1. Introduction
Human papillomavirus (HPV) is the most common sexually transmitted infection in the world (World Health Organization 2020; Brianti et al. 2017), affecting more than 600 million people worldwide (Gaspar et al. 2015). HPV can be transmitted through skin-to-skin contact or through contact between mucosal membranes (Brianti et al. 2017). HPV can cause a range of clinical diseases in the body, escalating in severity from benign warts to metastatic cancer. More than 200 types of HPV have been identified, which are broadly categorized into high-risk and low-risk types. Low-risk HPV types, such as HPV6 and 11, do not cause cancer. Instead, low-risk HPV types can generate genital warts around the anogenital region, known as condylomata acuminata, as well as benign tumors in the respiratory tract, known as recurrent respiratory papillomatosis or laryngeal papillomatosis. High-risk HPV types, including HPV16, 18, 31, 33, 35, 45, 51, 52, 56, 58, 59, and 68, can cause cancer and are often necessary for oncogenic transformation. Virtually, all cases of cervical cancer, 95% of cases of anal cancer, 70% of cases of oropharyngeal cancer, 65% of cases of vaginal cancer, 50% of cases of vulvar cancer, and 35% of cases of penile cancer are caused by high-risk HPV types. Specifically, HPV16 and 18 are associated with the majority of these cancers, including over 70% of all cervical cancers (Roden and Stern 2018), ~90% of anogenital cancers, and up to 75% of oropharyngeal cancers (Elrefaey et al. 2014; Walboomers et al. 1999; National Cancer Institute 2020).
It is estimated that around 5% of all cancers worldwide are caused by HPV (de Martel et al. 2012, 2017). Of the aforementioned HPV-associated diseases, cervical cancer accounts for the largest number of HPV-associated cancer cases (de Martel et al. 2017). Cervical cancer is the fourth most common cancer in women worldwide, and the second most common cancer in women living in low- and middle-income countries (LMICs). In 2018 alone, cervical cancer was responsible for over ~311,000 deaths (World Health Organization 2019; World Cancer Research Fund 2018). The global prevalence of cervical cancer has decreased since the 1950s largely due to early detection, improved HPV testing, prophylactic vaccination, and wider treatment availability; however, more than 85% of cervical cancer-associated deaths occurred in LMICs where infrastructure and access to preventions and treatments may be limited (World Health Organization 2018, 2019, Vaccarella et al. 2013). Additionally, we have seen global rates of HPV-associated oropharyngeal cancer in men on the rise, especially in North America and Northern Europe (Gillison et al. 2015). Unlike for cervical cancer in women, no routine screening tests exist for oropharyngeal cancers, largely due to the inability to detect precancerous lesions as well as subclinical or early-stage cancer (Roden and Stern 2018; Gillison et al. 2015; American Cancer Society 2018). This information highlights the need for methods to control HPV, especially oncogenic types.
The identification of HPV as the etiological factor for HPV-associated diseases has afforded the opportunity to manage these cancers through vaccination (Yang et al. 2016). Our increased understanding of the molecular biology of HPV has powered the development of HPV-targeted vaccines. HPV is a small, non-enveloped, double-stranded DNA virus belonging to the Papillomaviridae family. The HPV genome is comprised of ~8000 base pairs, which encode for eight major proteins, six early (E) genes and two late (L) genes (Yang et al. 2016; Graham 2010). Early genes, E1, E2, E4, E5, E6, and E7, contribute to the regulatory function of the viral genome, including DNA replication and transcription (Graham 2010). Late genes, L1 and L2, are known as the major and minor capsid proteins, respectively. The late genes comprise the viral capsid, which is responsible for viral transmission, spread, and survival (Graham 2010). Upon infection, the HPV viral genome is integrated into the host genome where it carries out processes necessary for viral replication and transcription. Specifically, E1 is involved in viral DNA replication, while E2 is involved in RNA transcription. E4 is involved in regulating the cytoskeleton network of infected cells, cell cycle arrest, and virion assembly. E5 is considered an oncogenic protein and is responsible for cell growth and differentiation as well as immune modulation (Yang et al. 2016; Graham 2010). Both E6 and E7 are oncoproteins expressed in transformed cells (Yang et al. 2016). E6 and E7 are responsible for the carcinogenesis of HPV-associated lesions and are necessary for the initiation and upkeep of HPV-associated malignancies. E6 inhibits apoptosis and differentiation through the degradation of the tumor suppressor gene p53 (Yim and Park 2005). E7 interacts with the Rb protein, a cell cycle regulator, rendering it inoperable. This interaction results in the unregulated proliferation of infected cells as well as the transformation into cancer (Yim and Park 2005). In most cases of HPV-associated cancer, the HPV viral DNA genome integrates into the host’s genome. The integration process leads to the deletion of early genes E1, E2, E4, and E5, and late genes L1 and L2. E2 is a negative transcriptional regulator for E6 and E7. The deletion of E2 leads to the disruption of normal cell cycle regulation by interacting with p53 and Rb, respectively. This results in the progression of HPV-associated cervical cancer (Yang et al. 2016). Further, the deletion of L1 and L2 during the integration process are what render prophylactic vaccines ineffective against established HPV-associated diseases (Yang et al. 2016).
HPV types are tissue-trophic and infect keratinocytes, preferentially propagating in epithelial mucosa (Egawa et al. 2015). Viral expression is associated with the differentiation of keratinocytes and viral shedding from superficial epithelial layers, which express L1 and L2 viral capsid proteins (Williams et al. 2011; Roden and Wu 2006). HPV infections are restricted to the basal epithelial cells, which are often shielded from circulating immune cells during surveillance. Because HPV infection often does not generate a host immune response, HPV DNA goes undetected, enabling the virus to continue to amplify, eventually spreading to and infecting neighboring cells. HPV is also non-lytic and does not generate an inflammatory response, meaning that individuals who are infected may not know their disease status. Only after HPV-associated tumor cell has been sufficiently amplified to a level where it can be detected by immune surveillance cells, does it mount an active immune response. Unfortunately, this often occurs during the later stages of HPV transformation, sometimes years after the initial HPV infection (Williams et al. 2011). Prophylactic vaccines have traditionally be used to prevent disease prior to infection. Current prophylactic HPV vaccines are used to deliver HPV L1 and/or L2 capsid antigens, which self-assemble to form a virus-like particle (VLP). These vaccines stimulate the immune generation of neutralizing antibodies against VLPs, which can prevent the acquisition of real HPV infection in healthy individuals. Unfortunately, neutralizing antibodies against HPV are incapable of controlling or killing existing HPV-infected and/or transformed cells. Instead, HPV antigen-specific cytotoxic T cells (CD8+ T cells) and helper T cells (CD4+ T cells) are necessary for the targeted killing of infected and/or transformed cells (Yang et al. 2016). Unlike prophylactic HPV vaccines, therapeutic HPV vaccines rely on T cell-mediated immune responses to target and kill infected cells. This is facilitated by antigen-presented cells (APCs), such as dendritic cells (DCs). DCs present HPV antigen through major histocompatibility class I (MHC-I) and class II (MHC-II) molecules for recognition by HPV antigen-specific CD8+ and CD4+ T cells, respectively.
When devising a therapeutic vaccine, the target antigen of choice requires significant consideration. Because L1 and L2 are deleted during the integration process of HPV into the host genome, they are not suitable target antigens for the development of therapeutic HPV vaccines. However, HPV oncoproteins E6 and E7 present as ideal targets for the development of therapeutic HPV vaccines. E6 and E7 are only expressed in transformed cells and are necessary for initiating and maintaining HPV-associated malignancies (Yang et al. 2016). Therefore, therapeutic HPV vaccines targeting E6 and E7 are safe and can circumvent immune tolerance against self-antigens (Yang et al. 2016).
2. Current Preventive HPV Vaccines
In the last decade, a total of three prophylactic HPV vaccines have been developed for commercial use, Cervarix™ (from GlaxoSmithKline), Gardasil®, Gardasil9® (from Merck). These prophylactic vaccines have provided an opportunity to prevent the acquisition of HPV infection in unexposed, healthy individuals. All three vaccines use an L1 VLP vaccine platform, which constitutes the non-infectious papillomavirus particles without the viral genome (Yang et al. 2016). Cervarix™ is a bivalent vaccine containing HPV16 and HPV18 VLPs produced in insect cells (Trichoplusia ni) using a baculovirus expression vector system. Cervarix™ also incorporates Adjuvant System 04 (comprised of monophosphoryl lipid A and an aluminum hydroxide salt) (U.S. Food and Drug Administraiton 2018) to enhance the body’s humoral immune responses after vaccination. Cervarix™ only protects against oncogenic HPV types HPV16 and HPV18; however, these HPV types are present in the majority of HPV-associated cancers (Elrefaey et al. 2014; National Cancer Institute 2019), and GlaxoSmithKline discontinued the marketing of Cervarix™ in the United States in 2016. Gardasil® is a recombinant quadrivalent vaccine prepared from HPV 6, 11, 16, and 18 VLPs. The L1 proteins are produced in yeast cells (Saccharomyces cerevisiae) and absorbed on an amorphous aluminum hydroxyphosphate sulfate adjuvant (U.S. Food and Drug Administration 2019). It provides protection against oncogenic types HPV16 and 18, as well as low-risk HPV types 6 and 11, which cause common genital warts.
Currently, around 15 oncogenic HPV types have been identified. While Gardasil® and Cervarix™ provide protection against the two most common oncogenic HPV types, HPV16 and HPV18, neither provide protection against any of the remaining ~13 oncogenic HPV types. To address this disparity in coverage, Gardasil®9 was developed to provide broader protection against more HPV types (Zhai and Tumban 2016; Manini and Montomoli 2018). Gardasil®9 is a recombinant nanovalent vaccine prepared from L1 VLPs of HPV6, 11, 16, 18, 31, 33, 45, 52, and 58. Gardasil®9 is produced using the same method as Gardasil® and contains an amorphous aluminum hydroxyphosphate sulfate adjuvant (U.S. Food and Drug Administration 2019) to enhance immunogenicity. Importantly, Gardasil®9 expanded existing vaccination coverage against HPV, protecting against the seven most common oncogenic HPV types (HPV16, 18, 31, 33, 45, 52, and 58) and two most common low-risk HPV types (HPV6 and 11) (Zhai and Tumban 2016; Immunization Action Coalition 2019). Gardasil®9 is currently the only HPV vaccine being distributed in the US and is licensed for females and males ages 9–45 (Immunization Action Coalition 2019). In fact, Cervarix™ and Gardasil® are no longer available for distribution or purchase in the US. Specifically, the sale of Cervarix™ was discontinued in the US due to low demand, while the sale of Gardasil® was discontinued in 2018, after the FDA-approved Gardasil®9 (Kaiser and Family Foundation 2018). However, Cervarix™ and Gardasil® are still widely used outside the use in both clinical practice and investigational trials.
In clinical testing, the efficacy of Gardasil® was assessed in 20,541 women and 4055 men ages 16–26. Vaccine efficacy, measured as protection from HPV types 6, 11, 16, and 18 after three doses, was evaluated in subjects who were HPV-naïve prior to the first vaccination dose. The results of this study showed that in both men and women aged 16–26 who were HPV-naïve, Gardasil® was effective at preventing the development of lesions caused by HPV6, 11, 16, and 18. Moreover, Gardasil® was shown to have: 98% efficacy against HPV16 and 18-associated cervical intraepithelial neoplasia grades 2 and 3 (CIN2/3) and adenomacarcinoma in situ (AIS); 100% efficacy against HPV16 and 18-associated vulvar intraepithelial neoplasia grades 2 and 3 (VIN2/3) and vaginal intraepithelial neoplasia grades 2–3 (VaIN2/3); 75% efficacy against HPV6, 11, 16, and 18-associated anal intraepithelial neoplasia grades 2–3 (AIN2/3); and 89% and 99% efficacy against HPV6 and 11-associated genital warts in males and females, respectively (Merck & Co. Inc. 2019).
Because Gardasil®9 was developed to protect against HPV strains not previously covered by the first generation of Gardasil®, a comparative clinical trial was led by Merck to empirically evaluate the efficacy of the two vaccines. The clinical study compared Gardasil® and Gardasil®9 in 14,204 women ages 16–26 worldwide. A total of 7099 women were randomized to receive Gardasil®9, while 7105 women were randomized to receive Gardasil®. Vaccine efficacy was evaluated in subjects who received three doses of vaccination and were HPV-naïve prior to the first vaccination dose. Compared to Gardasil®, Gardasil®9 demonstrated 97% clinical efficacy against HPV31, 33, 45, 52, and 58-associated CIN2/3, AIS, VIN2/3, and VaIN2/3, suggesting that Gardasil®9 provides protection against five more types of HPV (types 31, 33, 45, 52, and 58) than the first-generation vaccine Gardasil®. Moreover, since both vaccines are manufactured similarly and comprise four of the same HPV L1 VLPs the efficacy and effectiveness of Gardasil®9 against HPV6, 11, 16, and 18 were comparable to that of Gardasil® (Merck & Co. Inc. 2019). Notably, the efficacy of both Gardasil® and Gardasil®9 against oropharyngeal cancer was not tested in these trials, which could be attributed to the somewhat recent determination of the etiologic relationship between HPV and oropharyngeal cancer (Guo et al. 2016). An additional Phase III trial comparing Gardasil® to Gardasil®9 in 14,215 women confirmed the results of the first study, demonstrating that Gardasil®9 protected against 96.7% of CIN2/3, VIN2/3, and VaIN2/3 caused by HPV31, 33, 45, 52, and 58. Furthermore, the efficacies of Gardasil® and Gardasil®9 against HPV types 6, 11, 16, and 18 were also shown to be comparable. The investigators of this study also found that the geometric mean antibody titers (GMTs) one month after the third vaccine dose of Gardasil®9 were noninferior to Gardasil® for HPV6, 11, 16, and 18. Additionally, seroconversion for women in the Gardasil®9 group to all nine HPV types was >99% (Joura et al. 2015). Additional clinical trials have been developed to evaluate the efficacy of the described prophylactic HPV vaccines (Centers for Disease Control and Prevention 2015).
All three commercially available prophylactic HPV vaccines are administered intramuscularly in the arm muscle in a two-dose or three-dose regimen, spread out over the course of 6–12 months, depending on dose schedule (Centers for Disease Control and Prevention 2015; Merck & Co. Inc. 2017). However, in the last decade, recommended vaccination series have changed as a result of burgeoning data on dose recommendations as well as the development of Gardasil®9. Pre-adolescent girls (ages 9–15) now have the option to receive a two-dose HPV vaccination regimen at a 6-month or 12-month interval to protect against HPV. While this two-dose recommendation was first recommended by the World Health Organization (WHO) in 2015, a three-dose vaccine regimen is recommended for girls and women 15 years and older (Harper and DeMars 2017). The second scheduled vaccine dose is typically administered 1–2 months after the first dose, followed by the third dose 5–10 months later (Centers for Disease Control and Prevention 2015). Due to the novelty of Gardasil®9, there is little data to elucidate the ideal dose schedule for vaccination. In comparative studies, Gardasil®9 has demonstrated similar GMTs and seropositivity for anti-HPV6, 11, 16, and 18; however, the immunogenicity of two or three-dose HPV vaccine regimens are still being studied (Harper and DeMars 2017). To this end, a comparative phase III clinical trial is planned to determine the comparative immunogenicity of the two-dose to the three-dose vaccine schedule (Harper and DeMars 2017) (NCT02834637). Importantly, this study might not only uncover the optimal vaccination schedule for Gardasil®9, but it may also help inform future efforts in vaccine development to minimize necessary vaccine doses. Additional studies have studied the efficacy of a single dose of prophylactic HPV vaccine, demonstrating that it could provide similar protection when compared to two- to three-dose vaccination regimens (Safaeian et al. 2013; Kreimer et al. 2015). Several clinical trials investigating whether a single dose of HPV vaccine is efficacious in the prevention of HPV infection were recently completed or are ongoing (NCT03431246, NCT00635830, and NCT03675256, respectively).
3. Improving Preventive HPV Vaccine Development
The clinical efficacy of available prophylactic HPV vaccines represents a substantial improvement in the prevention of HPV and HPV-associated diseases for targeted-types, but problems in coverage and disease burden still remain. 85% of the burden of HPV and HPV-associated diseases occurs in LMICs, where infrastructure and access to HPV prevention, therapies, or treatments are often limited (World Health Organization 2018; Vaccarella et al. 2013). Furthermore, many persons living in LMICs face significant barriers to vaccination. The cost of the vaccine can pose a significant financial barrier at both the individual and institutional levels. Due to the cost associated with vaccination, many LMICs choose to fund vaccination for a single age group each year, rather than make the vaccine available to persons of all recommended age groups (Gallagher et al. 2018; Bruni et al. 2016). Likewise, the need for multiple doses (2–3 doses) of prophylactic HPV vaccines is a barrier for many, as persons may receive an incomplete vaccine schedule, which does not afford full immunologic protection. Another barrier to vaccination involves the physical access and availability of the vaccine. Many individuals in LMICs do not live in close proximity to a health clinic or a provider through which they can access and obtain the vaccine. Many LMICs also lack the infrastructure or capacity to store and distribute such vaccines (i.e., through cold chains) proving vaccine provision and dissemination a significant challenge.
Another challenge of prophylactic vaccines is limited cross-reactivity to multiple oncogenic HPV strains. Although individuals vaccinated with Gardasil®9 will be protected from seven of the most common oncogenic HPV types, including HPV16 and 18, Gardasil®9 still only covers fewer than half of the ~15 oncogenic HPV strains (Merck & Co. Inc. 2017), rendering vaccinated persons susceptible to subsequent infection. Because vaccination with the current prophylactic vaccine cannot protect close to 100% of all HPV infections, Pap smear screening or HPV testing for screening are still required. Although substantial progress has been made towards developing a more-protective HPV vaccine, continued efforts to improve prophylactic HPV vaccines are warranted. Broader, or even full-coverage, against oncogenic HPV types is a desirable attribute in future generations of prophylactic HPV vaccines that researchers should strive towards. Strategies to developed improved prophylactic HPV vaccines include, but are not limited to, the development of (1) L1-based capsomeres, (2) L2-based vaccines, and (3) chimeric L1–L2 based vaccines. Figure 1 summarizes the various strategies in the current, and the next generation of preventive HPV vaccines, including the newest multivalent VLP vaccine, Gardasil®9.
Fig. 1.
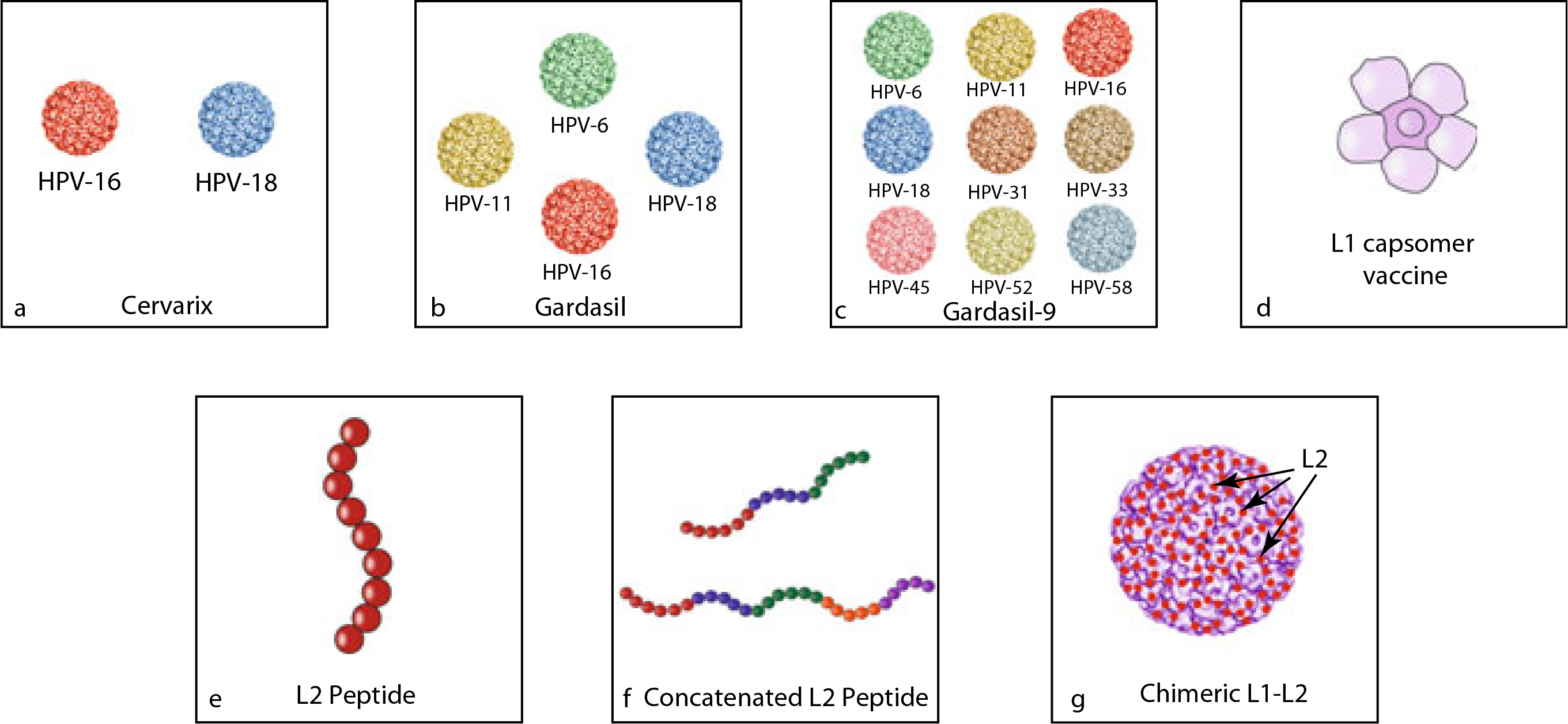
Schematic diagram to depict the current and next generation of preventive HPV vaccines. a Cervarix™ composed of HPV16 and HPV18 VLPs. b Gardasil® composed of HPV6, HPV11, HPV16, and HPV18 VLPs. c Gardasil®9 composed of HPV6, HPV11, HPV16, HPV18, HPV31, HPV33, HP-45, HPV52, and HPV58 VLPs. d L1 capsomer vaccine. e L2 peptide vaccine. f Concatenated L2 peptide vaccine. g Chimeric L1-L2 VLP vaccine with L2 on the surface
One potential method for creating new prophylactic HPV vaccines involves the development of L1 capsomere vaccines. Capsomeres are structural subunits, which self-assemble to form the virus capsid. HPV L1 VLPs are composed of 360 L1 monomers, which assemble into 72 pentavalent capsomeres (DiGiuseppe et al. 2017). L1 capsomeres can be purified from Escherichia coli, offering a cost-effective alternative to L1 VLP-based vaccines, which are produced in yeast cells (Schadlich et al. 2009). In preclinical models, L1 capsomere proteins produced in E. coli, attenuated measles virus, or recombinant Salmonella enterica serotype Typhi have successfully generated neutralizing antibodies against HPV-L1 and demonstrated immunogenicity comparable to that of VLPs (Barra et al. 2019). Thus, L1 capsomere vaccines may represent a potential lower-cost prophylactic HPV vaccine which provides comparable immunogenicity to existing VLP vaccines and potentially reduce the number of booster vaccinations currently required. Another potential approach to creating new prophylactic vaccines involves the use of the HPV minor capsid protein, L2-based vaccines, instead of L1 VLPs. The difficulties associated with manufacturing multivalent L1-VLP vaccines, such as Gardasil®9, limit the number of VLPs and hinder the vaccine’s protective capacity. Unlike L1, L2 is highly conserved between different HPV types and contains type-common epitopes. Therefore, the L2 from one HPV strain could potentially induce broader protection against multiple HPV types through cross-neutralizing antibodies, even across species (Roden and Stern 2018; Schellenbacher et al. 2017; Gambhira et al. 2006; Kaliamurthi et al. 2019). Currently, more than three preventive L2 VLP-based prophylactic HPV vaccines are underway for evaluation in early phase clinical trials (for review see Schellenbacher et al. 2017). One downside to L2-based vaccines is low immunogenicity. Antibody levels induced by L2 peptides are significantly inferior to those induced by L1 VLP vaccines. Hence, the development of L2-based vaccines necessitates strategies to enhance immunogenicity and antibody response in vivo (Schellenbacher et al. 2017).
In general, L2-based vaccines are less immunogenic than L1-based vaccines (Roden and Stern 2018). Additionally, the current gold standard for the detection of L1-specific antibodies cannot reliably detect an L2-specific immune response in vivo. To address this challenge, Day et al. created a test to detect L2-directed neutralizing antibodies with significantly improved sensitivity (Day et al. 2012). With an improved method of detection now available, several strategies have been implemented to enhance the immunogenicity of L2, many of which have shown great potential. For example, in a preclinical study, mice vaccinated with a combination of L2-peptides derived from eight HPV types displayed on the surface of PP& bacteriophage VLPs were protected from HPV pseudovirus challenge of all eight HPV types (Caldeira Jdo et al. 2010; Tumban et al. 2011). Other studies have also been used to enhance L2 immunogenicity, including the application of an E. coli-based concatenated multitype L2 fusion protein with multitype cross-neutralizing epitopes (Jagu et al. 2009), the oral administration of HPV16 L2 expressed on the surface of Lactobacillus casei (Yoon et al. 2012), and the administration of HPV16 L2 protein with bacterial thioredoxin, and T cell stimulator (Rubio et al. 2009). While L2-based vaccines have the potential to confer greater cross-reactivity, the immunogenicity of L2 proteins remain low. For this reason, more potent adjuvants and display methods continue to be explored. Currently, however, no L2-peptide-based prophylactic HPV vaccines have been approved for clinical trials (Kaliamurthi et al. 2019).
The potent immunogenicity of L1 vaccines and the broad cross-protection provided by L2 can also potentially be exploited through the combination in the form of chimeric L1/L2 VLPs. A single copy of the L2 protein is present in each L1 pentavalent capsomere, thus, each HPV virion contains 72 copies of the L2 protein (Kaliamurthi et al. 2019). L2 plays a critical role in the assembly of L1 into VLPs and has been demonstrated to facilitate the encapsulation of the viral genome (Kaliamurthi et al. 2019). Because L2 is less abundant than L1 and is predominantly found in the interior of the VLP, replacing some L1 immunodominant epitope regions of the VLP surface with a neutralizing epitope of L2 may generate stronger immunogenic, cross-protective immune responses against multiple HPV types. In L1-based vaccine models, the surface expression of the neutralizing epitope of L2 is necessary for the generation of L2 neutralizing antibodies. In a recent study, Kaliamurthi et al. constructed an L2-based chimeric HPV vaccine (SGD58) using two selected epitope sequences on the N-terminal region of the L2 sequence of HPV58 [the fourth most common high-risk HPV type in the world (Zhai and Tumban 2016)], two Toll-like receptors (TLR) adjuvants (Flagellin and RS09), and two T helper epitopes (PADRE and TpD) (Kaliamurthi et al. 2019). While this chimeric vaccine has not been tested in vivo, SGD58 demonstrated immunologic properties capable of producing both humoral and cellular immune responses against HPV through immunomics testing in vitro. The SGD58 vaccine also demonstrated cross-protection against 15 different high-risk HPV types (Kaliamurthi et al. 2019). Another candidate chimeric L1–L2 based vaccine uses the RG1 epitope, a single L2 epitope. RG1 can be incorporated into the capsid surface DE loop of HPV16 L1 or HPV18 L1 to create a chimeric L1–L2 based VLP vaccine. In preclinical studies, RG1-VLPs provided broad protection against heterologous high-risk HPV types (Schellenbacher et al. 2017; Boxus et al. 2016; Gambhira et al. 2007a, b). Specifically, chimeric RG1-VLP vaccines have demonstrated protection against challenge of high-risk HPV types 16, 18, 26, 32, 33, 34, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, and low-risk HPV types 6, 43, and 44 (Schellenbacher et al. 2013). Currently, chimeric RG1-VLPs are under cGMP production and are planned for testing in phase I clinical studies. RG1-VLPs offer a promising next-generation vaccine for wider protection against HPV (Schellenbacher et al. 2013, 2017). In short, the inclusion of the immunodominant neutralizing epitopes of L2, such as RG1, into the L1 VLPs offer a promising prophylactic HPV vaccine capable of inducing broad-spectrum neutralizing antibodies against different HPV types.
4. Strategies for Therapeutic HPV Vaccine Development
4.1. Introduction to Therapeutic HPV Vaccines
While prophylactic HPV vaccines have been hugely successful in averting HPV infections, they are incapable of treating or eliminating existing HPV infections or HPV-associated lesions. Given that HPV is the most common sexually transmitted infection (STI), virtually anyone who is sexually active is susceptible to HPV exposure or infection during their lifetime (Yang et al. 2016; Centers for Disease Control and Prevention 2017). Because HPV infection is the known etiologic factor for HPV-associated diseases, including nearly all cases of cervical cancer, therapeutic HPV vaccines represent an ideal method for the eradication of HPV-infected cells and HPV-associated tumors. Most individuals who develop an HPV infection will clear the viral infection naturally through their immune system. However, individuals who are unable to clear the infection can develop persistent HPV infections, which may progress into precancerous lesions and eventually, invasive cancer. The progression of an HPV infection into invasive cancer can take years, and remain asymptomatic. Due to the latent nature of the HPV virus, regular screening is recommended to track the progression or regression of the disease. In a prolonged chronic infection, there is a considerable window for secondary preventive treatment for infections caught by cytologic screening and HPV DNA testing. Currently, the US Preventive Services Task Force (USPSTF), American Cancer Society (ACS), and the American College of Obstetricians and Gynecologists (ACOG) recommend cytologic screening (Pap smears) every three years in women aged 21–65 (U.S. Preventive Services Task Force 2012; Centers for Disease Control and Prevention 2012). In 2014, the FDA approved an HPV screening test for primary cervical cancer screening. Primary HPV testing, as well as HPV co-testing (Pap smear and HPV testing), have become widely accepted and utilized in clinical practice (Cooper and Saraiya 2017). While methods of early detection for HPV-associated diseases have seen significant improvement, these detection and screening strategies are still limited or impossible in some settings. Furthermore, some HPV-associated diseases, such as oropharyngeal cancer, do not have methods for routine screening (American Cancer Society 2018). Therefore, efficacious therapeutic HPV vaccines that can selectively target HPV-infected cells during HPV transformation and carcinogenesis represent an ideal strategy to treat HPV infection or HPV-associated diseases, as well as prevent the development of advanced cancer. If therapeutic vaccines are able to eradicate transformed cells before disease progression, the disease burden of HPV and HPV-associated malignancies worldwide may see a drastic decline.
Many different platforms have been used to develop therapeutic HPV vaccines, which have been tested at various phases in preclinical and clinical trials. Characteristics of an ideal therapeutic vaccine include (1) safety; (2) ability to mount a potent HPV antigen-specific T cell-mediated immune response; (3) tumor-targeting specificity; (4) lasting efficacy; (5) cost-effective; and (6) minimal dose requirements. The ability of a therapeutic vaccine to elicit antigen-specific T cell-mediated killing is vital to the vaccine’s efficacy in recognizing and targeting transformed cells, and evading healthy cells. Reduced production and storage cost is also a highly desirable trait of any vaccine in order to increase access and availability of therapeutic vaccines for patients with persistent HPV infections or HPV-associated cancers worldwide. In addition, the need for fewer doses of the therapeutic HPV vaccine can potentially improve vaccination compliance and may help reinforce vaccination receipt in targeted populations. As described earlier, E6 and E7 are ideal targets for therapeutic HPV vaccines. To this end, several types of therapeutic HPV vaccines targeting E6 and/or E7 antigens have been developed and undergone preclinical and clinical studies, including live-vector-based, peptide-based, protein-based, dendritic cell-based, DNA-based, and combination vaccines. A graphic representation of how therapeutic HPV vaccines harness the host’s immune system to fight HPV infection and associated disease is provided in Fig. 2 (adapted from Cheng et al. 2018).
Fig. 2.
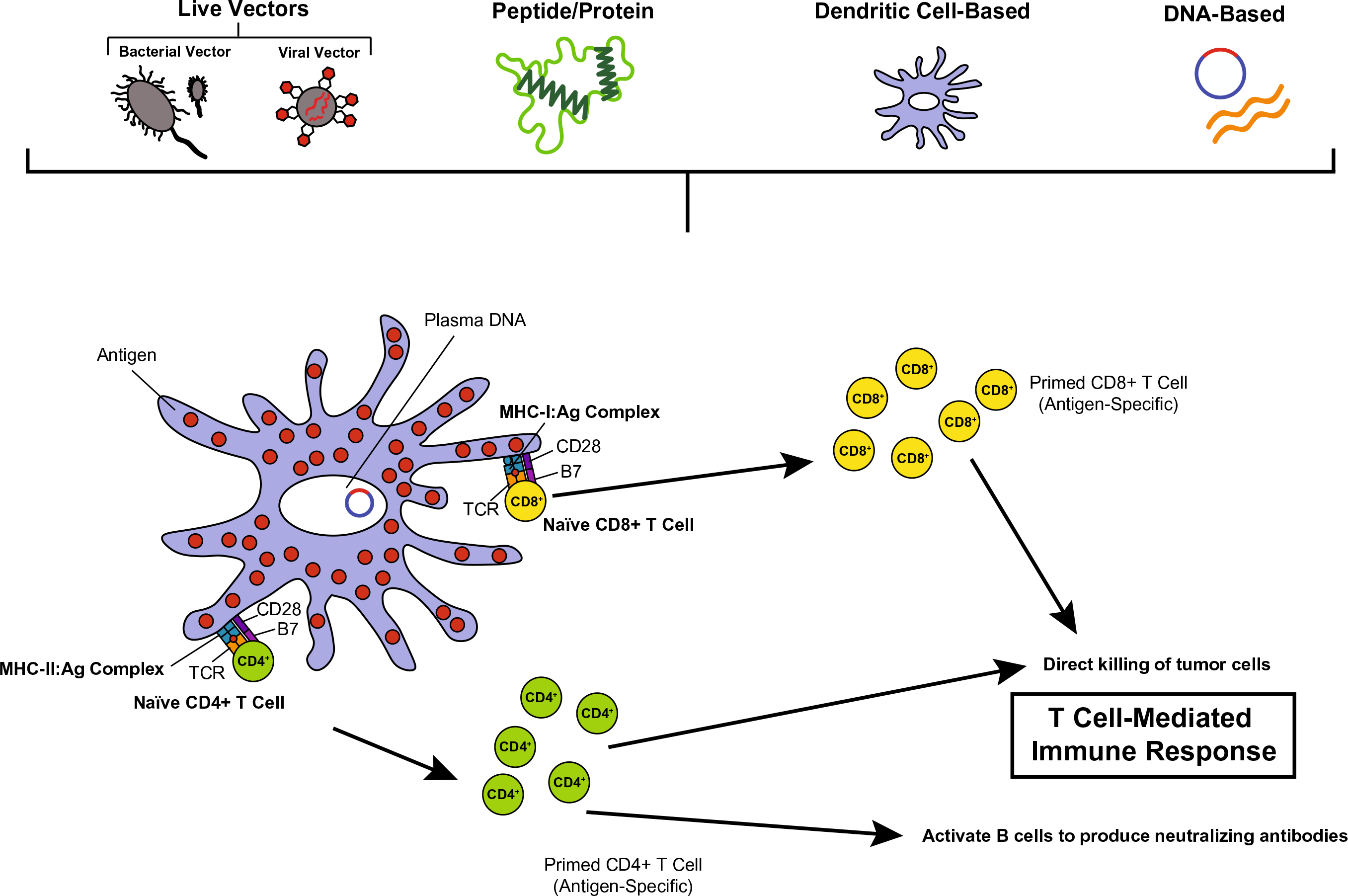
Therapeutic HPV vaccination schematic. Therapeutic vaccines activate the adaptive immune system by targeting E6 and/or E7 antigen(s), producing a cell-mediated immune response for the control or treatment of HPV infection or HPV-associated disease. Therapeutic HPV vaccination methods include, live-vector-based vaccines (bacterial vector or viral vector); peptide- or protein-based vaccines; dendritic cell-based vaccines; and DNA-based vaccines
4.2. Live Vector-Based Therapeutic HPV Vaccines
Live vector vaccines utilize live bacterial or viral vectors as a vehicle to deliver recombinant antigens. These live vectors infect the host, replicate in the body, and spread the antigen in order to mount an immune response. Live vector-based therapeutic HPV vaccines can deliver E6 and/or E7 antigens to APCs to stimulate antigen presentation to immune cells through the MHC-I MHC-II pathways. Live vector-based vaccines are highly immunogenic and capable of mounting robust humoral and cell-mediated immune responses. One challenge to live vector-based vaccines is the potential safety risk they pose, particularly to individuals who are immunocompromised (Yang et al. 2016; da Silva et al. 2014).
4.2.1. Bacterial Vectors
Several bacterial vectors have been explored for the development of therapeutic HPV vaccines. Among these, Listeria monocytogenes, a facultative gram-positive intracellular bacterium, has garnered significant attention as a particularly promising vector for the delivery of HPV antigens (Guirnalda et al. 2012). HPV vaccine antigens can be expressed through fusion to the pore-forming toxin, listeriolysin O (LLO). Fused vaccine antigens are then processed and presented through both the MHC-I and MHC-II pathways. Live L. monocytogenes-based vaccines have been shown to induce both antigen-specific CD8+ and CD4+ T cell responses (Kim and Kim 2017; Cory and Chu 2014; Wallecha et al. 2013). In preclinical studies, L. monocytogenes-based HPV E7 vaccines have been shown to stimulate a potent E7-specific CD8+ T cell response and were able to slow tumor growth and reduce tumor burden in both transgenic and wild-type mice (Gunn et al. 2001; Lin et al. 2002; Verch et al. 2004; Hussain and Paterson 2004; Sewell et al. 2004; Souders et al. 2007). In response to the demonstrated immunogenicity of L. monocytogenes-based HPV E7 vaccines in preclinical studies, this research has been translated into clinical studies. For example, the vaccine ADXS11–001 is a live, attenuated L. monocytogenes bacterial vector in which HPV16 E7 protein is fused to a modified LLO molecule (Cory and Chu 2014; Miles et al. 2017). In preclinical studies, ADXS11–001 mounted strong humoral and E7 antigen-specific CD8+ T cell immune responses. In phase I/II clinical trials, ADXS11–001 has demonstrated efficacy in women with cervical cancer (Yang et al. 2016; Cory and Chu 2014; Miles et al. 2017). Currently, two phase II clinical trials are ongoing to assess the safety and efficacy of ADXS11–001 in patients with HPV-associated head and neck (NCT02002182) and cervical cancer, respectively (NCT01266460) (Yang et al. 2016) (for review see Miles et al. 2017). Another phase II clinical trial evaluating the efficacy of ADXS11–001 in patients with anorectal cancer was recently finished recruitment (NCT02399813). While clinical trial data has not yet been reported, ADXS11–001 has shown promising antitumor activity in patients with HPV-associated diseases in multiple studies and was well-tolerated in patients. These findings, along with the ongoing clinical trial findings may lay the groundwork for phase III clinical trials and the potential introduction of ADXS11–001 in the clinic (Guirnalda et al. 2012; Miles et al. 2017). In addition, other attenuated bacterial vectors can also be used to deliver antigens of interest to APCs, including Salmonella (Buttaro and Fruehauf 2010; Le Gouellec et al. 2012), Shingella, and E. coli (Yang et al. 2016; Buttaro and Fruehauf 2010; Hitzeroth 2018).
4.2.2. Viral Vectors
In addition to bacterial vectors, live viral vector-based HPV vaccines have been studied in preclinical and clinical studies due to their high immunogenicity. Viral vectors, including adenoviruses, adeno-associated viruses, alphaviruses, lentiviruses, and vaccinia viruses have been used to deliver HPV E6, E7, and E2 antigens (Yang et al. 2016; Kim and Kim 2017; Hung et al. 2008). Of these viral vectors, vaccinia virus has demonstrated the greatest immunogenic potential in clinical studies. The vaccinia virus is an enveloped, double-stranded DNA virus in the Poxviridae family. In particular, vaccinia virus represents a promising viral vector for vaccine delivery because it has a large genome and is highly infectious (Yang et al. 2016). In the past two decades, several vaccinia-based therapeutic HPV vaccines have been studied in clinical trials. The first vaccinia-based vaccine trial was a phase I/II trial using a recombinant vaccinia virus expressing HPV16 and 18 E6/E7 proteins (TA-HPV) in patients with advanced cervical cancer (Borysiewicz et al. 1996). Subsequent phase I/II clinical trials have evaluated the safety and immunogenicity of TA-HPV in patients with early-stage cervical cancer (Kaufmann et al. 2002), VIN (Davidson et al. 2003), and VaIN (Baldwin et al. 2003). Through various clinical studies, TA-HPV was found to be safe and successful in mounting a vaccinia-specific antibody, and HPV antigen-specific CD8+T cell-mediated response (Borysiewicz et al. 1996; Kaufmann et al. 2002; Davidson et al. 2003; Baldwin et al. 2003).
Another vaccinia-based therapeutic HPV vaccine MVA-E2, is a modified vaccinia Ankara virus (MVA) encoding bovine papillomavirus type 1 (BPV-1) E2 protein (Corona Gutierrez et al. 2004; Vici et al. 2016). In several early phase I/II clinical trials, MVA-E2 led to significant therapeutic effects in patients with CIN1/2/3 lesions, including complete regression of precancerous lesions and generation of robust HPV antigen-specific immune responses (Corona Gutierrez et al. 2004; Garcia-Hernandez et al. 2006)). More recently, MVA-E2 was tested in a phase III clinical study for the treatment of anogenital HPV-associated intraepithelial lesions (Rosales et al. 2014) in a total of 1176 female and 180 male patients. Patients received MVA-E2 vaccination at the lesion-site and were then monitored using colposcopy (females only) and cytology for lesion progression/regression. After MVA-E2 vaccination, ~90% of female study patients and all-male study patients had complete elimination of intraepithelial lesions. Furthermore, all patients in the study treated with MVA-E2 developed MVA-E2-specific antibodies, as well as cell-mediated cytotoxic activity, specifically against HPV-transformed cells. The MVA-E2 vaccine was found to eliminate CIN1/2/3 lesions as well as most other anogenital lesions. The results of this study demonstrate the therapeutic potential of the MVA-E2 vaccine for the treatment of HPV-associated anogenital intraepithelial lesions in both males and females (Rosales et al. 2014).
Another MVA-based therapeutic HPV vaccine, TG4001, has been tested in clinical studies. TG4001 is a suspension of MVATG8042 vector particles. MVATG8042 vector particles consist of an attenuated recombinant MVA, encoding modified HPV16 E6 and E7 proteins, and human interleukin-2 (IL-2) (Yang et al. 2016; ***Brun et al. 2011). In phase II clinical trial, the safety and efficacy of TG4001 were evaluated in 21 patients with HPV16-associated CIN2/3. All patients received three weekly subcutaneous injections of TG4001. Regression of CIN2/3 lesions and clearance of HPV infection were monitored using cytology, colposcopy, and DNA/mRNA detection. After six months of vaccination, nearly half the women in the study had a clinical response, showing improvement in their infection or lesion regression. Out of the ten responders in this study, HPV16 DNA clearance was observed in eight individuals, HPV16 mRNA clearance in seven individuals. Further, no recurrence of high-grade lesions was observed for 12 months after treatment (Yang et al. 2016; Kim and Kim 2017; Brun et al. 2011). Another ongoing phase Ib/II clinical trial is assessing the safety and efficacy of TG4001 in combination with avelumab in patients with HPV16-associated oropharyngeal squamous cell carcinoma of the head and neck (NCT03260023). While live vector-based therapeutic HPV vaccines have shown promising results in clinical studies, they pose a potential safety risk, especially for individuals who may be immunocompromised. Due to the possibility of inducing vector-specific neutralizing antibodies and/or having pre-existing vector-specific immunity, live vectors are not able to be repeatedly administered.
4.3. Peptide-Based Therapeutic HPV Vaccines
Short peptides derived from HPV antigens can be delivered to DCs for processing and presentation on MHC-I molecules in order to activate an antigen-specific immune response. Production of peptide vaccines involves the prior identification of specific CD8+ cytotoxic and CD4+ helper T cell epitopes of HPV antigens. Peptide-based vaccines offer several advantages over other therapeutic vaccine types; they are safe, stable, and easy to produce (Cheng et al. 2018). However, peptide-based vaccines also suffer from low immunogenicity and MHC restriction, which ultimately affects their strength and efficacy (Yang et al. 2016; Lin et al. 2010).
Peptide-based vaccines often require lipids or other adjuvants, such as chemokines, cytokines, or TLR ligands to enhance immunogenicity (Yang et al. 2016). Specific adjuvants that have been previously employed to enhance the immunogenicity of peptide-based therapeutic HPV vaccines include aluminum adjuvants (Khong and Overwijk 2016), immunoglobulin G fragment (Qin et al. 2005), streptavidin fused to the extracellular domain of murine 4–1BBL (Sharma et al. 2009), DC stimulatory cytokine bryostatin (Yan et al. 2010), and TLR agonists (Khong and Overwijk 2016; Daftarian et al. 2006; Wu et al. 2010; Zhang et al. 2010; Zwaveling et al. 2002). Another limitation of peptide-based vaccines is that they are MHC-specific, which can lead to challenges for large-scale vaccine production and treatment of HPV-associated diseases (Yang et al. 2016; Su et al. 2010). One potential strategy to circumvent such a challenge is to employ the use of overlapping long-peptide vaccines. Numerous HPV16 synthetic long-peptide vaccines (e.g., HPV16-SLP) have been studied in clinical trials to evaluate therapeutic effect against HPV-associated disease. A phase II study in patients with low-grade abnormalities of the cervix demonstrated that two doses of the HPV16-SLP vaccine were capable of eliciting a robust HPV16-specific T cell response lasting for more than one year (de Vos van Steenwijk et al. 2014). In a more recent study, Welters et al. explored whether HPV16-SLP vaccination could be combined with standard chemotherapy to enhance immunogenicity in patients with advanced cervical cancer (Yang et al. 2016; Welters et al. 2016). From this study, HPV16-SLP vaccination was found to enhance T cell response levels in patients, which remained unchanged even after six cycles of chemotherapy. While this vaccination regimen did not demonstrate significant tumor regression, the HPV16-SLP vaccine was observed to be well-tolerated with minimal adverse effects. As a result of this study, additional phase I and II clinical trials have been designed to evaluate the therapeutic potential of HPV16-SLP vaccines in advanced or recurrent cervical cancer (NCT02128126), as well as in other HPV-associated malignancies (NCT01923116) (Welters et al. 2016). Another method to enhance therapeutic HPV vaccine potency is the administration of therapeutic HPV vaccines in combination with immune checkpoint blockade, which has become popularized in recent years. Recently, a clinical study investigating the safety of an HPV peptide vaccine (ISA101) and nivolumab was completed in patients with HPV16+ incurable/recurrent solid tumors. In this study, patients received nivolumab, an anti-programmed cell death 1 (PD-1) antibody and ISA101 peptide vaccine, a therapeutic HPV16-SLP vaccine containing nine HPV16 E6 synthetic peptides, and four HPV16 E7 synthetic peptides in order to determine whether ISA101 amplified the efficacy of nivolumab in patients with incurable HPV16+ cancer (NCT02426892). The results of this study showed that, out of 24 patients with oropharyngeal cancer, eight patients responded, resulting in an overall response rate of 33% and median overall survival of 17.5 months. Based on the results of this trial, future studies investigating the efficacy of ISA101 and immune checkpoint blockade are warranted (Massarelli et al. 2019).
Several additional studies have been conducted to evaluate the immunogenicity of various peptide-based therapeutic HPV vaccines. In a phase I dose-escalation study, 31 patients with high-grade squamous intraepithelial lesions (HSIL) received PepCan, a therapeutic HPV vaccine containing four cGMP-manufactured synthetic peptides covering HPV16 E6 and Candin® as an adjuvant. No dose-limiting toxicities were observed in this study (Coleman et al. 2016). Varying histologic regression rates were observed and correlated with increasing vaccination dose (Coleman et al. 2016). Due to the demonstrated safety of PepCan, a phase II clinical trial to evaluate the efficacy and safety of PepCan in patients with HSIL is ongoing (NCT02481414). Similarly, another phase I/II clinical trial is underway investigating the safety and efficacy of PepCan in patients with head and neck cancer (NCT03821272). In a phase I dose-escalation study of GL-0810, an HPV peptide-based vaccine with Montanide™ and granulocyte-macrophage colony-stimulating factor (GM-CSF) as adjuvants, patients with recurrent/metastatic squamous cell carcinoma of the head and neck (SCCHN) were shown to have an immune response of the vaccine, which was also well-tolerated (Zandberg et al. 2015). In addition, two more phase I clinical trials was designed to evaluate the safety and immunogenicity of PDS0101, a peptide-based therapeutic HPV vaccine. One of these trials was recently completed in women with high-risk HPV infection of CIN1 lesions (NCT02065973), while the other trial, a phase Ib/II trial in human leukocyte antigen (HLA)-A*02 positive patients with incurable HPV16-associated oropharyngeal, cervical, and anal cancer, is still ongoing (NCT02865135) (Yang et al. 2016).
4.4. Protein-Based Vaccines
Protein-based vaccines have also been developed as potential therapeutic vaccines for HPV-associated cancer. Not only are protein-based vaccines safer than live vector-based vaccines, but they also contain all HLA-binding epitopes, enabling protein-based vaccines to circumvent MHC restriction. However, one downside to protein-based vaccines is that they suffer from low immunogenicity. Additionally, most protein-based vaccines are presented through the MHC-II pathway resulting in the generation of antibody rather than antigen-specific cytotoxic T cell immune response (Su et al. 2010). Strategies to improve the immunogenicity and MHC-I processing and presentation of protein-based vaccines have been widely studied. To this end, numerous adjuvants, fusion proteins, and immunostimulating molecules have been tested, including the liposome-polycation-DNA (LPD) adjuvant (Cui and Huang 2005), saponin-based ISCOMATRIX (Frazer et al. 2004), and TLR agonist s (Kang et al. 2011).
One protein-based vaccine which has shown efficacy against HPV-associated diseases is TA-CIN, a fusion protein-based vaccine consisting of HPV16 E6, E7, and L2 (van der Burg et al. 2001). In several phase I/II clinical trials, TA-CIN has been shown to be safe and immunogenic against HPV infection. Additionally, in previous a phase II clinical trial, TA-CIN was administered in combination with imiquimod, a topical immunomodulator in patients with high-grade VIN. The combination treatment was well-tolerated in patients and these “responders” had increased levels of infiltrating CD4+ and CD8+ T cells both locally and in HPV-associated lesions (Daayana et al. 2010). Currently, a phase I clinical trial to determine the safety and feasibility of TA-CIN in patients with HPV16-associated cervical cancer is ongoing (NCT02405221). Another protein-based therapeutic HPV vaccine, which targets both HPV16 and 18 has undergone clinical studies (Van Damme et al. 2016). GTL001, is composed of recombinant HPV16 and 18 E7 proteins fused to catalytically inactive Bordetella pertussis CyaA expressed in E. coli (Yang et al. 2016; Van Damme et al. 2016). In a phase I clinical trial, GTL001 demonstrated tolerability and immunogenicity in women with HPV16 or 18 infections with normal cytology (Van Damme et al. 2016). A phase II clinical trial assessing the efficacy of GTL001 in women with HPV16 or 18 infections with normal cytology or atypical squamous cells of undetermined significance (ASC-US)/LSIL was recently completed; however, results have not yet been published (NCT01957878). Another phase II clinical trial to test the safety and efficacy of a different protein-based vaccine was recently completed. This phase IIa clinical trial was conducted to test the safety and efficacy of TVGV-1 vaccine construct, a fusion protein consisting of HPV16 E7 protein fused to pseudomonas aeruginosa exotoxin A (PE) and an endoplasmic reticulum retention signal (KDEL), to treat patients with cervical HSIL (NCT02576561). Data analysis from this trial is still ongoing.
4.5. Dendritic Cell-Based Vaccines
DCs are professional APCs that can induce an adaptive immune response by processing antigens to prime antigen-specific T cells. DCs play an important role in the regulation of the immune system and are commonly cited as the most efficient APCs (Yang et al. 2016; Lee et al. 2016). Autologous DCs can be pulsed ex vivo with peptides, proteins, or DNA-encoding antigens, and then reintroduced into the patient in order to elicit a cell-mediated immune response (Kim and Kim 2017). One benefit to DC-based vaccines is that DCs can serve as adjuvants to increase the strength of antigen-specific immunotherapies (Yang et al. 2016; Santin et al. 2005). Unfortunately, T cell-mediated apoptosis may limit the lifespan of DCs (Skeate et al. 2016); therefore, strategies to increase the immunogenicity and efficacy of DC-based vaccines, as well as prolong the survival of DCs are needed. Strategies have emerged, including the addition of adjuvants such as cholera toxin (Nurkkala et al. 2010) and TLR agonists (Chen et al. 2010). DCs have also been transfected with siRNAs, which target pro-apoptotic molecules to prevent the apoptosis of DCs (Kim et al. 2009; Peng et al. 2005; Ahn et al. 2015).
Several clinical trials have been developed to evaluate the therapeutic potential of DC-based therapeutic HPV vaccines. For example, in a phase I dose-escalation study to evaluate the safety, toxicity, and immunogenicity of DC-based vaccines in patients with stage IB or IIA cervical cancer, all patients developed CD4+ T cell and antibody responses to the DC-based vaccine. Furthermore, eight out of ten patients developed an E7-specific CD8+ T cell response. In their study, Santin et al. concluded that the HPV E7-loaded DC-based vaccine tested in this study was both safe and immunogenic (Santin et al. 2008). In another phase I clinical trial, the toxicity and immunogenicity of a DC-based vaccine was assessed in patients with HPV-associated, advanced, recurrent cervical cancer. The DC-based vaccine was shown to be well-tolerated by patients; however, it did not increase lymphocyte proliferation to a degree of statistical significance (Ramanathan et al. 2014). Nonetheless, there are several downsides to the use of DC-based vaccines. DC-based vaccines are technically difficult to manufacture and highly individualized, making them a poor vaccine choice for large-scale production. In addition, due to a lack of standard vaccine evaluation criteria and varying cell-culture techniques, vaccine quality can be inconsistent amongst batches, which is a particular challenge for large-scale production (Yang et al. 2016; Kim and Kim 2017; Skeate et al. 2016).
4.6. DNA-Based Vaccines
DNA-based vaccines have emerged as an attractive approach to therapeutic HPV vaccination as they offer several advantages over the other types of therapeutic vaccination. Specifically, DNA-based vaccines are safe, stable, and easy to produce on a large scale. DNA-based vaccination involves intramuscularly injecting a plasmid encoding the antigens of interest into the host’s cells for uptake and processing by APCs. DNA-based vaccines are an especially attractive method for therapeutic vaccination because they are capable of mounting both cell-mediated and humoral immune responses against the encoded antigen (Kim and Kim 2017; Vici et al. 2016). While DNA vaccines have fewer safety risks than other types of therapeutic HPV vaccines, genomic integration remains a potential issue. However, no evidence of genomic integration has been observed (Yang et al. 2016; Wang et al. 2004). Naked DNA is easy to manufacture and can maintain antigen expression inside target cells for a longer duration than other therapeutic vaccine types. Unlike vector-based vaccines, DNA-based vaccines do not elicit neutralizing antibodies; therefore, they can be safely administered multiple times for booster vaccinations (Yang et al. 2016; Kim and Kim 2017; Vici et al. 2016). While DNA-based vaccines offer many advantages to therapeutic HPV vaccination, they have limited immunogenicity as they are unable to amplify and spread to surrounding cells. For this reason, DNA-based vaccines require strategies to improve antigen delivery to DCs in order to mount a potent immune response. In general, strategies to enhance therapeutic HPV DNA-based vaccine immunogenicity have focused on: (1) increasing the uptake of HPV antigen by DCs; (2) improving HPV antigen expression, processing, and presentation in DCs; and (3) enhancing DC and T cell interaction (for review see Cheng et al. 2018).
Several strategies have been employed to enhance the number of antigen-expressing/antigen-loaded DCs after DNA-based vaccination including, delivery by gene gun, microencapsulation, electroporation, laser, and microsphere-/nanoparticle-based delivery systems (Kim and Kim 2017). After DNA plasmids encoding HVP antigens are taken up by DCs, they must be expressed, processed, and presented. Enhancing antigen expression in transfected DCs, amplifying translation of HPV mRNA, increasing MHC-I and MHC-II expression in DCs, enhancing antigen processing through the MHC-II pathway, and increasing cross-presentation of HPV antigen through the MHC-I pathway are all methods that have been used to improve HPV antigen expression, processing, and presentation by DCs. Additionally, several methods to enhance DC and T cell function, survival, and interaction have also been employed to improve the therapeutic HPV DNA-based vaccine response. These methods include improving DC function and survival, boosting DC and T cell interactions, promoting T cell function and survival, as well as eliminating immunosuppressive regulatory T cells in the tumor microenvironment (TME) (for review see Cheng et al. 2018).
Therapeutic HPV DNA-based vaccines have undergone numerous clinical trials to evaluate safety and efficacy. In addition, DNA-based vaccines have been tested in conjunction with different adjuvants to improve vaccine potency. An ongoing phase I clinical study is evaluating the safety, tolerability, and feasibility of heterologous prime-boost regimen with therapeutic HPV DNA-based vaccine, pNGVL4a-Sig/E7(detox)/HSP70, and TA-HPV vaccine (NCT00788164). In this study, patients with HPV16-associated CIN3 will receive pNGVL4a-sig/E7(detox)/HSP70 DNA vaccine and TA-HPV vaccine boost with or without imiquimod. Although this study is still ongoing, the preliminary results of this study suggest that this vaccine regimen can induce a localized immune response, which was largely responsible for the therapeutic effects observed in target lesions (Yang et al. 2016; Maldonado et al. 2014). Another phase I clinical trial, which assessed the efficacy and safety of various routes of administration of the pNGVL4a-CRT/E7 (detox) DNA vaccine in patients with HPV16-associated CIN2/3 was recently completed (NCT00988559). Patients were vaccinated with pNGVL4a-CRT/E7 (detox) either through intradermal, intramuscular, or intralesional injection. In this study, pNGVL4a-CRT/E7(detox) was shown to be well-tolerated by patients and elicited the most robust immune response when administered via intralesional injection (Alvarez et al. 2016). Many other clinical trials studying the efficacy of various therapeutic HPV DNA-based vaccines have been completed (NCT02139267) or are currently ongoing, including two early phase clinical studies evaluating the safety and efficacy of GX-188E DNA vaccine (Kim et al. 2014) (NCT02596243; NCT03444376) (for review see Yang et al. 2016). Additionally, more than ten clinical studies evaluating the safety and efficacy of HPV DNA vaccine VGX-3100 in patients with HPV-associated diseases have been completed (NCT03499795; NCT03180684; NCT03721978; NCT01188850; NCT03185013; NCT02163057; NCT00685412; NCT02172911; NCT03606213; NCT03439085) or are ongoing (NCT03603808). One such study, led by Trimble et al. in collaboration with Inovio Pharmaceuticals, found that half of the patients with HPV16/18 CIN2/3 who received VGX-3100 showed histopathological regression of their disease. This study is the first to demonstrate the therapeutic efficacy of VGX-3100 against HPV16/18-associated CIN2/3 as a potential alternative to surgical treatment (NCT01304524) (Trimble et al. 2015).
Numerous therapeutic HPV DNA-based vaccines have been developed or are under development for the control and treatment of HPV infections and HPV-associated diseases. As aforementioned, there are many benefits to the use of therapeutic HPV DNA-based vaccines and their adaptation in clinical studies has become favorable as novel methods to improve their immunogenicity have been generated and studied. Therapeutic HPV DNA-based vaccines also offer ample opportunity for the application of combinational treatment regimens to enhance vaccine potency. As described in Sect. 4.7, such as combinational regiments include prime-boost vaccination strategies, combination with conventional or standard-of-care cancer treatments such as chemotherapy and radiotherapy, as well as co-administration with different drugs (for review see Cheng et al. 2018). Thus, therapeutic HPV DNA-based vaccines are highly advantageous in the treatment of HPV infections and HPV-associated diseases, and continued efforts to develop more immunogenic DNA vaccines or DNA vaccine regimens are warranted for the amelioration of the global HPV-associated disease burden.
4.7. Combination Strategies
As briefly stated in Sect. 4.6, therapeutic HPV vaccines can be used in combination with other therapies to improve vaccine potency and host immune response. Combinatorial administration of therapeutic HPV vaccines along with established cancer treatment methods such as chemotherapy and/or radiation therapy may offer one method to improve the treatment of HPV-associated malignancies. Due to the growing popularity of HPV DNA-based vaccines, combination regimens of therapeutic HPV DNA-based vaccines have been explored in preclinical and clinical studies. The continued development of therapeutic HPV DNA-based vaccines largely relies on vaccine administration in combination with other treatment strategies to create synergistic effects capable of mounting potent CD8+ T cell-mediated immune responses (Cheng et al. 2018). For example, chemotherapy and radiotherapy are known to induce the apoptosis of tumor cells. In turn, this process releases HPV antigens into circulation where they can be more easily “seen” and up-taken by DCs traveling through the bloodstream. To this end, combination regimens of therapeutic HPV DNA-based vaccines in conjunction with chemotherapy or radiation therapy have been used to improve vaccine immunogenicity and demonstrated great potential. In preclinical studies, a therapeutic HPV DNA-based vaccine with calreticulin (CRT) fused to HPV16 E7 (CRT/E7) was administered in combination with chemotherapeutic agents cisplatin and bortezomib. This combinatorial approach was shown to generate a strong E7-specific CD8+ T cell responses in mice bearing an HPV16 E6/E7-expressing tumors (TC-1 tumor model) (Kim et al. 2014) compared to mice treated with CRT/E7 DNA vaccine only, or chemotherapy alone (Tseng et al. 2008a, b). Other chemotherapeutic agents have also been tested in preclinical studies, including 5,6-dimethylxanthenon-4-acetic acid (Peng et al. 2011), epigallocathechin-3-gallate (Kang et al. 2007), and 4′,5,7-trihydroxyflavone (Chuang et al. 2009) (for review see Cheng et al. 2018). Another method to enhance therapeutic HPV vaccine efficacy for the control and treatment of HPV-associated diseases is the combination of treatment with radiation therapy. In one such study, DNA vaccine with CRT linked to the modified form of HPV16 E7 antigen (CRT/E7(detox)) was administered in combination with radiotherapy in TC-1 tumor-bearing mice. Mice treated with CRT/E7(detox) and radiotherapy showed a significant increase in the number of E7-specific CD8+ T cells and had significantly better antitumor effects against E7-expressing tumors compared to mice treated with CRT/E7(detox) DNA vaccine, or radiotherapy alone (Tseng et al. 2009). The combination of chemotherapy and/or radiation therapy with therapeutic HPV vaccines has demonstrated promising antitumor results in preclinical studies and may offer significant benefits to patients with HPV-associated diseases.
Another example of a combinational strategy employed to enhance the potency of therapeutic HPV vaccines is prime-boost vaccination regimens. In previous studies, heterologous prime with DNA vaccine followed by boosting with recombinant protein has been shown to elicit both potent CD8+ and CD4+ T cell responses, as well as antibody responses. For example, in an ongoing clinical trial, a total of 12 patients with HPV16+ CIN2/3 were administered a heterologous DNA-prime-recombinant vaccinia vector-based boost vaccination regimen targeting HPV16 and HPV18 E6/E7 (NCT00788164). This prime-boost vaccination regimen was found to be safe and tolerable in patients with HPV16+ CIN2/3 lesions. The vaccination regimen included intramuscular prime vaccination with a DNA vaccine expressing HPV16 E7 (pNGVL4a-Sig/E7(detox)/HSP70), followed by a boost with TA-HPV, a recombinant vaccinia boost expressing HPV16 and HPV18 E6 and E7. This prime-boost vaccination strategy was also found to generate a more potent immune response than DNA vaccination alone (Cheng et al. 2018; Maldonado et al. 2014). Unfortunately, due to the risks associated with live vaccinia virus, TA-HPV may not be a suitable for administering prime-boost vaccination regimens in patients who are immune compromised (Peng et al. 2016). Additional studies have shown the therapeutic efficacy of prime-boost regimens using different recombinant proteins. For example, in a preclinical study, heterologous DNA-prime, TA-CIN protein boost was demonstrated to be safe and well-tolerated in both naïve and tumor-bearing mice (Peng et al. 2016). In this particular study, Peng et al. showed that heterologous DNA-prime with pNGVL4aCRTE6E7L2 DNA vaccine and TA-CIN protein boost regimen was capable of eliciting a more potent antigen-specific response than homologous DNA or protein vaccination alone. Specifically, the TA-CIN boosting regimen was able to generate an HPV16 L2, E6, and E7-specific immune response after heterologous DNA prime, representing the therapeutic efficacy of this regimen.
In addition to preclinical studies, several clinical studies have been conducted to evaluate the safety and efficacy of therapeutic HPV vaccines are prime-boost vaccination regimens in human patients. Currently, several ongoing clinical studies are testing the safety and tolerability of heterologous DNA prime vaccination with TA-CIN protein boost. Including a phase II clinical trial in patients with HPV16+ Atypical Squamous Cells of Undetermined Significance (ASC-US), which will evaluate the safety and tolerability of the prime-boost vaccination regimen with IM injection of pNGVL4a-Sig/E7(detox)/HSP70 DNA vaccine followed by IM TA-CIN protein boost (NCT03911076). In another phase I ongoing clinical study is assessing the safety and feasibility of IM administration of pNGVL4aCRTE6E7L2 DNA vaccine prime, TA-CIN protein boost in patients with persistent HPV16+ ASC-US/LSIL (NCT03913117). While these studies are ongoing, the potential therapeutic strategies of prime-boost vaccination regimens are promising and warrant further clinical investigation. Table 1 (adapted from Yang et al. 2017) lists several ongoing clinical trials evaluating the efficacy of various therapeutic HPV vaccines.
Table 1.
Ongoing Therapeutic HPV vaccine clinical trials for HPV-associated diseases
| Vaccine | Antigen(s) | Construct | Study sponsor | Trial design | Estimated date of trial completion | Clinicaltrials.gov identifier |
|---|---|---|---|---|---|---|
| Persistent HPV infection and Low-grade Squamous Intraepithelial Lesion (LSIL) | ||||||
| Ad26-HPV16, Ad26-HPV18, and MVA-HPV16/18 | HPV16/18 E6/E7 | Adenovims serotype 26 (Ad26)-human papillomavirus (HPV16 or HPV18) and Modified Vaccinia Ankara (MVA)-HPV16/18 | Janssen Vaccines and prevention B.V. | Phase I/IIa in healthy female patients with persistent HPV16 or HPV18 infection of the cervix (66 estimated patients) | September 2020 | NCT03610581 |
| Atypical Squamous Cells of Undetermined Significance (ASC-US) or atypical squamous cells, cannot rule out high-grade SIL | ||||||
| pNGVL4a-Sig/E7(detox)/HSP70 + TA-CIN (PVX-2) | HPV16 E6/E7 | Plasmid encoding mutated form of HPV16-E7 linked to Sig and HSP70 and HPV16 E6, E7, and L2 fusion protein | PapiVax Biotech Inc. | Phase II in female patients with confirmation of ASC-US, HSC-H or LSIL (122 estimated patients) | December 2021 | NCT03911076 |
| pNGVL4aCRTE6E7L2 + TA-CIN (PVX-6) | HPV16 E6/E7 | Plasmid encoding Calreticulin, HPV16 E6, E7, and L2, and HPV16 E6, E7, and L2 fusion protein | PapiVax Biotech Inc. | Phase I in female patients with persistent ASC-US/LSIL (30 estimated patients) | December 2021 | NCT03913117 |
| Cervical Intraepithelial Neoplasia (CIN)/High-Grade Squamous Intraepithelial Lesion (HSIL) | ||||||
| GX-188E | HPV16/18 E6/E7 | Plasmid encoding fusion protein of HPV 16/18 E6/E7 linked to Flt3L and tpa | Genexine, Inc | Phase II in female patients with HPV16/18+ CIN2, CIN2/3, or CIN3 (134 enrolled patients) | August 2018 | NCT02596243 |
| pNGVL4a-Sig/E7(detox)/HSP70 + TA-HPV | HPV16/18 E6/E7 | Plasmid encoding mutated form of HPV16-E7 linked to Sig and HSP70 and vaccinia virus with HPV16/18 E6/E7 | Sidney Kimmel Comprehensive Cancer Center | Phase I in female patients with HPV16+ CIN3 in combination with topical imiquimod (48 estimated patients) | June 2020 | NCT00788164 |
| TVGV-1 + GPI-0100 | HPV16 E7 | Fusion protein of HPV16 E7 and ER targeting sequence | THEVAX Genetics Vaccine Co. | Phase IIa in female patients with HPV16 or HPV16/18+ cervical HSIL (10 enrolled patients) | September 2018 | NCT02576561 |
| PepCan + Candin | HPV16 E6 | HPV16 E6 peptides combined with Candida skin testing reagent candin. | University of Arkansas | Phase II in female patients with cervical HSIL (125 estimated patients) | August 2020 | NCT02481414 |
| Gardasil®9 | hrHPV16, 18, 31, 33, 45, 52 and 58 | Recombinant vaccine purified VLPs of the major capsid protein (L1) of HPV6, 11, 16, 18, 31, 33, 45, 52 and 58 | AIDS Malignancy Consortium | Phase III trial in female patients with HIV-1 infection and co-infection with hrHPV16, 18, 31, 33, 35, 45, 52 or 58 (536 estimated patients) | October 2021 | NCT03284866 |
| Gardasil®9 + imiquimod | HPV6, 11, 16, 18, 31, 33, 45, 52 and 58 | Gardasil®9: Recombinant vaccine purified VLPs of the major capsid protein (L1) of HPV6, 11, 16, 18, 31, 33, 45, 52 and 58 Imiquimod: TLR7/8 agonist | Yale University | Phase II trial in female patients with untreated HPV+ CIN2/3 (138 patients estimated) | April 2021 | NCT02864147 |
| GX-188E + GX-I7 or imiquimod | HPV16/18 E6/E7 | GX-188E: Plasmid encoding fusion protein of HPV 16/18 E6/E7 linked to Flt3L and tpa GX-17: recombinant human IL-7-hybrid Fc NT-I7 Imiquimod: TLR7/8 agonist |
Seoul St. Mary’s Hospital | Safety and efficacy trial in female patients with HPV 16/18+ CIN3 (50 patients estimated) | October 2018 | NCT03206138 |
| Anal Intraepithelial Neoplasia (AIN) | ||||||
| VGX-3100 + Electroporation | HPV16/18 E6/E7 | Mixture of three plasmids encoding optimized consensus of E6 and E7 antigen of HPV 16 and 18 | AIDS Malignancy Consortium | Phase II trial in male and female patients with HPV 16/18+ anal HSIL (80 estimated patients) | September 2021 | NCT03603808 |
| HPV-associated incurable solid tumors | ||||||
| ISA101 (SLP-HPV-01; HPV16-SLP) + nivolumab | HPV16 E6/E7 | Combination of nine HPV-16 E6 and four HPV-16 E7 synthetic peptides with incomplete Freund’s adjuvant | MD Anderson Cancer Center | Phase II trial in male and female patients with HPV16+ incurable solid tumors (OPSCC, cervical, vulvar, vaginal, anal, and penile cancer) as combination therapy with Nivolumab (34 enrolled patients) | December 2019 | NCT02426892 |
| E7 TCR T cells (T cell-based vaccine) | HPV16 E7 | Genetically engineered T cells with a T cell receptor (TCR) targeting HPV16 E7 (E7 TCR) | National Cancer Institute | Phase I/II trial in male and female patients with incurable metastatic or refractory/recurrent HPV16+ cervical, vulvar, vaginal, penile, anal, and oropharyngeal cancer) (180 estimated patients) | January 2026 | NCT02858310 |
| DPX-E7 | HPV16 E7 | Synthetic HPV16 E7 peptides packed into liposomes, freeze dried, then re-suspended in oil | Dana-Farber Cancer Institute | Phase Ib/II trial in male and female patients positive for HLA-A*02 with incurable HPV16+ head and neck, cervical or anal cancer | May 2023 | NCT02865135 |
| HARE-40 | HPV16 E6/E7 | Anti-CD40 IS-Ab ChiLob7/4 | University of Southhampton | Phase I/II trial in male and female patients with advanced HPV+ head and neck, anogenital, penile, or cervical cancer | December 2020 | NCT03418480 |
| INO-3112 + durvalumab | HPV16/18 E6/E7 | Mixture of three plasmids encoding optimized consensus of E6 and E7 antigen of HPV 16 and 18 and proprietary immune activator expressing IL-12 | MD Anderson Cancer Center | Phase II trial in male and female patients with recurrent or metastatic HPV16/18+ cancers (anal, cervical, penial, vaginal, vulval) (77 estimated patients) | January 2020 | NCT03439085 |
| Head and neck cancer | ||||||
| ADXS11-001 (ADXS-HPV) | HPV16 E7 | prfA-defective Listeria monocytogenes strain transformed with plasmid encoding HPV-16 E7 antigen fused to a fragment of nonhemolytic listeriolysin O (LLO) | Advaxis, Inc. | Phase II trial in male and female patients with HPV+ OPSCC (stage I-IV) prior to robot-assisted resection (30 estimated patients) | August 2021 | NCT02002182 |
| PepCan | HPV16 E6 | HPV16 E6 peptides | University of Arkansas | Phase I/II trial in male and female patients with head and neck cancer (20 estimated participants) | December 2021 | NCT03821272 |
| MEDI0457 + durvalumab | HPV16/18 E6/E7 | Mixture of three plasmids encoding optimized consensus of E6 and E7 antigen of HPV 16 and 18 and proprietary immune activator expressing IL-12 | MedImmune LLC | Phase Ib/IIa trial in male and female patients with HPV-associated recurrent or metastatic head and neck cancer (50 estimated patients) | August 2020 | NCT03162224 |
| TG4001 + Avelumab | HPV16 E6/E7 | Suspension of MVATG8042 vector particles, which consists of an attenuated recombinant MVA encoding modified HPV16 E6 and E7, and human interleukin-2 (IL-2) | Transgene | Phase Ib/II trial in male and female patients with HPV 16+ recurrent or metastatic OPSCC of the head and neck (52 estimated patients) | December 2021 | NCT03260023 |
| Cervical cancer | ||||||
| ADXS11-001 (Lm-LLo-E7) | HPV16 E7 | prfA-defective Listeria monocytogenes strain transformed with plasmid encoding HPV-16 E7 antigen fused to a fragment of nonhemolytic listeriolysin O (LLO) | Advaxis, Inc. | Phase II trial in female patients with persistent or recurrent squamous or non-squamous cell carcinoma of the cervix (67 estimated patients) | October 2018 | NCT01266460 |
| ISA101/ISA101b (SLP-HPV-01; HPV16-SLP) | HPV16 E6/E7 | Combination of nine HPV-16 E6 and four HPV-16 E7 synthetic peptides with incomplete Freund’s adjuvant | ISA Pharmaceuticals | Phase I/II trial in female patients with HPV-16+ advanced or recurrent cervical cancer (100 estimated patients) | April 2021 | NCT02128126 |
| TA-CIN | HPV16 E6/E7/L2 | HPV16 E6, E7, L2 fusion protein | Sidney Kimmel Comprehensive Cancer Center | Phase I trial in female patients with a history of HPV16-associated cervical cancer (stage IB1-IV) (14 patients estimated) | November 2022 | NCT02405221 |
| GX-188E + pembrolizumab | HPV16/18 E6/E7 | Plasmid encoding fusion protein of HPV 16/18 E6/E7 linked to Flt3L and tpa | Genexine, Inc | Phase Ib/II trial in female patients with advanced, non-resectable HPV+ cervical cancer (46 estimated patients) | June 2023 | NCT03444376 |
| BVAC-C | HPV16/18 E6/E7 | B cell and monocyte-based vaccine transfected with recombinant HPV E6/E7 gene and loaded with alpha-glactosyl ceramide | Celid Co., Ltd. | Phase I/II trial in females with multiple metastatic progressive or recurrent HPV 16 or 18+ cervical cancer (30 estimated patients) | August 2020 | NCT02866006 |
| INO-3112 + durvalumab | HPV16/18 E6/E7 | Mixture of three plasmids encoding optimized consensus of E6 and E7 antigen of HPV 16 and 18 and proprietary immune activator expressing IL-12 | MD Anderson Cancer Center | Phase II trial in female patients with recurrent or metastatic HPV16/18+ cancers (77 estimated patients) | January 2020 | NCT03439085 |
AIN Anal intraepithelial Neoplasia; ASC-US Atypical Squamous Cells of Undetermined Significance; ASC-H Atypical Squamous Cells, Cannot Rule out High-Grade SIL; CIN Cervical intraepithelial neoplasia; ER Endoplasmic reticulum; HIV Human immunodeficiency virus; HPV Human papillomavirus; HSIL High-grade squamous intraepithelial lesion; LSIL Low-grade squamous intraepithelial lesion; OPSCC oropharyngeal squamous cell carcinoma
5. Conclusion
While HPV infections and HPV-associated diseases remain highly prevalent across the globe, the development of effective, and accessible methods for the control of HPV infections and HPV-associated diseases continue to pose a significant challenge for researchers, healthcare providers, and public health workers. The current, commercially available prophylactic vaccines represent a significant triumph in HPV research and public health; however, there is still an urgent need to improve methods for the control of HPV-associated disease. Methods to improve HPV prevention and the control of HPV-associated diseases include: (1) develop broader vaccine coverage against oncogenic HPV types; (2) reduce vaccine-related costs; (3) reduce minimum dose requirements; (4) improve vaccine stability; (5) make prophylactic HPV vaccines more widely accessible; and (6) employ our current understanding of the immunology of HPV infections to create successful and efficacious therapeutic HPV vaccines. Numerous innovative strategies have been employed in order to develop therapeutic HPV vaccines, including many which have resulted in phase I, II, and III clinical trials. In order to best identify suitable therapeutic HPV vaccine candidates for the control of established HPV infections and HPV-associated lesions or disease, we must continue to devise and implement clinical studies, and improve upon existing therapeutic strategies that have demonstrated promising clinical translatability. Additionally, the control of advanced HPV-associated diseases will likely necessitate further investigation into the ideal combinatorial approaches using therapeutic HPV vaccines in conjunction with conventional cancer therapies such as chemotherapy and radiation therapy. Continued efforts to advance both prophylactic and therapeutic HPV vaccines will undoubtedly relieve the global burden of HPV and help reduce incidence rates of HPV infection in millions of people each year around the world.
Acknowledgements
This review is not intended to be encyclopedic, and the authors apologize to those not cited. This work was funded by the National Institutes of Health Cervical Cancer SPORE (P50 CA098252).
Contributor Information
Emily Farmer, Department of Pathology, The Johns Hopkins School of Medicine, Cancer Research Building II, 1550 Orleans Street, Baltimore, MD 21287, USA.
Max A. Cheng, Department of Pathology, The Johns Hopkins School of Medicine, Cancer Research Building II, 1550 Orleans Street, Baltimore, MD 21287, USA
Chien-Fu Hung, Department of Pathology, The Johns Hopkins School of Medicine, Cancer Research Building II, 1550 Orleans Street, Baltimore, MD 21287, USA; Department of Oncology, The Johns Hopkins School of Medicine, Cancer Research Building II, 1550 Orleans Street, Baltimore, MD 21287, USA.
T.-C. Wu, Department of Pathology, The Johns Hopkins School of Medicine, Cancer Research Building II, 1550 Orleans Street, Baltimore, MD 21287, USA Department of Oncology, The Johns Hopkins School of Medicine, Cancer Research Building II, 1550 Orleans Street, Baltimore, MD 21287, USA; Department of Obstetrics and Gynecology, The Johns Hopkins School of Medicine, Cancer Research Building II, 1550 Orleans Street, Baltimore, MD 21287, USA; Department of Pathology, Oncology, Obstetrics and Gynecology, and Molecular Microbiology and Immunology, The Johns Hopkins Medical Institutions, Cancer Research Building II, Room 309, 1550 Orleans Street, Baltimore, MD 21287, USA.
References
- Ahn YH, Hong SO, Kim JH, Noh KH, Song KH, Lee YH, Jeon JH, Kim DW, Seo JH, Kim TW (2015) The siRNA cocktail targeting interleukin 10 receptor and transforming growth factor-beta receptor on dendritic cells potentiates tumour antigen-specific CD8(+) T cell immunity. Clin Exp Immunol 181(1):164–178. Epub 2015/03/11. 10.1111/cei.12620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez RD, Huh WK, Bae S, Lamb LS Jr., Conner MG, Boyer J, Wang C, Hung CF, Sauter E, Paradis M, Adams EA, Hester S, Jackson BE, Wu TC, Trimble CL (2016) A pilot study of pNGVL4a-CRT/E7(detox) for the treatment of patients with HPV16+ cervical intraepithelial neoplasia 2/3 (CIN2/3). Gynecol Oncol 140(2):245–252. Epub 2015/12/01. 10.1016/j.ygyno.2015.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Cancer Society (2018) Can oral cavity and oropharyngeal cancers be found early? Available from https://www.cancer.org/cancer/oral-cavity-and-oropharyngeal-cancer/detection-diagnosis-staging/detection.html. Updated 9 Mar 2018; Cited 2019
- Baldwin PJ, van der Burg SH, Boswell CM, Offringa R, Hickling JK, Dobson J, Roberts JS, Latimer JA, Moseley RP, Coleman N, Stanley MA, Sterling JC (2003) Vaccinia-expressed human papillomavirus 16 and 18 e6 and e7 as a therapeutic vaccination for vulval and vaginal intraepithelial neoplasia. Clin Cancer Res 9(14):5205–5213 [PubMed] [Google Scholar]
- Barra F, Maggiore ULR, Bogani G, Ditto A, Signorelli M, Martinelli F, Chiappa V, Lorusso D, Raspagliesi F, Ferrero S (2019) New prophylactics human papilloma virus (HPV) vaccines against cervical cancer. J Obstet Gynaecol 39(1):1–10. Epub 2018/10/30. 10.1080/01443615.2018.1493441. [DOI] [PubMed] [Google Scholar]
- Borysiewicz LK, Fiander A, Nimako M, Man S, Wilkinson GW, Westmoreland D, Evans AS, Adams M, Stacey SN, Boursnell ME, Rutherford E, Hickling JK, Inglis SC (1996) A recombinant vaccinia virus encoding human papillomavirus types 16 and 18, E6 and E7 proteins as immunotherapy for cervical cancer. Lancet 347(9014):1523–1527 [DOI] [PubMed] [Google Scholar]
- Boxus M, Fochesato M, Miseur A, Mertens E, Dendouga N, Brendle S, Balogh KK, Christensen ND, Giannini SL (2016) Broad cross-protection is induced in preclinical models by a human papillomavirus vaccine composed of L1/L2 chimeric virus-like particles J Virol 90 (14):6314–6325. Epub 2016/05/06. 10.1128/jvi.00449-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brianti P, De Flammineis E, Mercuri SR (2017) Review of HPV-related diseases and cancers. New Microbiol 40(2):80–85 [PubMed] [Google Scholar]
- Brun JL, Dalstein V, Leveque J, Mathevet P, Raulic P, Baldauf JJ, Scholl S, Huynh B, Douvier S, Riethmuller D, Clavel C, Birembaut P, Calenda V, Baudin M, Bory JP (2011) Regression of high-grade cervical intraepithelial neoplasia with TG4001 targeted immunotherapy (2011) Am J Obstet Gynecol 204(2):169 e1–e8. Epub 2011/02/03. 10.1016/j.ajog.2010.09.020. [DOI] [PubMed] [Google Scholar]
- Bruni L, Diaz M, Barrionuevo-Rosas L, Herrero R, Bray F, Bosch FX, de Sanjose S, Castellsague X (2016) Global estimates of human papillomavirus vaccination coverage by region and income level: a pooled analysis. Lancet Glob Health 4(7):e453–e463. Epub 2016/06/25. 10.1016/s2214-109x(16)30099-7. [DOI] [PubMed] [Google Scholar]
- Buttaro C, Fruehauf JH (2010) Engineered E. coli as vehicles for targeted therapeutics. Curr Gene Ther 10(1):27–33. [DOI] [PubMed] [Google Scholar]
- Caldeira Jdo C, Medford A, Kines RC, Lino CA, Schiller JT, Chackerian B, Peabody DS (2010) Immunogenic display of diverse peptides, including a broadly cross-type neutralizing human papillomavirus L2 epitope, on virus-like particles of the RNA bacteriophage PP7. Vaccine 28 (27):4384–4393. Epub 2010/05/04. 10.1016/j.vaccine.2010.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2012) Cervical cancer screening guidelines for average-risk womena. Available from https://www.cdc.gov/cancer/cervical/pdf/guidelines.pdf. Cited 2019
- Centers for Disease Control and Prevention (2015) Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. Available from https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6411a3.htm. Updated 27 Mar 2015; Cited 2019 [PMC free article] [PubMed]
- Centers for Disease Control and Prevention (2017) Genital HPV infection—Fact sheet. Available from https://www.cdc.gov/std/hpv/stdfact-hpv.htm. Updated 16 Nov 2017; Cited 2019
- Chabeda A, Yanez RJR, Lamprecht R, Meyers AE, Rybicki EP, Hitzeroth II (2018) Therapeutic vaccines for high-risk HPV-associated diseases. Papillomavirus Res 5:46–58. Epub 2017/12/27. 10.1016/j.pvr.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XZ, Mao XH, Zhu KJ, Jin N, Ye J, Cen JP, Zhou Q, Cheng H (2010) Toll like receptor agonists augment HPV 11 E7-specific T cell responses by modulating monocyte-derived dendritic cells. Arch Dermatol Res 302(1):57–65. Epub 2009/07/07. 10.1007/s00403-009-0976-0. [DOI] [PubMed] [Google Scholar]
- Cheng MA, Farmer E, Huang C, Lin J, Hung CF, Wu TC (2018) Therapeutic DNA vaccines for human papillomavirus and associated diseases. Hum Gene Ther 29(9):971–976. Epub 2018/01/11. 10.1089/hum.2017.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang CM, Monie A, Wu A, Hung CF (2009) Combination of apigenin treatment with therapeutic HPV DNA vaccination generates enhanced therapeutic antitumor effects. J Biomed Sci 16:49. Epub 2009/05/29. 10.1186/1423-0127-16-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman HN, Greenfield WW, Stratton SL, Vaughn R, Kieber A, Moerman-Herzog AM, Spencer HJ, Hitt WC, Quick CM, Hutchins LF, Mackintosh SG, Edmondson RD, Erickson SW, Nakagawa M (2016) Human papillomavirus type 16 viral load is decreased following a therapeutic vaccination. Cancer Immunol Immunother 65(5):563–573. Epub 2016/03/17. 10.1007/s00262-016-1821-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper CP, Saraiya M (2017) Primary HPV testing recommendations of US providers, 2015. Prev Med 105:372–377. Epub 2017/10/24. 10.1016/j.ypmed.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona Gutierrez CM, Tinoco A, Navarro T, Contreras ML, Cortes RR, Calzado P, Reyes L, Posternak R, Morosoli G, Verde ML, Rosales R (2004) Therapeutic vaccination with MVA E2 can eliminate precancerous lesions (CIN 1, CIN 2, and CIN 3) associated with infection by oncogenic human papillomavirus. Hum Gene Ther 15(5):421–431. Epub 2004/05/18. 10.1089/10430340460745757. [DOI] [PubMed] [Google Scholar]
- Cory L, Chu C (2014) ADXS-HPV: a therapeutic Listeria vaccination targeting cervical cancers expressing the HPV E7 antigen. Hum Vaccin Immunother 10(11):3190–3195. Epub 2014/12/09. 10.4161/hv.34378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Z, Huang L (2005) Liposome-polycation-DNA (LPD) particle as a carrier and adjuvant for protein-based vaccines: therapeutic effect against cervical cancer. Cancer Immunol Immunother 54(12):1180–1190. Epub 2005/04/23. 10.1007/s00262-005-0685-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva AJ, Zangirolami TC, Novo-Mansur MT, Giordano Rde C, Martins EA (2014) Live bacterial vaccine vectors: an overview. Braz J Microbiol 45(4):1117–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daayana S, Elkord E, Winters U, Pawlita M, Roden R, Stern PL, Kitchener HC (2010) Phase II trial of imiquimod and HPV therapeutic vaccination in patients with vulval intraepithelial neoplasia. Br J Cancer 102(7):1129–1136. Epub 2010/03/18. 10.1038/sj.bjc.6605611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daftarian P, Mansour M, Benoit AC, Pohajdak B, Hoskin DW, Brown RG, Kast WM (2006) Eradication of established HPV 16-expressing tumors by a single administration of a vaccine composed of a liposome-encapsulated CTL-T helper fusion peptide in a water-in-oil emulsion. Vaccine 24(24):5235–5244. Epub 2006/05/06. 10.1016/j.vaccine.2006.03.079. [DOI] [PubMed] [Google Scholar]
- Davidson EJ, Boswell CM, Sehr P, Pawlita M, Tomlinson AE, McVey RJ, Dobson J, Roberts JS, Hickling J, Kitchener HC, Stern PL (2003) Immunological and clinical responses in women with vulval intraepithelial neoplasia vaccinated with a vaccinia virus encoding human papillomavirus 16/18 oncoproteins. Cancer Res 63(18):6032–6041 [PubMed] [Google Scholar]
- Day PM, Pang YY, Kines RC, Thompson CD, Lowy DR, Schiller JT (2012) A human papillomavirus (HPV) in vitro neutralization assay that recapitulates the in vitro process of infection provides a sensitive measure of HPV L2 infection-inhibiting antibodies. Clin Vaccine Immunol 19(7):1075–1082. Epub 2012/05/18. 10.1128/cvi.00139-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, Plummer M (2012) Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol 13(6):607–615. Epub 2012/05/12. 10.1016/s1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- de Martel C, Plummer M, Vignat J, Franceschi S (2017) Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer 141(4):664–670. Epub 2017/04/04. 10.1002/ijc.30716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vos van Steenwijk PJ, van Poelgeest MI, Ramwadhdoebe TH, Lowik MJ, Berends-van der Meer DM, van der Minne CE, Loof NM, Stynenbosch LF, Fathers LM, Valentijn AR, Oostendorp J, Osse EM, Fleuren GJ, Nooij L, Kagie MJ, Hellebrekers BW, Melief CJ, Welters MJ, van der Burg SH, Kenter GG (2014) The long-term immune response after HPV16 peptide vaccination in women with low-grade pre-malignant disorders of the uterine cervix: a placebo-controlled phase II study. Cancer Immunol Immunother 63(2):147–160. Epub 2013/11/16. 10.1007/s00262-013-1499-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGiuseppe S, Bienkowska-Haba M, Guion LGM, Keiffer TR, Sapp M (2017) Human papillomavirus major capsid protein L1 remains associated with the incoming viral genome throughout the entry process. J Virol Epub 2017/06/02. 10.1128/jvi.00537-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egawa N, Egawa K, Griffin H, Doorbar J (2015) Human papillomaviruses; epithelial tropisms, and the development of neoplasia. Viruses 7(7):3863–3890. Epub 2015/07/21. 10.3390/v7072802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elrefaey S, Massaro MA, Chiocca S, Chiesa F, Ansarin M (2014) HPV in oropharyngeal cancer: the basics to know in clinical practice. Acta Otorhinolaryngol Ital 34(5):299–309. [PMC free article] [PubMed] [Google Scholar]
- Frazer IH, Quinn M, Nicklin JL, Tan J, Perrin LC, Ng P, O’Connor VM, White O, Wendt N, Martin J, Crowley JM, Edwards SJ, McKenzie AW, Mitchell SV, Maher DW, Pearse MJ, Basser RL (2004) Phase 1 study of HPV16-specific immunotherapy with E6E7 fusion protein and ISCOMATRIX adjuvant in women with cervical intraepithelial neoplasia. Vaccine 23 (2):172–181. Epub 2004/11/09. 10.1016/j.vaccine.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Gallagher KE, LaMontagne DS, Watson-Jones D (2018) Status of HPV vaccine introduction and barriers to country uptake. Vaccine 36(32 Pt A):4761–4767. Epub 2018/03/28. 10.1016/j.vaccine.2018.02.003. [DOI] [PubMed] [Google Scholar]
- Gambhira R, Gravitt PE, Bossis I, Stern PL, Viscidi RP, Roden RB (2006) Vaccination of healthy volunteers with human papillomavirus type 16 L2E7E6 fusion protein induces serum antibody that neutralizes across papillomavirus species. Cancer Res 66(23):11120–11124. Epub 2006/12/06. 10.1158/0008-5472.can-06-2560. [DOI] [PubMed] [Google Scholar]
- Gambhira R, Jagu S, Karanam B, Gravitt PE, Culp TD, Christensen ND, Roden RB (2007a) Protection of rabbits against challenge with rabbit papillomaviruses by immunization with the N terminus of human papillomavirus type 16 minor capsid antigen L2. J Virol 81(21):11585–11592. Epub 2007/08/24. 10.1128/jvi.01577-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambhira R, Karanam B, Jagu S, Roberts JN, Buck CB, Bossis I, Alphs H, Culp T, Christensen ND, Roden RB (2007b) A protective and broadly cross-neutralizing epitope of human papillomavirus L2. J Virol 81(24):13927–13931. Epub 2007/10/12. 10.1128/jvi.00936-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Hernandez E, Gonzalez-Sanchez JL, Andrade-Manzano A, Contreras ML, Padilla S, Guzman CC, Jimenez R, Reyes L, Morosoli G, Verde ML, Rosales R (2006) Regression of papilloma high-grade lesions (CIN 2 and CIN 3) is stimulated by therapeutic vaccination with MVA E2 recombinant vaccine. Cancer Gene Ther 13(6):592–597. Epub 2006/02/04. 10.1038/sj.cgt.7700937. [DOI] [PubMed] [Google Scholar]
- Gaspar J, Quintana SM, Reis RK, Gir E (2015) Sociodemographic and clinical factors of women with HPV and their association with HIV. Rev Lat Am Enfermagem 23(1):74–81. Epub 2015/03/26. 10.1590/0104-1169.3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillison ML, Chaturvedi AK, Anderson WF, Fakhry C (2015) Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J Clin Onco l33(29):3235–3242. Epub 2015/09/10. 10.1200/jco.2015.61.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham SV (2010) Human papillomavirus: gene expression, regulation and prospects for novel diagnostic methods and antiviral therapies. Future Microbiol 5(10):1493–1506. Epub 2010/11/16. 10.2217/fmb.10.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guirnalda P, Wood L, Paterson Y (2012) Listeria monocytogenes and its products as agents for cancer immunotherapy. Adv Immunol 113:81–118. Epub 2012/01/17. 10.1016/b978-0-12-394590-7.00004-x. [DOI] [PubMed] [Google Scholar]
- Gunn GR, Zubair A, Peters C, Pan ZK, Wu TC, Paterson Y (2001) Two Listeria monocytogenes vaccine vectors that express different molecular forms of human papilloma virus-16 (HPV-16) E7 induce qualitatively different T cell immunity that correlates with their ability to induce regression of established tumors immortalized by HPV-16. J Immunol 167(11):6471–6479 [DOI] [PubMed] [Google Scholar]
- Guo T, Eisele DW, Fakhry C (2016) The potential impact of prophylactic human papillomavirus vaccination on oropharyngeal cancer. Cancer 122(15):2313–2323. Epub 2016/05/07. 10.1002/cncr.29992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper DM, DeMars LR (2017) HPV vaccines—A review of the first decade. Gynecol Oncol 146 (1):196–204. Epub 2017/04/27. 10.1016/j.ygyno.2017.04.004. [DOI] [PubMed] [Google Scholar]
- Hung CF, Ma B, Monie A, Tsen SW, Wu TC (2008) Therapeutic human papillomavirus vaccines: current clinical trials and future directions. Expert Opin Biol Ther 8(4):421–439. Epub 2008/03/21. 10.1517/14712598.8.4.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain SF, Paterson Y (2004) CD4+CD25+ regulatory T cells that secrete TGFbeta and IL-10 are preferentially induced by a vaccine vector. J Immunother 27(5):339–346 [DOI] [PubMed] [Google Scholar]
- Immunization Action Coalition (2019) Human papillomavirus (HPV). Available from http://www.immunize.org/askexperts/experts_hpv.asp. Updated 4 Mar 2019; Cited 2019 [Google Scholar]
- Jagu S, Karanam B, Gambhira R, Chivukula SV, Chaganti RJ, Lowy DR, Schiller JT, Roden RB (2009) Concatenated multitype L2 fusion proteins as candidate prophylactic pan-human papillomavirus vaccines. J Natl Cancer Inst 101(11):782–792. Epub 2009/05/28. 10.1093/jnci/djp106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joura EA, Giuliano AR, Iversen OE, Bouchard C, Mao C, Mehlsen J, Moreira ED Jr., Ngan Y, Petersen LK, Lazcano-Ponce E, Pitisuttithum P, Restrepo JA, Stuart G, Woelber L, Yang YC, Cuzick J, Garland SM, Huh W, Kjaer SK, Bautista OM, Chan IS, Chen J, Gesser R, Moeller E, Ritter M, Vuocolo S, Luxembourg A (2015) Broad spectrum HPVVS. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med 372(8):711–723. Epub 2015/02/19. 10.1056/nejmoa1405044. [DOI] [PubMed] [Google Scholar]
- Kaiser KJ, Family Foundation (2018) The HPV vaccine: access and use in the U.S. Available from https://www.kff.org/womens-health-policy/fact-sheet/the-hpv-vaccine-access-and-use-in-the-us/. Updated 9 Oct 2018; Cited 2019 [Google Scholar]
- Kaliamurthi S, Selvaraj G, Chinnasamy S, Wang Q, Nangraj AS, Cho WC, Gu K, Wei DQ (2019) Exploring the papillomaviral proteome to identify potential candidates for a chimeric vaccine against cervix papilloma using immunomics and computational structural vaccinology. Viruses 11(1). Epub 2019/01/18. 10.3390/v11010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang TH, Lee JH, Song CK, Han HD, Shin BC, Pai SI, Hung CF, Trimble C, Lim JS, Kim TW, Wu TC (2007) Epigallocatechin-3-gallate enhances CD8+ T cell-mediated antitumor immunity induced by DNA vaccination. Cancer Res 67(2):802–811. Epub 2007/01/20. 10.1158/0008-5472.can-06-2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang TH, Monie A, Wu LS, Pang X, Hung CF, Wu TC (2011) Enhancement of protein vaccine potency by in vivo electroporation mediated intramuscular injection. Vaccine 29(5):1082–1089. Epub 2010/12/07. 10.1016/j.vaccine.2010.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann AM, Stern PL, Rankin EM, Sommer H, Nuessler V, Schneider A, Adams M, Onon TS, Bauknecht T, Wagner U, Kroon K, Hickling J, Boswell CM, Stacey SN, Kitchener HC, Gillard J, Wanders J, Roberts JS, Zwierzina H (2002) Safety and immunogenicity of TA-HPV, a recombinant vaccinia virus expressing modified human papillomavirus (HPV)-16 and HPV-18 E6 and E7 genes, in women with progressive cervical cancer. Clin Cancer Res 8 (12):3676–3685 [PubMed] [Google Scholar]
- Khong H, Overwijk WW (2016) Adjuvants for peptide-based cancer vaccines. J Immunother Cancer 4:56. Epub 2016/09/24. 10.1186/s40425-016-0160-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Kim HJ (2017) Current status and future prospects for human papillomavirus vaccines. Arch Pharm Res 40(9):1050–1063. Epub 2017/09/07. 10.1007/s12272-017-0952-8. [DOI] [PubMed] [Google Scholar]
- Kim JH, Kang TH, Noh KH, Bae HC, Kim SH, Yoo YD, Seong SY, Kim TW (2009) Enhancement of dendritic cell-based vaccine potency by anti-apoptotic siRNAs targeting key pro-apoptotic proteins in cytotoxic CD8(+) T cell-mediated cell death. Immunol Lett 122 (1):58–67. Epub 2009/01/13. 10.1016/j.imlet.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Kim TJ, Jin HT, Hur SY, Yang HG, Seo YB, Hong SR, Lee CW, Kim S, Woo JW, Park KS, Hwang YY, Park J, Lee IH, Lim KT, Lee KH, Jeong MS, Surh CD, Suh YS, Park JS, Sung YC (2014) Clearance of persistent HPV infection and cervical lesion by therapeutic DNA vaccine in CIN3 patients. Nat Commun 5:5317. Epub 2014/10/31. 10.1038/ncomms6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreimer AR, Struyf F, Del Rosario-Raymundo MR, Hildesheim A, Skinner SR, Wacholder S, Garland SM, Herrero R, David MP, Wheeler CM, Costa Rica Vaccine Trial Study Group A, Gonzalez P, Jimenez S, Lowy DR, Pinto LA, Porras C, Rodriguez AC, Safaeian M, Schiffman M, Schiller JT, Schussler J, Sherman ME, Authors PSG, Bosch FX, Castellsague X, Chatterjee A, Chow SN, Descamps D, Diaz-Mitoma F, Dubin G, Germar MJ, Harper DM, Lewis DJ, Limson G, Naud P, Peters K, Poppe WA, Ramjattan B, Romanowski B, Salmeron J, Schwarz TF, Teixeira JC, Tjalma WA, Collaborators HPPIC-PI, Group GSKVCSS (2015) Efficacy of fewer than three doses of an HPV-16/18 AS04-adjuvanted vaccine: combined analysis of data from the costa rica vaccine and PATRICIA trials. Lancet Oncol. 16(7):775–786. Epub 2015/06/14. 10.1016/s1470-2045(15)00047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gouellec A, Chauchet X, Polack B, Buffat L, Toussaint B (2012) Bacterial vectors for active immunotherapy reach clinical and industrial stages. Hum Vaccin Immunother 8(10):1454–1458. Epub 2012/08/17. 10.4161/hv.21429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Yang A, Wu TC, Hung CF (2016) Immunotherapy for human papillomavirus-associated disease and cervical cancer: review of clinical and translational research. J Gynecol Oncol 27 (5):e51. Epub 2016/06/23. 10.3802/jgo.2016.27.e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CW, Lee JY, Tsao YP, Shen CP, Lai HC, Chen SL (2002) Oral vaccination with recombinant Listeria monocytogenes expressing human papillomavirus type 16 E7 can cause tumor growth in mice to regress. Int J Cancer 102(6):629–637. Epub 2002/11/26. 10.1002/ijc.10759. [DOI] [PubMed] [Google Scholar]
- Lin K, Doolan K, Hung CF, Wu TC (2010) Perspectives for preventive and therapeutic HPV vaccines. J Formos Med Assoc 109(1):4–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado L, Teague JE, Morrow MP, Jotova I, Wu TC, Wang C, Desmarais C, Boyer JD, Tycko B, Robins HS, Clark RA, Trimble CL (2014) Intramuscular therapeutic vaccination targeting HPV16 induces T cell responses that localize in mucosal lesions. Sci Transl Med 6 (221):221ra13. Epub 2014/01/31. 10.1126/scitranslmed.3007323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manini I, Montomoli E (2018) Epidemiology and prevention of Human Papillomavirus. Ann Ig 30 (4 Suppl 1):28–32. Epub 2018/08/01. 10.7416/ai.2018.2231. [DOI] [PubMed] [Google Scholar]
- Massarelli E, William W, Johnson F, Kies M, Ferrarotto R, Guo M, Feng L, Lee JJ, Tran H, Kim YU, Haymaker C, Bernatchez C, Curran M, Zecchini Barrese T, Rodriguez Canales J, Wistuba I, Li L, Wang J, van der Burg SH, Melief CJ, Glisson B (2019) Combining immune checkpoint blockade and tumor-specific vaccine for patients with incurable human papillomavirus 16-related cancer: a phase 2 clinical trial. JAMA Oncol 5(1):67–73. Epub 2018/09/30. 10.1001/jamaoncol.2018.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merck & Co. Inc. (2017) Gardasil 9 human papillomavirus 9-valent vaccine, recombinatnt. Available from https://www.gardasil9.com/. Updated 2017; Cited 2019
- Merck & Co. Inc. (2019) Efficacy of GARDASIL 9. Available from https://www.merckvaccines.com/Products/Gardasil9/efficacy#add5Types. Updated Feb 2019; Cited 2019
- Miles BA, Monk BJ, Safran HP (2017) Mechanistic insights into ADXS11–001 human papillomavirus-associated cancer immunotherapy. Gynecol Oncol Res Pract 4:9. Epub 2017/06/08. 10.1186/s40661-017-0046-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cancer Institute (2020) HPV and cancer. Available from: https://www.cancer.gov/about-cancer/causes-prevention/risk/infectious-agents/hpv-and-cancer. Updated 1 Mar 2019; Cited 2019 [Google Scholar]
- Nurkkala M, Wassen L, Nordstrom I, Gustavsson I, Slavica L, Josefsson A, Eriksson K (2010) Conjugation of HPV16 E7 to cholera toxin enhances the HPV-specific T-cell recall responses to pulsed dendritic cells in vitro in women with cervical dysplasia. Vaccine 28(36):5828–5836. Epub 2010/07/06. 10.1016/j.vaccine.2010.06.068. [DOI] [PubMed] [Google Scholar]
- Peng S, Kim TW, Lee JH, Yang M, He L, Hung CF, Wu TC (2005) Vaccination with dendritic cells transfected with BAK and BAX siRNA enhances antigen-specific immune responses by prolonging dendritic cell life. Hum Gene Ther 16(5):584–593. Epub 2005/05/27. 10.1089/hum.2005.16.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S, Monie A, Pang X, Hung CF, Wu TC (2011) Vascular disrupting agent DMXAA enhances the antitumor effects generated by therapeutic HPV DNA vaccines. J Biomed Sci 18:21. Epub 2011/03/10. 10.1186/1423-0127-18-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S, Qiu J, Yang A, Yang B, Jeang J, Wang JW, Chang YN, Brayton C, Roden RBS, Hung CF, Wu TC (2016) Optimization of heterologous DNA-prime, protein boost regimens and site of vaccination to enhance therapeutic immunity against human papillomavirus-associated disease. Cell Biosci 6:16. Epub 2016/02/27. 10.1186/s13578-016-0080-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Wang XH, Cui HL, Cheung YK, Hu MH, Zhu SG, Xie Y (2005) Human papillomavirus type 16 E7 peptide(38–61) linked with an immunoglobulin G fragment provides protective immunity in mice. Gynecol Oncol 96(2):475–83. Epub 2005/01/22. 10.1016/j.ygyno.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Ramanathan P, Ganeshrajah S, Raghanvan RK, Singh SS, Thangarajan R (2014) Development and clinical evaluation of dendritic cell vaccines for HPV related cervical cancer—A feasibility study. Asian Pac J Cancer Prev 15(14):5909–5916 [DOI] [PubMed] [Google Scholar]
- Roden RBS, Stern PL (2018) Opportunities and challenges for human papillomavirus vaccination in cancer. Nat Rev Cancer. 18(4):240–254. Epub 2018/03/03. 10.1038/nrc.2018.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roden R, Wu TC (2006) How will HPV vaccines affect cervical cancer? Nat Rev Cancer 6 (10):753–763. Epub 2006/09/23. 10.1038/nrc1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosales R, Lopez-Contreras M, Rosales C, Magallanes-Molina JR, Gonzalez-Vergara R, Arroyo-Cazarez JM, Ricardez-Arenas A, Del Follo-Valencia A, Padilla-Arriaga S, Guerrero MV, Pirez MA, Arellano-Fiore C, Villarreal F (2014) Regression of human papillomavirus intraepithelial lesions is induced by MVA E2 therapeutic vaccine. Hum Gene Ther 25 (12):1035–1049. Epub 2014/10/03. 10.1089/hum.2014.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio I, Bolchi A, Moretto N, Canali E, Gissmann L, Tommasino M, Muller M, Ottonello S (2009) Potent anti-HPV immune responses induced by tandem repeats of the HPV16 L2 (20–38) peptide displayed on bacterial thioredoxin. Vaccine 27(13):1949–1956. Epub 2009/04/17. 10.1016/j.vaccine.2009.01.102. [DOI] [PubMed] [Google Scholar]
- Safaeian M, Porras C, Pan Y, Kreimer A, Schiller JT, Gonzalez P, Lowy DR, Wacholder S, Schiffman M, Rodriguez AC, Herrero R, Kemp T, Shelton G, Quint W, van Doorn LJ, Hildesheim A, Pinto LA, Group CVT (2013) Durable antibody responses following one dose of the bivalent human papillomavirus L1 virus-like particle vaccine in the Costa Rica Vaccine Trial. Cancer Prev Res (Phila). 6(11):1242–1250. Epub 2013/11/06. 10.1158/1940-6207.capr-13-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santin AD, Bellone S, Roman JJ, Burnett A, Cannon MJ, Pecorelli S (2005) Therapeutic vaccines for cervical cancer: dendritic cell-based immunotherapy. Curr Pharm Des 11(27):3485–3500 [DOI] [PubMed] [Google Scholar]
- Santin AD, Bellone S, Palmieri M, Zanolini A, Ravaggi A, Siegel ER, Roman JJ, Pecorelli S, Cannon MJ (2008) Human papillomavirus type 16 and 18 E7-pulsed dendritic cell vaccination of stage IB or IIA cervical cancer patients: a phase I escalating-dose trial. J Virol 82(4):1968–1979. Epub 2007/12/07. 10.1128/jvi.02343-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schadlich L, Senger T, Gerlach B, Mucke N, Klein C, Bravo IG, Muller M, Gissmann L (2009) Analysis of modified human papillomavirus type 16 L1 capsomeres: the ability to assemble into larger particles correlates with higher immunogenicity. J Virol 83(15):7690–7705. Epub 2009/05/22. 10.1128/jvi.02588-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellenbacher C, Kwak K, Fink D, Shafti-Keramat S, Huber B, Jindra C, Faust H, Dillner J, Roden RBS, Kirnbauer R (2013) Efficacy of RG1-VLP vaccination against infections with genital and cutaneous human papillomaviruses. J Invest Dermatol 133(12):2706–2713. Epub 2013/06/12. 10.1038/jid.2013.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellenbacher C, Roden RBS, Kirnbauer R (2017) Developments in L2-based human papillomavirus (HPV) vaccines. Virus Res 231:166–175. Epub 2016/11/28. 10.1016/j.virusres.2016.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewell DA, Shahabi V, Gunn GR 3rd, Pan ZK, Dominiecki ME, Paterson Y (2004) Recombinant Listeria vaccines containing PEST sequences are potent immune adjuvants for the tumor-associated antigen human papillomavirus-16 E7. Cancer Res 64(24):8821–8825. Epub 2004/12/18. 10.1158/0008-5472.can-04-1958. [DOI] [PubMed] [Google Scholar]
- Sharma RK, Elpek KG, Yolcu ES, Schabowsky RH, Zhao H, Bandura-Morgan L, Shirwan H (2009) Costimulation as a platform for the development of vaccines: a peptide-based vaccine containing a novel form of 4–1BB ligand eradicates established tumors. Cancer Res 69 (10):4319–4326. Epub 2009/05/14. 10.1158/0008-5472.can-08-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeate JG, Woodham AW, Einstein MH, Da Silva DM, Kast WM (2016) Current therapeutic vaccination and immunotherapy strategies for HPV-related diseases. Hum Vaccin Immunother 12(6):1418–1429. Epub 2016/02/03. 10.1080/21645515.2015.1136039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souders NC, Sewell DA, Pan ZK, Hussain SF, Rodriguez A, Wallecha A, Paterson Y (2007) Listeria-based vaccines can overcome tolerance by expanding low avidity CD8+ T cells capable of eradicating a solid tumor in a transgenic mouse model of cancer. Cancer Immun 7:2. [PMC free article] [PubMed] [Google Scholar]
- Su JH, Wu A, Scotney E, Ma B, Monie A, Hung CF, Wu TC (2010) Immunotherapy for cervical cancer: Research status and clinical potential. BioDrugs 24(2):109–129. Epub 2010/03/05. 10.2165/11532810-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble CL, Morrow MP, Kraynyak KA, Shen X, Dallas M, Yan J, Edwards L, Parker RL, Denny L, Giffear M, Brown AS, Marcozzi-Pierce K, Shah D, Slager AM, Sylvester AJ, Khan A, Broderick KE, Juba RJ, Herring TA, Boyer J, Lee J, Sardesai NY, Weiner DB, Bagarazzi ML (2015) Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2b trial. Lancet 386(10008):2078–2088. Epub 2015/09/21. 10.1016/s0140-6736(15)00239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng CW, Hung CF, Alvarez RD, Trimble C, Huh WK, Kim D, Chuang CM, Lin CT, Tsai YC, He L, Monie A, Wu TC (2008a) Pretreatment with cisplatin enhances E7-specific CD8+ T-cell-mediated antitumor immunity induced by DNA vaccination. Clin Cancer Res 14 (10):3185–3192. Epub 2008/05/17. 10.1158/1078-0432.ccr-08-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng CW, Monie A, Wu CY, Huang B, Wang MC, Hung CF, Wu TC (2008b) Treatment with proteasome inhibitor bortezomib enhances antigen-specific CD8+ T-cell-mediated antitumor immunity induced by DNA vaccination. J Mol Med (Berl). 86(8):899–908. Epub 2008/06/11. 10.1007/s00109-008-0370-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng CW, Trimble C, Zeng Q, Monie A, Alvarez RD, Huh WK, Hoory T, Wang MC, Hung CF, Wu TC (2009) Low-dose radiation enhances therapeutic HPV DNA vaccination in tumor-bearing hosts. Cancer Immunol Immunother 58(5):737–748. Epub 2008/09/26. 10.1007/s00262-008-0596-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumban E, Peabody J, Peabody DS, Chackerian B (2011) A pan-HPV vaccine based on bacteriophage PP7 VLPs displaying broadly cross-neutralizing epitopes from the HPV minor capsid protein, L2. PLoS One 6(8):e23310. Epub 2011/08/23. 10.1371/journal.pone.0023310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration (2018) Cervarix. Available from https://www.fda.gov/biologicsbloodvaccines/vaccines/approvedproducts/ucm186957.htm. Updated 26 Feb 26 2018; Cited 2019
- U.S. Food and Drug Administration (2019) Package insert—Gardasil 9. Available from https://www.fda.gov/downloads/biologicsbloodvaccines/vaccines/approvedproducts/ucm111263.pdf
- U.S. Preventive Services Task Force (2012) Cervical cancer: screening. Available from https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cervical-cancer-screening. Updated Mar 2012; Cited 2019
- Vaccarella S, Lortet-Tieulent J, Plummer M, Franceschi S, Bray F (2013) Worldwide trends in cervical cancer incidence: impact of screening against changes in disease risk factors. Eur J Cancer 49(15):3262–3273. Epub 2013/06/12. 10.1016/j.ejca.2013.04.024. [DOI] [PubMed] [Google Scholar]
- Van Damme P, Bouillette-Marussig M, Hens A, De Coster I, Depuydt C, Goubier A, Van Tendeloo V, Cools N, Goossens H, Hercend T, Timmerman B, Bissery MC (2016) GTL001, A therapeutic vaccine for women infected with human papillomavirus 16 or 18 and normal cervical cytology: results of a phase I clinical trial. Clin Cancer Res 22(13):3238–3248. Epub 2016/06/03. 10.1158/1078-0432.ccr-16-0085. [DOI] [PubMed] [Google Scholar]
- van der Burg SH, Kwappenberg KM, O’Neill T, Brandt RM, Melief CJ, Hickling JK, Offringa R (2001) Pre-clinical safety and efficacy of TA-CIN, a recombinant HPV16 L2E6E7 fusion protein vaccine, in homologous and heterologous prime-boost regimens. Vaccine 19(27):3652–3660 [DOI] [PubMed] [Google Scholar]
- Verch T, Pan ZK, Paterson Y (2004) Listeria monocytogenes-based antibiotic resistance gene-free antigen delivery system applicable to other bacterial vectors and DNA vaccines. Infect Immun 72(11):6418–6425. Epub 2004/10/27. 10.1128/iai.72.11.6418-6425.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vici P, Pizzuti L, Mariani L, Zampa G, Santini D, Di Lauro L, Gamucci T, Natoli C, Marchetti P, Barba M, Maugeri-Sacca M, Sergi D, Tomao F, Vizza E, Di Filippo S, Paolini F, Curzio G, Corrado G, Michelotti A, Sanguineti G, Giordano A, De Maria R, Venuti A (2016) Targeting immune response with therapeutic vaccines in premalignant lesions and cervical cancer: hope or reality from clinical studies. Expert Rev Vaccines 15(10):1327–1336. Epub 2016/04/12. 10.1080/14760584.2016.1176533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Munoz N (1999) Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 189(1):12–19. Epub 1999/08/19. . [DOI] [PubMed] [Google Scholar]
- Wallecha A, Wood L, Pan ZK, Maciag PC, Shahabi V, Paterson Y (2013) Listeria monocytogenes-derived listeriolysin O has pathogen-associated molecular pattern-like properties independent of its hemolytic ability. Clin Vaccine Immunol 20(1):77–784. Epub 2012/11/09. 10.1128/cvi.00488-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Troilo PJ, Wang X, Griffiths TG, Pacchione SJ, Barnum AB, Harper LB, Pauley CJ, Niu Z, Denisova L, Follmer TT, Rizzuto G, Ciliberto G, Fattori E, Monica NL, Manam S, Ledwith BJ (2004) Detection of integration of plasmid DNA into host genomic DNA following intramuscular injection and electroporation. Gene Ther 11(8):711–721. Epub 2004/01/16. 10.1038/sj.gt.3302213. [DOI] [PubMed] [Google Scholar]
- Welters MJ, van der Sluis TC, van Meir H, Loof NM, van Ham VJ, van Duikeren S, Santegoets SJ, Arens R, de Kam ML, Cohen AF, van Poelgeest MI, Kenter GG, Kroep JR, Burggraaf J, Melief CJ, van der Burg SH (2016) Vaccination during myeloid cell depletion by cancer chemotherapy fosters robust T cell responses. Sci Transl Med 8(334):334ra52. Epub 2016/04/15. 10.1126/scitranslmed.aad8307. [DOI] [PubMed] [Google Scholar]
- Williams VM, Filippova M, Soto U, Duerksen-Hughes PJ (2011) HPV-DNA integration and carcinogenesis: putative roles for inflammation and oxidative stress. Future Virol 6(1):45–57. Epub 2011/02/15. 10.2217/fvl.10.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Cancer Research Fund (2018) Worldwide cancer data. Available from: https://www.wcrf.org/dietandcancer/cancer-trends/worldwide-cancer-data. Cited 2019
- World Health Organization (2018) Human papillomavirus (HPV). Available from: https://www.who.int/immunization/diseases/hpv/en/. Cited 2019
- World Health Organization (2020) Human papillomavirus (HPV) and cervical cancer. Available from https://www.who.int/immunization/diseases/hpv/en/. Updated 24 Jan 2019; Cited 2019
- Wu CY, Monie A, Pang X, Hung CF, Wu TC (2010) Improving therapeutic HPV peptide-based vaccine potency by enhancing CD4+ T help and dendritic cell activation. J Biomed Sci 17:88. Epub 2010/11/26. 10.1186/1423-0127-17-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W, Chen WC, Liu Z, Huang L (2010) Bryostatin-I: a dendritic cell stimulator for chemokines induction and a promising adjuvant for a peptide based cancer vaccine. Cytokine 52(3):238–244. Epub 2010/09/28. 10.1016/j.cyto.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Yang A, Farmer E, Wu TC, Hung CF (2016) Perspectives for therapeutic HPV vaccine development. J Biomed Sci 23(1):75. Epub 2016/11/05. 10.1186/s12929-016-0293-9. PubMed PMID: 27809842; PMCID: PMC5096309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang A, Farmer E, Lin J, Wu TC, Hung CF (2017) The current state of therapeutic and T cell-based vaccines against human papillomaviruses. Virus Res 231:148–615. Epub 2016/12/10. 10.1016/j.virusres.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim EK, Park JS (2005) The role of HPV E6 and E7 oncoproteins in HPV-associated cervical carcinogenesis. Cancer Res Treat 37(6):319–324. Epub 2005/12/01. 10.4143/crt.2005.37.6.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon SW, Lee TY, Kim SJ, Lee IH, Sung MH, Park JS, Poo H (2012) Oral administration of HPV-16 L2 displayed on Lactobacillus casei induces systematic and mucosal cross-neutralizing effects in Balb/c mice. Vaccine 30(22):3286–3294. Epub 2012/03/20. 10.1016/j.vaccine.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Zandberg DP, Rollins S, Goloubeva O, Morales RE, Tan M, Taylor R, Wolf JS, Schumaker LM, Cullen KJ, Zimrin A, Ord R, Lubek JE, Suntharalingam M, Papadimitriou JC, Mann D, Strome SE, Edelman MJ (2015) A phase I dose escalation trial of MAGE-A3- and HPV16-specific peptide immunomodulatory vaccines in patients with recurrent/metastatic (RM) squamous cell carcinoma of the head and neck (SCCHN). Cancer Immunol Immunother 64(3):367–379. Epub 2014/12/30. 10.1007/s00262-014-1640-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai L, Tumban E (2016) Gardasil-9: a global survey of projected efficacy. Antiviral Res.130:101–109. Epub 2016/04/05. 10.1016/j.antiviral.2016.03.016. [DOI] [PubMed] [Google Scholar]
- Zhang YQ, Tsai YC, Monie A, Hung CF, Wu TC (2010) Carrageenan as an adjuvant to enhance peptide-based vaccine potency. Vaccine 28(32):5212–5219. Epub 2010/06/15. 10.1016/j.vaccine.2010.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaveling S, Ferreira Mota SC, Nouta J, Johnson M, Lipford GB, Offringa R, van der Burg SH, Melief CJ (2002) Established human papillomavirus type 16-expressing tumors are effectively eradicated following vaccination with long peptides. J Immunol. 169(1):350–358 [DOI] [PubMed] [Google Scholar]


