Abstract
Objective
The purpose of this study was to describe adverse events (AEs) and dropouts (DOs) in randomized controlled trials of therapeutic exercise for hip osteoarthritis (HOA) and to identify whether Consolidated Standards of Reporting Trials (CONSORT) guidelines were followed.
Methods
The Cochrane Library, Embase, PubMed, and CINAHL databases were searched. Randomized controlled trials of therapeutic exercise for HOA published in English from January 1, 1980 to August 1, 2020 were included. Studies were excluded if other interventions were provided, if participants had previous hip arthroplasty, or if AEs and DOs for HOA participants were not reported separately. The internal validity of each study (Physiotherapy Evidence Database [PEDro] scoring) was assessed, participant and intervention characteristics were extracted, and the existence of a clear statement and reasons for AEs and DOs was reported. Descriptive statistics characterized results. Data heterogeneity prohibited the use of meta-analysis.
Results
Fourteen studies (mean PEDro score = 7.4; range = 6-10) from 10 countries were included, with 707 participants exercising. Exercise intensity was unspecified in 72.2% of exercise arms. Six studies (42.9%) included a statement of AEs, and 32 AEs were reported. All studies had a DO statement, but 29.0% of DOs occurred for unknown reasons. Six studies (42.9%) gave reasons for DOs that could be classified as AEs in 9 participants; 41 participants (5.8%) experienced exercise-related AEs.
Conclusion
Reports of AEs were inconsistent, some DOs were potentially misclassified, and primary components of exercise interventions were frequently unreported. Despite these limitations, the overall low number of nonserious AEs suggests that the exercise-related risk of harm is minimal for individuals with HOA.
Impact
Understanding the risk of harm associated with exercise for HOA can better inform safe dosing of exercise, clinical implementation, and replicability. Informative, consistent reporting of AEs, DOs, and exercise is needed. Greater use of the CONSORT harms-reporting checklist is warranted.
Keywords: Adult, Exercise Therapy, Hip Osteoarthritis, Rehabilitation, Risk Assessment
Introduction
Hip osteoarthritis (HOA) is associated with impaired physical function, pain, and economic burden.1 The lifetime risk of developing symptomatic HOA has been reported to be 18.5% for men and 28.6% for women.2 Over the past few decades, the prevalence of total hip arthroplasty has grown 8-fold.3 Whereas the effectiveness of total hip arthroplasty is high, it is equally important to identify effective and noninvasive treatments to manage HOA symptoms and reduce disease progression, as well as delay the need for or time to total hip arthroplasty.
Several professional rheumatology, osteoarthritis, and health organizations recognize therapeutic exercise as an effective approach to manage HOA and to improve outcomes related to pain, physical function, mobility, and quality of life.4–6 Structured therapeutic exercise programs vary greatly depending on patient goals, function, likes and dislikes, and the environment. Therefore, proper conventions for exercise prescription should be followed to include the specification of frequency, intensity, duration, mode of exercise, and level of supervision.7 A comprehensive description of these elements enables clear interpretation of the dose, and facilitates clinical implementation, replicability, and advancements for therapists and researchers alike.8,9
Importantly, the advocacy of therapeutic exercise for HOA is largely based on its effectiveness in individuals with knee osteoarthritis, given the limited number of studies that examine therapeutic exercise specifically for the hip alone.10 As researchers are being encouraged to shift their focus toward identifying the ideal therapeutic exercise prescription for different stages or severities of HOA,11,12 an understanding of the potential risk of harm is vital to safely optimize the benefits of the intervention. However, the risks of harm, or adverse events (AEs), associated with therapeutic exercise for HOA have not yet been comprehensively evaluated.
Under the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) harms statement, AEs are broadly defined as any unfavorable outcome that occurs during or after an intervention, where the event can reasonably be attributed to the intervention itself.13 In this regard, participant dropouts (DOs) have the potential to be misclassified. Understanding why a participant discontinues an exercise intervention may help inform clinicians and researchers about the feasibility, efficacy, or harms related to that particular mode or dose of exercise. Taken together, knowledge of the risk of exercise-related AEs and DOs enables clinicians to provide patients with explicit instruction, appropriate expectations regarding harmless and harmful symptoms associated with exercise, and an opportunity to elucidate the characteristics of participants who may be at greater risk of exercise-related AEs. Additionally, a statement of adherence to therapeutic exercise is also informative because it may be related to AEs, exercise dose, and/or feasibility.
Prior systematic reviews and meta-analyses have examined the occurrence of AEs in relation to exercise.14–17 These reviews generally conclude that participating in exercise is safe; however, different exercise interventions and definitions of AEs are operationalized in populations so heterogeneous that it may not be appropriate to extrapolate the conclusions specifically to HOA. Moreover, these reviews did not assess the exercise-related risk of harm with the additional consideration of reasons for DOs.
In an effort to improve the standardization and transparency of reporting the design and conduct of clinical trials, the Consolidated Standards of Reporting Trials (CONSORT) statement recommends that authors report AEs according to a 10-item harms checklist. This checklist includes, but is not limited to, data such as the specific definition of AE used, how and when the AE information was collected, relatedness to the intervention, as well as the reasons and severity of the AE.
In this study, we aimed to identify how AEs and DOs are defined and reported in clinical trials for therapeutic exercise interventions used to treat adults with HOA. We also aimed to identify the harm associated with therapeutic exercise for HOA by describing the exercise frequencies, intensities, durations, modes, levels of supervision, and adherence. We hypothesized that the classification of AEs and DOs would be inconsistent across identified randomized controlled trials and that interventions with direct supervision of therapeutic exercise would report fewer AEs.
Methods
Data Sources and Searches
The research team included the authors (K.A.J., J.v.H., and M.D.I.), responsible for all procedures detailed below. The American Physical Therapy Association (APTA) definition of therapeutic exercise18 was used to conduct a systematic review of randomized controlled trials of therapeutic exercise for managing HOA symptoms. The Cochrane Library, CINAHL, PubMed, and Embase databases were searched for peer-reviewed randomized controlled trials conducted in adults diagnosed with HOA and published in English from January 1, 1980 to August 1, 2020. The following search terms for HOA were applied: (hip osteoarthritis OR hip osteoarthritides OR coxarthrosis OR coxarthroses OR ((degenerative joint disease) AND hip)) OR (“osteoarthritis, hip”[Mesh]) OR ((“hip”[Mesh] OR “hip joint”[Mesh]) AND “osteoarthritis”[Mesh]))). This search was combined with the following search terms to identify therapeutic exercise interventions: ((“exercise”[Mesh] OR “Physical Fitness”[Mesh] OR “Exercise Therapy”[Mesh]) OR (exercise OR exercise therapy OR therapeutic exercise OR dynamic OR static OR aerobic OR anaerobic OR resistance OR resistance training OR strength OR strength training OR physical therapy OR physical activity OR physical activities OR acute OR isometric OR isotonic OR isokinetic)). Lastly, the following search terms related to AEs were applied: (harm* OR “risk of harm*” OR “adverse event*” OR “safety” OR “risk”).
These search terms were used in an effort to include every relevant (international and/or anachronistic) naming convention of HOA, types of therapeutic exercise, and AEs over the past 4 decades. All modes of exercise were included so long as the APTA definition of therapeutic exercise was met.18 Studies that compared different nonpharmacological interventions were eligible as long as the therapeutic exercise arm included only therapeutic exercise. The exception to this criterion was if “education” was the additional component, because this would not pose any additional risks. For example, a study that prescribed exercise plus pharmaceuticals would be excluded, whereas a study that prescribed exercise plus education would be included. In addition, studies were excluded if they enrolled participants with a history of arthroplasty or nonspecified hip pain and if they enrolled participants with other forms of arthritis (knee osteoarthritis, rheumatoid arthritis) and did not report separate outcomes for participants based on their diagnosis. The PRISMA harms checklist13 was followed in the development and reporting of this systematic review, which was registered with and approved by PROSPERO,19 the international prospective register of systematic reviews.
Study Selection
Research team members individually examined the title and abstract of each study and eliminated studies that did not meet the inclusion criteria. The reference list for each chosen article was also screened for eligible studies. If the eligibility of the study was unclear, reviewers deliberated whether the study met inclusion/exclusion criteria and came to a consensus regarding inclusion. Next, the team members individually reviewed the full text of articles to determine whether the study met the inclusion criteria. The search results and process of study elimination are summarized in the PRISMA flow diagram (Figure). The search was performed again prior to final data analysis to determine if any relevant articles had been missed or recently published.
Figure.
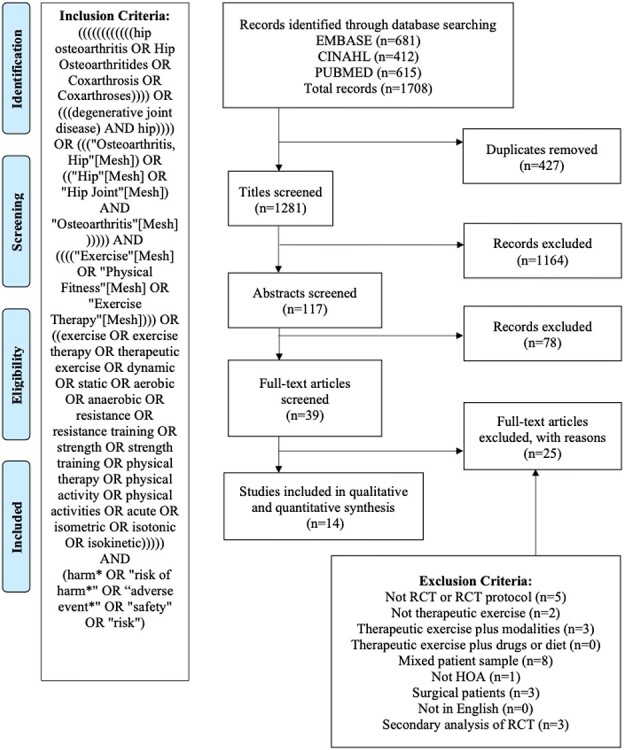
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart.
Data Extraction and Risk of Bias
After identifying all articles that met the inclusion criteria, data were extracted using a standardized form. Specific data elements included: year of publication; country where study was conducted; total number of participants in exercise and comparison arms; participant demographics and clinical characteristics (eg, age, sex, body mass index, disease severity); program length in weeks; frequency, intensity, duration, and type of intervention; the amount of exercise supervision; whether there was a clear statement of AEs and DOs; whether there was a clear reason for AEs and DOs; the severity of AEs; and the number of intervention- and nonintervention-related AEs and DOs.
We extracted the authors’ a priori definitions and protocol, thresholds for reporting AEs (eg, symptoms must be felt for ≥2 days to be reported as an AE), and descriptions of the attributions of AEs to define expectedness and relatedness to the intervention. We also identified whether authors reported methods used to identify AEs (ie, instruments used, frequency of identification). When DOs were reported in a reference to a symptom that could be considered an AE (eg, musculoskeletal pain), the DO was reclassified as an AE. When authors did not assign a severity level, identified and reclassified AEs were categorized as “severe” or “nonserious”.20 Severe AEs were defined by fracture, permanent damage, disability, or death. Nonserious AEs were defined by muscle strain, soreness, or a fall not related to the exercise program. Reclassified AEs (ie, misclassified DOs) were denoted as “related to the intervention” unless the authors specifically reported an alternative reason. The results section of each article was thoroughly annotated to ensure that ambiguously reported AEs were not overlooked.
Researchers independently assessed the risk of bias in included articles using the Physiotherapy Evidence Database (PEDro) scoring method.21 The PEDro scoring scale includes 11 items that are used to rate randomized controlled trials based on the internal validity and sufficiency of statistical information provided in order to inform clinical decisions. One or no points were given for the presence or absence of the following data elements: random allocation, concealed allocation, baseline comparability, participant masked, therapist masked with regard to allocation, assessor masked with regard to allocation, measures of key outcomes, intention to treat, results comparison, and point estimate of variability. Presence of eligibility criteria was assessed but not included in the score. Points were then summed to create a single score for each article ranging from 0 to 10. When discordant PEDro scores occurred between raters, a normative group process was used to reach a consensus.
Data Synthesis and Analysis
The team conducted a narrative synthesis of the studies. First, the team recorded the presence of a statement (yes/no) regarding AEs and DOs along with the number of exercise- and non–exercise-related AEs. If a statement was not made in-text, the participant flow diagram was checked to determine whether AEs and DOs were acknowledged. Next, the team recorded the number of DOs that occurred during the intervention within the exercise and comparison arms of each study. DOs were not recorded if they occurred during a follow-up period, after the exercise intervention was completed, or if studies noted that the DO occurred after randomization but prior to beginning the exercise intervention. DOs were recorded as “unspecified” in studies where it was defined as such, or if the reasons for DOs were reported in the aggregate (eg, across all study groups and could not be isolated to the exercise group). We then calculated the percentage of DOs among all exercisers and participants in the comparison arms and made a determination regarding whether the DO should be considered an AE (eg, withdrawal due to increased or unspecified pain).
Therapeutic exercise programs were evaluated by recording the frequency of exercise (days per week), intensity of exercise, duration of individual exercise sessions (minutes) and total duration of the programs (weeks), modes of exercise, level of supervision (partial or complete), and mean adherence [(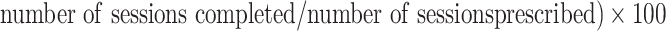 ]. Intensity of exercise was synthesized by aerobic and strength training only (guidelines for other modes have not been determined). Modes of exercise were categorized as follows: strengthening alone; strengthening plus balance, flexibility or range of motion; strengthening plus balance or flexibility plus aerobic; strengthening plus aerobic; and aerobic alone. In cases where a range was provided for any of these variables, we took the average or rounded up to the next category. Last, we calculated the percentage of studies by continent/region.
]. Intensity of exercise was synthesized by aerobic and strength training only (guidelines for other modes have not been determined). Modes of exercise were categorized as follows: strengthening alone; strengthening plus balance, flexibility or range of motion; strengthening plus balance or flexibility plus aerobic; strengthening plus aerobic; and aerobic alone. In cases where a range was provided for any of these variables, we took the average or rounded up to the next category. Last, we calculated the percentage of studies by continent/region.
Role of Funding Source
There was no specific funding for this work. M.D.I. was partially supported by an NIH grant (R03 AR057133–0) during the conduct of this study. The funder played no role in the design, conduct, or reporting of this study.
Results
Study Characteristics
We identified 1708 articles from our initial literature search. Of these, 14 studies from 10 countries (64.3% from Europe, 21.4% from North America, 14.3% from Asia-Pacific) met the inclusion criteria (Figure).22–35 There were 707 and 436 participants in the exercise intervention and comparison groups, respectively (Tab. 1). The participant mean age was 62.4 years, and there was a higher proportion of women (67.0%) than men. The comparison groups were slightly older on average (mean [SD] age = 64.2 [9.0] years), and there was a smaller proportion of women (60.1%). The majority of studies reported an average body mass index that fell in the “overweight” or “obese” categories. Four studies (26.7%) targeted individuals with end-stage HOA24,25,32,33; otherwise, the disease severity of the sample varied greatly. The median numbers of participants in the therapeutic exercise and comparison arms were 36 (range = 16-70) and 46 (range = 13-65), respectively.
Table 1.
Summary of Study Characteristics and Associated Numbers of Dropouts (DOs), Adverse Events (AEs), and DOs for Which the Reason Could Be Considered a Harma
| Study (year) | Country | Inclusion Criteria | Sample Size | Ageb | No. (%) Women | Group | Intervention Descriptionc | AE Statement | No. of AEsd | No. of DOs | No. of DOs for Which a Reason Could Be Considered a Harm |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bearne et al22 (2011) | England | Clinical diagnosis of HOA based on ACR criteria | 24 | 65.0 (52-76)e | 15 (62.5) | Exercise | 5-wk progressive strength, aerobic, flexibility, and balance program with full physical therapist supervision; prescribed 2 times/wk for 45 min at an unspecified intensity | No statement provided | 0 | 2 | 0 |
| 24 | 67.0 (53-78)e | 19 (79.2) | Usual care | Routine management prescribed by general practitioner | 0 | 6 | 0 | ||||
| Bennell et al23 (2018) | Australia | Clinical diagnosis of HOA based on ACR criteria | 67 | 61.2 (7.2) | 45 (67.2) | Pain-coping skills training | 16-wk progressive strength and flexibility program with partial physical therapist supervision; prescribed 3 times/wk for 30 min at an individualized, RPE-based intensity; HOA education and pain-coping skills training provided | AE statement, definition, and severity provided | 15 | 2 | 0 |
| 70 | 61.3 (7.1) | 37 (52.9) | Comparison | Same exercise protocol as described above except that no pain-coping skills training was provided | 16 | 5 | 0 | ||||
| Bieler et al28 (2017) | Denmark | Clinical diagnosis of HOA based on ACR criteria | 42 | 70.0 (6.3) | 33 (78.6) | Nordic walking | 16-wk progressive Nordic walking program with partial physical therapist supervision; prescribed 3 times/wk for 60 min at a moderate intensity (12-14 RPE) | No statement provided | 0 | 7 | 2 |
| 49 | 69.6 (5.4) | 34 (69.4) | Strength training | 16-wk progressive strength program with partial physical therapist supervision; prescribed 3 times/wk for 60 min at 75% 1RM | 0 | 1 | 0 | ||||
| 52 | 69.3 (6.4) | 36 (69.2) | Home-based exercise | 16-wk unsupervised home-based program including hip range of motion, stretching, and strengthening exercises with unspecified prescription details | 0 | 9 | 7 | ||||
| Fernandes et al29 (2010) | Norway | Hip pain for >3 mo; radiographic presence of HOA; Harris Hip Score of 60-95 | 55 | 58.4 (10.0) | 31 (56.4) | Exercise + education | 12-wk progressive strength and flexibility program with partial physical therapist supervision; prescribed 2 or 3 times/wk for an unspecified duration at 70%-80% 1RM; HOA education provided | AE statement provided; no definition | 1 | 1 | 0 |
| 54 | 57.2 (9.8) | 28 (51.9) | Education | HOA education provided | 0 | 0 | 0 | ||||
| French et al30 (2013) | Ireland | Diagnosis of HOA based on ACR clinical and radiographic criteria | 66 | 62.4 (9.1) | 44 (66.7) | Exercise | 8-wk progressive strength, aerobic, and flexibility program with partial supervision; supervised and home-based exercises were prescribed 1 or 2 times/wk and daily, respectively, for 30 min at an unspecified intensity | No statement provided | 0 | 3 | 0 |
| 65 | 61.4 (10.8) | 40 (61.5) | Exercise + manual therapy | 6-8 physical therapist sessions over 8 wk, including exercise program described above + up to 15 min of ≤5 manual therapy techniques | 0 | 5 | 0 | ||||
| Fukumoto et al31 (2014) | Japan | Bilateral or unilateral HOA; unspecified classification criteria | 23 | 52.4 (9.2) | 23 (100.0) | High-velocity exercise | 8-wk progressive strength program with partial physical therapist supervision; prescribed daily for an unspecified duration at a “somewhat hard” intensity; the concentric phase of exercise was performed as fast as possible | No statement provided | 0 | 4 | 2 |
| 23 | 52.5 (10.1) | 23 (100.0) | Low-velocity exercise | Same as described above, except that the concentric phase of exercise was performed for 3 s | 0 | 3 | 0 | ||||
| Gocen et al32 (2004) | Turkey | Patients scheduled for THA | 30 | 46.9 (11.5) | 13 (43.3) | Exercise | 8-wk strength and flexibility program with partial physical therapist supervision; prescribed daily for an unspecified duration at an unspecified intensity | No statement provided | 0 | 1 | 0 |
| 30 | 55.5 (14.4) | 8 (26.6) | Control | No exercise or education provided | 0 | 0 | 0 | ||||
| Hermann et al33 (2016) | Denmark | Patients scheduled for THA | 39 | 70.0 (7.7) | 27 (69.2) | Exercise | 10-wk progressive strength program with full physical therapist supervision; prescribed 2 times/wk for 60 min at moderate to high intensity | AE statement and definition provided | 0 | 3 | 0 |
| 40 | 70.8 (7.5) | 25 (62.5) | Control | Usual care, including a handout recommending low-intensity, home-based training with unspecified exercises or prescription details | 0 | 2 | 0 | ||||
| Hoeksma et al34 (2004) | The Netherlands | Clinical diagnosis of HOA based on ACR criteria | 53 | 71.0 (6.0) | 38 (71.7) | Exercise | 5-wk strength, aerobic, and flexibility program with full physical therapist supervision; prescribed 2 times/wk for 25 min at an unspecified intensity | AE statement provided; unclear definition | 0 | 3 | 2 |
| 56 | 72.0 (7.0) | 38 (67.9) | Manual therapy | 5-wk stretching, traction, and traction manipulation program performed 2 times/wk | 0 | 3 | 3 | ||||
| Østerås et al35 (2017) | Norway | Hip pain for >3 mo and radiographic presence of HOA | 16 | 62.8 (7.2) | 11 (68.8) | High-dose exercise | 8-wk progressive strength and aerobic program with full physical therapist supervision; prescribed 3 times/wk at 60%-80% HRmax and 12-14 RPE, respectively; high-dose strength and aerobic exercises totaled 240 repetitions/session (unspecified duration) and 40 min/session, respectively | No statement provided | 0 | 2 | 1 |
| 17 | 63.0 (10.6) | 10 (58.8) | Low-dose exercise | Same as described above, except that low-dose strength and aerobic exercises totaled 80 repetitions/session (unspecified duration) and 10-20 min/session, respectively | 0 | 0 | 0 | ||||
| Rooks et al24 (2006) | United States | Patients scheduled for THA | 32 | 65.0 (11.0) | 2 (62.5) | Exercise | 6-wk progressive strength, aerobic, and flexibility program with full physical therapist supervision; prescribed 3 times/wk for 30-60 min at a moderate intensity | AE statement provided; no definition | 0 | 4 | 0 |
| 31 | 59.0 (7.0) | 16 (51.6) | Education | No exercise; preoperative education provided | 0 | 5 | 0 | ||||
| Shrier et al25 (2008) | Canada | Patients scheduled for THA | 19 | 65.1 (8.0)f | 7 (87.5)f | Exercise | 8-wk progressive strength and balance program with partial supervision; prescribed daily for 10-20 min at an unspecified intensity | No statement provided | 0 | 11 | 0 |
| 17 | 65.6 (10.7)f | 4 (57.1)f | Control | No intervention provided | 0 | 10 | 0 | ||||
| Tak et al26 (2005) | The Netherlands | Clinical diagnosis of HOA; unspecified classification criteria | 55 | 67.4 (7.6) | 29 (64.0) | Exercise | 8-wk progressive strength and aerobic program with partial physical therapist supervision; prescribed 1 time/wk for 60 min at an unspecified intensity | No statement provided | 0 | 10 | 2 |
| 54 | 68.9 | 35 (71.0)f | Control | No intervention provided except for self-initiated contact with a general practitioner | 0 | 5 | 0 | ||||
| Thompson et al27 (2020) | United States | Radiographic evidence of HOA | 28 | 59.7 (8.2) | 9 (32.1) | Exercise | 12-wk progressive strength, aerobic, and flexibility program with partial physical therapist supervision; prescribed daily for 60 min; intensity was unspecified for strength but was prescribed at a moderate level for aerobic exercise | AE statement and definition provided | 0 | 7 | 0 |
| 13 | 60.8 (9.1) | 7 (70.0) | Control | The wait-list control group was offered the intervention described above after the study was completed | 0 | 3 | 0 |
a ACR = American College of Radiology; HOA = hip osteoarthritis; HRmax = maximum heart rate; RPE = rating of perceived exertion; THA = total hip arthroplasty; 1RM = 1 repetition maximum.
b Reported as mean (SD) unless otherwise indicated.
c DOs and AEs were extracted between baseline and the first follow-up time point; therefore, the exercise intervention durations reported are a reflection of that time window.
d All reported AEs (n = 32) and DOs for which the reason could be considered a harm (total n = 19; exercise n = 9; comparison n = 10) were categorized as “nonserious” in level of severity.
e Reported as mean (range).
f Only baseline characteristics for “completers” were reported.
Risk of Bias
The mean PEDro score of the studies was 7.4 (range = 6-10) (Tab. 2). Half of the studies used concealed allocation (50.0%), and the majority used assessor masking (71.4%). Two studies (14.3%) were awarded a point for participant masking because they specifically reported that participants were unaware of the hypothesis and/or purpose of the comparison arms.23,31
Table 2.
Risk-of-Bias Assessment of Included Randomized Trials Using the Physiotherapy Evidence Database (PEDro) Scoring Formata
| Study | Random Allocation | Concealed Allocation | Baseline Comparability | Participant Masked | Therapist Masked | Assessor Masked | Measures of Key Outcome (>85% Allocated) | Intention to Treat | Results Comparisons | Point Estimate Variability |
|---|---|---|---|---|---|---|---|---|---|---|
| Bearne et al22 (2011) | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 |
| Bennell et al23 (2018) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Bieler et al28 (2017) | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 |
| Fernandes et al29 (2010) | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 |
| French et al30 (2013) | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 |
| Fukumoto et al31 (2014) | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 |
| Gocen et al32 (2004) | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 |
| Hermann et al33 (2016) | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 |
| Hoeksma et al34 (2004) | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 |
| Østerås et al35 (2017) | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 |
| Rooks et al24 (2006) | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 |
| Shrier et al25 (2008) | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 |
| Tak et al26 (2005) | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 |
| Thompson et al27 (2020) | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 |
a Measures of at least 1 key outcome were obtained from more than 85% of the participants initially allocated to the groups. The PEDro score contains 11 components; the eligibility score is not calculated in the total score and therefore is not shown in the table.
Attributes of Therapeutic Exercise
Three of 14 studies compared more than 1 type of exercise intervention (eg, high vs low dose; high vs low velocity; strength vs aerobic training),28,31,35 and 1 study compared 2 groups with the same exercise dose but different education programs,23 yielding 18 exercise arms total. Table 3 summarizes the exercise prescription characteristics among all exercise arms. Most exercise interventions (50.0%) were more than 6 weeks up to 12 weeks in duration, and the common frequencies prescribed were 3 times per week (44.4%) and 5-7 d/wk (33.3%). The majority of interventions (94.4%) included strengthening as at least 1 component. The most common duration of each exercise session was at least 45 to 60 minutes, inclusive (27.8%). In approximately one-third of the exercise interventions, the duration of each session was either unreported or could not clearly be determined. Exercise intensity was not specified in more than half of the intervention arms. Considered together, 10 studies (71.4%) did not report at least 1 of the essential components of therapeutic exercise prescription.
Table 3.
Summary of Therapeutic Exercise Intervention Durations, Frequencies, Intensities, Modes, and Levels of Supervision in Exercise Arms in Included Studies (n = 18)
| Category | No. (%) | Reference(s) |
|---|---|---|
| Total duration of exercise training | ||
| 5 or 6 wk | 3 (16.7) | 22 , 24 , 34 |
| >6 to <12 wk | 9 (50.0) | 25 , 26 , 30–33 , 35 |
| ≥12 to <52 wk | 6 (33.3) | 23 , 27–29 |
| Frequency | ||
| 1 or 2 d/wk | 4 (22.2) | 22 , 26 , 33 , 34 |
| 3 d/wk | 8 (44.4) | 23 , 24 , 28 , 29 , 35 |
| >5 to 7 d/wk | 6 (33.3) | 25 , 27 , 30–32 |
| Duration of individual exercise sessions | ||
| Unspecified | 6 (33.3) | 29 , 31 , 32 , 35 |
| <30 min | 2 (11.1) | 25 , 34 |
| ≥30 to ≤45 min | 4 (22.2) | 22–24 |
| >45 to ≤60 min | 5 (27.8) | 26–28 , 33 |
| >60 min | 1 (5.6) | 30 |
| Intensity of exercisea | ||
| Aerobic exercise (n = 10) | ||
| Unspecified | 5 (50.0) | 22 , 26 , 30 , 34 |
| Perceived exertion scale | 3 (30.0) | 24 , 27 , 28 |
| Percentage heart rate reserve or maximal heart rate | 2 (20.0) | 35 |
| Strength exercise (n = 18) | ||
| Unspecified | 8 (44.4) | 22 , 25–27 , 30 , 32 , 34 , 35 |
| Perceived exertion scale | 8 (44.4) | 23 , 24 , 31 , 33 |
| Percentage 1 repetition maximum test | 2 (11.1) | 28 , 29 |
| Mode of exercise | ||
| Strengthening alone (progressive or not) | 4 (22.2) | 28 , 31 , 33 |
| Strengthening + balance, flexibility, or range of motion | 5 (27.8) | 23 , 25 , 29 , 32 |
| Strengthening + balance or flexibility + aerobic | 5 (27.8) | 22 , 24 , 27 , 30 , 34 |
| Strengthening + aerobic | 3 (16.7) | 26 , 35 |
| Aerobic alone | 1 (5.6) | 28 |
| Supervised sessions | ||
| No | 0 (0.0) | |
| Partially | 12 (66.7) | 23 , 25–32 |
| Yes | 6 (33.3) | 22 , 24 , 33–35 |
a Intensity is reported per mode to reflect therapeutic exercise interventions with more than 1 mode.
All interventions had in-person supervision (33.3%) or at least partial supervision (66.7%) during the exercise program. Our hypothesis, that fewer AEs would be reported among therapeutic exercise arms that were directly supervised, could not be tested because the majority of AEs were reported from 1 study.23 Among 4 exercise intervention groups with direct supervision,22,33,35 the reported level of adherence ranged from 81% to 93%. In addition, 1 study did not report adherence by study group,24 2 studies reported adherence as “complete” or “incomplete” (ie, adherence rate was not provided),27,33 and 1 study did not report adherence levels at all.34 Five exercise intervention groups with partial supervision primarily used training diaries to report adherence levels, which ranged from 53.3% to 93%.23,29–31 Among groups that used training diaries, 1 study reported that compliance with completing the training log was low (24%).30 Adherence could not be determined in 5 exercise arms because it was either partially reported (eg, either supervised or home-based session adherence was reported, but not both), not isolated to those with HOA, or not reported at all.24–26,32,34
Reporting of AEs and DOs
Tables 1, 4 and 5 summarize the characteristics of AEs and DOs reported. Less than half (42.9%) of the studies included a statement of AEs.23,24,27,29,33,34 Only 1 study provided an a priori definition for AEs, threshold for reporting, and methods for collecting AEs (ie, patient-report log and online survey at the end of the exercise intervention).23 Two studies reported predefined pain thresholds that were used to adjust exercise intensity (not to define AEs),28,33 and 2 other studies provided an a priori definition for AEs27,33; otherwise no other reporting characteristics or methods were mentioned.
Table 4.
Summary of Reporting of Adverse Events (AEs) and Dropouts (DOs) in Randomized Controlled Trials of Therapeutic Exercise for Hip Osteoarthritis (n = 14)
| Category | No. (%) | References |
|---|---|---|
| Total participants exercising | 707 | |
| Total exercise arms across all studies | 18 | |
| Studies with a clear statement of AEs | 6 (42.9) | 23 , 24 , 27 , 29 , 33 , 34 |
| Participants in studies that had a clear statement of AEsa | 344 (48.7) | |
| AEs reportedb | 32 (4.5) | 23 , 29 |
| Studies with a clear statement of DOs | 14 (100.0) | 22–35 |
| Studies that reported DOsb | 13 (92.9) | 22–35 |
| DOs reportedb | 69 (9.8) | |
| Studies that reported DOs without clear reasons | 8 (57.1) | 22 , 23 , 26 , 27 , 30 , 33 , 35 |
| DOs reported with unclear reasons | 20 (29.0) | |
| Studies where reasons for DOs could be AEs (eg, increased pain) | 6 (42.9) | 26 , 28 , 30 , 31 , 34 , 35 |
| DOs with reasons that could be AEs | 9 (13.0) | |
| Participants who dropped out where reason could be considered an AEb | 9 (1.3) | |
| Participants who dropped out where reason could be considered an AE plus total number of AEs reportedb | 41 (5.8) |
a All AEs were related to the therapeutic exercise intervention.
b Among all participants allocated to the exercise intervention.
Table 5.
Types of Reported Adverse Events (AEs) and Study Dropouts (DOs) Among Participants in Exercise and Comparison Arms
| Exercise Arms | Comparison Arms | |||
|---|---|---|---|---|
| Types of AEs and DOs Reported | No. (%) | Reference(s) (No. of AEs or DOs) | No. (%) | Reference(s) (No. of AEs or DOs) |
| AEs reported | 32 (100.0) | 0 | ||
| Other pain | 15 (46.9) | 1523 | 0 | |
| Hip paina | 13 (40.6) | 1223; 129 | 0 | |
| Muscle soreness | 3 (9.4) | 323 | 0 | |
| Numbness | 1 (3.1) | 123 | 0 | |
| DOs for which reason could be considered a harm | 9 (100.0) | 10 (100.0) | ||
| Hip pain | 2 (30.0) | 231 | 0 (0.0) | |
| Unspecified pain | 5 (50.0) | 2 each26,28; 135 | 7 (70.0) | 722 |
| Complaints | 2 (20.0) | 234 | 3 (30.0) | 334 |
| DOs unrelated to exercise | 39 (100.0) | 19 (100.0) | ||
| Hip surgery | 8 (20.5) | 625; 226 | 8 (42.1) | 625; 1 each26,30 |
| Unrelated illness | 7 (17.9) | 1 each25, 26,28,29,32–34 | 2 (10.5) | 1 each26,30 |
| Lack of motivation/time | 9 (23.1) | 4 each27,31; 122 | 2 (10.5) | 1 each22,33 |
| Poor communication | 3 (7.7) | 325 | 2 (10.5) | 225 |
| Family reasons | 3 (7.7) | 1 each25,28,30 | 0 (0.0) | |
| Moved | 2 (5.1) | 226 | 1 (5.3) | 122 |
| Did not like the physical therapist | 1 (2.6) | 126 | 0 (0.0) | |
| No benefit | 1 (2.6) | 128 | 0 (0.0) | |
| Too hard | 1 (2.6) | 128 | 1 (5.3) | 122 |
| Unsatisfied | 1 (2.6) | 126 | 0 (0.0) | |
| Weather | 1 (2.6) | 128 | 0 (0.0) | |
| Not suitable for research | 1 (2.6) | 123 | 3 (15.8) | 226; 125 |
| Low back painb | 1 (2.6) | 131 | 0 (0.0) | |
| DOs with unclear reasons | 20 (100.0) | 19 (100.0) | ||
| Lost to follow-up | 11 (55.0) | 623; 327; 233 | 10 (52.6) | 522; 327; 1 each30,33 |
| Unspecified | 5 (25.0) | 230; 1 each22,26,35 | 4 (21.1) | 230; 1 each25,26 |
| Reasons aggregated | 4 (20.0) | 424 | 5 (26.3) | 524 |
a One participant withdrew because of the AE.
b Not considered a harm because the authors specifically indicated that the DO was unrelated to exercise.
Two studies reported 32 AEs (ie, muscle soreness, numbness) in participants assigned to exercise arms.23,29 Of these 2 studies, 1 reported 31 of the 32 AEs,23 and all were classified as “transient and minor” or “nonserious” using our definition.20 The other study with the single AE (ie, hip pain) did not provide an a priori definition and the severity was not classified29; therefore, we used our definition to classify it as “nonserious.”20 No AEs were reported in the comparison arms.
All 14 studies had a clear statement regarding DOs. All studies reported DOs that occurred during the exercise intervention period, and all used an intention-to-treat approach for analysis. Among those exercising, 1 study (with 4 DOs total)24 did not report DO reasons by group, and 16 DOs from 7 studies22,23,26,27,30,33,35 were unspecified (eg, reasons were either unreported altogether or participants were lost to follow-up), yielding 20 total DOs (29.0%) that occurred for unknown reasons. Most reasons for DOs were unrelated to the intervention (Tab. 5). Nine participants withdrew from 3 studies due to lack of motivation or time, and this was the most common specified reason for dropping out.22,27,31
Nine exercisers from 5 studies26,28,31,34,35 and 10 comparison participants from 2 studies22,34 gave reasons for DOs that we believe could be classified as AEs (Tab. 5). This reclassification increased the occurrence of nonserious AEs from 2 exercise arms to 8 exercise arms. One exerciser withdrew due to low back pain that was specifically cited as unrelated to the intervention; therefore this was not reclassified as an AE.31 Therefore, 41 of 707 exercisers (5.8%) and 10 of 436 comparison participants (2.3%) experienced intervention-related AEs.
Discussion
The clinical benefits of therapeutic exercise for HOA are well established. Less is known about the optimal dose, mode, intensity, duration, and frequency, and how these characteristics may impact the effectiveness of exercise.11 As future research shifts toward addressing this gap in knowledge, consideration should be given to the associated risk of exercise-related harms. This study determined how AEs and DOs were reported in clinical trials of therapeutic exercise interventions for adults with HOA in order to better understand the exercise-related risk for harm. We assessed 14 studies with a low risk of bias and participants who reflected a typical HOA population in terms of demographic (ie, age and sex) and clinical characteristics. Overall, there were substantial inconsistencies in the reporting and classification of AEs and DOs across the studies included in this review. Moreover, the heterogeneity and incomplete reporting of therapeutic exercise interventions made it difficult to draw accurate conclusions about the association of AEs with specific components of exercise.
Less than half of the identified studies made a clear statement that acknowledged the potential risk for exercise-related harms, occurrence of AEs, or lack thereof. In contrast, all 14 studies made a statement regarding DOs, reported either in the participant flow diagram (as recommended by CONSORT), in-text, or both. Our results are consistent with another systematic review that found AEs to be underreported in progressive strength training16 and tai chi interventions17 in older adults. To broaden this concept, we found that some studies disclosed DOs attributed to increased pain and complaints but did not report any AEs. Based on the PRISMA definition of an AE, we identified these occurrences as AEs where the reason could be considered a harm (ie, misclassified AEs). Although the overall number of AEs remained low, this reclassification underscores a critical oversight of the expectedness of associated risk of harms of therapeutic exercise for HOA.
Only 3 studies reported their a priori definition, or expectedness, of an AE.23,27,33 Bennell et al23 considered an AE to be “any problem that participants believed to be caused by treatment that interfered with function lasting ≥2 days and/or requiring medication or seeking a health professional”; Thompson and colleagues27 defined AEs as “falls or musculoskeletal injury (eg, fracture, ligament sprain, muscle strain)”; and Hermann et al33 reported AEs as “medical illness, musculoskeletal injury or cancelled sessions due to pain and/or injury.” These authors highlight the concept that hip pain is not necessarily the only manifestation of exercise-related AEs worth considering in individuals with HOA. For example, other identified nonserious AEs included low back pain, nonspecified pain not located at the hip, muscle cramping/soreness, and numbness.23 The high prevalence of comorbidities in individuals with HOA may also contribute to the wide range of potential AEs and restrictions to certain therapeutic exercises.36 Interestingly, the majority of identified AEs were reported by Bennell et al,23 who used a broad (eg, “any problem”) and participant-focused definition of AE. This type of definition may facilitate a more comprehensive assessment of potential exercise-related harms for HOA because it may capture events specific to the musculoskeletal system and beyond.
Considering how infrequently studies defined AEs, it is not surprising that supplemental details related to severity, how, and when AEs were reported were largely omitted. In our review, Bennell et al23 provided the most comprehensive information regarding AE reporting, because their study was the only one to assign a severity level, define a reporting threshold for AEs, or explicitly indicate that AEs were reported by the participant. Specifically, 27 nonserious incidences of participant-reported pain met the predefined criteria for AEs in this strength training intervention. In contrast, Bieler et al28 and Hermann et al33 both referenced predefined thresholds of “acceptable,” “safe,” and “high-risk” pain based on a numeric scale from 0 to 10 (where 0 = no pain and 10 = worst imaginable pain) but used the ratings to adjust exercise intensity rather than to define AEs. Another study mentioned that they eliminated measures of hip extension, adduction, and abduction strength due to increased pain, but protocols for AEs and pain monitoring were not reported.25 These discrepancies, alongside the general paucity of detail of reporting AEs, reflects the need for a professional consensus on how transient symptoms of pain should be reported in therapeutic exercise interventions for HOA.
The reporting of DOs was generally more consistent than the reporting of AEs. The reasons for DOs varied and were not found to be associated with any particular characteristic of therapeutic exercise. This is likely due to the wide variety in exercise doses among a small sample of studies. However, about one-third of the reported DOs occurred for unknown reasons. Participants in clinical trials are not always required to share their reasons for dropping out. However, unknown reasons for withdrawal, in addition to the overall lack of reporting of AEs, makes it impossible to fully interpret the exercise-related risk of harm. Admittedly, the CONSORT statement for harm may be more directly applicable to clinical trials in pharmacology. Nonetheless, if therapeutic exercise for HOA is to be prescribed as “medicine,” then clinical trials should not be excused from the high standards of reporting harm.
Level of supervision may play an important role in the relation between therapeutic exercise adherence and potential risk of harm. Supervision can ensure that exercises are being performed safely and may facilitate the individualization and modification of exercise programs to improve adherence and clinical benefits. For example, transient pain flares after exercise are common among new exercisers with hip pain and can influence adherence,37,38 as demonstrated by the identification of 7 of the misclassified DOs (attributed to pain) among exercisers. As such, we hypothesized that studies with direct supervision would report fewer AEs and expected adherence to be lower in studies with partial supervision. However, we were unable to examine this relation due the low number of AEs, which were primarily reported from a single study,23 combined with inconsistent reporting of adherence in all exercise intervention arms. Of note, we reclassified 7 DOs (ie, increased pain) as AEs from 1 comparison arm that prescribed unsupervised, home-based exercise.28 This group was considered to be a comparison arm because their intervention did not meet the APTA definition for therapeutic exercise. Compared with the 2 partially supervised exercise arms in this particular study, the unsupervised, home-based arm had 5 more pain-related DOs and underscores the potential importance of supervision in relation to risk of harm.
Two studies reported baseline characteristics of participants stratified by those who completed the therapeutic exercise intervention and those who dropped out.24,31 Although these particular DOs could not be attributed to AEs, this level of specificity is encouraged because it enables clinicians to identify the exercise-related risk-benefit ratio in patients with varying demographics or HOA phenotypes. Taken together, clear and consistent reporting of AEs, DOs, and adherence may lend weight to the safety and feasibility of a particular exercise dose that could be further leveraged by direct supervision.
Finally, this review calls attention to the inconsistencies and heterogeneity of exercise intervention descriptions for adults with HOA. Assessment of harm in relation to specific components of therapeutic exercise is not possible if researchers do not provide specific details of the intervention itself. The majority of identified studies did not specifically detail at least 1 of the major components of exercise prescription (ie, frequency, intensity, time, or types of exercise). Phase 1 and 2 pharmacological trials are purposefully designed to simultaneously estimate efficacy and risk of harm to ensure adequate dose instructions.39 A comparable standard does not exist for nonpharmacological therapeutic exercise trials; yet, health care providers are expected to be cognizant of the appropriate exercise dose along with its associated risk of harm. Therapeutic exercise researchers may want to consider using the modified Consensus on Exercise Reporting Template (CERT), a 16-item checklist that guides a comprehensive description of the intervention.7 The CERT checklist can be used with the CONSORT harm statement to improve the clarity, transparency, and interpretability of a study’s methods and results.
Limitations and Strengths
In contrast to knee osteoarthritis, the sample of clinical trials of therapeutic exercise for adults with HOA is much smaller. This sample may be further limited by excluding articles not written in English. Inconsistencies in reporting harms and exercise interventions limited our ability to conduct inferential statistics. Additionally, our interpretation of “AE” differs from those operationalized by the identified studies, introducing the possibility of misclassification bias. For example, participant-reported pain could be a reflection of osteoarthritis itself, independent of the prescribed exercise. However, we used a consistent method in our classifications given the ambiguity of DO definitions. Discrepant coding was discussed until reviewers agreed on the specific data element, rather than assessing interrater reliability. The rigor of study eligibility criteria ensured our identification of AEs in adults with HOA was attributed to therapeutic exercise alone. Finally, multiple databases with a comprehensive set of search terms were employed to account for alternative terminology used in various regions and time periods.
Conclusions
Essential elements of exercise dosing and the CONSORT harms statement are frequently unavailable or poorly defined in current randomized controlled trials for therapeutic exercise in adults with HOA. This suggests that the true risks associated with certain aspects of therapeutic exercise for adults with HOA may not be reflected in the current literature. Despite this limitation, the available data (ie, small number of nonserious AEs) suggest that therapeutic exercise, overall, has minimal risk of harm for individuals with HOA. A professional consensus is warranted in regard to the operational definition of AEs and DOs associated with therapeutic exercise. The use of standard definitions along with specific reporting conventions consistent with the CERT and CONSORT guidelines may provide a deeper understanding of appropriate exercise dosing and prescription, as well as incidence of AEs due to therapeutic exercise in adults with HOA.
Contributor Information
Khara A James, Department of Physical Therapy, Movement and Rehabilitation Sciences, Northeastern University, Boston, Massachusetts, USA.
Johan von Heideken, Department of Women’s and Children’s Health, Karolinska Intitutet, Stockholm, Sweden.
Maura D Iversen, Department of Physical Therapy, Movement and Rehabilitation Sciences, Northeastern University, Boston, Massachusetts, USA; Department of Women’s and Children’s Health, Karolinska Intitutet, Stockholm, Sweden; Section of Clinical Sciences, Division of Rheumatology, Immunology & Allergy, Brigham & Women’s Hospital, Department of Medicine, Harvard Medical School, Boston, Massachusetts, USA; College of Health Professions, Sacred Heart University, Fairfield, Connecticut, USA.
Author Contributions
Concept/idea/research design: M.D. Iversen
Writing: K.A. James, J. von Heideken, M.D. Iversen
Data collection: K.A. James, M.D. Iversen
Data analysis: K.A. James, J. von Heideken, M.D. Iversen
Providing institutional liaisons: M.D. Iversen
Consultation (including review of manuscript before submitting): M.D. Iversen
Ethics Approval
None reported (the PRISMA harms checklist was followed in the development and reporting of this systematic review).
Funding
There was no specific funding for this study. M. Iversen was partially supported by a National Institutes of Health grant (R03 AR057133–0) during the conduct of this study.
Systematic Review Registration
The protocol for this systematic review was registered on PROSPERO (CRD42019136454).
Disclosures
The authors completed the ICMJE Form for Disclosure of Potential Conflicts of Interest and reported no conflicts of interest.
References
- 1. Litwic A, Registrar S, Edwards M, et al. Epidemiology and burden of osteoarthritis. Br Med Bull. 2013;44:185‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Murphy LB, Helmick CG, Schwartz TA, et al. One in four people may develop symptomatic hip osteoarthritis in his or her lifetime. Osteoarthr Cartil. 2011;18:1372‐1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kremers HM, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2014;97:1386‐1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr Cartil. 2019;27:1578‐1589. [DOI] [PubMed] [Google Scholar]
- 5. Rausch Osthoff AK, Niedermann K, Braun J, et al. 2018 EULAR recommendations for physical activity in people with inflammatory arthritis and osteoarthritis. Ann Rheum Dis. 2018;77:1251‐1260. [DOI] [PubMed] [Google Scholar]
- 6. Kolasinski SL, Neogi T, Hochberg MC, et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2020;72:149‐162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Slade SC, Dionne CE, Underwood M, et al. Consensus on exercise reporting template (CERT): explanation and elaboration statement. Br J Sports Med. 2016;50:1428‐1437. [DOI] [PubMed] [Google Scholar]
- 8. Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687. [DOI] [PubMed] [Google Scholar]
- 9. Iversen MD. Managing hip and knee osteoarthritis with exercise: what is the best prescription? Ther Adv Musculoskelet Dis. 2010;2:279‐290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goh SL, Persson MSM, Stocks J, et al. Efficacy and potential determinants of exercise therapy in knee and hip osteoarthritis: a systematic review and meta-analysis. Ann Phys Rehabil Med. 2019;62:356‐365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kraus VB, Sprow K, Powell KE, et al. Effects of physical activity in knee and hip osteoarthritis: a systematic umbrella review. Med Sci Sports Exerc. 2019;51:1324‐1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Morgan PJ, Young MD, Smith JJ, et al. Exercise as medicine during the course of hip osteoarthritis. Exerc Sport Sci. 2021;49:77‐87. [DOI] [PubMed] [Google Scholar]
- 13. Zorzela L, Loke YK, Ioannidis JP, et al. PRISMA harms checklist: improving harms reporting in systematic reviews. BMJ. 2016;352:i157. [DOI] [PubMed] [Google Scholar]
- 14. Bricca A, Harris LK, Jäger M, et al. Benefits and harms of exercise therapy in people with multimorbidity: a systematic review and meta-analysis of randomised controlled trials. Ageing Res Rev. 2020;63:101166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Niemeijer A, Lund H, Stafne SN, et al. Adverse events of exercise therapy in randomised controlled trials: a systematic review and meta-analysis. Br J Sports Med. 2020;54:1073‐1080. [DOI] [PubMed] [Google Scholar]
- 16. Liu CJ, Latham N. Adverse events reported in progressive resistance strength training trials in older adults: 2 sides of a coin. Arch Phys Med Rehabil. 2010;91:1471‐1473. [DOI] [PubMed] [Google Scholar]
- 17. Wayne PM, Berkowitz DL, Litrownik DE, et al. What do we really know about the safety of tai chi? A systematic review of adverse event reports in randomized trials. Arch Phys Med Rehabil. 2014;95:2470‐2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. American Physical Therapy Association . APTA guide to physical therapist practice 3.0. 2014. Accessed May 3, 2020 .https://guide.apta.org/.
- 19. Iversen MD, von Heideken J, James KA,, et al. Reporting of adverse events in clinical trials of therapeutic exercise in patients with hip osteoarthritis: a systematic review. PROSPERO 2019; CRD42019136454. Accessed November 2, 2019. https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=136454. [Google Scholar]
- 20. Vincent KR, Vasilopoulos T, Montero C, et al. Eccentric and concentric resistance exercise comparison for knee osteoarthritis. Med Sci Sports Exerc. 2019;51:1977‐1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maher C, Sherrington C, Herbert R, et al. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83:713‐721. [PubMed] [Google Scholar]
- 22. Bearne LM, Walsh NE, Jessep S, et al. Feasibility of an exercise-based rehabilitation programme for chronic hip pain. Musculoskeletal Care. 2011;9:160‐168. [DOI] [PubMed] [Google Scholar]
- 23. Bennell KL, Nelligan RK, Rini C, et al. Effects of internet-based pain coping skills training before home exercise for individuals with hip osteoarthritis (HOPE trial): a randomised controlled trial. Pain. 2018;159:1833‐1842. [DOI] [PubMed] [Google Scholar]
- 24. Rooks DS, Huang J, Bierbaum BE, et al. Effect of preoperative exercise on measures of functional status in men and women undergoing total hip and knee arthroplasty. Arthritis Care Res (Hoboken). 2006;55:700‐708. [DOI] [PubMed] [Google Scholar]
- 25. Shrier I, Zukor D, Boivin JF, et al. The feasibility of a randomized trial using a progressive exercise program in patients with severe hip osteoarthritis. J Musculoskelet Pain. 2008;16:309‐317. [Google Scholar]
- 26. Tak E, Staats P, Van Hespen A, et al. The effects of an exercise program for older adults with osteoarthritis of the hip. J Rheumatol Res. 2005;32:1106‐1113. [PubMed] [Google Scholar]
- 27. Thompson AR, Christopherson Z, Marshall LM, et al. A pilot randomized controlled trial for aerobic and strengthening exercises on physical function and pain for hip osteoarthritis. PM R. 2020;12:229‐237. [DOI] [PubMed] [Google Scholar]
- 28. Bieler T, Siersma V, Magnusson SP, Kjaer M, Christensen HEBN. In hip osteoarthritis, Nordic walking is superior to strength training and home-based exercise for improving function. Scand J Med Sci Sports. 2017;27:873‐886. [DOI] [PubMed] [Google Scholar]
- 29. Fernandes L, Storheim K, Sandvik L, et al. Efficacy of patient education and supervised exercise vs patient education alone in patients with hip osteoarthritis: a single blind randomized controlled trial. Osteoarthr Cartil. 2010;18:1237‐1243. [DOI] [PubMed] [Google Scholar]
- 30. French HP, Cusack T, Brennan A, et al. Exercise and manual physiotherapy arthritis research trial (EMPART) for osteoarthritis of the hip: a multicenter randomized controlled trial. Arch Phys Med Rehabil. 2013;94:302‐314. [DOI] [PubMed] [Google Scholar]
- 31. Fukumoto Y, Tateuchi H, Ikezoe T, et al. Effects of high-velocity resistance training on muscle function, muscle properties, and physical performance in individuals with hip osteoarthritis: a randomized controlled trial. Clin Rehabil. 2014;28:48–58. [DOI] [PubMed] [Google Scholar]
- 32. Gocen Z, Sen A, Unver B, Karatosun VGI. The effect of preoperative physiotherapy and education on the outcome of total hip replacement: a prospective randomized controlled trial. Clin Rehabil. 2004;18:353‐358. [DOI] [PubMed] [Google Scholar]
- 33. Hermann A, Holsgaard-Larsen A, Zerahn B, et al. Preoperative progressive explosive-type resistance training is feasible and effective in patients with hip osteoarthritis scheduled for total hip arthroplasty—a randomized controlled trial. Osteoarthr Cartil. 2016;24:91‐98. [DOI] [PubMed] [Google Scholar]
- 34. Hoeksma HL, Dekker J, Karel Ronday H, et al. Comparison of manual therapy and exercise therapy in osteoarthritis of the hip: a randomized controlled trial. Arthritis Care Res (Hoboken). 2004;51:722‐729. [DOI] [PubMed] [Google Scholar]
- 35. Østerås H, Paulsberg F, Olsen SE, et al. Effects of medical exercise therapy in patients with hip osteoarthritis: a randomized controlled trial with six months follow-up. A pilot study. J Bodyw Mov Ther. 2017;21:284‐289. [DOI] [PubMed] [Google Scholar]
- 36. Marks R, Allegrante JP. Comorbid disease profiles of adults with end-stage hip osteoarthritis. Med Sci Monit. 2002;8:CR305-9. [PubMed] [Google Scholar]
- 37. Fleng Sandal L, Bloch Thorlund J, Roos EM. No difference in muscle strength and functional performance in middle-aged individuals with knee or hip pain undergoing 8 weeks of neuromuscular exercise therapy or resistance training. Osteoarthr Cartil. 2016;24:S470. [DOI] [PubMed] [Google Scholar]
- 38. Sandal LF, Roos EM, Bøgesvang SJ, et al. Pain trajectory and exercise-induced pain flares during 8 weeks of neuromuscular exercise in individuals with knee and hip pain. Osteoarthr Cartil. 2016;24:589‐592. [DOI] [PubMed] [Google Scholar]
- 39. Friedman LM, Furberg C, DeMets DL. Fundamentals of Clinical Trials. 4th ed. Springer; 2010.


