ABSTRACT
The relative importance of antimuscarinic anticholinergic medications for Parkinson's disease (PD) declined after the introduction of levodopa, such that anticholinergic medications are now much more likely to be prescribed for clinical indications other than parkinsonism. Recent studies have found an association between anticholinergic medication exposure and future risk of dementia in older individuals and those with PD. These findings provide a further reason to avoid the use of anticholinergic medications to treat motor symptoms of PD. More importantly, they raise the question of whether one of the goals of PD treatment should be to deprescribe all medications with anticholinergic properties, regardless of their indication, to reduce dementia risk. In this review, we discuss the use of anticholinergic medications in PD, the evidence supporting the association between anticholinergic medications and future dementia risk, and the potential implications of these findings for clinical care in PD.
Keywords: Parkinson's disease, anticholinergic medications, Parkinson's disease dementia, deprescribing, neurotoxicity
Antimuscarinic anticholinergic compounds, such as trihexyphenidyl hydrochloride, procyclidine hydrochloride, diphenhydramine hydrochloride, and benztropine mesylate, were the first widely used medications to treat the motor symptoms of Parkinson's disease (PD). Levodopa and other dopaminergic drugs subsequently became first‐line therapy because of superior efficacy and fewer side effects 1 , 2 ; however, anticholinergic medications continue to be used today for PD, albeit in a limited capacity. Separately, drugs with anticholinergic properties have become popular treatment choices for common cardiovascular, gastrointestinal, genitourinary, and psychiatric conditions. As a result, anticholinergic medications are prescribed to over half of PD patients, most often for clinical indications other than parkinsonism. 3 , 4 , 5
The potential negative effects of anticholinergic medications on cognition include both immediate and long‐term effects. Acute delirium associated with anticholinergic drug exposure has been described. 6 In recent studies, chronic anticholinergic medication use has been associated with an increased risk of dementia in the general older adult population. 7 , 8 , 9 , 10 , 11 , 12 , 13 Given that PD itself is associated with a high risk of dementia, these data require a reassessment of the use of anticholinergic anti‐PD medications and whether there should be efforts to deprescribe all medications with anticholinergic properties in persons with PD. Here, we review the use of anticholinergic medications in PD and the evidence supporting the association between anticholinergic medications and future dementia risk. Last, we discuss the potential implications of these findings for clinical care in PD.
Antimuscarinic Anticholinergics for Motor Symptoms in PD
Until the introduction of levodopa in the late 1960s, antimuscarinic anticholinergic compounds were the principal pharmacological treatment for PD. The use of anticholinergic drugs for PD is often traced to Charcot's reported use of hyoscyamine, atropine, and scopolamine in 1868, 14 but herbs containing anticholinergic compounds were prescribed for conditions resembling PD far earlier in China in the 12th Century A.D. 15 From the late 19th Century until the development of synthetic antimuscarinic anticholinergic agents, antimuscarinic alkaloids were promoted as the primary treatment for PD by European neurologists. 16 By 1945, several synthetic antimuscarinics were made available for PD, including trihexyphenidyl hydrochloride, benztropine mesylate, ethopropazine, cycrimine, and biperiden, some of which are still used today. 16
Anticholinergic medications for the treatment of motor symptoms were eclipsed by levodopa in the late 1960s. A series of studies from 1997 to 2012 report that 5% to 8% of PD patients are prescribed anticholinergic PD drugs (Table 1). More recently, the use of antiparkinsonian anticholinergics was observed to decline from 4.8% in 2010 to 3.6% in 2017 in the United States (US). 17 Nevertheless, they have continued to be used for PD symptoms and even advocated for patients with motor fluctuations or as a levodopa‐sparing strategy when that was a more common goal of early symptomatic therapy. 18 , 19 , 20 Anticholinergics are generally thought to exert a preferential benefit on tremor in PD over bradykinesia and rigidity, 21 , 22 and this observation (although inconsistent) has informed their use for PD symptoms in the post‐levodopa era. 23 , 24 Considering the available evidence, the 2018 International Parkinson and Movement Disorder Society review of treatments for PD motor symptoms concluded that anticholinergic medications are likely efficacious and clinically useful. 25 The mechanism by which anticholinergic medications exert their beneficial effect in PD is incompletely understood. It is presumed that anticholinergics exert their beneficial effect on PD tremor and dystonia via blockade of muscarinic M4 receptors on aspiny cholinergic neurons in the striatum. 26 , 27
TABLE 1.
Percentage of PD patients prescribed anticholinergic medications for motor symptoms
Amantadine, the most commonly used PD medication with anticholinergic effects, is thought to reduce classic motor symptoms through its antimuscarinic effect, enhancement of dopaminergic transmission, and non‐selective NMDA receptor antagonism. 28 The most common indication for amantadine in PD today is to treat levodopa‐induced dyskinesias likely via NMDA receptor antagonism. 25
Adverse Effects of Anticholinergic Medications in PD Patients
Anticholinergic drugs are believed to exert their antiparkinsonian effects by unclear mechanisms, perhaps by antagonizing the increase in striatal acetylcholine that results from reduced nigrostriatal dopaminergic stimulation (eg, trihexyphenidyl, benztropine), 29 and in some cases blocking uptake of dopamine by dopaminergic neurons (ie, benztropine). 30 The mechanisms by which antimuscarinic anticholinergic medications cause adverse effects, however, are better understood. Anticholinergic‐mediated inhibition of smooth muscle results in blurred vision and urinary retention (from impaired ocular accommodation and bladder contraction). Slowing of gastrointestinal tract motility by antimuscarinic drugs produces constipation, may delay the absorption of levodopa in the small intestine and may change the gut microbiome in PD patients. 31 , 32 Anticholinergic medications also inhibit exocrine gland secretion, which results in reduced sweating and salivation. In fact, it was by trialing anticholinergics in parkinsonian patients to reduce salivation that Charcot discovered their antiparkinsonian properties. 33
In addition to peripheral side effects, anticholinergic medications have multiple central nervous system effects in persons with PD, most notably cognitive dysfunction and hallucinations. These adverse effects are mediated through reduced cholinergic transmission from the basal forebrain. Cholinergic basal forebrain degeneration increases with age and having PD causes further basal forebrain degeneration and cortical cholinergic deficiency. 34 Therefore, treatment approaches for PD motor symptoms have recommended avoiding use of anticholinergic medications for the treatment of older (ages 70+) PD patients 19 or those with frank cognitive impairment. 35 Differences in anticholinergic drug metabolism may also predispose those with PD to the negative consequences of anticholinergic medications. In PD, there is evidence of impaired cytochrome P450 2D6 (CYP2D6) function, which has a role in the transformation of precursors into serotonin and dopamine and is central to drug metabolism. Specifically, CYP2D6 is responsible for the metabolism of more anticholinergic drugs than any other drug‐metabolizing enzyme that is widely expressed in the brain (See Fig. 1). Mann et al 36 found that PD individuals had 40% lower CYP2D6 levels in the frontal cortex, cerebellum, and hippocampus than controls. Lower levels of CYP2D6 may reduce the ability of PD individuals to metabolize anticholinergic drugs in the liver and brain, thereby contributing to increased adverse drug events and long‐term neurotoxicity.
FIG. 1.
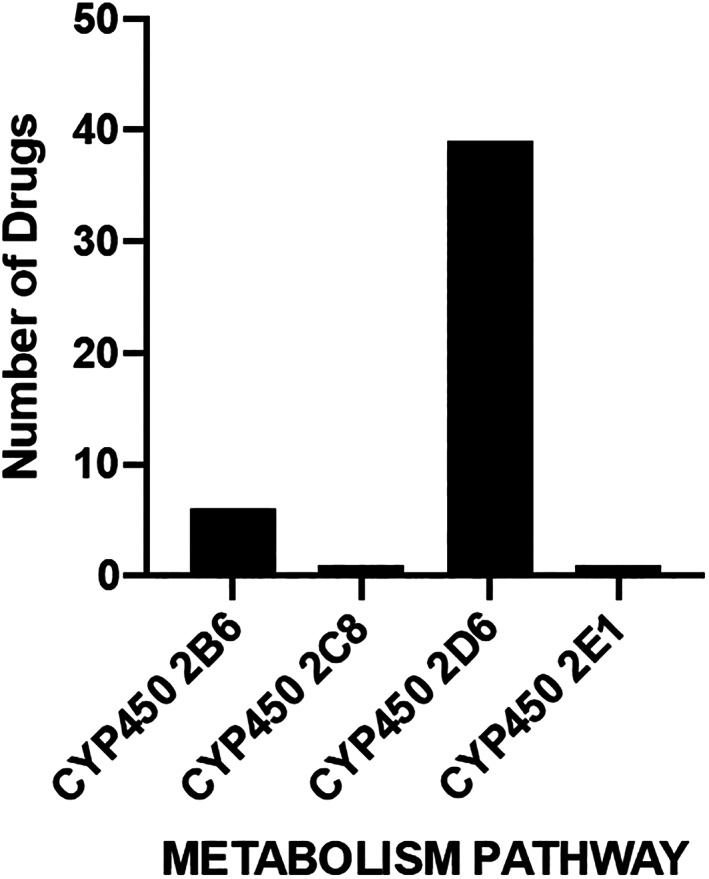
The role of broadly brain expressed CYP450 enzymes on anticholinergic drug metabolism. This figure displays the number of anticholinergic medications included in the Anticholinergic Cognitive Burden scale that are metabolized by each cytochrome P450 enzyme.
Current data suggest that not all cognitive domains are affected equally by anticholinergics; in PD they induce impairment in executive function, 37 , 38 memory, 34 , 39 , 40 , 41 and attention. 42 One study demonstrated that at least some of the memory deficits could be reversible as withdrawal of long‐term anticholinergic medications were associated with improvement in short‐term memory. 43 In early PD, perhaps at a time when the cholinergic basal forebrain system is relatively intact, use of anticholinergic medications has not always been associated with changes in cognition. 44 Increasing evidence from population health and experimental studies suggests that drugs with anticholinergic properties can cause physical frailty. 45 , 46 Mach et al 46 conducted the first preclinical study showing anticholinergic drug withdrawal reversed physical impairment and determined that type and dose of the anticholinergic drug caused more adverse effects than chronic polypharmacy. The effects of anticholinergic drugs on physical frailty remain unexplored in PD.
Anticholinergic drug exposure is associated with negative health events and excess health care use in PD patients. Reduced baseline central cholinergic function related to age and cholinergic basal forebrain degeneration predisposes PD patients to develop anticholinergic delirium. 6 Anticholinergic medications can exacerbate psychotic symptoms in PD, therefore, treatment approaches for PD psychosis recommend discontinuing these medications first. 47 , 48 Loss of cholinergic function also plays a role in gait dysfunction in PD. 49 , 50 It is not surprising that a study of US inpatients with PD found those taking anticholinergic medications were more likely to have fractures or delirium and visits to the emergency department or readmissions within 30 days of discharge. 51
Anticholinergics and Risk of Dementia
Recent studies suggest that medications with anticholinergic properties not only carry a risk of immediate side effects, but may also have long‐term consequences. Over the last decade, an increasing number of studies have found that medications with anticholinergic properties are associated with cognitive decline 7 , 52 , 53 and increased risk of future cognitive impairment or dementia in older adults. 7 , 8 , 9 , 10 , 11 , 12 , 13 This relationship has been found to be dose‐dependent, because greater cumulative anticholinergic use and use of agents with greater anticholinergic activity were associated with greater risk of dementia. 8 , 9 , 10 , 12
Because cholinergic basal forebrain degeneration is already more common in PD, 54 , 55 , 56 , 57 the negative effects of medications with anticholinergic properties may compound already present cholinergic deficits in PD, generating worse immediate negative effects on cognition and psychotic symptoms, but also potentially producing long‐term negative effects on cognition. Although not nearly as robust as the number and quality of studies showing increased risk of future cognitive impairment in individuals taking anticholinergic drugs, a few studies have reported increased risk of cognitive impairment in PD patients taking medications with anticholinergic properties. 58 , 59 , 60 , 61
In a 1996 study of 70 PD patients, the duration of anticholinergic medication usage was associated with an increased risk of dementia. 58 A community‐based cohort of 235 PD patients found that 40% used anticholinergics at baseline, and use of anticholinergics was associated with a lower Mini‐Mental State Examination (MMSE) score. After 8 years, use of medications with anticholinergic properties, greater anticholinergic drug burden, and longer duration of use were all associated with a greater decline in MMSE score. 59 Because depression is a recognized risk factor for dementia, these findings may have been confounded by the fact that anticholinergic use was associated with a greater likelihood of depression at baseline. Two recent studies using administrative data found that PD patients prescribed antiparkinsonian anticholinergics or other medications with anticholinergic properties had an increased risk of future dementia compared to those not prescribed anticholinergics or those who had a low cumulative dose of anticholinergic medications. 60 , 61 Specifically, a high cumulative dose of anticholinergic drugs increased the risk of being diagnosed with dementia by 3‐fold compared to having a low cumulative dose. 61
Two prior studies failed to show an association between anticholinergic medication use and dementia, but in neither study was this a primary objective. In an analysis of the DATATOP cohort, use of anticholinergics was not associated with increased risk of cognitive impairment at a median of 6.5 years after enrollment, although the cumulative incidence of cognitive impairment was low as only 5.8% of 740 participants met criteria for cognitive impairment after 5 years. 62 Similarly, anticholinergic medication usage was not associated with cognitive decline in the Parkinson's Progression Markers Initiative (PPMI) study, but like DATATOP, this is an early PD cohort with only 3.6% of PD participants determined to have dementia at 5 years. 63
After finding that duration of anticholinergic medication usage was associated with dementia, Pondal et al 58 concluded in 1996 that anticholinergic medications should be “avoided in PD patients with some cognitive decline.” This idea is also expressed in the 2018 International Parkinson and Movement Disorder Society review of treatments for motor symptoms, but the authors went further: the “use (of anticholinergics for motor symptom management) should generally be limited to young and cognitively intact patients because of their unfavorable neuropsychiatric adverse effect profile and the long‐term risk of memory impairment.” 25
Use of Anticholinergic Medications for Symptoms Other than Parkinsonism in PD
Anticholinergic exposure in PD patients has shifted from motor symptom management to non‐motor and comorbid disease management (Table 2). A review of inpatient records from 2011 to 2012 in the Basque region of Spain found that 53.6% of PD patients were prescribed at least 1 anticholinergic drug. 3 The drugs were most often antidepressants, antipsychotics, urological drugs, opiates, or antihistamines. A similarly high anticholinergic burden in PD was found in 2 subsequent studies. A study of US inpatients found that 57.8% were prescribed at least 1 anticholinergic medication not used to treat parkinsonism, 51 and in a French prescription database, 58.2% of PD patients were taking at least 1 anticholinergic medication. 5 This latter study emphasized that most of the anticholinergic medications (93.8%) were not prescribed for their anticholinergic properties. 5 Interestingly, in 1 study of elderly patients, PD was associated with reduced risk of taking anticholinergic medications. 64 A recent analysis of a study cohort receiving specialty care found that 33.6% of early PD patients were taking medications with anticholinergic properties at baseline, and 51.4% of PD patients were taking medications with anticholinergic properties after 5 years. 63 This suggests that the total anticholinergic burden in PD increases over time, even in specialty care centers, under current practice parameters. A population‐based study uncovered high rates of persistent concurrent prescribing of a cholinesterase inhibitor and medications with anticholinergic properties, a particularly troubling finding, because these drug classes have opposing mechanisms of action. 65 Although anticholinergic medication use for motor PD treatment is declining, there are no guidelines for neurologists addressing whether and how to consider and manage the total anticholinergic burden in PD as a part of PD management. Considering the potential negative immediate and long‐term effects of anticholinergic medications for PD patients, the question arises whether neurologists should attempt to deprescribe medications with anticholinergic properties for indications other than parkinsonism.
TABLE 2.
Percentage of PD patients prescribed anticholinergic medications for any indication
Areas for Future Anticholinergic Medication Research in PD
In PD care, there are several barriers that limit clinicians' enthusiasm for taking neuroprotective action when it comes to total anticholinergic medication exposures. These barriers primarily relate to (1) historical importance and continued use of anticholinergic medications to treat PD motor symptoms; (2) uncertainty about determining anticholinergic medication burden; (3) historical discovery‐implementation gaps in PD research and care; and (4) underdeveloped clinical structure and processes to support deprescribing. Additional cohort studies with appropriate follow‐up periods and clinical trials of deprescribing are required to help guide the neurologist in considering the relative risks and benefits of medications with anticholinergic activity and their impact on PD patients' long‐term outcomes.
Determination of Anticholinergic Medication Burden
The first step to reducing anticholinergic burden in PD patients with cognitive impairment or psychosis is to properly identify medications with anticholinergic properties. However, there is no gold standard measure that characterizes anticholinergic exposure and subsequent neurological harm. Studies showing a relationship between anticholinergic medication use and risk of developing dementia have relied on compiled lists or scales that grade the severity of the anticholinergic effect, such as the American Geriatrics Society Beers Criteria and the Anticholinergic Cognitive Burden Scale. 66 , 67 , 68 The Anticholinergic Cognitive Burden (ACB) scale is the most used measure in pharmacoepidemiology and drug safety assessments and includes medications frequently prescribed by neurologists (eg, quetiapine, paroxetine, carbamazepine, trazodone, amitriptyline, and bupropion), but the majority of the medications are for non‐neurological indications. 67 The ACB scale applies scores based on the strength of evidence for an anticholinergic effect. Medications are scored 1 for possible anticholinergic effects (eg, trazodone and bupropion), and 2 (eg, carbamazepine) and 3 (eg, quetiapine, paroxetine, and diphenhydramine) for clinically‐relevant anticholinergic effects. Other commonly used scales are the Anticholinergic Drug Scale (ADS) and the Anticholinergic Risk Scale (ARS), and all the aforementioned scales have measurable differences in categorizations. 69 , 70 Beyond the scoring scheme in these scales, there is little to no data currently about the differences between specific agents or neurochemical classes as it pertains to long‐term negative effects on cognition.
Mechanisms of Neurotoxicity of Anticholinergic Medications in PD
At this time, the mechanism of anticholinergic medication neurotoxicity is not understood. One potential mechanism of anticholinergic medication neurotoxicity in PD is via initiation or acceleration of Alzheimer's disease (AD) pathology. 71 A retrospective pathological study found that PD patients with long‐term treatment with anticholinergic medications had significantly greater senile plaques and neurofibrillary tangles compared with PD patients who had only short‐term or no exposure to anticholinergic medications. 72 Because AD pathology is a contributor to dementia in a significant number of PD patients, 73 it is important to understand whether anticholinergic medications increase dementia risk via facilitation of AD pathology.
Implementation and Determination of the Efficacy of Deprescribing Interventions Anticholinergic Medications
Although there are substantial data in the general geriatric population, there is no clear evidence that deprescribing anticholinergics is protective against neurocognitive decline in PD. Such data will be collected as part of a deprescribing trial for neuroprotection in PD study (A.W.W.). In addition to demonstrating an overall benefit of lowering anticholinergic medication exposure after PD diagnosis, if one exists, the critical time window for exposure reduction needs to be identified and whether the negative effects of anticholinergic exposure may sometimes be irreversible needs to be determined. Further, there may be class and dose effects of anticholinergic medications that need to be explored. Producing data on the efficacy of reducing anticholinergic medication burden for cognitive decline may not be sufficient, however. PD historically has a large discovery‐implementation gap. For example, significant data support the use of physiotherapy, deep brain stimulation, and dopaminergic medications in PD, but population‐level studies suggest the use of these treatments ranges from very low to modest. 74 , 75
Medication discontinuation involves collaboration between patients and providers. PD individuals often have multiple providers (ie, primary care physicians, internists, neurologists, psychiatrists, urologists, and other specialists), which requires an interprofessional approach to deprescribing. Although providers and patients often recognize the importance of reducing harmful medications, there are numerous provider‐patient‐level barriers to actualizing this process. 76 , 77 Some of the most common barriers for providers include lack of awareness of neuropharmacology and drug‐disease interactions for PD, lack of knowledge and self‐efficacy to implement de‐prescribing, and prescribing inertia related to the fear of unknown consequences of medication changes or lack of belief in the efficacy of deprescribing. 77 , 78 Provider level barriers can be addressed through training and subsequent real‐world experience. 78 Research is needed to understand how training programs and implementation of medication risk tools such as the ADS, ACB, and ARS in the clinical setting will change prescribing of anticholinergic medications to PD patients.
There is a great unmet need for more treatment options for non‐motor symptoms and dyskinesias in PD. For many, treatment with amantadine or quetiapine is a highly effective means of controlling dyskinesias or psychosis, resulting in improved patient function and quality of life, and is more appropriate and accessible than deep brain stimulation or other psychosis treatments. Even when an anticholinergic medication is necessary, efforts should be made to use anticholinergic medications that are less likely to cross the blood–brain barrier and to minimize the total anticholinergic drug burden.
Conclusion
The difficult question is whether movement disorders neurologists should strive to deprescribe medications with anticholinergic properties that they did not prescribe in all PD patients, even those who are younger and without cognitive impairment. This would be the most drastic conclusion to draw from recent studies demonstrating a relationship between medications with anticholinergic properties and future dementia risk, both in PD and non‐PD populations. Assuming these findings are accurate, the impact of deprescribing anticholinergics in PD is perhaps more significant for 3 specific reasons. First, PD patients have a significant anticholinergic burden: baseline anticholinergic prevalence is 7%–57% in non‐PD populations, whereas more than half of PD patients are prescribed medications with anticholinergic properties. Second, cholinergic degeneration is common in PD, on par with Alzheimer's disease, which may make PD patients more vulnerable to the negative effects of anticholinergics, both short‐term and long‐term. Third, 80% of PD patients will develop dementia, 79 thus it is critical to identify and mitigate modifiable risk factors of dementia. Many of the recognized contributors to dementia in PD are currently not thought to be modifiable. 80 Robust randomized studies are needed to provide evidence for deprescribing anticholinergic medications or limiting anticholinergic exposures in PD patients. The risk of using anticholinergic medications for symptomatic management of PD, however, will always need to be balanced against their potential benefit in a range of clinical contexts.
Author Roles
(1) Research project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
M.J.B.: 1A, 1B, 1C, 3A
L.S.: 1C, 3B
H.N.: 1C, 3B
D.W.: 1C, 3B
E.T.P.: 1C, 3B
A.W.W.: 1A, 1B, 1C, 3B
Disclosures
Ethical Compliance Statement
The authors confirm that the approval of an institutional review board was not required for this work. The authors confirm that patient consent was not required for this work. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest
A.W.W. and D.W. received funding from the National Institutes of Health (NIH) (R01 NS099129‐03). The authors declare that there are no conflicts of interest relevant to this work.
Financial Disclosures for the Previous 12 Months
M.J.B. has received funding from the VCU Center for Clinical and Translational Research and served on advisory boards for Kyowa Kirin and Eisai. L.S. reports no disclosures. D.W. has received research funding or support from the Michael J. Fox Foundation for Parkinson's Research, Alzheimer's Therapeutic Research Initiative (ATRI), Alzheimer's Disease Cooperative Study (ADCS), the International Parkinson and Movement Disorder Society (IPMDS), and National Institute on Aging (NIA); honoraria for consultancy from Acadia, CHDI Foundation, Clintrex LLC (Alkahest, Aptinyx, Avanir, Otsuka), Eisai, Great Lake Neurotechnologies, Janssen, Sage, Scion, Signant Health, and Sunovion; and license fee payments from the University of Pennsylvania for the QUIP and QUIP‐RS. E.T.P. reports no disclosures. A.W.W. reports no disclosures.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Oertel WH, Quinn NP. Parkinson's disease: drug therapy. Baillieres Clin Neurol 1997;6:89–108. [PubMed] [Google Scholar]
- 2. Bhatia K, Brooks DJ, Burn DJ, et al. Guidelines for the management of Parkinson's disease. The Parkinson's Disease Consensus Working Group. Hosp Med 1998;59:469–480. [PubMed] [Google Scholar]
- 3. Lertxundi U, Isla A, Solinis MA, Domingo‐Echaburu S, Hernandez R, Peral‐Aguirregoitia J, Medrano J. Anticholinergic burden in Parkinson's disease inpatients. Eur J Clin Pharmacol 2015;71:1271–1277. [DOI] [PubMed] [Google Scholar]
- 4. Crispo JAG, Fortin Y, Thibault DP, et al. Trends in inpatient antiparkinson drug use in the USA, 2001‐2012. Eur J Clin Pharmacol 2015;71:1011–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. De Germay S, Montastruc J‐L, Rousseau V, et al. Atropinic (anticholinergic) burden in Parkinson's disease. Mov Disord 2016;31:632–636. [DOI] [PubMed] [Google Scholar]
- 6. Dawson AH, Buckley NA. Pharmacological management of anticholinergic delirium ‐ theory, evidence and practice. Br J Clin Pharmacol 2016;81:516–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carriere I, Fourrier‐Reglat A, Dartigues J‐F, et al. Drugs with anticholinergic properties, cognitive decline, and dementia in an elderly general population: the 3‐city study. Arch Intern Med 2009;169:1317–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gray SL, Anderson ML, Dublin S, et al. Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study. JAMA Intern Med 2015;175:401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jessen F, Kaduszkiewicz H, Daerr M, et al. Anticholinergic drug use and risk for dementia: target for dementia prevention. Eur Arch Psychiatry Clin Neurosci 2010;260(Suppl 2):S111–S115. [DOI] [PubMed] [Google Scholar]
- 10. Richardson K, Fox C, Maidment I, et al. Anticholinergic drugs and risk of dementia: case‐control study. BMJ 2018;361:k1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Campbell NL, Boustani MA, Lane KA, et al. Use of anticholinergics and the risk of cognitive impairment in an African American population. Neurology 2010;75:152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Coupland CAC, Hill T, Dening T, Morriss R, Moore M, Hippisley‐Cox J. Anticholinergic drug exposure and the risk of dementia: a nested case‐control study. JAMA Intern Med 2019;179:1084–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chuang Y‐F, Elango P, Gonzalez CE, Thambisetty M. Midlife anticholinergic drug use, risk of Alzheimer's disease, and brain atrophy in community‐dwelling older adults. Alzheimers Dement (N Y) 2017;3:471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ordenstein L. Sur la paralsie agitante et la sclérose en plaques généralisées. Paris: Delahaye; 1868. [Google Scholar]
- 15. Zhang Z‐X, Dong Z‐H, Roman GC. Early descriptions of Parkinson disease in ancient China. Arch Neurol 2006;63:782–784. [DOI] [PubMed] [Google Scholar]
- 16. Fahn S. The medical treatment of Parkinson disease from James Parkinson to George Cotzias. Mov Disord 2015;30:4–18. [DOI] [PubMed] [Google Scholar]
- 17. Dubaz OM, Wu S, Cubillos F, Miao G, Simuni T. Changes in prescribing practices of dopaminergic medications in individuals with Parkinson's disease by expert care centers from 2010 to 2017: the Parkinson's Foundation Quality Improvement Initiative. Mov Disord Clin Pract 2019;6:687–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hurtig HI. Anticholinergics for Parkinson disease. Ann Neurol 1980;7:495. [DOI] [PubMed] [Google Scholar]
- 19. Silver DE, Ruggieri S. Initiating therapy for Parkinson's disease. Neurology 1998;50:S18–S18, S22. [DOI] [PubMed] [Google Scholar]
- 20. Jankovic J. Parkinson's disease therapy: tailoring choices for early and late disease, young and old patients. Clin Neuropharmacol 2000;23:252–261. [DOI] [PubMed] [Google Scholar]
- 21. Katzenschlager R, Sampaio C, Costa J, Lees A. Anticholinergics for symptomatic management of Parkinson's disease. Cochrane Database Syst Rev 2003;2(2):CD003735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lang AE, Lees A. Anticholinergic therapies in the treatment of Parkinson's disease. Mov Disord 2002;17(Suppl 4):S7–S12. [DOI] [PubMed] [Google Scholar]
- 23. Calne DB. Early idiopathic parkinsonism: initiation and optimization of treatment. Clin Neuropharmacol 1994;17(Suppl 2):S14–S18. [PubMed] [Google Scholar]
- 24. Scharre DW, Mahler ME. Parkinson's disease: making the diagnosis, selecting drug therapies. Geriatrics 1994;49:14–13. [PubMed] [Google Scholar]
- 25. Fox SH, Katzenschlager R, Lim S‐Y, et al. International Parkinson and movement disorder society evidence‐based medicine review: update on treatments for the motor symptoms of Parkinson's disease. Mov Disord 2018;33:1248–1266. [DOI] [PubMed] [Google Scholar]
- 26. Betz AJ, McLaughlin PJ, Burgos M, Weber SM, Salamone JD. The muscarinic receptor antagonist tropicamide suppresses tremulous jaw movements in a rodent model of parkinsonian tremor: possible role of M4 receptors. Psychopharmacology (Berl) 2007;194:347–359. [DOI] [PubMed] [Google Scholar]
- 27. Moehle MS, Conn PJ. Roles of the M4 acetylcholine receptor in the basal ganglia and the treatment of movement disorders. Mov Disord 2019;34:1089–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Deleu D, Northway MG, Hanssens Y. Clinical pharmacokinetic and pharmacodynamic properties of drugs used in the treatment of Parkinson's disease. Clin Pharmacokinet 2002;41:261–309. [DOI] [PubMed] [Google Scholar]
- 29. Pisani A, Bernardi G, Ding J, Surmeier DJ. Re‐emergence of striatal cholinergic interneurons in movement disorders. Trends Neurosci 2007;30:545–553. [DOI] [PubMed] [Google Scholar]
- 30. Kim HJ, Jeon BS, Jenner P. Hallmarks of treatment aspects: Parkinson's disease throughout centuries including l‐Dopa. Int Rev Neurobiol 2017;132:295–343. [DOI] [PubMed] [Google Scholar]
- 31. Bianchine JR, Sunyapridakul L. Interactions between levodopa and other drugs: significance in the treatment of Parkinson's disease. Drugs 1973;6:364–388. [DOI] [PubMed] [Google Scholar]
- 32. Hill‐Burns EM, Debelius JW, Morton JT, et al. Parkinson's disease and Parkinson's disease medications have distinct signatures of the gut microbiome. Mov Disord 2017;32:739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lang AE, Blair RD. Anticholinergic drugs and amantadine in the treatment of Parkinson's disease. In: Calne DB, editor. Drugs for the Treatment of Parkinson's Disease. Berlin, Heidelberg: Springer‐Verlag; 1989:307–323. [Google Scholar]
- 34. Dubois B, Danze F, Pillon B, Cusimano G, Lhermitte F, Agid Y. Cholinergic‐dependent cognitive deficits in Parkinson's disease. Ann Neurol 1987;22:26–30. [DOI] [PubMed] [Google Scholar]
- 35. Stacy M, Brownlee HJ. Treatment options for early Parkinson's disease. Am Fam Physician 1996;53:1281–1287. [PubMed] [Google Scholar]
- 36. Mann A, Miksys SL, Gaedigk A, Kish SJ, Mash DC, Tyndale RF. The neuroprotective enzyme CYP2D6 increases in the brain with age and is lower in Parkinson's disease patients. Neurobiol Aging 2012;33:2160–2171. [DOI] [PubMed] [Google Scholar]
- 37. Dubois B, Pilon B, Lhermitte F, Agid Y. Cholinergic deficiency and frontal dysfunction in Parkinson's disease. Ann Neurol 1990;28:117–121. [DOI] [PubMed] [Google Scholar]
- 38. Bedard MA, Pillon B, Dubois B, Duchesne N, Masson H, Agid Y. Acute and long‐term administration of anticholinergics in Parkinson's disease: specific effects on the subcortico‐frontal syndrome. Brain Cogn 1999;40:289–313. [DOI] [PubMed] [Google Scholar]
- 39. Syndulko K, Gilden ER, Hansch EC, Potvin AR, Tourtellotte WW, Potvin JH. Decreased verbal memory associated with anticholinergic treatment in Parkinson's disease patients. Int J Neurosci 1981;14:61–66. [DOI] [PubMed] [Google Scholar]
- 40. Sadeh M, Braham J, Modan M. Effects of anticholinergic drugs on memory in Parkinson's disease. Arch Neurol 1982;39:666–667. [DOI] [PubMed] [Google Scholar]
- 41. Nishiyama K, Sugishita M, Kurisaki H, Sakuta M. Reversible memory disturbance and intelligence impairment induced by long‐term anticholinergic therapy. Intern Med 1998;37:514–518. [DOI] [PubMed] [Google Scholar]
- 42. Cooper JA, Sagar HJ, Doherty SM, Jordan N, Tidswell P, Sullivan EV. Different effects of dopaminergic and anticholinergic therapies on cognitive and motor function in Parkinson's disease. A follow‐up study of untreated patients. Brain 1992;115(Pt 6):1701–1725. [DOI] [PubMed] [Google Scholar]
- 43. van Herwaarden G, Berger HJ, Horstink MW. Short‐term memory in Parkinson's disease after withdrawal of long‐term anticholinergic therapy. Clin Neuropharmacol 1993;16:438–443. [DOI] [PubMed] [Google Scholar]
- 44. Yarnall AJ, Lawson RA, Duncan GW, et al. Anticholinergic load: is there a cognitive cost in early Parkinson's disease? J Parkinsons Dis 2015;5:743–747. [DOI] [PubMed] [Google Scholar]
- 45. Sargent L, Nalls M, Amella EJ, et al. Anticholinergic drug induced cognitive and physical impairment: results from the InCHIANTI study. J Gerontol A Biol Sci Med Sci 2020;75:995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mach J, Gemikonakli G, Logan C, et al. Chronic polypharmacy with increasing Drug Burden Index (DBI) exacerbates frailty and impairs physical function, with effects attenuated by deprescribing, in aged mice. J Gerontol A Biol Sci Med Sci 2020;76:1010–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Weintraub D, Stern MB. Psychiatric complications in Parkinson disease. Am J Geriatr Psychiatry 2005;13:844–851. [DOI] [PubMed] [Google Scholar]
- 48. Truong DD, Bhidayasiri R, Wolters E. Management of non‐motor symptoms in advanced Parkinson disease. J Neurol Sci 2008;266:216–228. [DOI] [PubMed] [Google Scholar]
- 49. Karachi C, Grabli D, Bernard FA, et al. Cholinergic mesencephalic neurons are involved in gait and postural disorders in Parkinson disease. J Clin Invest 2010;120:2745–2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bohnen NI, Frey KA, Studenski S, et al. Gait speed in Parkinson disease correlates with cholinergic degeneration. Neurology 2013;81:1611–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Crispo JAG, Willis AW, Thibault DP, et al. Associations between anticholinergic burden and adverse health outcomes in Parkinson disease. PLoS One 2016;11:e0150621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fox C, Richardson K, Maidment ID, et al. Anticholinergic medication use and cognitive impairment in the older population: the medical research council cognitive function and ageing study. J Am Geriatr Soc 2011;59:1477–1483. [DOI] [PubMed] [Google Scholar]
- 53. Shah RC, Janos AL, Kline JE, et al. Cognitive decline in older persons initiating anticholinergic medications. PLoS One 2013;8:e64111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gaspar P, Gray F. Dementia in idiopathic Parkinson's disease. A neuropathological study of 32 cases. Acta Neuropathol 1984;64:43–52. [DOI] [PubMed] [Google Scholar]
- 55. Whitehouse PJ, Hedreen JC, White CL 3rd, Price DL. Basal forebrain neurons in the dementia of Parkinson disease. Ann Neurol 1983;13:243–248. [DOI] [PubMed] [Google Scholar]
- 56. Candy JM, Perry RH, Perry EK, Irving D, Blessed G, Fairbairn AF, Tomlinson BE. Pathological changes in the nucleus of Meynert in Alzheimer's and Parkinson's diseases. J Neurol Sci 1983;59:277–289. [DOI] [PubMed] [Google Scholar]
- 57. Perry EK, Curtis M, Dick DJ, et al. Cholinergic correlates of cognitive impairment in Parkinson's disease: comparisons with Alzheimer's disease. J Neurol Neurosurg Psychiatry 1985;48:413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pondal M, Del Ser T, Bermejo F. Anticholinergic therapy and dementia in patients with Parkinson's disease. J Neurol 1996;243:543–546. [DOI] [PubMed] [Google Scholar]
- 59. Ehrt U, Broich K, Larsen JP, Ballard C, Aarsland D. Use of drugs with anticholinergic effect and impact on cognition in Parkinson's disease: a cohort study. J Neurol Neurosurg Psychiatry 2010;81:160–165. [DOI] [PubMed] [Google Scholar]
- 60. Hong C‐T, Chan L, Wu D, Chen W‐T, Chien L‐N. Antiparkinsonism anticholinergics increase dementia risk in patients with Parkinson's disease. Parkinsonism Relat Disord 2019;65:224–229. [DOI] [PubMed] [Google Scholar]
- 61. Sheu J‐J, Tsai M‐T, Erickson SR, Wu C‐H. Association between anticholinergic medication use and risk of dementia among patients with Parkinson's disease. Pharmacotherapy 2019;39:798–808. [DOI] [PubMed] [Google Scholar]
- 62. Uc EY, McDermott MP, Marder KS, et al. Incidence of and risk factors for cognitive impairment in an early Parkinson disease clinical trial cohort. Neurology 2009;73:1469–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Weintraub D, Caspell‐Garcia C, Simuni T, et al. Neuropsychiatric symptoms and cognitive abilities over the initial quinquennium of Parkinson disease. Ann Clin Transl Neurol 2020;7:449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lee EK, Lee YJ. Prescription patterns of anticholinergic agents and their associated factors in Korean elderly patients with dementia. Int J Clin Pharmacol 2013;35:711–718. [DOI] [PubMed] [Google Scholar]
- 65. Mantri S, Fullard M, Gray SL, Weintraub D, Hubbard RA, Hennessy S, Willis AW. Patterns of dementia treatment and frank prescribing errors in older adults with Parkinson disease. JAMA Neurol 2019;76:41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. American Geriatrics Society Beers Criteria Update Expert Panel . American Geriatrics Society 2019 updated AGS beers criteria(R) for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2019;67:674–694. [DOI] [PubMed] [Google Scholar]
- 67. Boustani M, Campbell N, Munger S, Maidment I, Fox C. Impact of anticholinergics on the aging brain: a review and practical application. Aging Health 2008;4:311–320. [Google Scholar]
- 68. Andre L, Gallini A, Montastruc F, Montastruc JL, Piau A, Lapeyre‐Mestre M, Gardette V. Association between anticholinergic (atropinic) drug exposure and cognitive function in longitudinal studies among individuals over 50 years old: a systematic review. Eur J Clin Pharmacol 2019;75:1631–1644. [DOI] [PubMed] [Google Scholar]
- 69. Naples JG, Marcum ZA, Perera S, et al. Concordance between anticholinergic burden scales. J Am Geriatr Soc 2015;63:2120–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Valladales‐Restrepo LF, Duran‐Lengua M, Castro‐Osorio EE, Machado‐Alba JE. Consistency between anticholinergic burden scales in the elderly with fractures. PLoS One 2020;15:e0228532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yoshiyama Y, Kojima A, Itoh K, Isose S, Koide M, Hori K, Arai K. Does anticholinergic activity affect neuropathology? Implication of neuroinflammation in Alzheimer's disease. Neurodegener Dis 2015;15:140–148. [DOI] [PubMed] [Google Scholar]
- 72. Perry EK, Kilford L, Lees AJ, Burn DJ, Perry RH. Increased Alzheimer pathology in Parkinson's disease related to antimuscarinic drugs. Ann Neurol 2003;54:235–238. [DOI] [PubMed] [Google Scholar]
- 73. Halliday GM, Leverenz JB, Schneider JS, Adler CH. The neurobiological basis of cognitive impairment in Parkinson's disease. Mov Disord 2014;29:634–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Willis AW, Schootman M, Kung N, Wang X‐Y, Perlmutter JS, Racette BA. Disparities in deep brain stimulation surgery among insured elders with Parkinson disease. Neurology 2014;82:163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Fullard ME, Thibault DP, Hill A, et al. Utilization of rehabilitation therapy services in Parkinson disease in the United States. Neurology 2017;89:1162–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Linsky A, Simon SR, Bokhour B. Patient perceptions of proactive medication discontinuation. Patient Educ Couns 2015;98:220–225. [DOI] [PubMed] [Google Scholar]
- 77. Linsky A, Simon SR, Marcello TB, Bokhour B. Clinical provider perceptions of proactive medication discontinuation. Am J Manag Care 2015;21:277–283. [PubMed] [Google Scholar]
- 78. Zimmerman KM, Linsky AM, Donohoe KL, Hobgood SE, Sargent L, Salgado TM. An Interprofessional workshop to enhance de‐prescribing practices among health care providers. J Contin Educ Health Prof 2020;40:49–57. [DOI] [PubMed] [Google Scholar]
- 79. Hely MA, Reid WGJ, Adena MA, Halliday GM, Morris JGL. The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Mov Disord 2008;23:837–844. [DOI] [PubMed] [Google Scholar]
- 80. Aarsland D, Batzu L, Halliday GM, Geurtsen GJ, Ballard C, Ray Chaudhuri K, Weintraub D. Parkinson disease‐associated cognitive impairment. Nat Rev Dis Primers 2021;7:47. [DOI] [PubMed] [Google Scholar]
- 81. Leoni O, Martignoni E, Cosentino M, et al. Drug prescribing patterns in Parkinson's disease: a pharmacoepidemiological survey in a cohort of ambulatory patients. Pharmacoepidemiol Drug Saf 2002;11:149–157. [DOI] [PubMed] [Google Scholar]
- 82. Wei Y‐JJ, Stuart B, Zuckerman IH. Use of antiparkinson medications among elderly Medicare beneficiaries with Parkinson's disease. Am J Geriatr Pharmacother 2010;8:384–394. [DOI] [PubMed] [Google Scholar]
- 83. Swarztrauber K, Koudelka C, Brodsky MA. Initial pharmacotherapy in a population of veterans with Parkinson disease. Neurology 2006;66:1425–1426. [DOI] [PubMed] [Google Scholar]
- 84. Dahodwala N, Willis AW, Li P, Doshi JA. Prevalence and correlates of anti‐Parkinson drug use in a nationally representative sample. Mov Disord Clin Pract 2017;4:335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]


