Abstract
Purpose.
Supporting the health of cancer survivors and their families from diagnosis through survivorship is a recognized priority. However, the extent to which health promotion efforts after the completion of acute treatment attend to the needs of adult survivors and families is unclear. This systematic scoping review summarizes the key characteristics of post-treatment lifestyle interventions aiming to improve diet, physical activity, and/or weight-related outcomes for adult cancer survivors and family members.
Methods.
We retrieved relevant studies from six databases using keywords. Studies were appraised for quality and limited to English-language, peer-reviewed journal articles published in or after 2005.
Results.
A total of 2,376 articles were obtained from the databases; 14 main articles (and 2 supplemental articles) representing 14 unique interventions were retained for our analysis. Most interventions were designed to modify aspects of participant diet and physical activity (in combination) or physical activity alone; cited Social Cognitive Theory as a guiding or interpretative framework; typically included survivors of multiple cancer types; and were limited to one type of familial relationship (e.g., spouse/partner, sister). Where reported, intervention samples were predominantly White.
Conclusions.
Few post-treatment interventions concurrently target cancer survivor and family member’s positive lifestyle behaviors. Positive findings highlight the potential for expanding this area of intervention research and increasing understanding of individual and familial factors that contribute to successful post-treatment family interventions.
Implications for Cancer Survivors.
Promoting cancer survivors’ healthy behaviors within the family context could capitalize on existing support networks and improve the health of family members in supportive roles.
Keywords: Cancer survivors, Family intervention, Post-treatment, Physical activity, Diet, Weight
INTRODUCTION
Many individuals make renewed commitments to health and well-being after a cancer diagnosis [1, 2]. These efforts often include a desire to increase engagement in behaviors that promote and sustain their health [3–7]. Research has shown that regular physical activity, a nutritious diet, and maintaining a healthy weight can have a positive impact on cancer survivor outcomes, such as quality of life and mental health (e.g., depression, distress) [8, 9]. The Cancer Survivorship Quality Framework [10] identifies health promotion (particularly weight management, diet, and physical activity) as a key individual factor and family/caregiver relationships as a key interpersonal factor that influences survivor health outcomes. Promoting survivors’ healthy behaviors within the family context could capitalize on interpersonal resources found in existing social networks [11, 12].
Family members are often a primary source of informal support for individuals during and after cancer treatment [13–15]. Common areas of family support include patient-provider communication [16], medical decision-making [17, 18], and symptom management [19, 20]. While providing this support, many family members may be managing health conditions of their own and working to maintain or improve their engagement in health-promoting behaviors [21–23]. Consequently, it is useful to understand the degree to which interventions have focused simultaneously on improving survivor and family member healthy behavior engagement.
Each phase of the cancer continuum provides challenges and opportunities for promoting well-being among individuals and family members. Previous systematic reviews have identified low adherence to diet, physical activity, and other recommended lifestyle behaviors among cancer survivors [24]; opportunities to enhance interventions for families at higher risk of early-onset breast cancer [25]; flexible and innovative ways to support survivors and intimate partners through online mechanisms [26]; and, mixed evidence on associations between social support (e.g., perceptions of support, functional support, relationship quality) and cancer survivor physical activity [27]. Other relevant reviews of broader areas of literature that include, but do not specifically focus on, cancer survivors have synthesized information in areas such as exercise in spouse caregiver/care recipient dyads (multiple health conditions, including cancer) [28] and interventions for chronically ill adults and their caregivers delivered via technology (e.g., text messaging, internet) [29]. To our knowledge, there has not been a systematic examination of diet, physical activity and weight-related interventions specifically for cancer survivors and family members after the completion of acute cancer treatment (i.e., surgery, radiation, chemotherapy). There is a need for greater understanding of interventions that focus on health promotion in this population [30, 31].
Purpose
The purpose of this scoping review is to summarize the characteristics and outcomes of lifestyle interventions aimed to improve diet, physical activity, or weight management outcomes for post-treatment adult cancer survivors and family members. Data from interventions with a broad range of research designs (e.g., randomized controlled trials (RCTs), quasi-experimental, pre-experimental) are included to capture the scope of intervention approaches. Identified strengths, challenges, and gaps in current lifestyle intervention research with survivors and family members can highlight opportunities to build upon existing evidence and pinpoint future directions for this research.
METHODS
Study Design
A scoping review is a type of knowledge synthesis designed to meticulously explore the scope of a field of evidence for heterogeneous studies in a possibly comprehensive manner. We used the methodological framework and guidelines for scoping reviews published by Joanna Briggs Institute (JBI) based on earlier recommendations described by Arksey and O’Malley [32] and subsequent updates proposed by Levac and colleagues [33]. The five stages of the scoping review were: 1) identifying the research question; 2) locating relevant studies with a focus on breadth and depth (i.e., search strategy); 3) selecting studies based on established criteria (i.e., data management and study selection); 4) retrieving relevant data from selected studies (i.e., data extraction), and 5) synthesizing and reporting the results and conducting a quality appraisal. These stages incorporate an iterative method with inclusion and exclusion criteria to search the literature extensively, a quality assessment of included articles, descriptive analysis of article data, and qualitative content review. Our reporting aligns with the adapted Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR) [34]. The methodology selected for our study reflects an inclusive approach that provides a rich understanding of the state of the existing evidence on lifestyle interventions for cancer survivors and family members.
Search Strategy
An experienced librarian (DN), in consultation with a health science informationist, conducted comprehensive literature searches in CINAHL and PsycInfo (both on the EBSCOhost platform), PubMed, Embase, Scopus, and the Web of Science using keywords and database-specific controlled vocabulary terms where available. The protocol for the search was registered with Open Science Framework (OSF, 2020). The searches were conducted between January 14 and February 17 of 2020. Results were limited to English-language peer-reviewed journal articles published in or after 2005. This starting date coincides with the publication of several reports drawing attention to the critical need to support the health and well-being of the growing cancer survivor population: “A National Action Plan for Cancer Survivorship: Advancing Public Health Strategies” published by the Centers for Disease Control and Prevention (CDC) and the Lance Armstrong Foundation in 2004 [35]; “Living Beyond Cancer” a 2004 summary of work by the President’s Cancer Panel [36]; and, “From Cancer Patient to Cancer Survivor: Lost in Transition” originally published by the Institute of Medicine and National Research Council 2005 and based on expert committee work conducted between 2004 and 2005 [37]. Since that time, survival rates have improved [38] and the number of individuals living with a history of cancer in the United States has increased from approximately 10 million to 16 million people [39].
Searches incorporated extensive terms to capture the concepts of diet, human body weight, physical activity, family support, cancer, and survivorship: for example, diet, healthy diet, vegetables, raw foods, nutrients, fruits, portion size, serving size, food preferences, healthy eating, exercise, fitness program, exercise therapy, physical conditioning, endurance training, exercise tolerance, muscle stretching exercises, gardening, swimming, running, walking, dancing, yoga, sports, body weight changes, weight reduction programs, weight gain, weight loss, body weight maintenance, overweight, obesity, aftercare, rehabilitation, continuity of patient care, cancer survivors, postoperative period, post-treatment, quality of life, family, social support, caregivers, spouses, nuclear family, family relations, psychosocial support systems, cancer, and neoplasms.
Data Management and Study Selection
We downloaded articles meeting our search criteria into Endnote. We also created a database in Microsoft Excel for data entry and management, which we used to monitor the process of tracking and removing inner and external duplicates from the pool of our data. Once we had a de-duplicated Endnote library, we uploaded the library to DistillerSR to complete title and abstract screening and full text review. The title and abstract of every retrieved literature were independently reviewed and screened by two study team members to determine studies to be assessed further. When discrepancies among team members occurred, discussion between the team members or a third study team member resolved discrepancies. We repeated the same process to screen the full text of all potentially relevant articles.
Article selected for inclusion in the review met the following criteria: 1) the study was peer-reviewed; 2) the article was published from 2005 onward; 3) the sample included adult cancer survivors ≥18 years of age and family members of any age; 4) the intervention sought to address weight or weight management, improve physical activity, and/or improve nutrition after the end of curative treatment; 5) intervention outcomes included at least one of the following: (a) anthropometric assessment, for example, body weight, body mass index (BMI), body fat, waist measurements; (b) weight-, physical activity-, or nutrition-related constructs (e.g., knowledge, behavior, efficacy, beliefs); and, 6) an English language version of the full text of the article was available. We conducted a pilot test for our study selection process from a random sub-sample of articles; a high level of inter-rater reliability (97.5%) was achieved, which demonstrated consistency and validity of our screening process.
Data Extraction and Quality Appraisal
The study team developed and used a data extraction and quality appraisal spreadsheet in Google Sheets to provide a clear and systematic description of the articles included in the study. The extracted data included but was not limited to study characteristics (e.g., author, publication year), study location, demographics, cancer type, the presence of clarifying commonly used terms such as ‘survivors’ and ‘supporters’, outcome measures, intervention findings. We also created a study codebook to help guide the collection of data to answer the research questions.
The data extraction and quality assessment spreadsheet was refined through pilot testing among team members (KE, DR, MA, MO, CA). To assess reliability, the team independently extracted data, which was followed by a team meeting for discussion and feedback on discrepancies. Upon completing the pilot tests, a high level of inter-rater reliability was achieved for data extraction and quality appraisal (89.69% and 83.33%, respectively). According to the studies reported by Belur et al. 2018, coder agreement above 80% is generally viewed as acceptable [40].
Several steps were undertaken if data being extracted for the review were determined to be missing from the source articles (i.e., main study article). First, the study team created a separate table that noted all the missing characteristics, and the lead author (KRE) verified whether data were missing from the main study article. Next, with the assistance of the experienced librarian (DN), two study team members (KRE & DR) searched for the missing data in other manuscripts referenced in the main study article, reviewed supplemental materials provided with the article (if available), articles that cited the main study article, and articles citing the same grant/funding source. Additional articles were included as supplemental articles if they provided information that complimented and/or added to information provided in the main study article. If data were not located, attempts were made to contact the corresponding author. In these requests, the study team prioritized missing data focused on sample and intervention characteristics. The study team made two attempts to contact the corresponding author via email and request the missing data.
Quality appraisal was conducted following best practices for scoping reviews [32]. We assessed study quality using a 12-point checklist, with one point awarded for each of the criteria met. Our quality assessment checklist was created by modifying Jinks and colleagues’ (2011) quality assessment checklist to fit the purposes of this review [41]. Our modifications included assessment criteria that reflected the description of study designs in our review, such as intervention settings, strengths and limitations, and the likelihood of biases. A detailed summary of the checklist is provided in Table 3.
Table 3:
Quality assessment
| Article (Author, Year) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Assessment (1=present; 0=absent) |
Anton et al. 2013 | Barber 2013 | Cadmus-Bertram et al. 2019 |
Conlon et al. 2015
|
Demark-Wahnefried et al. 2014 | James et al. 2015 |
Knobf et al. 2018
|
Manne et al. 2019 |
Pisu et al. 2017
|
Porter et al. 2018
|
Ross Zahavich et al. 2012 | St. George et al. 2019 | Stoutenberg et al. 2016 | Winters-Stone et al. 2016 |
| Research question/objectives/hypothesis are clear and appropriate | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Clear overview of intervention is given with use of appropriate outcome measures | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Sample size is given | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Randomization method is used in sample selection | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 |
| Attrition rate from the intervention is recorded | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 |
| Data analysis is adequately described and rigorous | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Outcomes of the interventions are clearly described | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Ethical issues mentioned | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Description of intervention setting where contents are delivered | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Strengths AND limitations mentioned or acknowledged | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| The likelihood of biases mentioned | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 |
| Conclusions supported by the results | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Quality Score (out of 12) | 4 | 6 | 11 | 11 | 12 | 12 | 9 | 12 | 9 | 12 | 10 | 11 | 10 | 12 |
RESULTS
The search strategy yielded 2,376 studies. A total of 73 abstracts were obtained from the databases that met our inclusion criteria based on title and abstract review. After examining full text articles and supplementary materials (as applicable), 14 main study articles and 2 supplemental articles representing 14 interventions were retained for our study. A detailed summary of the search and study selection process is in Figure 1. Sample and intervention characteristics are summarized in Tables 1 & 2. Quality assessment information can be found in Table 3. The information included in the tables is discussed below.
Figure 1.
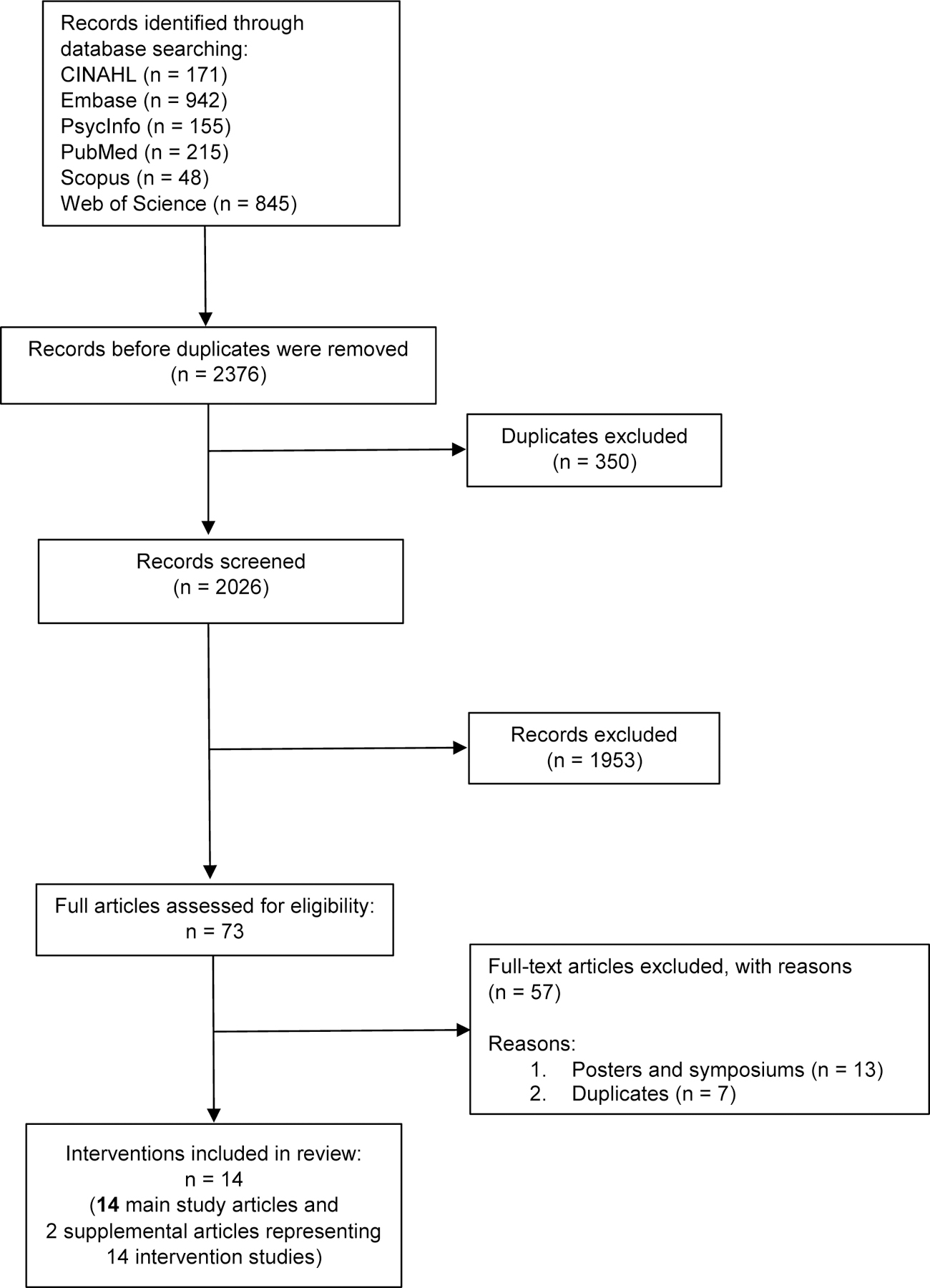
Flow chart of study selection process
Table 1.
Study & Intervention Characteristics
| Author/ Year | Intervention Name | Intervention Setting |
Sample Characteristics | Cancer Type and Stage | Study Focus | Purpose | ||
|---|---|---|---|---|---|---|---|---|
| Diet | Physical Activity | Weight | ||||||
| Anton et al. 2013 | Strong Survivors Nutrition and Exercise Program for Cancer Survivors and Caregivers (SS) | Not reported (NR) |
Sample Size 12 participants (NR survivors, NR family members) Sample Size -Mean age: NR -Sex: NR -Race: NR Family members -Relationship type(s): spouses/partners -Age: Range 27–73 -Sex: 50% male, 50% female -Race: NR |
Type(s) Uterine, prostate, breast, leukemia, testicular Stage 0-IIIB |
X | X | Explore cancer caregivers’ perceptions of participating in a structured exercise and nutrition program with cancer survivors. | |
| Barber 2013 | FitSTEPS for Life® (FSFL) | Community-based exercise site |
Sample Size 101 participants (62 survivors, 39 caregivers) Survivors -Mean age: 65.2 -Sex: 79% female, 21% male -Race: White=81%, African American=7%, Hispanic/Latino=5%, Asian/other=5% Family members -Relationship type(s): spouses, parents, children, siblings, relative -Mean age: 62.1 -Medical conditions: 17 hypertension, 15 high cholesterol, 9 arthritis, 3 diabetes, 3 heart disease -Sex: 74% female, 26% male -Race: White=83%, African American =11%, Hispanic/Latino=6% (n=36 reporting) |
Type(s) Breast, lung, cervical, endometrial, colorectal, liver, gastric, lymphoma, myeloma, leukemia, melanoma, skin, sarcoma, head, neck, kidney, prostate, brain Stage I-IV |
X | Examine associations between cancer survivor and caregiver social support, self-efficacy for physical activity, physical activity behavior, and quality of life. | ||
| Cadmus-Bertram et al. 2019 | NR | Clinic; home; internet application, online, web-based platform; smartphone app; work site; email | 25 dyads (25 survivor, 25 support partner dyads) Survivors -Mean age: 54.4 -Sex: 96% female -Race: White=94%, Hispanic=2%, Black=2%, Multiracial=2% Family members -Family relationship type(s): spouses, relative (broad reference) |
Breast and colorectal cancer. Stage I-III |
X | Test the feasibility of augmenting survivorship care planning with a multi-level physical activity intervention. | ||
| Conlon et al. 2015 | Bronx Oncology Living Daily (BOLD) Healthy Living (BHL) Program | Church; clinic; community-based |
Sample Size 83 participants (66 cancer survivors, 17 cancer co-survivors) Survivors -Mean age: 59.0 -Sex: 93.9% female -Race: African American=45.4%, White=10.6%, Hispanic=31.8%, Other=12.1% Family members -Relationship type(s): NR (study reported co-cancer survivors as family members, friends, anyone who acts as support) -Mean age: 66.4 -Sex: 100% female -Race: African American=94.1%, Hispanic=5.9% |
Type(s) Breast, gynecological, lung. Stage NR |
X | X | Facilitate behavior change among cancer survivors to reduce diabetes risk and improve health-related quality of life through nutrition education and physical activity. | |
| Demark-Wahnefried et al. 2014 | DAMES (Daughters And MothErS Against Breast Cancer) | Home, print materials |
Sample Size 68 dyads (68 mother, 68 daughter dyads) Survivors -Mean age: 61.3 -Sex: 100% female -Race: Non-Hispanic White=74%, African American=18%, Hispanic White=7%, Asian=1% Family members -Relationship type(s): mother/daughter -Mean age: 32.9 -Sex: 100% female -Race: NR |
Type(s) Breast Stage 0-III |
X | X | X | Explore the feasibility of a mother-daughter weight loss intervention and evaluate individual approach vs. team-based approaches to achieving diet and exercise goals and reductions in body mass index (BMI). |
|
James et al. 2015 Supplemental article: Stacey et al. 2016 |
The Exercise and Nutrition Routine Improving Cancer Health (ENRICH) | Home, group based sessions (community) |
Sample Size 133 participants (96 survivors, 24 caregivers, 12 both cancer survivors and caregivers, 1 missing) Survivors -Mean age: control group 58.1 and intervention group 56.2 -Sex: control 74.1% female, intervention 56.2% female -Race: NR Family members -Relationship type(s): spouses, relative/friend -Mean age & sex: NR -Race: NR |
Type(s) Breast, prostate, colorectal, melanoma, non-Hodgkins lymphoma, leukemia, ovarian, and thyroid Stage NR |
X | X | X | Report findings of a theory-based, group-delivered, face-to-face multiple health behavior change intervention on behavioral outcomes among survivors and caregivers. |
| Knobf et al. 2018 | Healthy Sisters - Intervention for Women of Color Breast Cancer Survivors (WCBCS) | NR |
Sample Size 40 participants (30 survivors, 10 partners) Survivors -Mean age: 55.7 -Sex: 100% female -Race: African American=87.5%, White=2.5%, Hispanic=10% Family members -Relationship type(s): siblings -Mean age: 55.7 -Sex: 100% female -Race: African American=80%, White=10%, Hispanic=10% |
Type(s) Breast Stage NR |
X | X | Examine feasibility and preliminary efficacy of a culturally grounded lifestyle intervention focused on functional ability, quality of life, and health behaviors. | |
|
Manne et al. 2019 Supplemental article: Myers-Virtue et al. 2015 |
General Health and Wellness (GHW) | Clinic |
Sample Size 132 couples (132 survivors, 132 spouses) Survivors -Mean age: 60.7 -100% male -Race: White=78%, African American=17%, Hispanic=3%, Asian=0.8%, Other=0.8% Family members -Relationship type(s): spouse/partners -Mean age: 57.1 -Sex: 100% female -Medical conditions: elevated levels of cancer-specific distress -Race: White=76%, African American=16%, Hispanic=5%, Asian=2%, Other=0.8% |
Type(s) Prostate Stage I-III |
X | X | Explore the degree of correspondence between physical activity and fruit and vegetable consumption among prostate cancer survivors and spouses using baseline data from an RCT, from which the GHW intervention was a comparison study arm. | |
| Pisu et al. 2017 | Restoring Health in You (and Your Partner) through Movement (RHYTHM) | Community-based dance studio |
Sample Size 31 couples (31 survivors, 31 partners) Survivors -Mean age: 57.9 -Sex: 100% female -Race: White=77.4%, African American=22.6% Family members -Relationship type(s): spouses/partners -Mean age: 60.7% -Sex: 100% male -Race: White=77.4%, African American=22.6% |
Type(s) Endometrial, ovarian, breast, colorectal Stage NR |
X | Assess feasibility, acceptability and impact of a ballroom dance intervention on improving quality of life and relationship outcomes in cancer survivors and their partners. | ||
| Porter et al. 2018 | Move2Health | Home-based via video-conference |
Sample Size 20 dyads (20 survivors, 20 partners) Survivors -Mean age: 63.0 -Sex: 70% female -Race (intervention): White=44.4%, African American=22.2%, More than 1 race=33.3%. (control): White=100% Family members -Relationship type(s): spouses/partners -Mean age: 62.8 -Sex: 35% female -Race (intervention): White=55.6%, African American =11.1%, More than one race=33.3%. (control): White=81.8%, More than 1 Race=18.2% |
Type(s) Breast and prostate Stage I-IV |
X | Determine the acceptability of a couple-based PA intervention delivered via videoconference and the feasibility of conducting a randomized controlled trial testing the intervention among breast and prostate cancer survivors and their partners. | ||
| Ross Zahavich et al. 2012 | Yoga Thrive | Class-based (location not specified) |
Sample Size 25 participants (15 survivors, 10 support persons: 9 spouse, 1 friend) Survivors -Mean age: 64.5 -Sex: 100% male -Race: NR Family members -Relationship type(s): spouse/friend -Mean age: 60.6 -Sex: 100% female -Race: NR |
Type(s) Prostate Stage NR |
X | Examine the feasibility and benefit of a therapeutic yoga program offered to prostate cancer survivors and their support persons. | ||
| St. George et al. 2019 | NR | Mobile device use (digital health intervention) |
Sample Size 46 participants (a subset of 21 participants completed individual interviews) Survivors -Mean age: 66.1 -Sex: 100% female -Race: Hispanic=34%, non-Hispanic black=33%, non-Hispanic white=33% Family members -Relationship type(s): parent/adult child, grandparent/grandchild -Mean age (adult children): 39.9; (grandchildren): 12.7 -Sex: NR -Race (adult children): Mean Hispanic=2.2, Mean non-Hispanic Black=2.5, Mean non-Hispanic White=2.2. (grandchildren): Mean Hispanic=3.6, Mean non-Hispanic Black=5.6; Mean non-Hispanic White=2.3 |
Type(s) Breast, endometrial, or ovarian Stage I-III |
X | X | X | Design a multigenerational digital lifestyle intervention to improve physical activity, diet and weight among racially and ethnically diverse overweight and obese women cancer survivors by encouraging positive healthy behaviors in their family members (children and grandchildren). |
| Stoutenberg et al. 2015 | Integrated Wellness Program | Clinic |
Sample Size 20 participants (16 survivors, 4 caregivers) Survivors -Mean age: 62.5 -Sex: 75% female, 25% male -Race: White=75%, African American=10%, Hispanic=15% Family members -Relationship type(s): NR -Mean age: data combined with survivors -Sex: NR -Race: NR |
Type(s) Breast, prostate, gastric, multiple myeloma, pancreatic, and more than one cancer type ( i.e., multiple cancer) Stage NR |
X | X | Assess the effectiveness of the Integrative Wellness Program on improving physical activity, nutrition, sleep hygiene, and quality of life program completers. | |
| Winters-Stone et al. 2016 | The Exercise Together Project | Oregon Health & Science University (OHSU) |
Sample Size 64 couples (64 survivors, 64 spouses) Survivors -Mean age: Intervention (70.6), control (72.9) -Sex: 100% male -Race: Intervention 91%=Caucasian, 97%=Non-Hispanic, Control 94%=Caucasian, 94%=Non-Hispanic Family members -Family relationship type(s): spouses -Mean age: Intervention (66.5), control (69.7) -Sex: 100% female -Race: Intervention 91%=Caucasian, 97%=Non-Hispanic, Control 94%=Caucasian, 94%=Non-Hispanic |
Type(s) Prostate Stage NR |
X | X | Determine the feasibility and preliminary efficacy of a couples-based approach to exercise training for couples coping with prostate cancer. | |
| Author/Year | Intervention Name | Study Design |
Intervention Duration & Follow-Up Period | Brief Description of Intervention Content |
|---|---|---|---|---|
| Anton et al. 2013 | Strong Survivors Nutrition and Exercise Program for Cancer Survivors and Caregivers (SS) | Pre-experimental |
Duration 12 weeks Follow-Up 6 months |
Basic nutrition and exercise to benefit the physical and psychosocial health of cancer survivors and cancer caregivers. |
| Barber 2013 | FitSTEPS for Life® (FSFL) | Quasi-experimental |
Duration NR Follow-Up 1 month |
Individually tailored and supervised community-based exercise program for cancer survivors and caregivers. |
| Cadmus-Bertram et al. 2019 | NR | Randomized controlled pilot trial |
Duration 12 weeks Follow-Up NR |
The control group received a care plan, printed copy of national dietary guidelines, and standardized emails during an 8-week period. The intervention group received a care plan, 12-week physical activity module and Fitbit trackers. |
| Conlon et al. 2015 | Bronx Oncology Living Daily (BOLD) Healthy Living (BHL) Program | Pre-experimental; pilot program |
Duration 1 year Follow-Up NR |
Weekly group nutrition education and exercise classes (60 to 75 minutes) for 4 weeks or 12 weeks. At-home class materials and physical activities provides to people who missed group sessions. |
| Demark-Wahnefried et al. 2014 | DAMES (Daughters And MothErS Against Breast Cancer) | Three-arm randomized controlled trial |
Duration 1 year Follow-Up 6, 12 months |
Arm 1: tailored diet and exercise intervention delivered to individuals in dyads Arm 2: tailored diet and exercise intervention emphasizing a team-based approach among dyads Arm 3: Standardized diet and exercise materials All arms received a personalized workbook, informational mailings on a bimonthly basis over the course of a year on exercise and diet (e.g., portion control, nutritious diets, aerobic activity, strength training). |
|
James et al. 2015 Supplemental article: Stacey et al. 2016 |
The Exercise and Nutrition Routine Improving Cancer Health (ENRICH) | Randomized controlled trial |
Duration 8 weeks Follow-Up 20 weeks, 12 months |
Information and activities focused on healthy eating, maintaining a healthy weight, a home-based walking program and resources, and a home based resistance training program with information and resources using a mix of didactic information delivery and practical activities. |
| Knobf et al. 2018 | Healthy Sisters - Intervention for Women of Color Breast Cancer Survivors (WCBCS) | Feasibility study |
Duration 6 weeks Follow-Up 3, 6 months |
Symptom identification and management; physical activity benefits, recommendations, and safety; nutrition choices and strategies; group empowerment and peer support, community resources; health goals; prayer at all sessions. |
|
Manne et al. 2019 Supplemental article: Myers-Virtue et al. 2015 |
General Health and Wellness (GHW) | Randomized controlled trial |
Duration 5 weeks Follow-Up 3, 6 months |
Audio-recorded couples’ sessions and a booster call focused on healthy lifestyles, including: dietary assessment, setting goals, adopting a plant-based diet, relaxation, and increasing regular activity. |
| Pisu et al. 2017 | Restoring Health in You (and Your Partner) through Movement (RHYTHM) | Randomized controlled trial with waitlist control |
Duration 12 weeks (avg - 14.7 weeks; max - 19 weeks) Follow-Up NR |
Couples were taught dances (e.g., Foxtrot, Waltz, Cha-Cha and East Coast Swing) and were expected to practice 5 times per week to increase their physical activity. |
| Porter et al. 2018 | Move2Health | Randomized controlled trial with waitlist control |
Duration 2 months Follow-Up 2 months |
Videoconference sessions that included training in communication skills to help couples identify and use effective social support strategies, engage in joint decision making about goals and plans for increasing physical activity, and work through barriers to behavior change. |
| Ross Zahavich et al. 2012 | Yoga Thrive | Feasibility study including an adherence and maintenance phase |
Duration 14 weeks Follow-Up 7, 14 weeks |
Class-based therapeutic yoga program offered to all prostate cancer survivor participants and their support persons and a maintenance phase involving self-selected physical activity. |
| St. George et al. 2019 | NR | Pre-experimental design using the Integrate, Design, Assess, and Share (IDEAS) framework to guide intervention development. |
Duration 12 months Follow-Up NR |
Didactic content for improving diet and physical activity behaviors (e.g., recipes, exercises), setting and monitoring goals, and enhancing communication. Integrated behavior change and intrinsic motivation-related elements from social cognitive theory (e.g., goal setting, self-regulation, autonomy) and characteristics of positive family relationships from family systems theory (e.g., communication). |
| Stoutenberg et al. 2015 | Integrated Wellness Program | Pre-experimental; pilot program |
Duration 1 year Follow-Up NR |
Knowledge about the benefits of different health practices such as resistance and cardiovascular exercises, eating habits, and mindfulness technique and applied experiences (i.e., practicing exercises). |
| Winters-Stone et al. 2016 | The Exercise Together Project | Randomized controlled trial |
Duration 6 months Follow-Up 3, 6 months |
Couples attend group strength-training exercise sessions led by an exercise physiologist with spouse/partners as training partners. |
Table 2.
Intervention Goals, Measures and Findings
| Author/Year | Intervention Name | Intervention Goals | Measures | Key Findings |
|---|---|---|---|---|
| Anton et al. 2013 | Strong Survivors Nutrition and Exercise Program for Cancer Survivors and Caregivers (SS) |
Diet Provide education on basic nutrition in the management of many physical and psychosocial issues related to cancer survivorship and cancer caregiving Physical Activity Educate survivors and caregivers on the techniques and importance of exercise in the management of the many physical and psychosocial issues related to cancer survivorship and cancer caregiving |
Diet & Physical Activity Semi-structured interview guide, with questions regarding participant experience in the program, including benefits and drawbacks; physical activity and nutrition behaviors, barriers, facilitators and changes; and influence of program on caregiving experience |
Caregivers Caregivers reported that the program helped them spend more quality time with the care recipient; caregivers derived physical and psychological benefits from the program; the program served as an additional source of support for caregivers; and, the program helped to improve caregiver skills and abilities. Survivors Not reported |
| Barber 2013 | FitSTEPS for Life® (FSFL) |
Physical Activity Improve self-efficacy for physical activity, physical activity behavior, and perceptions of social support for physical activity participation |
Physical Activity
-Social Support and Exercise Survey (Sallis) -Exercise Confidence Survey (Sallis) -8-Foot Up-and-Go -Medical Outcomes Survey Short Form 8 (SF-8) |
Survivors and Caregivers Higher physical QoL among caregivers; No significant differences in social support, self-efficacy for PA, PA, or mental QOL in cancer survivors or their caregivers at the one-month follow-up visit. |
| Cadmus-Bertram et al. 2019 | NR |
Physical Activity
To increase survivors’ and supporters’ physical activity Weight: Though not part of the specific study goal, weight change was reported |
Physical Activity ActiGraph GT3X+ accelerometer Weight Digital scale |
Survivors and Support Partners* Increase in physical activity, daily steps among survivors and support partners in the intervention; Intervention group lost 1.8kg compared with the comparison group. *results not separated by group |
| Conlon et al. 2015 | Bronx Oncology Living Daily (BOLD) Healthy Living (BHL) Program |
Diet & Physical Activity To support behavior change that reduces cancer survivor diabetes risk and improves their health-related quality of life and increase community capacity to support the health of survivors transitioning from active treatment. |
Quality of Life 2 items from 36-Item Short Form Health Survey (SF-36) Weight Anthropometric measurements of height, weight, and waist circumference |
Survivors and Family Members There were no statistically significant changes in BMI; however, mean waist circumference significantly improved among participants in the 12-week program. Most participants reported that the program helped them to meet their short-term goals. |
| Demark-Wahnefried et al. 2014 | DAMES (Daughters And MothErS Against Breast Cancer) |
Weight Promote weight loss in overweight and obese mothers recently diagnosed with breast cancer and their daughters who were also overweight or obese. |
Diet -24-hour dietary recalls using the interactive Nutrition Data System Revised software -Healthy Eating Index Physical Activity -Cardiopulmonary exercise test (CPET) -Leisure-Time Exercise Questionnaire Clinical Assessments Height, weight, BMI, systolic and diastolic blood pressure, cardiopulmonary exercise test to assess exercise capacity (VO2peak), oxyhemoglobin saturation. Health-Related Quality of Life 36-Item Short Form Health Survey (SF-36) Self-Efficacy 2 items developed by study team. Social Support Sallis et al. validated scales measuring social support for diet and exercise change. |
Survivors (Mothers) There were significant reductions in BMI, weight and waist circumference among mothers in the individual arm vs. the control arm. Mothers in the individual arm lost a clinically-significant amount of weight. Daughters There were significant reductions in waist circumference among daughters in the individual vs. the control arm. Daughters in the individual arm lost a clinically-significant amount of weight. Dyads Compared to dyads in the control group, dyads in the individual and team arms reported more physical activity per week and exercise capacity. |
|
James et al. 2015 Supplemental article: Stacey et al. 2016 |
The Exercise and Nutrition Routine Improving Cancer Health (ENRICH) |
Diet, Physical Activity, & Weight To improve pedometer-assessed PA, weight, and subsequently body mass index, and vegetable consumption |
Diet Food frequency questionnaire (FFQ) Physical Activity Sealed pedometer to measure step counts during various types of physical activity. Self-Efficacy 9-item scale (Plotnikoff, et al.) Behavior Goals 1-item scale (Courneya, et al.) Outcome Expectations 5-item scale (Plotnikoff, et al.) Impediments 5-item scale (Plotnikoff, et al.) Social Support 2-item scale (Courneya, et al.) |
Survivors and Carers* There was a significant increase in mean daily steps and daily vegetable consumption. Higher step count at follow-ups vs. baseline intervention group; lower step count at follow-ups vs. baseline in control groups. Weight loss was also reported at follow-ups. Behavioral goals were a significant mediator. *results not separated by group |
| Knobf et al. 2018 | Healthy Sisters - Intervention for Women of Color Breast Cancer Survivors (WCBCS) |
Physical Activity & Diet Improve functional ability, quality of life, and health lifestyle behaviors, including physical activity and nutrition. |
Physical Activity & Diet
The Health Promoting Lifestyle Profile II (assesses health responsibility, physical activity, nutrition, interpersonal relations, spiritual growth, and stress management) Functional Ability Medical Outcomes Short Form (MOS-SF36) Quality of Life The Functional Assessment of Cancer Therapy-Breast (Version 4) (FACT-B) Empowerment Self-Efficacy for Exercise Scale |
Survivors There were significant increases in self-efficacy to engage in physical activity, health lifestyle behaviors (overall), physical activity, nutrition, and stress management. Family Members There were significant increases in healthy lifestyle behaviors (overall), nutrition, and health responsibility. |
|
Manne et al. 2019 Supplemental article: Myers-Virtue et al. 2015 |
General Health and Wellness (GHW) |
Diet & Physical Activity To improve the adoption and maintenance of a healthy lifestyle through nutrition education and goal setting, and engaging in regular physical activity. |
Diet An 8-item self-report assessment (Borger, et al.) Physical Activity Godin Leisure Time Exercise Questionnaire Partner Support A 5-item measure (Butterfield and Lewis, 2002) Cancer-Related Distress The Impact of Events Scale (IES; Horwitz, et al.) Depression The Patient Health Questionnaire-9 (PHQ-9) General Psychological Adjustment The Mental Health Inventory-38 (Veit & Ware,) |
Survivors and Partners Baseline findings* showed significant adherence for meeting physical activity guidelines but in terms of fruit and vegetable guidelines, there was no statistical significance. *baseline findings from the supplemental article were not as a result of intervention effects. Manne et al, on the other hand, did not report intervention effects for lifestyle behaviors. |
| Pisu et al. 2017 | Restoring Health in You (and Your Partner) through Movement (RHYTHM) | Physical Activity Increase physical activity and improve relationship functioning and quality of life. |
Physical Activity
Godin Leisure Time Exercise Questionnaire Functional Capacity 6 Minute Walk Test Quality of Life 36-Item Short Form Health Survey (SF-36) Relationship Functioning -Dyadic Trust Scale -Dyadic Adjustment Scale (DAS-7) -3-item Perceived Self-Disclosure Scale |
Survivors Improved physical activity, functional capacity, mental quality of life, vitality, social functioning, and mental health among survivors in treatment vs. control group. Partners No significant differences between treatment and control groups. |
| Porter et al. 2018 | Move2Health |
Physical Activity
Increase physical activity by leveraging partner social support for behavior change. |
Physical Activity Godin Leisure-Time Exercise Questionnaire Partner Support A 15-item scale that measures partner support for exercise habits (Sallis, et al.) Quality of Life Functional Assessment of Cancer Therapy-General (FACT-G physical wellbeing subscale; survivors) |
Survivors and Partners Dyadic communication skills and communal coping enhanced greater participation in physical activity. Survivors and partners reported improved physical activity and physical well-being. |
| Ross Zahavich et al. 2012 | Yoga Thrive |
Physical Activity Increase physical activity adherence by incorporating social support |
Physical Activity Godin’s Leisure Score Index (LSI) of the Godin Leisure Time Exercise Questionnaire Quality of Life -Functional Assessment of Cancer Therapy–Prostate Scale (FACT-P; survivors) -Short Form Health Survey (SF-12; support persons) Fatigue -Functional Assessment of Cancer Therapy–Fatigue scale (FACT-F; survivors) -Fatigue Severity Scale (FSS; support persons) Perceived Social Support Social Provisions Scale (SPS; survivors only) Fitness Canadian Physical Activity, Fitness and Lifestyle Approach (CPAFLA; survivors only) Stress, Mood & Fatigue Thermometer Thermometer Scale |
Survivors and Support Persons Improved stress, fatigue and mood; no significant changes in quality of life or fatigue. Higher perceived social support among survivors when survivor and support person participated together. |
| St. George et al. 2019 | NR |
Diet Dietary intake of at least 2.5 cups per day of fruits and vegetables Physical Activity Engage in regular physical activity (≥ 150 minutes of moderate activity per week) Weight Achieve and maintain a healthy weight (i.e., “normal” BMI) |
Diet Block Fruit/Vegetable/Fiber and Fat Intake Screeners Physical Activity -International Physical Activity Questionnaire – Short Form (IPAQ-SF) -Metabolic equivalent of task (MET) scores for moderate and vigorous physical activity Weight Body-mass index (BMI) Family Functioning Family Environment Scale (FES) |
Survivors Low fruit intake, moderate vegetable intake, and high levels of moderate physical activity was reported among survivors. Family Members Not reported |
| Stoutenberg et al. 2015 | Integrated Wellness Program |
Diet
To engage in an active learning process that improves participants’ knowledge and self-efficacy in making healthy dietary decisions. Physical Activity: To improve participants’ knowledge and self-efficacy in making healthy decisions to engage in physical activity. |
Diet -Self-Efficacy and Eating Habits Survey -Starting the Conversation (STC, an eight-item simplified food frequency instrument) Physical Activity -Godin Leisure–Time Survey -Self-Efficacy and Exercise Habits Survey Sleep The Pittsburgh Sleep Quality Index (PSQI) Quality of Life Short-Form Health Survey (SF-36) |
Survivors and Caregivers* Increased self-efficacy in adopting healthy dietary behaviors; no significant result was reported in physical activity attitudes. *results not separated by group |
| Winters-Stone et al. 2016 | The Exercise Together Project |
Physical Activity & Weight To facilitate partnered exercise as an activity for improving muscle strength, bone mass, body composition, and relationship quality. |
Physical Activity
-Physical Performance Battery (PPB) -CHAMPS questionnaire -1-repetition maximum test (1-RM) Weight Body composition (lean and fat mass) Fatigue Piper Fatigue Scale Depression Center for Epidemiological Studies-Depression (CES-D) Mental & Physical Health Short-Form 36 |
Survivors -Increase in moderate-vigorous intensity physical activity Partners -No significant increase in physical activity was reported -Slight increase in lean mass was reported There was an overall decrease in percentage body fat* *result not separated by group |
Sample Characteristics
Sample Size
The number of families (i.e., survivor and family member enrolled) included in the studies ranged from 9 to 132. Some interventions allowed individuals to enroll alone or with a family member, while others required a survivor and a family member. The number of survivors represented in studies ranged from 16 to 132; the number of family members represented ranged from 4 to 132. The number of family member types (i.e., siblings, children, spouses, etc.) included in the interventions ranged from 1 to 5. Where reported, the mean age ranged from 54.4 to 72.9 years for survivors and 12.7 to 69.7 years for family members. Females made up the majority of the survivor sample in 71.5% of interventions (n=10) [42–52]; in 50% (n=7) of interventions, family members were primarily female [42, 44–46, 53–56].
Family Relationships
In half of the studies (50%, n=7), familial relationships between survivors and family members were limited to one type of familial relationship such as spouse/partner or mother/daughter [45–48, 53, 55–57], while others included mixed types of relationships (37.7%, n=5) [42, 43, 49, 50, 52, 54]. Overall, most studies (64.3%, n=9) included spouses or partners [42, 43, 47, 48, 50, 52–57]. Parent/child (14.3%, n=2) [42, 45], sibling (14.3%, n=2) [42, 46], and grandparent/ grandchild relationships (7.1%, n=1) [49] were also observed. Relationship type was not specified in two interventions (14.3%, n=2) [44, 51]. Though not a focus of this scoping review, a few studies (21.4%, n=3) [44, 50, 52, 54] also included non-family members (i.e., friends).
Cancer Type, Stage, and Time Since Treatment
Five of the interventions focused on a single cancer type, either breast cancer (14.3%, n=2) [45, 46] or prostate cancer (21.4%, n=3) [53–56]. In a majority of interventions, however, multiple cancer types were represented (64.3%, n=9) [42–44, 47–49, 51, 52, 57, 58]. Survivors of breast cancer were included in most of the studies (78.6%, n=11) [42–52, 57]; other cancer types represented were colorectal (28.6%, n=4) [42, 43, 47, 50, 52], endometrial (21.4%, n=3) [42, 47, 49], leukemia (21.4%, n=3;) [42, 50, 52, 57], ovarian (21.4%, n=3) [47, 49, 50, 52], myeloma (14.3%, n=2) [42, 51], lung (14.3%, n=2) [42, 44], gastric (14.3%, n=2) [42, 51], lymphoma (14.3%, n=2) [42, 50, 52], melanoma (14.3%, n=2) [42, 50, 52], uterine (7.1%, n=1) [57], testicular (7.1%, n=1) [57], cervical (7.1%, n=1) [42], liver (7.1%, n=1) [42], skin (7.1%, n=1) [42], sarcoma (7.1%, n=1) [42], head/neck (7.1%, n=1) [42], kidney (7.1%, n=1) [42], brain (7.1%, n=1) [42], thyroid (7.1%, n=1) [50, 52], pancreatic (7.1%, n=1) [51], and unspecified gynecological cancers (7.1%, n=1) [44]. The stage of cancer across all studies ranged from 0-IV; cancer stage was not reported in half of the studies (50%, n=7) [44, 46, 47, 51, 52, 54, 55, 59]. Time since treatment was reported in three studies (21.4%, n=3) [53, 54, 56, 57]; it ranged from 6 to 12 months and overall time since diagnosis was reported in 5 studies ranging from 24 months to 60 months (37.5%, n=5) [44, 45, 47, 50, 52, 54].
Family Medical History
Very few studies reported physical and/or mental health conditions of family members (14.3%, n=2). Of these two studies, one reported cancer-related distress of family members [53, 56]. The other reported the prevalence of hypertension, high cholesterol, arthritis, diabetes, heart disease among family members [42].
Race and Ethnicity
Racial and ethnic composition was reported for cancer survivors and/or family members in most of the intervention studies (78.6%, n=11) [42–49, 53, 55, 56]. All studies had more than one racial or ethnic group; however, many studies included a primary racial group, representing over 50% of the sample. For example, in 50% (n=7) studies, the population sample for survivors and family members were predominantly White (50%, n=7) [42, 43, 45, 47, 48, 51, 53, 56]; in 14.3% (n=2) the study sample was predominantly African American [44, 46]; in 7.1% (n=1) the study sample was predominantly Hispanic (excluding non-Hispanic White or non-Hispanic Black race) [49]; and, in 7.1% (n=1) the study sample was predominantly non-Hispanic, non-White [55].
Study Design
Study Type & Theoretical Framework
Half of the intervention study design types were RCTs (50%, n=7) [43, 45, 47, 48, 50, 52, 53, 55, 56] and one study employed a quasi-experimental design (7.1%, n=1) [42]. A theoretical framework or model was discussed in most of the intervention studies (71.4%, n=10) [42, 44–53, 56]. The theories included: social cognitive theory (35.7%, n=5) [42, 45, 49–52]; interdependence theory (21.4%, n=3) [45, 48, 53, 56]; theory of communal coping (14.3%, n=2) [45, 48]; socio-ecological framework (7.1%, n=1) [44]; transtheoretical model of behavior change (7.1%, n=1) [45]; cognitive interaction (7.1%, n=1) [47]; health belief model (7.1%, n=1) [51]; intimacy model (7.1%, n=1) [47]; self-determination theory (7.1%, n=1) [49]; chronic disease self-management model (7.1%, n=1) [50, 52]; and, family systems theory (7.1%, n=1) [49]. Most of the studies reported more than one theory in their study designs (42.9%, n=6) [45, 47–52], and a few others reported one theory (28.6%, n=4) [42, 44, 46, 53, 56].
Measurement
A variety of measures captured social support and social interactions believed to be associated with the health behavior engagement, including interpersonal trust in relationships, relationship quality, level of agreement, cohesion, expressiveness, and conflict, among others. Most studies measured perceived social support in some capacity (57.1%, n=8) [42, 46–49, 53–56]. Measures used included the Social Support and Exercise Survey (14.3%, n=2) [42, 48], Dyadic Trust and Dyadic Adjustment Scale (7.1%, n=1) [47], Spiritual Growth and Interpersonal Relations Subscale (7.1%, n=1) [46], Social Provisions Scale (7.1%, n=1) [54], a 5-item measure adapted from Butterfield and Lewis (7.1%, n=1) [53, 56], a 15-item Mutuality Scale (7.1%, n=1) [55], and the Family Environment Scale (7.1%, n=1) [49].
Intervention Characteristics
Settings
Intervention settings were varied and in some instances, included multiple locations. Interventions occurred at academic settings (7.1%, n=1) [55], medical clinics (35.7%, n=5) [43, 44, 47, 51, 53, 56], participant homes (28.6%, n=4) [43, 45, 48, 52, 58], via internet or other web-based platforms (14.3%, n=2) [43, 49], church (7.1%, n=1) [44] and other community-based locations (28.6%, n=4) [42, 44, 47, 54]. Some or all of the settings where interventions occurred were not reported or unclear in a few cases (14.3%, n=2) [46, 57].
Content, Modality & Duration
Intervention content included nutrition and exercise programs or classes either as an individual or as a group (28.6%, n=4) [42, 44, 47, 57]; some targeted improving knowledge about healthy eating habits using dietary guidelines, and the delivery of informational materials/sessions about healthy eating habits in combination with physical activity (28.6%, n=4) [43, 45, 50–52]. Other modalities included audio/video-recorded sessions with a focus on dietary assessment and setting goals for increasing physical activity (14.3%, n=2) [48, 53, 56]; exercise sessions only (14.3%, n=2) [54, 55]; a face-to-face interactive design (7.1%, n=1) [46]; and, digital health intervention design (7.1%, n=1) [51]. The average duration of an intervention was 23.5 weeks, with a range of 6 to 52 weeks (based on available data from 13/14 studies).
Outcomes
Several studies were designed to modify aspects of participant diet and physical activity in combination (35.7%, n=5) [44, 46, 51, 53, 57] and three studies examined diet, physical activity and weight (21.4%, n=3)[45, 49, 50, 52]. A number of studies also focused exclusively on physical activity-related outcomes (35.7%, n=5) [42, 43, 47, 48, 54]. No studies focused on diet alone.
Most of the studies reported an increase in knowledge and self-efficacy, leading to significant improvements in physical activity, weight management, and/or improved diet quality (64.3%, n=9) [42, 43, 47, 48, 50–54, 56, 57]. A study (7.1%, n=1) [44] focused on behavior changes to reduce diabetes risk in cancer survivors through nutrition education and physical activity showed no statistically significant changes in body mass index but reported improved mean waist circumference in those completing a 12-week (vs. 4-week) program.
One study focused on improved physical functioning (e.g., decreased fatigue, increased muscle strength) and body composition as measured by the total amount of muscle and percent body fat (7.1%, n=1) [55]. This study reported similar improvements in upper and lower body strength for both survivors and partners, but only survivors reported an increase in moderate-vigorous intensity physical activity [55]. Another study focused on promoting a nutrient-rich, low-energy density diet; improving physical activity by encouraging exercise; and improved weight-related outcomes as measured by body mass index, overall weight, and waist circumference in overweight or obese women (7.1%, n=1) [45]. This study showed improvements in diet quality and decreased daily caloric intake that were associated with better weight-related outcomes for survivors, while diet quality was not significantly associated with any weight-related outcomes for family members [45]. Survivors in the Knobf study (2018) reported improvements in physical activity and nutrition and partners showed an increase in nutrition and significant changes in total healthy lifestyle [46].
Quality Assessment
Table 3 presents the quality assessment of 14 articles included in this review. Quality assessment scores ranged from 4 to 12 (of 12 possible points) with a mean score of 10.1. The majority of the studies scored 10 or greater (71.4%, n=10) [43–45, 48, 49, 51, 52, 54–56]. Across all studies, the lowest scoring area of quality assessment was use of a randomization method (50%, n=7) [43, 45, 48, 49, 52, 55, 56], followed by reporting of the intervention attrition rate (64.3%, n=9) [42, 44–46, 48, 51, 52, 55, 56], and discussing the likelihood of study bias (78.6%, n=11) [42–45, 48, 49, 52, 54–57]. Clear and appropriate research questions, objectives, and/or hypotheses was a noted strength of all studies in this review.
DISCUSSION
This review summarized information on study design, sample characteristics, measures, intervention content and duration, quality assessment, and outcomes of lifestyle interventions for post-treatment cancer survivors and family members. Regular physical activity, maintaining a healthy weight, and consuming diets rich in fruits and vegetables have been associated with better physical functioning throughout the continuum of cancer care and improved quality of life for cancer survivors [8, 9]. Cancer is not usually experienced in isolation [60]; cancer survivors often deal with the challenges of cancer diagnosis and treatment alongside a family member [61]. Thus, lifestyle interventions centered within the family context can facilitate mutual engagement in health-promoting behaviors.
In the United States, cancers of the breast and prostate are the most prevalent among women and men, respectively [62]. In this review, five of the fourteen intervention studies (35.7%) focused on either breast cancer or prostate cancer (alone), and more than half of the included survivors of more than one type of cancer. Thus, the survivor populations included in this review reflect broader prevalence data. Very few studies provided information on the health/medical history of the family members included in the intervention, so it is unclear whether family members in the study resemble broader population trends in physical and mental health. Most studies reported improvements in physical activity, weight management, and/or improved diet quality. When reviewing studies, it was often not clear whether results differed based on survivor or family member role. Where reported, a few studies showed different outcomes for cancer survivors and family members (21.4%, n=3) [45, 46, 55]. More information about the medical history of family members, and similarities and differences in intervention outcomes for survivors and family members, could be useful for informing future interventions.
While the concept of family is often broadly defined, its operationalization in intervention research with adult cancer survivors remains narrow. The primary familial relationship observed in our review was the spouse/intimate partner relationship; other relationships such as parent/child and sibling relationships were represented to a lesser extent. Research shows that adult cancer survivors in marital or committed relationships may cope better with the challenges of a cancer diagnosis and treatment than their unmarried counterparts [60, 63]. These findings are often attributed to the support for managing cancer-related challenges provided within a spousal/intimate partner relationship. For example, a recent systematic review of couple-based intervention studies for couples coping with cancer found that interventions focusing on facilitating coping through communication yielded promising results [64].
Though few, studies in this review with non-spousal family members highlight how researchers have moved beyond spousal relationships when designing and implementing lifestyle interventions. Frameworks and models like the socioecological model and family health model recognize the family as health producers [65]. The influence of family on individual health outcomes is attributed to shared values, behaviors, routines, and decisions not exclusive to spouse/partner dyads [66, 67]. As this work continues, it will be helpful to determine how relationship type influences mechanisms of change (e.g., communication, type and availability of support) and how to best tailor intervention components to reflect the spousal and non-spousal relationship dynamics.
One rationale for family-based interventions is that close or intimate relationships provide support to help or facilitate control of behaviors and individual self-management [68]. Social support, a predictor of improved health outcomes [69], was commonly assessed across intervention studies by different measures specific to physical activity, dietary change, and cancer type (e.g., the Sallis Social Support and Exercise Survey). Of the five interventions in this review that included spouse/partner relationships (exclusively), two reported associations between partner support and physical activity [47, 48] and one reported associations between spouse/partner trust and physical activity and diet [53, 56]. Understanding the degree to which relationship functioning – beyond social support - may influence participation in interventions and impact the effectiveness is critical [70]. Characteristics of relationship functioning such as trust, happiness, cohesion, conflict, communication, or expressiveness were not consistently measured in the studies included in this review. Differences in relationship characteristics among families (e.g., communication or lack thereof, cohesion, etc.) may help explain inconsistencies in the results of family-based interventions. For example, poor marital satisfaction or marital adjustment among a couple is less likely to lead to collaborative or supportive behaviors to illness management [71]. Moving forward, interventions should consider targeting constructs that strengthen relationship quality and measure relationship quality-related constructs to identify if interventions have influenced these constructs and to evaluate the association between the constructs and the health outcome.
Several interventions in the review sought to support physical activity, nutrition and/or weight management with family members and other supporters identified as family caregivers [42, 50–52, 57]. Cancer survivors and their family caregivers may experience increased depression, uncertainty, and anxiety [72]. There is increased awareness of the challenges associated with cancer caregiving, even after acute treatment ends. As often happens during treatment, cancer caregiver support for survivors after the completion of cancer treatment may be complicated by other competing priorities [73]. Several studies have indicated that the burdens associated with caregiving can contribute to employment-related problems, time-constraints, and financial pressures [74], all of which can influence caregivers’ ability to engage in lifestyle behaviors that support their health and well-being. Interventions that attend to the needs of survivors and those in caregiving roles have the potential to mitigate negative behavioral, psychological, and physiological effects that cancer/caregiving can have on this population [73].
Racial and ethnic disparities in cancer survivorship, specifically poorer outcomes among populations of color, are well documented [62]. Yet communities of color remain underrepresented in cancer interventions, and few studies incorporate culturally appropriate strategies to address cancer inequities [30, 75] or the concurrent health needs of family members [76]. In our review, where reported, half of the studies included predominantly White samples (50%, n=7). Only a few studies included predominantly non-White samples; these samples were primarily African American (14.3%, n=2), Hispanic/Latino (7.1%, n=1), or non-Hispanic/Latino, non-White (7.1%, n=1). Kreuter and colleagues [77] argue that programs using culturally appropriate strategies that are peripheral (e.g., package materials that appeal to the group), evidential (e.g., information, data, experiences specific to that group), linguistic (e.g., words commonly used by the specific group), constituent-involving (e.g., using experiences of a member of the target group through lay helpers or patient navigators), and sociocultural (e.g., concepts that are meaningful and reflect the knowledge of the group’s culture) can help address inequities in cancer care [77].
A few studies in our review documented how their program design attended to issues of cultural appropriateness. Conlon and colleagues [44] included cultural tailoring in the curriculum modules to address the psychosocial needs of minority survivors using informational, practical, supportive, and spiritual strategies. Knobf and colleagues [46] reported that cultural tailoring within their 6-week healthy lifestyle intervention focused information, support and addressing barriers to improved physical and emotional survivorship outcomes. This intervention included prayers at each weekly session (i.e., sociocultural strategy), presentations about breast cancer and women of color (i.e., informational strategy), and an African American exercise physiologist who lived in the local community to give information about physical activity and local resources (i.e., informational and constituent-involving strategy). While they did not explicitly evaluate these cultural strategies, Knobf et al. [46] suggested that these approaches likely contributed to improvements in the participants adopting healthy lifestyle behaviors. Other studies [42, 43] in our review mentioned individual-level tailoring of intervention components but did not describe any aspects of cultural tailoring. Moving forward, increased focus on the cultural appropriateness of interventions can help to ensure that interventions designed are relevant, appropriate, and applicable to the needs, contexts, and experiences of the target population, particularly populations of color [77, 78].
Limitations
Our search strategy was limited to articles published in the English language, so we may have overlooked non-English articles with potential findings relevant for more generalizable conclusions. The search was also limited to six databases (CINAHL, PubMed, PsycInfo, Web of Science, Scopus, and Embase), and it is possible that other relevant work could have been identified from other sources. Finally, our review focused on peer-reviewed journal articles to the exclusion of reports, white papers, or other methods of dissemination that programs – particularly those in non-academic settings – may use to share their findings.
Conclusion
Cancer is a significant life event for individuals who are diagnosed and their loved ones. Family members, often the primary support givers, may be experiencing their own health issues. A family-focused approach to cancer survivorship could improve key lifestyle behaviors such as physical activity, diet, and weight management associated with cancer and chronic disease for survivors and other members of their family system. The majority of studies reviewed included dyads (i.e., two family members) and did not extend to larger family systems. As a whole, studies show promise for improving lifestyle behaviors in the short-term; however, future interventions should aim to sustain behavior change through relevant individual and family-level processes. In addition, the majority of studies included White participants. If equity in cancer outcomes is to be achieved, more studies focused on non-White populations, many of whom are disproportionately burdened by most cancer, are needed. These studies would likely benefit from employing known strategies to enhance cultural appropriateness. Taken together, programs that target survivors and their family members can capitalize on existing social networks while promoting health and well-being for all involved.
REFERENCES
- 1.Demark-Wahnefried W, Peterson B, McBride C, Lipkus I, Clipp E. Current health behaviors and readiness to pursue life-style changes among men and women diagnosed with early stage prostate and breast carcinomas. Cancer 2000;88(3):674–84. [PubMed] [Google Scholar]
- 2.Satia JA, Campbell MK, Galanko JA, James A, Carr C, Sandler RS. Longitudinal Changes in Lifestyle Behaviors and Health Status in Colon Cancer Survivors. Cancer Epidemiol Biomarkers Prev 2004;13(6):1022. [PubMed] [Google Scholar]
- 3.Denlinger CS, Engstrom PF. Colorectal Cancer Survivorship: Movement Matters. Cancer Prev Res (Phila) 2011;4(4):502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mosher CE, Sloane R, Morey MC, Snyder DC, Cohen HJ, Miller PE, et al. Associations between lifestyle factors and quality of life among older long-term breast, prostate, and colorectal cancer survivors. Cancer 2009;115(17):4001–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morey MC, Snyder DC, Sloane R, Cohen HJ, Peterson B, Hartman TJ, et al. Effects of Home-Based Diet and Exercise on Functional Outcomes Among Older, Overweight Long-term Cancer Survivors: RENEW: A Randomized Controlled Trial. JAMA 2009;301(18):1883–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paxton RJ, Phillips KL, Jones LA, Chang S, Taylor WC, Courneya KS, et al. Associations among physical activity, body mass index, and health-related quality of life by race/ethnicity in a diverse sample of breast cancer survivors. Cancer 2012;118(16):4024–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blanchard C, Courneya K, Stein K. Cancer Survivors’ Adherence to Lifestyle Behavior Recommendations and Associations With Health-Related Quality of Life: Results From the American Cancer Society’s SCS-II. J Clin Oncol 2008;26:2198–204. [DOI] [PubMed] [Google Scholar]
- 8.Iyer NS, Osann K, Hsieh S, Tucker JA, Monk BJ, Nelson EL, et al. Health Behaviors in Cervical Cancer Survivors and Associations with Quality of Life. Clin Ther 2016;38(3):467–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hyland KA, Jacobs JM, Lennes IT, Pirl WF, Park ER. Are cancer survivors following the national comprehensive cancer network health behavior guidelines? An assessment of patients attending a cancer survivorship clinic. J Psychosoc Oncol 2018;36(1):64–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nekhlyudov L, Mollica MA, Jacobsen PB, Mayer DK, Shulman LN, Geiger AM. Developing a Quality of Cancer Survivorship Care Framework: Implications for Clinical Care, Research, and Policy. JNCI: Journal of the National Cancer Institute 2019;111(11):1120–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gruber KJ, Haldeman LA. Using the family to combat childhood and adult obesity. Prev Chronic Dis 2009;6(3):A106. [PMC free article] [PubMed] [Google Scholar]
- 12.Zimmerman RS, Connor C. Health Promotion in Context: The Effects of Significant Others on Health Behavior Change. Health education quarterly 16(1):57–75. [DOI] [PubMed] [Google Scholar]
- 13.Kim Y, Given BA. Quality of life of family caregivers of cancer survivors. Cancer 2008;112(S11):2556–68. [DOI] [PubMed] [Google Scholar]
- 14.Kim Y, Kashy DA, Wellisch DK, Spillers RL, Kaw CK, Smith TG. Quality of Life of Couples Dealing with Cancer: Dyadic and Individual Adjustment among Breast and Prostate Cancer Survivors and Their Spousal Caregivers. Ann Behav Med 2008;35(2):230. [DOI] [PubMed] [Google Scholar]
- 15.Northouse L, Williams A-l, Given B, McCorkle R. Psychosocial Care for Family Caregivers of Patients With Cancer. J Clin Oncol 2012;30(11):1227–34. [DOI] [PubMed] [Google Scholar]
- 16.Surbone A Cultural aspects of communication in cancer care. Support Care Cancer 2008;16(3):235–40. [DOI] [PubMed] [Google Scholar]
- 17.Hobbs GS, Landrum MB, Arora NK, Ganz PA, van Ryn M, Weeks JC, et al. The role of families in decisions regarding cancer treatments. Cancer 2015;121(7):1079–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Polacek GNLJ, Ramos MC, Ferrer RL. Breast cancer disparities and decision-making among U.S. women. Patient Educ Couns 2006;65(2):158–65. [DOI] [PubMed] [Google Scholar]
- 19.Given BA, Given CW, Kozachik S. Family Support in Advanced Cancer. CA Cancer J Clin 2001;51(4):213–31. [DOI] [PubMed] [Google Scholar]
- 20.Applebaum AJ, Breitbart W. Care for the cancer caregiver: A systematic review. Palliat Support Care 2013;11(3):231–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellis KR, Janevic MR, Kershaw T, Caldwell CH, Janz NK, Northouse L. The influence of dyadic symptom distress on threat appraisals and self-efficacy in advanced cancer and caregiving. Support Care Cancer 25(1):185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ellis KR, Janevic MR, Kershaw T, Caldwell CH, Janz NK, Northouse L. Engagement in health-promoting behaviors and patient–caregiver interdependence in dyads facing advanced cancer: an exploratory study. J Behav Med 2017;40(3):506–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Litzelman K, Kent EE, Rowland JH. Interrelationships between health behaviors and coping strategies among informal caregivers of cancer survivors. Health Educ Behav 2018;45(1):90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tollosa DN, Tavener M, Hure A, James EL. Adherence to multiple health behaviours in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv 2019;13(3):327–43. [DOI] [PubMed] [Google Scholar]
- 25.Kehm RD, Lloyd SE, Terry MB. Reducing Breast Cancer Risk Across Generations Through Family-Based Interventions. Current Epidemiology Reports 2020;7(3):132–8. [Google Scholar]
- 26.Vanstone R, Fergus KD. Online couple interventions in cancer. Current Opinion in Supportive and Palliative Care 2020;14(1). [DOI] [PubMed] [Google Scholar]
- 27.McDonough MH, Beselt LJ, Daun JT, Shank J, Culos-Reed SN, Kronlund LJ, et al. The role of social support in physical activity for cancer survivors: A systematic review. Psychooncology 2019;28(10):1945–58. [DOI] [PubMed] [Google Scholar]
- 28.Doyle KL, Toepfer M, Bradfield AF, Noffke A, Ausderau KK, Andreae S, et al. Systematic Review of Exercise for Caregiver–Care Recipient Dyads: What Is Best for Spousal Caregivers—Exercising Together or Not at All? The Gerontologist 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Irani E, Niyomyart A, Hickman RL. Systematic Review of Technology-Based Interventions Targeting Chronically Ill Adults and Their Caregivers. West J Nurs Res 2020:0193945919897011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Northouse LL, Katapodi MC, Song L, Zhang L, Mood DW. Interventions with Family Caregivers of Cancer Patients: Meta-Analysis of Randomized Trials. CA Cancer J Clin 2010;60(5):317–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frazelle ML, Friend PJ. Optimizing the Teachable Moment for Health Promotion for Cancer Survivors and Their Families. Journal of the Advanced Practitioner in Oncology 2016;7(4):422–33. [PMC free article] [PubMed] [Google Scholar]
- 32.Arksey H, O’Malley L. Scoping studies: towards a methodological framework. International journal of social research methodology 2005;8(1):19–32. [Google Scholar]
- 33.Levac D, Colquhoun H, O’Brien KK. Scoping studies: advancing the methodology. Implement Sci 2010;5(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med 2018;169(7):467–73. [DOI] [PubMed] [Google Scholar]
- 35.Control CfD, Prevention. A national action plan for cancer survivorship: advancing public health strategies Washington, DC: US Dept of Health and Human Services. 2004. [Google Scholar]
- 36.Reuben S Living beyond cancer: finding a new balance. President’s Cancer Panel 2003–2004 Annual Report 2004. [Google Scholar]
- 37.Hewitt M, Greenfield S, Stovall E, National Research C, Institute of M, National Cancer Policy B, et al. From Cancer Patient to Cancer Survivor : Lost in Transition Washington, D.C., UNITED STATES: National Academies Press; 2005. [Google Scholar]
- 38.Seer.cancer.gov. All Cancer Sites Combined Recent Trends in SEER Relative Survival Rates, 2000–2016. All Stages By Survival Time, Both Sexes, All Races (includes Hispanic), All Ages In: SEER*Explorer, editor.: NIH; National Cancer Institute; 2020. [Google Scholar]
- 39.Seer.cancer.gov. All Cancer Sites Combined; People Alive with Cancer (U.S. Prevalence) on January 1, 2017. In: SEER*Explorer, editor. 2017 cancer prevalence proportions from the SEER 13 Areas (excluding the Alaska Native Registry) and 1/1/2017 US population estimates based on the average of 2016 and 2017 population estimates from the US Bureau of the Census: NIH; National Cancer Institute; 2020. [Google Scholar]
- 40.Belur J, Tompson L, Thornton A, Simon M. Interrater Reliability in Systematic Review Methodology. Sociol Method Res 4912411879937. [Google Scholar]
- 41.Jinks A, Cotton A, Rylance R. Obesity interventions for people with a learning disability: an integrative literature review. J Adv Nurs 67(3):460–71. [DOI] [PubMed] [Google Scholar]
- 42.Barber FD. Effects of social support on physical activity, self-efficacy, and quality of life in adult cancer survivors and their caregivers. Oncol Nurs Forum 2013;40(5):481–9. [DOI] [PubMed] [Google Scholar]
- 43.Cadmus-Bertram L, Tevaarwerk AJ, Sesto ME, Gangnon R, Van Remortel B, Date P. Building a physical activity intervention into clinical care for breast and colorectal cancer survivors in Wisconsin: a randomized controlled pilot trial. J Cancer Surviv 2019;13(4):593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Conlon BA, Kahan M, Martinez M, Isaac K, Rossi A, Skyhart R, et al. Development and Evaluation of the Curriculum for BOLD (Bronx Oncology Living Daily) Healthy Living: a Diabetes Prevention and Control Program for Underserved Cancer Survivors. J Cancer Educ 2015;30(3):535–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Demark-Wahnefried W, Jones LW, Snyder DC, Sloane RJ, Kimmick GG, Hughes DC, et al. Daughters and Mothers Against Breast Cancer (DAMES): Main outcomes of a randomized controlled trial of weight loss in overweight mothers with breast cancer and their overweight daughters. Cancer 2014;120(16):2522–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Knobf MT, Erdos D, Jeon S. Healthy Sisters: A Feasibility study of a health behavior intervention for women of color breast cancer survivors. J Psychosoc Oncol 2018;36(5):597–608. [DOI] [PubMed] [Google Scholar]
- 47.Pisu M, Demark-Wahnefried W, Kenzik KM, Oster RA, Lin CP, Manne S, et al. A dance intervention for cancer survivors and their partners (RHYTHM). J Cancer Surviv 2017;11(3):350–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Porter LS, Gao X, Lyna P, Kraus W, Olsen M, Patterson E, et al. Pilot randomized trial of a couple-based physical activity videoconference intervention for sedentary cancer survivors. Health Psychol 2018;37(9):861–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.St George SM, Esquives BN, Agosto Y, Kobayashi M, Leite R, Vanegas D, et al. Development of a multigenerational digital lifestyle intervention for women cancer survivors and their families. Psychooncology 2020;29(1):182–94. [DOI] [PubMed] [Google Scholar]
- 50.Stacey FG, James EL, Chapman K, Lubans DR. Social cognitive theory mediators of physical activity in a lifestyle program for cancer survivors and carers: Findings from the ENRICH randomized controlled trial. Int J Behav Nutr Phy 2016;13(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stoutenberg M, Sogor A, Arheart K, Cutrono S, Kornfeld J, Cutrono SE. A Wellness Program for Cancer Survivors and Caregivers: Developing an Integrative Pilot Program with Exercise, Nutrition, and Complementary Medicine. J Cancer Educ 2016;31(1):47–54. [DOI] [PubMed] [Google Scholar]
- 52.James E, Stacey F, Chapman K, Boyes A, Burrows T, Girgis A, et al. Impact of a nutrition and physical activity intervention (ENRICH: Exercise and Nutrition Routine Improving Cancer Health) on health behaviors of cancer survivors and carers: a pragmatic randomized controlled trial. BMC Cancer 2015;15(1):710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Myers Virtue S, Manne SL, Kashy D, Heckman CJ, Zaider T, Kissane DW, et al. Correspondence of physical activity and fruit/vegetable consumption among prostate cancer survivors and their spouses. Eur J Cancer Care (Engl) 2015;24(6):827–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ross Zahavich AN, Robinson JA, Paskevich D, Culos-Reed SN. Examining a Therapeutic Yoga Program for Prostate Cancer Survivors. Integr Cancer Ther 2012;12(2):113–25. [DOI] [PubMed] [Google Scholar]
- 55.Winters-Stone KM, Lyons KS, Dobek J, Dieckmann NF, Bennett JA, Nail L, et al. Benefits of partnered strength training for prostate cancer survivors and spouses: Results from a randomized controlled trial of the Exercising Together project. J Cancer Surviv 2016;10(4):633–44. [DOI] [PubMed] [Google Scholar]
- 56.Manne SL, Kashy DA, Zaider T, Kissane D, Lee D, Kim IY, et al. Couple-focused interventions for men with localized prostate cancer and their spouses: A randomized clinical trial. Br J Health Psychol 2019;24(2):396–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Anton PM, Partridge JA, Morrissy MJ. Cancer caregivers’ perceptions of an exercise and nutrition program. Support Care Cancer 2013;21(3):803–10. [DOI] [PubMed] [Google Scholar]
- 58.Stacey FG, James EL, Chapman K, Lubans DR. Social cognitive theory mediators of physical activity in a lifestyle program for cancer survivors and carers: findings from the ENRICH randomized controlled trial. International Journal of Behavioral Nutrition & Physical Activity 2016;13:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.D’Andrea AP, Fernandez CA, Tannenbaum SL, Clarke TC, McClure LA, LeBlanc WG, et al. Correlates of leisure time physical activity compliance in colorectal cancer survivors. Prev Med 2014;62:78–82. [DOI] [PubMed] [Google Scholar]
- 60.Badr H New frontiers in couple-based interventions in cancer care: refining the prescription for spousal communication. Acta Oncol 56(2):139–45. [DOI] [PubMed] [Google Scholar]
- 61.Manne S, Badr H. Intimacy and relationship processes in couples’ psychosocial adaptation to cancer. Cancer 2008;112(S11):2541–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Society AC. Cancer Facts & Figures for African Americans 2019-2021 American Cancer Society; Atlanta, GA; 2019. [Google Scholar]
- 63.Aizer AA, Chen M-H, McCarthy EP, Mendu ML, Koo S, Wilhite TJ, et al. Marital Status and Survival in Patients With Cancer. J Clin Oncol 2013;31(31):3869–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li Q, Loke AY. A systematic review of spousal couple-based intervention studies for couples coping with cancer: direction for the development of interventions. Psychooncology 2014;23(7):731–9. [DOI] [PubMed] [Google Scholar]
- 65.Hanson CL, Crandall A, Barnes MD, Magnusson B, Novilla MLB, King J. Family-Focused Public Health: Supporting Homes and Families in Policy and Practice. Frontiers in public health 2019;7:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Novilla MLB, Barnes MD, De La Cruz NG, Williams PN, Rogers J. Public Health Perspectives on the Family: An Ecological Approach to Promoting Health in the Family and Community. Fam Community Health 29(1):28–42. [DOI] [PubMed] [Google Scholar]
- 67.Poland BD, Green K, McCall. Settings for Health Promotion: An Analytic Framework to Guide Intervention Design and Implementation. Health Promot Pract 2009;10(4):505–16. [DOI] [PubMed] [Google Scholar]
- 68.Martire LM, Helgeson VS. Close relationships and the management of chronic illness: Associations and interventions. Am Psychol 2017;72(6):601–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wright K Social Networks, Interpersonal Social Support, and Health Outcomes: A Health Communication Perspective. Front Commun 2016;1. [Google Scholar]
- 70.Cohen S, Underwood LG, Gottlieb BH. Social Support Measurement and Intervention Cary: Oxford University Press, Incorporated; 2000. [Google Scholar]
- 71.Qadir F, Khalid A, Haqqani S, Zill e H, Medhin G. The association of marital relationship and perceived social support with mental health of women in Pakistan. BMC Public Health 13(1):1150-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Badger T, Segrin C, Dorros SM, Meek P, Lopez AM. Depression and anxiety in women with breast cancer and their partners. Nurs Res 2007;56(1):44–53. [DOI] [PubMed] [Google Scholar]
- 73.Bevans M, Sternberg EM. Caregiving Burden, Stress, and Health Effects Among Family Caregivers of Adult Cancer Patients. JAMA 2012;307(4):398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gaugler JE, Linder J, Given CW, Kataria R, Tucker G, Regine WF. The proliferation of primary cancer caregiving stress to secondary stress. Cancer Nurs 2008;31(2):116–23. [DOI] [PubMed] [Google Scholar]
- 75.Kagawa-Singer M, Valdez Dadia A, Yu MC, Surbone A. Cancer, Culture, and Health Disparities: Time to Chart a New Course? CA Cancer J Clin 2010;60(1):12–39. [DOI] [PubMed] [Google Scholar]
- 76.Ellis KR, Hecht HK, Young TL, Oh S, Thomas S, Hoggard LS, et al. Chronic Disease Among African American Families: A Systematic Scoping Review. Prev Chronic Dis 2020;17:E167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kreuter MW, Lukwago SN, Bucholtz DC, Clark EM, Sanders-Thompson V. Achieving Cultural Appropriateness in Health Promotion Programs: Targeted and Tailored Approaches. Health Educ Behav 2003;30(2):133–46. [DOI] [PubMed] [Google Scholar]
- 78.Sheppard VB, Williams KP, Harrison TM, Jennings Y, Lucas W, Stephen J, et al. Development of decision-support intervention for Black women with breast cancer. Psychooncology 2010;19(1):62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]


