Abstract
Context:
The coronavirus disease 2019 (COVID-19) is a viral respiratory illness which was first detected in Wuhan, Hubei Province, China. A few case studies demonstrated that 14–53% of the cases of COVID-19 reported abnormal levels of liver enzymes during disease progression. Patients with severe COVID-19 seem to have higher rates of hepatic dysfunction.
Aims:
Our aim was to investigate the changes in the liver function test in COVID-19 patients admitted to hospital and its association with the severity of the disease, length of hospital stay, and outcome of patients.
Settings and Design:
This was a cross-sectional study involving 678 COVID-19 patients, who were admitted at AIIMS, Bhopal.
Methods and Material:
The case records of 678 patients were evaluated by the research team from the Department of Medicine, AIIMS, Bhopal, and data were analyzed. All laboratory data were obtained. The liver function tests (LFT) including alanine aminotransferase, aspartate aminotransferase, and total bilirubin values were recorded, and liver dysfunction defined as any parameter having more than the upper limit of normal value.
Results:
From April 2020 to September 2020, a total of 678 COVID-19 patients were screened, and 600 were assessed for eligibility; 78 were excluded due to either significant alcohol history or due to prior liver disease. Among the 600 patients, 265 patients (44.16%) had liver dysfunction while 335 patients (55.83%) had a normal liver function. The patients having a severe disease were more affected by liver dysfunction than the mild disease patients. The average hospital stay was more in those patients having liver dysfunction than in those patients with normal liver function. Among the patients with normal LFT on admission, 97.9% got cured while 2.1% died. Among the patients with liver dysfunction, 80.4% got cured and 19.6% died.
Conclusions:
Hepatic injury is common in severe COVID-19 patients, which may be caused by direct injury to the bile duct cells by a virus or indirectly by a cytokine storm. The liver function should be evaluated in all symptomatic COVID-19 patients. In patients with pre-existing liver diseases, special attention should be paid to monitoring and treatment.
Keywords: COVID-19, liver injury, morbidity
Introduction
The coronavirus disease 2019 (COVID-19) is a viral respiratory illness which was first detected in Wuhan, Hubei Province, China. This disease has spread worldwide since then, and in March 2020, it was declared a pandemic by WHO. Nearly 6 months after the peak of the first wave of COVID-19 in September 2020, COVID-19 cases in India once again started rising from the first week of March leading to a deadlier second wave of COVID-19 in the country. As of June 11, 2021, more than 29 million cases have been confirmed in India. More than 300,000 deaths have been attributed to COVID-19 in India, making it one of the deadliest pandemics in history.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes primarily respiratory illness but can lead to multiorgan failure and death. The progression to severe COVID-19 is particularly more in 11 elderly and immune-compromised individuals. It is a beta-coronavirus belonging to the same subgenus as the virus causing severe acute respiratory syndrome and Middle East respiratory syndrome. The symptoms appear within 11.5 days in over 95% of the infected persons with a median incubation period of about 5.5 days.[1] This disease can cause venous and arterial thrombosis which can lead to stroke, myocardial infarction as well as pulmonary embolism. Severe COVID-19 has been associated with a systemic reaction characterized by raised serum ferritin and hyper-inflammation due to cytokines storm which eventually leads to multiorgan failure.[2] The angiotensin-converting enzyme 2 (ACE-2) receptor is found to be used by the SARS-CoV-2 for cellular entry. The latest study has shown that ACE-2 receptors are highly expressed in type II alveolar epithelial cells and bile duct cells.[3] Huang C, et al.[4] studied the liver biopsy specimen of COVID-19 patients and found moderate microvascular steatosis and mild lobular inflammation. These observations suggest that SARS-CoV-2 may cause hepatic injury.
A few case studies indicated that 14–53% of the cases of COVID-19 reported derangement of liver enzymes during disease progression. Patients with severe COVID-19 had higher rates of hepatic dysfunction.[5,6,7,8,9]
Our aim was to investigate the changes in the liver function test in COVID-19 patients admitted to hospital and its association with the severity of the disease, length of hospital stay, and outcome of patients.
Subjects and Methods
Study design
It was a cross-sectional study involving 678 COVID-19 patients, who were admitted at AIIMS, Bhopal.
Subjects who met the following criteria were included in the study:
Male or non-pregnant, non-lactating female aged ≥18 and ≤75 years (both inclusive)
Reverse transcription–polymerase chain reaction (RT-PCR) confirmed diagnosis of COVID-19
The following subjects were excluded from the study:
Asymptomatic patients
Patients with pre-existing liver diseases like cirrhosis, positive hepatitis B surface antigen, positive antibody against hepatitis C virus, patients using/have used hepatotoxic drugs in the last 6 months, men who consume >140 g and women who consume >70 g of alcohol per week
Pregnant or lactating females
The cases were classified as mild COVID-19 if they had minor symptoms and no pneumonia in imaging. Moderate COVID-19 patients had a fever, respiratory tract symptoms, and imaging showing pneumonia, but no respiratory distress. The patients were labeled as severe COVID-19 if they had (a) respiratory distress, respiratory rate ≥30 breaths/min; (b) means oxygen saturation ≤93% at rest; (c) arterial blood oxygen partial pressure/oxygen concentration ≤300 mmHg; (d) radiological imaging showing more than 50% lung involvement.
Study methodology
The case records of 678 patients were evaluated by the research team from the Department of Medicine, AIIMS, Bhopal, and data were analyzed. All laboratory data were obtained. Liver function tests including alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total bilirubin values were recorded and liver dysfunction was defined as any parameter having more than the upper limit of normal value. Categorical variables were recorded as percentages and frequency. The continuous variables were recorded as mean and standard deviation. Student's t-test or Mann– Whitney test was applied on continuous variables. For categorical variables, the Chi-square test and Fisher's exact tests were used. If the P value was < 0.05, then the difference between various parameters was considered statistically significant. All data were analyzed with the software SPSS 26.0. Ethical clearance was obtained from the Institutional Ethical Committee before the start of the study (IHEC-LOP/2020/PG/Jan/10).
Results
From April 2020 to September 2020, a total of 678 COVID-19 patients were screened, and 600 were assessed for eligibility; 78 were excluded due to either significant alcohol history or due to prior liver disease [Figure 1]. Among the 600 patients, 412 were males while 188 were females [Figure 2].
Figure 1.
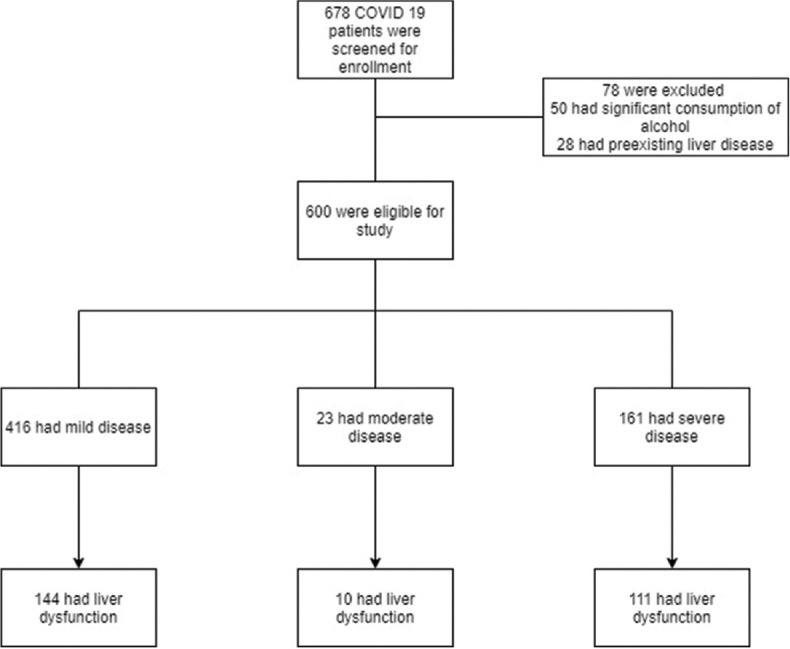
Flow diagram illustrating COVID-19 patients enrolled in the study
Figure 2.
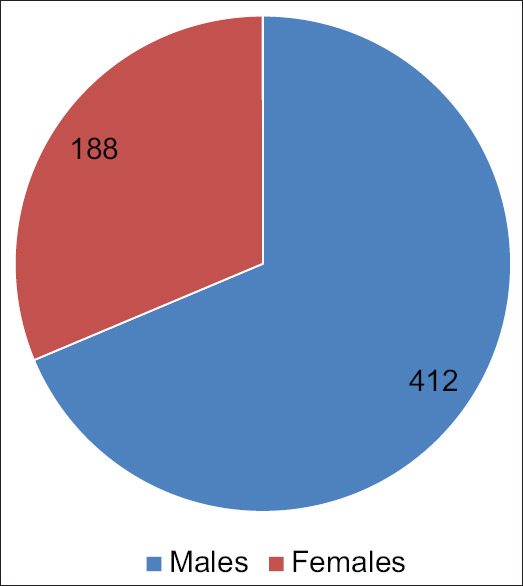
Gender distribution of patients
Among the 600 patients, 265 patients had liver dysfunction while 335 patients had a normal liver function. The male patients (50.2%) had more liver dysfunction than the female patients (30.9%) (P value < 0.01). Among the 600 patients, 416 patients had mild disease, 23 patients had moderate disease, and 161 patients had severe disease [Table 1 and Figure 3]. The patients having severe disease had more liver dysfunction than the mild disease patients (68.8% vs. 34.6%, P value < 0.01) [Figure 4]. The average hospital stay was more in the patients having liver dysfunction than the patients with normal liver function (13 ± 10 vs. 8 ± 2, P value < 0.01). Among the patients with normal LFTs on admission, 97.9% got cured while 2.1% died. For those with abnormal LFTs, there was a cure in 80.4% and 19.6% died. There is a statistically significant difference between the outcome in the two groups with P value of < 0.01.
Table 1.
Outcome summary table
| Abnormal LFTs | Normal LFTs | P | |
|---|---|---|---|
| Total Number of Patients (n) | 265 (44.2%) | 335 (55.8%) | |
| Number of Males | 207 (50.2%) | 205 (49.8%) | |
| Number of Females | 58 (30.9%) | 130 (69.1%) | |
| Disease Severity | |||
| Mild Disease | 144 (34.6%) | 272 (65.4%) | |
| Moderate Disease | 10 (43.5%) | 13 (56.5%) | |
| Severe Disease | 111 (68.9%) | 50 (31.1%) | <0.01 |
| Average Hospital Stay in Days [Mean (SD)] | 13 (10) | 8 (2) | <0.01 |
| Mortality | 52 (19.6%) | 7 (2.1%) | <0.01 |
Figure 3.
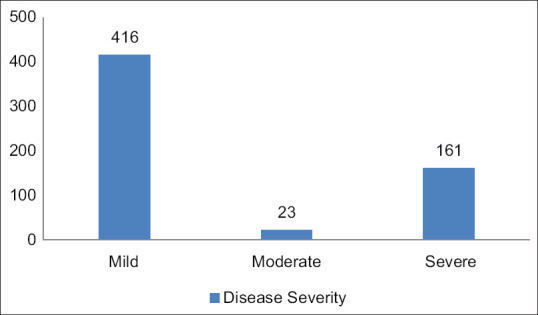
Classification of patients on the basis of disease severity
Figure 4.
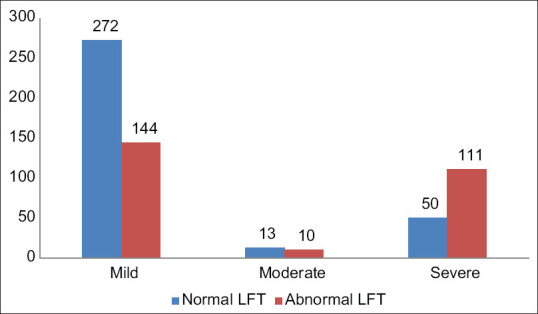
Number of patients with abnormal LFTs in different categories of COVID-19
Discussion
Our study has shown that 44.2% of COVID-19 patients had liver dysfunction and it has increased to 68.9% in severe cases. The males were affected more than the females. Several studies have shown that the male gender, age more than 60, presence of comorbidities (diabetes, hypertension, and cardiovascular illness), and secondary acute respiratory distress syndrome were associated with a poor prognosis in COVID-19 patients.[10,6] Cai Q, et al. have shown that there was a significant increase in serum bilirubin, liver enzymes, and other liver-related parameters in severe COVID-19 patients. They also demonstrated that gradually the liver parameters became normal with the recovery of patients.[11] In a recent systematic review and meta-analysis, it has been shown that 33.3% of the COVID-19 cases had elevated AST and 24.1% had elevated ALT.[12] A correlation between the severity of liver dysfunction and the severity of COVID-19 has also been reported by other investigators.[13,14,15] In a study, one COVID-19 patient had severe hepatitis with liver enzymes more than 200 times than normal.[5] However, in our study, none of the patients had liver enzymes more than four times the normal value. In a study done in Shanghai, 50.7% of the total patients in the study had deranged LFT at the time of hospitalization. Interestingly, a large number of these patients had severe symptoms when compared with the patients with normal LFT (44% vs. 27.4%; P = 0.035).[10] Only a few patients with mild symptoms experienced abnormal LFT values. In a systematic review and meta-analysis by Xin Zhao,[16] a total of 57 review articles from China were included. They have studied 9,889 confirmed cases of COVID-19. The results showed that in patients with mild COVID-19, the incidence of liver dysfunction was 24.7% (95% CI, 23.4–26.4%). The liver dysfunction was found in a larger number of severe patients as compared to the mild patients (risk ratio 2.07 [95% CI, 1.77–2.43]). Further analysis showed that as the severity of COVID-19 increases, the level of AST, ALT, total bilirubin, glutamyl transpeptidase (GGT), and alkaline phosphatase (ALP) increases and the level of albumin reduces. These studies support the concept that the degree of liver injury also increases in proportion to the severity of COVID-19. Thus, patients with severe coronavirus infection or pre-existing liver conditions should undergo surveillance and therapy should be individually tailored to avoid initiation or progression of hepatic injury.
In a majority of clinical studies, regardless of pre-existing chronic liver disease, COVID-19 was associated with mild-to-moderate liver failure. Many patients had raised transaminase levels, raised GGT and ALP levels (less frequently), low protein levels, and prolongation of prothrombin time.[17,18] Deranged transaminases, especially in men, lead to a severe course of disease compared to others. Moreover, hypoalbuminemia has been identified as an independent marker of severe COVID-19, with a poor prognosis and higher chances of mortality.[19,20,21,22] Baroiu L, et al.[23] Studied 849 patients of COVID-19 treatment and found that 388 patients had abnormal liver enzymes. They also observed that patients with liver dysfunction were older, had comorbidities, and had a more severe course.
In the current pandemic, there is a need to reduce travel and limit visits to specialists. To facilitate decentralized care for these patients, primary care physicians should manage mild cases of liver injury in COVID-19 patients. Primary care physicians should stratify patients according to the severity. Mild liver dysfunction in COVID-19 is often temporary and returns to normal without special treatment. However, when a severe liver injury occurs, these patients should be referred to a hepatologist.
Conclusion
Hepatic injury is frequent in patients with SARS-CoV-2 infection, which may be due to viral infection of the bile duct cells or cytokine storms. In the absence of any definite treatment and prophylactic measures, hepatic injury in COVID-19 is expected to persist. This necessitates the preparedness of health organizations to respond to the possible increase in liver disorders during and after the pandemic. Liver function should be evaluated in all symptomatic COVID-19 patients. In patients with pre-existing liver diseases, special attention should be paid to monitoring and treatment.
Key Message
Abnormal hepatic enzymes are found in about one-third of the patients with symptomatic COVID-19. Marked derangement of liver function may increase the risk of multiorgan dysfunction, prolong the duration of hospitalization, and worsen the prognosis. Abnormal liver function test results may be used as a predictor of the severity of COVID-19.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng Q, Meradith HR, et al. The incubation period of Coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: Estimation and application. Ann Intern Med. 2020;172:577–82. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colafrancesco S, Alessandri C, Conti F, Priori R. COVID-19 gone bad: A new character in the spectrum of the hyperferritinemic syndrome? Autoimmun Rev. 2020;19(Suppl 7):102573. doi: 10.1016/j.autrev.2020.102573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. bioRxiv. 2020. Available at: https://doi.org/10.1101/2020.020.03.931766 .
- 4.Huang C, Wang Y, Li X, Lili R, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395:507–13. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–9. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: A descriptive study. Lancet Infect Dis. 2020;4:425–34. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu X-W, Wu X-X, Jiang X-G, Xu K-J, Ying L-J, Ma C-L, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: Retrospective case series. BMJ. 2020;2020:368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–81. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan Z, Chen L, Jun LI, Cheng X, Yang J, Tian C, et al. Clinical features of COVID-19-related liver damage. Clin Gastroenterol Hepatol. 2020;18:1561–6. doi: 10.1016/j.cgh.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai Q, Huang D, Ou P, Yu H, Zhu Z, Xia Z, et al. COVID-19 in a designated infectious diseases Hospital Outside Hubei Province, China. Allergy. 2020;75:1742–52. doi: 10.1111/all.14309. [DOI] [PubMed] [Google Scholar]
- 12.Wang JT, Sheng WH, Fang CT, Chen YC, Wang JL, Yu CJ, et al. Clinical manifestations, laboratory findings, and treatment outcomes of SARS patients. Emerg Infect Dis. 2004;10:818–24. doi: 10.3201/eid1005.030640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang C, Shi L, Wang FS. Liver injury in COVID-19: Management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428–30. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li L, Li S, Xu M, Yu P, Zheng S, Duan Z, et al. Risk factors related to hepatic injury in patients with corona virus disease 2019. medRxiv. 2020 https://doi.org/10.1101/2020.02.28.20028514. [Google Scholar]
- 15.Guan W-J, Ni Z-Y, Hu Y, Liang W-H, Ou C-Q, He J-X, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao X, Lei Z, Gao F, Xie Q, Jang K, Gong J. The impact of coronavirus disease 2019 (COVID-19) on liver injury in China: A systematic review and meta-analysis. Medicine (Baltimore) 2021;100:e24369. doi: 10.1097/MD.0000000000024369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deidda S, Tora L, Firinu D, Del Giacco S, Campagna M, Meloni F, et al. Gastrointestinal coronavirus disease 2019: Epidemiology, clinical features, pathogenesis, prevention, and management. Expert Rev Gastroenterol Hepatol. 2021;15:41–50. doi: 10.1080/17474124.2020.1821653. [DOI] [PubMed] [Google Scholar]
- 18.Ghoda A, Ghoda M. Liver injury in COVID-19 infection: A systematic review. Cureus. 2020;12:e9487. doi: 10.7759/cureus.9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu W, Tao ZW, Wang L, Yuan ML, Liu K, Zhou L, et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J (Engl) 2020;133:1032–8. doi: 10.1097/CM9.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trevenzoli M, Guarnaccia A, Alberici I, Fassan M, Di Meco E, Farinati F, et al. SARS-CoV-2 and hepatitis. J Gastrointestin Liver Dis. 2020;29:473–5. doi: 10.15403/jgld-2747. [DOI] [PubMed] [Google Scholar]
- 21.Gholizadeh P, Safari R, Marofi P, Zeinalzadeh E, Pagliano P, Ganbarov K, et al. Alteration of liver biomarkers in patients with SARS-CoV-2 (COVID-19) J Inflamm Res. 2020;13:285–92. doi: 10.2147/JIR.S257078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cichoż-Lach H, Michalak A. Liver injury in the era of COVID-19. World J Gastroenterol. 2021;27:377–90. doi: 10.3748/wjg.v27.i5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baroiu L, Dumitru C, Iancu A, Leşe AC, Drăgănescu M, Baroiu N, et al. COVID-19 impact on the liver. World J Clin Cases. 2021;9:3814–25. doi: 10.12998/wjcc.v9.i16.3814. [DOI] [PMC free article] [PubMed] [Google Scholar]


