of the host's defences, so that subsequent infections are either alleviated (Rodrigues et al., 2010) or prevented altogether (Ratcliff et al., 1999; Syller and Grupa, 2016). Prior infection can also sequester within-host resources, which will then alter the developmental trajectory of late-arriving parasites (Graham, 2008). Although traditionally used in the context of species assemblages and community structures (Connell and Slatyer, 1977; Wilbur and Alford, 1985), this notion of priority effects has since been widely applied to the study of sequential infections (Hoverman et al., 2013; Wuerthner et al., 2017; Clay et al., 2018; Carpenter et al., 2021). Incidentally, a majority of studies have reported negative effects on later arriving parasites (reviewed in Karvonen et al., 2019; but see also Ezenwa et al., 2010, Lohr et al., 2010b).
Over the past decade, water fleas of the genus Daphnia (Crustacea: Cladocera) and their microparasites have emerged as an ecologically relevant system for testing the outcome of interspecific coinfections (Ben-Ami et al., 2011; Lange et al., 2014; Sánchez et al., 2019). As common inhabitants and crucial agents in the stability of freshwater ecosystems (Carpenter et al., 1985; Lampert, 2006, 2011), Daphnia are known to harbour a functionally and taxonomically diverse range of parasite species, including microsporidia, fungi, ichthyosporea, bacteria (Ebert, 1995; Stirnadel and Ebert, 1997; Wolinska, et al., 2009; Goren and Ben-Ami, 2013) and viruses (Toenshoff et al., 2018). For example, the gut microsporidium Ordospora colligata (Microsporidia: Ordosporidae, hereafter referred to as Ordospora) can be found in northern and western European ponds (Ebert, 2005), where high prevalences have been recorded in populations of its only host, Daphnia magna (Ebert et al., 2001; Decaestecker et al., 2005). In temperate ponds, the prevalence of microsporidian parasites increases from late spring to early summer, before waning back in the autumn and winter (Ebert, 1995; Larsson et al., 1997). Epidemics usually start from infectious spore banks contained in the sediment, although transmission stages are also able to disperse in the water, where they can be encountered as free-floating spores (Mangin et al., 1995; Kirk et al., 2018). Microsporidian spores exhibit high survivability outside their hosts, allowing the parasite to overwinter and survive periods of host diapause (Ebert, 1995). Another common parasite of Daphnia, the waterborne yeast Metschnikowia bicuspidata (Ascomycota: Saccharomycetales, hereafter referred to as Metschnikowia) is a generalist capable of infecting several zooplankton species (Ebert, 2005; Dallas et al., 2016). In temperate freshwater bodies of the Northern Hemisphere, epidemics of Metschnikowia typically peak in the late summer to early autumn (Duffy et al., 2009; Hall et al., 2011; Penczykowski et al., 2014), although it has been found to overlap with gut microsporidia in the summer period (Ebert, 1995; Stirnadel and Ebert, 1997) or during the rainy season in Mediterranean to semi-arid climates of the Middle East (Goren and Ben-Ami, 2013). Transmission is also horizontal, although infective propagules are only released from dead hosts (i.e. obligate killer), and thus mostly restricted to the sediment (Duffy, 2009; Duffy and Hunsberger, 2019).
Due to their overlapping distribution, coinfections of D. magna involving both taxa are likely to occur. However, these phylogenetically distant species have been shown to differ greatly in their overall reproductive strategy: while infections by Ordospora typically reduce host lifespan by up to 20% (Ebert et al., 2000), Metschnikowia is a highly virulent parasite, producing lethal infections under 2-to-3 weeks (Ebert, 2005). Because virulence in coinfection generally aligns with the amount of damage induced by the more virulent parasite (Ben-Ami et al., 2008; Ben-Ami and Routtu, 2013), coinfection by an obligate killer may drastically reduce the timespan available to efficiently exploit host resources for growth (Lohr et al., 2010b; Clay et al., 2019). Furthermore, within-host competition for resources may be particularly relevant for parasites that colonize distinct niches within the host (Ben-Ami et al., 2011). The intracellular Ordospora ensures reproduction by hijacking energy (i.e. ATP molecules) within the cytoplasm of epithelial cells (Tsaousis et al., 2008), which serves both as a barrier and interface between the gut lumen and the haemolymph. Meanwhile, development of Metschnikowia takes place in the body cavity (Codreanu and Codreanu-Balcescu, 1981), which is in turn alimented by direct trophic exchanges along these compartments.
In addition to their contrasting reproductive strategies, the exact sequence in which parasites succeed each other within one host may further complicate such interactions (Hood, 2003; de Roode et al., 2005; Jäger and Schjørring, 2006). The documented phenology of both parasites suggests that infections are likely to overlap in late summer, with a predicted prior presence of Ordospora in sympatric populations. Incidentally, some studies of priority effects have been conducted using Metschnikowia, along with ichtyhosporean (Lohr et al., 2010b) and bacterial (Clay et al., 2019) parasites of Daphnia, in which it was shown to consistently experience impaired transmission under prior residency. However, the literature is currently lacking such experimental assays for microsporidian parasites of Daphnia. In their exploratory study, Mangin et al. (1995) reported successful transmission of Ordospora to individuals previously infected with the microsporidium Tuzetia sp. (now referred to as Hamiltosporidium magnivora, Haag et al., 2011). Nevertheless, systematic assays of sequential exposure using Ordospora have not been documented.
Here, we sequentially exposed the host D. magna to the parasites Metschnikowia and Ordospora. Experimental treatments were designed to reflect two possible scenarios of parasite succession: one in which a gut microsporidium (Ordospora) encounters the host after prior establishment of a fungal parasite in the haemolymph (Metschnikowia), and a second, opposite scenario in which the haemolymph-infecting yeast encounters the host after prior establishment of the gut parasite. We aimed to determine (i) whether sequential infections differ from single infections in terms of parasite transmission traits, specifically addressing the following questions: (a) how does Metschnikowia respond to later arrival of Ordospora; (b) how does Metschnikowia respond to prior infection by Ordospora; (c) how does Ordospora respond to later arrival of Metschnikowia and (d) how does Ordospora respond to prior infection by Metschnikowia; and (ii) whether opposite scenarios of parasite succession influence host fitness in diverging ways.
Materials and methods
Study system
Daphnia magna is commonly found in lakes and temporary freshwater bodies of the Northern Hemisphere (Ebert, 2005). Due to its large size (i.e. up to 5 mm) and efficient filtering rate, D. magna is particularly prone to multiple infections in general, as compared with smaller sympatric species (Stirnadel and Ebert, 1997). One clonal line of D. magna was used as the focal host for this experiment (clone NO-V-7, isolated from Norway; Haag et al., 2020). This single genotype was selected on the basis of having the highest compatibility with both strains of parasites used in this study, as reported by preliminary infectivity assays.
A single strain of the yeast Metschnikowia was used, isolated from Ammersee, Germany and later propagated on lab-reared D. magna (clone E17:07). Spores are needle-shaped and puncture the gut epithelium to reach the haemolymph, where fungal development takes place (Codreanu and Codreanu-Balcescu, 1981; Stewart Merrill and Cáceres, 2018). Infection symptoms are clearly visible after 9–10 days, when the host's body cavity starts to fill with elongated asci (Stewart Merrill and Cáceres, 2018).
A single strain of Ordospora was used, isolated and maintained on lab-reared cultures of the experimental host (NO-V-7). Late stages of infection are characterized by the presence of several dozens of spore clusters in the gut epithelium, which are mostly confined to the upper half of the gut epithelium (Refardt and Ebert, 2006) and notably visible in the ‘angular’ sections of the digestive tract, such as the anterior diverticuli (Ebert, 2005). Spore release can occur from live host after 3 days (Mangin et al., 1995; Refardt and Ebert, 2007), although reliable detection of infection is usually possible after 11 days, due to the exponential increase in parasite spore load throughout the infection (Kirk et al., 2019).
Experimental setup
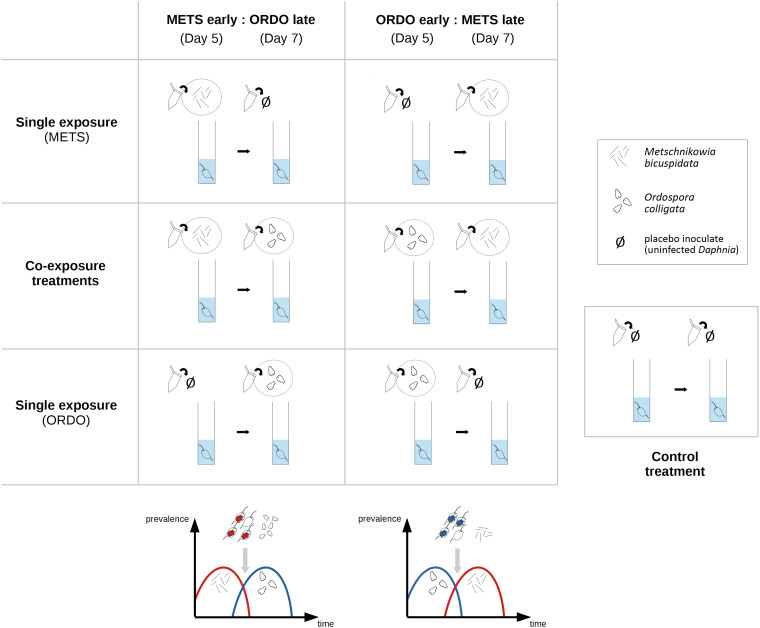
The experimental design included four single-exposure treatments (‘METS early’: exposed to spores of Metschnikowia on day 5; ‘METS late’: exposed to spores of Metschnikowia on day 7; ‘ORDO early’: exposed to spores of Ordospora on day 5; ‘ORDO late’: exposed to spores of Ordospora on day 7), two co-exposure treatments (‘CO:METS early:ORDO late’: exposed to spores of Metschnikowia on day 5 and Ordospora on day 7; ‘CO:ORDO early:METS late’: exposed to spores of Ordospora on day 5 and Metschnikowia on day 7) and one control treatment (exposed to crushed tissue of uninfected D. magna on both days). On the day which did not feature exposure to the parasite, all single infection treatments were exposed to the same placebo as the control. Forty replicates (individual Daphnia) were used for each treatment, yielding a total of 280 experimental units (Fig. 1).
Fig. 1.
Graphical representation of the six exposure treatments, corresponding to two possible scenarios of parasite succession. On the left, the haemolymph parasite Metschnikowia bicuspidata arrives ‘early’ and the gut parasite Ordospora colligata arrives ‘late’. On the right, the gut parasite O. colligata arrives ‘early’ and the haemolymph parasite M. bicuspidata arrives ‘late’. Single-exposure treatments within each scenario follow the same timing of infection as the co-exposure treatment, to allow proper comparison of parasite and host fitness parameters across single and co-exposure settings. The control treatment received the same placebo inoculate (obtained from crushed uninfected Daphnia) as single-exposure treatments, albeit on both inoculation days.
Inoculation process
Juvenile Daphnia born within a 24-h time span (i.e. day 1) were transferred to individual jars containing 5 mL of synthetic culture medium (SSS-medium, Saebelfeld et al., 2017). Daphnia were maintained at a constant temperature of 19°C, under a 12:12 light–dark photoperiod and fed three times per week with 1 mg C L−1 of Scenedesmus obliquus (green algae, maintained in WC algal medium). On day 5, spore solutions were prepared for both parasites. Infected individuals were gathered in Eppendorf tubes and crushed with a plastic pestle. The equivalent of ten adult Daphnia were crushed per 40 replicates, ensuring a balanced amount of host tissue was introduced in all seven treatments. To prepare the stock solution of Metschnikowia, the appropriate number of infected Daphnia (clone E17:07) was crushed to achieve a target dose of 17 500 spore per recipient Daphnia (3500 spores mL−1) across 80 replicates in two treatments (METS early; CO:METS early:ORDO late). This dose was comparably higher than previous studies utilizing the same system (Hesse et al., 2012), in order to maximize chances of successful coinfection in the co-exposure treatments. The solution was completed by crushing additional uninfected individuals up to a total of 20. To prepare the stock solution of Ordospora, 20 Daphnia (clone NO-V-7) presenting signs of late stage infection (large amount of spore clusters in the gut) were crushed to achieve a target dose of approximately 38 000 spores per recipient Daphnia (7600 spores mL−1) across 80 replicates in two treatments (ORDO early; CO:ORDO early:METS late). Repeated counts from stock cultures were shown to provide the required number of spores from 20 individuals (CI95% of average spore yield per inoculation dose: 38 100 ± 6.5%). These spore solutions were then distributed across all replicates of their respective treatments. Single infection treatments that did not receive spores on day 5 (METS late; ORDO late), as well as the control treatment were exposed to a placebo inoculate, prepared by crushing uninfected individuals (clone NO-V-7) using the same ratio of ten adult Daphnia for each treatment of 40 replicates.
After an exposure period of 2 days allocated to the first parasite, all Daphnia were transferred to 5 mL of clean medium, and the inoculation process was repeated on day 7. This delay was chosen to ensure that either Metschnikowia (Stewart Merrill and Cáceres, 2018) or Ordospora (Mangin et al., 1995; Refardt and Ebert, 2007) would reach their target compartment, before exposing the host to the second parasite (consistent with the definition of sequential infection as following establishment of the prior parasite; Marchetto and Power, 2018). Spore solutions were prepared anew, using the same methods as described for day 5, and inoculated into their respective treatments (METS late; CO:ORDO early:METS late; ORDO late; CO:METS early:ORDO late). Daphnia were not fed on either exposure day, in order to promote spore uptake (Hall et al., 2007). On experimental day 9 (i.e. the end of the exposure period allocated to the second parasite), Daphnia were transferred to 20 mL of fresh, spore-free medium.
From day 9 onwards (both exposure periods having been completed), dead individuals were collected and fixed in 3.7% formaldehyde. Samples were kept at 4°C until the assessment of spore production (see below). Juveniles were removed and counted daily, and Daphnia were transferred to fresh medium (20 mL) every 4 days. The experiment was terminated on day 81, when the last surviving Daphnia in the control treatment had died.
Recorded parameters
Parasite fitness
Individual Daphnia from all treatments were assigned a binary value for host viability (0 = early death, 1 = viable host). Viable hosts were described as individual Daphnia having survived until the first possible detection of infection symptoms (i.e. presence of spores from crushed individuals), which was determined as 9 days post-exposure for Metschnikowia (Stewart Merrill and Cáceres, 2018) and 11 days post-exposure for Ordospora (Kirk et al., 2019). Individuals from the six exposure treatments were assigned a separate value for parasite infectivity (0 = non infected, 1 = infected). Infected hosts were described as individual Daphnia in which spores of either parasite were detected (among those considered viable). Individuals which did not survive until at least both inoculation events had occurred (i.e. beyond experimental day 7) were excluded from both calculations, as these could not be properly attributed to their intended treatments (Appendix, Table S1). All retrieved samples (except for the control) were blinded to ensure reliable assessment of spore yield upon host death across single and co-exposure treatments. Samples were crushed in 0.3 mL, homogenized and loaded with 10 μL in a Neubauer Improved chamber. Samples were first screened for detection and quantification of needle-shaped Metschnikowia spores, under a Nikon SMZ25 stereomicroscope (200× magnification). For identification and quantification of Ordospora, samples were observed under a Nikon Ti Eclypse inverted microscope, using phase contrast and UV exposure (200× magnification); for each sample, 2 μL of Calcofluor-White (1 mg mL−1) were added to the counting chamber to generate blue fluorescence, thereby staining the chitin-rich wall of pyriform spores (Krebs et al., 2017).
Parasite growth (i.e. the rate of spore production) was computed as the ratio of spore yield over the number of days survived by the host post-exposure. A comprehensive measure of parasite fitness, the net spore output per exposed host, was computed as an estimation of overall transmission success. Here, in addition to individuals that produced a detectable spore yield, those that scored ‘0’ for either host viability or parasite infectivity were also included, and recorded as a having a ‘net’ spore output of zero. This was done to reflect the probability of each encounter with an exposed host leading to subsequent reproduction of the parasite, which may differ across experimental treatments, independently of parasite growth (Manzi et al., 2020).
Host fitness
Host fitness was recorded via three variables: host lifespan post-exposure was defined as the number of days survived by individual Daphnia, following the completion of both exposure events (i.e. beyond experimental day 7). Total offspring production per individual was used as a comprehensive measure of the host's reproductive success. Finally, the rate of offspring production was computed as the ratio of total offspring production over host lifespan post-exposure.
Data analysis
Data were analysed using R version 4.0.4 (R Core Team, 2021). Graphical outputs were produced using the ‘ggplot2’ (Wickham, 2016), ‘Hmisc’ (Harrell and Harrell, 2019) and ‘patchwork’ (Pedersen, 2020) packages. Analyses of variance (F-test or χ2 test) were performed with the ‘car’ package (Fox and Weisberg, 2019).
Parasite fitness
Parasite fitness variables were analysed separately for each parasite and compared across single and co-exposure treatments with the same timing of infection. Host viability (0 = early death, 1 = viable host) and parasite infectivity (0 = non infected, 1 = infected) were analysed using a binary logistic regression with ‘exposure’ as explanatory variable (i.e. a factor with up to six possible levels). Additionally, host viability was compared to baseline mortality in the control treatment (Appendix, Table S2). In co-exposure treatments, infectivity of a given parasite included the total number of cases in which spores of that parasite were detected, either in single or coinfection. Parasite growth and the net spore output per exposed host were analysed with ‘exposure’ as explanatory variable in a linear model, assuming a normal distribution of residuals. Only successful infections (i.e. detection of a non-zero number of spores) were included in the analysis of parasite growth. All individuals which survived until at least both exposure events had occurred (i.e. beyond experimental day 7) were included in the analysis of net spore output. Normal distribution and homoscedasticity of the residuals were verified by visual inspection of quantile–quantile plots and residuals against fitted values.
Host fitness
Host fitness variables (namely lifespan post-exposure, rate of offspring production and total offspring production) were analysed using linear models, assuming a normal distribution of residuals, with ‘exposure’ as the explanatory variable (i.e. a factor with seven levels, including the control treatment). Only individuals successfully infected by either one (single exposure) or both parasites (co-exposure) were included in the non-control treatments. One individual from the control treatment was lost due to handling error and was thus excluded from these analyses. Post-hoc pairwise comparisons (Tukey's HSD test) were performed with the ‘multcomp’ package (Hothorn et al., 2008).
Results
Parasite fitness
How does Metschnikowia respond to later arrival of Ordospora?
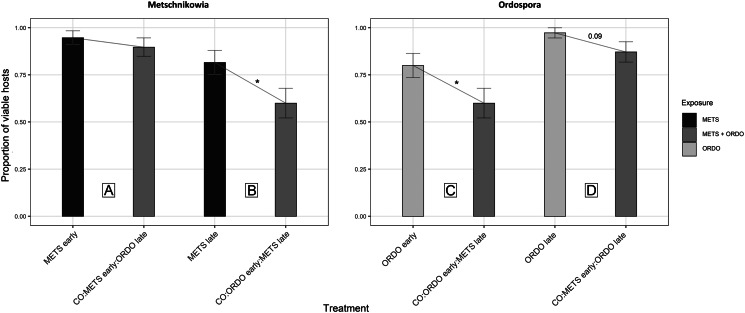
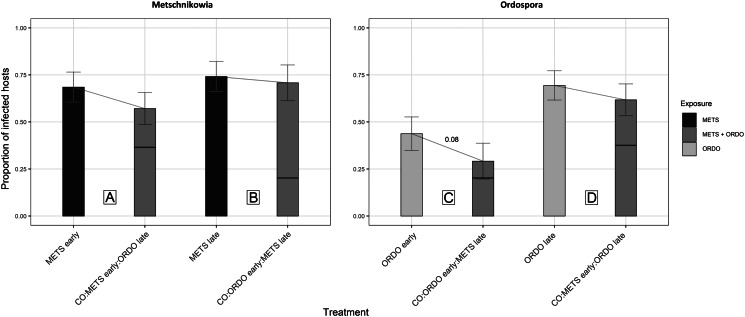
Under prior arrival of Metschnikowia, the viability of experimental Daphnia did not differ between the single and co-exposure treatments, with 94.7% (METS early) and 89.7% (CO:METS early:ORDO late) of hosts surviving until day 9 post-exposure (Fig. 2A, Table 1). Among hosts considered viable, the probability of successful infection did not differ significantly between single (68.6%) and co-exposure (57.1%) treatments (Fig. 3A, Table 1). Parasite growth was comparable between single and co-exposure treatments (Fig. 4A, Table 1). Thus, the net output of Metschnikowia did not differ significantly across single and co-exposure treatments (Fig. 5A, Table 1).
Fig. 2.
Graphical representation of the proportion of Daphnia considered viable hosts, i.e. which survived until at least 9 days post-exposure (Metschnikowia) or 11 days post-exposure (Ordospora), allowing either parasite to produce detectable levels of infection (i.e. presence of spores in crushed individuals). Host viability was compared between single and co-exposure treatments, to answer the following: (A) How does Metschnikowia respond to later arrival of Ordospora? (B) How does Metschnikowia respond to prior infection by Ordospora? (C) How does Ordospora respond to later arrival of Metschnikowia? (D) How does Ordospora respond to prior infection by Metschnikowia? Individuals which did not survive until at least both inoculation events had occurred were excluded from these calculations. Error bars depict the standard error of the mean (calculated from binary values assigned to individual Daphnia: 0 = early death, 1 = viable host). Significance levels are provided by logistic regression performed across single and co-exposure treatments with shared timing of infection: *P ⩽ 0.05.
Table 1.
Analysis of variance (F-test or χ2 test) was performed across single and co-exposure treatments with shared timing of infection, to answer the following: (a) How does Metschnikowia respond to later arrival of Ordospora? (b) How does Metschnikowia respond to prior infection by Ordospora? (c) How does Ordospora respond to later arrival of Metschnikowia? (d) How does Ordospora respond to prior infection by Metschnikowia?
| Response variable | Degree of freedom | χ2/F value | P value |
|---|---|---|---|
| (a) METS early | CO:METS early:ORDO late | |||
| Host viability | 75 | 0.6807 | 0.4093 |
| Parasite infectivity | 68 | 0.9820 | 0.3217 |
| Parasite growth | 42 | 0.5758 | 04522 |
| Net output per exposed host | 75 | 0.8109 | 0.3708 |
| (b) METS late | CO:ORDO early:METS late | |||
| Host viability | 76 | 4.4597 | 0.0347 |
| Parasite infectivity | 53 | 0.0768 | 0.7817 |
| Parasite growth | 38 | 5.7688 | 0.0213 |
| Net output per exposed host | 76 | 0.4945 | 0.0291 |
| (c) ORDO early | CO:ORDO early:METS late | |||
| Host viability | 78 | 3.8652 | 0.0493 |
| Parasite infectivity | 58 | 2.9877 | 0.0839 |
| Parasite growth | 19 | 0.1618 | 0.6920 |
| Net output per exposed host | 78 | 6.0996 | 0.0157 |
| (d) ORDO late | CO:METS early:ORDO late | |||
| Host viability | 74 | 2.9155 | 0.0877 |
| Parasite infectivity | 70 | 1.2013 | 0.2731 |
| Parasite growth | 43 | 1.2613 | 0.2676 |
| Net output per exposed host | 73 | 1.2907 | 0.2596 |
A generalized linear model was used, assuming a binomial distribution of residuals for host viability of individual Daphnia (0 = early death, 1 = viable host) and infection status of individual Daphnia (0 = non infected, 1 = infected). A general linear model was used, assuming a normal distribution of residuals for parasite growth (rate of spore production per infected host) and the net spore output per exposed host. Significant P values (⩽0.05) are highlighted in bold.
Fig. 3.
Graphical representation of the proportion of Daphnia successfully infected by the parasites Metschnikowia and Ordospora. Parasite infectivity was compared between single and co-exposure treatments, to answer the following: (A) How does Metschnikowia respond to later arrival of Ordospora? (B) How does Metschnikowia respond to prior infection by Ordospora? (C) How does Ordospora respond to later arrival of Metschnikowia? (D) How does Ordospora respond to prior infection by Metschnikowia? The horizontal section of the bar in co-exposure treatments represents the contribution of coinfections to the overall number of successful infections by the focal parasite. Individuals which did not survive until the earliest possible observation of parasite symptoms were excluded from the analysis of infectivity; reported proportions are computed amongst the remaining number of individuals considered viable. Error bars depict the standard error of the mean (calculated from binary values assigned to individual Daphnia: 0 = non infected, 1 = infected). Significance levels are provided by logistic regression performed across single and co-exposure treatments with shared timing of infection; none of the pairwise comparisons were significant.
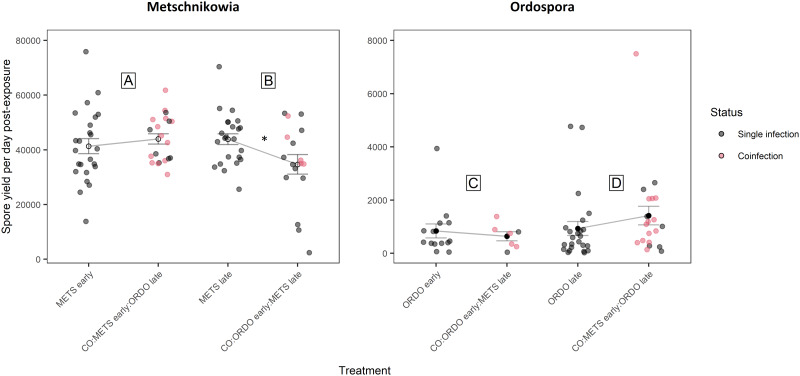
Fig. 4.
Graphical representation of parasite growth (computed as the ratio of spore yield upon host death and the number of days survived by the host, post-second exposure event) for the parasites Metschnikowia and Ordospora. Parasite growth was compared between single and co-exposure treatments, to answer the following: (A) How does Metschnikowia respond to later arrival of Ordospora? (B) How does Metschnikowia respond to prior infection by Ordospora? (C) How does Ordospora respond to later arrival of Metschnikowia? (D) How does Ordospora respond to prior infection by Metschnikowia? Coloured dots depict individuals which were confirmed to be coinfected by Metschnikowia and Ordospora. Error bars depict the standard error of the mean, which was computed by pooling singly and coinfected individuals in the co-exposure treatments. Significance levels are provided by analysis of variance (F-test) across single and co-exposure treatments with shared timing of infection: *P ⩽ 0.05.
Fig. 5.
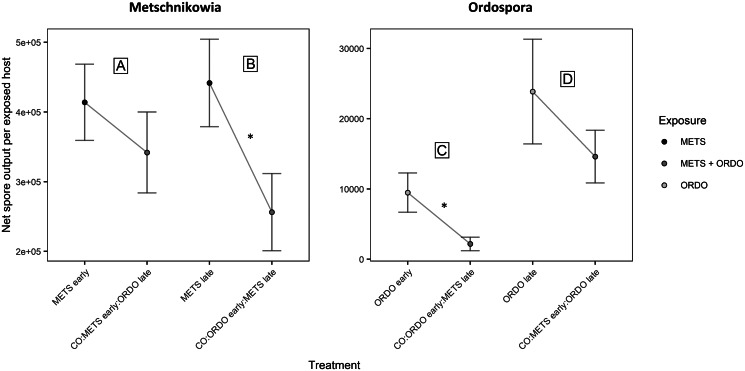
Graphical representation of the net spore output (per exposed host) for the parasites Metschnikowia and Ordospora, as compared between single and co-exposure treatments, to answer the following: (A) How does Metschnikowia respond to later arrival of Ordospora? (B) How does Metschnikowia respond to prior infection by Ordospora? (C) How does Ordospora respond to later arrival of Metschnikowia? (D) How does Ordospora respond to prior infection by Metschnikowia? Error bars depict the standard error of the mean. Significance levels are provided by analysis of variance (F-test) across single and co-exposure treatments with shared timing of infection: *P ⩽ 0.05.
How does Metschnikowia respond to prior infection by Ordospora?
Under late arrival of Metschnikowia, individuals which were first exposed to Ordospora suffered significant mortality during the early days of the experiment, with only 60.0% of hosts remaining viable (CO:ORDO early:METS late), as opposed to 81.6% in the single-exposure treatment (METS late) (Fig. 2B, Table 1). Infectivity did not differ significantly between the single (74.2%) and co-exposure (70.8%) treatments (Fig. 3B, Table 1). Parasite growth was significantly reduced in the co-exposure treatment (Fig. 4B, Table 1). Consequently, the net output of Metschnikowia in co-exposure was only half of that in the corresponding single-exposure treatment (Fig. 5B, Table 1).
How does Ordospora respond to later arrival of Metschnikowia?
Under prior arrival of Ordospora, the viability of experimental Daphnia was significantly reduced in the co-exposure treatment, with only 60.0% of hosts remaining viable (CO:ORDO early:METS late) compared to 80.0% in single exposure (ORDO early) (Fig. 2C, Table 1). There was a tendency towards higher infectivity in single exposure (43.8%) compared with the co-exposure treatment (29.2%) (Fig. 3C, Table 1). Parasite growth did not differ between the single and co-exposure treatments (Fig. 4C, Table 1). However, the net output of Ordospora was still 3-fold lower in co-exposure than in the single-exposure treatment (Fig. 5C, Table 1).
How does Ordospora respond to prior infection by Metschnikowia?
Under late arrival of Ordospora, there was a tendency towards higher viability in single exposure, with respectively 97.3% (ORDO late) and 87.2% (CO:METS early:ORDO late) of surviving hosts (Fig. 2D, Table 1). Infectivity did not differ between these treatments, with respectively 69.4% in single exposure and 61.8% in co-exposure (Fig. 3D, Table 1). Parasite growth did not differ either between those treatments (Fig. 4D, Table 1). Consequently, the net output of Ordospora did not differ significantly between single and co-exposure (Fig. 5D, Table 1).
Host fitness
Exposure had a significant effect on host lifespan (F6,135 = 138.4; P < 0.001) and total offspring production (F6,135 = 74.46; P < 0.001). On average, control Daphnia lived 56 days post-exposure (CI95% ± 2.19; Fig. 6A) and produced 33 offspring (CI95% ±2.28; Fig. 6B). In comparison, hosts singly infected by Ordospora lived 38 days post-exposure (±2.89; Fig. 6A) and produced 23 offspring (±1.62; Fig. 6B), while those singly infected by Metschnikowia lived 17 days (±1.54; Fig. 6A) and produced only ten offspring (±1.56; Fig. 6B). Single-exposure treatments with opposite timing of infection did not differ significantly from each other (Appendix, Table S3). The reduction in host lifespan and total offspring production induced by coinfection was comparable to that of single infections by Metschnikowia, but much stronger overall than the effect of single infections by Ordospora. Post-hoc analyses of the rate of offspring production indicate that such differences in fecundity were mostly driven by lifespan (Fig. 6C). While exposure had a significant effect on the rate of offspring production (F6,135 = 2.376; P = 0.033), the only significant pairwise comparison occurred between the METS early and METS late treatments, with the former reducing host fecundity to a greater extent (Tukey's HSD: t-value: 3.315, P = 0.018; Appendix, Table S3).
Fig. 6.
Graphical representation of (A) lifespan post-exposure, (B) total offspring production and the resulting (C) rate of offspring production (number of offspring per day post-exposure) compared for individual Daphnia across the control and all six exposure treatments. Only individuals successfully infected by one (single exposure) or both parasites (co-exposure) were included in the non-control treatments. Error bars depict the standard error of the mean.
Discussion
By exposing the host D. magna to sequential infections of the gut-dwelling microsporidium, O. colligata and the haemolymph-infecting yeast, M. bicuspidata, we investigated the potential for priority effects at the within-host level, in a system of sympatric species. We simulated two orders of arrival, designed to reflect contrasting patterns of parasite succession. In sequential exposures where Metschnikowia arrived first (CO:METS early:ORDO late), parasite transmission traits (parasite infectivity, parasite growth) did not differ significantly from single exposures. However, in sequential exposures where Ordospora arrived first (CO:ORDO early:METS late), parasite growth was reduced for the fungal parasite. Though infectivity was not significantly impacted, there was also higher host mortality in this treatment, which contributed to a decrease in the net spore output of both parasites (i.e. a comprehensive measure of parasite fitness).
Performance of Metschnikowia under single vs sequential infections
Under prior residency of Metschnikowia, sequential exposures were not shown to influence its transmission potential, as none of the recorded parameters differed between single exposure (METS early) and co-exposure (CO:METS early:ORDO late). This apparent lack of effect was unexpected, as it somewhat contradicts previous findings involving this parasite. When pitting Metschnikowia against the ichthyosporean gut parasite Caullerya mesnili, Lohr et al. (2010b) found that given prior residency, Metschnikowia took longer to develop, and produced fewer spores in coinfection. Similarly, Clay et al. (2019) observed lower production of fungal spores in coinfected hosts, when Metschnikowia was first to arrive against the bacterium Pasteuria ramosa, as opposed to the treatment where it arrived second. Both studies suggest that Metschnikowia generally does not fare well under prior residency. However, the authors co-exposed Daphnia hosts to parasites that are considerably more virulent than Ordospora. Both C. mesnili and P. ramosa are known to induce complete castration of their hosts (Bittner et al., 2002; Ebert et al., 2004; Jensen et al., 2006; Lohr et al., 2010a). Parasites that shut down reproduction entirely (i.e. parasitic castration) are thought to redirect considerable amount or resources, that would normally support reproductive effort of the host, towards increased growth or survivorship instead (Baudoin, 1975). This difference in exploitation strategy may partly explain why Metschnikowia would experience strong priority effects against such virulent parasites, while demonstrating no apparent response to the later establishment of Ordospora.
By contrast, we found evidence for reduced transmission of Metschnikowia, when it was preceded by the gut parasite. Sequential exposure resulted in a 2-fold reduction of Metschnikowia's net spore output, which was seemingly driven by two parameters of parasite fitness. First, parasite growth of Metschnikowia was slightly reduced in sequential exposure (CO:ORDO early:METS late), as opposed to the single-exposure treatment (METS late). This effect may be attributed to prior resource sequestration by the gut parasite. Intracellular microsporidian parasites ensure within-host growth by scavenging ATP molecules from host cells, through the activity of nucleotide transporters (Tsaousis et al., 2008; Smith, 2009) and further interactions with host mitochondria (Terry et al., 1997). Considering that infection by Ordospora takes place in the gut epithelium, prior sequestration of resources at the interface between the gut lumen and the haemolymph (i.e. where Metschnikowia completes its development and reproduction cycle) seems plausible. Second, a significant reduction of host viability was recorded in hosts that were first exposed to Ordospora, prior to Metschnikowia (CO:ORDO early:METS late), which resulted in a large proportion of co-exposed hosts not progressing towards successful reproduction of Metschnikowia.
While the mechanism responsible for such high mortality is difficult to infer from our results, this pattern is reminiscent of the ultrainfection phenomenon first described by Sofonea et al. (2015). Ultrainfection occurs when two parasites display adaptive levels of virulence and growth in single infection, while double infection triggers ‘explosive’ levels of host mortality, that are normally not found in each respective species. For this reason, coinfections are often hidden in the population, as cases that do occur only exist for a brief span of time, quickly interrupted by host death (Sofonea et al., 2017). With regards to the present study, the CO:ORDO early:METS late treatment did result in excessive host mortality, which also contributed to a very low number of successfully coinfected hosts. A similar phenomenon has been described in nature, where interspecific coinfection of an insect host generates lethal levels of damage from a viral pathogen that is otherwise considered avirulent (Nazzi et al., 2012).
Additionally, it has been observed that prior infection by a gut parasite can modify the structural integrity of the gut in Daphnia, which in turn modulates the probability of fungal spores successfully crossing into the haemolymph (T. Stewart Merrill, personal communication). Thus, we suspected prior colonization of epithelial cells by Ordospora could have altered susceptibility to Metschnikowia; however, parasite infectivity did not differ from single exposure in this treatment.
Performance of Ordospora under single vs sequential infections
In single-exposure treatments, the overall infection success of Ordospora was lower when it was inoculated on day 5. Although we suspect possible heterogeneity between spore solutions may have contributed to this observation (as different parasite inoculates were used on days 5 and 7), age and body size-related effects could have further influenced infectivity (Izhar and Ben-Ami, 2015; Garbutt and Little, 2017). For instance, filtering rate and permeability of the gut epithelium (i.e. thickness of cell wall) in Daphnia have been shown to directly correlate with age and size class (Burns, 1969; Stewart Merrill et al., 2019). As D. magna can reach maturity starting from 7 days at 20°C (Lampert, 1993), the initial exposure of pre-adults Daphnia (i.e. from days 5–7) as opposed to potentially mature individuals (i.e. from days 7–9) may have influenced the parasite's infection success (Ben-Ami, 2019).
Independent of this observation, sequential exposure reduced transmission of Ordospora, when it was first to infect the host (CO:ORDO early:METS late). Contrary to our observations on Metschnikowia, these results seem to have been driven mostly by increased mortality of co-exposed hosts, as parasite growth did not differ between the single and co-exposure treatments. While our method for quantifying spores did not allow us to monitor the continuous shedding of propagules from live hosts, the number of spore clusters recorded in the gut of infected individuals increases exponentially throughout the course of infection (Mangin et al., 1995; Kirk et al., 2018), with each cluster bearing up to 60 infective stages (Kirk et al., 2019). This suggests that spore yield recorded upon fixation of the host can be used to approximate the parasite's progression along the gut epithelium (i.e. infection intensity) and overall reproductive success. Although previous coinfection experiments using Ordospora were not available for comparison, C. mesnili benefited from an increase in spore production, when it was first to arrive in coinfection with Metschnikowia (Lohr et al., 2010b). As mentioned above, the contrasting priority effects observed here may stem from distinct strategies of host exploitation and varying degrees of fitness impairment, as Ordospora is one of the least virulent endoparasites commonly found in Daphnia (Ebert, 2005).
Due to external factors, such as selective predation on infected individuals (Duffy et al., 2005; Johnson et al., 2006; Goren and Ben-Ami, 2017), Daphnia in their natural habitat may not experience such long lifespans as those observed in controlled conditions (instead, rarely surviving beyond 20 days; Lampert, 1993). In the present study, individuals which were successfully coinfected by both parasites experienced similar lifespan as those singly infected by Metschnikowia, but lived only half the span of those singly infected by Ordospora (Fig. 6A). Therefore, coinfections in nature may contribute fewer infective propagules to the overall transmission of Ordospora, especially when no benefit to coinfection was observed, that would help compensate this reduction in host lifespan.
From parasite phenology to sequential exposure
The phenology of symbionts often varies, causing them to emerge among a host population sequentially (Schmidt et al., 2007; Dumbrell et al., 2011). Because the probability of being the first to infect directly correlates with a parasite's prior prevalence in the population (Clay et al., 2018), differences in species emergence patterns may in turn facilitate the occurrence of priority effects at the within-host level. While Ordospora may reach very high prevalence in natural populations of D. magna (Ebert, 2001), reportedly nearing 40% in shallow eutrophic ponds (Decaestecker et al., 2005), much lower prevalences have been recorded for Metschnikowia in similar environments (<10%, Stirnadel and Ebert, 1997). Thus, co-occurrence of these two species could imply that a significant proportion of the host population may have already encountered Ordospora, around the time when Metschnikowia increases to peak prevalence (i.e. in the late summer).
Additionally, spores of these two parasites are likely to be found in separate locations of the water column. While epidemics of Ordospora typically start from infectious spore banks, following a period of inactivity from host populations (Mangin et al., 1995), subsequent infections are likely to result in the continuous shedding of spores from live hosts. Because infective stages are able to disperse in the water (Mangin et al., 1995; Kirk et al., 2018), these may be encountered as free-floating spores across the upper parts of the water column. By contrast, spores of Metschnikowia gradually build up in the sediment, where infected hosts sink to and decompose after succumbing to infection (Duffy and Hunsberger, 2019). However, selective predation of spore-bearing individuals may contribute to the occasional resuspension of the parasite in the water column, as non-damaged asci can remain infectious following their passage through a fish's digestive tract (Duffy, 2009). Due to particularly strong diel vertical migration behaviour in D. magna (De Meester, 1992), this species is especially prone to contamination from infectious spore banks (Decaestecker et al., 2002, 2004). However, differences in the likelihood of spore encounter may also be driven by individual variability in phototactic behaviour, which exhibits strong genotypic variation among clones of D. magna (De Meester, 1989; De Meester et al., 1994). For instance, positively phototactic genotypes may recruit a higher proportion of free-floating microsporidian spores during the day, while being exposed to buried spore banks during the night. Finally, it has been shown that D. magna individuals infected with Ordospora exhibit much deeper position than uninfected ones in artificial mesocosms (Fels et al., 2004). This suggests that prior infection by Ordospora may also influence host behaviour in such a way that secondary infections (e.g. by Metschnikowia) are facilitated in nature.
Within-host interactions between symbionts may scale up to influence host-parasite dynamics at the community level (Mordecai et al., 2016; Marchetto and Power, 2018; Karvonen et al., 2019), a phenomenon that has been demonstrated experimentally (Halliday et al., 2017). For instance, mechanisms of positive or negative frequency dependence may arise from system-specific priority effects (Clay et al., 2018). The unilateral priority effects highlighted in this study (i.e. reduced transmission under prior arrival of Ordospora) are likely to occur in populations where both parasites are sympatric. These may be of particular importance during the early phase of parasite emergence, when every successful infection helps to kick-start a parasite's successful outbreak in the environment. For instance, species that usually emerge later in the season (e.g. Metschnikowia) are effectively starting in an environment where most – if not all – available hosts may have previously encountered a competing parasite species (e.g. Ordospora). Parasites that tend to suffer from late residency might face a ‘critical early point’ in their epidemic curve, during which most infections with previously infected hosts could result in a suboptimal outcome, potentially slowing – if not preventing – their successful establishment and emergence in the environment.
Concluding remarks
Our results suggest that specific patterns of parasite succession, with prior emergence of the microsporidium Ordospora over the yeast Metschnikowia (i.e. a plausible scenario in natural populations) may limit the transmission of both species, due to (i) impaired spore production of the yeast and (ii) maladaptive levels of host mortality that are not found in single infections. We also highlight the inherent specificity of priority effects among common parasites of Daphnia, showing that contrasting responses to sequential infections can be observed across a microsporidian gut parasite and functionally similar species. Thus, we encourage further research to consider other assemblages of ecologically relevant parasites, while monitoring temporal succession patterns that are observed in the field. Changes in parasite phenology could be especially relevant in light of climate change: distinct species may react differently to specific environmental triggers – such as light, temperature or nutrient availability – that are known to stimulate the emergence of resting stages, transmission and within-host reproduction (e.g. Overholt et al., 2012; Kirk et al., 2018). Elevated freshwater temperatures may cause asymmetric shifts between the overlapping epidemic curves of waterborne parasites, which could have implications for the likelihood of sequential infections at the within-host level.
Acknowledgements
We would like to thank two anonymous reviewers for their support and helpful comments. We also thank Ursula Newen for the maintenance of Daphnia cultures, Mark Phillipo for linguistic help, Sabrina Gattis for providing the Ordospora cultures and Florent Sylvestre for advice on statistical analyses.
Author contributions
All authors conceived and designed the study. FM, LS and JW conducted data gathering. FM analysed the data. FM wrote the article with input from JW, FBA and SH. All authors approved the final version of the manuscript.
Financial support
This work was supported by a joint German–Israeli project (WO 1587/8-1 to JW, 0604317501 to FBA) funded by the German Science Foundation (DFG).
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0031182021001384.
click here to view supplementary material
Data
The data supporting this study can be found at https://doi.org/10.5281/zenodo.5223097
Conflict of interest
None.
References
- Alizon S, de Roode JC and Michalakis Y (2013) Multiple infections and the evolution of virulence. Ecology Letters 16, 556–567. [DOI] [PubMed] [Google Scholar]
- Baudoin M (1975) Host castration as a parasitic strategy. Evolution 29, 335–352. [DOI] [PubMed] [Google Scholar]
- Ben-Ami F (2019) Host age effects in invertebrates: epidemiological, ecological, and evolutionary implications. Trends in Parasitology 35, 466–480. [DOI] [PubMed] [Google Scholar]
- Ben-Ami F and Routtu J (2013) The expression and evolution of virulence in multiple infections: the role of specificity, relative virulence and relative dose. BMC Evolutionary Biology 13, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ami F, Mouton L and Ebert D (2008) The effects of multiple infections on the expression and evolution of virulence in a Daphnia-endoparasite system. Evolution 62, 1700–1711. [DOI] [PubMed] [Google Scholar]
- Ben-Ami F, Rigaud T and Ebert D (2011) The expression of virulence during double infections by different parasites with conflicting host exploitation and transmission strategies. Journal of Evolutionary Biology 24, 1307–1316. [DOI] [PubMed] [Google Scholar]
- Bittner K, Rothhaupt KO and Ebert D (2002) Ecological interactions of the microparasite Caullerya mesnili and its host Daphnia galeata. Limnology and Oceanography 47, 300–305. [Google Scholar]
- Burns CW (1969) Relation between filtering rate, temperature, and body size in four species of Daphnia. Limnology and Oceanography 14, 693–700. [Google Scholar]
- Carpenter SR, Kitchell JF and Hodgson JR (1985) Cascading trophic interactions and lake productivity. BioScience 35, 635–639. [Google Scholar]
- Carpenter SA, Vannatta JT and Minchella DJ (2021) Host exposure history and priority effects impact the development and reproduction of a dominant parasite. International Journal for Parasitology. doi: 10.1016/j.ijpara.2021.03.007 [DOI] [PubMed] [Google Scholar]
- Clay PA, Cortez MH, Duffy MA and Rudolf VH (2018) Priority effects within coinfected hosts can drive unexpected population-scale patterns of parasite prevalence. Oikos 128, 571–583. [Google Scholar]
- Clay PA, Dhir K, Rudolf VH and Duffy MA (2019) Within-host priority effects systematically alter pathogen coexistence. The American Naturalist 193, 187–199. [DOI] [PubMed] [Google Scholar]
- Codreanu R and Codreanu-Balcescu D (1981) On two Metschnikowia yeast species producing hemocoelic infections in Daphnia magna and Artemia salina (Crustacea, Phyllopoda) from Romania. Journal of Invertebrate Pathology 37, 22–27. [Google Scholar]
- Connell JH and Slatyer RO (1977) Mechanisms of succession in natural communities and their role in community stability and organization. The American Naturalist 111, 1119–1144. [Google Scholar]
- Dallas T, Holtackers M and Drake JM (2016) Costs of resistance and infection by a generalist pathogen. Ecology and Evolution 6, 1737–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaestecker E, De Meester L and Ebert D (2002) In deep trouble: habitat selection constrained by multiple enemies in zooplankton. Proceedings of the National Academy of Sciences 99, 5481–5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaestecker E, Lefever C, De Meester L and Ebert D (2004) Haunted by the past: evidence for dormant stage banks of Daphnia. Limnology & Oceanography 49, 1355–1364. [Google Scholar]
- Decaestecker E, Declerck S, De Meester L and Ebert D (2005) Ecological implications of parasites in natural Daphnia populations. Oecologia 144, 382–390. [DOI] [PubMed] [Google Scholar]
- De Meester L (1989) An estimation of the heritability of phototaxis in Daphnia magna Straus. Oecologia 78, 142–144. [DOI] [PubMed] [Google Scholar]
- De Meester L (1992) The phototactic behaviour of male and female Daphnia magna. Animal Behaviour 43, 696–698. [Google Scholar]
- De Meester L, Vandenberghe J, Desender K and Dumont HJ (1994) Genotype-dependent daytime vertical distribution of Daphnia magna in a shallow pond. Belgian Journal of Zoology 124, 3–9. [Google Scholar]
- De Roode JC, Helinski ME, Anwar MA and Read AF (2005) Dynamics of multiple infection and within-host competition in genetically diverse malaria infections. The American Naturalist 166, 531–542. [DOI] [PubMed] [Google Scholar]
- Duffy MA (2009) Staying alive: the post-consumption fate of parasite spores and its implications for disease dynamics. Limnology and Oceanography 54, 770–773. [Google Scholar]
- Duffy MA and Hunsberger KK (2019) Infectivity is influenced by parasite spore age and exposure to freezing: do shallow waters provide Daphnia a refuge from some parasites? Journal of Plankton Research 41, 12–16. [Google Scholar]
- Duffy MA, Hall SR, Tessier AJ and Huebner M (2005) Selective predators and their parasitized prey: are epidemics in zooplankton under top-down control? Limnology and Oceanography 50, 412–420. [Google Scholar]
- Duffy MA, Hall SR, Cáceres CE and Ives AR (2009) Rapid evolution, seasonality, and the termination of parasite epidemics. Ecology 90, 1441–1448. [DOI] [PubMed] [Google Scholar]
- Dumbrell AJ, Ashton PD, Aziz N, Feng G, Nelson M, Dytham C, Fitter AH and Helgason T (2011) Distinct seasonal assemblages of arbuscular mycorrhizal fungi revealed by massively parallel pyrosequencing. New Phytologist 190, 794–804. [DOI] [PubMed] [Google Scholar]
- Ebert D (1995) The ecological interactions between a microsporidian parasite and its host Daphnia magna. Journal of Animal Ecology 64, 361–369. [Google Scholar]
- Ebert D (2005) Ecology, Epidemiology, and Evolution of Parasitism in Daphnia. Bethesda, MD: National Library of Medicine. [Google Scholar]
- Ebert D, Lipsitch M and Mangin KL (2000) The effect of parasites on host population density and extinction: experimental epidemiology with Daphnia and six microparasites. The American Naturalist 156, 459–477. [DOI] [PubMed] [Google Scholar]
- Ebert D, Hottinger JW and Pajunen VI (2001) Temporal and spatial dynamics of parasite richness in a Daphnia metapopulation. Ecology 82, 3417–3434. [Google Scholar]
- Ebert D, Joachim Carius H, Little T and Decaestecker E (2004) The evolution of virulence when parasites cause host castration and gigantism. The American Naturalist 164, S19–S32. [DOI] [PubMed] [Google Scholar]
- Ezenwa VO, Etienne RS, Luikart G, Beja-Pereira A and Jolles AE (2010) Hidden consequences of living in a wormy world: nematode-induced immune suppression facilitates tuberculosis invasion in African buffalo. The American Naturalist 176, 613–624. [DOI] [PubMed] [Google Scholar]
- Fels D, Lee VA and Ebert D (2004) The impact of microparasites on the vertical distribution of Daphnia magna. Archiv für Hydrobiologie 161, 65–80. [Google Scholar]
- Fox J and Weisberg S (2019) Package ‘Car’. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- Garbutt JS and Little TJ (2017) Bigger is better: changes in body size explain a maternal effect of food on offspring disease resistance. Ecology and Evolution 7, 1403–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goren L and Ben-Ami F (2013) Ecological correlates between cladocerans and their endoparasites from permanent and rain pools: patterns in community composition and diversity. Hydrobiologia 701, 13–23. [Google Scholar]
- Goren L and Ben-Ami F (2017) To eat or not to eat infected food: a bug's dilemma. Hydrobiologia 798, 25–32. [Google Scholar]
- Graham AL (2008) Ecological rules governing helminth–microparasite coinfection. Proceedings of the National Academy of Sciences 105, 566–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haag KL, Larsson JI, Refardt D and Ebert D (2011) Cytological and molecular description of Hamiltosporidium tvaerminnensis gen. et sp nov., a microsporidian parasite of Daphnia magna, and establishment of Hamiltosporidium magnivora comb. nov. Parasitology 138, 447–462. [DOI] [PubMed] [Google Scholar]
- Haag KL, Pombert JF, Sun Y, de Albuquerque NRM, Batliner B, Fields P, Lopes TF and Ebert D (2020) Microsporidia with vertical transmission were likely shaped by nonadaptive processes. Genome Biology and Evolution 12, 3599–3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SR, Sivars-Becker L, Becker C, Duffy MA, Tessier AJ and Cáceres CE (2007) Eating yourself sick: transmission of disease as a function of foraging ecology. Ecology Letters 10, 207–218. [DOI] [PubMed] [Google Scholar]
- Hall SR, Becker CR, Duffy MA and Cáceres CE (2011) Epidemic size determines population-level effects of fungal parasites on Daphnia hosts. Oecologia 166, 833–842. [DOI] [PubMed] [Google Scholar]
- Halliday FW, Umbanhowar J and Mitchell CE (2017) Interactions among symbionts operate across scales to influence parasite epidemics. Ecology Letters 20, 1285–1294. [DOI] [PubMed] [Google Scholar]
- Halliday FW, Penczykowski RM, Barrès B, Eck JL, Numminen E and Laine AL (2020) Facilitative priority effects drive parasite assembly under coinfection. Nature Ecology & Evolution 4, 1510–1521. [DOI] [PubMed] [Google Scholar]
- Harrell FE Jr and Harrell MFE Jr (2019) Package ‘Hmisc’. CRAN R, 235-6.
- Hesse O, Engelbrecht W, Laforsch C and Wolinska J (2012) Fighting parasites and predators: how to deal with multiple threats? BMC Ecology 12, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood ME (2003) Dynamics of multiple infection and within-host competition by the anther-smut pathogen. The American Naturalist 162, 122–133. [DOI] [PubMed] [Google Scholar]
- Hothorn T, Bretz F and Westfall P (2008) Simultaneous inference in general parametric models. Biometrical Journal 50, 346–363. [DOI] [PubMed] [Google Scholar]
- Hoverman JT, Hoye BJ and Johnson PTJ (2013) Does timing matter? How priority effects influence the outcome of parasite interactions within hosts. Oecologia 173, 1471–1480. [DOI] [PubMed] [Google Scholar]
- Izhar R and Ben-Ami F (2015) Host age modulates parasite infectivity, virulence and reproduction. Journal of Animal Ecology 84, 1018–1028. [DOI] [PubMed] [Google Scholar]
- Jäger I and Schjørring S (2006) Multiple infections: relatedness and time between infections affect the establishment and growth of the cestode Schistocephalus solidus in its stickleback host. Evolution 60, 616–622. [DOI] [PubMed] [Google Scholar]
- Jensen KH, Little TJ, Skorping A and Ebert D (2006) Empirical support for optimal virulence in a castrating parasite. PLoS Biology 4, 1265–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PT, Stanton DE, Preu ER, Forshay KJ and Carpenter SR (2006) Dining on disease: how interactions between infection and environment affect predation risk. Ecology 87, 1973–1980. [DOI] [PubMed] [Google Scholar]
- Karvonen A, Jokela J and Laine AL (2019) Importance of sequence and timing in parasite coinfections. Trends in Parasitology 35, 109–118. [DOI] [PubMed] [Google Scholar]
- Kirk D, Jones N, Peacock S, Phillips J, Molnár PK, Krkošek M and Luijckx P (2018) Empirical evidence that metabolic theory describes the temperature dependency of within-host parasite dynamics. PLoS Biology 16, e2004608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk D, Luijckx P, Stanic A and Krkošek M (2019) Predicting the thermal and allometric dependencies of disease transmission via the metabolic theory of ecology. The American Naturalist 193, 661–676. [DOI] [PubMed] [Google Scholar]
- Krebs M, Routtu J and Ebert D (2017) QTL mapping of a natural genetic polymorphism for long-term parasite persistence in Daphnia populations. Parasitology 144, 1686–1694. [DOI] [PubMed] [Google Scholar]
- Lampert W (1993) Phenotypic plasticity of the size at first reproduction in Daphnia: the importance of maternal size. Ecology 74, 1455–1466. [Google Scholar]
- Lampert W (2006) Daphnia: model herbivore, predator and prey. Polish Journal of Ecology 54, 607–620. [Google Scholar]
- Lampert W (2011) Daphnia: Development of a Model Organism in Ecology and Evolution. Excellence in Ecology: Book 21. Oldendorf/Luhe, Germany: International Ecology Institute. [Google Scholar]
- Lange B, Reuter M, Ebert D, Muylaert K and Decaestecker E (2014) Diet quality determines interspecific parasite interactions in host populations. Ecology and Evolution 4, 3093–3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson JR, Ebert D and Vavra J (1997) Ultrastructural study and description of Ordospora colligata gen. et sp. nov. (Microspora, Ordosporidae fam. nov.), a new microsporidian parasite of Daphnia magna (Crustacea, Cladocera). European Journal of Protistology 33, 432–443. [Google Scholar]
- Levin S and Pimentel D (1981) Selection of intermediate rates of increase in parasite-host systems. The American Naturalist 117, 308–315. [Google Scholar]
- Lohr JN, Laforsch C, Koerner H and Wolinska J (2010a) A Daphnia parasite (Caullerya mesnili) constitutes a new member of the ichthyosporea, a group of protists near the animal–fungi divergence. Journal of Eukaryotic Microbiology 57, 328–336. [DOI] [PubMed] [Google Scholar]
- Lohr JN, Yin M and Wolinska J (2010b) Prior residency does not always pay off–co-infections in Daphnia. Parasitology 137, 493–500. [DOI] [PubMed] [Google Scholar]
- Mangin KL, Lipsitch M and Ebert D (1995) Virulence and transmission modes of two microsporidia in Daphnia magna. Parasitology 111, 133–142. [Google Scholar]
- Manzi F, Agha R, Lu Y, Ben-Ami F and Wolinska J (2020) Temperature and host diet jointly influence the outcome of infection in a Daphnia-fungal parasite system. Freshwater Biology 65, 757–767. [Google Scholar]
- Marchetto KM and Power AG (2018) Coinfection timing drives host population dynamics through changes in virulence. The American Naturalist 191, 173–183. [DOI] [PubMed] [Google Scholar]
- Mordecai EA, Gross K and Mitchell CE (2016) Within-host niche differences and fitness trade-offs promote coexistence of plant viruses. The American Naturalist 187, E13–E26. [DOI] [PubMed] [Google Scholar]
- Nazzi F, Brown SP, Annoscia D, Del Piccolo F, Di Prisco G, Varricchio P, Della Vedova G, Cattonaro F, Caprio E and Pennacchio F (2012) Synergistic parasite-pathogen interactions mediated by host immunity can drive the collapse of honeybee colonies. PLoS Pathogens 8, e1002735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak MA and May RM (1994) Superinfection and the evolution of parasite virulence. Proceedings of the Royal Society of London. Series B: Biological Sciences 255, 81–89. [DOI] [PubMed] [Google Scholar]
- Overholt EP, Hall SR, Williamson CE, Meikle CK, Duffy MA and Cáceres CE (2012) Solar radiation decreases parasitism in Daphnia. Ecology Letters 15, 47–54. [DOI] [PubMed] [Google Scholar]
- Pedersen TL (2020) Patchwork: The composer of plots (R package version 1.1.1). Available at https://CRAN.R-project.org/package=patchwork.
- Penczykowski RM, Hall SR, Civitello DJ and Duffy MA (2014) Habitat structure and ecological drivers of disease. Limnology and Oceanography 59, 340–348. [Google Scholar]
- Poulin R and Morand S (2000) The diversity of parasites. The Quarterly Review of Biology 75, 277–293. [DOI] [PubMed] [Google Scholar]
- R Core Team (2021) R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. ISBN 3-900051-07-0. Available at http://www.R-project.org/. [Google Scholar]
- Ratcliff FG, MacFarlane SA and Baulcombe DC (1999) Gene silencing without DNA: RNA-mediated cross-protection between viruses. The Plant Cell 11, 1207–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read AF and Taylor LH (2001) The ecology of genetically diverse infections. Science (New York, N.Y.) 292, 1099–1102. [DOI] [PubMed] [Google Scholar]
- Refardt D and Ebert D (2006) Quantitative PCR to detect, discriminate and quantify intracellular parasites in their host: an example from three microsporidians in Daphnia. Parasitology 133, 11–18. [DOI] [PubMed] [Google Scholar]
- Refardt D and Ebert D (2007) Inference of parasite local adaptation using two different fitness components. Journal of Evolutionary Biology 20, 921–929. [DOI] [PubMed] [Google Scholar]
- Rodrigues J, Brayner FA, Alves LC, Dixit R and Barillas-Mury C (2010) Hemocyte differentiation mediates innate immune memory in Anopheles gambiae mosquitoes. Science (New York, N.Y.) 329, 1353–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saebelfeld M, Minguez L, Griebel J, Gessner MO and Wolinska J (2017) Humic dissolved organic carbon drives oxidative stress and severe fitness impairments in Daphnia. Aquatic Toxicology 182, 31–38. [DOI] [PubMed] [Google Scholar]
- Sánchez KF, Huntley N, Duffy MA and Hunter MD (2019) Toxins or medicines? Phytoplankton diets mediate host and parasite fitness in a freshwater system. Proceedings of the Royal Society B: Biological Sciences 286, 20182231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt SK, Costello EK, Nemergut DR, Cleveland CC, Reed SC, Weintraub MN, Meyer AF and Martin AM (2007) Biogeochemical consequences of rapid microbial turnover and seasonal succession in soil. Ecology 88, 1379–1385. [DOI] [PubMed] [Google Scholar]
- Seifi A, Nonomura T, Matsuda Y, Toyoda H and Bai Y (2012) An avirulent tomato powdery mildew isolate induces localized acquired resistance to a virulent isolate in a spatiotemporal manner. Molecular Plant-Microbe Interactions 25, 372–378. [DOI] [PubMed] [Google Scholar]
- Smith JE (2009) The ecology and evolution of microsporidian parasites. Parasitology 136, 1901–1914. [DOI] [PubMed] [Google Scholar]
- Sofonea MT, Alizon S and Michalakis Y (2015) From within-host interactions to epidemiological competition: a general model for multiple infections. Philosophical Transactions of the Royal Society B: Biological Sciences 370, 20140303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofonea MT, Alizon S and Michalakis Y (2017) Exposing the diversity of multiple infection patterns. Journal of Theoretical Biology 419, 278–289. [DOI] [PubMed] [Google Scholar]
- Stewart Merrill TE and Cáceres CE (2018) Within-host complexity of a plankton-parasite interaction. Ecology 99, 2864–2867. [DOI] [PubMed] [Google Scholar]
- Stewart Merrill TE, Hall SR, Merrill L and Cáceres CE (2019) Variation in immune defense shapes disease outcomes in laboratory and wild Daphnia. Integrative and Comparative Biology 59, 1203–1219. [DOI] [PubMed] [Google Scholar]
- Stirnadel HA and Ebert D (1997) Prevalence, host specificity and impact on host fecundity of microparasites and epibionts in three sympatric Daphnia species. Journal of Animal Ecology 66, 212–222. [Google Scholar]
- Swanson SJ, Neitzel D, Reed KD and Belongia EA (2006) Coinfections acquired from Ixodes ticks. Clinical Microbiology Reviews 19, 708–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syller J and Grupa A (2016) Antagonistic within-host interactions between plant viruses: molecular basis and impact on viral and host fitness. Molecular Plant Pathology 17, 769–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry RS, Dunn AM and Smith JE (1997) Cellular distribution of a feminizing microsporidian parasite: a strategy for transovarial transmission. Parasitology 115, 157–163. [DOI] [PubMed] [Google Scholar]
- Toenshoff ER, Fields PD, Bourgeois YX and Ebert D (2018) The end of a 60-year riddle: identification and genomic characterization of an iridovirus, the causative agent of white fat cell disease in zooplankton. G3: Genes, Genomes, Genetics 8, 1259–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsaousis AD, Kunji ER, Goldberg AV, Lucocq JM, Hirt RP and Embley TM (2008) A novel route for ATP acquisition by the remnant mitochondria of Encephalitozoon cuniculi. Nature 453, 553–556. [DOI] [PubMed] [Google Scholar]
- Wickham H (2016) ggplot2-Elegant Graphics for Data Analysis. Cham, Switzerland: Springer International Publishing. [Google Scholar]
- Wilbur HM and Alford RA (1985) Priority effects in experimental pond communities: responses of Hyla to Bufo and Rana. Ecology 66, 1106–1114. [Google Scholar]
- Wolinska J, Giessler S and Koerner H (2009) Molecular identification and hidden diversity of novel Daphnia parasites from European lakes. Applied and Environmental Microbiology 75, 7051–7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuerthner VP, Hua J and Hoverman JT (2017) The benefits of coinfection: trematodes alter disease outcomes associated with virus infection. Journal of Animal Ecology 86, 921–931. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0031182021001384.
click here to view supplementary material
Data Availability Statement
The data supporting this study can be found at https://doi.org/10.5281/zenodo.5223097