Abstract
Background:
Over the past decade, the number of experimental and clinical studies using theta-burststimulation (TBS) protocols of transcranial magnetic stimulation (TMS) to modulate brain activity has risen substantially. The use of TBS is motivated by the assumption that these protocols can reliably and lastingly modulate cortical excitability despite their short duration and low number of stimuli. However, this assumption, and thus the experimental validity of studies using TBS, is challenged by recent work showing large inter- and intra-subject variability in response to TBS protocols.
Objectives:
To date, the reproducibility of TBS effects in humans has been exclusively assessed with motor evoked potentials (MEPs), which provide an indirect and limited measure of cortical excitability. Here we combined TMS with electroencephalography (TMS-EEG) and report the first comprehensive investigation of (1) direct TMS-evoked cortical responses to intermittent (iTBS) and continuous TBS (cTBS) of the human motor cortex, and (2) reproducibility of both iTBS- and cTBS-induced cortical response modulation against a robust sham control across repeat visits with commonly used cortical responsivity metrics.
Results:
We show that although single pulse TMS generates stable and reproducible cortical responses across visits, the modulatory effects of TBS vary substantially both between and within individuals. Overall, at the group level, most measures of the iTBS and cTBS-induced effects were not significantly different from sham-TBS. Most importantly, none of the significant TBS-induced effects observed in visit1 were reproduced in visit-2.
Conclusions:
Our findings suggest that the generally accepted mechanisms of TBS-induced neuromodulation, i.e. through changes in cortical excitability, may not be accurate. Future research is needed to determine the mechanisms underlying the established therapeutic effects of TBS in neuropsychiatry and examine reproducibility of TBS-induced neuromodulation through oscillatory response dynamics.
Keywords: TMS-EEG, Theta burst stimulation, Transcranial evoked potentials (TEPs), Reliability
Introduction
Repetitive Transcranial Magnetic Stimulation (rTMS) involves the application of sequences of TMS pulses at different patterns and frequencies to induce a lasting modulation of brain activity, presumably via Hebbian synaptic plasticity mechanisms [1,2]. A variety of different rTMS protocols have been developed, with the goal of reliably potentiating or suppressing cortical activity in the stimulated brain region [3,4]. These rTMS protocols have been increasingly used in neuroscience research to gain insights into causal relations between brain activity and behavior [5,6], to study brain networks [7], explore their dynamics, and characterize mechanisms of brain plasticity [8]. Furthermore, a number of therapeutic rTMS protocols have already been developed and cleared by the Food and Drug Administration for treatment of medication-resistant depression [9], obsessive compulsive disorder [10], and migraine [11]. Although randomized controlled clinical trials [9–11] have provided robust evidence on the therapeutic effects of rTMS, most uses of rTMS in basic neurophysiological research are predicated on the premise that rTMS modulates neural excitability in the stimulated region and/or its associated brain networks, and that these modulatory effects are reliable and predictable.
Theta-Burst Stimulation (TBS) is a particular form of rTMS inspired by invasive animal studies [12] reporting synaptic longterm-potentiation (LTP) in hippocampal axons in response to patterned electrical stimulation with high-frequency gamma bursts (50hz) repeated at the theta frequency (4–7 Hz). The first translation of this protocol into humans reported that when TBS was applied as continuous (cTBS) trains with a total of 600 gamma pulses in 40 s over the hand region of the primary motor cortex, it suppressed corticospinal excitability for up to 60 min [13], as indexed by decreased motor-evoked potentials (MEPs) recorded from the contralateral hand muscles. Conversely, if the same amount of gamma pulses were applied as 2 s of intermittent trains (iTBS) with 8 s of inter-stimulus intervals, corticospinal excitability is facilitated for up to 20 min. Because of their low stimulation intensities, shorter durations, and reported durability of effects [13], TBS protocols are often considered among the most effective, safe and practical approach to noninvasive neuromodulation [14]. Traditionally, TBS has been applied to motor cortex to modulate corticospinal excitability and evaluate the mechanisms of plasticity [15,16]. Further, a growing body of behavioral and clinical research has employed TBS to non-motor regions to modulate local cortical function and network-level connectivity in different non-motor cortices [17,18], and claimed therapeutic effects with specific brain-behavior relationships through presumed mechanisms of TBS in modulating cortical excitability [9,19–21].
Despite its increasingly widespread experimental and clinical use, however, the effects of TBS on cortical activity have not been clearly established. While the time course of TBS-induced neuromodulation has been extensively studied at the corticospinal level, MEPs are indirect readouts of cortical excitability, and thus precisely how cortical circuits are being modulated remains unclear. Direct epidural recordings from the cervical region of the spinal cord have revealed that while cTBS suppresses early I-waves [22], iTBS preferentially potentiates later I-waves [23]. These results suggest that TBS-induced changes observed in MEPs may reflect modulation of local and specific subpopulations of neuronal circuits in the stimulated motor region. Given the limitations of MEPs, TMS in combination with EEG (TMS-EEG) can be used to characterize TBS-induced neuromodulation directly at the cortical level, and thus may provide a more comprehensive and thorough examination of TBS effects across the cortex [24]. To date, few studies have reported TBS-induced changes in TMS evoked potentials (TEPs) from a single session [25–27] with conflicting results. No systematic research has been performed to evaluate reproducibility of TBS-induced effects in cortical responses using different TBS protocols with a robust sham-control. Furthermore, even the impact of TBS on corticospinal excitability is called into question by accumulating evidence showing high variability between individuals [28,29]. In particular, several studies [30–34] with large sample sizes have failed to produce consistent and expected response patterns, with only ~50% of subjects showing the expected neuromodulation in response to TBS protocols. Consequently, TBS protocols used in majority of these studies [30,31,33] did not show any significant neuromodulation at the group level. Recent research has also reported a considerable degree of intra-subject variability in TBS effects, such that the MEP response to TBS can be dramatically different (e.g. suppression versus facilitation) across day-to-day measurements for a given individual [35]. Most importantly, relatively few studies included sham TBS protocols, and thus the extent to which observed changes in excitability can be attributed to the TMS stimulation rather than other aspects of the experimental design remains unclear.
Here, we aimed to address these fundamental knowledge gaps by first (1) investigating the effects of iTBS, cTBS and sham stimulation of primary motor cortex (M1) on single-pulse TMS-evoked cortical (EEG) and motor (MEP) responses in a single session, and then (2) examining the reproducibility of TBS-induced neuromodulation with identical repeat sessions performed at least one month apart. For corticospinal responses, we expected significant facilitation of MEPs following iTBS, suppression following cTBS, and no significant change following sham TBS. Given the paucity of research on TEP responses to different TBS protocols, we did not predict directional differences between active TBS (iTBS vs cTBS) protocols on specific TEP elements, but rather hypothesized that both iTBS and cTBS would significantly modulate TEP responses to TMS, while sham-TBS would not induce any significant TEP modulation. We also hypothesized that such TBS-induced MEP and TEP modulations would be reliable across sessions.
Methods
Participants:
Twenty-four right-handed healthy volunteers (16 males; mean ± SD age = 29.67 ± 10.60 years, range = 18 to 49) participated in this study. None of the participants had self-reported history of psychiatric or neurological diseases. In accordance with the Declaration of Helsinki, experimental protocols and voluntary participation procedures were explained to all participants before they gave their written informed consent to the study. All questionnaires and procedures were approved by the Institutional Review Board of the Beth Israel Deaconess Medical Center, Boston, MA.
Experimental Procedures:
A T1-weighted (T1w) anatomical MRI scan was obtained from all participants at the beginning of the study and used for neuronavigation during TMS visits. In each visit, TMS-EEG and TMS-EMG data were collected synchronously. Details of MRI scanning, TMS, EEG and EMG systems are provided in the supplementary methods.
Each participant completed a total of six visits: two visits for iTBS, two visits for cTBS and two visits for sham TBS conditions. The order of first visits for each TBS condition was randomized between subjects and the same order was kept for repeat visits. First visits of TBS conditions were spaced at least 2 days apart to minimize carry over effects, and repeat visits of each TBS condition were performed at least 1 month after the first set of visits. Visits for a given participant were scheduled at the same time of the day as much as possible.
The general layout of our experimental design for TBS protocols is summarized in Fig. 1A. At the beginning of each visit, the motor hotspot was determined over the hand region of left motor cortex (L-M1) for eliciting MEPs in the right FDI muscle. The hotspot was defined as the region where single-pulse TMS elicited consistent MEPs in the FDI muscle. Following International Federation of Clinical Neurophysiology (IFCN) guidelines, resting motor threshold (RMT) was determined on the FDI hotspot as the minimum stimulation intensity eliciting at least five MEPs (≥50 μV) out of ten pulses in the relaxed FDI using monophasic (posterioranterior in the brain) current waveforms [36,37]. In compliance with the IFCN safety recommendations, participants were asked to wear earplugs during hotspot and RMT trials to protect their hearing, and to minimize external noise [38,39]. Active motor threshold was determined by again asking participants to flex their right index finger to engage the FDI muscle to approximately 20% of maximum voluntary contraction (ΜVC) and determined at the lowest intensity to produce MEPs of ≥200μV at least 5/10 times. TMS was administered with a thin layer of foam placed under the coil to minimize somatosensory contributions to TEPs. To minimize auditory evoked potentials related to the TMS coil click, auditory white noise masking was used throughout the TMS stimulation.
Fig. 1.
Experimental protocol and TMS-EEG metrics. A: Overall layout of a representative TMS-EEG session. B: TMS evoked potentials from 63 channels (upper panel) with selected peaks (colored vertical lines). Lower panel in B shows topographical distribution of selected peaks (blue: n15, red: P30). C: Computation of GMFP (upper panel), LMFP in left motor cortex (middle panel) within pre-defined time-windows (Early responses: 15–75 ms and Late responses: 76–300 ms following TMS pulse), and selected TEP peaks (lower panel) extracted from C3 electrode before (red line) and after (black line) iTBS at T20 in a representative subject.
Following determination of motor thresholds in each session, 120 single pulses of TMS (spTMS) at 120% of RMT were delivered to the motor hotspot as a baseline measure of corticospinal (MEPs) and cortical (TEPs) excitability. The TBS protocol for that session was then applied, followed by 4 blocks of 60 spTMS at 120% of RMT at 5 (T5), 10 (T10), 20 (T20), and 30 (T30) minutes after the TBS protocol (Fig. 1A). This manuscript primarily focuses on TBS effects on cortical excitability as assessed via TEPs; MEP effects are presented briefly here and are reported in detail in a separate manuscript (Boucher et al., submitted).
TBS Procedures:
TBS was applied to the motor hotspot at 80% of AMT. All TBS protocols were delivered as 3 pulse bursts at 50 Hz with 200 ms between bursts (600 pulses total). This pattern was delivered continuously in cTBS and in a 2 sec–on, 8 sec–off pattern for iTBS. Participants were randomly assigned to receive either sham using either the cTBS or iTBS pattern, which was maintained across both sham visits. Sham cTBS and iTBS protocols were administered on the motor hot spot from the placebo side of the Cool-B65 A/P coil with a 3D printed 3 cm spacer additionally attached to the placebo side (MagVenture A/S, Farum, Denmark). Both active and sham-TBS protocols also included delivery of weak current pulses (between 2 and 4 mA and proportional to the intensity of actual TMS pulse) via surface electrodes (Ambu Neuroline 715 12/Pouch) placed approximately 1 cm below the inion bump and synchronized with the TBS trains to produce scalp sensations during both active and sham TBS conditions. This was done with the intention of blinding participants as to what kind of stimulation they were receiving when the direct somatosensory sensations of active TBS were not present during the sham stimulation.
EEG preprocessing
All EEG data pre-processing was performed offline using EEGLAB 18.1 [40], and customized scripts running in Matlab R2017b (Math-Works Inc., USA). For TEP analyses, 120 single-pulse M1 block (Fig. 1A) before TBS were used as baseline (PreTBS) measures. 60 single pulse TEPs following TBS from both T5 and T10 were merged to create a single block of 120 trials and labeled as T5, while TEPs from T20 and T30 were merged to create another single block and labeled as T20 to measure TBS effects at two separate time points (Fig. 1A, red blocks). All trials within each block were then segmented into 3000 ms epochs, each starting 1000 ms before (pre-stimulus) and ending 2000 ms (post-stimulus) following TMS pulse, respectively. Baseline correction was performed by subtracting the mean pre-stimulus (−900 to −100) signal amplitude from the rest of the epoch in each channel. Following baseline correction, data were visually inspected to identify noisy channels (2.5 ± 1.5 channels were deleted on average; range 0–4 out of 63). Zero-padding between −2 ms and 14 ms time range were then applied to remove the early TMS pulse artifact from the EEG data. All zero padded epochs were then tagged based on voltage (≥100 μV), kurtosis (≥3), and joint probability (Single channel-based threshold ≥ 3.5sd; All channel-based threshold ≥ 5sd) metrics to identify excessively noisy epochs. Visual inspection was performed on the tagged epochs for the final decision for the removal of noisy epochs (18 ± 6 epochs were deleted on average; range 2–39 out of 120). Next, an initial round of fast independent component analysis (fICA) was performed to identify and remove components with early TMS evoked high amplitude electrode and EMG artifacts (1 ± 1 components were removed; range 0–3 out of 63). After the first round of fICA, the EEG data were interpolated for previously zero-padded time window around TMS pulse (−2 ms–14 ms) using linear interpolation, band pass filtered using a forward-backward 4th order butterworth filter from 1 to 100 Hz, notch filtered between 57 and 63 Hz, and referenced to global average. Subsequently, a second round of fICA was run to manually remove all remaining artifact components including eye movement/blink, muscle noise (EMG), single electrode noise, TMS evoked muscle, cardiac beats (EKG), as well as auditory evoked potentials. Fig. 2 shows representative topographies, single trial amplitudes and averaged time series of ICA components removed from the data in second round of fICA.
Fig. 2.
ICA-based cleaning of TMS-EEG. Topography (upper panels), sorted single trial amplitudes (middle panels) and averaged times series (lower panels) representing pulse, eye, muscle, EKG artifacts and auditory evoked potential (AEPs) components from a representative subject. These components were removed from the data using fICA (see methods for details of EEG preprocessing and supplementary materials for details of AEP identification).
Details for identifying and validating AEP components were provided in supplementary materials (see supplementary methods and Supplementary Fig. 1). In both rounds of fICA, a semiautomated artifact detection algorithm incorporated into the open source TMS-EEG Signal Analyzer (TESA v0.1.0-beta [41]) extension for EEGLAB was used to classify and visually inspect components based on their frequency, activity power spectrum, amplitude, scalp topography, and time course (http://nigelrogasch.github.io/TESA/). Finally, the data were low pass filtered with a 4th order Butterworth filter at 50 Hz, and interpolated for missing/ removed channels using spherical interpolation.
TMS-EEG and TMS-EMG metrics:
Details for computing Global Mean Field Power (GMFP) and Local Mean Field Power (LMFP) as TMS-EEG metrics are provided in the supplementary methods. 60 single pulse MEPs and TEPs following TBS from both T5 and T10 were merged to create a single block of 120 trials and labeled as T5, while MEPs and TEPs from T20 and T30 were merged to create another single block and labeled as T20 to measure TBS effects at two separate time points (Fig. 1A, red blocks).
Both for GMFP and LMFP, we took the integral of the time series and computed area under the curve (AUC) as the amount of global (GMFP) and local (LMFP) cortical response measure in two separate time windows. The first AUC window ranges from 15 to 75 ms and represents the sum of low amplitude and “early responses”, while the second AUC window ranges from 76 to 300 ms and represents the sum of large and “late responses” (Fig. 1C, second and third panels from the top). AUC values at each postTBS time point (T5 and T20) were normalized to baseline (PreTBS) by taking the percentage of “postTBS/PreTBS” for each subject. These AUC ratios were used to classify postTBS response of each subject into three categories: A “Facilitation” response was defined as a minimum of ≥10% increase, while a “Suppression” response is defined as a minimum of 10% decrease in AUC values at postTBS time points (T5 and T20). This 10% change threshold in postTBS is chosen to ensure a clear TBS effect accounting possible random fluctuations around baseline levels [42]. Therefore, subjects showing less than 10% increase or decrease in their responses at postTBS were classified with a “No Change” response type. In addition to AUC ratios, time series analyses were also performed both for GMFP and LMFP responses to examine significant differences between TBS blocks (Baseline vs T5 and Baseline vs T20) at the highest temporal resolution.
TEP peaks for each EEG channel were extracted by using predetermined time windows. The first negative peak N15 (14–24 ms following TMS), the first positive peak P30 (25–45 ms following TMS) and a second negative peak N100 (80–120 ms following TMS) were identified using these time windows, and the voltage amplitudes within each time range were averaged to compute TEP peaks (Fig. 1C, bottom panel).
MEPs were computed as the absolute amplitude difference between minimum and maximum voltage peaks from 20 to 50 ms following TMS for each trial. For a given block, individual MEPs greater than 2.5 standard deviations (SDs) from the mean were considered outliers and rejected from further analyses. Similar to GMFP and LMFP, mean MEP amplitudes for each post stimulation time-point were expressed as the percentage change from baseline.
Statistical analyses
To evaluate the stability of baseline measurements across visits, preTBS GMFP, and LMFP were entered into a repeated-measure analyses of variance (Rm-ANOVA) with Visit (V1, V2, V3, V4, V5, V6) as a within-subject factor. To test overall reproducibility of cortical responses to spTMS, intra-class correlation coefficients (ICCs) were computed for all metrics (GMFP, LMFP AUC, and TEP peaks) to calculate absolute agreement across all visits [43]. For the neuromodulatory effects of TBS, our goal was to examine if there is any “TBS-condition” main or “TBS-condition x Block” interaction effect in the first visit (Visit-1) and, if so, whether we can reproduce these effects in repeat data sets with an independent set of analyses (Visit-2). Thus, GMFP, and LMFP AUC, values from the 1st and 2nd visit of each condition were entered into separate Rm-ANOVAs with TBS-condition (iTBS, cTBS, Sham) and Block (PreTBS, T5, T20) as within-subject factors. In addition, to assess the effects of TBS on more conventional measures of corticospinal excitability, similar analyses were conducted for MEP values. We also performed preliminary analyses to examine the relationships between the TBS-induced changes in early cortical responses (early LMFP, 15–75 ms) and baseline measures of corticospinal (MEPs) and cortical (early LMFP) excitability. For this aim, early LMFP responses following each TBS condition were grouped as facilitation (post-TBS LMFP > pre-TBS LMFP) and suppression (post-TBS LMFP < pre-TBS LMFP) at T5, and baseline MEP and early LMFP amplitudes were retrospectively compared between groups using independent t-test statistics with Bonferroni correction for multiple comparisons. Further, we used bivariate Pearson correlations to examine the relationships between changes in MEPs and early LMFP responses, and between changes in MEPs and the early TEP peaks (N15, P30).
Cluster-based permutation paired sample t-test statistics were performed to compare GMFP and LMFP time series at each time point across blocks (PreTBS, T5, T20) for each TBS-condition and visit. First, we ran paired sample t-tests at each sample to determine significant time points between PreTBS and T5, and between PreTBS and T20 separately. We then computed the length of adjacent significant time points and sum of t-scores for significant time points to determine (1) cluster size and (2) cluster magnitude in the main analyses, respectively. Following main analyses, we performed permutation t-tests [44] (n = 1000) by randomly shuffling 50% of subjects across compared blocks (i.e., 50% of subjects shifted from PreTBS to T5 or vice versa) and determined cluster size and magnitude of significant adjacent time points at each iteration. Finally, we re-compute p-values of significant clusters in the main analyses by calculating the probability of their size and magnitude in the permutation analyses. A cluster in the main analyses is considered to survive permutation, and thus significant, only if both the size and magnitude of a given cluster is above 95% of all cluster sizes and magnitudes derived from permutation tests. Similarly, cluster-based permutation paired sample t-test statistics with multiple corrections were performed separately for each TEP peak (N15, P30 and N100) to identify significant cluster of channels across comparisons (PreTBS vs T5 and PreTBS vs T20). A significant cluster in main analyses is defined as at least two neighboring channels at T5 or T20 that significantly differ from baseline measurements (PreTBS) and survived permutation (n = 1000).
ICCs were computed for all metrics to examine test-retest reliability of TBS-induced effects. We first normalized all metrics (GMFP, LMFP, TEP and MEPs) to baseline assessments by taking the ratio of each metric at T5 and T20 in reference to preTBS. We then performed ICCs on the ratio scores across all visits and between pairs of identical visits for T5 and T20 separately. ICCs < 0.25 were considered as “very low”, between 0.25 and 0.50 were considered as “low”, between 0.50 and 0.75 were considered as moderate, and > 0.75 were considered as “high” reproducibility, respectively [45]. Data from iTBS in one participant, from cTBS in another participant, and from a sham sessions in two participants were excluded from the final analyses due to insufficient TMS-EEG and TMS-EMG data sets following the respective TBS protocols.
Results
Baseline TMS-evoked cortical responses are stable over time
We first examined how baseline measurements of TMS evoked cortical responses varied across repeated sessions (Fig. 3). Rm-ANOVA revealed no significant main effect of Visit for both GMFP (p > 0.05) and LMFP (p > 0.05) responses, indicating statistically stable spTMS evoked global and local responses across all visits (Fig. 3A, Supplementary Table 1). ICC analyses for GMFP and LMFP in L-M1 showed significant correlations both for early (p < 0.00) and late (p < 0.00) responses with moderate reproducibility (0.50 ≤ r < 0.75) in late responses across visits (Fig. 3A, right panels). For TEPs, the highest reproducibility was achieved for the N100 peaks, followed by P30 and N15 peaks (Fig. 3B). For N15 peaks, ICCs were not significant for one electrode (C3) on the stimulation site and four other (FT7, T8, TP8 and TP10) temporal electrodes (Fig. 3B, left panel). All electrodes for the P30 (Fig. 1B, middle panel) and N100 (Fig. 3B, right panel) peaks were significantly correlated across visits.
Fig. 3.
Reproducibility of TMS-EEG metrics at baseline (Pre-TBS) measurements. A: Group average AUC values for GMFP (upper panels) and LMFP responses (Early responses: left panels and Late responses: right panels) at baseline (PreTBS) measurements of each visit. Error bars show one unit of standard error. B: Topographical distribution of intra-class correlation coefficients of each TEP peaks across all visits at the electrode level. ICCs are not significant for red colored electrodes, and significant for black colored electrodes.
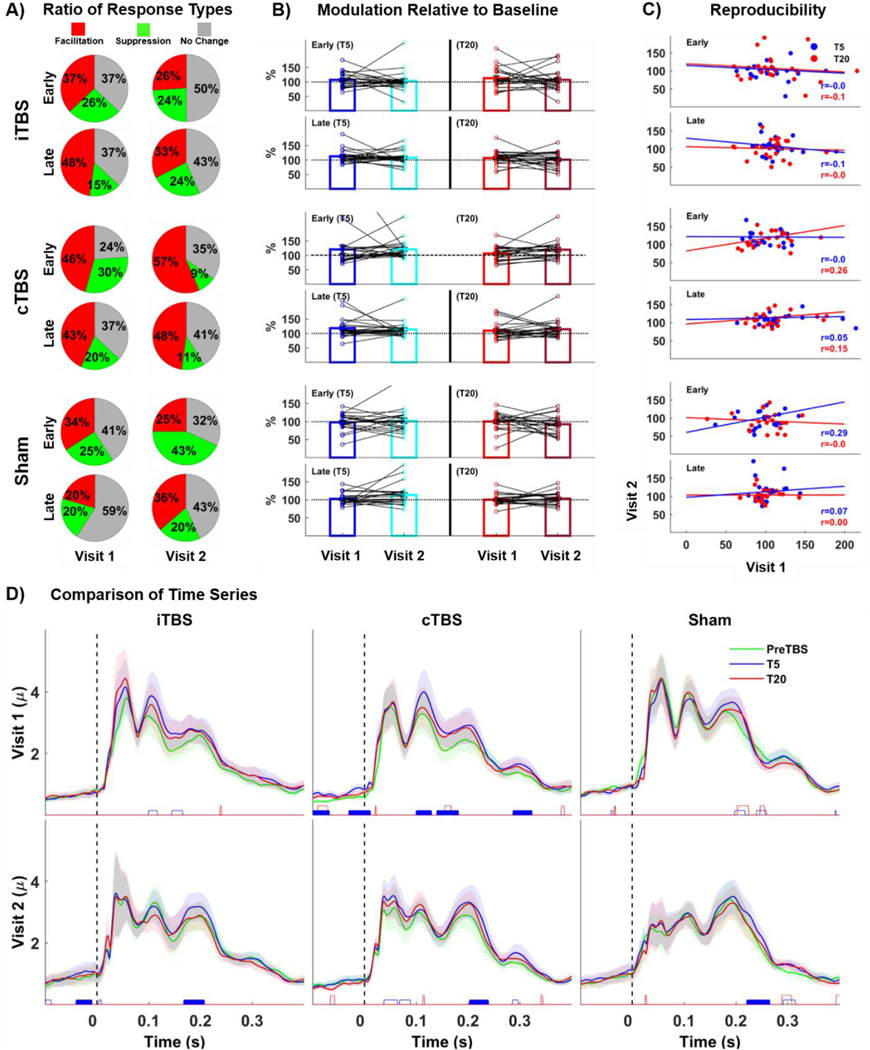
Descriptive classification of global and local cortical responses to TBS
Results of GMFP and LMFP from L-M1 responses to TBS are summarized in Fig. 4 and Fig. 5, respectively. For GMFP, only 35–44% of participants showed excitatory iTBS response (Facilitation: post TBS ratios > 110%) with increased ratios across visits (Fig. 4A, upper panel), while 31–52% of participants did not show changes with stable GMFPs (No change: post TBS ratios between 90 and 110%) and 9–22% of participants showed inhibitory responses with decreased GMFPs (Suppression: post TBS ratios < 90%). GMFP responses to cTBS were also highly variable with 9–40% of participants having inhibitory response with suppressed GMFP, while 23–59% and 33–46% of participants showed no change or facilitation, respectively. Fig. 4B illustrates both the inter-and intra-subject variability to TBS conditions across visits. While the majority of group-averaged responses are around 100% of baseline, individual responses show high variability across subjects, and generally without any clear tendency towards facilitation versus suppression. Furthermore, when each subject’s own responses are connected across visits, substantial changes in the direction of individual responses are observed such that individual responses in the first session (i.e. facilitation versus no-response versus suppression) are not clearly associated with the response in the second session. Similar to GMFPs, LMFPs from L-M1 (ipsilateral to the stimulation) showed high variability in response classifications for all TBS-conditions (Fig. 5A), and high inter- and intra-subject variability in TBS-induced modulation within and across visits (Fig. 5B).
Fig. 4.
TBS effects on GMFP. A: Classification of subjects according to response types to TBS in ratios (Red: increased response following TBS/facilitation, Yellow: decreased response following TBS/suppression and Gray: no change in response following TBS/no response). B: Bars show group averaged response ratios normalized to baseline (PreTBS) at T5 (left panels) and T20 (right panels) both for early (upper panels) and late (lower panels) responses for each visit. Colored dots superimposed over group average bars represent individual responses and black lines connecting dots over group bars track individual response changes across visits. C: Scatter plots, regression lines and ICCs of GMFP ratios both early and late responses (Blue: reproducibility of responses at T5 and Red: at T20) across visits. D: Statistical comparison GMFP responses at the millisecond level for both visit-1 (upper panels) and visit-2 (lower panels). Colored lines represent group-averaged responses to TBS conditions while shaded regions show standard error of measurement (1 unit). Colored Blocks at the bottom of each panel show significant cluster of time-points between post-TBS (Blue: T5 and Red: T20) and baseline (PreTBS) measurements (Empty Blocks: Significant clusters for bivariate comparisons but did not survive permutation tests and Filled Blocks: Significant clusters survived permutation tests.
Fig. 5.
TBS effects on LMFP (Left Motor Cortex). A: Classification of subjects according to response types to TBS in ratios B: Bars show group averaged response ratios normalized to baseline for each visit. Colored dots superimposed over group average bars represent individual responses and black lines connecting dots over group bars track individual response changes across visits. C: Scatter plots, regression lines and ICCs of LMFP ratios both early and late responses (Blue: reproducibility of responses at T5 and Red: at T20) across visits. D: Statistical comparison LMFP responses at the millisecond level for both visit-1 (upper panels) and visit-2 (lower panels).
TBS effects on TMS-evoked cortical and motor responses are not reproducible
Rm-ANOVA for GMFP values revealed no main effects of TBS-condition (p > 0.05) or Block (p > 0.05), and no TBS-condition by Block interaction for early responses in both visits (Supplementary Table 1). As for late GMFP responses, we found a significant main effect of Block both for visit-1 (F(2,38) = 6.06, p = 0.001, = 0.24, power = .892) and visit-2 (F(2,38) = 4.521, p = 0.010, = 0.20, power = .754), but no significant main effect of TBS-condition and no significant TBS-condition by Block interaction (p > 0.05). Follow up pairwise comparisons revealed significant GMFP increase at T5 compared to PreTBS block for both visits (Visit-1: F(1,19) = 8.322, p = 0.009, = 0.305, power = .881; Visit-2: F(1,19) = 10.706, p = 0.004, = 0.360, power = .911). Altogether, these results suggest that the late GMFP response show nonspecific increases at T5 across all TBS-conditions.
ICCs revealed low reproducibility for only early responses at T5 for Sham condition (r = 0.38, p = 0.02), while all other TBS-conditions showed very low to no reproducibility both for early and late responses, with ICCs ranging from r = −0.17–0.20 (Fig. 4C). Cluster based permutation t-tests for the TEP time series at T5 (Fig. 4D) showed significant clusters between 141 and 169 ms for iTBS, and between 141–668 and 290–332 ms for cTBS in Visit-1. However, none of these clusters were reproduced in Visit-2, suggesting lack of reproducibility for the TBS effects on GMFP responses in the temporal dimension (Fig. 4D). No significant differences that survived permutations were found between preTBS and T20 (Fig. 4D).
Similar to GMFPs, Rm-ANOVA for LMFP revealed no significant main effects of TBS-condition (p > 0.05) or Block (p > 0.05), and no TBS-condition by Block interaction (p > 0.05) for early responses in both Visit-1 and Visit-2. For late responses, we found a significant main effect of Block both in visit-1 (F(2,38) = 7.352, p = 0.002, = 0.28, power = .920) and Visit-2 (F(2,38) = 4.048, p = 0.025, = 0.18, power = .689), but no significant main effect of TBS-condition and no significant “TBS-condition by Block interaction. Follow up pairwise comparisons revealed significant LMFP increase at T5 compared to PreTBS block for both visits (Visit-1: F(1,19) = 9.112, p = 0.016, = 0.342, power = .843; Visit-2: F(1,19) = 6.762, p = 0.024, = 0.305, power = .718), suggesting that the late LMFP values show nonspecific increases at T5 across all TBS-conditions (Supplementary Table 1).
Intra-class correlations revealed very low reproducibility both for early and late responses for iTBS and cTBS with ICCs ranging from r = −0.18 to 0.26 (Fig. 5C). Early responses at T5 for Sham TBS showed the highest ICCs (r = 0.29, p = 0.048). Although, cluster based permutations for time series analyses revealed significant differences between PreTBS and T5 for cTBS at multiple time windows in Visit-1 (Fig. 5D middle upper panel), none of these clusters were reproduced in Visit-2 (Fig. 5D middle lower panel). LMFPs from R-M1 also showed significant effect of Block for both early (F(2,38) = 5.938, p = 0.001, = 0.24, power = .895) and late (F(2,38) = 3.590, p = 0.031, = 0.16, power = .714) responses in Visit 2 only, but no main effect of TBS-condition and no TBS-condition by Block interaction effect (See Supplementary Table 1 for statistics and Supplementary Fig. 4 for details). No significant main effects or interactions were found for V1 (See Supplementary Table 1 for statistics and Supplementary Fig. 5 for details).
TBS effects on MEPs are summarized in Fig. 6. Briefly, similar to GMFP and LMFP, individual responses show high variability across subjects, and within subjects across visits for all TBS conditions (Fig. 6A.) Rm-ANOVA revealed no main effects of TBS-condition (p > 0.05) and Block (p > 0.05), and no TBS-condition by Block interaction (p > 0.05) in Visit-1 (Supplementary Table 1). In Visit-2, we found a significant main effect of Block (F(2,38) = 5.51, p = 0.001, = 0.22, power = .823), but no significant main effect of TBS-condition (p > 0.05) and no significant TBS-condition by Block interaction (p > 0.05). Follow up pairwise comparisons revealed significant increase in MEP amplitudes at T20 compared to PreTBS block (F(1,19) = 14.712, p = 0.001, = 0.436, power = .953), indicating nonspecific MEP increases at T20 across all TBS-conditions (Supplementary Table 1). ICCs revealed negative correlations (Fig. 6B, upper panel) with very low reproducibility for iTBS both at T5 (r = 0.24, p = 0.84) and T20 (r = 0.14, p = 0.73). For cTBS, however ICCs were positive across sessions (Fig. 6B, middle panel) with low reproducibility at T5 (r = 0.39, p = 0.02), and very low reproducibility at T20 (r = 0.11, p = 0.30). While MEPs from shamTBS were positively correlated at T5 (Fig. 6B, lower panel) with low reproducibility (r = 0.28, p = 0.08), no correlation pattern was observed at T20 (r = −0.02, p = 0.64). MEP effects are explored with substantial detail in Boucher et al. (submitted).
Fig. 6.
TBS effects on MEPs. A: Bars show group averaged response ratios normalized to baseline (PreTBS) at T5 (left panels) and T20 (right panels) for both visits. Colored dots superimposed over group average bars represent individual responses and black lines connecting dots over group bars track individual response changes across visits. B: Scatter plots, regression lines and ICCs of MEP ratios at T5 (Blue) and T20 (Red).
TBS effects on TEP components are not reproducible
Group averaged topographies for each TEP peak across TBS conditions, blocks and visits are shown in Supplementary Fig. S6. Cluster based permutation t-test statistics revealed significantly reduced N15 amplitude (p < 0.05) for electrodes over left sensorimotor cortices at T5 for Sham TBS (Fig. 7A, upper-right panel). As for P30 peaks, a series of mid-frontal electrodes showed increased P30 response while left parietal electrodes showed decreased P30 response only for cTBS at T5 (Fig. 7A, middle panel). iTBS showed increased (more negative) N100 response in four electrodes over left sensorimotor cortices, while cTBS showed increased N100 response over a series of left parietal and posterior parietal region as well as a reduced (more positive) N100 response over right frontal and temporal regions at T5 (Fig. 7A, lower-left and middle panels). Importantly, however, none of these significant clusters was reproduced at Visit-2, but different patterns of significant modulations were observed for N15 and P30 peaks after cTBS and Sham conditions, respectively (Fig. 7B, upper and middle panels). Similarly, significant set of clusters in each TEP peak observed at T20 for Visit-1 were generally not reproduced in Visit-2 for all TBS-conditions (Fig. 8A, and Fig. 8B). Sham TBS was the only condition with reproducible modulation for a subset of electrodes for P30 peaks across visits (Fig. 8A and B middle-right panels).
Fig. 7.
TEP responses to different TBS at T5. Topoplots show statistical comparison (t values) of TEP peaks between baseline (PreTBS) and postTBS measurements at T5 for each TBS protocol and TEP peak both in visit-1 (A) and visit-2 (B). Significant cluster of electrodes that survived permutation analyses are shown in red. Negative t values for N15 and N100 peaks represents increased amplitude (more negative peak) while positive t values represent decreased amplitudes (less negative peak). Negative t values for P30 peak represents decreased amplitude (less positive peak) and positive t values represent increased amplitude (more positive peak).
Fig. 8.
TEP responses to different TBS at T20.
Next, we tested whether modulations induced by iTBS and cTBS were significant compared to Sham TBS and, if so, whether these significant differences from Sham TBS were reproducible across visits (Fig. 9). Both iTBS and cTBS had significantly larger (more negative) N15 amplitudes over left motor cortex when compared to sham TBS at T5 for Visit-1 (Fig. 9A, Visit-1 upper panels). Only significant cTBS differences from sham in left motor cortex were partially reproduced in Visit-2, but within a substantially larger cluster extending from left parietal to left temporal cortex observed in visit 2 (Fig. 9A, Visit-2 upper panels). Significant differences between active and sham TBS conditions observed for N100 response in Visit-1 were also not reproduced in Visit-2 (Fig. 9A, visit-1 and visit-2 lower panels). Similarly for T20, significant differences between active and sham TBS conditions observed in Visit-1 were not reproduced in Visit-2, except partially for larger (more negative) N15 amplitudes over left motor cortex in cTBS condition (Fig. 9B, visit-1 and visit-2 upper panels).
Fig. 9.
Statistical comparison of active TBS effects with sham control. TBS effects for each TBS condition is calculated by subtracting PostTBS (T5 and T20) TEPs from Baseline (PreTBS) TEPs. These differences were then statistically compared between active TBS (iTBS vs Sham and cTBS vs Sham) and sham control both at T5 (A) and T20 (B) separately for both visits (Visit-1 and Visit-2). Significant cluster of electrodes that survived permutation analyses are shown in red. Negative t values for N15 and N100 peaks represents increased amplitude in active TBS as compared to sham control (more negative peak in active TBS), while positive t values represent decreased amplitudes amplitude in active TBS as compared to sham control (less negative peak in active TBS). Negative t values for P30 peak represents decreased amplitude (less positive peak in active TBS as compared to sham) and positive t values represent increased amplitude (more positive peak in active TBS as compared to sham).
We also tested the reproducibility of TBS effects on TEPs at the electrode level by computing ICCs for each channel across TEP peaks and TBS conditions (Fig. 10). There was no clear pattern on the spatial distribution and direction of ICCs, with different electrodes both negatively and positively weakly correlated across visits (r = −0.5–0.5).
Fig. 10.
Scalp distribution of ICCs for each TEP peak across identical TBS sessions. Higher values (>0) represent positive relationships while lower values (<0) represent negative correlations between electrode pairs across visits.
Role of baseline excitability on TBS effects and relationship between changes in MEPs and TEPs
Here, we performed preliminary analyses to better understand whether (1) baseline corticospinal and cortical excitability indexed by MEPs and early LMFPs, respectively, differ in subjects with facilitatory versus suppressive cortical responses to TBS, and, if so, (2) whether TBS-induced modulation of corticospinal and cortical responses are related (Fig. 11B and Fig. 11C). We found that individuals with facilitatory LMFP responses (n = 14) following iTBS at T5 had significantly lower (t(1,22) = −5.80, p = 0.000) MEPs at baseline than those with a suppressive response (n = 9) in visit-1 (Fig. 11A, left panel) suggesting that the facilitatory effects of iTBS on cortical reactivity are seen primarily in those with lower corticospinal excitability at baseline. Contrary to iTBS, cTBS further suppressed cortical reactivity (n = 12) in individuals with low baseline corticospinal excitability (t(1,22) = 2.44, p = 0.035) in Visit1. However, although a similar pattern was observed for iTBS, baseline MEP comparisons were not significant (p > 0.05) in visit-2 for both iTBS and cTBS indicating poor reproducibility. No significant baseline MEP differences were found (p > 0.05) between facilitated versus suppressed LMFP responses following sham for both visits. Furthermore, we found no statistical difference in baseline LMFP comparisons (p > 0.05) between facilitation (LMFP+) and suppression (LMFP-) cortical responses to TBS.
Fig.11.
Corticospinal excitability and TBS effects on cortical responses: A: Comparison of baseline MEP amplitudes as a function of early LMFP response type to TBS conditions across visits. Blue bars show baseline MEP means for individuals with facilitation response (+LMFP), while red bars show baseline MEPs means for suppressive response to (-LMFP) each TBS protocol in left-M1 at T5. Error bars represent standard error of measurements (1 unit) and horizontal lines with asterisks over the bars show significant differences (p < 0.05) in baseline MEPs between facilitation and suppression responses to TBS. B: Scatter plots, regression lines and ICCs for the relationship between pre-post TBS changes in MEPs and early LMFP both for visit-1 (blue) and visit-2 (red). Changes in MEP and early LMFP amplitudes are expressed as response ratios normalized to baseline (PreTBS) at T5. C: Scalp distribution of bivariate Pearson correlations between changes in MEP and TEP responses for N15 (upper panels) and P30 peaks (lower panels) across visits and TBS conditions. Higher values (>0) represent positive relationships while lower values (<0) represent negative correlations at the electrode level.
Next, we examined relationships between changes in MEP and LMFP responses following each TBS condition and visit (Fig. 11B). We found significant positive correlations between MEP and early LMFP ratios following iTBS both for visit-1 (r = 0.44, p = 0.005) and visit-2 (r = 0.40, p = 0.019). Although cTBS was associated with negative correlations between MEP and LMFP ratios, the magnitude of correlations were low (visit-1: r = −0.17, visit-2: r = −0.03) and not significant (p > 0.05) for both visits. There was no significant correlation between MEP and LMFP changes with sham stimulation. Finally, we examined changes in MEPs and early TEP components (Fig. 11C) to further understand specific contributions of each TEP component to the relationship between corticospinal and local cortical neuromodulation observed in Fig. 11B. We found that changes in MEP responses were positively and consistently correlated with changes in P30 component over the stimulated region following iTBS in both visits (Fig. 11C left lower panels). On the other hand, cTBS resulted in weak negative correlations between MEP and TEP changes over the stimulated cortical region only in visit-1 but not in visit-2. Taken together, these results suggest that the P30 component reliably reflects relationships between corticospinal and local cortical response modulations following iTBS.
Discussion
TBS is an rTMS protocol that is widely used with the goal of modulating cortical activity for both scientific and therapeutic aims. The initial study establishing these protocols reported increased corticospinal excitability (assessed with TMS-elicited MEPs) lasting up to 20 min following iTBS, and decreased corticospinal excitability lasting up to 60 min following cTBS [13,46]. Since then, both iTBS and cTBS have been conceptualized as “excitatory” and “inhibitory” rTMS protocols, respectively. Today these TBS protocols have been widely used to induce lasting brain plasticity and modulate human behavior and cognition. Numerous studies, for example, have applied TBS outside the motor cortex to disrupt [19,20,47] or enhance normal behaviors [7,48], as well as to recover impaired behaviors [49,50] and treat neuropsychiatric conditions [9]. Observed changes in behavior, cognition or neuropsychiatric conditions are attributed to TBS-induced brain plasticity over the stimulated networks. The vast majority of these studies have inferred mechanisms based on the presumed “excitatory” or “inhibitory” effects of the particular TBS without independent neurophysiologic measurements of the direct cortical responses to TBS. However, a fundamental limitation inherent to the use of TBS is that the presumed effects on cortical excitability have not been adequately established. More specifically, the neuromodulatory effects of TBS have typically been assessed with MEPs, which provide at best an indirect and limited measure of local cortical excitability in the stimulated primary motor region, and cannot be used to probe overall neurophysiologic changes across brain regions. Furthermore, the reliability of the TBS-induced effects on cortical excitability have not been studied.
Here, we examined the impact and reproducibility of TBS-induced neuromodulation directly at the cortical level by TMS-EEG with a robust sham-controlled test-retest design. We found that although spTMS of the motor cortex generates stable and reproducible cortical responses across sessions [44,51], the neuromodulatory effects of TBS on TEPs were substantially variable between individuals at the cortical level. Thus, both iTBS and cTBS-induced changes for global (GMFP) and local (LMFP) cortical responses were not significantly different from sham control at the group level. When assessing the effects of TBS on individual TEP peaks, we found that the modulation of the N15 peaks over the stimulated motor cortex following iTBS and cTBS, and the modulation of the N100 peaks within a large cluster of electrodes over frontal, premotor, and parietal regions following iTBS, were significantly different from Sham TBS in Visit-1. However, none of the significant sham-controlled active TBS effects observed in Visit-1 were reproduced in Visit-2. Importantly, we noticed that all TBS conditions also suffered from high intra-subject variability across visits, such that a given person with increased cortical excitability in Visit-1 may show an opposite response pattern to TBS with decreased cortical excitability in Visit-2. Overall, there results indicate very poor reproducibility for TBS-effects on cortical excitability in healthy individuals, and suggest that brain-behavior relationships established in behavioral research paradigms through the suggested “excitatory” or “inhibitory” mechanisms of TBS-induced neuromodulation may need to be reevaluated. In particular, our findings strongly suggest that future studies applying TBS with the aim of modulating human behavior and cognition need to employ techniques such as TMS-EEG to characterize the TBS-induced changes in cortical activity to understand the underlying brain-behavior relationships.
One critical finding of this study is the importance of repeat (test-retest) sessions to draw valid conclusions on the effects of TBS-induced neuromodulation. To date, direct cortical responses following cTBS of the primary motor cortex have only been assessed in two previous reports, using either a single electrode [26] or a sub-group of electrodes [52] over the stimulated cortex. With regard to TBS effects in other brain regions, Chung and colleagues employed iTBS over the prefrontal cortex in a series of recent studies [27,53,54], and reported significant increases in selected TEP components. Although our results from Visit-1 are partially in line with these studies, our reliability analyses showed that none of these results were observed in Visit-2, demonstrating poor reproducibility across sessions, and thus suggesting the critical importance of validating TBS-induced neuromodulation with repeat sessions.
A large body of prior research [13,55–59] also reported significant modulation of corticospinal excitability following TBS protocols using MEPs as the outcome measure. A recent meta-analysis [15] and a large-scale analysis [16] pooling data from several previous reports also supported these prior findings by showing significant modulation of corticospinal excitability, with increased MEPs up to 30-min following iTBS and decreased MEPs at 5–10 or 60-min following cTBS. Our results for MEPs and TEPs did not corroborate those findings. One major factor may be the lack of sham control in the majority of the previous studies included in these reports. For example, out of 87 studies re-examined in Chung et al.’s meta-analysis [15] only 3 studies performed a sham control in their TBS protocols, and out of 22 studies included in Corp et al.’s large-scale analysis [16] only 1 study included a sham control. It is critical to note that we could also reach similar conclusions in our study by ignoring results from the sham TBS, as both iTBS and cTBS produced significant differences in all of our metrics (GMFP, LMFP, TEP peaks and MEPs at T20) compared to baseline values. However, we noticed that most of the TBS effects on cortical responses (GMFP, LMFP and P30 at T5, p30 and N100 at T20) and MEPs were not significant when compared to sham effects, supporting the recent evidence showing no significant differences between active TBS and primed sham conditions [35].
TMS of M1 generates unique response dynamics compared to TMS of non-motor regions, as M1 stimulation activates corticospinal pathways in parallel to cortico-cortical and thalamo-cortical tracts. The early local cortical activation following TMS primarily propagates through corticospinal tracts, elicits a motor response in the targeted muscle, and a following sensory return to somatosensory cortex suggesting dynamic relationships among local cortical and corticospinal excitability and evoked sensory responses in the brain. Accordingly, many recent studies [60,61] reported that MEPs are related with early TEP components (P25/30) and delayed oscillatory responses following spTMS [62], and that neuromodulatory TBS interventions induces correlated changes in MEPs and early TEP peaks following iTBS [25,57] and cTBS [26,57]. A recent study [60] suggested modulation of pre-stimulus oscillatory power in the alpha band following transcranial direct current stimulation as a possible mechanism for correlated changes in MEPs and TEPs. Our preliminary results from visit-1 showing positive correlations between changes in MEPs and P30 component following iTBS and negative correlations following cTBS localized to the stimulated cortex are in line with these findings; however, only the correlation for iTBS was reliable across sessions. As iTBS and cTBS were previously shown to modulate different components of descending corticospinal volleys [22,23], further studies with simultaneous epidural cervical and scalp EEG recordings would be highly useful to establish underlying mechanisms of correlated changes in MEPs and TEPs following iTBS. Taken together, these findings suggest that interventions that increase corticospinal excitability reliably increase cortical excitability as well, but that the effects of other interventions may not be as consistent. Additionally, we showed that neuromodulatory effects of iTBS on cortical responses might be influenced by baseline corticospinal excitability; however, these effects have poor reproducibility.
Contrary to our initial prediction, we observed significant deviations from baseline measurements following sham TBS (See N15 and P30 peaks in Figs. 6 and 7). One methodological reason for this could be the measurement of TBS neuromodulation through repeated blocks of spTMS over time. A recent study by Pellicciari and colleagues [63] showed that spTMS, without having any changes in stimulation parameters, can induce cumulative increases in corticospinal excitability across multiple stimulation blocks within the session. Our results from MEPs in both visits confirm these findings as MEP amplitudes increased following shamTBS both at T5 and T20 compared to baseline (Fig. 5A, Lower panels). Accordingly, significant sham effects on P30 peaks we observed at T20 in both visits (Fig. 6A and B) are in line with increased MEP amplitudes reported in Pellicciari et al. [63] and may extend their findings to cumulative effects of spTMS to direct cortical responses. Another important aspect to consider for significant sham responses would be the possible placebo effects of TBS. A growing body of evidence suggests that the magnitude of sham effects for therapeutic applications of rTMS in different clinical populations has considerably increased over the years, possibly due to growing awareness on the potential of TMS in public and media, adoption of more realistic sham protocols, and sophisticated experimental set-ups with neuronavigated TMS-EEG designs [64]. An alternative and highly plausible explanation would be mutually interacting effects of repeated blocks of spTMS and placebo effects of TBS. Given the lack of experimental evidence, understanding possible neurophysiological and environmental mechanisms of significant sham responses in TMS research clearly warrants future systematic investigations.
Certain limitations of the current study should also be acknowledged. First, our results are limited to stimulation of the primary motor cortex, and thus do not provide direct evidence for reproducibility of TBS-induced neuromodulation over non-motor cortices. Understanding direct cortical responses to TBS and, more importantly, examining reproducibility of such cortical responses outside the motor cortex is essential for validating previously suggested brain-behavior relationships through the mechanism of TBS-induced neuromodulation. Secondly, our results do not generalize to other widely used rTMS protocols. In general, low frequency rTMS (1 Hz) and high frequency rTMS (10 Hz, 20 Hz) have been used as “inhibitory” and “excitatory” protocols respectively to modulate brain activity [65,66]. However, the effects of these rTMS protocols on cortical excitability as assessed via EEG, and the reproducibility of rTMS-induced neuromodulatory effects against a robust sham control have not been clearly established. Thirdly, our results are limited to analyses of TEPs, and thus do not generalize to other measures of cortical responsivity and modulation. A series of recent studies [27,53,67], for example, reported significant TBS-induced modulations in TMS-evoked oscillatory responses in the frequency domain, but did not evaluate the reproducibility of these oscillatory responses. In this study, we limited our analyses to TEPs, GMFPs and LMFPs in the temporal domain, as they are the most commonly used EEG metrics for measuring cortical response modulation in the current TMS-EEG literature. Clearly, cortical responsivity can be measured with variety of other EEG metrics including connectivity, perturbational complexity index, and time-frequency responses. Future research is needed to address TBS-induced neuromodulation and its reproducibility with these measures. Importantly, our study is not designed to systematically examine methodological and subject-related factors and their effects on the observed high inter and intra-subject response variability to TBS. Although a number of methodological (i.e, stimulation duration, intensity, coil orientation, time of the day), inter-subject (genetics, age, variability in neural circuits activated by TMS) and intra-subject (brain state, prior sleep, baseline cortico-spinal excitability) factors have been reported to contribute to the response variability to TBS protocols [14], no systematic evaluation have been performed to examine their causal effects on reproducibility of TBS responses. Furthermore, significant but inconsistent cortical responses following active- and sham-TBS could potentially be attributed to presence of TMS-induced sensory potentials, such as somatosensory and auditory evoked potentials, in TEPs. As these non-transcranially evoked potentials have large amplitudes with stereotyped spatial-temporal dynamics, possible spurious fluctuations across blocks or visits may confound our ability to detect TBS-induced neuromodulation and its’ reproducibility. To eliminate potential effects of sensory evoked responses in our analyses we followed a structured ICA approach to carefully identify and remove these non-transcranial responses from TEPs (Fig. 2). We then showed reproducibility of baseline TEPs (Fig. 3) across sessions, suggesting that even if any residual sensory-evoked potentials remain in our post-processed TEPs, they are stable across sessions. However, to clearly understand and eliminate any possible confounding effects of TBS on sensory-evoked potentials, future studies could integrate single pulse sham-TMS designs into their TBS protocols. In the current study, a sham control for spTMS was not feasible for our post TBS measurements, since we could only administer either active spTMS or sham spTMS at any one time point (e.g. T5) following TBS. Given the effects of TBS are not stable over time; it would not be valid to compare the effects of iTBS on real spTMS at T5 with the effects on sham spTMS at T15. When advances in TMS technology permit interleaved sham and real spTMS, sham spTMS stimulation should be included in future studies to parcel out the simultaneous effects of TBS on the transcranial and non-transcranial sensory-evoked components. Finally, as in most of the previous literature, the experimenters were not blinded to the applied stimulation protocol. Although currently it is not a standard experimental design in basic neurophysiological research, future studies could clearly benefit from double blind methods to minimize the risk of experimenter bias and ensure the robustness of replication designs.
Conclusions and future directions
After almost 30 years of experimental research, the large response variability to rTMS protocols still stands as a major obstacle for the clinical utility and experimental validity of rTMS-induced neuromodulation. This study reports the first comprehensive examination of (1) the direct cortical EEG responses to iTBS and cTBS of human motor cortex and (2) the reproducibility of both iTBS- and cTBS-induced modulation of cortical excitability against a sham control across repeat sessions. We find that the neuromodulatory effects of TBS are substantially variable both between and within individuals, with no significant group effects over sham control, and with very poor reproducibility across sessions. Our results suggest three key messages for future studies. First, a sham control has to be an integral part of any experimental TBS design to rule out cumulative effects of spTMS, as well as to control possible placebo effects of rTMS interventions. Secondly, studies applying TBS with the goal of establishing brain-behavior relationships through the mechanisms of TBS neuromodulation should validate expected TBS effects by measuring direct cortical (EEG) responses to TBS. Finally, significant neuromodulatory and behavioral effects of TBS over sham control should be confirmed with either repeat sessions or independent data sets to establish the reproducibility and robustness of observed effects. Further randomized controlled trials with double blind methods are urgently needed to minimize the risk of bias and unravel the underlying reasons for such substantial response variability to rTMS by experimentally evaluating the causal role of possible methodological, inter- and intra-subject factors on rTMS-induced neuromodulation.
Supplementary Material
Acknowledgment
We are grateful to all research assistants who helped to run these study visits. We are grateful to the gracious funding from the MIT-Harvard Broad institute (6600024–5500000895) directly supporting the study and our line of research on brain plasticity biomarkers. Dr. Santarnecchi is supported by the Beth Israel Deaconess Medical Center (BIDMC) via the Chief Academic Officer (CAO) Award 2017, the Defence Advanced Research Projects Agency (DARPA) via HR001117S0030, the NIH (P01 AG031720-06A1, R01 MH117063-01, R01 AG060981-01) and ADDF (ADDF-FTD GA201902–2017902). Dr. Pascual-Leone is supported by grants from the National Institutes of Health (R24AG06142, and P01 AG031720), the National Science Foundation, and the Barcelona Brain Health Initiative funded primarily by La Caixa. Dr. Shafi is supported by the Football Players Health Study (FPHS) at Harvard University, and the NIH (R01 MH115949, R01AG060987, P01 AG031720-06A1). The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of Harvard University and its affiliated academic health care centres, or the National Institutes of Health.
Declaration of competing interest
Dr. A. Pascual-Leone is a co-founder of Linus Health and TI Solutions AG; serves on the scientific advisory boards for Starlab Neuroscience, Neuroelectrics, Magstim Inc., Nexstim, Cognito, and MedRhythms; and is listed as an inventor on several issued and pending patents on the real-time integration of noninvasive brain stimulation with electroencephalography and magnetic resonance imaging.
Footnotes
CRediT authorship contribution statement
Recep A. Ozdemir: Methodology, conceptualized the framework, Data curation, preprocessed the TMS-EEG and EMG data. Pierre Boucher: Data curation, preprocessed the TMS-EEG and EMG data. Peter J. Fried: Methodology. Davide Momi: Data curation. Ali Jannati: performed statistical analyses for reproducibility of GMFP and LMFPs, oversaw study conduction and edited the first draft. Alvaro Pascual-Leone: Methodology, oversaw study conduction and edited the first draft. Emiliano Santarnecchi: Methodology. Mouhsin M. Shafi: Methodology, conceptualized the framework, oversaw study conduction and edited the first draft, All authors critically reviewed the manuscript for content and approved the final version for publication.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.brs.2021.05.013.
References
- [1].Ziemann U, Muellbacher W, Hallett M, Cohen LG. Modulation of practice-dependent plasticity in human motor cortex. Brain 2001;124:1171–81. [DOI] [PubMed] [Google Scholar]
- [2].Klomjai W, Katz R, Lackmy-Vallee A. Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS). Ann Phys Rehab Med 2015;58: 208–13. [DOI] [PubMed] [Google Scholar]
- [3].Lefaucheur J-P, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol 2014;125:2150–206. [DOI] [PubMed] [Google Scholar]
- [4].Di Lazzaro V, et al. Modulation of motor cortex neuronal networks by rTMS: comparison of local and remote effects of six different protocols of stimulation. J Neurophysiol 2011;105:2150–6. [DOI] [PubMed] [Google Scholar]
- [5].Hilgetag CC, Theoret H, Pascual-Leone A. Enhanced visual spatial attention ipsilateral to rTMS-induced’virtual lesions’ of human parietal cortex. Nat Neurosci 2001;4:953–7. [DOI] [PubMed] [Google Scholar]
- [6].Tavor I, et al. Task-free MRI predicts individual differences in brain activity during task performance. Science 2016;352:216–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wang JX, et al. Targeted enhancement of cortical-hippocampal brain networks and associative memory. Science 2014;345:1054–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ziemann U, et al. Consensus: motor cortex plasticity protocols. Brain Stimul 2008;1:164–82. [DOI] [PubMed] [Google Scholar]
- [9].Blumberger DM, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet2018;391:1683–92. [DOI] [PubMed] [Google Scholar]
- [10].Sachdev PS, Loo CK, Mitchell PB, McFARQUHAR TF, Malhi GS. Repetitive transcranial magnetic stimulation for the treatment of obsessive compulsive disorder: a double-blind controlled investigation. Psychol Med 2007;37:1645. [DOI] [PubMed] [Google Scholar]
- [11].Brighina F, et al. rTMS of the prefrontal cortex in the treatment of chronic migraine: a pilot study. J Neurol Sci 2004;227:67–71. [DOI] [PubMed] [Google Scholar]
- [12].Larson J, Lynch G. Theta pattern stimulation and the induction of LTP: the sequence in which synapses are stimulated determines the degree to which they potentiate. Brain Res 1989;489:49–58. [DOI] [PubMed] [Google Scholar]
- [13].Huang Y-Z, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron 2005;45:201–6. [DOI] [PubMed] [Google Scholar]
- [14].Suppa A, et al. Ten years of theta burst stimulation in humans: established knowledge, unknowns and prospects. Brain Stimul 2016;9:323–35. [DOI] [PubMed] [Google Scholar]
- [15].Chung SW, Hill AT, Rogasch NC, Hoy KE, Fitzgerald PB. Use of theta-burst stimulation in changing excitability of motor cortex: a systematic review and meta-analysis. Neurosci Biobehav Rev 2016;63:43–64. [DOI] [PubMed] [Google Scholar]
- [16].Corp DT, et al. Large-scale analysis of interindividual variability in theta-burst stimulation data: results from the ‘Big TMS Data Collaboration’. Brain Stimul 2020;13(5):1476–88. 10.1016/j.brs.2020.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lowe CJ, Manocchio F, Safati AB, Hall PA. The effects of theta burst stimulation (TBS) targeting the prefrontal cortex on executive functioning: a systematic review and meta-analysis. Neuropsychologia 2018;111:344–59. [DOI] [PubMed] [Google Scholar]
- [18].Demirtas-Tatlidede A, Vahabzadeh-Hagh AM, Pascual-Leone A. Can noninvasive brain stimulation enhance cognition in neuropsychiatric disorders? Neuropharmacology 2013;64:566–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Maier MJ, et al. Forgiveness and cognitive controleProvoking revenge via theta-burst-stimulation of the DLPFC. Neuroimage 2018;183:769–75. [DOI] [PubMed] [Google Scholar]
- [20].Verbruggen F, Aron AR, Stevens MA, Chambers CD. Theta burst stimulation dissociates attention and action updating in human inferior frontal cortex. Proc Natl Acad Sci Unit States Am 2010;107:13966–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Stefan K, Gentner R, Zeller D, Dang S, Classen J. Theta-burst stimulation: remote physiological and local behavioral after-effects. Neuroimage 2008;40: 265–74. [DOI] [PubMed] [Google Scholar]
- [22].Di Lazzaro V, et al. Theta-burst repetitive transcranial magnetic stimulation suppresses specific excitatory circuits in the human motor cortex. J Physiol 2005;565:945–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Di Lazzaro V, et al. The physiological basis of the effects of intermittent theta burst stimulation of the human motor cortex. J Physiol 2008;586:3871–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hill AT, Rogasch NC, Fitzgerald PB, Hoy KE. TMS-EEG: a window into the neurophysiological effects of transcranial electrical stimulation in non-motor brain regions. Neurosci Biobehav Rev 2016;64:175–84. [DOI] [PubMed] [Google Scholar]
- [25].Gedankien T, Fried PJ, Pascual-Leone A, Shafi MM. Intermittent theta-burst stimulation induces correlated changes in cortical and corticospinal excitability in healthy older subjects. Clin Neurophysiol 2017;128:2419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Vernet M, et al. Insights on the neural basis of motor plasticity induced by theta burst stimulation from TMSeEEG. Eur J Neurosci 2013;37:598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chung SW, et al. Demonstration of short-term plasticity in the dorsolateral prefrontal cortex with theta burst stimulation: a TMS-EEG study. Clin Neurophysiol 2017;128:1117–26. [DOI] [PubMed] [Google Scholar]
- [28].Ziemann U, Siebner HR. Inter-subject and inter-session variability of plasticity induction by non-invasive brain stimulation: boon or bane? Brain Stimul 2015;8:662–3. [DOI] [PubMed] [Google Scholar]
- [29].Huang Y-Z, et al. Plasticity induced by non-invasive transcranial brain stimulation:a position paper. Clin Neurophysiol 2017;128:2318–29. [DOI] [PubMed] [Google Scholar]
- [30].Hamada M, Murase N, Hasan A, Balaratnam M, Rothwell JC. The role of interneuron networks in driving human motor cortical plasticity. Cerebr Cortex 2013;23:1593–605. [DOI] [PubMed] [Google Scholar]
- [31].Lopez-Alonso V, Cheeran B, Río-Rodríguez D, Fernandez-del-Olmo M. Interindividual variability in response to non-invasive brain stimulation paradigms. Brain Stimul 2014;7:372–80. [DOI] [PubMed] [Google Scholar]
- [32].Lowe CJ, Hall PA. Reproducibility and sources of interindividual variability in the responsiveness to prefrontal continuous theta burst stimulation (cTBS). Neurosci Lett 2018;687:280–4. [DOI] [PubMed] [Google Scholar]
- [33].Schilberg L, Schuhmann T, Sack AT. Interindividual variability and intraindividual reliability of intermittent theta burst stimulation-induced neuroplasticity mechanisms in the healthy brain. J Cognit Neurosci 2017;29: 1022–32. [DOI] [PubMed] [Google Scholar]
- [34].Vallence A-M, et al. Inter-and intra-subject variability of motor cortex plasticity following continuous theta-burst stimulation. Neuroscience 2015;304: 266–78. [DOI] [PubMed] [Google Scholar]
- [35].Perellon-Alfonso R, et al. Similar effect of intermittent theta burst and sham stimulation on corticospinal excitability: a 5-day repeated sessions study. Eur J Neurosci 2018;48:1990–2000. [DOI] [PubMed] [Google Scholar]
- [36].Rothwell JC, et al. Magnetic stimulation: motor evoked potentials. Electroencephalogr Clin Neurophysiol Suppl 1999;52:97–103. [PubMed] [Google Scholar]
- [37].Rossini PM, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application. An updated report from an IFCN Committee. Clin Neurophysiol 2015;126:1071–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Rossi S, Hallett M, Rossini PM, Pascual-Leone A, Group, S. of T. C. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 2009;120: 2008–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Rossi S, et al. Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: expert Guidelines. Clin Neurophysiol 2021;132(1):269–306. 10.1016/j.clinph.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of singletrial EEG dynamics including independent component analysis. J Neurosci Methods 2004;134:9–21. [DOI] [PubMed] [Google Scholar]
- [41].Rogasch NC, et al. Analysing concurrent transcranial magnetic stimulation and electroencephalographic data: a review and introduction to the open-source TESA software. Neuroimage 2017;147:934–51. [DOI] [PubMed] [Google Scholar]
- [42].Nettekoven C, et al. Inter-individual variability in cortical excitability and motor network connectivity following multiple Blocks of rTMS. Neuroimage 2015;118:209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].McGraw KO, Wong SP. Forming inferences about some intraclass correlation coefficients. Psychol Methods 1996;1:30. [Google Scholar]
- [44].Ozdemir RA, et al. Individualized perturbation of the human connectome reveals reproducible biomarkers of network dynamics relevant to cognition. Proc Natl Acad Sci Unit States Am 2020. 10.1073/pnas.1911240117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Portney LG, Watkins MP. Foundations of clinical research: applications to practice, vol. 892. Upper Saddle River, NJ: Pearson/Prentice Hall; 2009. [Google Scholar]
- [46].Wischnewski M, Schutter DJ. Efficacy and time course of theta burst stimulation in healthy humans. Brain Stimul 2015;8:685–92. [DOI] [PubMed] [Google Scholar]
- [47].Rounis E, Maniscalco B, Rothwell JC, Passingham RE, Lau H. Theta-burst transcranial magnetic stimulation to the prefrontal cortex impairs metacognitive visual awareness. Cognit Neurosci 2010;1:165–75. [DOI] [PubMed] [Google Scholar]
- [48].Debarnot U, et al. Intermittent theta burst stimulation over left BA10 enhances virtual reality-based prospective memory in healthy aged subjects. Neurobiol Aging 2015;36:2360–9. [DOI] [PubMed] [Google Scholar]
- [49].Talelli P, Greenwood RJ, Rothwell JC. Exploring Theta Burst Stimulation as an intervention to improve motor recovery in chronic stroke. Clin Neurophysiol 2007;118:333–42. [DOI] [PubMed] [Google Scholar]
- [50].Koch G, et al. Theta-burst stimulation of the left hemisphere accelerates recovery of hemispatial neglect. Neurology 2012;78:24–30. [DOI] [PubMed] [Google Scholar]
- [51].Kerwin LJ, Keller CJ, Wu W, Narayan M, Etkin A. Test-retest reliability of transcranial magnetic stimulation EEG evoked potentials. Brain Stimul 2018;11:536–44. [DOI] [PubMed] [Google Scholar]
- [52].Rocchi L, et al. Variability and predictors of response to continuous theta burst stimulation: a TMS-EEG study. Front Neurosci 2018;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Chung SW, et al. The effects of individualised intermittent theta burst stimulation in the prefrontal cortex: a TMS-EEG study. Hum Brain Mapp 2019;40: 608–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Chung SW, Rogasch NC, Hoy KE, Fitzgerald PB. The effect of single and repeated prefrontal intermittent theta burst stimulation on cortical reactivity and working memory. Brain Stimul 2018;11:566–74. [DOI] [PubMed] [Google Scholar]
- [55].Huang Y-Z, Rothwell JC, Edwards MJ, Chen R-S. Effect of physiological activity on an NMDA-dependent form of cortical plasticity in human. Cerebr Cortex 2008;18:563–70. [DOI] [PubMed] [Google Scholar]
- [56].Gentner R, Wankerl K, Reinsberger C, Zeller D, Classen J. Depression of human corticospinal excitability induced by magnetic theta-burst stimulation: evidence of rapid polarity-reversing metaplasticity. Cerebr Cortex 2008;18: 2046–53. [DOI] [PubMed] [Google Scholar]
- [57].Vernet M, et al. Reproducibility of the effects of theta burst stimulation on motor cortical plasticity in healthy participants. Clin Neurophysiol 2014;125: 320–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Jannati A, Block G, Oberman LM, Rotenberg A, Pascual-Leone A. Interindividual variability in response to continuous theta-burst stimulation in healthy adults. Clin Neurophysiol 2017;128:2268–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Fried PJ, et al. Humans with type-2 diabetes show abnormal long-term potentiation-like cortical plasticity associated with verbal learning deficits. J Alzheim Dis 2017;55:89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ahn S, Frohlich F. Pinging the brain with transcranial magnetic stimulation€ reveals cortical reactivity in time and space. Brain Stimul 2021;14(2):304–15. 10.1016/j.brs.2021.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].M€aki H, Ilmoniemi RJ. The relationship between peripheral and early cortical activationinduced by transcranial magnetic stimulation. Neurosci Lett 2010;478:24–8. [DOI] [PubMed] [Google Scholar]
- [62].Fecchio M, et al. The spectral features of EEG responses to transcranial magnetic stimulation of the primary motor cortex depend on the amplitude of the motor evoked potentials. PloS One 2017;12:e0184910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Pellicciari MC, Brignani D, Miniussi C. Excitability modulation of the motor system induced by transcranial direct current stimulation: a multimodal approach. Neuroimage 2013;83:569–80. [DOI] [PubMed] [Google Scholar]
- [64].Burke MJ, Kaptchuk TJ, Pascual-Leone A. Challenges of differential placebo effects in contemporary medicine: the example of brain stimulation. Ann Neurol 2019;85:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Fitzgerald PB, Fountain S, Daskalakis ZJ. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol 2006;117:2584–96. [DOI] [PubMed] [Google Scholar]
- [66].Fried PJ, Elkin-Frankston S, Rushmore RJ, Hilgetag CC, Valero-Cabre A. Characterization of visual percepts evoked by noninvasive stimulation of the human posterior parietal cortex. PloS One 2011;6:e27204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Chung SW, et al. Impact of different intensities of intermittent theta burst stimulation on the cortical properties during TMS-EEG and working memory performance. Hum Brain Mapp 2018;39:783–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.