Abstract
This study used litchi (Heiye) wine and distilled spirit as raw experimental materials to analyze the volatile aroma compounds. Qualitative and quantitative determination of aromatic components was studied using stir bar sportive extraction (SBSE) and gas chromatography coupled to mass spectrometry (GC/MS). Results indicated that a total of 128 different types of aroma compounds were observed, which belonged to six chemical groups, including 39 esters, 16 alcohols, 16 acids, 22 terpenes, 17 aldehydes and ketones, and 18 other compounds. In particular, esters were the highest among all six categories and represented approximately 52% of the total flavor component content in litchi distilled spirit. The odor activity values (OAVs) revealed 22 types of aroma compounds with OAVs >1 in this test. It is possible that the produced litchi distilled spirit had a stronger varietal character due to the increased concentrations and OAVs of β‐damascenone, linalool, ethyl butyrate, ethyl isovalerate, ethyl caproate, trans‐rose oxide, and cis‐rose oxide. Taking the OAVs into account, we evaluated the characteristic aromas for litchi wine and litchi distilled spirit.
Keywords: litchi distilled spirit, litchi wine, OAVs, SBSE‐GC/MS, volatile compounds
It is possible that the produced litchi distilled spirit had a stronger varietal character due to the increased concentrations and OAVs of β‐damascenone, linalool, ethyl isovalerate, ethyl butyrate, ethyl caproate, trans‐rose oxide, and cis‐rose oxide since these compounds are known to play a decisive fruit and flower aroma in the distilled spirit.

Highlights.
The naturally occurring litchi (Heiye) has potential applications in wine products, particularly in distilled spirit.
128 different types of aroma compounds were identified in this study.
Alcoholic fermentation and distilled spirit process lead to a series of by‐products.
It is possible that the produced litchi distilled spirit had a stronger varietal character.
1. INTRODUCTION
Litchi (Litchi chinensis Sonn.) is an important fruit crop originated in China and has earned its popularity worldwide due mainly to its charming aroma, unique taste, and possible health benefits. In recent years, litchi cultivation has been distributed primarily for countries in Southeast Asia, such as China, India, Thailand, and Vietnam which are the leading litchi‐producing countries in the world, among which China is the largest producing country (Pareek, 2016). Several cultivars in the west of Guangdong region have a long history of cultivation and account for approximately one‐third of all fruit exports in China, while others are relatively new (Emanuele et al., 2017; Xiong et al., 2018). Litchi is suitable for producing fruit wine, with its high sugar content (up to 19.2%) and rose‐floral and citrus‐like aroma (Chen et al., 2014). Various physiological functions have also been reported for litchi, such as cancer preventive, antioxidant activity, antimicrobial, anti‐inflammatory activities, and so on (Emanuele et al., 2017; Kilari & Putta, 2016; Varzakas et al., 2016). These days, the fermented fruit alcohol industry shows increasing interest in functional food, which displays an additional function related to disease prevention or health promotion. The naturally occurring litchi is a prime candidate. A wide range of litchi cultivars and growing climates in addition to orchard management practices allow for countless variables in starting fruit material. Likewise, prefermentation treatments of fruit and juice, fermentation management, and postfermentation treatments of litchi wine will also have impacts on final product quality. Li et al. (2012) found a 3.2‐fold difference in phenolic content between the highest and lowest litchi varieties, Heiye and Chanchutou, respectively. However, litchi pericarp browning and pulp decay lead to its short shelf life; it is liable to lose its attractive feature and rapidly goes unpleasant once harvested from trees (Zhao et al., 2020). Therefore, much attention has been paid to postharvest quality preservation via various approaches and further processing, including the production of litchi wine and distilled spirit, aiming to keep its excellent characteristics for a more extended period.
Today, the consumption of distilled spirit has become increasingly and wildly popular in the world. Globally, famous distilled spirits such as whiskey, brandy, and rum occupy most of the domestic alcohol market because of recognition as high‐quality products (Lee et al., 2018). And there have been numerous studies regarding the volatile aroma compounds in fermented wine and distilled spirit. Sánchez‐Palomo et al. (2019) identified 77 volatile compounds in Chelva wines and the Chelva grapes variety cultivated in La Mancha region presents an excellent aroma potential and a complex sensory profile. Chen et al. (2019) found more aroma‐active areas with flavor dilution (FD) factors of ≥64 in the aged rice wine than in the young rice wine, and the odor activity values (OAVs) revealed 33 aroma compounds with OAVs of ≥1 in young or aged rice wine. Lee et al. (2018) analyzed the flavor of aged spirits made from sweet potato and rice by gas chromatography‐mass spectrometry. Zheng et al. (2014) studied the fundamental volatile aroma compound changes in sweet Hongqu glutinous rice wine (a select type of rice wine) by headspace solid‐phase microextraction (HS‐SPME) and gas chromatography‐olfactometry (GC‐O). Wang et al. (2018) detected 71 kinds of aroma components in five distilled spirit (made from main grape varieties). Among them, the contents of esters, alcohols, and acids were higher. Yi et al. (2010) analyzed volatile compounds in liquor distilled from mash produced using Koji or Nuruk under reduced or atmospheric pressure. Tang et al. (2019) observed a total of 97 volatiles (38 in common) in lychee wine during fermentation, including 39 terpenoids, 5 alcohols, 22 esters, 4 acids, 7 alkenes, 4 ketones and ethers, 3 sulfur compounds, and others. Chen and Liu (2016) found 84 volatiles and 88 volatiles in the AF (alcoholic fermentation) and MLF (malolactic fermentation) litchi wines, respectively. However, there is little research regarding the flavor of litchi distilled spirit, particularly those made from litchi (Heiye) as one of the raw materials.
The purpose of this study was to explain the difference in aroma compounds between litchi (cv. Heiye) wine and distilled spirit by SBSE‐GC/MS technology and to objectively evaluate the contribution of aroma compounds to litchi wine and distilled spirit through the calculation of OAVs. Based on these results, the study may improve our understanding of the essence of the aroma difference between litchi wine and litchi distilled spirit. It can provide a substantial basis for further research into the control of flavor during the distilled process for litchi distilled spirit.
2. MATERIALS AND METHODS
2.1. Litchi materials
Two varieties of litchi (cvs. Heiye an Guiwei) were from Maoming, Guangdong Province, China, 2013 vintage, at their optimum point of maturity and sound quality without the disease. Fresh litchi fruits were kept at −20°C until processing and analysis.
2.2. Winemaking processes
The method of Lee et al. (2018) was used with some modifications. 70 kg of raw materials was divided, peeled, destemmed, and crushed, and then all placed in 20‐L stainless steel tanks, and 60 mg/L sulfur dioxide was added for preservation. Physicochemical characteristics of litchi juice were as follows: total sugar, 125 g/L; titratable acidity (expressed as tartaric acid), 2.5 g/L. Afterward, the modified litchi juice was added with 20 mg/L pectinase and then kept at 5°C for 24 hr for clarification. As a starter, commercial yeast (Saccharomyces cerevisiae EC‐1118, Chr.‐Han, Horsholm, Denmark) 200 mg/L activated in advance (hydration in 5% of the glucose solution at 37°C for 30 min). The starter was added into the clear juice to start fermentation. And alcoholic fermentation was maintained at 25°C. Cap punching was performed three times a day during fermentation. When the residual sugar was lower than 2 g/L, the wines were centrifuged at 1,500 × g for 20 min, and the supernatants were transferred to the clean jars, treated with 60 mg/L sulfur dioxide, and stored at 4°C for six months. During the storage, a cold treatment at −4°C for 15 days was used to stabilize, and three times general racking was performed to clarify.
The distilled spirit was gained by alcoholic distillation with some modifications (Paolo et al., 1997). 200 ml of litchi wine, at 12% alcohol (v/v), was distilled in a copper distiller (Hoga Co., Salvaterra de Mino, Spain). A fraction of 50 ml at 43.9% alcohol (v/v) was collected. Four of these fractions were redistilled together, and a second 50 ml fraction at 83% alcohol was collected. The body of distillation was selected for testing; samples were recorded as distilled spirit.
Samples were collected at three different phases: raw juice, litchi wine, and litchi distilled spirit. Each sample was made in triplicate.
2.3. Chemicals and reagents
Analytical chemicals included tartaric acid, sodium chloride, anhydrous ethanol, and other reagents (Xi'an Chemical Industry Co. Ltd., China). The internal standard and chromatographically pure standards included ethyl caprylate, ethyl acetate, ethyl caprate, ethyl butyrate, isobutyl acetate, phenethyl acetate, diethyl succinate, ethyl laurate, octanoic acid, 1‐propanol, benzyl alcohol, phenethyl alcohol, 3‐hexen‐1‐ol, 2,3‐butanediol, isobutyric acid, isovaleric acid, phenylacetic acid, benzaldehyde, phenylacetaldehyde, geraniol, linalool, citronellol, 4‐terpineol, and 3,4‐dimethyl phenol. All volatile standards used for identification and quantification in this study were HPLC quality or GC grade, and their purities and commercial sources are listed in Table 1.
TABLE 1.
Manufacturers, purities, quantitative ions, and emendation factors to 2‐octanol of the volatile standards used for the characterization of litchi wine and distilled spirit
| No. | Compounds | Manufacturers a | Purity |
Quantitative ions |
Emendation factor A | ||
|---|---|---|---|---|---|---|---|
| 1% | 11% | 28% | |||||
| 1 | Ethyl caprylate | Sigma | 99% | 43 + 61+45 | 13.632 | 10.185 | 15.445 |
| 2 | Ethyl acetate | Sigma | 98% | RIC‐(35–400) | 4.222 | 8.291 | 7.260 |
| 3 | Ethyl caprate | Sigma | 98% | RIC‐(35–400) | 1.114 | 1.287 | 1.576 |
| 4 | Ethyl butyrate | Sigma | 98% | RIC‐(35–400) | 1.385 | 0.998 | 0.786 |
| 5 | Isobutyl acetate | Sigma | 97% | RIC‐(35–400) | 1.230 | 1.578 | 1.923 |
| 6 | Phenethyl acetate | Aldrich | 98% | RIC‐(35–400) | 2.114 | 3.927 | 4.407 |
| 7 | Diethyl succinate | Sigma | 98% | 101 + 29+129 | 11.456 | 10.232 | 8.793 |
| 8 | Ethyl laurate | Aldrich | 97% | 88 + 101+43 | 0.472 | 0.371 | 1.233 |
| 9 | Octanoic acid | Aldrich | 98% | RIC‐(35–400) | 3.212 | 3.065 | 4.219 |
| 10 | 1‐Propanol | Sigma | 98% | 73 + 43 | 4.241 | 2.334 | 3.788 |
| 11 | Benzyl alcohol | Aldrich | 97% | 68 + 59 | 3.221 | 6.882 | 5.327 |
| 12 | Phenethyl alcohol | Aldrich | 98% | RIC‐(35–400) | 2.114 | 1.008 | 0.653 |
| 13 | 1‐Propanol | Sigma | 99% | 31 + 29+27 | 13.493 | 14.375 | 14.770 |
| 14 | 2‐Octanol | Sigma | 98% | RIC‐(35–400) | 1 | 1 | 1 |
| 15 | 2,3‐Butanediol | Aldrich | 97% | 45 + 29 | 6.782 | 8.325 | 10.376 |
| 16 | cis−3‐Hexen−1‐ol | Aldrich | 98% | 67 + 41+39 | 10.845 | 14.397 | 15.436 |
| 17 | 4‐Terpineol | Aldrich | 97% | 71 + 111+93 | 1.556 | 1.247 | 2.117 |
| 18 | Benzyl alcohol | Sigma | 98% | RIC‐(35–400) | 17.189 | 17.322 | 13.376 |
| 19 | Phenyl ethanol | Aldrich | 99% | RIC‐(35–400) | 19.434 | 28.445 | 21.32 |
| 20 | Benzaldehyde | Aldrich | 97% | RIC‐(35–400) | 0.492 | 0.755 | 1.331 |
| 21 | Phenylacetaldehyde | Sigma | 97% | RIC‐(35–400) | 2.339 | 3.129 | 3.785 |
| 22 | Geraniol | Sigma | 98% | 94 + 129+112 | 4.120 | 4.313 | 8.998 |
| 23 | Linalool | Sigma | 98% | 93 + 121+136 | 1.398 | 2.287 | 3.221 |
| 24 | Citronellol | Aldrich | 98% | 93 + 124+112 | 0.768 | 2.834 | 2.137 |
| 25 | 3,4‐Dimethyl phenol | Aldrich | 97% | RIC‐(35–400) | 0.435 | 0.821 | 1.211 |
A: Emendation factors to 2‐octanol (internal standard) of each volatile standard were measured in litchi wine and litchi distilled spirit containing 1%, 11%, and 28% (v/v) ethanol separately.
Manufacturers: Sigma‐Aldrich, St. Louis, MO, USA; Aldrich, Milwaukee, WI.
2.4. Volatile compounds analysis
The volatile compounds of the samples were isolated by stir bar sportive extraction (SBSE) according to the modified method proposed by Aguirre et al. (2014), and the volatile compounds of the samples were analyzed by GC‐MS as previously described by Lin et al. (2019).
2.4.1. Extraction of volatile compounds
About 10 ml of each sample was placed in a 15‐ml airtight vial containing a magnetic stirrer bar (SBSE, 20 mm × 0.5 mm, Gerstel Co. Ltd, Germany), including 50 µl of 2‐octanol (0.234 mg/ml water, internal standard) and 2 g NaCl. Then, the airtight vial was placed on a magnetic stirrer (PC‐420D, Corning Co. Ltd, USA) and extracted for 90 min at 40°C with stirring (1,100 rpm). After the extraction, the stir bar was taken out with tweezers, washed it until there is no residual sample with distilled water, and dried with filter paper. Finally, it was inserted into the GC injector for 3 min to desorb analytes. The desorption temperature is 250°C. Each sample was extracted in triplicate.
2.4.2. GC‐MS analysis
The separation, detection, and quantification of volatile compounds were performed on an Agilent TRACE GC (Agilent Technologies Inc., Santa Clara, CA, USA) coupled with Xcalibur V.3.0 mass spectrometry system (Thermo Fisher Scientific, Waltham, MA) and equipped with a DB‐WAXetr capillary column (30 m × 0.25 mm inner diameter, 0.25 μm film thickness, Agilent Technologies, USA). The conditions of GC‐MS in this study were applied as previously reported with some modifications (Yang et al., 2019). Ultrapure helium was used as the carrier gas at 1.0 ml/min. The initial column temperature was set at 40°C for 3 min. Afterward, the temperature was raised to 160°C at a rate of 5°C/min, then 7°C/min to 230°C, and held at 230°C for 8 min. Mass detector conditions were as follows: voltage of electron impact was 70 eV, scan range was m/z 30–350, and scanning frequency in full scan mode was 5.27 times/s.
2.4.3. Quantification analysis of volatile compounds
Volatiles were identified by matching the obtained mass spectra with the Wiley libraries and by comparing the retention indices (RI) to those of the compounds reported in the NIST 2.0 and the literature. According to the method proposed by Wu et al. (2016), the quantification procedure was carried out with the internal standard quantification method with light modification. 2‐Octanol was employed as the internal standard compound. Three synthetic matrixes with 1%, 11%, and 28% (v/v) ethanol were prepared in distilled water, each contained 7.0 g/L tartaric acid, and the pH was adjusted to 3.6 with NaOH. Volatile standards were then extracted and analyzed under the same conditions as litchi juice, litchi wine, and distilled spirit samples to obtain their internal standard emendation factors. They are listed in Table 1. The emendation factors in the 1% standard solution were used to quantify volatile compounds in litchi juice. Those obtained in the 11% and 28% standard solution were used for volatile compounds in litchi wine and distilled spirit samples. The concentration of volatile compounds for which there was no pure reference available was estimated using the same emendation factor (obtained in the same standard solution) as one of the compounds with the most similar chemical structure.
2.4.4. Determination of odor activity values
Odor activity value (OAV) is a parameter frequently used to assess each volatile compound's contribution to wine's aroma. Compounds existing in greater concentrations than their odor threshold (OAVs >1) are defined as aroma impact compounds and contribute individually to the wine aroma (Zhu et al., 2014). The influence of each aromatic compound on the scent of litchi wine and distilled spirit was determined by the odor activity values (OAVs), which were calculated as the ratio between the concentration and the odor threshold of the individual aroma compounds (Velázquez et al., 2015).
2.5. Statistical analysis
The data were reported as means ± standard deviation of three triplicates and were analyzed statistically by one‐way ANOVA. Means were compared by Duncan's multiple range test. Differences with p values <0.05 were considered statistically significant. The statistical software utilized was SPSS 17.0 (SPSS Inc., Chicago, IL, USA). To highlight the similarities and differences between wine samples and volatile compounds, principal component analysis (PCA) was carried out on the analytical data.
3. RESULTS AND DISCUSSION
3.1. Esters
Esters are of primary industrial interest because they have very low thresholds and can directly affect wine flavor and via complex synergistic interactions (Dzialo et al., 2017; Lytra et al., 2016). In this study, esters were the largest group in terms of the composition and concentration of aroma compounds. The esters' concentration ranged from 581.06 (litchi juice) to 117,121.99 µg/L (litchi distilled spirit), which was the highest among all six categories and represented approximately 52% of the total flavor component content (Figure 2). Similarly, Zhang et al. (2019) reported that esters represented about 40% of the total flavor component content (w/w) in hulless barley wine. Thirty‐nine different types of esters were identified (Table 2). The most inadequate variety of esters was observed in litchi juice; there were only seven esters, but 15 and 28 kinds of esters in litchi wine and distilled spirit (Figure 1), respectively. All esters those detected in litchi juices showed the increasing trend in the fermented ones except diisopropyl adipate, diethyl phthalate, and dibutyl phthalate (Table 2). In general, these compounds had a similar trend with a sharp increase during the winemaking and distilling process. Since esters are one of the most important by‐products of alcoholic fermentation and mainly produced by yeast as secondary products of sugar metabolism (Zhang et al., 2019). Our research also illustrates that 30 of 39 esters were newly generated during the winemaking process. Seven esters were just detected in litchi wine, including isoamyl acetate, isobutyl acetate, ethyl valerate, citronellyl acetate, diethyl succinate, ethyl 9‐decenoate, and 3‐hydroxytridecanoate ethyl. Some of these esters have also been found to be dominant in strawberry wine (Kafkas et al., 2006) and orange wine (Selli, 2007). These esters faded away in litchi distilled spirit. Conversely, another five esters (ethyl butyrate, ethyl caproate, ethyl caprylate, ethyl caprate, and ethyl palmitate) increased sharply at the end of the distilling process. Ethyl acetate (1973.52 ± 142.78 µg/L), ethyl caprylate (1,296.28 ± 110.93 µg/L), and isoamyl acetate (545.70 ± 45.02 µg/L) were the most abundant esters in litchi wine. Similarly, Ayestarán et al. (2019) also found the highest isoamyl acetate levels in Tempranillo Blanco wines. These aroma compounds might be significant contributors to the characteristic aroma of litchi wine. In the finished litchi distilled spirit, ethyl palmitate was most abundant among esters and represented approximately 65% of the total ester content (256.2 mg/L), followed by ethyl linoleate (19,815.3 ± 1,232.1 µg/L), ethyl linolenate (15,981.76 ± 32.00 µg/L), and ethyl caprylate (14,288.9 ± 737.72 µg/L). In particular, the increase in ethyl acetate concentration in litchi wine and especially in litchi distilled spirit with respect to litchi juice was possibly caused by the action of Saccharomyces cerevisiae (Erasmus et al., 2004), as it was emphasized by another author who found a similar trend in durian pulp wine (Lu et al., 2017). Based on the odor activity values (Table 3), 12 types of esters showed OAVs >1 in three samples. Litchi wine presented a total of 6 esters with OAVs >1. Among them, ethyl caproate (OAV of 29.62), ethyl caprylate (OAV of 5.18), and ethyl butyrate (OAV of 4.47) were presented with relatively high OAVs and might be significant contributors to the aroma of litchi wine. However, these esters' OAVs increased sharply in distilled spirit except isoamyl acetate and were 5 to 11 times higher in distilled spirit than in litchi wine. Additionally, five types of new esters were presented in distilled spirit with OAVs >1, including cis‐whiskey lactone (OAV of 4.46), citronellyl acetate (OAV of 4.2), ethyl laurate (OAV of 5.07), ethyl myristate (OAV of 1.81), ethyl palmitate (OAV of 1.81). It is noteworthy that the OAVs of ethyl butyrate (OAV of 22), ethyl isovalerate (OAV of 16.6), and ethyl caproate (OAV of 206) were much higher than its odor thresholds in the distilled spirit. These aroma compounds might explain the fruit aroma in the distilled spirit.
FIGURE 2.
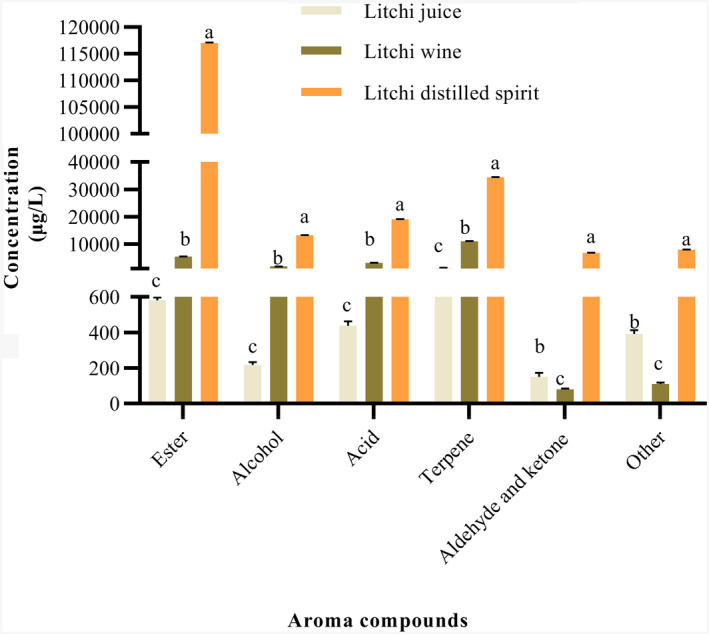
Comparison of the concentration (μg/L) of aroma compounds in litchi juice, wine, and distilled spirit. Values in the same column with different superscripted alphabet are significantly different at p < .05
TABLE 2.
Concentration (μg/L) of volatile compounds detected in litchi juice, litchi wine, and distilled spirit
| Code | Aroma compound | Concentration (μg/L) | ||
|---|---|---|---|---|
| Litchi juice | Litchi wine | Litchi distilled spirit | ||
| Esters | ||||
| E1 | Ethyl acetate | 49.52 ± 7.21c | 1973.52 ± 142.78b | 3,933.12 ± 267.09a |
| E2 | Diisopropyl adipate | 81.21 ± 10.54a | nd | nd |
| E3 | cis‐Whiskey lactone | nd | nd | 298.72 ± 42.22a |
| E4 | Isobutyl acetate | nd | 91.67 ± 9.54a | nd |
| E5 | Ethyl butyrate | nd | 89.49 ± 5.09b | 448.08 ± 52.34a |
| E6 | Ethyl isovalerate | nd | 8.73 ± 0.65b | 49.78 ± 4.87a |
| E7 | Isoamyl pyruvate | nd | nd | 1,294.46 ± 111.02a |
| E8 | linalyl caproate | nd | nd | 846.38 ± 68.77a |
| E9 | 5‐Oxohexanethioic acid,s‐t‐butyl ester | nd | nd | 1,254.26 ± 143.09a |
| E10 | Isoamyl acetate | nd | 545.70 ± 45.02a | nd |
| E11 | Ethyl valerate | nd | 2.18 ± 0.03a | nd |
| E12 | Ethyl caproate | nd | 414.72 ± 36.82b | 2,887.64 ± 276.34a |
| E13 | Ethyl caprylate | nd | 1,296.28 ± 110.93b | 14,288.9 ± 737.72a |
| E14 | Terpinyl formate | nd | nd | 746.80 ± 87.57a |
| E15 | Ethyl caprate | nd | 408.18 ± 38.78b | 13,293.2 ± 462.38a |
| E16 | Citronellyl acetate | nd | 185.54 ± 20.13a | nd |
| E17 | Diethyl succinate | nd | 43.66 ± 5.11a | nd |
| E18 | Ethyl 9‐decenoate | nd | 54.57 ± 4.37a | nd |
| E19 | Ethyl−3‐hydroxytridecanoate | nd | 34.92 ± 2.13a | nd |
| E20 | Terpinyl formate | nd | nd | 746.80 ± 87.57a |
| E21 | Geranyl isovalerate | 40.28 ± 5.11b | nd | 799.08 ± 50.89a |
| E22 | Citronellyl acetate | nd | nd | 2,688.5 ± 21.42a |
| E23 | Ethyltrans−4‐decenoate | nd | nd | 2,688.5 ± 307.34a |
| E24 | Ethyl laurate | nd | nd | 497.87 ± 34.87a |
| E25 | Ethyl myristate | nd | nd | 7,617.44 ± 568.32a |
| E26 | Methyl Z−11‐tetradecenoate | nd | nd | 3,634.46 ± 643.99a |
| E27 | Pentadecanoic acid, ethyl ester | nd | nd | 647.24 ± 58.44a |
| E28 | Methyl 14‐methylpentadecanoate | nd | nd | 1513.41 ± 20.90a |
| E29 | Ethyl 9‐hexadecenoate | nd | nd | 6,771.06 ± 703.28a |
| E30 | 4‐Nitrophenylhexadecanoate | nd | nd | 5,327.24 ± 92.11a |
| E31 | Ethyl palmitate | nd | 117.87 ± 5.39b | 75,880.12 ± 123.2a |
| E32 | Ethyl 15‐methylheptadecanoate | nd | nd | 6,024.90 ± 78.89a |
| E33 | Ethyl oleate | nd | nd | 63.66 ± 5.92a |
| E34 | Ethyl linoleate | nd | nd | 19,815.3 ± 1,232.1a |
| E35 | Ethyl linolenate | nd | nd | 15,981.76 ± 32.00a |
| E36 | Dimethyl phthalate | 80.87 ± 9.08b | 102.53 ± 7.43a | nd |
| E37 | Diethyl phthalate | 91.67 ± 9.23a | nd | nd |
| E38 | Diisobutyl phthalate | 182.59 ± 21.22b | nd | 2,539.14 ± 347.23a |
| E39 | Dibutyl phthalate | 54.92 ± 8.34a | nd | nd |
| Alcohols | ||||
| A1 | 2‐Methyl−3‐buten−2‐ol | 17.83 ± 1.79b | nd | 297.37 ± 36.04a |
| A2 | 1‐Pentanol | 30.37 ± 2.46c | 763.98 ± 48.90b | 4,132.34 ± 320.90a |
| A3 | Isoamyl alcohol | nd | 255.39 ± 28.31b | 2,837.88 ± 247.28a |
| A4 | 3‐Methyl−3‐buten−1‐ol | 9.90 ± 0.08b | nd | 149.36 ± 28.47a |
| A5 | Hexyl alcohol | 15.85 ± 1.78a | 2.18 ± 0.07b | nd |
| A6 | 3‐Octanol | nd | 85.13 ± 6.09b | 248.94 ± 18.99a |
| A7 | 1‐Octen−3‐ol | 52.29±0.4.38a | 56.75 ± 7.26a | nd |
| A8 | 2‐Ethylhexanol | 23.11 ± 0.38b | 37.11 ± 4.31a | nd |
| A9 | 3‐Methylthiopropanol | 52.29±0.4.38a | 45.84 ± 2.80a | nd |
| A10 | 7‐Methyl−3‐methylene−6‐Octen−1‐ol | nd | 82.95 ± 3.42a | nd |
| A11 | 2‐(4‐methylene‐cyclohexyl)−2‐propen−1‐ol | 23.11 ± 0.38b | 61.12 ± 7.00a | nd |
| A12 | Phenethyl alcohol | nd | 464.94 ± 78.26a | nd |
| A13 | trans‐p−2,8‐Menthadien−1‐ol | 27.78 ± 4.39a | nd | nd |
| A14 | Furfuryl alcohol | 22.97 ± 2.08a | nd | nd |
| A15 | 2,7‐Octadien−4‐ol,2‐methyl−6‐methylene‐ | 27.28 ± 1.19a | nd | nd |
| A16 | 7‐Methyl−3‐methylene−6‐octen−1‐ol | 6.94 ± 0.03c | 82.95 ± 4.56b | 5,476.75 ± 10.23a |
| A17 | 2,6‐Octadiene−1,8‐diol,2,6‐dimethyl‐ | 27.24 ± 3.24a | nd | nd |
| Acids | ||||
| AC1 | 2‐Ethylexanoic acid | 49.68 ± 3.87a | nd | nd |
| AC2 | Ethylboronic acid | 30.98 ± 4.32c | 63.30 ± 3.19b | 497.86 ± 66.32a |
| AC3 | Methylenecyclopropane−2‐carboxylic acid | nd | 13.10 ± 0.09a | nd |
| AC4 | Acetic acid | nd | 96.04 ± 7.23a | nd |
| AC5 | Hexanoic acid | 71.04 ± 9.46a | 52.38 ± 4.90b | nd |
| AC6 | Octanoic acid | 138.36 ± 14.68c | 964.80 ± 81.21b | 3,335.74 ± 289.07a |
| AC7 | Nonanoic acid | 63.04 ± 6.54a | nd | nd |
| AC8 | 3,7‐dimethyl−6‐Octenoic acid | nd | 189.90 ± 34.78a | nd |
| AC9 | Decanoic acid | 50.75 ± 6.89c | 993.17 ± 100.78b | 3,476.10 ± 198.33a |
| AC10 | 3,7‐dimethyl−6‐octadienoic acid | nd | 373.26 ± 45.87a | nd |
| AC11 | Lauric acid | nd | 106.96 ± 11.34a | nd |
| AC12 | Myristic acid | nd | 133.15 ± 18.35b | 3,485.10 ± 378.62a |
| AC13 | Linoleic acid | nd | 91.68 ± 5.32a | nd |
| AC14 | Pentadecanoic acid | nd | nd | 3,026.80 ± 276.55a |
| AC15 | 14‐Pentadecenoic acid | nd | nd | 1,045.52 ± 87.22a |
| AC16 | Diethylenetriaminepentaacetic acid | 42.74 ± 3.98a | nd | nd |
| Terpenes | ||||
| T1 | cis‐Rose oxide | 169.34 ± 18.42c | 635.19 ± 48.09b | 5,053.40 ± 48.88a |
| T2 | trans‐Rose oxide | 75.32 ± 7.21c | 279.40 ± 34.21b | 1867.02 ± 198.34a |
| T3 | (1S)‐(1)‐beta‐Pinene | nd | 6.54 ± 0.98b | 127.56 ± 14.08a |
| T4 | D‐Sylvestrene | nd | 8.73 ± 0.34b | 248.94 ± 16.87a |
| T5 | Terpinolene | nd | 10.91 ± 0.79a | nd |
| T6 | p‐Mentha−3,8‐diene | nd | nd | 199.14 ± 21.22a |
| T7 | 2,6‐Dimethyl−1,3,5,7‐octatetrene | nd | nd | 297.86 ± 31.24a |
| T8 | 5‐Caranol | 21.37 ± 2.91c | nd | 2,788.08 ± 300.90a |
| T9 | b‐Guaiene | nd | nd | 995.74 ± 120.38a |
| T10 | Carveol | 8.03 ± 0.05b | 54.57 ± 8.39a | ‐ |
| T11 | Linalool | 85.18 ± 8.25c | 772.71 ± 37.77b | 4,381.28 ± 381.34a |
| T12 | 4‐Terpineol | 28.84 ± 1.19a | nd | nd |
| T13 | p‐Menth−1‐en−8‐ol | 71.58 ± 6.32b | 1672.02 ± 129.02a | nd |
| T14 | cis‐Carveol | 33.65 ± 3.13a | nd | nd |
| T15 | D‐Citronellol | 263.89 ± 30.67c | 5,662.18 ± 438.70b | 8,488.72 ± 789.32a |
| T16 | cis‐Geraniol | 33.12 ± 3.12c | 528.24 ± 36.90b | 1,344.26 ± 78.99a |
| T17 | trans‐Carveol | nd | 91.68 ± 8.73a | nd |
| T18 | Neroloxide | nd | 67.67 ± 8.32b | 2,937.44 ± 264.19a |
| T19 | iso‐Geraniol | 50.21 ± 6.51c | 870.74 ± 76.32b | 3,534.88 ± 200.15a |
| T20 | Geraniol | 397.44 ± 46.88c | 870.74 ± 48.77b | 1593.22 ± 07.32a |
| T21 | Elemicin | 87.07 ± 4.32a | nd | nd |
| T22 | cis‐Nerolidol | nd | nd | 597.44 ± 48.41a |
| Aldehydes and ketones | ||||
| AK1 | Hexanal | 18.69 ± 3.21a | nd | nd |
| AK2 | Acetal | nd | nd | 2,489.36 ± 235.21a |
| AK3 | trans−2‐Hexenal | 10.56 ± 1.99a | nd | nd |
| AK4 | 2‐Isopropylidene−5‐methyhex−4‐enal | 20.83 ± 3.01a | nd | nd |
| AK5 | 3,7‐dimethylnona−2,6‐dienal | nd | 10.91 ± 0.29a | nd |
| AK6 | 3‐Furaldehyde | nd | 41.47 ± 7.21a | nd |
| AK7 | 1,3‐Dioxolane−4‐methanol | nd | 4.58 ± 0.92a | nd |
| AK8 | 2‐Methyl−3‐octanone | nd | 8.73 ± 0.89a | nd |
| AK9 | 2‐Octanone | 20.84 ± 2.00a | 10.91 ± 0.77b | nd |
| AK10 | Acetophenone | 27.07 ± 1.54a | nd | nd |
| AK11 | Cyclohexanone, 2‐cyclohexyl‐ | nd | nd | 99.58 ± 10.32a |
| AK12 | 2‐Methyltetrahydrothiophen−3‐one | nd | nd | 398.28 ± 28.45a |
| AK13 | 3,6,6‐Trimethylundecane−2,5,10‐trione | nd | nd | 3,186.38 ± 273.89a |
| AK14 | β‐Damascenone | nd | nd | 647.24 ± 77.46a |
| AK15 | 3‐Isopropylidene−5‐methyhex−4‐en−2‐one | 7.25 ± 0.08a | nd | nd |
| AK16 | 1‐Butanone,1‐(2,4,6‐trihydroxyphenyl) | 49.24 ± 2.87a | nd | nd |
| AK17 | 6‐Methyl−5‐hepten−2‐one | 22.45 ± 2.58a | nd | nd |
| Others | ||||
| S1 | 2,6‐methyl−2‐octene | 176.94 ± 12.35a | nd | nd |
| S2 | Durenol | 33.68 ± 1.90a | nd | nd |
| S3 | 1,4‐Benzenediol, dimethyl‐ | 18.70 ± 0.05a | nd | nd |
| S4 | 2,4,6‐Triisopropylphenol | 43.27 ± 2.42a | nd | nd |
| S5 | 4,6‐Di‐tert‐butyl−2‐methylphenol | 46.87 ± 3.78a | nd | nd |
| S6 | Pentylcyclopropane | 48.61 ± 4.21 | nd | nd |
| S7 | 1,1‐Diethoxy−2‐methylbutane | nd | 4.37 ± 0.48a | 53.44 ± 7.45a |
| S8 | 1,1‐Diethoxy−3‐methylbutane | nd | nd | 99.58 ± 6.31a |
| S9 | 1‐(1‐Ethoxyethoxy)‐pentane | nd | 4.39 ± 0.92a | 298.72 ± 33.28a |
| S10 | 2‐Pentylfuran | 13.21 ± 0.09a | 8.73 ± 0.75a | 34.85 ± 5.32a |
| S11 | Trimethylhydrazine | 26.18 ± 3.23a | nd | nd |
| S12 | Mesylazide | 9.90 ± 4.11a | nd | nd |
| S13 | 2‐Ethyl‐p‐xylene | nd | nd | 149.36 ± 17.23a |
| S14 | Verbenyl ethyl ether | nd | nd | 1692.76 ± 187.33a |
| S15 | Styrene, 3,4‐dimethyl‐ | nd | nd | 1842.12 ± 88.89a |
| S16 | Benzene,1,2,3,5‐tetramethyl‐ | nd | nd | 1593.18 ± 123.89a |
| S17 | 2,4‐di‐tert‐butylphenol | nd | nd | 2041.26 ± 253.12a |
Data are expressed as mean ± standard deviation (n = 3). a‐c means with different lowercase letters in the same row indicate significant differences (p <.05);
“–”: Not detected.
FIGURE 1.
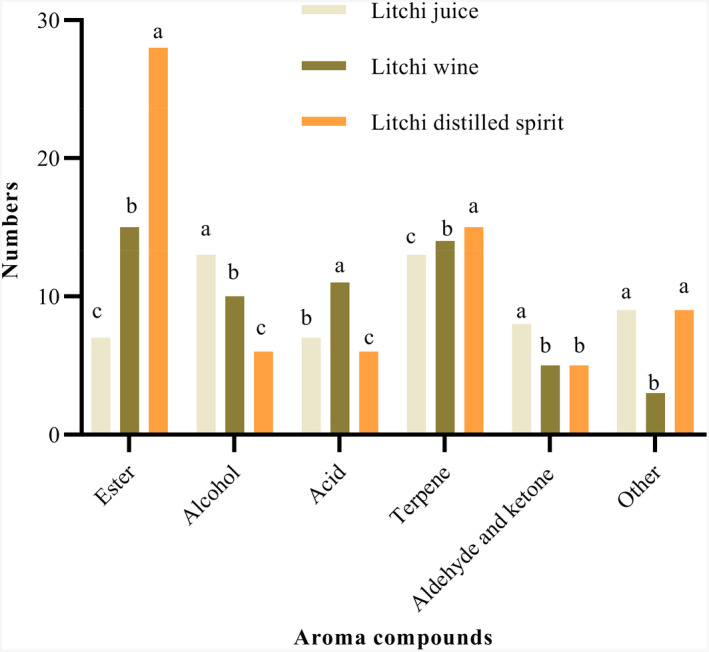
Comparison of the numbers of aroma compounds in litchi juice, wine, and distilled spirit. Values in the same column with different superscripted alphabet are significantly different at p < .05
TABLE 3.
Odor description, odor threshold, and odor activity value of main volatile compounds found in litchi juice, litchi wine, and distilled spirit
| Code | Volatile compound | Odor description a | Odor threshold (μg/L) b | Odor activity value | ||
|---|---|---|---|---|---|---|
| Litchi juice | Litchi wine | Distilled spirit | ||||
| E1 | Ethyl acetate | Pineapple | 7,500 | 0.006 | 0.26 | 0.52 |
| E3 | cis‐Whiskey lactone | Fruit, cocoa | 6 | 0.0 | 0.0 | 4.46 |
| E4 | Isobutyl acetate | Fruit, apple, banana | 1,600 | 0.0 | 0.06 | 0.0 |
| E5 | Ethyl butyrate | Apple, banana | 20 | 0.0 | 4.47 | 22 |
| E6 | Ethyl isovalerate | Fruit | 3 | 0.0 | 2.91 | 16.6 |
| E10 | Isoamyl acetate | Banana | 200 | 0.0 | 2.72 | 0.0 |
| E12 | Ethyl caproate | Apple | 14 | 0.0 | 29.62 | 206 |
| E13 | Ethyl caprylate | Sweet | 250 | 0.0 | 5.18 | 57.15 |
| E15 | Ethyl caprate | Pineapple, flower | 200 | 0.0 | 2.04 | 66 |
| E17 | Diethyl succinate | Wine, fruit | 1,200 | 0.0 | 0.04 | 0.0 |
| E18 | Ethyl 9‐decenoate | Fat | 100 | 0.0 | 0.55 | 0.0 |
| E22 | Citronellyl acetate | Pineapple, flower | 640 | 0.0 | 0.3 | 4.2 |
| E24 | Ethyl laurate | Leaf | 1,500 | 0.0 | 0.0 | 5.07 |
| E25 | Ethyl myristate | Ether | 2000 | 0.0 | 0.0 | 1.81 |
| E31 | Ethyl palmitate | Apple, sweet | 1,500 | 0.0 | 0.08 | 50.59 |
| A2 | 1‐Pentanol | Alcohol, pungent | 80,000 | 0.0003 | 0.009 | 0.05 |
| A3 | Isoamyl alcohol | Wine, solvent, bitter | 7,000 | 0.0 | 0.04 | 0.4 |
| A4 | 3‐Methyl−3‐butene−1‐ol | Apple | 600 | 0.02 | 0.0 | 0.2 |
| A5 | Hexyl alcohol | Resin, flower, green | 8,000 | 0.002 | 0.0002 | 0.0 |
| A7 | 1‐Octen−3‐ol | Mushroom | 18 | 2.91 | 3.15 | 0.0 |
| A8 | 2‐Ethylhexanol | Rose, green | 900 | 0.03 | 0.04 | 0.0 |
| A9 | 3‐Methylthiopropanol | Sweet potato | 1,000 | 0.05 | 0.05 | 0.0 |
| A11 | Phenethyl alcohol | Rose | 1,100 | 0.0 | 0.42 | 0.0 |
| AC4 | Acetic acid glacial | Vinegar | 200 mg/L | 0.0 | 0.0005 | 0.0 |
| AC5 | Hexanoic acid | Barbecue, cheese | 420 | 0.2 | 0.12 | 0.0 |
| AC6 | Octanoic acid | Sweat, cheese | 500 | 0.14 | 1.93 | 6.7 |
| AC9 | Decanoic acid | Rancid, fat | 1,000 | 0.05 | 1 | 3.5 |
| AC11 | Lauric acid | Metal | 1,500 | 0.0 | 0.07 | 0.0 |
| T1 | cis‐Rose oxide | Rose, flower | 20 | 8.5 | 3.2 | 252.67 |
| T2 | trans‐Rose oxide | Rose, flower | 20 | 3.8 | 1.4 | 93.36 |
| T11 | Linalool | Floral | 15 | 5.7 | 51.51 | 292.09 |
| T12 | 4‐Terpineol | Turpentine, nutmeg, must | 110 | 0.26 | 0.0 | 0.0 |
| T13 | p‐Menth−1‐en−8‐ol | Fruit, flower | 250 | 0.3 | 6.68 | 0.0 |
| T15 | D‐Citronellol | Clove | 100 | 2.63 | 56.62 | 84.88 |
| T16 | cis‐Geraniol | Rose | 300 | 0.11 | 1.76 | 4.48 |
| T18 | Neroloxide | Rose | 6,000 | 0.0 | 0.01 | 0.49 |
| T20 | Geraniol | Rose | 130 | 3.1 | 3.44 | 12.25 |
| AK9 | 2‐Octanone | Hot milk, peanut, green | 250 | 0.0 | 0.04 | 0.0 |
| AK14 | β‐Damascenone | Apple | 0.5 | 0.0 | 0.0 | 1,294.48 |
3.2. Alcohol
Alcohols, also called ‘fusel alcohols’, which are the main group of volatile metabolites synthesized by yeast during alcoholic fermentation, whereas only tiny quantities derive from the litchi (Dzialo et al., 2017; Ma et al., 2017). The greatest amount of alcohols was observed in the distilled spirit, in which the variety of alcohols was actually the lowest (Figure 1 and Figure 2). Conversely, the litchi juice showed the greatest variety with a minimum of alcohols concentration. Four alcohols were just detected in litchi juice (Table 2), namely trans‐p‐2,8‐menth‐adien‐1‐ol (27.78 ± 4.39 µg/L), 2,6‐octadiene‐1,8‐diol,2,6‐dimethyl‐ (27.24 ± 3.24 µg/L), 2,7‐octadien‐4‐ol,2‐methyl‐6‐methylene‐ (27.78 ± 4.39 µg/L), and furfuryl alcohol (22.97 ± 2.08 µg/L). These alcohols faded away during alcoholic fermentation. Another 2 alcohols, hexyl alcohol and 3‐methylthiopropanol, had a higher concentration in litchi juice but decreased and were only detected in litchi wine. Similar to previous results reported by Feng et al. (2018), who also observed hexyl alcohol in raw Sweetheart lychee. On the contrary, alcohols, such as 1‐pentanol, 1‐octen‐3‐ol, 2‐ethylhexanol, 2‐(4‐methylene‐cyclohexyl)‐2‐propen‐1‐ol, 7‐methyl‐3‐methylene‐6‐octen‐1‐ol, increased along with the fermentation. The amounts of the major alcohols 1‐pentanol and isoamyl alcohol and 3‐octanol increased in the distilled spirit in relation to litchi wine, what is similar to results reported by Chen and Liu (2016), who also reported the higher isoamyl alcohol in litchi (Litchi chinensis Sonn. var. Nuomi Ci) wine. The highest concentration of 7‐methyl‐3‐methylene‐6‐octen‐1‐ol (5,476.75 ± 10.23 µg/L) was found in litchi distilled spirit, followed by 1‐pentanol (4,132.34 ± 320.90 µg/L) and isoamyl alcohol (2,837.88 ± 247.28 µg/L). 1‐octen‐3‐ol presenting OAV >1 was only found in litchi wine (Table 3), which is different from the study of Tang et al. (2019), who did not find 1‐octen‐3‐ol in litchi (Huaizhi) wine.
3.3. Acids
Some acids in litchi distilled spirit are naturally present in litchi juice, while others are by‐products of fermentation (Venkatachalam et al., 2018). This group of volatiles could actively contribute to wine flavor at low levels. The widest variety of acids was observed in litchi wine, and there were 11 kinds of acids, but 7 and 6 kinds of acids in litchi juice and litchi distilled spirit, respectively. The concentration of total acids ranged from 446.59 (litchi juice) to 19058.24 µg/L (litchi distilled spirit) (Figure 2). Higher concentrations of octanoic acid (964.80 ± 81.21 µg/L) and decanoic acid (993.17 ± 100.78 µg/L) were recorded in litchi wine (Table 2); these values were higher than the values reported previously in lychee wine (319.58 ± 9.33 µg/L and 56.74 ± 0.87 µg/L) (Tang et al., 2019), but they were much lower than the amount at the end of alcohol fermentation of litchi wine (8,339.0 ± 794.2 µg/L and 10,711.9 ± 3,499.5 µg/L) (Wu et al., 2011). The apparent discrepancy may reflect differences in the type of raw litchi or the fermentation process's duration. Myristic acid (3,485.10 ± 378.62 µg/L), decanoic acid (3,476.10 ± 198.33 µg/L), octanoic acid (3,335.74 ± 289.07 µg/L), and pentadecanoic acid (3,026.80 ± 276.55 µg/L) were presented in higher concentration in distilled spirit. Only two types of acids showed OAVs >1 in our study (Table 3), namely decanoic acid and octanoic acid. The OAVs of octanoic acid and decanoic acid increased sharply during the distilled process. They were 3.5 times higher in distilled spirit than in litchi wine, consistent with the trends of their concentrations. However, unpopular sweat and rancid aromas might not be generated in distilled spirit because the OAVs of octanoic acid (OAV of 6.7) and decanoic acid (OAV of 3.5) were much lower than its odor thresholds.
3.4. Terpenes
Terpenes, synthesized from glucose by the isoprenoid pathway, provide a powerful floral and fruity aroma to berries (Venkatachalam et al., 2018; Wu et al., 2016). In the present study, 22 terpenes were detected in three treatments (Table 2). The kinds of total aroma compounds did not differ significantly among the three treatments (Figure 1). In contrast, the total contents of aroma compounds significantly differed among these treatments. Thirteen terpenes were found in litchi juice, namely cis‐rose oxide (169.34 ± 18.42 µg/L), trans‐rose oxide (75.32 ± 7.21 µg/L), 5‐caranol (21.37 ± 2.91 µg/L), carveol (8.03 ± 0.05 µg/L), linalool (85.18 ± 8.25 µg/L), 4‐terpineol (28.84 ± 1.19 µg/L), p‐menth‐1‐en‐8‐ol (71.58 ± 6.32 µg/L), cis‐carveol (33.65 ± 3.13 µg/L), D‐citronellol (263.89 ± 30.67 µg/L), cis‐geraniol (33.12 ± 3.12 µg/L), iso‐geraniol (50.21 ± 6.51 µg/L), geraniol (397.44 ± 46.88 µg/L), and elemicin (87.07 ± 4.32 µg/L) (Table 2). Except for 4‐terpineol, cis‐carveol, and elemicin, which were typically present in litchi juice but faded away in litchi wine and distilled spirit, these terpenes were sharply increased during the whole winemaking and distilling process. Geraniol, linalool, and cis‐rose oxide were higher than other terpenes in litchi juice; this trend was similar to the results reported by Chen and Liu (2016) in litchi juice. Geraniol could be metabolized into cis‐rose oxide by some yeast strains (Steyer et al., 2012). Simultaneously, cis‐rose oxide had a positive effect on the rose aroma of wine because of its high odor activity (Guth, 1997). Linalool is produced by many flowers and spice plants with a pleasant floral scent and can also contribute sweet and citrus scents to the overall aroma profile of litchi. Among these various terpenes, D‐citronellol (5,662.18 ± 438.70 µg/L) and p‐menth‐1‐en‐8‐ol (1672.02 ± 129.02 µg/L) were predominant in litchi wine; in contrast, D‐citronellol (8,488.72 ± 789.32 µg/L), cis‐rose oxide (5,053.40 ± 48.88 µg/L), linalool (4,381.28 ± 381.34 µg/L), and iso‐Geraniol (3,534.88 ± 200.15 µg/L) were also predominant in litchi distilled spirit. These terpenes, including cis‐rose oxide, trans‐rose oxide, linalool, geraniol, citronellol, and carveol, were previously reported in litchi wine (Chen & Liu, 2016; Tang et al., 2019; Wu et al., 2011). A total of 7 and 6 terpenes were presented with OAVs higher than 1 in litchi juice and distilled spirit (Table 3), respectively. Among them, D‐citronellol (OAV of 56.62), linalool (OAV of 51.51), p‐menth‐1‐en‐8‐ol (OAV of 6.68), geraniol (OAV of 3.44), and cis‐rose oxide (OAV of 3.2) were presented with relatively high OAVs and might be important contributors to the aroma of litchi wine. Moreover, linalool (OAV of 292.09), cis‐rose oxide (OAV of 252.67), and trans‐rose oxide (OAV of 93.36) had the highest OAVs in distilled spirit, and the OAVs were much higher than its odor thresholds. These aroma compounds might explain the unique rose and flower aroma in the distilled spirit.
3.5. Aldehydes and ketones
Aldehydes and ketones could confer a more abundant, more elegant, and unique aroma to wine (Nyanga et al., 2013). The widest variety of aldehydes and ketones was observed in litchi juice. There were eight kinds of aldehydes and ketones, but 5 and 5 kinds of aldehydes ketones in litchi wine and litchi distilled spirit (Table 2 and Figure 1), respectively. Conversely, the highest total concentration of aldehydes and ketones was found in litchi distilled spirit (6,820.84 µg/L). The kinds of aroma compounds were absolutely different among the three treatments except for the 2‐octanone, which is detected in litchi juice and litchi wine with peanut and green aroma. Among the various aldehydes and ketones, 3‐furaldehyde (41.47 ± 7.21 µg/L), 3,7‐dimethylnona‐2,6‐dienal (10.91 ± 0.29 µg/L), and 2‐octanone (10.91 ± 0.77 µg/L) were predominant in litchi wine, while 3,6,6‐trimethylundecane‐2,5,10‐trione (3,186.38 ± 273.89 µg/L) and acetal (2,489.36 ± 235.21 µg/L) were predominant in distilled spirit, which represented approximately 83% of the total aldehydes and ketones content. β‐damascenone presented the most robust odor (OAV of 1,294.48), increased significantly during the distilling process, and was only found in the distilled spirit. Therefore, apple and plum odor could be expected in the distilled spirit, as suggested by Genovese et al. (2007) and Lukić et al. (2016), who observed a similar trend in wines made from overripe grapes.
3.6. Others
The variety and content of others in litchi wine were actually the lowest, while the total contents of others in litchi distilled spirit represented approximately 94%. Nine aroma compounds of others were identified in litchi juice. Among these aroma compounds, 2,6‐methyl‐2‐octene, 2,4,6‐triisopropylphenol, dureno, 1,4‐benzenediol, dimethyl‐, pentylcyclopropane, trimethylhydrazine, mesylazide were just found in litchi juice and fade away in litchi wine. In particular, 2‐pentylfuran was the only one and presented in the whole winemaking and distilling process. 2‐Pentylfuran was also determined in cv. Dimrit grape seed oil. These author also reported that presence of 2‐pentylfuran may be associated with the applied high temperature during the oil extraction procedure (Sevindik et al., 2020). Six aroma compounds were newly generated during the distilling process. In the finished litchi distilled spirit, 2,4‐di‐tert‐butylphenol (2041.26 ± 253.12 µg/L) was most abundant and represented approximately 26% of the total contents, followed by benzene,1,2,3,5‐tetramethyl‐(1593.18 ± 123.89 µg/L), styrene,3,4‐dimethyl‐ (1842.12 ± 88.89 µg/L), and verbenyl ethyl ether (1692.76 ± 187.33 µg/L).
3.7. Analysis of the characteristic volatiles by principal component analysis (PCA) with OAVs (>1)
PCA was conducted to understand the correlation and segregation among those volatile compounds, which are significantly different among the three samples. Twenty‐two essential volatile compounds were selected for PCA to determine the contribution with OAVs of >1 in Table 3. The richest variety of OAVs >1 was observed in litchi distilled spirit; there were 19 kinds of volatile aroma compounds, but 6 and 16 kinds of aroma compounds in litchi juice and litchi wine, respectively. As shown in Figure 3, the three samples were differentiated by their main aroma profiles. The compound cis‐rose oxide (T1) was more related to litchi juice. Four kinds of volatile compounds may be related to litchi wine, including E5 (ethyl butyrate), T13 (p‐menth‐1‐en‐8‐ol), T15 (D‐citronellol), and A7 (1‐octen‐3‐ol). And six kinds of volatile compounds were more related to litchi distilled spirit, including E12 (ethyl caproate), E6 (ethyl isovalerate), T2 (trans‐rose oxide), T11 (linalool), and AK14 (β‐damascenone).
FIGURE 3.
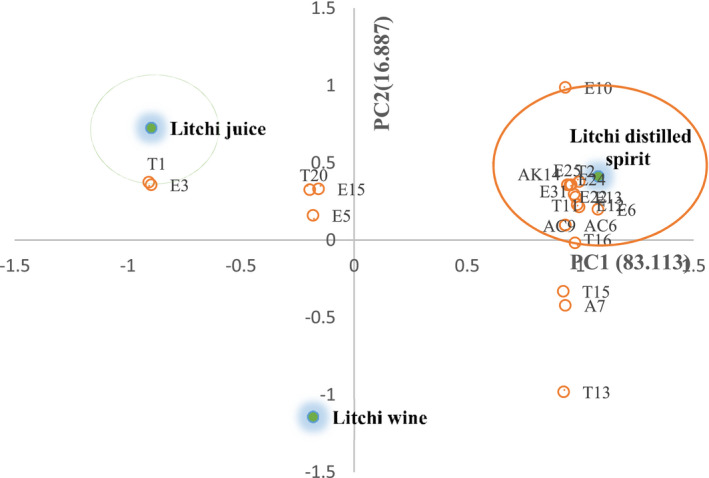
Principal components analysis for the main volatiles (OAVs >1) in litchi juice, wine, and distilled spirit
The kinds of total volatile aroma compounds appeared only in distilled spirit were highest (Figure 4 and Table 2), and there were 41 kinds of compounds in distilled spirit, but 25 and 23 in litchi juice and litchi wine, respectively. This illustrated fermentation and distilled spirit process lead to a series of by‐products. They include esters, alcohols, acids, terpenes, and so on, all of which influence the final wine's quality. The concentration of the by‐products can vary widely from a few g/L to hundreds of mg/L. Conversely, five kinds of volatile aroma compounds were disappeared in the alcohol fermentation process, and 33 disappeared in the distilled spirit process. Fourteen kinds of volatile aroma compounds detected in litchi juice showed increasing trends during alcoholic fermentation and distilled spirit process. Notably, some volatile aroma compounds detected in distilled spirit were ten times higher than litchi wine, such as ethyl caprylate (11.02), ethyl caprate (32.57), ethyl palmitate (643.76), isoamyl alcohol (11.11), 7‐methyl‐3‐methylene‐6‐octen‐1‐ol (66.02), myristic acid (26.17), (1S)‐(1)‐beta‐pinene (19.50), D‐sylvestrene (28.52), neroloxide (43.41), 1,1‐diethoxy‐2‐methylbutane (12.23) and 1‐(1‐ethoxyethoxy)‐pentane (68.05). According to the determined numbers of total aroma components (Figure 1), the proportion of esters in litchi distilled spirit was higher, while terpenes in litchi wine was higher, suggesting the difference of aroma in litchi wine and distilled spirit.
FIGURE 4.
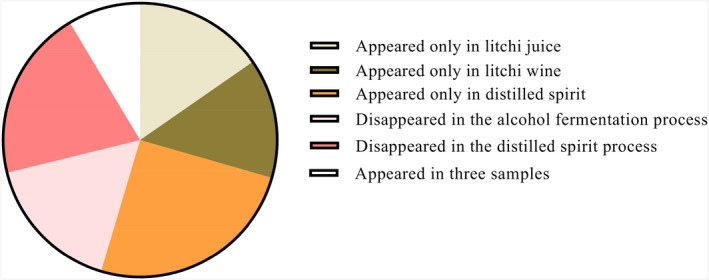
Changes of the numbers of aroma compounds in winemaking process
The presence of larger amounts of bioactive compounds in litchi assures its considerable nutritional value. Concentration (µg/L) of volatile compounds detected in litchi (Heiye and Guiwei) is shown in Table S1. 1‐Pentanol (30.37 µg/L), 3‐methyl‐3‐butene‐1‐ol (9.90 µg/L), 1‐octen‐3‐ol (52.29 µg/L), linalool (85.18 µg/L), 4‐terpineol (28.84 µg/L), trans‐2‐hexenal (18.56 µg/L) and 6‐methyl‐5‐hepten‐2‐one (22.45 µg/L) have higher concentration in litchi (Heiye) than litchi (Guiwei). Pieces of literature also indicate that litchi (Heiye) mainly includes higher total phenolics, total flavonoids, chlorogenic acid, and gallic acid than litchi (Guiwei) (Zhang et al., 2013; Zhao et al., 2020). Moreover, cis‐rose oxide, trans‐2‐hexenal, linalool, and geraniol had the highest OAVs in litchi (Heiye and Guiwei), much higher than its odor thresholds (Table S1). These aroma compounds might be important contributors to the unique aroma of litchi, agreeing with previous reports by Tang et al. (2019). They also observed higher OAVs of linalool (OAV of 43.270 ± 0.767), cis‐rose oxide (OAV of 99.969 ± 9.009), and geraniol (OAV of 1.866 ± 0.071) in litchi (Huaizhi). Notably, for the first time, the volatile aroma compounds in litchi distilled spirit (Heiye) were identified in this study. Compared with the Gewürztraminer wine, the concentrations of ethyl butyrate, cis‐rose oxide, linalool, and β‐damascenone were 403 ± 34 µg/L, 4.6 ± 0.8 µg/L, 35.9 ± 1.3 µg/L, and 2.0 ± 0.3 µg/L, respectively, and litchi distilled spirit possessed higher concentrations of ethyl butyrate (448.08 ± 52.34 µg/L), cis‐rose oxide (5,053.40 ± 48.88 µg/L), linalool (4,381.28 ± 381.34 µg/L), β‐damascenone (647.24 ± 77.46 µg/L) (Lukić et al., 2016). It is possible that the produced litchi distilled spirit had a stronger varietal character due to the increased concentrations and OAVs of β‐damascenone, linalool, ethyl isovalerate, ethyl butyrate, ethyl caproate, trans‐rose oxide, and cis‐rose oxide (Table 2 and Table 3) since these compounds are known to play a decisive fruit and flower aroma in the distilled spirit. Similar to previous results reported by Wu et al. (2011), who also observed higher OAVs of ethyl butyrate (OAV of 69.1 ± 4.0), cis‐rose oxide (OAV of 62.7 ± 0.1), and trans‐rose oxide (OAV of 20.6 ± 0.4) in litchi wine.
4. CONCLUSION
This study provides the first comprehensive characterization of the volatile aroma compounds contributing to the aroma profile of litchi distilled spirit (Heiye). One twenty‐eight different aroma compounds were identified in this study, which belonged to 6 chemical groups, including 39 esters, 16 alcohols, 16 acids, 22 terpenes, 17 aldehydes and ketones, and 18 other compounds. According to the determined concentrations of total aroma components, they were ranked in the following order (highest to lowest concentration): esters; terpenes; acids; alcohols; others, and aldehydes and ketones. The concentrations and kinds of total volatile aroma compounds were higher in distilled spirit than litchi wine. The richest variety of OAVs >1 was also observed in litchi distilled spirit; there were 19 kinds of volatile aroma compounds, but only 16 kinds of them in litchi wine. β‐damascenone, linalool, ethyl butyrate, ethyl isovalerate, ethyl caproate, trans‐rose oxide, and cis‐rose oxide were the essential aroma‐active compounds and played a decisive fruit and flower aroma in litchi distilled spirit. These findings provided comprehensive knowledge on the aroma character of litchi wine and distilled spirit.
5. INFORMED CONSENT
Written informed consent was obtained from all study participants.
CONFLICTS OF INTEREST
The authors declare no competing financial interests.
AUTHOR CONTRIBUTIONS
Zhao Lili: Data curation (equal); Writing‐original draft (lead). Ruan Shili: Methodology (equal); Resources (supporting). Yang XiangKe : Software (lead); Validation (equal). Chen QiLing : Formal analysis (equal); Resources (lead). Shi Kan: Conceptualization (lead); Validation (equal). Lu Ke: Data curation (equal); Investigation (lead). Ling He: Methodology (equal); Validation (lead). Yangbo Song: Writing‐original draft (equal); Writing‐review & editing (lead). Shuwen Liu: Project administration (lead); Writing‐review & editing (supporting).
ETHICAL APPROVAL
This study does not involve any human or animal testing.
Supporting information
Table S1
ACKNOWLEDGMENTS
This study was supported by the National Key R&D Program of China (Nos. 2019YFD1002503) and the Natural Science Foundation of Guangdong province (Nos. 2011A01003). We thank the staff at the Fermentation Engineering Laboratory, College of Enology, Northwest A & F University, for their technical assistance.
Lili Z, Shili R, Xiangke Y, et al. Characterization of volatile aroma compounds in litchi (Heiye) wine and distilled spirit. Food Sci Nutr. 2021;9:5914–5927. 10.1002/fsn3.2361
Contributor Information
Shuwen Liu, Email: liushuwen@nwsuaf.edu.cn.
Yangbo Song, Email: liushuwen@nwsuaf.edu.cn, Email: ys792@cornell.edu.
REFERENCES
- Aguirre, J. , Bizkarguenaga, E. , Iparraguirre, A. , Fernández, L. Á. , Zuloaga, O. , & Prieto, A. (2014). Development of stir‐bar sorptive extraction‐thermal desorption‐gas chromatography‐mass spectrometry for the analysis of musks in vegetables and amended soils. Analytica Chimica Acta, 812, 74–82. 10.1016/j.aca.2013.12.036 [DOI] [PubMed] [Google Scholar]
- Ayestarán, B. , Martínez‐Lapuente, L. , Guadalupe, Z. , Canals, C. , Adell, E. , & Vilanova, M. (2019). Effect of the winemaking process on the volatile composition and aromatic profile of Tempranillo Blanco wines. Food Chemistry, 276, 187–194. 10.1016/j.foodchem.2018.10.013 [DOI] [PubMed] [Google Scholar]
- Chen, D. , Jing, Y. C. , & Liu, S. Q. (2014). Impact of addition of aromatic amino acids on non‐volatile and volatile compounds in lychee wine fermented with Saccharomyces cerevisiae MERIT.ferm. International Journal of Food Microbiology, 170, 12–20. 10.1016/j.ijfoodmicro.2013.10.025 [DOI] [PubMed] [Google Scholar]
- Chen, D. , & Liu, S. Q. (2016). Transformation of chemical constituents of lychee wine by simultaneous alcoholic and malolactic fermentations. Food Chemistry, 196, 988–995. 10.1016/j.foodchem.2015.10.047 [DOI] [PubMed] [Google Scholar]
- Chen, S. , Wang, C. , Qian, M. , Li, Z. , & Xu, Y. (2019). Characterization of the key aroma compounds in aged Chinese rice wine by comparative aroma extract dilution analysis, quantitative measurements, aroma recombination, and omission studies. Journal of Agricultural and Food Chemistry 67, 4876–4884. 10.1021/acs.jafc.9b01420 [DOI] [PubMed] [Google Scholar]
- Dzialo, M. C. , Park, R. , Steensels, J. , Lievens, B. , & Verstrepen, K. J. (2017). Physiology, ecology and industrial applications of aroma formation in yeast. FEMS Microbiology Reviews, 41, S95–S128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuele, S. , Lauricella, M. , Calvaruso, G. , D’Anneo, A. , & Giuliano, M. (2017). Litchi chinensis as a functional food and a source of antitumor compounds: An overview and a description of biochemical pathways. Nutrients, 9, 992. 10.3390/nu9090992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erasmus, D. J. , Cliff, M. , & van Vuuren, H. J. J. (2004). Impact of yeast strain on the production of acetic acid, glycerol, and the sensory attributes of icewine. American Journal of Enology and Viticulture, 55, 371–378. [Google Scholar]
- Feng, S. , Huang, M. , Crane, J. H. , & Wang, Y. (2018). Characterization of key aroma‐active compounds in lychee (Litchi chinensis Sonn.). Journal of Food and Drug Analysis, 26, 497–503. 10.1016/j.jfda.2017.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese, A. , Gambuti, A. , Piombino, P. , & Moio, L. (2007). Sensory properties and aroma compounds of sweet Fiano wine. Food Chemistry, 103, 1228–1236. 10.1016/j.foodchem.2006.10.027 [DOI] [Google Scholar]
- Guth, H. (1997). Quantitation and sensory studies of character impact odorants of different white wine varieties. Journal of Agricultural and Food Chemistry, 45, 3027–3032. 10.1021/jf970280a [DOI] [Google Scholar]
- Kafkas, E. , Cabaroglu, T. , Selli, S. , Bozdogan, A. , Kurkcuoglu, M. , Paydas, S. , & Baser, K. H. C. (2006). Identification of volatile aroma compounds of strawberry wine using solid‐phase microextraction techniques coupled with gas chromatography‐mass spectrometry. Flavour and Fragrance Journal, 21, 68–71. 10.1002/ffj.1503 [DOI] [Google Scholar]
- Kilari, E. K. , & Putta, S. (2016). Biological and phytopharmacological descriptions of Litchi chinensis. Pharmacognosy Reviews, 10, 60. 10.4103/0973-7847.176548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S. J. , Kwon, H. S. , Shin, W. C. , Choi, J. Y. , & Noh, B. S. (2018). Analysis of the flavor of aged spirits made from sweet potato and rice by gas chromatography‐mass spectrometry. Food Science and Biotechnology, 27, 313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, N. , Shu, T. , Liang, Y. , Duan, Q. , Li, H. , & Wang, H. (2018). Effect of different harvest times on aroma components of Ecolly wines and distillations. Food Science, 39, 215–221. [Google Scholar]
- Li, W. , Liang, H. , Zhang, M. W. , Zhang, R. F. , Deng, Y. Y. , Wei, Z. C. , Zhang, Y. , & Tang, X. J. (2012). Phenolic profiles and antioxidant activity of litchi (Litchi Chinensis Sonn.) fruit pericarp from different commercially available cultivars. Molecules, 17, 14954–14967. 10.3390/molecules171214954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, X. , Hu, X. , Wu, W. , Liu, S. , & Li, C. (2019). Evaluation of the volatile profile of wax apple (Syzygium samarangense) wines fermented with different commercial Saccharomyces cerevisiae strains. Food Science and Biotechnology, 28, 657–667. 10.1007/s10068-018-0511-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, Y. Y. , Chua, J. Y. , Huang, D. J. , & Liu, S. Q. (2017). Chemical consequences of three commercial strains of Oenococcus oeni co‐inoculated with Torulaspora delbrueckii in durian wine fermentation. Food Chemistry, 215, 209–218. 10.1016/j.foodchem.2016.07.158 [DOI] [PubMed] [Google Scholar]
- Lukić, I. , Radeka, S. , Grozaj, N. , Staver, M. , & Peršurić, Đ. (2016). Changes in physico‐chemical and volatile aroma compound composition of Gewürztraminer wine as a result of late and ice harvest. Food Chemistry, 196, 1048–1057. 10.1016/j.foodchem.2015.10.061 [DOI] [PubMed] [Google Scholar]
- Lytra, G. , Tempere, S. , Marchand, S. , de Revel, G. , & Barbe, J. C. (2016). How do esters and dimethyl sulphide concentrations affect fruity aroma perception of red wine? Demonstration by dynamic sensory profile evaluation. Food Chemistry, 194, 196–200. 10.1016/j.foodchem.2015.07.143 [DOI] [PubMed] [Google Scholar]
- Ma, L. , Huang, S. , Du, L. , Tang, P. , & Xiao, D. (2017). Reduced production of higher alcohols by Saccharomyces cerevisiae in red wine fermentation by simultaneously overexpressing BAT1 and deleting BAT2. Food Chemistry, 65, 6936–6942. [DOI] [PubMed] [Google Scholar]
- Nyanga, L. K. , Nout, M. J. , Smid, E. J. , Boekhout, T. , & Zwietering, M. H. (2013). Fermentation characteristics of yeasts isolated from traditionally fermented masau (Ziziphus mauritiana) fruits. International Journal of Food Microbiology, 166, 426–432. 10.1016/j.ijfoodmicro.2013.08.003 [DOI] [PubMed] [Google Scholar]
- Paolo, C. , Alberto, A. , Vicenzo, L. G. , & Filippo, M. P. (1997). Pesticides in the distilled spirits of wine and its byproducts. Journal of Agriculture and Food Chemistry, 45, 2248–2251. 10.1021/jf960457l [DOI] [Google Scholar]
- Pareek, S. (2016). Nutritional and biochemical composition of lychee (Litchi chinensis Sonn.) cultivars. Nutritional composition of fruit cultivars, (395–418). Amsterdam, Netherlands: Elsevier. 10.1016/B978-0-12-408117-8.00017-9 [DOI] [Google Scholar]
- Sánchez‐Palomo, E. , Delgado, J. A. , Ferrer, M. A. , & Viñas, M. G. (2019). The aroma of La Mancha Chelva wines: Chemical and sensory characterization. Food Research International, 119, 135–142. 10.1016/j.foodres.2019.01.049 [DOI] [PubMed] [Google Scholar]
- Selli, S. (2007). Volatile constituents of orange wine obtained from moro oranges (Citrus sinensis [L.] Osbeck). Journal of Food Quality, 30, 330–341. 10.1111/j.1745-4557.2007.00124.x [DOI] [Google Scholar]
- Sevindik, O. , Guclu, G. , Bombai, G. , Rombola, A. D. , Kelebek, H. , & Selli, S. (2020). Volatile compounds of cvs Magliocco Canino and Dimrit grape seed oils. Journal of Raw Materials to Processed Foods, 1, 47–54. [Google Scholar]
- Steyer, D. , Ambroset, C. , Brion, C. , Claudel, P. , Delobel, P. , Sanchez, I. , & Legras, J. L. (2012). QTL mapping of the production of wine aroma compounds by yeast. BMC Genomics, 13, 573. 10.1186/1471-2164-13-573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, Z. S. , Zeng, X. A. , Brennan, M. A. , Han, Z. , Niu, D. , & Huo, Y. (2019). Characterization of aroma profile and characteristic aromas during lychee wine fermentation. Journal of Food Processing and Preservation, e14003. 10.1111/jfpp.14003 [DOI] [Google Scholar]
- Varzakas, T. , Zakynthinos, G. , & Verpoort, F. (2016). Plant food residues as a source of nutraceuticals and functional foods. Foods, 5, 88. 10.3390/foods5040088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velázquez, R. , Zamora, E. , Álvarez, M. L. , Hernández, L. M. , & Ramírez, M. (2015). Effects of new Torulaspora delbrueckii killer yeasts on the must fermentation kinetics and aroma compounds of white table wine. Frontiers in Microbiology, 6, 1222. 10.3389/fmicb.2015.01222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam, K. , Techakanon, C. , & Thitithanakul, S. (2018). Impact of the ripening stage of wax apples on chemical profiles of juice and cider. ACS Omega, 3, 6710–6718. 10.1021/acsomega.8b00680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X. , Liang, Y. Y. , Li, N. N. , Du, H. , Yang, C. L. , Li, H. , & Wang, H. (2018). Analysis of the aroma components of distilled spirits from main grape varieties in Yangling area. Chin. Brew., 37, 161–167. [Google Scholar]
- Wu, Y. , Duan, S. , Zhao, L. , Gao, Z. , Luo, M. , Song, S. , & Wang, S. (2016). Aroma characterization based on aromatic series analysis in table grapes. Scientific Reports, 6, 31116. 10.1038/srep31116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Y. , Zhu, B. , Tu, C. , Duan, C. , & Pan, Q. (2011). Generation of volatile compounds in litchi wine during winemaking and short‐term bottle storage. Journal of Agricultural and Food Chemistry, 59, 4923–4931. 10.1021/jf2001876 [DOI] [PubMed] [Google Scholar]
- Xiong, J. , Lin, R. , Liu, Z. , He, Z. , Tang, L. , Yang, Z. , & Zou, X. (2018). The recognition of litchi clusters and the calculation of picking point in a nocturnal natural environment. Biosystems Engineering, 166, 44–57. 10.1016/j.biosystemseng.2017.11.005 [DOI] [Google Scholar]
- Yang, Y. , Jin, G. J. , Wang, X. J. , Kong, C. L. , Liu, J. , & Tao, Y. S. (2019). Chemical profiles and aroma contribution of terpene compounds in Meili (Vitis vinifera L.) grape and wine. Food Chemistry, 284, 155–161. 10.1016/j.foodchem.2019.01.106 [DOI] [PubMed] [Google Scholar]
- Yi, H. C. , Moon, S. H. , Park, J. S. , Jung, J. W. , & Hwang, K. T. (2010). Volatile compounds in liquor distilled from mash produced using koji or nuruk under reduced or atmospheric pressure. Journal of the Korean Society of Food Science and Nutrition, 39, 880–886. 10.3746/jkfn.2010.39.6.880 [DOI] [Google Scholar]
- Zhang, K. , Yang, J. , Qiao, Z. , Cao, X. , Luo, Q. , Zhao, J. , & Zhang, W. (2019). Assessment of β‐glucans, phenols, flavor and volatile profiles of hulless barley wine originating from highland areas of China. Food Chemistry, 293, 32–40. 10.1016/j.foodchem.2019.04.053 [DOI] [PubMed] [Google Scholar]
- Zhang, R. , Zeng, Q. , Deng, Y. , Zhang, M. , Wei, Z. , Zhang, Y. , & Tang, X. (2013). Phenolic profiles and antioxidant activity of litchi pulp of different cultivars cultivated in Southern China. Food Chemistry, 136, 1169–1176. 10.1016/j.foodchem.2012.09.085 [DOI] [PubMed] [Google Scholar]
- Zhao, L. , Wang, K. , Wang, K. , Zhu, J. , & Hu, Z. Y. (2020). Nutrient components, health benefits, and safety of litchi (Litchi chinensis Sonn.): A review. Compr. Rev. Food Sci. F., 19(4). [DOI] [PubMed] [Google Scholar]
- Zheng, C. Y. , Gong, L. T. , Huang, Z. Q. , Liu, Z. B. , Zhang, W. , & Ni, L. (2014). Analysis of the key volatile aroma compounds in sweet Hongqu glutious rice wine. Journal of Chinese Institute of Food Science and Technology, 14, 209–214. [Google Scholar]
- Zhu, J. C. , Niu, Y. W. , Feng, T. , Liu, S. J. , Cheng, H. X. , Xu, N. , & Xiao, Z. B. (2014). Evaluation of the formation of volatiles and sensory characteristics of persimmon (Diospyros kaki Lf) fruit wines using different commercial yeast strains of Saccharomyces cerevisiae. Natural Product Research, 28, 1887–1893. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


