Abstract
Purpose:
To characterize the impact of slowed processing speed on the efficiency of broader cognitive function in temporal lobe epilepsy (TLE).
Methods:
Participants included 100 patients with TLE and 89 healthy controls (mean ages 36.8 and 33.6, respectively) administered a neuropsychological battery consisting of 15 cognitive metrics. Confirmatory factor analysis using structural equation modeling (SEM) latent variable modeling demonstrated a cognitive structure representing the domains of verbal intelligence, immediate memory, delayed memory, executive function, working memory, and processing speed. Furthermore, the latent variable measurement model determined the direct and indirect relationships of verbal intelligence and processing speed with immediate memory, delayed memory, executive function, and working memory.
Results:
Following SEM of hypothesized structural models, the results demonstrated that, among controls, intelligence had a direct and unmediated (by processing speed) relationship with all identified cognitive domains. In contrast, among participants with TLE, processing speed mediated the relationship between verbal intelligence and performance across all cognitive domains.
Conclusion:
Slowing of cognitive/psychomotor processing speed appears to play a critical mediating role in the broader cognitive status of participants with TLE and may serve as a target through which to attempt to exert a broad positive impact on neuropsychological status.
Keywords: Temporal lobe epilepsy, Cognition, Processing speed, Neuropsychology, Structural equation modelling, Intelligence
1. Introduction
Cognitive impairment is a major comorbidity of the epilepsies and an understanding of its etiology has been a classic neuropsychological pursuit [1–8]. Throughout this literature a core focus has been the cognitive consequences associated with the fundamental features of the epilepsies; that is, those clinical factors related to the cause, course, characteristics, and treatment of epilepsy in developing, mature, and aging patients [9].
Much less examined as contributors of specific cognitive impairments are abnormalities in other cognitive domains upon which successful performance may depend. For example, abnormalities in executive function can adversely impact learning and memory performance [10,11] as may anomalies in language [12]. Aspects of executive dysfunction including working memory [13] and attentional vigilance [14] can adversely impact academic performance. Problems in motor development are associated with reading problems in Rolandic epilepsy [15] and executive function mediates the effect of antiseizure medication load on intelligence [16].
In this context, very understudied is the potential impact of slowed cognitive and/or psychomotor processing speed (PS) on the efficiency of other cognitive processes. Slowed PS is known to be prevalent in children and adults with epilepsy and, while known to be related to medication treatment [17–20], slowed PS has been reported in drug naïve new-onset pediatric and adult patients with epilepsy [21–23] implicating the impact of other intrinsic causative factors. The course of PS can be problematic in that it is among the most prominent cognitive declines over the progressive course of epilepsy [24,25]. Interestingly, slowing of PS can remain evident even in those whose epilepsy remits spontaneously or following treatments such as epilepsy surgery [26–28]. More generally, prospective studies of youth with new-onset epilepsies have shown that slowing of PS at baseline predicts an increased risk of behavior problems 3 years later [29] and is also associated with abnormal development of large-scale neural networks over a two-year course [30]. Application of machine learning analytics has shown slowed PS to be the cognitive ability with the most power to discriminate patients with epilepsy from controls with underlying associated anomalies in brain structure and connectivity [31]. Given the presence, course, persistence, neurobiology, and predictive significance of slowed PS, it is surprising that the degree to which it impacts performance across other critical cognitive domains such as memory or executive function has been largely uninvestigated. Addressing this gap represents the primary focus of this investigation.
This task, however, requires broader consideration of the positive relationships that exist across diverse cognitive metrics [32], especially the positive associations of intelligence with multiple specific ability areas [32]. The need to consider the potential influence of intelligence is furthermore highlighted by population, community-based, and clinical studies that have demonstrated a leftward (lower) distributional shift of intelligence compared to controls in both children and adults with epilepsy [33–38]. Indeed, neuropsychological differences between patients with epilepsy and controls are significantly attenuated when intelligence, lower in patients with epilepsy, has been covaried [39,40], raising the question of intelligence-independent findings and the advisability of considering the impact of intelligence in epilepsy neuropsychology research.
To that end, this investigation undertook the following aims. First, the cognitive structure underlying a targeted neuropsychological battery in individuals with chronic TLE and controls was determined via confirmatory factor analysis (i.e., the measurement model). Second, the relationships of PS and intelligence to other specific cognitive domains (immediate and delayed memory, executive function, working memory) were assessed and compared in epilepsy and control groups. Third, tested in both the epilepsy and control groups was whether PS mediated the relationship between intelligence and performance in the target cognitive domains. If slowed PS mediated such relationships, it would highlight the role of this common cognitive complication and suggest a potential target for intervention that could conceivably impact general cognitive status in epilepsy in an overall positive fashion.
2. Material and methods
2.1. Participants and procedures
Participants included 100 individuals with TLE and 89 healthy controls. Initial selection criteria for the participants with epilepsy included: (a) chronological age between 18 and 63 years, (b) Weschler Adult Intelligence Scale-3rd Edition (WAIS-III) IQ > 69, (c) complex partial seizures of definite or probable TLE based on consensus conference review, (d) no MRI abnormalities other than atrophy on clinical review, and (e) no other neurological disorder. Consensus review included all available interictal and/or continuous video/EEG monitoring, clinical semiology, clinical neuroimaging, and developmental and clinical history.
Initial selection criteria for the controls included: (a) chronological age between 18 and 63, (b) WAIS-III Full-Scale IQ (FSIQ) > 69, (c) either a friend, relative, or spouse of the participant with epilepsy, (d) no current substance abuse, or medical or psychiatric condition that could affect cognitive functioning, and (e) no episode of loss of consciousness greater than five minutes, identified developmental learning disorder, or repetition of a grade in school. This project was reviewed and approved by the University of Wisconsin School of Medicine and Public Health Institutional Review Board, and all participants were informed of the nature and purposes of this investigation, their questions were answered, and signed informed consent was obtained.
Table 1 provides information regarding the baseline characteristics of epilepsy and control participants. Notably, participants with epilepsy had a significantly lower FSIQ than controls, although still within the average range (t(180) = 5.94, p < .001).
Table 1.
Means and Standard Deviations of Demographic Variables by Group
| TLE (N = 100) | Controls (N = 82) | t | |
|---|---|---|---|
| Age | 36.8 (11.5) | 33.6 (12.6) | ns |
| Gender | |||
| Male % | 33.3 % | 40.2 % | ns |
| Female % | 66.7 % | 59.8 % | ns |
| Years of Education | 12.97 (2.33) | 13.60 (2.39) | ns |
| Handedness | |||
| Right | 87 % | 87.8 % | ns |
| Left | 11 % | 12.2 % | ns |
| Mixed | 2 % | 0 % | ns |
| Full Scale IQ (FSIQ) | 92.7 (15.98) | 106.4 (14.3) | 6.05** |
| Verbal IQ (VIQ) | 91.5 (14.9) | 103.5 (14.2) | 5.52** |
| Performance IQ (PIQ) | 95.5 (17.0) | 109.6 (14.6) | 5.94** |
| Seizure Characteristics | |||
| Age of First Seizure (Months) | 175.75 (127.52) | ||
| Epilepsy Duration (Months) | 267.53 (144.16) | ||
| Number of AEDs | 1.80 (.72) | ||
| Time Since Most Recent | |||
| Seizure (Days) | 5.86 (16.75) | ||
| Seizure Frequency (Past Year) | |||
| Simple Partial Seizure | |||
| None | 33% | ||
| Daily | 8% | ||
| Weekly | 16% | ||
| Monthly | 24% | ||
| Yearly | 13% | ||
| Unknown | 6% | ||
| Complex Partial Seizure | |||
| None | 18% | ||
| Daily | 1% | ||
| Weekly | 23% | ||
| Monthly | 40% | ||
| Yearly | 12% | ||
| Unknown | 6% | ||
| Secondary Generalized Seizure | |||
| None | 68% | ||
| Daily | 1% | ||
| Weekly | 1% | ||
| Monthly | 7% | ||
| Yearly | 14% | ||
| Unknown | 9% |
Note.
p < .05 (two-tailed).
p<.01 (two-tailed).
Nonsignificant (ns).
2.2. Neuropsychological measures
The focus here was a cognitive battery that included 15 metrics from 8 different tests from the domains of intelligence, immediate and delayed memory, executive function, working memory, and cognitive/psychomotor processing speed (Table 2). These domains were assessed by at least two measures in order to allow for latent variables in subsequent SEM modeling. Supplemental Table 3 provides baseline means and standard deviations of the cognitive scores by group which we have reported in detail previously [41]. In the analyses, we focused on verbal IQ given the speed-based contribution of several performance IQ tests. For the analyses to be described, the test metrics used for controls and patients with TLE were raw scores except for Wechsler-based tests.
Table 2.
Neuropsychological Tests and Domains
| Verbal Intelligence |
| WAIS-III Information |
| WAIS-III Similarities |
| Immediate Memory |
| WMS-III Immediate Memory Index |
| Verbal Selective Reminding Test Total |
| Nonverbal Selective Reminding Test Total |
| Delayed Memory |
| WMS-III General Memory Index |
| Verbal Selective Reminding Test Long-term Recall |
| Nonverbal Selective Reminding Test Long-term Recall |
| Executive Function |
| Trail Making Test – B |
| Wisconsin Card Sorting Test |
| Categories |
| Perseverative Errors |
| Working Memory |
| WAIS-III Digit Span |
| WAIS-III Spatial Span |
| Processing Speed |
| Trail Making Test – A |
| Stroop Test (Color Naming) |
| Grooved Pegboard |
| Dominant |
| Nondominant |
3. Results
3.1. Analytic overview
Descriptive statistics and baseline group comparisons were investigated using IBM SPSS Statistics v26.0. Missing data were addressed using full information maximum likelihood estimation. Structural equation modeling (SEM) was conducted using IBM SPSS Amos v26 to test whether processing speed mediates the relationship between (verbal) IQ and other cognitive domains. Structural equation modeling allows use of latent variable modeling of the cognitive constructs [42] while also reducing measurement error [43]. Latent variables in the current study were based on theoretical domains associated with neuropsychological tests.
3.2. Initial correlations
Supplemental Table 2 provides initial correlations of baseline neuropsychological characteristics of the epilepsy and control participants. As expected, results demonstrate a pattern of broad significant associations across the neuropsychological measures in both groups. In general, the correlations were higher in participants with epilepsy.
3.3. SEM models
3.3.1. Measurement model
An initial measurement model examined the factor structure for the various latent variables/domains. This consisted of a simple confirmatory factor analysis for both control and participants with epilepsy combined. Fit indices provided by SEM were used to determine model fit. An acceptable fit is reflected in a nonsignificant chi-square (χ2), root mean square error of approximation (RMSEA) ≤0.05, comparative fit index (CFI) ≥0.95, and Tucker-Lewis Index (TLI) ≥0.95 [44,45].
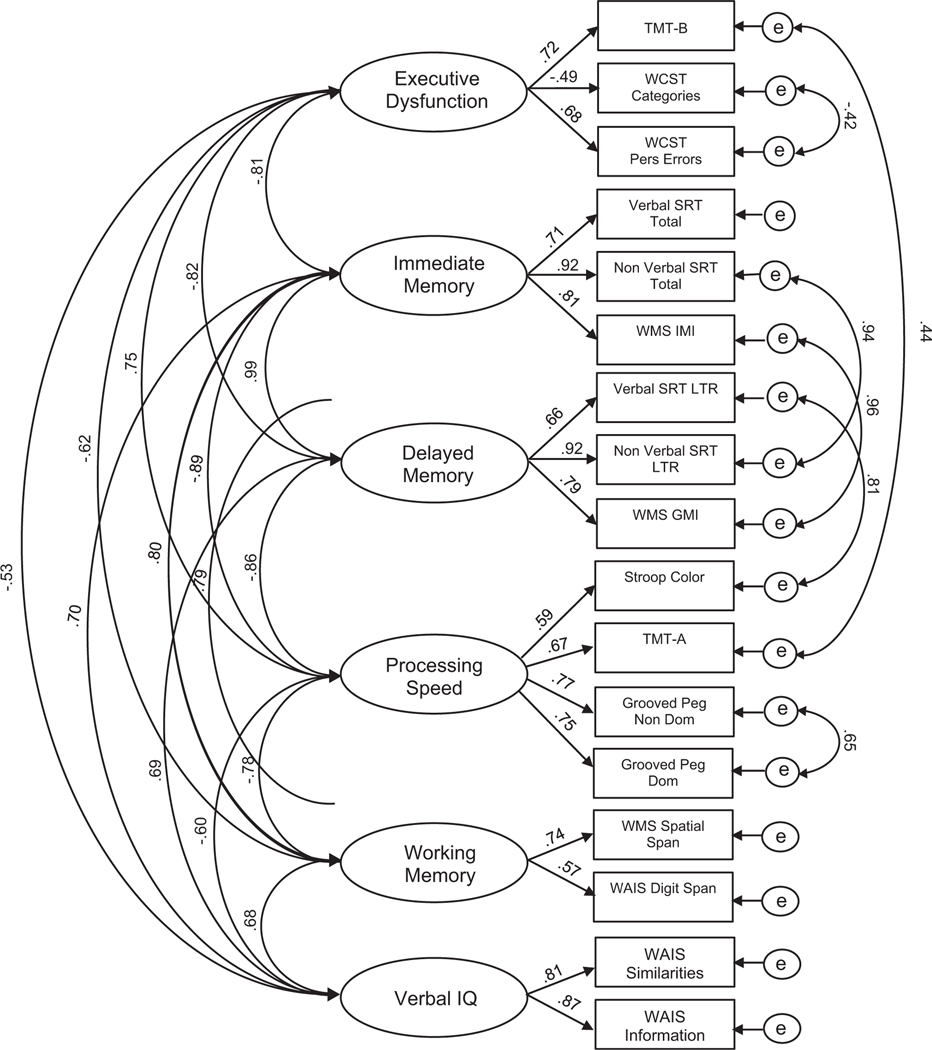
The measurement model (Fig. 1) delineates the estimation of the 6 latent variables from the administered tests. All cognitive domains included at least 2 tests within each latent variable including: Verbal IQ (WAIS-III Information, WAIS-III Similarities); Executive Function (Trail Making Test – B (TMT-B), WCST categories, WCST perseverations); Immediate Memory (Verbal Selective Reminding Test (SRT) total recall, nonverbal SRT total recall, Weschler Memory Scale – 3rd Edition (WMS-III) immediate memory index); Delayed Memory (Verbal and Nonverbal SRT long-term retrieval, WMS-III general memory index); Processing Speed (Stroop-Color, Trail Making Test – A (TMT-A), Grooved Pegboard dominant hand and nondominant hand); and Working Memory (WMS-III spatial span total, WAIS-III total digit span). Correlated errors were derived from theory and reflected similar constructs or measures across domains. Tests measuring different cognitive function domains, but were administered in close succession or as part of the same subtest (e.g., TMT-A and TMT-B), likely have shared error variance; therefore, errors were correlated, which also improved model fit.
Fig. 1.
Standardized estimates for the initial measurement model.
Fit indices for the measurement model were generally strong [χ2 (98, N = 182) = 141.64, p = .003; CFI = 0.98; TLI = 0.98; RMSEA = 0.05 (CI: 0.03–0.07); AIC: 285.64]. The statistically significant χ2 value was not surprising given the sensitivity of the test; however, all other incremental and absolute fit indices (RMSEA, CFI, TLI) indicated good fit, and therefore, the measurement of these underlying constructs was acceptable. For clarity, it should be noted that factor loadings indicated that in this model and subsequent models, Executive Function actually reflected Executive Dysfunction (i.e., higher scores on this latent variable reflected lower executive functioning skills).
To ensure that memory was captured accurately, an additional measurement model was considered that replaced Immediate and Delayed Memory latent variables with Verbal Memory (WMS-III auditory immediate and delayed memory indices, Verbal SRT total recall and retrieval) and Visual Memory (WMS-III immediate and delayed memory indices, Nonverbal SRT total recall and retrieval). Although fit indices for the model were also generally strong [χ2 (130, N = 182) = 201.09, p < .001; CFI = 0.98; TLI = 0.97; RMSEA = 0.06 (CI: 0.04–0.07); AIC: 359.09], the model fit was worse than the previous model. Therefore, the initial measurement model was used for further structural model analyses (Fig. 1).
3.3.2. Initial structural models
The structural model examines the effects of Verbal IQ on other cognitive domains, directly and indirectly (through PS) for both controls and the TLE group. The initial model was tested as a multigroup analysis (TLE and control), but with all parameters free to vary between groups. Notably, the abnormalities for Immediate Memory and Delayed Memory were allowed to correlate. While these are measured as separate cognitive domains, they are strongly related theoretically, and in fact, the model fit significantly declined when they were not allowed to be correlated in the structural model. Fit indices for this initial structural model suggested good fit [χ2 (206) = 253.83, p = .01; CFI = 0.98; TLI = 0.97; RMSEA = 0.04 (CI: 0.02–0.05); AIC: 521.83]. For initial structural model total, direct, and indirect effects by group, see Supplemental Tables 4, 5, and 6.
Our primary interest was in the mediated effect of PS on other domains of cognitive function. For initial structural model standardized direct effects of Verbal IQ on the various cognitive domains by group, see Supplemental Table 5. For controls (see Supplemental Fig. 1), for every one standard deviation (SD) increase in Verbal IQ, Executive Dysfunction decreased by 0.28 SDs (indicating better executive function); Immediate Memory increased by 0.57 SDs; and Delayed Memory increased by 0.58 SDs.
For participants with epilepsy (see Supplemental Fig. 2), Verbal IQ only had a significant effect on PS. For every one SD increase in Verbal IQ, PS decreased by 0.67 SDs (indicating better processing speed).
To determine whether PS mediated the effect of Verbal IQ on other domains of cognitive function, the indirect effect of Verbal IQ through PS was examined. For controls, while PS appeared to have strong effects on Executive Dysfunction (0.50), Immediate Memory (−0.33), Delayed Memory (−0.26), and Working Memory (−0.35), Verbal IQ did not have a significant direct effect on PS (−0.09). Consequently, Verbal IQ had minimal indirect effects on Executive Dysfunction (−0.05), Immediate Memory (0.03), Delayed Memory (0.02), and Working Memory (0.03). Poor PS does affect other cognitive functions; however, it is not a mediator of Verbal IQ for controls.
For the TLE group Verbal IQ did, however, have a significant direct effect on PS (−0.67), with better processing speed seen for those with higher Verbal IQ. Furthermore, PS had strong effects on Executive Dysfunction (0.79), Immediate Memory (−0.87), Delayed Memory (−0.84), and Working Memory (−0.59). In other words, PS had positive effects on domains of cognitive functioning. As a result, Verbal IQ had moderate-to-large indirect effects on Executive Dysfunction (−0.52), Immediate Memory (0.58), Delayed Memory (0.56), and Working Memory (0.39). This suggests that PS mediates the effects of Verbal IQ on other domains of cognitive function for participants with epilepsy. Importantly, these findings suggest that the mediated relationship between Verbal IQ and all other domains of cognitive functioning may be present only in the TLE group. As shown in Supplemental Table 3, the TLE group has lower PS in general compared to controls and as a result, the TLE groups are more likely to experience negative impacts on cognitive performance due to slowed processing speed compared to controls.
3.3.3. Subsequent structural models
To better test the equivalence of this path across both groups, follow-up analyses were conducted for three subsequent structural models (See Table 3) where constraints were added subsequently to each model. Constraints specify whether the factor loadings and/or effects are the same across both groups. First, to characterize the nature of the mediation, all paths between latent variables were constrained for both groups, except for the path between Verbal IQ and PS. Next, all paths between latent variables were constrained, including the path between Verbal IQ and PS. Lastly, to make the model more parsimonious, all paths between latent variables and factor loadings were constrained to represent a fully constrained model. The Akaike Information Criterion (AIC) was estimated and reported in addition to a χ2 difference test, and ΔAIC was used to compare competing models [42,46].
Table 3.
Fit indices for structural models
| Model | χ 2 | df | Δχ2 | Δdf | p | AIC | ΔAIC | TLI | CFI | RMSEA (90% CI) |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| 1. Initial Structural | 253.83 | 206 | 0.01* | 521.83 | 0.97 | 0.98 | 0.04 (0.02–0.05) |
|||
| 2. Partially Constrained | 260.00 | 214 | 6.17 | 8 | 0.63 | 512.00 | 9.83 | 0.97 | 0.98 | 0.04 (0.02–0.05) |
| 3. Full Factor Constrained | 284.14 | 215 | 24.14 | 1 | 0.00* | 534.14 | 22.14 | 0.96 | 0.97 | 0.04 (0.03–0.06) |
| 4. All Constrained Paths | 317.52 | 226 | 33.38 | 11 | 0.00* | 545.42 | 11.28 | 0.95 | 0.96 | 0.05 (0.04–0.06) |
Note.
p < .05.
The initial structural model provided evidence suggesting PS may mediate the relationship between Verbal IQ and all other cognitive domains in participants with epilepsy, but not controls (Supplemental Figs. 1 and 2). The next step was to test for group differences in the nature of this mediation. To do so, we first constrained all paths between the latent variables to be equal for both groups, with the exception of the path from Verbal IQ to PS that was allowed to vary (Table 3). Adding these constraints did not significantly reduce the fit from the initial, unconstrained structural model (χ2(8) = 6.17, p = 0.63, ΔAIC = −9.83). This suggests that, with the exception of the effect of Verbal IQ on PS, all other effects between latent constructs are the same for the epilepsy group and controls.
An important follow-up analysis that additionally constrained the path from Verbal IQ to PS to be equal across groups was conducted. Adding this constraint resulted in a significantly poorer model fit [Δχ2(1) = 24.14, p < .001, ΔAIC = 22.14] compared to the previous, Partially Constrained model (See Table 3). Not only was the Δχ2 significant, but it also resulted in a larger AIC, further suggesting that unlike the other effects, the specific relationship between Verbal IQ and PS is different for those with TLE versus controls.
In an effort to make the structural model even more parsimonious, a fully constrained competing model in which all paths and factor loadings were equal across groups was compared to the previous Full Factor Constrained model (See Table 3). While fit indices for the resulting overall model suggest good fit, results from the Δχ2 difference test and AICs indicated a significantly poorer fit (Δχ2(11) = 33.38, p < .001, ΔAIC = 11.28) than the previous, Full Factor Constrained model. Therefore, the Partially Constrained model should be used as it is the best representation of the relationships among the variables and will be discussed in the subsequent section.
3.3.4. Partially constrained pathway model
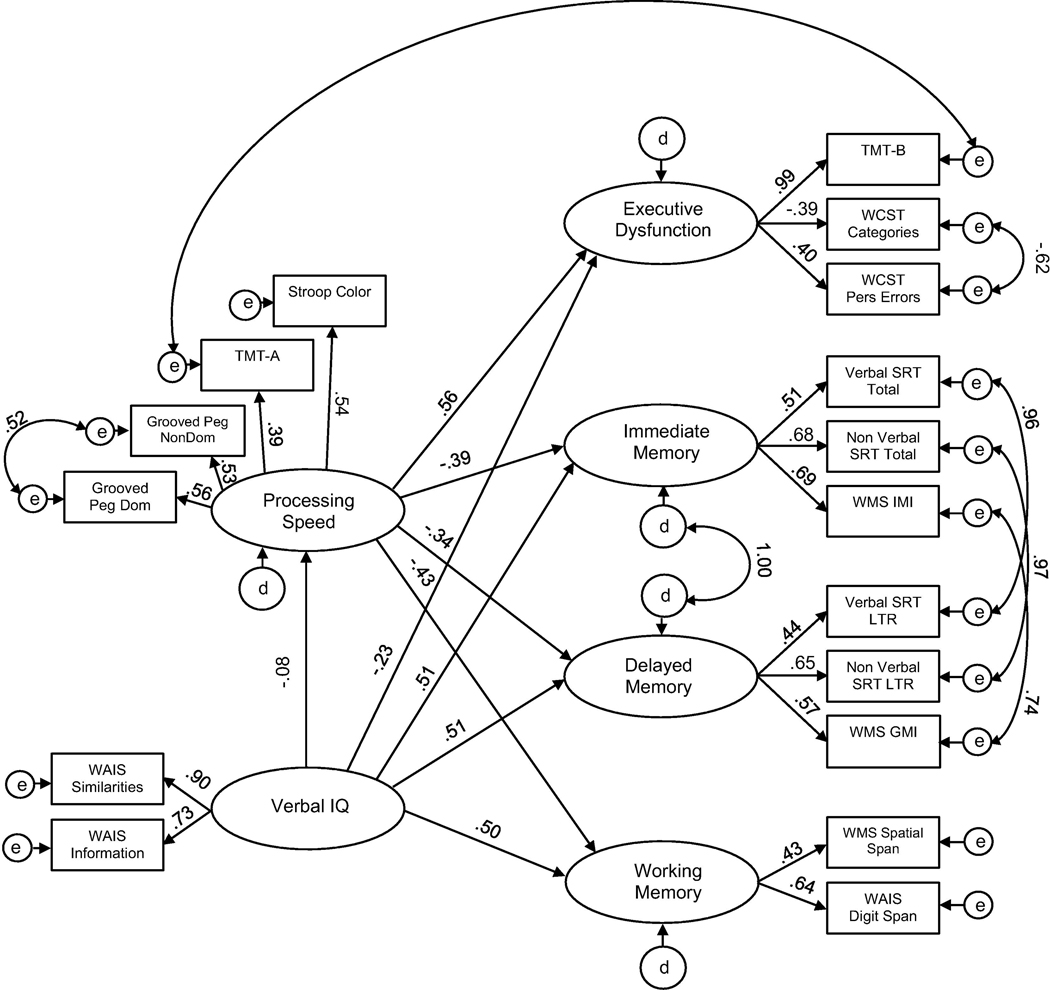
After comparing competing models, the Partially Constrained model is the best model to characterize the impact of processing speed on other cognitive domains for both control and TLE groups. Therefore, the Verbal IQ to PS path was allowed to vary between groups in this model. Fit indices for the partially constrained model suggested a good fit to the data [χ2 (214) = 260.00, p = .02; CFI = 0.98; TLI = 0.97; RMSEA = 0.04 (CI: 0.02–0.05); AIC: 512.00]. For total, direct, and indirect effects for this model by group, please see Supplemental Tables 7, 8, and 9.
For controls, the direct pathway between Verbal IQ and PS for controls (−0.08) remained nonsignificant (Fig. 2) in this partially constrained model. Again, examination of indirect effects revealed that PS does not appear to mediate the relationship between Verbal IQ and other cognitive domains for controls. Verbal IQ had minimal indirect effects on Executive Dysfunction (−0.04), Immediate Memory (0.03), Delayed Memory (0.03), and Working Memory (0.03). PS does not mediate the relationship between Verbal IQ and cognitive function for controls.
Fig. 2.
Standared estimates for the partially constrained model, control group. Results pertain to the model in which all paths between latent variables, with exception of the effect of Verbal IQ and Processing Speed, are constrained. Factor loadings are free to vary.
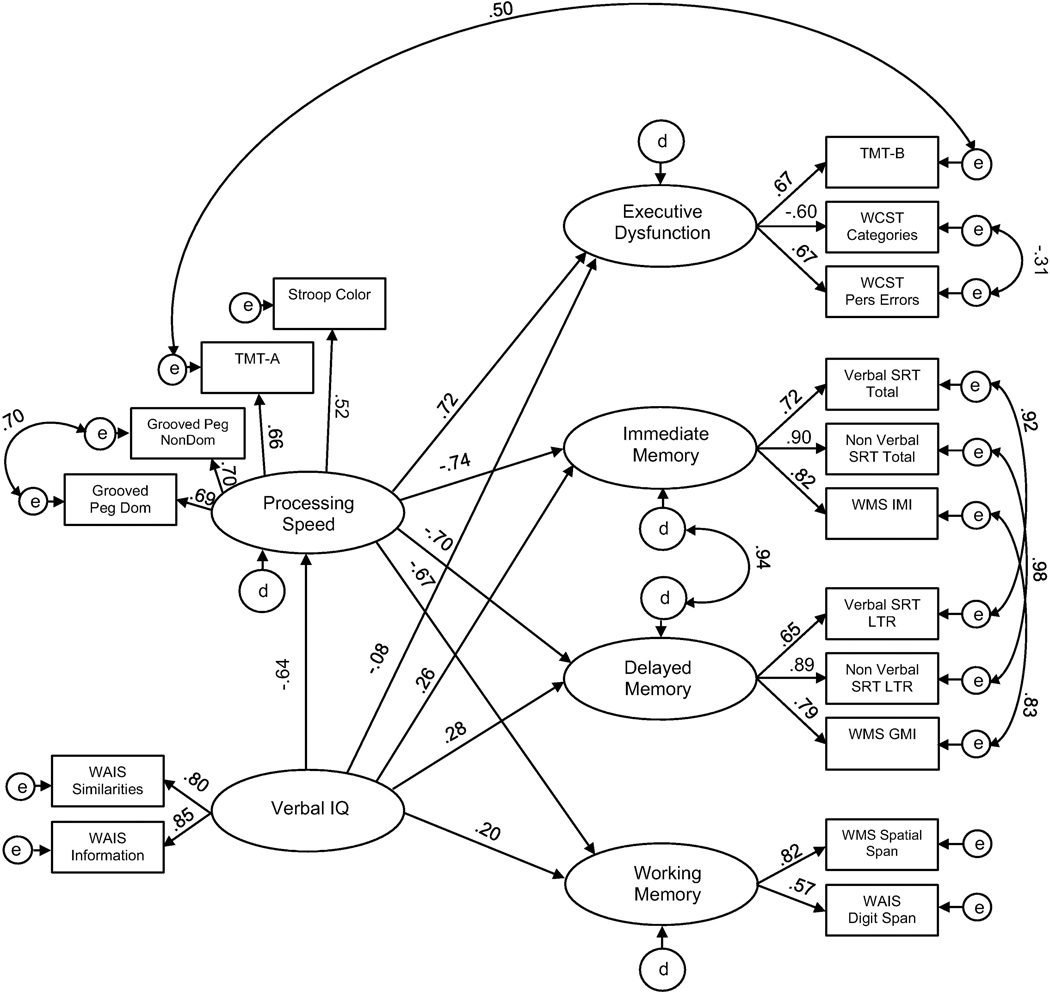
Consistent with the previous model, the pathway between Verbal IQ and PS (−0.64) for the TLE group remained statistically significant (See Fig. 3). Further examination of indirect effects revealed that Verbal IQ had moderate-to-large effects on Executive Dysfunction (−0.47), Immediate Memory (0.48), Delayed Memory (0.45), and Working Memory (0.43) when hypothesized pathways were constrained. Therefore, this model with pathways that were partially constrained further solidifies the main finding: PS mediates the relationship between Verbal IQ and performance across all other cognitive domains among individuals with TLE.
Fig. 3.
Standared estimates for the partially constrained model, TLE group. Results pertain to the model in which all paths between latent variables, with exception of the effect of Verbal IQ and Processing Speed, are constrained. Factor loadings are free to vary.
After comparing competing models, results indicate that only in the TLE group does PS mediate the relationships between Verbal IQ and other domains of cognitive functioning (Executive Dysfunction, Immediate Memory, Delayed Memory, and Working Memory). Furthermore, the partially constrained model (which constrained the pathways between latent variables, with exception of Verbal IQ and PS) solidifies that the direct effect of Verbal IQ and PS is indeed different between controls and the epilepsy group.
3.3.5. Clinical seizure variables and processing speed correlations
Lastly, Supplemental Table 1 provides correlations between clinical seizure variables (seizure onset, epilepsy duration, number of antiepileptic drugs (AEDs), and time since most recent seizure prior to the neuropsychological evaluation) and processing speed measures. Overall, epilepsy duration and number of AEDs were significantly associated with most measures of processing speed.
4. Discussion
Three core findings emerged from this investigation. First, confirmatory factor analysis revealed a conceptually expected cognitive structure for the epilepsy and control participants characterized by domains of verbal intelligence, immediate memory, delayed memory, executive dysfunction, working memory, and processing speed. The specific cognitive tests loading on the factors were similarly conceptually and clinically reassuring. Second, the final multigroup model indicated that there was a specific and significant relationship between slowing and intelligence to the other cognitive domains in both groups. Third, and most critically, psychomotor speed mediated the relationship between verbal intelligence and the other cognitive domains in the epilepsy group, whereas among the controls, verbal intelligence exhibited a direct and unmediated relationship with all other cognitive domains. These findings and their implications are discussed below.
4.1. Cognitive structure
A fundamental issue addressed in this investigation was the degree to which slowed cognitive and psychomotor processing speed in epilepsy, a known cognitive morbidity, impacted performance across other cognitive tests and domains. The first step in this process was to examine the underlying structure of the administered tests via confirmatory factor analysis. These analyses revealed a solution (Fig. 1) represented by the domains of verbal intelligence, processing speed, executive dysfunction, immediate memory, delayed memory, and working memory. Of note was the structure of immediate and delayed memory, each of which subsumed both verbal and visual memory tests as opposed to separate verbal and visual memory domains.
4.2. Intelligence and slowing and their relationship to other cognitive domains
As anticipated, there were significant associations between verbal intelligence and the other cognitive factors in both the epilepsy and control groups, again pointing to a symmetry of effect (Figs. 2 and 3) with verbal intelligence demonstrating a broad positive relationship with various cognitive domains. In the current study, the TLE group had significantly lower PS compared to controls. Reduced processing speed is not only a recognized comorbidity among the epilepsies [1–8], but it has also been shown to be a salient measure in discriminating control and TLE groups [31].
4.3. The mediating impact of processing speed in epilepsy
The critical finding was that in the TLE group, PS mediated the relationship between verbal intelligence across all examined cognitive factors including executive function, immediate and delayed memory, and working memory. In direct contrast, among the controls there was no such mediating effect observed, with a direct relationship between verbal intelligence and the other cognitive domains (with exception of Working Memory). These results suggest that slowed PS plays a central mediating role in the cognitive efficiency of patients with epilepsy, disrupts the known effects of intelligence with other cognitive abilities, and as such may serve as a central target through which it may be possible to exert a positive impact on cognition should such treatment become available. Potentially related to this hypothesis, recently Adams and colleagues demonstrated that administration of low-dose methylphenidate resulted in a generalized improvement in cognition, conceivably consistent with an important central role of processing speed which is known to be impacted by methylphenidate [47,48].
4.4. Processing speed in epilepsy
Complicating the situation in epilepsy, as well as general processing speed research, is that cognitive and psychomotor PS has been assessed through a diversity of measures that include simple and complex reaction time, finger tapping, mental scanning, motor assembly tasks, and other methods [49,50]. The generalizability of findings across studies using different PS metrics is a persisting concern. At its most basic level, processing speed can be defined as the time required to complete a cognitive task or the amount of work that can be completed in a finite amount of time [51]. A commonly used metric of “processing speed”, examined in epilepsy and various clinical disorders, is the digit symbol substitution or number symbol substitution test. This measure has been used to examine speeded performance in schizophrenia [52–55], bipolar disorder [56], multiple sclerosis [57], chronic fatigue [58], other clinical groups [53] as well as normal aging [59,60].
Among adults with TLE, research has demonstrated a linkage between white matter volume and the Sternberg task [61] and occipital-parietal-temporal cortical thickness and resting-state fMRI using the Pattern Comparison Processing Speed test from the NIH Toolbox Cognitive battery [31]. In youth with mixed epilepsies, digit symbol performance is linked to diffusely distributed patterns of cortical gyrification [62] and patterns of altered large-scale cortical-subcortical networks [30]. Clearly important going forward is greater consensus regarding the optimal metric or composite measure based on specific speed-based tasks. Here the measure of speed was a composite determined by factor analysis.
4.5. Limitations
This project has limitations that include, but are not limited to, its cross-sectional nature, modest sample size of the epilepsy and control participants, examination of individuals with epilepsy attending a specialized center as opposed to a population-based sample, and use of a traditional neuropsychological battery as opposed to purer behavioral markers of discrete cognitive domains. Nonetheless, the presence and potential impact of slowed processing speed has been “hiding in plain sight” for decades. Future studies should include non-speed measure of core language and perceptual skills to determine whether similar relationships, as observed here, exist for those domains. Additional research is also needed to clarify the underlying etiology of slowed processing speed. Our preliminary correlational analysis of clinical epilepsy variables with processing speed measures (Supplemental Table 1) showed associations of slowing with longer duration of epilepsy and the expected relationship with the number of medications. Future research to identify treatable factors will be important. Despite our limitations, the central messages appear clear and suggest that future investigation into processing speed abnormalities may be of theoretical and clinical significance.
5. Conclusions
Slowing of cognitive and psychomotor processing speed, a long recognized cognitive complication of the epilepsies, exhibits an impressive mediational role across several critical domains of higher cognitive function including immediate memory, delayed memory, executive function, and working memory. This role is selective for epilepsy and is not observed in controls, and as such may offer an interventional target through which to attempt to broadly impact cognition in persons with chronic epilepsy.
Supplementary Material
Acknowledgments
We greatly appreciate the dedication of the epilepsy and control participants to this research project as well as the contributions of our research coordinators (Michelle Szomi, Dace Almane), student psychometrists, and Department of Neurology colleagues (Drs. Brian Bell, Paul Rutecki, Raj Sheth) to this project.
Funding Source Declaration
This work was supported by the National Institutes of Health (NINDS 2RO1-37738).
Footnotes
Declaration of Interest
None.
Ethical Approval
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Appendix A. Supplementary data
Conflicts of Interest
The authors have no significant relationship with, or financial interest in, any commercial companies pertaining to this article.
References
- [1].Harrower-Erickson M. Psychological studies of patients with epileptic seizures. In: Penfield W, Erickson TC, editors. Epilepsy and cerebral localization: a study of the mechanism, treatment and prevention of epilepstic seizures. Springfield: Charles C. Thomas; 1941. p. 546–74. [Google Scholar]
- [2].Collins AL. Epileptic intelligence. J Consult Psychol 1951;15(5):392–9. [DOI] [PubMed] [Google Scholar]
- [3].Lennox WG, Lennox-Buchthal MA. Epilepsy and related disorders/by William Gordon Lennox, with the collaboration of Margaret A. Lennox. Boston: Little, Brown; 1960. [Google Scholar]
- [4].Brown SW, Reynolds EH. Cognitive impairment in epileptic patients. In: Trimble MR, Reynolds EH, editors. Epilepsy and psychiatry. Edinburgh: Churchill Livingstone; 1981. [Google Scholar]
- [5].Dodrill CB, Matthews CG. The role of neuropsychology in the assessment and treatment of persons with epilepsy. Am Psychol 1992;47(9):1139–42. [DOI] [PubMed] [Google Scholar]
- [6].Baxendale S, Thompson P. The new approach to epilepsy classification: Cognition and behavior in adult epilepsy syndromes. Epilepsy Behav 2016;64(Pt A):253–6. [DOI] [PubMed] [Google Scholar]
- [7].Elger CE, Helmstaedter C, Kurthen M. Chronic epilepsy and cognition. Lancet Neurol 2004;3(11):663–72. [DOI] [PubMed] [Google Scholar]
- [8].Tarter RE. Intellectual and adaptive functioning in epilepsy. A review of 50 years of research. Dis Nerv Syst 1972;33(12):763–70. [PubMed] [Google Scholar]
- [9].Lin JJ, Mula M, Hermann BP. Uncovering the neurobehavioural comorbidities of epilepsy over the lifespan. Lancet 2012;380(9848):1180–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rzezak P, Guimarães CA, Fuentes D, Guerreiro MM, Valente KD. Memory in children with temporal lobe epilepsy is at least partially explained by executive dysfunction. Epilepsy Behav 2012;25(4):577–84. [DOI] [PubMed] [Google Scholar]
- [11].Sepeta LN, Casaletto KB, Terwilliger V, Facella-Ervolini J, Sady M, Mayo J, et al. The role of executive functioning in memory performance in pediatric focal epilepsy. Epilepsia 2017;58(2):300–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hermann BP, Seidenberg M, Haltiner A, Wyler AR. Adequacy of language function and verbal memory performance in unilateral temporal lobe epilepsy. Cortex 1992;28(3):423–33. [DOI] [PubMed] [Google Scholar]
- [13].Danguecan AN, Smith ML. Academic outcomes in individuals with childhood-onset epilepsy: mediating effects of working memory. J Int Neuropsychol Soc 2017;23(7):594–604. [DOI] [PubMed] [Google Scholar]
- [14].Srnka K, Seidenberg M, Hermann B, Jones J. Intraindividual variability in attentional vigilance in children with epilepsy. Epilepsy Behav 2018;79:42–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Overvliet GM, Aldenkamp AP, Klinkenberg S, Nicolai J, Vles JSH, Besseling RMH, et al. Correlation between language impairment and problems in motor development in children with rolandic epilepsy. Epilepsy Behav 2011;22 (3):527–31. [DOI] [PubMed] [Google Scholar]
- [16].Helmstaedter C, Witt JA, Hoppe C. Evaluating the mediating role of executive functions for antiepileptic drugs’ effects on IQ in children and adolescents with epilepsy. Epilepsy Behav 2019;96:98–103. [DOI] [PubMed] [Google Scholar]
- [17].Vermeulen J, Aldenkamp AP. Cognitive side-effects of chronic antiepileptic drug treatment: a review of 25 years of research. Epilepsy Res 1995;22 (2):65–95. [DOI] [PubMed] [Google Scholar]
- [18].Eddy CM, Rickards HE, Cavanna AE. The cognitive impact of antiepileptic drugs. Ther Adv Neurol Disord 2011;4(6):385–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Loring DW, Marino S, Meador KJ. Neuropsychological and behavioral effects of antiepilepsy drugs. Neuropsychol Rev 2007;17(4):413–25. [DOI] [PubMed] [Google Scholar]
- [20].Park SP, Kwon SH. Cognitive effects of antiepileptic drugs. J Clin Neurol 2008;4 (3):99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Oostrom KJ, Smeets-Schouten A, Kruitwagen CLJJ, Peters ACB, Jennekens-Schinkel A. Not only a matter of epilepsy: early problems of cognition and behavior in children with “epilepsy only”–a prospective, longitudinal, controlled study starting at diagnosis. Pediatrics 2003;112(6):1338–44. [DOI] [PubMed] [Google Scholar]
- [22].Prevey ML, Delaney, Cramer JA, Mattson RH Complex partial and secondarily generalized seizure patients: cognitive functioning prior to treatment with antiepileptic medication. VA Epilepsy Cooperative Study 264 Group. Epilepsy Res 1998;30(1):1–9. [DOI] [PubMed] [Google Scholar]
- [23].Taylor J, Kolamunnage-Dona R, Marson AG, Smith PEM, Aldenkamp AP, Baker GA, et al. Patients with epilepsy: cognitively compromised before the start of antiepileptic drug treatment? Epilepsia 2010;51(1):48–56. [DOI] [PubMed] [Google Scholar]
- [24].Baker GA, Taylor J, Aldenkamp AP, SANAD group. Newly diagnosed epilepsy: cognitive outcome after 12 months. Epilepsia 2011;52(6):1084–91. [DOI] [PubMed] [Google Scholar]
- [25].Hermann BP, Seidenberg M, Dow C, Jones J, Rutecki P, Bhattacharya A, et al. Cognitive prognosis in chronic temporal lobe epilepsy. Ann Neurol 2006;60 (1):80–7. [DOI] [PubMed] [Google Scholar]
- [26].Aldenkamp AP, Alpherts WC, Blennow G, Elmqvist D, Heijbel J, Nilsson HL, et al. Withdrawal of antiepileptic medication in children–effects on cognitive function: The Multicenter Holmfrid Study. Neurology 1993;43(1):41–50. [DOI] [PubMed] [Google Scholar]
- [27].Aldenkamp AP, Alpherts WCJ, Sandstedt P, Blennow G, Elmqvist D, Heijbel J, et al. Antiepileptic drug-related cognitive complaints in seizure-free children with epilepsy before and after drug discontinuation. Epilepsia 1998;39 (10):1070–4. [DOI] [PubMed] [Google Scholar]
- [28].Berg AT, Langfitt JT, Testa FM, Levy SR, DiMario F, Westerveld M, et al. Residual cognitive effects of uncomplicated idiopathic and cryptogenic epilepsy. Epilepsy Behav 2008;13(4):614–9. [DOI] [PubMed] [Google Scholar]
- [29].Austin JK, Perkins SM, Johnson CS, Fastenau PS, Byars AW, deGrauw TJ, et al. Behavior problems in children at time of first recognized seizure and changes over the following 3 years. Epilepsy Behav 2011;21(4):373–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Garcia-Ramos C, Dabbs K, Meyerand E, Prabhakaran V, Hsu D, Jones J, et al. Psychomotor slowing is associated with anomalies in baseline and prospective large scale neural networks in youth with epilepsy. Neuroimage Clin 2018;19:222–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hwang G, Dabbs K, Conant L, Nair VA, Mathis J, Almane DN, et al. Cognitive slowing and its underlying neurobiology in temporal lobe epilepsy. Cortex 2019;117:41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Deary IJ. Intelligence. Annu Rev Psychol 2012;63(1):453–82. [DOI] [PubMed] [Google Scholar]
- [33].Sokka A, Olsen P, Kirjavainen J, Harju M, Keski-Nisula L, Räisänen S, et al. Etiology, syndrome diagnosis, and cognition in childhood-onset epilepsy: A population-based study. Epilepsia Open 2017;2(1):76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Høie B, Mykletun A, Sommerfelt K, Bjørnæs H, Skeidsvoll H, Waaler PE. Seizure-related factors and non-verbal intelligence in children with epilepsy. A population-based study from Western Norway. Seizure 2005;14(4):223–31. [DOI] [PubMed] [Google Scholar]
- [35].Rantanen K, Nieminen P, Eriksson K. Neurocognitive functioning of preschool children with uncomplicated epilepsy. J Neuropsychol 2010;4(Pt 1):71–87. [DOI] [PubMed] [Google Scholar]
- [36].Berg AT, Langfitt JT, Testa FM, Levy SR, DiMario F, Westerveld M, et al. Global cognitive function in children with epilepsy: a community-based study. Epilepsia 2008;49(4):608–14. [DOI] [PubMed] [Google Scholar]
- [37].Glosser G, Cole LC, French JA, Saykin AJ, Sperling MR. Predictors of intellectual performance in adults with intractable temporal lobe epilepsy. J Int Neuropsychol Soc 1997;3(3):252–9. [PubMed] [Google Scholar]
- [38].Nolan MA, Redoblado MA, Lah S, Sabaz M, Lawson JA, Cunningham AM, et al. Intelligence in childhood epilepsy syndromes. Epilepsy Res 2003;53(1–2):139–50. [DOI] [PubMed] [Google Scholar]
- [39].Rzezak P, Moschetta SP, Mendonça M, Paiva MLMN, Coan AC, Guerreiro C, et al. Higher IQ in juvenile myoclonic epilepsy: Dodging cognitive obstacles and “masking” impairments. Epilepsy Behav 2018;86:124–30. [DOI] [PubMed] [Google Scholar]
- [40].Rzezak P, Guimarães CA, Guerreiro MM, Valente KD. The impact of intelligence on memory and executive functions of children with temporal lobe epilepsy: Methodological concerns with clinical relevance. Eur J Paediatr Neurol 2017;21(3):500–6. [DOI] [PubMed] [Google Scholar]
- [41].Oyegbile TO, Dow C, Jones J, Bell B, Rutecki P, Sheth R, et al. The nature and course of neuropsychological morbidity in chronic temporal lobe epilepsy. Neurology 2004;62(10):1736–42. [DOI] [PubMed] [Google Scholar]
- [42].Keith T. Multiple regression and beyond: an introduction to multiple regression and structural equation modeling. New York: Routledge; 2019. [Google Scholar]
- [43].Aiken LS, Stein JA, Bentler PM. Structural equation analyses of clinical subpopulation differences and comparative treatment outcomes: characterizing the daily lives of drug addicts. J Consult Clin Psychol 1994;62 (3):488–99. [DOI] [PubMed] [Google Scholar]
- [44].Hu L-T, Bentler PM. Fit indices in covariance structure modeling: Sensitivity to underparameterized model misspecification. Psychol Methods 1998;3 (4):424–53. [Google Scholar]
- [45].Hu L-T, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct Equ Model 1999;6(1):1–55. [Google Scholar]
- [46].Loehlin JC, Beaujean AA. Latent variable models: an introduction to factor, path, and structural equation analysis. New York: Routledge; 2017. [Google Scholar]
- [47].Adams J, Alipio-Jocson V, Inoyama K, Bartlett V, Sandhu S, Oso J, et al. Methylphenidate, cognition, and epilepsy: A 1-month open-label trial. Epilepsia 2017;58(12):2124–32. [DOI] [PubMed] [Google Scholar]
- [48].Adams J, Alipio-Jocson V, Inoyama K, Bartlett V, Sandhu S, Oso J, et al. Methylphenidate, cognition, and epilepsy: A double-blind, placebo-controlled, single-dose study. Neurology 2017;88(5):470–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Grevers E, Breuer LEM, IJff DM, Aldenkamp AP. Mental slowing in relation to epilepsy and antiepileptic medication. Acta Neurol Scand 2016;134 (2):116–22. [DOI] [PubMed] [Google Scholar]
- [50].Martin TA, Bush SS. Assessment tools and research methods for human information processing speed. In: Information processing speed in clinical populations. Philadelphia, PA, US: Taylor & Francis; 2008. p. 29–51. [Google Scholar]
- [51].Deluca J. Information processing speed: How fast, how slow, and how come? In: Information processing speed in clinical populations. Philadelphia, PA, US: Taylor & Francis; 2008. p. 265–73. [Google Scholar]
- [52].Dickinson D, Ramsey ME, Gold JM. Overlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Arch Gen Psychiatry 2007;64(5):532–42. [DOI] [PubMed] [Google Scholar]
- [53].DeLuca J, Kalmar JH. Processing speed and the Digit Symbol Substitution Test in schizophrenia. In: Information processing speed in clinical populations. Philadelphia, PA, US: Taylor & Francis; 2008. p. 125–51. [Google Scholar]
- [54].Knowles EEM, David AS, Reichenberg A. Processing speed deficits in schizophrenia: reexamining the evidence. Am J Psychiatry 2010;167 (7):828–35. [DOI] [PubMed] [Google Scholar]
- [55].Morrens M, Hulstijn W, Sabbe B. Psychomotor slowing in schizophrenia. Schizophr Bull 2007;33(4):1038–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Morsel AM, Temmerman A, Sabbe B, Hulstijn W, Morrens M. Unraveling psychomotor slowing in bipolar disorder. Neuropsychobiology 2015;71 (4):234–40. [DOI] [PubMed] [Google Scholar]
- [57].Batista S, Zivadinov R, Hoogs M, Bergsland N, Heininen-Brown M, Dwyer MG, et al. Basal ganglia, thalamus and neocortical atrophy predicting slowed cognitive processing in multiple sclerosis. J Neurol 2012;259(1):139–46. [DOI] [PubMed] [Google Scholar]
- [58].Demaree HA, Frazier TW, Johnson CE. Information processing speed: Measurement issues and its relationships with other neuropsychological constructs. In: Information processing speed in clinical populations. Philadelphia, PA, US: Taylor & Francis; 2008. p. 53–77. [Google Scholar]
- [59].Salthouse TA. The role of memory in the age decline in digit-symbol substitution performance. J Gerontol 1978;33(2):232–8. [DOI] [PubMed] [Google Scholar]
- [60].Salthouse TA. Aging and measures of processing speed. Biol Psychol 2000;54 (1–3):35–54. [DOI] [PubMed] [Google Scholar]
- [61].Dow C, Seidenberg M, Hermann B. Relationship between information processing speed in temporal lobe epilepsy and white matter volume. Epilepsy Behav 2004;5(6):919–25. [DOI] [PubMed] [Google Scholar]
- [62].Bobholz SA, Dabbs K, Almane D, Jones JE, Hsu DE, Stafstrom CE, et al. Neurobiological substrates of processing speed in childhood epilepsy. Brain Imaging Behav 2019;13(6):1719–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.