Abstract
Background:
Frailty is an important physiologic factor in studies of influenza and influenza vaccines carried out in older adults and hospitalized populations. Unfortunately, comprehensive assessments of frailty requiring physical assessments and extensive medical record review are not often feasible in time- and resource-limited settings common to studies of influenza and influenza vaccines.
Methods:
We developed a 5-question frailty short interview, and implemented it in a multicenter, hospital-based study of influenza over two years. Frailty status defined by the frailty short interview was compared to a validated frailty index based on medical record review of 59 parameters. Agreement between the two frailty measures was assessed, and multivariable linear regression models were used to explore differences between the measures. The association between each frailty measure and likelihood of influenza vaccination was also assessed.
Results:
During the 2015–2016 and 2016–2017 influenza seasons, 2070 adult patients hospitalized with acute respiratory illness were enrolled and included in analyses. Frailty was frequently identified in the study population; 43% of participants were defined as frail by the frailty short interview and 32% by frailty index. Responses to the frailty short interview were only moderately correlated with the frailty index, and agreement between the two frailty measures was low. Women were more likely to be defined as frail by the frailty short interview than men. White individuals were more likely than other races to be defined as frail by the frailty index. Increasing frailty index was associated with increased likelihood of influenza vaccination, but the frailty short interview was not associated with vaccination.
Conclusions:
The frailty short interview provided a feasible and consistent measure of frailty across study hospitals and study years. However, its modest correlation with the frailty index and differential association with likelihood of influenza vaccination highlight differences in the conceptualization of frailty.
Keywords: Influenza, Influenza vaccination, Frailty, Hospitalization, Adults
Frailty is a prevalent physiological condition in older adults that is associated with increased risk of adverse health outcomes such as hospitalization and death [1,2]. As a result of conceptual debate on how to define frailty, a variety of measurement methods are used both clinically and in research [2,3]. One approach defines frailty as an accumulation of deficits over time. This definition is typically operationalized by calculation of a frailty index that quantifies the presence of chronic conditions, functional disabilities, cognitive deficits, and other signs and symptoms associated with aging [4]. An alternative approach, developed by Fried et al, defines frailty as a clinical syndrome, distinct from comorbidity and disability, characterized by unintentional weight loss, exhaustion, weakness, difficulty walking, and physical inactivity [5]. This definition is typically operationalized through a combination of physical assessments of grip strength and gait speed, and patient report of weight loss, energy level, and physical activity, which may not be feasible or representative when a patient is acutely ill.
Influenza is recognized as an important pathogen in older adults contributing to substantial annual morbidity and mortality in this population. Past studies of influenza and influenza vaccines have demonstrated associations between frailty and likelihood of influenza vaccination [6,7], poor immune response to vaccination [8,9], reduced vaccine effectiveness [10–12], risk of influenza infection [8,13], and risk of severe outcomes following influenza infection [11,14,15]. As in the broader field of frailty research, there is no consensus regarding the definition of frailty in studies of influenza and influenza vaccines, and the term has often been used interchangeably with comorbidity, disability, and functional status. Furthermore, studies of influenza and influenza vaccines often require rapid, prospective enrollment of a large population of acutely ill individuals during hospitalization or outpatient medical visits. Comprehensive assessments of frailty requiring physical assessments and extensive medical record review are not often feasible in these time- and resource-limited settings common to studies of influenza and influenza vaccines.
To address the need for a frailty assessment that can be implemented quickly when a patient is acutely ill, we developed a short interview based on the five components of the frailty phenotype defined by Fried et al.: weight loss, exhaustion, weakness, difficulty walking, and physical inactivity. Validation of this short interview could improve the feasibility of including frailty assessments in studies of influenza and influenza vaccines. In addition, self-reported measures of health may contribute additional important information beyond what can be obtained by medical record review. Because of this, and varying conceptual definitions, different measures of frailty often substantially disagree in the individuals they define as frail [16–19]. It is unclear which definition is most appropriate for studies of influenza and influenza vaccines.
We implemented this short interview in a hospital-based study of influenza over two years and compared the results to a validated frailty index based on detailed medical record review of 59 parameters [4]. The agreement between the frailty short interview and frailty index was assessed, associations between each measure of frailty and the likelihood of influenza vaccination were estimated, and a predictive model was developed to explore differences between the frailty index and the frailty short interview.
1. Methods
1.1. Study design
The US Hospitalized Adult Influenza Vaccine Effectiveness Network (HAIVEN), established in 2015, is a network of four US institutions. The primary goal of HAIVEN is to provide annual estimates of influenza vaccine effectiveness (VE) in preventing laboratory-confirmed influenza-associated hospitalizations among US adult populations. Two of the four participating sites, the University of Michigan and Vanderbilt University Medical Center, carried out this HAIVEN sub-study. Briefly, adults ≥18 years of age who are hospitalized for treatment of acute respiratory illness are enrolled according to a standard case definition each influenza season. Participants or their authorized representatives provided informed consent and completed an enrollment interview. As part of the enrollment interview, participants were asked to report their age, sex, race and ethnicity, smoking history, influenza vaccination status, any supplemental oxygen use at home, and elements of the frailty short interview described in the following section. Influenza infection status was determined by RT-PCR, using protocols, primers, and probes developed by the CDC Influenza Division.
The study was reviewed approved by the institutional review boards at the University of Michigan, St Thomas Hospitals (Sterling) and Vanderbilt University Medical Center.
1.2. Frailty and health status assessments
The frailty index described below was adapted from the method of Mitnitski et al. to measure frailty based on review of the medical record [4]. This index has been validated against a variety of outcomes including mortality, declining health, and institutionalization [20]. Medical record review for participants enrolled during the 2015–2016 and 2016–2017 influenza seasons was carried out by reviewers at each site to assess 59 frailty index elements including chronic conditions, functional disabilities, cognitive deficits, and other signs and symptoms associated with aging for calculation of a frailty index. Detailed chart abstraction methods are described in the supplementary materials. The frailty index was calculated as the number of elements indicating presence of a condition, disability, or deficit divided by the number of non-missing elements. Values of the frailty index have classified as non-frail (≤0.1), pre-frail (>0.1 – ≤0.21), frail (>0.21 – ≤0.45), and most frail (>0.45) based on validation against hospital-related outcomes [21], and have been used in prior studies of influenza [10,22]. The full list and description of the elements included in the frailty index is included in the supplemental materials.
During the enrollment interview, each participant was asked five questions comprising the frailty short interview based on the components of the frailty phenotype defined by Fried et al. [5]. The specific questions (Table 1) were adapted for use during acute illness from the Cardiovascular Health Study [5], the Survey of Health, Ageing and Retirement in Europe [23], and the National Health Interview Survey [24].
Table 1.
Elements of the frailty short interview.
| Element of Fried Phenotype [5] | Frailty Short Interview Question | Adapted From |
|---|---|---|
| Weight Loss | “In the last year, before this current illness, have you lost more than 10 lb unintentionally (i.e., not due to dieting or exercise)?” [YES;NO] |
[5] |
| Exhaustion | “In the last month, before this current illness, have you had too little energy to do the things you wanted to do?” [YES;NO] |
[23] |
| Weakness | “Before this current illness, how difficult was it for you to lift or carry something as heavy as 10 lb, such as a full bag of groceries, by yourself, and without using any special equipment?” [NO DIFFICULTY; A LITTLE…; SOME…; A LOT…; UNABLE TO DO] |
[24] |
| Difficulty Walking | “Before this current illness, did you have difficulty walking 100 yards (around the size of a football field) because of a health problem?” [NO DIFFICULTY; A LITTLE…; SOME…; A LOT…; UNABLE TO DO] |
[23] |
| Physical Inactivity | “Before this current illness, how often did you engage in activities that require a low or moderate level of energy such as gardening, cleaning the car, or going for a walk?” [>ONCE PER WEEK; ONCE PER WEEK; 1–3 TIMES PER MONTH; HARDLY EVER/NEVER] |
[23] |
Responses to the Weakness, Difficulty Walking, and Physical Inactivity questions were recoded as dichotomous variables with at least some difficulty or engaging in physical activity less than once per week indicating positive responses indicative of frailty. The sum of positive responses was divided by the total number of questions (5) to create a frailty short interview score ranging from 0 to 1. Presence of limitations in at least 3 of the 5 content areas (frailty short interview score ≥0.6) was considered to indicate frail status.
1.3. Statistical analysis
We compared the proportion of individuals defined as frail by each measure by subject characteristics using chi-square tests. We also assessed the sensitivity, specificity, and agreement of frailty status defined by the frailty short interview using the frailty index as a gold standard. The frailty index was selected as the gold standard because it has been previously validated and has been used in previous influenza studies. The correlation between the frailty short interview and frailty index scores was calculated using the Spearman method and the correlation coefficient was reported. To further investigate differences between the frailty short interview and frailty index, we built a multivariable linear regression model to predict frailty index from the frailty short interview score and other covariates. Model covariates, including age at enrollment, sex, race, ethnicity, supplemental oxygen use at home, smoking status and study hospital, were selected a priori based on presumed relationships with frailty status. Internal model validation of the predictive model was performed using bootstrapping method with 150 samples. The prediction model built from the 2015–2016 study year data was applied to data from the 2016–2017 study year to predict frailty index and a temporal validation was carried out.
Finally, we evaluated the associations between likelihood of influenza vaccination and each of the two frailty assessment methods using multivariable logistic regression models. Separate models were performed for each frailty outcome stratified by study year (2015–2016 or 2016–2017). Regression models were adjusted for the pre-specified covariates listed above based on their presumed association with both frailty and likelihood of vaccination. Odds ratios with 95% confidence intervals (CIs) were reported.
All analysis was done using R version 3.5.0.
2. Results
In total, 2087 participants were enrolled from Vanderbilt University and University of Michigan hospitals during the 2015–2016 and 2016–2017 influenza seasons. Complete frailty short interview data were missing from 17 individuals who were excluded from analyses. Among the 2070 individuals included in analyses, 798 were enrolled during the 2015–2016 study year and 1272 were enrolled during the 2016–2017 study year (Supplementary Table 1). Across both years, 1294 were enrolled from VU hospitals and 776 were enrolled from UM hospitals. Participant ages ranged from 18 to 102 (median 60) years, 53% were female, 77% were white, and 19% were black. Across both study years, 72% of participants reported receiving an influenza vaccine and 13% had an influenza infection.
Frailty was prevalent in the study population. Across both study seasons, the median frailty short interview was 0.4 (IQR: 0.2, 0.8). The most common positive responses to elements of the frailty short interview were exhaustion (63%) and difficulty walking (51%) (Table 2). Similarly, the median frailty index was 0.17 (IQR: 0.11, 0.24) across both study seasons, a value consistent with pre-frail status. Median frailty short interview and frailty index scores were similar across enrollment hospitals and study years (Supplementary Table 2).
Table 2.
Distribution of responses to each element of the frailty short interview.
| Positive Response Indicative of Frailty | ||
|---|---|---|
| Frailty Short Interview Element | Yes | No |
| Weight loss – N (%) | 644 (31%) | 1407 (68%) |
| Exhaustion – N (%) | 1304 (63%) | 744 (36%) |
| Weakness – N (%) | 812 (39%) | 1244 (61%) |
| Difficulty walking – N (%) | 1048 (51%) | 1012 (49%) |
| Physical inactivity – N (%) | 708 (35%) | 1336 (65%) |
There were differences in the distributions of each frailty measure by participant characteristics (Table 3). Frailty, as defined by both measures, increased in prevalence with increasing age (P < 0.001) and was more prevalent among those who used supplemental oxygen at home (P < 0.001). Women were more likely to be defined as frail by the frailty short interview than men (49% vs 36%, P < 0.001), but the proportion of women and men defined as frail by the frailty index was comparable (34% vs 31%). Compared with men, women were significantly (P < 0.001) more likely to report difficulty on each element of the frailty short interview except for weight loss. Race was only significantly associated with frailty as defined by the frailty index with white participants having the highest prevalence of frailty. Current and former smokers both had higher prevalence of frailty defined by the frailty short interview compared to never smokers, but only former smokers had a higher prevalence of frailty defined by the frailty index. Frailty defined by the frailty short interview was not associated with influenza vaccination, but participants defined as frail by the frailty index were more likely to have been vaccinated.
Table 3.
Distributions of frailty short interview and frailty index by participant characteristics.
| Frailty Short Interview | Frailty Index | |||||
|---|---|---|---|---|---|---|
| Fraila No. (row %) | Non-Frail No. (row %) | P-valueb | Frailc No. (row %) | Non-Frail No. (row %) | P-valueb | |
| Age group – % (N) | ||||||
| 18–49 | 203 (34) | 395 (66) | <0.001 | 111 (19) | 487 (81) | <0.001 |
| 50–64 | 294 (43) | 384 (57) | 190 (28) | 488 (72) | ||
| 65–74 | 210 (49) | 219 (51) | 188 (44) | 241 (56) | ||
| 75+ | 189 (52) | 176 (48) | 186 (51) | 179 (49) | ||
| Sex – % (N) | ||||||
| Male | 352 (36) | 615 (64) | <0.001 | 303 (31) | 664 (69) | 0.25 |
| Female | 544 (49) | 559 (51) | 372 (34) | 731 (66) | ||
| Race – % (N) | ||||||
| White | 676 (43) | 908 (57) | 0.36 | 539 (34) | 1045 (66) | 0.04 |
| Black | 184 (46) | 213 (54) | 113 (28) | 284 (72) | ||
| Other | 36 (40) | 53 (60) | 23 (26) | 66 (74) | ||
| Hispanic – % (N)d | ||||||
| No | 885 (44) | 1142 (56) | 0.05 | 662 (33) | 1365 (67) | 0.80 |
| Yes | 11 (28) | 28 (72) | 12 (31) | 27 (69) | ||
| Home oxygen use – % (N) | ||||||
| No | 603 (38) | 1004 (62) | <0.001 | 474 (29) | 1133 (71) | <0.001 |
| Yes | 292 (63) | 168 (37) | 201 (44) | 259 (56) | ||
| Smoking History – % (N) | ||||||
| Never smoked | 316 (37) | 536 (63) | <0.001 | 251 (29) | 601 (71) | <0.001 |
| Current smoker | 204 (49) | 216 (51) | 105 (25) | 315 (75) | ||
| Quit ≥6 months ago | 374 (47) | 420 (53) | 316 (40) | 478 (60) | ||
| Self-reported vaccine receipt – % (N) | ||||||
| No | 223 (41) | 320 (59) | 0.25 | 120 (22) | 423 (78) | <0.001 |
| Yes | 652 (44) | 833 (56) | 534 (36) | 951 (64) | ||
| Influenza infection status – % (N) | ||||||
| Negative | 789 (44) | 1009 (56) | 0.22 | 593 (33) | 1205 (67) | 0.35 |
| Positive | 106 (40) | 160 (60) | 80 (30) | 186 (70) | ||
Frailty short interview score ≥0.6.
Chi-square test.
Frailty Index >0.21.
Hispanic ethnicity was unknown for 4 individuals, home oxygen use was unknown for 3 individuals, smoking history was unknown for 4 individuals, and influenza vaccination status was unknown for 42 individuals.
There was a moderate positive correlation between frailty short interview and frailty index (Fig. 1) (Spearman correlation; r = 0.43; P < 0.001). However, more individuals were characterized as frail by the frailty short interview (896 [43%] had frailty short interview scores ≥ 0.6) than by the frailty index (675 [33%] had scores >0.21), and overall agreement was low (Table 4). Consistent with low agreement, the sensitivity (64%) and specificity (67%) of the frailty short interview compared to the frailty index gold standard were also low overall. Sensitivity was slightly lower and specificity slightly higher among subjects <50 years relative to those ≥50 years.
Fig. 1.
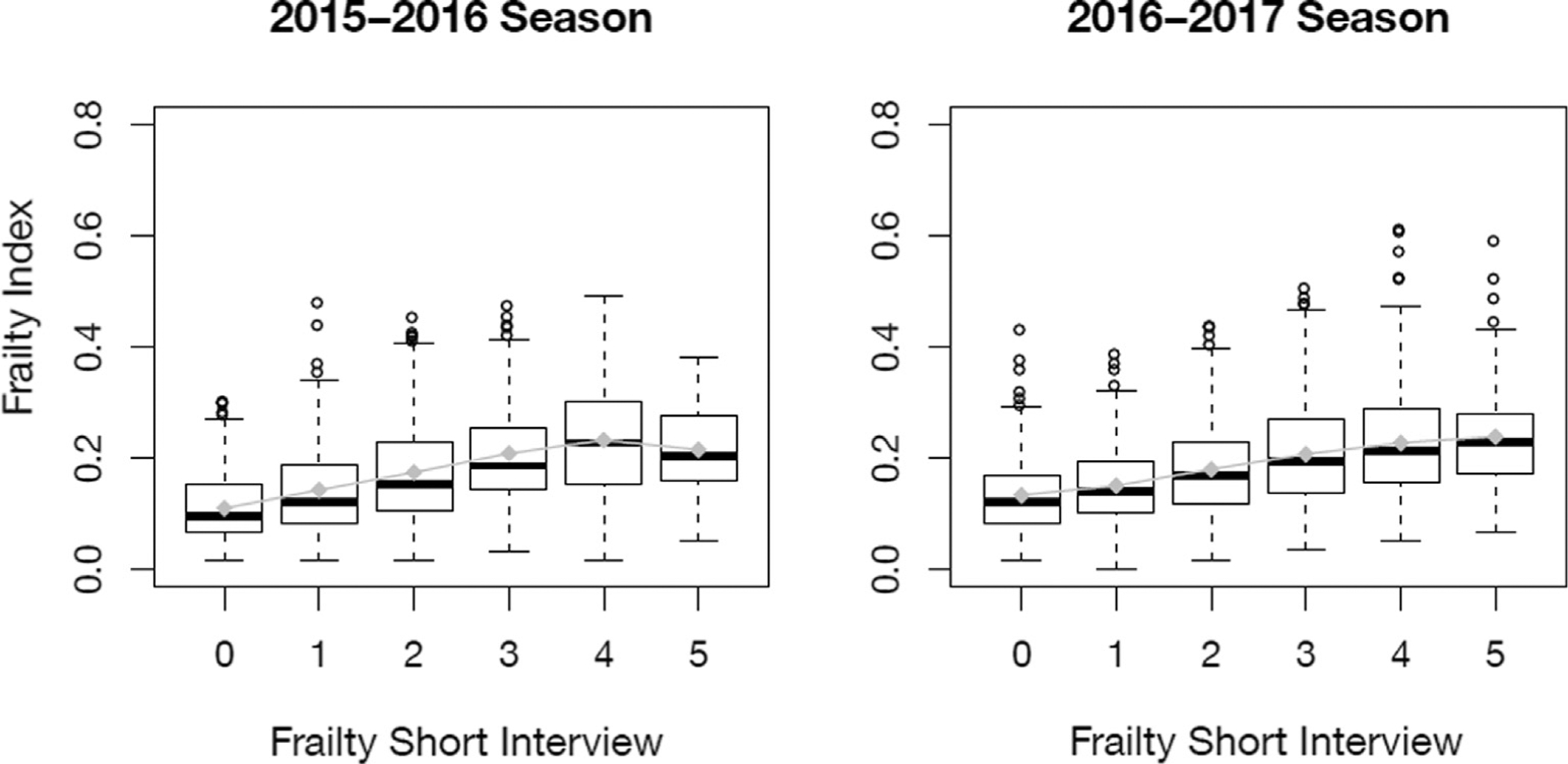
Association between frailty short interview and frailty index by study year. Boxes represent the interquartile range of the frailty index at each level of frailty short interview score with the median indicated by the thick black bar. Connected gray diamonds represent the mean frailty index at each level of frailty short interview score.
Table 4.
Agreement between frailty short interview and frailty index.
| Frailty Index | ||||||
|---|---|---|---|---|---|---|
| Frailb | Non-Frail | Kappa | McNemar | Sensitivity | Specificity | |
| Frailty Short Interview | ||||||
| <50 years | 0.23 | <0.001 | 58.6% | 71.7% | ||
| Fraila | 65 | 138 | ||||
| Non-Frail | 46 | 349 | ||||
| ≥50 years | 0.29 | <0.001 | 65.4% | 64.3% | ||
| Fraila | 369 | 324 | ||||
| Non-Frail | 195 | 584 | ||||
| All adults | 0.29 | <0.001 | 64.3% | 66.9% | ||
| Fraila | 434 | 462 | ||||
| Non-Frail | 241 | 933 | ||||
Presence of limitations in at least 3 of the 5 frailty short interview content areas.
Frailty Index >0.21.
We constructed a multivariable linear regression model to predict frailty index from the frailty short interview and other available covariates. The model was initially built using data from the 2015–2016 study year only. Frailty short interview score, age, sex, race, Hispanic ethnicity, home oxygen use, smoking status, and enrollment site were included in the final model. Increasing frailty short interview score, increasing age, and home oxygen use were all positively predictive of frailty index, while current smoking was predictive of lower frailty index relative to non-smokers; the effects of all other included variables did not reach statistical significance (Supplementary Table 3). The temporal validity of the model built from the 2015–2016 data was assessed using data from the 2016–2017 study year. In the 2016–2017 study year, the mean predicted frailty index (0.186) did not significantly differ from the mean observed frailty index (0.187; P = 0.46). Although the model successfully predicted the mean frailty index, there was substantial variation between observed and predicted values (Fig. 2). R-square values for the predictive linear model were 0.29 in the 2015–2016 training data, and 0.22 in 2016–2017 validation data.
Fig. 2.
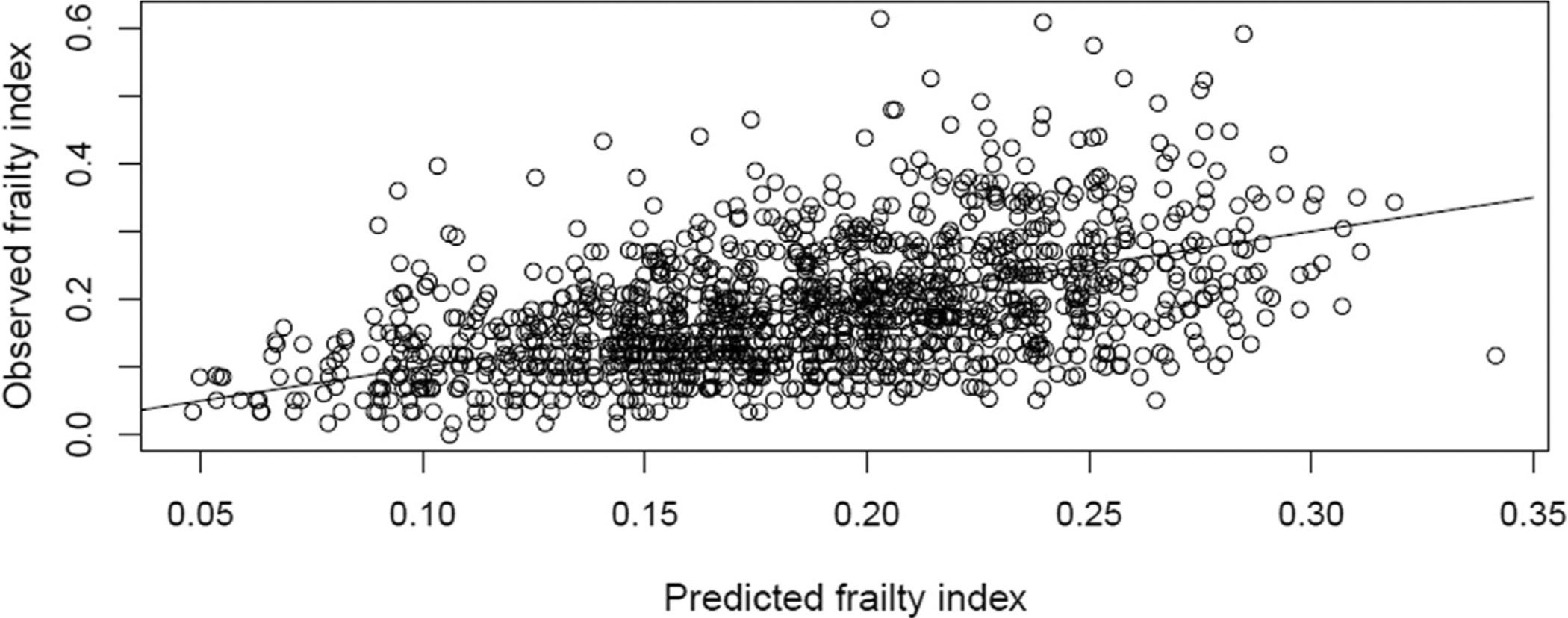
Scatter plot of observed frailty index scores vs those predicted in multivariable linear regression models including frailty short interview score, age at enrollment, gender, race, ethnicity, supplemental oxygen use at home, smoking status and study hospital.
The relationship between each of the two frailty measures and influenza vaccination were measured in logistic regression models. Those with higher frailty index were more likely to be vaccinated; each 0.1 unit increase in frailty index was associated with a 31% increase in likelihood of vaccination (OR: 1.31; 95% CI: 1.22, 1.42). In contrast, the frailty short interview score was not associated with influenza vaccination (OR per 0.1 unit increase: 1.02; 95% CI: 0.99, 1.05). After covariate adjustment, the association between frailty index and likelihood of vaccination was slightly attenuated, but remained statistically significant (aOR per 0.1 unit increase: 1.23; 95% CI: 1.09, 1.40). Frailty short interview score remained unassociated with likelihood of influenza vaccination after covariate adjustment (aOR per 0.1 unit increase: 1.00; 95% CI: 0.96, 1.03). The association between frailty index and influenza vaccination was slightly stronger for adults <50 years (aOR per 0.1 unit increase: 1.38; 95% CI: 1.10, 1.75) than for adults ≥50 (aOR per 0.1 unit increase: 1.18; 95% CI: 1.01, 1.36). However, the association between frailty short interview score and vaccination was nearly identical in younger (aOR per 0.1 unit increase: 1.01; 95% CI: 0.95, 1.07) and older (aOR per 0.1 unit increase: 0.99; 95% CI: 0.95, 1.03) adults.
3. Discussion
We developed a 5-question interview to assess frailty in a large-scale study of influenza in adults with acute respiratory illness requiring a short enrollment interview. Responses to this frailty short interview were only moderately correlated with an established and validated frailty index that requires extensive manual chart abstraction [4]. The ability to predict frailty index scores from responses to the frailty short interview remained relatively low even after inclusion of additional important variables, such as age, supplemental oxygen use at home, and smoking status in multivariable models. Less than 30% of the variation in frailty index was explained by these models in each study year. Differences between the two frailty measures were also highlighted by distinct patterns of frailty prevalence by participant characteristics. Further, only frailty as defined by the frailty index was associated with increased likelihood of influenza vaccination.
There were likely multiple explanations for the differences we observed between the two frailty measures. First, there are multiple frameworks of frailty used in aging research [2,3]. The two instruments that we used each conceptualize frailty in different ways. The frailty short interview was based on the Fried frailty phenotype, which conceptualizes frailty as a clinical syndrome characterized by weight loss, exhaustion, weakness, difficulty walking, and physical inactivity [5]. In contrast, the frailty index is based on the accumulation of deficits model which does not make as clear of distinctions between frailty, comorbidity, and functional status [4]. The level of disagreement between the two measures is consistent with a recent study that found a median kappa coefficient value of 0.40 for the frailty phenotype relative to 34 other frailty measures [16].
The methods by which our two measures were implemented could have contributed to differences in their associations with each other and other key variables including sex, race, and smoking status. Because the frailty index was defined by information abstracted from the medical record, a diagnosis of a condition, disability, or deficit must have been made and recorded in the medical record for it to have been captured. Differences in healthcare seeking behavior by sex and race, for example, could therefore contribute to the observed differences. In addition, our finding that the frailty short interview defined more women than men as frail is consistent with several studies demonstrating that women tend to live longer than men, but have poorer health status and be more frail [25,26]. However, the components of frailty may differ between men and women [27], potentially explaining the observed differences between the two frailty instruments.
There has recently been some debate regarding the degree to which frailty confounds estimates of influenza vaccine effectiveness [10,11,22,28,29]. In this study, we found that the association between frailty and influenza vaccination, a necessary requirement for confounding, varied by the instrument used to measure frailty. Specifically, only those with higher frailty index were more likely to be vaccinated while frailty short interview scores were not associated with vaccination. We speculate that this could be because the frailty index incorporates comorbid conditions that could increase the likelihood that vaccination is recommended to a patient. In addition, we did not observe an association between either frailty instrument and influenza infection status. While prior studies have found reduced immune response to influenza vaccination and increased infection risk among frail individuals, infection risk also depends on contact patterns related to exposure. The relationship between frailty and these contact patterns is complex and likely varies by population. These results, and the debate over the definition of frailty in the broader field of aging research, highlight the importance of the instrument chosen to measure frailty.
Frailty is often considered to be limited to elderly adults. While the frailty is most prevalent in elderly patients, frailty in younger adults with chronic conditions or disabilities has been described [30–32]. In this study, we enrolled hospitalized adults 18 years of age or older with the vast majority having at least one chronic condition [12]. While the prevalence of frailty increased with age, substantial proportions of adults 18–49 years of age were identified as frail by each of the two frailty assessments (frailty short interview: 34%; frailty index: 19%), and agreement between the two measures did not vary substantially by age. There was also a slightly stronger association between frailty index and influenza vaccination for younger compared to older adults. This suggests that frailty should be considered as an important factor in studies of acute respiratory illness-related hospitalization in adults of all ages. However, researchers should be mindful that frailty may be physiologically different in geriatric and younger adults [32].
This study benefitted from a relatively large sample size and from being carried out in two geographically diverse hospitals over two influenza seasons. The consistency of results across study hospitals and study years suggests that the frailty short interview may be a generalizable measure of the Fried frailty phenotype across populations. This along with its ease of implementation make it an attractive option for measurement of this specific conceptualization of frailty during acute respiratory illness. Unfortunately, we did not measure functional status as part of the enrollment interview and therefore could not assess whether it might have explained some of the differences between the frailty short interview and the frailty index, which does include elements of functional status. Self-reported measures of influenza vaccination and the frailty short interview used in this study could be subject to recall bias. In addition, we assessed frailty during acute respiratory illness. Although we asked participants to report the level of difficulty they had with each element of the frailty short interview prior to their current illness, it is not possible to determine how their acute illness may have affected their responses without base-line measures of frailty.
The degree to which frailty and functional status confound measures of influenza vaccine effectiveness, and the best ways to conceptualize and measure these health states, remain open questions. To facilitate resolution of these issues, continued development and assessment of short, easily implemented instruments for measuring frailty and functional status at the time of an acute illness is needed. Such tools would benefit not only studies of influenza vaccine effectiveness but also any research or clinical setting where frailty and functional status are of interest and time and resources are limited.
Supplementary Material
Acknowledgements
This work was supported by the Centers for Disease Control and Prevention (grant numbers U01 IP000974-01 and U01 IP000979-01).
Declaration of Competing Interest
E.T.M. has received grant support from Merck and Pfizer for work unrelated to this report. A.S.M. has received grant support from Sanofi Pasteur and consultancy fees from Sanofi, GSK and Novavax for work unrelated to this report. H.K.T. has received research funding from Sanofi Pasteur and Gilead and has served as an advisor for Seqirus. All authors reported no potential conflicts.
Abbreviations:
- CDC
Centers for Disease Control and Prevention
- CI
confidence interval
- HAIVEN
Hospitalized Adult Influenza Vaccine Effectiveness Network
- IQR
interquartile range
- US
United States
- VE
vaccine effectiveness
Footnotes
Publisher's Disclaimer: Disclaimer
Publisher's Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2019.05.051.
References
- [1].Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc 2010;58 (4):681–7. [DOI] [PubMed] [Google Scholar]
- [2].Xue Q-L. The frailty syndrome: definition and natural history. Clin Geriatr Med 2011;27(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fulop T, Larbi A, Witkowski JM, et al. Aging, frailty and age-related diseases. Biogerontology 2010;11(5):547–63. [DOI] [PubMed] [Google Scholar]
- [4].Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. Sci World J 2001;1:323–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fried LP, Tangen CM, Walston J, et al. Frailty in older adults evidence for a phenotype. J Gerontol Ser A 2001;56(3):M146–57. [DOI] [PubMed] [Google Scholar]
- [6].Jackson LA, Jackson ML, Nelson JC, Neuzil KM, Weiss NS. Evidence of bias in estimates of influenza vaccine effectiveness in seniors. Int J Epidemiol. 2006;35 (2):337–44. [DOI] [PubMed] [Google Scholar]
- [7].Jackson LA, Nelson JC, Benson P, et al. Functional status is a confounder of the association of influenza vaccine and risk of all cause mortality in seniors. Int J Epidemiol 2006;35(2):345–52. [DOI] [PubMed] [Google Scholar]
- [8].Yao X, Hamilton RG, Weng N, et al. Frailty is associated with impairment of vaccine-induced antibody response and increase in post-vaccination influenza infection in community-dwelling older adults. Vaccine 2011;29(31):5015–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].McElhaney JE, Zhou X, Talbot HK, et al. The unmet need in the elderly: How immunosenescence, CMV infection, co-morbidities and frailty are a challenge for the development of more effective influenza vaccines. Vaccine 2012;30 (12):2060–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Andrew MK, Shinde V, Ye L, et al. The importance of frailty in the assessment of influenza vaccine effectiveness against influenza-related hospitalization in elderly people. J Infect Dis 2017;216(4):405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].McElhaney JE, Andrew MK, McNeil SA. Estimating influenza vaccine effectiveness: evolution of methods to better understand effects of confounding in older adults. Vaccine 2017;35(46):6269–74. [DOI] [PubMed] [Google Scholar]
- [12].Petrie JG, Ohmit SE, Cheng CK, et al. Influenza vaccine effectiveness against antigenically drifted influenza higher than expected in hospitalized adults: 2014–2015. Clin Infect Dis Off Publ Infect Dis Soc Am 2016;63(8):1017–25. [DOI] [PubMed] [Google Scholar]
- [13].Haq K, McElhaney JE. Ageing and respiratory infections: The airway of ageing. Immunol Lett 2014;162(1, Part B):323–8. [DOI] [PubMed] [Google Scholar]
- [14].Barker WH, Borisute H, Cox C. A study of the impact of influenza on the functional status of frail older people. Arch Intern Med 1998;158(6):645–50. [DOI] [PubMed] [Google Scholar]
- [15].Hak E, Wei F, Nordin J, Mullooly J, Poblete S, Nichol KL. Development and validation of a clinical prediction rule for hospitalization due to pneumonia or influenza or death during influenza epidemics among community-dwelling elderly persons. J Infect Dis 2004;189(3):450–8. [DOI] [PubMed] [Google Scholar]
- [16].Aguayo GA, Donneau A-F, Vaillant MT, et al. Agreement between 35 published frailty scores in the general population. Am J Epidemiol 2017;186(4):420–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].de Vries NM, Staal JB, van Ravensberg CD, Hobbelen JSM, Olde Rikkert MGM, Nijhuis-van der Sanden MWG. Outcome instruments to measure frailty: a systematic review. Ageing Res Rev 2011;10(1):104–14. [DOI] [PubMed] [Google Scholar]
- [18].Bouillon K, Kivimaki M, Hamer M, et al. Measures of frailty in population-based studies: an overview. BMC Geriatr 2013;13(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Theou O, Brothers TD, Mitnitski A, Rockwood K. Operationalization of frailty using eight commonly used scales and comparison of their ability to predict all-cause mortality. J Am Geriatr Soc. 2013;61(9):1537–51. [DOI] [PubMed] [Google Scholar]
- [20].Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci 2007;62(7):722–7. [DOI] [PubMed] [Google Scholar]
- [21].Hoover M, Rotermann M, Sanmartin C, Bernier J. Validation of an index to estimate the prevalence of frailty among community-dwelling seniors. Health Rep 2013;24(9):10–7. [PubMed] [Google Scholar]
- [22].Talbot HK, Nian H, Chen Q, Zhu Y, Edwards KM, Griffin MR. Evaluating the case-positive, control test-negative study design for influenza vaccine effectiveness for the frailty bias. Vaccine 2016;34(15):1806–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Romero-Ortuno R, Walsh CD, Lawlor BA, Kenny RA. A frailty instrument for primary care: findings from the Survey of Health, Ageing and Retirement in Europe (SHARE). BMC Geriatr 2010;10:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Centers for Disease Control and Prevention (CDC). NHIS questionnaire – adult; 2014. [Internet]. [cited 2018 Jul 13]. Available from: ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/Survey_Questionnaires/NHIS/2014/english/qadult.pdf.
- [25].Gordon EH, Peel NM, Samanta M, Theou O, Howlett SE, Hubbard RE. Sex differences in frailty: a systematic review and meta-analysis. Exp Gerontol 2017;89:30–40. [DOI] [PubMed] [Google Scholar]
- [26].Hubbard RE. Sex differences in frailty. Frailty Aging 2015;41:41–53. [DOI] [PubMed] [Google Scholar]
- [27].Alexandre Tda S, Corona LP, Brito TRP, Santos JLF, Duarte YAO, Lebrão ML. Gender differences in the incidence and determinants of components of the frailty phenotype among older adults: findings from the SABE study. J Aging Health 2018;30(2):190–212. [DOI] [PubMed] [Google Scholar]
- [28].Skowronski DM, Chambers C, De Serres G. Selection bias in the assessment of frailty and its role in influenza vaccine effectiveness evaluation among elderly adults. J Infect Dis 2017;217(1). 168–168. [DOI] [PubMed] [Google Scholar]
- [29].Sullivan SG, Cowling BJ, Greenland S. Frailty and influenza vaccine effectiveness. Vaccine 2016;34(39):4645–6. [DOI] [PubMed] [Google Scholar]
- [30].Bagshaw M, Majumdar SR, Rolfson DB, Ibrahim Q, McDermid RC, Stelfox HT. A prospective multicenter cohort study of frailty in younger critically ill patients. Crit Care Lond Engl 2016;20(1):175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Smart R, Carter B, McGovern J, et al. Frailty exists in younger adults admitted as surgical emergency leading to adverse outcomes. J Frailty Aging 2017;6 (4):219–23. [DOI] [PubMed] [Google Scholar]
- [32].Kehler DS, Ferguson T, Stammers AN, et al. Prevalence of frailty in Canadians 18–79 years old in the Canadian Health Measures Survey. BMC Geriatr 2017;17:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


