Abstract
Plantar fasciitis is the most common cause of chronic heel pain in adults, affecting both young active patients and older sedentary individuals. It results from repetitive stress to the plantar fascia at its origin on the medial tubercle of the calcaneus and is often associated with gastrocnemius tightness. The diagnosis can be made clinically with a focused history and physical examination; imaging is reserved for atypical presentations and those that do not respond to initial treatment. The most common presenting symptom is aching plantar heel pain, which is worst with first step in the morning or after periods of rest. Diagnosis is confirmed with point tenderness at the origin of the plantar fascia on the medial tubercle of the calcaneus. Initial treatment consists of activity modification, anti-inflammatory medication, gastrocnemius and plantar fascia stretching, and an in-shoe orthosis that lifts and cushions the heel. These nonoperative treatments lead to complete resolution of pain in 90% of patients but can take 3-6 months. Patients who remain symptomatic despite a 6-month trial of nonoperative therapy may be considered for minimally invasive treatment or surgery. Platelet-rich plasma injections and therapeutic ultrasound are among a number of minimally invasive treatments that stimulate the body’s healing response. Corticosteroid injections temporarily relieve pain, but may increase the risk of plantar fascia rupture and fat pad atrophy. Botulinum toxin injections relax the calf muscles, which decreases the stress in the plantar fascia. Operative treatments include gastrocnemius recession and medial head of gastrocnemius release, which decrease the stress on the plantar fascia and partial planter fasciotomy, which stimulates a healing response.
Level of Evidence:
Level V, expert opinion.
Keywords: tendinosis, tendon disorders, fasciosis, plantar fascia
Introduction
Chronic plantar fasciitis (CPF) is the most common cause of chronic heel pain in adults, affecting both young active patients and older more sedentary individuals. 39 It results from chronic overload of the plantar fascia. It can be due to overuse, as seen in runners and military personnel, or due to excessive loading, as seen in obese (body mass index >30) sedentary individuals and those who stand for prolonged periods of time. 1,6,42 Plantar fasciitis (PF) occurs more frequently in individuals with structural foot deformities, including pes planus, pes cavus, and leg length discrepancies, each of which are associated with tightness of the intrinsic foot muscles or heel cord. 39,42 PF more often affects only one foot, although approximately 30% of patients have bilateral symptoms. 39
Structure and Function
The plantar fascia is a thick band of fibrous connective tissue spanning the arch from the posterior tuberosity of the calcaneus to the bases of the proximal phalanges. Histologically, the plantar fascia bears resemblance to both tendon and ligament with a relatively inelastic extracellular matrix composed of collagen fibers in a wavy or crimpled pattern, produced by elongated fibrocytes embedded in longitudinal rows. Progressive degenerative change in this matrix structure leads to PF. 49 The plantar fascia originates at the medial tubercle of the calcaneus and inserts at 3 locations in the forefoot, creating 3 distinct bands: medial, central, and lateral (Figure 1). The medial band overlies and inserts onto the muscles of the hallux, and the lateral band inserts on the base the fifth metatarsal. 23 These 2 bands are infrequently involved in PF. The central band (aka the plantar aponeurosis) is the thickest, strongest, and most often involved in PF. It divides into five bundles at the midtarsal level, with each band attaching to the plantar plate of one of the proximal phalanges, which in combination with the osseous structures of the arch effectively creates a truss. The plantar fascia is responsible for raising and stabilizing the arch during gait via the windlass mechanism. Dorsiflexion of the toes that occurs during terminal stance leads to tightening of the central band of the plantar fascia, which in turn pulls the metatarsal heads closer to the calcaneus, increasing the height of the arch (Figure 2). 23
Figure 1.
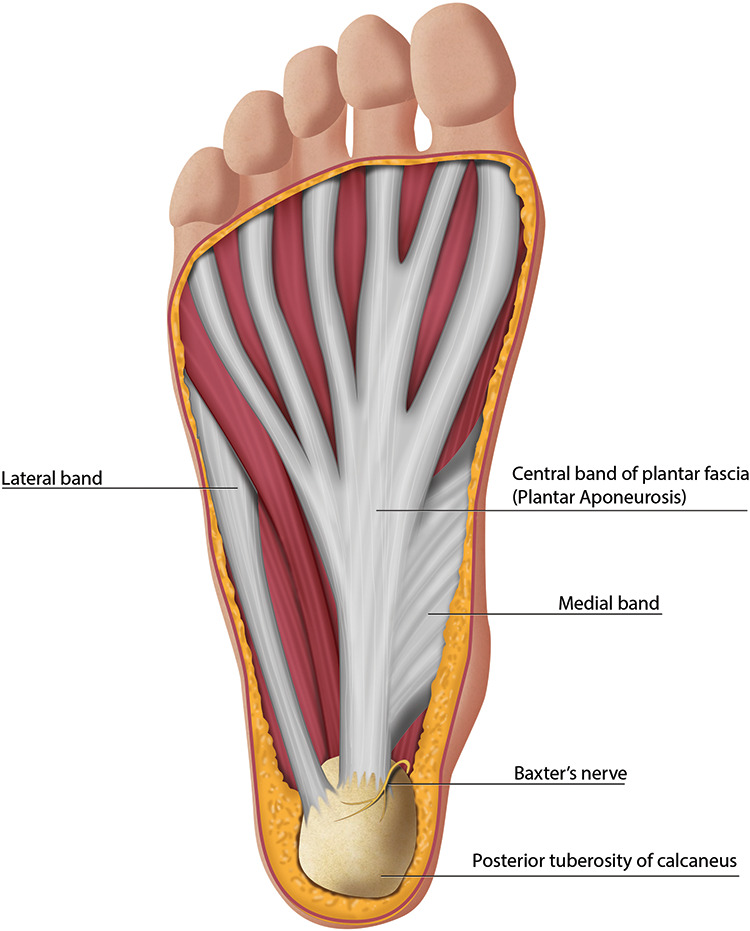
Anatomy of the plantar aspect of the foot demonstrating the bands of the plantar fascia.
Figure 2.
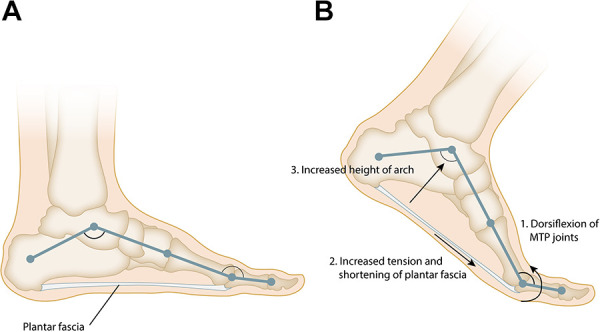
(A) The plantar fascia and the longitudinal arch of the foot form a truss. (B) Dorsiflexion of the toes during the late stance phase of gait tensions the plantar fascia around the metatarsal heads leading to an increase in the height and stability of the longitudinal arch of the foot, this effect is known as the “Windlass” mechanism.
There are a number of foot deformities that predispose individuals to develop PF through induction of pathologic biomechanical stresses. For example, a tight Achilles tendon restricts ankle dorsiflexion during the late stance phase of gait, thus blocking forward progression of the center of mass. Patients may compensate for the lack of ankle dorsiflexion by increasing motion through the subtalar axis, which is oriented obliquely relative to the long axis of the foot, resulting in increased dorsiflexion, but also valgus of the hindfoot and abduction of the forefoot. This overprotonation leads to increased stress in, and eventually attenuation of, the plantar fascia and the other musculotendinous structures supporting the arch leading to the development of PF and pes planovalgus (flatfoot). In contrast, the high-arched foot of pes cavus has restricted mobility through the transverse tarsal joints, leading to an inability to dissipate shock from ground strike, thus increasing the load in the plantar fascia, and leading to plantar fascia overload. 3
Pathophysiology
Classically, PF was thought to occur from a mechanical injury in which excessive tensile strain within the plantar fascia produces microscopic tears leading to chronic inflammation. 49 However, current understanding is that PF occurs through a degenerative rather than an inflammatory process, that is, a “fasciosis,” rather than a fasciitis, where tensile strain is the key feature in the pathogenesis. 49 Specifically, the increased fascial load is sensed by the gap junctions between fibrocytes (mechanotransduction), which then mediate changes in the extracellular matrix, resulting in myxoid degeneration and fragmentation of the plantar fascia and perifascial structures.
Evaluation
History
The evaluation of a patient with heel pain should begin with a focused history to elicit the timing, onset, character, location, and intensity of the pain. Typically, patients report a dull aching or throbbing pain, which is localized to the area around the origin of the plantar fascia on the calcaneus. This pain is usually worst with the first step in the morning and when getting up from sitting. It often gets better with activity, but then worsens as the activity becomes prolonged.
When a patient does not report these classic findings, the physician should be open and attune to the possibility of, and should search for, other causes of heel pain (Table 1). Heel pain that is described as burning or an electric shock is often neurologic in origin—either from peripheral neuropathy or a compression neuropathy. The compression can occur at a number of locations along the peripheral nerves supplying sensation to the heel. Clues as to the site of compression can be found in the history and elicited on physical examination. Compression at the L5/S1 neural foramen is relatively common and may be caused by either degenerative disk disease or facet hypertrophy leading to neural foraminal stenosis. Compression at the level of the nerve root is often associated with back pain, radicular leg pain, or sensory loss in the foot or leg. Compression can also occur within the tarsal tunnel due to the mass effect from varicose veins, tenosynovitis, exostosis, or a soft tissue tumor. Tarsal tunnel syndrome typically presents with burning or shocking pain along the plantar aspect of the foot and is often associated with numbness along the plantar surface. Baxter neuroma (aka Baxter neuritis or Baxter neuropathy) is an uncommon compressive neuropathy of the first branch of the lateral plantar nerve characterized by burning across the heel pad. Baxter nerve is a mixed nerve supplying both motor innervation to abductor digiti minimi, flexor digitorum brevis, quadratus plantae and sensory innervation to the calcaneal periosteum, long plantar ligament, and skin on the lateral aspect of the sole of the foot. 28 The compression occurs at the plantar medial heel as the nerve crosses under the abductor hallucis muscle belly. The presence of a suspected compressive neuropathy may be confirmed by imaging and/or electromyography, but should be initially evaluated with a focused physical examination. In the case of Baxter neuritis, the diagnosis is confirmed by magnetic resonance imaging (MRI) demonstrating atrophy of the abductor digiti quinti minimi muscle belly.
Table 1.
| Etiology | Disorder |
|---|---|
| Neurologic | Peripheral neuropathy (idiopathic, diabetic, nutritional) |
| L5/S1 neural foraminal impingement or lumbar spinal stenosis | |
| Tarsal tunnel syndrome | |
| Baxter neuroma (neuritis) | |
| Traumatic | Plantar fascia tear / rupture |
| Calcaneal stress fracture | |
| Rheumatologic | Rheumatoid arthritis |
| Reactive arthritis Psoriatic Arthritis |
|
| Seronegative spondyloarthropathy | |
| Degenerative | Fat pad atrophy |
| Plantar fasciitis (fasciopathy) |
Traumatic causes of plantar heel pain include plantar fascia tear or rupture and calcaneus stress fracture. The pain of a plantar fascia tear is often located within the midportion of the plantar fascia as opposed to at the insertion onto the calcaneus. The presence of a calcaneus stress fracture should be suspected in a patient who has a history of osteopenia, other stress fractures, or fragility fractures. Patients with stress fractures typically report aching pain throughout the posterior tuberosity of the calcaneus that is related to activity, but not to the first step in the morning. Patients with fat pad atrophy also tend to have aching that is activity related.
Finally, the presence of bilateral heel pain, heel pain concurrent with multiple painful or stiff joints, or a stiff spine suggest a rheumatologic etiology. 19 The seronegative spondyloarthropathies occur most commonly in young men as opposed to CPF, which is most common in middle age and more often in women than men.
Physical Examination
The physical examination of patients with heel pain should focus on determining the precise location of the pain, evaluating for tightness of the heel cord, and ruling out other potential causes of heel pain. The physical examination begins with an evaluation of standing alignment and gait. Particular attention should be paid to the alignment of the hindfoot and the midfoot as individuals with pes planus or pes cavus are predisposed to the development of PF. The plantar fascia and its origin at the calcaneus should be palpated, to elicit tenderness. Tenderness at the origin of the PF is the hallmark of CPF while tenderness along the length of the central band of the PF is more commonly seen in distal PF (an acute injury that normally resolves with stretching and arch supports). The dorsiflexion range of motion of the ankle should be assessed both with the knee flexed and extended (Figure 3). Less than 10 degrees of dorsiflexion with the knee extended or more than a 10-degree difference between dorsiflexion with the knee flexed and extended are signs of gastrocnemius equinus (ie, a positive Silverskiold test). Special tests should include a Tinel test along the distal portion of the tibial nerve to rule out tarsal tunnel syndrome and a calcaneus squeeze test (Figure 4) to rule out calcaneal stress fracture.
Figure 3.
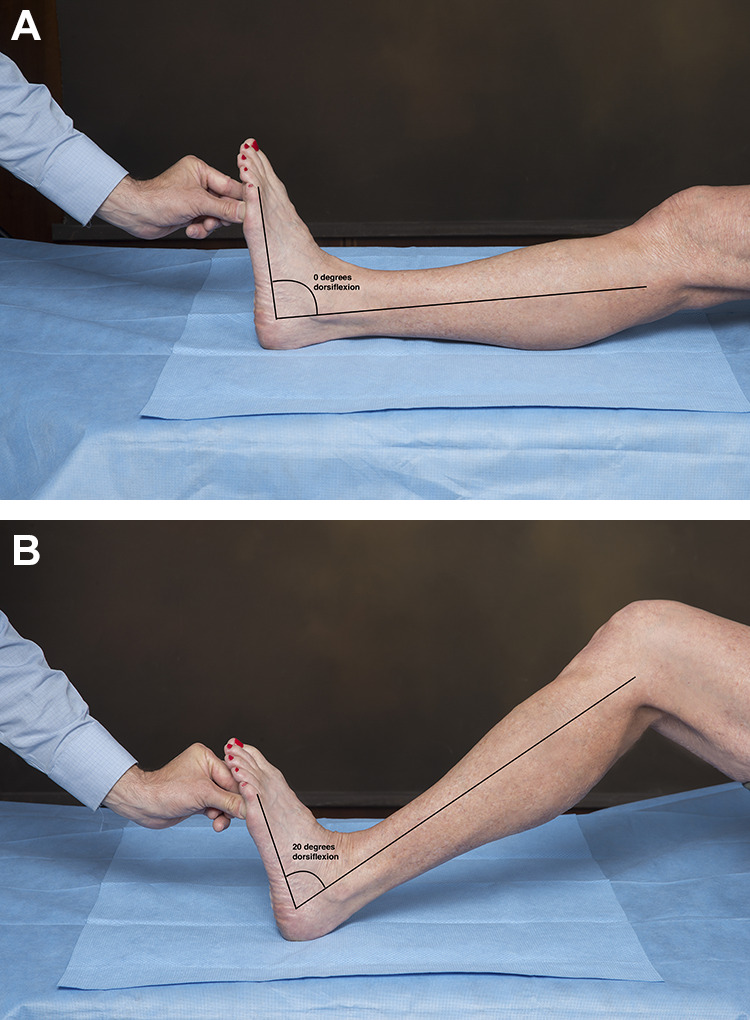
The Silverskiold test is used to assess for gastrocnemius equinus. The maximum passive ankle dorsiflexion is compared with the knee (A) extended to (B) flexed. The difference between dorsiflexion in these 2 positions is the contribution of the gastrocnemius to the equinus contracture because the gastrocnemius crosses both the ankle and the knee joints whereas the other plantarflexors of the ankle do not. Dorsiflexion of less than 10 degrees with the knee extended or a difference of greater than 10 degrees confirms the presence of gastrocnemius equinus contracture.
Figure 4.
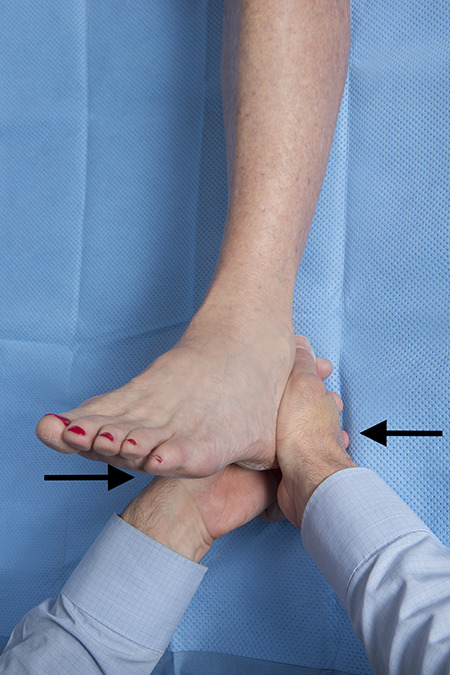
In the calcaneus squeeze test, the examiner’s fingers are interlaced, the thenar or hypothenar eminences are then squeezed together compressing the posterior tuberosity of the calcaneus. The test is positive if this elicits the patient’s pain and is more painful than pressing plantarly at the insertion of the plantar fascia.
Imaging and Laboratory Evaluation
Weight-bearing radiographs of the foot are often obtained at presentation to evaluate foot alignment, exclude bony lesions, and determine the presence of a plantar heel spur (Figure 5). The heel spur is a sign of calcification at the origin of the flexor digitorum brevis muscle, which develops in response to chronic tightness of the heel cord. It is seen in a number of disorders but is of no functional significance, as neither the shape of the spur nor its size correlates with symptoms of PF. 2 It has been suggested that radiographs are not needed in the initial evaluation of heel pain as the vast majority are either normal or show only a heel spur. 30
Figure 5.
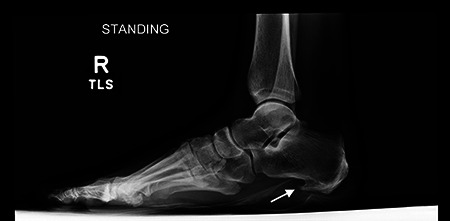
Weightbearing lateral radiograph of the foot demonstrating a large plantar calcaneal spur consistent with chronic heel cord tightness.
Soft tissue imaging is not routinely required in the diagnosis of CPF but is instead performed in patients with heel pain refractory to several months of conservative therapy. The goals of soft tissue imaging are to confirm the diagnosis of PF and to exclude other causes of heel pain such as inflammatory arthropathy (spondyloarthropathy), Baxter neuritis, and calcaneus stress fracture. 39
Ultrasound provides a rapid and cost effective means to confirm the diagnosis of CPF. 31 A plantar fascia thickness >4.5 mm and the presence of hypoechoic areas are specific for PF (Figure 6). Whereas, the presence of subcalcaneal bone spurs (24%), peritendinous edema (5%), subcalcaneal bone erosion (4%), intratendinous calcification (3%), and retrocalcaneal bursitis are associated with PF but are not specific. 26,31 Diagnostic ultrasonography can also be used to evaluate treatment response. The extent of reduction of fascia thickness is an objective measure of treatment efficacy, as thicker plantar fascia correlates with increased symptoms and patient-reported pain scores on VAS. 20
Figure 6.
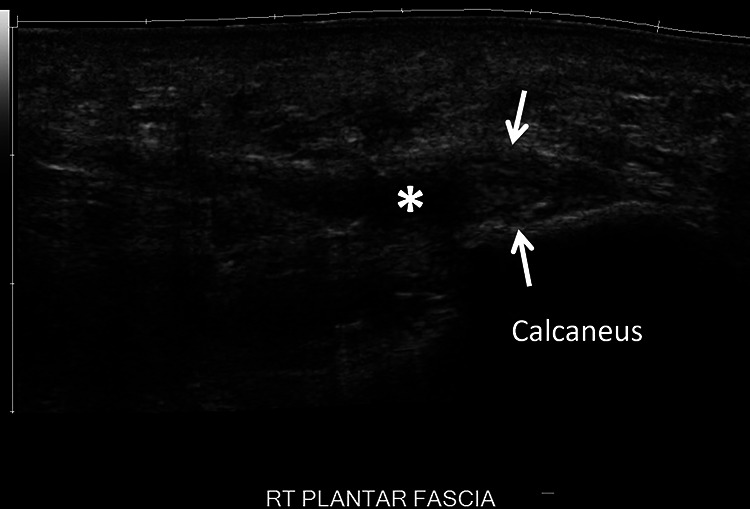
Long axis ultrasound imaging of the plantar fascia demonstrates thickening (arrows) of the proximal medial/central band, the right plantar fascia measuring up to 6 mm. There is a small region of hypoechogenicity (asterisk) within the medial aspect of the central band consistent with a moderate-grade partial-thickness tear.
MRI can be also be used to confirm the presence of PF with characteristic findings of increased signal intensity and proximal plantar fascia thickening (Figure 7) on T2-weighted and short tau inversion recovery images. 18 However, MRI is most useful in excluding the presence of other causes of heel pain such as calcaneus stress fracture, Baxter neuritis, tarsal tunnel syndrome, and insertional Achilles tendinopathy. Baxter neuritis is suspected when atrophy of the abductor digiti quinti minimi muscle belly is seen on T1-weighted images (Figure 8). Calcaneus stress fracture is diagnosed in the presence of a linear low T1 and high T2 signal in the posterior tuberosity of the calcaneus (Figure 9).
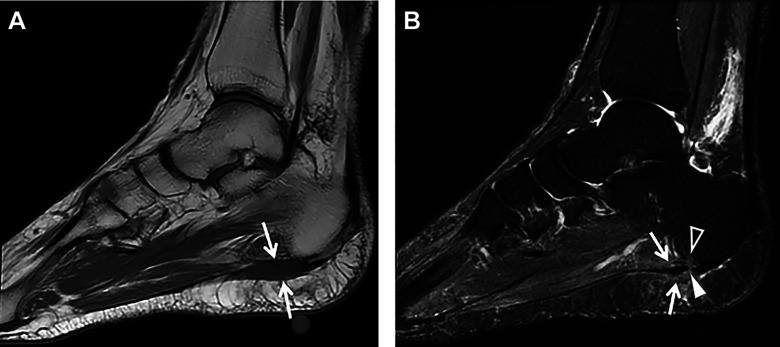
Figure 7.
(A) Sagittal T1-weighted MRI showing intermediate signal intensity in the region of the origin of the central band of the plantar fascia (arrows). (B) Sagittal STIR MRI showing heterogeneous increased signal intensity involving the proximal central limb of the plantar fascia adjacent to the vitamin E marker (arrows) consistent with plantar fasciitis. A full-thickness partial-width tear of the proximal central band of the plantar fascia is seen (arrowhead) as well as associated perifascial subcutaneous and deep soft tissue edema and a small focus of mild focal bone marrow edema in the adjacent calcaneal tuberosity (open arrowhead). MRI, magnetic resonance imaging; STIR, short tau inversion recovery.
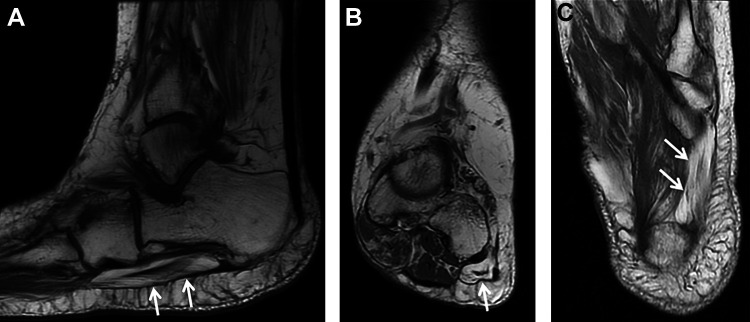
Figure 8.
(A) Sagittal, (B) coronal, and (C) axial T1-weighted MRI showing severe atrophy of the abductor digiti minimi muscle (arrows) consistent with Baxter neuropathy with entrapment of the inferior calcaneal nerve. MRI, magnetic resonance imaging.
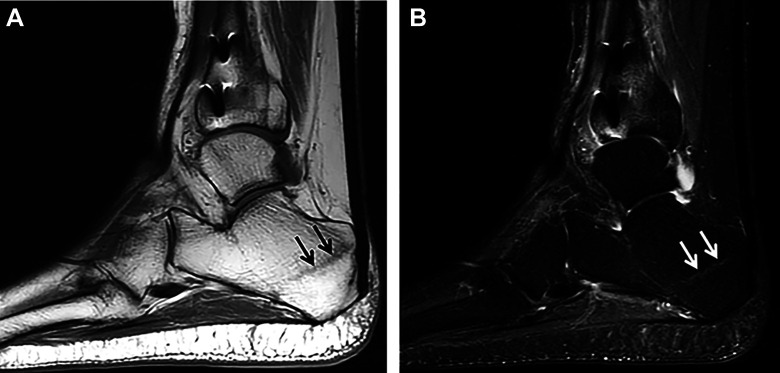
Figure 9.
(A) Sagittal T1-weighted MRI of the ankle demonstrating an oblique curvilinear hypointense marrow signal in the posterior tuberosity of the calcaneus (arrows) consistent with a stress fracture (B) Sagittal STIR MRI showing high signal calcaneal fracture line (arrows) with minimal surrounding bone marrow edema, consistent with a healing/healed stress/insufficiency fracture. MRI, magnetic resonance imaging; STIR, short tau inversion recovery.
Electromyography (EMG) can be used to evaluate suspected neurologic causes of heel pain in patients presenting with sensory disturbances or proximal or distal radiation. Chronic neurologic heel pain secondary to local nerve entrapment commonly occurs with the first branch of the lateral plantar nerve and medial calcaneal nerve branches. In these cases, EMG helps to localize the site of entrapment, but is user dependent and not always sensitive for compression within in the foot. 32
Laboratory evaluation may be used to exclude the inflammatory arthropathies in patients presenting with systemic symptoms, bilateral involvement, multiple joint involvement (especially small joints), and/or morning stiffness greater than 30 minutes. The workup should include a complete blood count with differential, renal and hepatic function tests, serum testing for rheumatoid factor, anti-citrullinated peptide antibodies, C-reactive protein, and erythrocyte sedimentation rate.
Nonoperative Treatment
Noninvasive Treatments
PF is usually a self-limiting condition, with more than 90% of patients achieving symptomatic relief with 3-6 months of conservative treatment. 39 Initial treatment should consist of nonsteroidal anti-inflammatories (NSAIDs), stretching of the gastrocnemius and the plantar fascia, and the use of an orthosis (heel pads, heel cups, arch supports, or night splints). Successful treatment is defined as a decrease in pain and improvement in function.
Nonsteroidal anti-inflammatory medications
NSAIDs, either taken orally or applied locally, reduce pain by inhibiting the cyclooxygenase pathway, which suppresses activation of downstream inflammatory mediators. They may produce short-term pain relief and decrease disability when used in combination with other modalities in a conservative regimen such as rest, activity modification, and stretching. 15 However, there is little evidence supporting their efficacy when used in isolation or for the purpose of reducing inflammation.
Orthotics, heel wedges, and heel cups
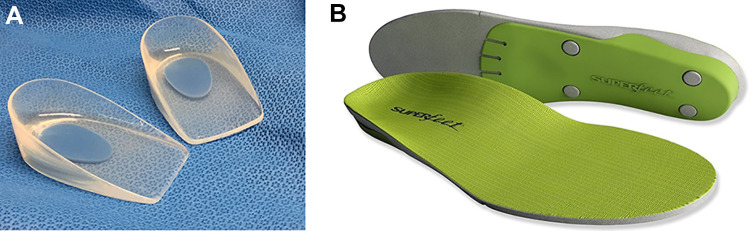
Computational modeling and cadaveric biomechanical studies of the PF have shown that it experiences maximal tension during the late-stance phase of gait, with the peak stress located near the medial calcaneal tubercle. Moreover, the stress within the PF is proportional to the Achilles tendon force, which is proportional to the magnitude of heel rise during gait. 27 Orthotics decrease heel rise and Achilles tendon force during gait, leading to pain reduction. Both prefabricated and custom-fitted orthotics have been shown to reduce pain and improve function in the short term with few risks or side effects. Prefabricated foot orthotics such as gel heel cups, longitudinal arch supports, and full-length shoe insoles (Figure 10) have been shown to be more effective than custom shoe inserts. 40
Figure 10.
In-shoe orthoses used in the treatment of plantar fasciitis: (A) gel heel cups, (B) over-the-counter arch supports. Image used with permission of Superfeet Worldwide, Inc.
Immobilization: Night splints and CAM walker boots
The night splint is an orthosis that prevents plantar fascia contracture by maintaining a neutral position of the ankle during sleep (Figure 11). Night splinting is most effective in patients whose chief complaint is morning pain. Their use is often limited by poor compliance stemming from discomfort leading to sleep disturbance. Moreover, there is conflicting data concerning their efficacy. Although some studies have reported no difference in heel pain and flexibility among splinted patients and controls, others have concluded that splinting offers significant short-term reduction of heel pain 41 in patients undergoing treatment for PF for the first time but has no significant effect on preventing recurrence. 47 A Cochrane review concluded that night splints used in conjugation with custom foot orthotics produced significant pain relief. 11 Controlled ankle motion (CAM) walking boots or walking casts are another alternative to surgery in patients with chronic recalcitrant plantar heel pain, with symptoms present for at least 1 year. Their proposed mechanism of action involves partial unloading of the heel and constant stretching of the fascia that occurs within the boot. Improvement is reported in more than 40% of patients. 21
Figure 11.
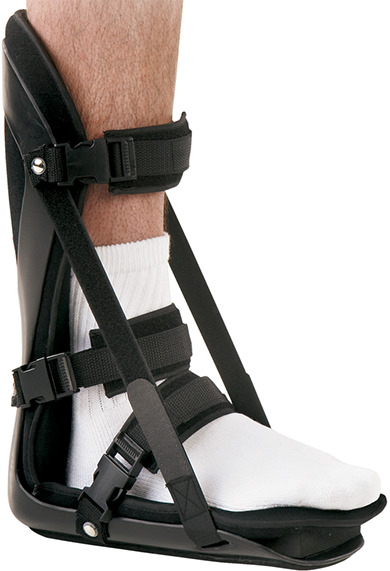
Night splint. Image used with permission of Breg, Inc.
Physical therapy: Therapeutic exercise and therapeutic modalities
A physical therapist can assist with the treatment of PF in a number of ways including reducing pain, guiding stretching exercises, supervising strengthening exercises, and providing an additional point of contact for the patient during the long recovery process. In addition to stretching, the therapist can use manual therapy, consisting of joint and soft tissue mobilization, to improve lower extremity flexibility, to decrease pain, and improve function. 32 The physical therapist can also use therapeutic modalities to reduce pain and inflammation and speed healing, although there is little evidence to support their use. 32 Specifically, the use of phonophoresis with ketoprofen gel and the use of low-level laser are weakly supported in the literature, while the efficacy of iontophoresis with dexamethasone gel has conflicting evidence. 32
Stretching of the gastrocnemius muscle is a mainstay of treatment of PF. This stretching can be either self-directed or guided by a physical therapist. Moreover, the stretching can be performed either seated or standing, but it must be done with the knee extended to isolate the gastrocnemius (Figure 12). Stretching of the PF (Figure 13) also plays a crucial role and has been shown, by itself, to be effective in treating CPF. 14 Regardless of the specific type of stretching, adherence to a daily regimen is the key to success. 32
Figure 12.

In the gastrocnemius stretch, the hands are placed against the wall, the leg being stretched is slid posteriorly with the knee bent as far as it will go and then the knee is straightened.
Figure 13.
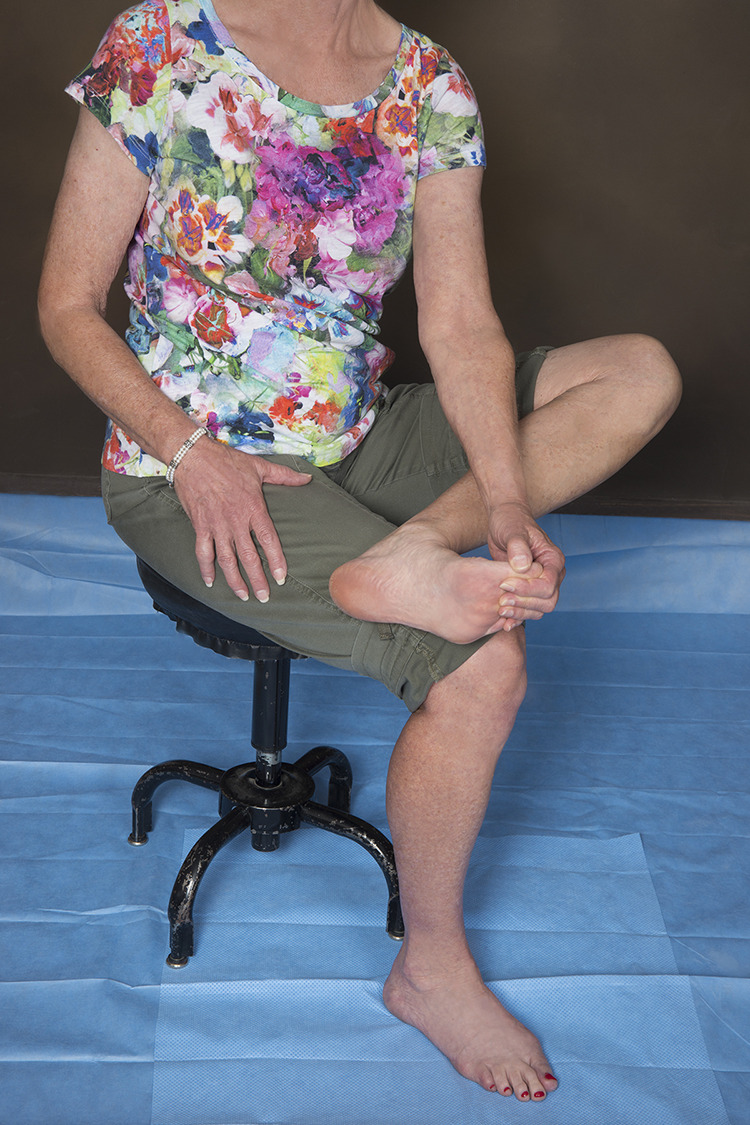
In the plantar fascia stretch, the foot to be stretched is placed on top of the contralateral knee, the ankle is maximally dorsiflexed, and then the toes are pulled up, tensioning the plantar fascia. The plantar fascia is then gently massaged along its length.
Minimally Invasive Treatments
Patients with heel pain for 6 months or more that is recalcitrant to the nonoperative treatments may undergo minimally invasive procedures that relieve pain (corticosteroid injection), decrease heel cord tightness (botulinum toxin injection), or stimulate the body’s healing response (platelet rich plasma [PRP] injection, dry needling, extracorporeal shock wave therapy (ESWT), intense therapeutic ultrasound). 34
Corticosteroid injection
Corticosteroid injections (CSIs) are often used to treat the pain associated with PF; however, their use is controversial. A number of studies have demonstrated short-term pain relief with CSI; some have shown longer-lasting pain relief than ESWT whereas others have found mixed results in comparison to PRP injection. 13,24,36 The rationale underlying the use of CSI in the treatment of PF has come into question because of the improved understanding of PF as a degenerative rather than an inflammatory process. The therapeutic benefit of corticosteroids is dependent on their anti-inflammatory properties, which provide significant pain reduction; however, they also act to inhibit fibroblast proliferation and ground substance protein expression. These effects can produce atrophy of the plantar fascia and plantar fat pad and can lead to complete plantar fascia rupture, both of which are difficult to treat. A number of studies have demonstrated the association between steroid injections and plantar fascia rupture. 13,29 In summary, steroid injections may provide temporary symptomatic relief but are associated with an increased risk of developing persistent pain, local tissue atrophy, or plantar fascia rupture. Thus, if they are used it should be with caution and patients should be advised of the risks and benefits prior to injection.
Botulinum toxin
Botulinum toxin A (BTX), the active component of Botox injections, inhibits acetylcholine release at the neuromuscular junction and induces skeletal muscle paralysis. Injections of BTX into the plantar fascia and gastrocnemius-soleus muscles reduce tension in the plantar fascia and may have an analgesic effect and produce muscle relaxation without the risk of perifascial atrophy or rupture that occurs with heel cord–lengthening procedures. BTX into the gastrocnemius combined with plantar fascia stretching exercises has been shown to produce greater pain relief and functional improvement compared with CSI. 2 It is unclear whether BTX alone is better than placebo as one study found improvements in gait and decrease in plantar fascia thickness, but another found no differences. 2,16 BTX injection might benefit patients with CPF associated with gastrocnemius contracture, but more evidence is needed.
Platelet-rich plasma
PRP has grown in popularity for treating a number of orthopedic conditions, including CPF. PRP is extracted from autologous whole blood and contains a high concentration of endogenous growth factors. These growth factors are thought to activate intrinsic cellular mechanisms that restore the plantar fascia. There is minimal literature regarding its effectiveness in CPF. One study found no benefit to PRP compared to CSI, whereas another found longstanding improvements in pain. A meta-analysis found no differences between CSI and PRP in pain relief at most time points, but a potential longer benefit with PRP. 24,36 PRP appears to be a safe treatment option, but its unproven benefit may not justify its cost.
Shock-wave therapy and intense therapeutic ultrasound
ESWT delivers low-frequency high-energy acoustic waves to the plantar fascia, which induces microtrauma that activates interstitial and extracellular responses leading to tissue regeneration, angiogenesis, increased blood flow, and nutrient delivery. Systematic reviews have concluded satisfactory short-term pain relief and functional outcomes, including decreased morning and activity pain, and pain associated with walking, although long-term efficacy remains unknown because of lack of long-term data. 9 Significant improvements in patient-reported outcome measures have been observed in patients receiving ESWT compared with those who received corticosteroid injections.
In contrast, intense therapeutic ultrasound uses high-frequency high-energy ultrasound to create small thermal injury zones inside soft tissue without damage to surrounding structures. Intense therapeutic ultrasound has been shown to initiate a tissue repair cascade and promote collagen generation. 4 It is approved by the FDA for nonoperative brow lifts and has been demonstrated to produce a greater and more rapid reduction in heel pain and perifascial lesion size when compared to sham control. 45
Operative Treatment
Operative treatment is indicated when pain and functional limitations persist despite an adequate nonoperative trial lasting at least 6 months. In practice, nonoperative therapy is often attempted for even longer as evidenced by a survey of foot and ankle fellowship-trained orthopedic surgeons, which found that only 55% would proceed with operative intervention after 10 months of recalcitrant heel pain. 12 This same study found that there was no consensus on preferred operative technique as there are a variety of procedures that have been used in the operative treatment of CPF and none has demonstrated superior results. Moreover, each carries some risk of persistent or worsening pain due to alterations in foot structure.
Partial Plantar Fasciotomy
Partial plantar fasciotomy is the traditional procedure used in the treatment of CPF. It involves partial transection of the PF to stimulate a healing response. This procedure can be performed either through an open or endoscopic approach. It can be combined with Baxter nerve decompression for medial heel pain where compression of Baxter nerve is suspected. Despite the popularity of plantar fascia release, there is only weak evidence to support its use. Retrospective evaluations of partial plantar fasciotomy have reported rates of success, ranging from 57% to 96%. 7,8,43,46,48 All of these studies have had substantial limitations and variability limiting any definitive conclusions on the benefit of operative release. Many of these studies report worsening and/or persistent heel pain in an unacceptably high percentage of patients. 46,48
Minimally invasive partial plantar fasciotomy can be performed using either ultrasound-guided percutaneous or endoscopic techniques. Both require extensive specialized training to prevent injury to neurovascular structures or over-resection of the plantar fascia. A number of retrospective reviews have demonstrated success with these techniques ranging from 40% to 100%, but none have demonstrated clear superiority over the open technique and they both have rates of persistent heel pain similar to open surgery. 5,6,25,38,43
It is often difficult to distinguish between PF and nerve compression. The history, physical examination, and imaging provide essential clues, but cannot always differentiate between them. Thus, many surgeons choose to simultaneously perform both a plantar fasciotomy and local nerve decompression. Simultaneous nerve decompression and partial fasciotomy has not shown any obvious difference in outcomes compared to release alone, with varied postoperative satisfaction rates from 48.8% to 87%. 10,12,13,37,44 The addition of simultaneous calcaneal drilling has been shown in a single randomized study to have a potential benefit compared with nerve release alone for recalcitrant heel pain. 10,12,37,44,46 These varied results demonstrate the importance of making the most accurate possible diagnosis and not simply performing a routine “all encompassing” surgery for patients with recalcitrant heel pain.
Gastrocnemius Lengthening
The strong association between gastrocnemius contracture and chronic heel pain has led many surgeons to believe that operative release of the gastrocnemius could result in improved heel pain. Moreover, the mixed results and potential risks (including persistent heel pain and loss of height in the longitudinal arch) of plantar fasciotomy make a proximal release of the gastrocnemius an attractive alternative. Gastrocnemius-lengthening procedures (gastrocnemius recession) carry minimal operative complication rates and some studies have shown promising results compared to plantar fascial release procedures. 1,17,22,33,35 Proximal release of the medial head of the gastrocnemius is an alternative technique for lengthening the gastrocnemius. It has been shown to be as effective as gastrocnemius recession and with fewer complications.
Summary
PF is a common condition that is self-limiting in the majority of patients. Evaluation consists of a focused history and physical examination. Initial treatment with anti-inflammatories, activity modification, plantar fascia stretching, gastrocnemius stretching, and the use of a heel lift, gel heel cushion, arch support, or night splint is effective in more than 90% of patients. Diagnostic imaging with weight-bearing radiographs is usually performed and MRI or ultrasound is performed for atypical presentations and in cases refractory to initial treatment. When symptoms persist despite 6 months of conservative treatment, either minimally invasive treatments or surgery may be considered. The minimally invasive treatments, including corticosteroid injection, botulinum toxin, PRP injection, and therapeutic ultrasound, are aimed at relieving pain (steroid injection) or stimulating the body’s healing response. Operative treatments include partial plantar fasciotomy and gastrocnemius lengthening performed through a variety of approaches. There is a lack of consensus supporting one operative technique over another.
Supplemental Material
Supplemental Material, FAO896763-ICMJE for Evaluation and Treatment of Chronic Plantar Fasciitis by L. Daniel Latt, David Eric Jaffe, Yunting Tang and Mihra S. Taljanovic in Foot & Ankle Orthopaedics
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. ICMJE forms for all authors are available online.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: L. Daniel Latt, MD, PhD,  https://orcid.org/0000-0002-9925-3440
https://orcid.org/0000-0002-9925-3440
References
- 1. Abbassian A, Kohls-Gatzoulis J, Solan MC. Proximal medial gastrocnemius release in the treatment of recalcitrant plantar fasciitis. Foot Ankle Int. 2012;33(1):14–19. [DOI] [PubMed] [Google Scholar]
- 2. Ahmad J, Ahmad SH, Jones K. Treatment of plantar fasciitis with botulinum toxin: a randomized, controlled study. Foot Ankle Int. 2017;38(1):1–7. [DOI] [PubMed] [Google Scholar]
- 3. Alshami AM, Babri AS, Souvlis T, Coppieters MW. Biomechanical evaluation of two clinical tests for plantar heel pain: the dorsiflexion-eversion test for tarsal tunnel syndrome and the windlass test for plantar fasciitis. Foot Ankle Int. 2007;28(4):499–505. [DOI] [PubMed] [Google Scholar]
- 4. Barton JK, Rice PS, Howard CC, et al. Structural and functional assessment of intense therapeutic ultrasound effects on partial Achilles tendon transection. In: Advanced Biomedical and Clinical Diagnostic and Surgical Guidance Systems XVI 2018, San Francisco, United States, January 28-30, 2018. Bellingham, WA: SPIE; 2018. [Google Scholar]
- 5. Bazaz R, Ferkel RD. Results of endoscopic plantar fascia release. Foot Ankle Int. 2007;28(5):549–556. [DOI] [PubMed] [Google Scholar]
- 6. Boyle RA, Slater GL. Endoscopic plantar fascia release: a case series. Foot Ankle Int. 2003;24(2):176–179. [DOI] [PubMed] [Google Scholar]
- 7. Brown JN, Roberts S, Taylor M, Paterson RS. Plantar fascia release through a transverse plantar incision. Foot Ankle Int. 1999;20(6):364–367. [DOI] [PubMed] [Google Scholar]
- 8. Brugh AM, Fallat LM, Savoy-Moore RT. Lateral column symptomatology following plantar fascial release: a prospective study. J Foot Ankle Surg. 2002;41(6):365–371. [DOI] [PubMed] [Google Scholar]
- 9. Buchbinder R, Ptasznik R, Gordon J, Buchanan J, Prabaharan V, Forbes A. Ultrasound-guided extracorporeal shock wave therapy for plantar fasciitis: a randomized controlled trial. JAMA. 2002;288(11):1364–1372. [DOI] [PubMed] [Google Scholar]
- 10. Conflitti JM, Tarquinio TA. Operative outcome of partial plantar fasciectomy and neurolysis to the nerve of the abductor digiti minimi muscle for recalcitrant plantar fasciitis. Foot Ankle Int. 2004;25(7):482–487. [DOI] [PubMed] [Google Scholar]
- 11. Crawford F, Thomson CE. Interventions for treating plantar heel pain. Cochrane Database Syst Rev. 2003;(3):CD000416. [DOI] [PubMed] [Google Scholar]
- 12. Davies MS, Weiss GA, Saxby TS. Plantar fasciitis: how successful is surgical intervention? Foot Ankle Int. 1999;20(12):803–807. [DOI] [PubMed] [Google Scholar]
- 13. DiGiovanni BF, Moore AM, Zlotnicki JP, Pinney SJ. Preferred management of recalcitrant plantar fasciitis among orthopaedic foot and ankle surgeons. Foot Ankle Int. 2012;33(6):507–512. [DOI] [PubMed] [Google Scholar]
- 14. Digiovanni BF, Nawoczenski DA, Malay DP, et al. Plantar fascia-specific stretching exercise improves outcomes in patients with chrnic plantar fasciitis. A prospective clinical trial with two-year follow-up. J Bone Joint Surg Am. 2006;88(8):1775–1781. [DOI] [PubMed] [Google Scholar]
- 15. Donley BG, Moore T, Sferra J, Gozdanovic J, Smith R. The efficacy of oral nonsteroidal anti-inflammatory medication (NSAID) in the treatment of plantar fasciitis: a randomized, prospective, placebo-controlled study. Foot Ankle Int. 2007;28(1):20–23. [DOI] [PubMed] [Google Scholar]
- 16. Elizondo-Rodriguez J, Araujo-Lopez Y, Moreno-Gonzalez JA, Cardenas-Estrada E, Mendoza-Lemus O, Acosta-Olivo C. A comparison of botulinum toxin A and intralesional steroids for the treatment of plantar fasciitis: a randomized, double-blinded study. Foot Ankle Int. 2013;34(1):8–14. [DOI] [PubMed] [Google Scholar]
- 17. Ficke B, Elattar O, Naranje SM, Araoye I, Shah AB. Gastrocnemius recession for recalcitrant plantar fasciitis in overweight and obese patients. Foot Ankle Surg. 2018;24(6):471–473. [DOI] [PubMed] [Google Scholar]
- 18. Gamba C, Sala-Pujals A, Perez-Prieto D, et al. Relationship of plantar fascia thickness and preoperative pain, function, and quality of life in recalcitrant plantar fasciitis. Foot Ankle Int. 2018;39(8):930–934. [DOI] [PubMed] [Google Scholar]
- 19. Geppert MJ, Mizel MS. Management of heel pain in the inflammatory arthritides. Clin Orthop Relat Res. 1998;349:93–99. [DOI] [PubMed] [Google Scholar]
- 20. Gibbon WW, Long G. Ultrasound of the plantar aponeurosis (fascia). Skeletal Radiol. 1999;28(1):21–6. [DOI] [PubMed] [Google Scholar]
- 21. Gill LH, Kiebzak GM. Outcome of nonsurgical treatment for plantar fasciitis. Foot Ankle Int. 1996;17(9):527–532. [DOI] [PubMed] [Google Scholar]
- 22. Harris RC, 3rd, Strannigan KL, Piraino J. Comparison of the complication incidence in open versus endoscopic gastrocnemius recession: a retrospective medical record review. J Foot Ankle Surg. 2018;57(4):747–752. [DOI] [PubMed] [Google Scholar]
- 23. Hedrick MR. The plantar aponeurosis. Foot Ankle Int. 1996;17(10):646–649. [DOI] [PubMed] [Google Scholar]
- 24. Jain SK, Suprashant K, Kumar S, Yadav A, Kearns SR. Comparison of plantar fasciitis injected with platelet-rich plasma vs corticosteroids. Foot Ankle Int. 2018;39(7):780–786. [DOI] [PubMed] [Google Scholar]
- 25. Jerosch J, Schunck J, Liebsch D, Filler T. Indication, surgical technique and results of endoscopic fascial release in plantar fasciitis (E FRPF). Knee Surg Sports Traumatol Arthrosc. 2004;12(5):471–477. [DOI] [PubMed] [Google Scholar]
- 26. Kane D, Greaney T, Shanahan M, et al. The role of ultrasonography in the diagnosis and management of idiopathic plantar fasciitis. Rheumatology (Oxford). 2001;40(9):1002–1008. [DOI] [PubMed] [Google Scholar]
- 27. Kogler GF, Veer FB, Verhulst SJ, Solomonidis SE, Paul JP. The effect of heel elevation on strain within the plantar aponeurosis: in vitro study. Foot Ankle Int. 2001;22(5):433–439. [DOI] [PubMed] [Google Scholar]
- 28. Lareau CR, Sawyer GA, Wang JH, DiGiovanni CW. Plantar and medial heel pain: diagnosis and management. J Am Acad Orthop Surg. 2014;22(6):372–380. [DOI] [PubMed] [Google Scholar]
- 29. Lee HS, Choi YR, Kim SW, Lee JY, Seo JH, Jeong JJ. Risk factors affecting chronic rupture of the plantar fascia. Foot Ankle Int. 2014;35(3):258–263. [DOI] [PubMed] [Google Scholar]
- 30. Levy JC, Mizel MS, Clifford PD, Temple HT. Value of radiographs in the initial evaluation of nontraumatic adult heel pain. Foot Ankle Int. 2006;27(6):427–430. [DOI] [PubMed] [Google Scholar]
- 31. Mahowald S, Legge BS, Grady JF. The correlation between plantar fascia thickness and symptoms of plantar fasciitis. J Am Podiatr Med Assoc. 2011;101(5):385–389. [DOI] [PubMed] [Google Scholar]
- 32. Martin RL, Davenport TE, Reischl SF, et al. Heel pain-plantar fasciitis: revision 2014. J Orthop Sports Phys Ther. 2014;44(11):A1–A33. [DOI] [PubMed] [Google Scholar]
- 33. Maskill JD, Bohay DR, Anderson JG. Gastrocnemius recession to treat isolated foot pain. Foot Ankle Int. 2010;31(1):19–23. [DOI] [PubMed] [Google Scholar]
- 34. Miller LE, Latt DL. Chronic plantar fasciitis is mediated by local hemodynamics: implications for emerging therapies. North Am J Med Sci. 2015;7:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Monteagudo M, Maceira E, Garcia-Virto V, Canosa R. Chronic plantar fasciitis: plantar fasciotomy versus gastrocnemius recession. Int Orthop. 2013;37(9):1845–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Monto RR. Platelet-rich plasma efficacy versus corticosteroid injection treatment for chronic severe plantar fasciitis. Foot Ankle Int. 2014;35(4):313–318. [DOI] [PubMed] [Google Scholar]
- 37. Mook WR, Gay T, Parekh SG. Extensile decompression of the proximal and distal tarsal tunnel combined with partial plantar fascia release in the treatment of chronic plantar heel pain. Foot Ankle Spec. 2013;6(1):27–35. [DOI] [PubMed] [Google Scholar]
- 38. Morton TN, Zimmerman JP, Lee M, Schaber JD. A review of 105 consecutive uniport endoscopic plantar fascial release procedures for the treatment of chronic plantar fasciitis. J Foot Ankle Surg. 2013;52(1):48–52. [DOI] [PubMed] [Google Scholar]
- 39. Neufeld SK, Cerrato R. Plantar fasciitis: evaluation and treatment. J Am Acad Orthop Surg 2008;16(6):338–346. [DOI] [PubMed] [Google Scholar]
- 40. Pfeffer G, Bacchetti P, Deland J, et al. Comparison of custom and prefabricated orthoses in the initial treatment of proximal plantar fasciitis. Foot Ankle Int. 1999;20(4):214–221. [DOI] [PubMed] [Google Scholar]
- 41. Powell M, Post WR, Keener J, Wearden S. Effective treatment of chronic plantar fasciitis with dorsiflexion night splints: a crossover prospective randomized outcome study. Foot Ankle Int. 1998;19(1):10–18. [DOI] [PubMed] [Google Scholar]
- 42. Riddle DL, Pulisic M, Pidcoe P, Johnson RE. Risk factors for plantar fasciitis: a matched case-control study. J Bone Joint Surg Am. 2003;85(5):872–877. [DOI] [PubMed] [Google Scholar]
- 43. Rizk AS, Kandel WA, Tabl EAE, Kandil MI. Mid-sole release of the plantar fascia combined with percutaneous drilling of the calcaneus for treatment of resistant heel pain. Foot Ankle Int. 2017;38(11):1271–1277. [DOI] [PubMed] [Google Scholar]
- 44. Sammarco GJ, Helfrey RB. Surgical treatment of recalcitrant plantar fasciitis. Foot Ankle Int. 1996;17(9):520–526. [DOI] [PubMed] [Google Scholar]
- 45. Slayton MH, Baravarian B, Amodei RC, et al. Intense therapeutic ultrasound for pain relief in the treatment for chronic plantar fasciopathy. Foot Ankle Orthop. 2019;4(3). doi:10.1177/2473011419862228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tountas AA, Fornasier VL. Operative treatment of subcalcaneal pain. Clin Orthop Relat Res. 1996;332:170–178. [DOI] [PubMed] [Google Scholar]
- 47. Wapner KL, Sharkey PF. The use of night splints for treatment of recalcitrant plantar fasciitis. Foot Ankle. 1991;12(3):135–137. [DOI] [PubMed] [Google Scholar]
- 48. Woelffer KE, Figura MA, Sandberg NS, Snyder NS. Five-year follow-up results of instep plantar fasciotomy for chronic heel pain. J Foot Ankle Surg. 2000;39(4):218–223. [DOI] [PubMed] [Google Scholar]
- 49. Zhang J, Nie D, Rocha JL, Hogan MV, Wang JH-C. Characterization of the structure, cells, and cellular mechanobiological response of human plantar fascia. J Tissue Eng. 2018;9:2041731418801103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, FAO896763-ICMJE for Evaluation and Treatment of Chronic Plantar Fasciitis by L. Daniel Latt, David Eric Jaffe, Yunting Tang and Mihra S. Taljanovic in Foot & Ankle Orthopaedics