Abstract
Aims/Introduction
To analyze the associations and interactions of the genetic susceptibility and family history of diabetes with lifestyle factors in relation to diabetes among Chinese adults.
Materials and Methods
We constructed a genetic risk score of 34 single‐nucleotide polymorphisms in 11,596 participants from Songnan and Youyi communities, Baoshan District, Shanghai, China. We determined a healthy lifestyle by a normal body mass index (<24 kg/m2), adequate fruit and vegetable intake (≥4.5 cups/day), never smoked or quit smoking >1 year prior, sufficient physical activity (≥600 metabolic equivalent minutes per week), and a sleep duration of ≥6 to ≤8 h/day. Logistic regression models were used to examine the associations and interactions between heritability and lifestyle on diabetes.
Results
A healthier lifestyle was associated with a lower prevalence of diabetes within any heritable risk groups categorized by the genetic risk score and family history of diabetes. In the combined communities, the odds ratio (95% confidence interval) for diabetes associated with each additional healthy lifestyle factor was 0.83 (0.77–0.89) among participants with a low genetic risk score and 0.86 (0.81–0.91) among participants with a high genetic risk score (P interaction = 0.66). Similar interaction patterns of family history (P interaction = 0.15) and the combination of family history and the genetic risk score with healthy lifestyle (P interaction = 0.55) on diabetes were observed.
Conclusions
A healthier lifestyle was associated with a significantly lower prevalence of diabetes regardless of heritable risk groups, highlighting the importance of adhering to a healthy lifestyle for diabetes prevention among the entire population.
Keywords: Heritability, Lifestyle, Diabetes
In this study of 11,596 middle‐aged and elderly Chinese adults, a healthier lifestyle was associated with a substantially lower prevalence of diabetes regardless of heritable risk categories determined by the genetic risk score and family history of diabetes, highlighting the importance of adhering to a healthy lifestyle for diabetes prevention among the entire population.

INTRODUCTION
Type 2 diabetes imposes a substantial public health burden by virtue of its high prevalence and the difficulty of effective management 1 . Diabetes is mainly caused by the interplay between genetic and environmental factors. To date, genome‐wide association studies (GWASs) have identified more than 500 independent single‐nucleotide polymorphisms (SNPs) associated with diabetes 2 . These risk alleles, when aggregated into a genetic risk score (GRS), could provide a continuous and quantitative measure of the genetic susceptibility to diabetes 3 , 4 . Before the GWASs, family history (FH) of diabetes used to be the primary surrogate measure of heritable contribution to diabetes 5 . Compared with people without a diabetic family member, people who have either one parent or one full sibling with diabetes have approximately a twofold elevated risk of diabetes 6 . FH not only reflects heritability, but also reflects potential shared environmental risk factors among relatives. The GWAS‐identified variants collectively can explain approximately 20% of the overall heritability for diabetes 2 , which suggests that FH might still contain unidentified shared genetic and environmental information that is complementary to the genetic variations 5 .
Previous studies of people of European descent have manifested that lifestyle factors, such as diet, physical activity and body mass index (BMI), might modulate the genetic susceptibility to diabetes 7 , 8 , 9 . However, data on the interplay between the genetic variations and lifestyle factors on diabetes are limited in Chinese adults. In addition, given the considerable popularity of FH of diabetes, a comprehensive analysis of the GRS and FH might obtain more comprehensive information about the heritable risk of diabetes.
In the present study, we determined the heritable risk factors of diabetes by a GRS and FH of diabetes, and examined the associations and interactions between healthy lifestyle factors and these heritable factors on diabetes in Chinese adults, with particular interest in assessing whether the association between a healthy lifestyle and diabetes could be modified by heritable risk factors.
MATERIALS AND METHODS
Study populations
The present study was a part of an ongoing investigation of community‐dwelling Chinese adults aged ≥40 10 , 11 . The participants of the present study were recruited from two nearby communities, Songnan and Youyi, Baoshan district of Shanghai, China, in 2011 and 2013. A total of 11,935 participants (6,552 in Songnan community and 5,383 in Youyi community) were recruited in this study, in which 339 participants (181 in Songnan and 158 in Youyi) who had more than two missing SNPs were excluded. Thus, 11,596 participants (6,371 in Songnan and 5,225 in Youyi) with qualified genotype information and complete information on lifestyle, FH of diabetes and diabetes measurements were included in the study (flowchart see Figure S1). This study was approved by the Medical Ethics Committee of Ruijin Hospital, Shanghai Jiao Tong University (Ethics committee reference number: [2011] Lin Lun Shen No. [14]; Approval date: 10 March 2011). All study participants provided written informed consent.
Anthropometric and laboratory measurements
Trained physicians administered standard questionnaires to collect socioeconomic and demographic information, FH of diseases, and lifestyle factors including smoking status and sleep duration. Education level was categorized as less than high school (<9 years) and high school or further education (≥9 years). Smoking status was classified as non‐smoker or quit smoking more than 1 year prior and current smoker or quit smoking not more than 1 year 12 . Sleep duration was obtained by adding nightly sleep duration and daytime snap duration, and was divided into 6–8 h/day and ≥0 to <6 or >8 h/day 13 . Physical activity was assessed based on the International Physical Activity Questionnaire Short Form 14 . Metabolic equivalent minutes per week (MET‐min/week) was calculated 15 , and physical activity was categorized as ≥0 to <600 MET‐min/week and ≥600 MET‐min/week. A food frequency questionnaire was used to collect fruit and vegetable intake by asking the consumption frequency and amount per serving during the previous 12 months. Fruit and vegetable intake was categorized as ≥0 to <4.5 cups/day and ≥4.5 cups/day 12 .
Height and bodyweight were measured by trained physicians. BMI was calculated as bodyweight in kilograms divided by height squared in meters (kg/m2). Systolic blood pressure and diastolic blood pressure were measured in triplicate on the same day after at least 10‐min rest using an automated electronic device (OMRON Model HEM‐752 FUZZY, Omron Co., Dalian, China), and the average value of the three measurements was used for analysis. Hypertension was diagnosed as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or use of antihypertensive medications.
All participants underwent a 75‐g oral glucose tolerance test (OGTT) after an overnight fast of at least 10 h, and blood samples were collected at 0 and 2‐h during the OGTT. Plasma glucose was measured using the glucose oxidase method on an autoanalyzer (ADVIA‐1650 Chemistry System; Bayer Corp., Leverkusen, Germany). Glycated hemoglobin was determined by high‐performance liquid chromatography (Bio‐Rad, Hercules, CA, USA). Serum concentrations of total cholesterol, triglycerides, high‐density lipoprotein cholesterol and low‐density lipoprotein cholesterol were measured with an autoanalyzer (ADVIA‐1650 Chemistry System; Bayer Corp., Leverkusen, Germany). Dyslipidemia was defined as total cholesterol ≥6.22 mmol/L (240 mg/dL), triglycerides ≥2.26 mmol/L (200 mg/dL), high‐density lipoprotein cholesterol <1.04 mmol/L (40 mg/dL), low‐density lipoprotein cholesterol ≥4.14 mmol/L (160 mg/dL) or use of lipid‐lowering medications 16 .
FH of diabetes and the GRS construction
In the present study, heritable risk factors of diabetes were determined by a GRS and FH of diabetes. FH of diabetes was defined as having at least one first‐degree relative with diabetes. White blood cells were collected for deoxyribonucleic acid extractions using a commercial blood genomic deoxyribonucleic acid extraction kit (OSRM102‐T1, TIANGEN BIOTECH Co., Ltd., Beijing, China) on an automated nucleic acid extraction instrument (OSEM48, TIANGEN BIOTECH Co., Ltd., Beijing, China) according to the manufacturer’s standard protocol. Specific assays were designed using the MassARRAY assay design software package (v3.1) (https://www.agenacx.com/Home). Mass determination was carried out with the MALDI‐TOF mass spectrometer, and data acquisition was carried out using MassARRAY Typer 4.0 software (Sequenom, CapitalBio Corp., Beijing, China). The minimum call rate was 98.7%. The concordance rate was >99% based on 100 duplicates genotyping 17 .
Given the ethnicity specificity of genetic backgrounds, we selected 34 SNPs that were discovered in European individuals and replicated in East Asian individuals, and those identified and validated in meta‐analysis including GWASs from East Asian individuals 18 , 19 . All SNPs reached a genome‐wide significance level (P < 5 × 10−8) in the association with diabetes and were not in linkage disequilibrium (r 2 = 0.000, except it was 0.055 between rs10906115 and rs12779790 in CDC123/CAMK1D) according to the data of East Asian ancestries in the International HapMap release 21. As for the GRS construction, we assumed the addictive genetic model for each SNP, applying a linear weighing of 0, 1 and 2 to genotypes containing 0, 1 or 2 risk alleles, respectively 20 . The GRS was calculated on the basis of the 34 diabetes‐associated SNPs by a weighted method (Table S1): GRS = (β1 × SNP1 + β2 × SNP2+…+β34 × SNP34) × (34/sum of the β‐coefficients), where SNPi is the risk allele number of each SNP associated with diabetes, and βi (the natural log of the odds ratio [OR]) is the effect size of each SNP associated with diabetes summarized in the literature 18 , 19 . As to participants who had one or two missing SNPs, we assigned them the average GRS. In the present study, the GRS ranged from 21.0 to 49.4, with a higher score indicating a higher risk of diabetes. We categorized participants into high and low GRS groups by the median value of the GRS.
Healthy lifestyle factors and the healthy lifestyle score
We determined a healthy lifestyle score consisting of five risk factors based on a prior knowledge and public health recommendations: a normal BMI (<24 kg/m2), adequate fruit and vegetable intake (≥4.5 cups/day), never smoked or quit smoking >1 year prior, sufficient physical activity (≥600 MET‐min/week), and a sleep duration of ≥6 to ≤8 h/day. We assigned a score (0 for unhealthy and 1 for healthy) for each lifestyle factor and summed the scores of the five factors. The healthy lifestyle score ranged from 0 (the least healthy) to 5 (the healthiest).
Definition of diabetes
According to the American Diabetes Association 2010 criteria, diabetes was defined as: fasting plasma glucose ≥7.0 mmol/L (126 mg/dL), OGTT‐2h plasma glucose ≥11.1 mmol/L (200 mg/dL), glycated hemoglobin A1c ≥6.5%, or a self‐reported previous diagnosis of diabetes by health‐care professionals 21 .
Statistical analysis
Characteristics of study participants were presented by low and high GRS levels. Categorical variables were summarized as numbers with percentages, and continuous variables were summarized as means with standard deviations. Differences in characteristics between low and high GRS levels were compared by the χ2‐test for categorical variables and analysis of variation for continuous variables.
The healthy lifestyle score was categorized into low (scored 0, 1 or 2), intermediate (scored 3) and high (scored 4 or 5) levels. Multivariable logistic regression models were used to evaluate the ORs and 95% confidence intervals (CIs) of diabetes associated with individual SNPs included in the GRS, heritable factors (FH of diabetes and the GRS) and lifestyle factors (individual lifestyle factors and the healthy lifestyle score). We used attributable risk percentage (AR%) to estimate the risk of diabetes attributable to high GRS and FH of diabetes. The AR% was calculated by dividing the AR (subtracting the prevalence in the unexposed from the prevalence in the exposed) by the prevalence in the exposed and then multiplying the product by 100 to obtain a percentage. Multiplicative interactions of the GRS and FH with lifestyle factors on diabetes were tested by including the product term (e.g., GRS × lifestyle score category) in the models. To further evaluate whether the association between the healthy lifestyle score and diabetes persists across different heritable risk levels, participants were categorized into four groups according to the combination of the GRS and FH of diabetes: low GRS without FH, low GRS with FH, high GRS without FH and high GRS with FH. The interaction between heritable risk levels and healthy lifestyle score in relation to diabetes was examined. We carried out the analysis in Songnan and Youyi communities, and pooled the findings across the two communities by means of inverse variance weighted fixed effects meta‐analysis. Statistical analysis was carried out by SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). A two‐sided P‐value <0.05 was the threshold for statistical significance.
RESULTS
Basic characteristics of the study participants according to the GRS levels in Songnan and Youyi communities were summarized in Table 1. Participants with a high GRS had a higher proportion of FH of diabetes, and had higher concentrations of fasting plasma glucose, OGTT‐2h plasma glucose and glycated hemoglobin than participants with a low GRS in both Songnan and Youyi. No significant differences were observed in age, education attainment, fruit and vegetable intake, smoking status, physical activity, sleep duration, blood pressures, and lipid profiles between low and high levels of the GRS in the two communities.
Table 1.
Basic characteristics of study participants according to the genetic risk score in two communities
| Characteristic | Overall | Genetic risk score | P‐value | |
|---|---|---|---|---|
| Low | High | |||
| Songnan community | ||||
| No. participants | 6,371 | 3,191 | 3,180 | |
| Age (years) | 62.2 (9.5) | 62.1 (9.5) | 62.2 (9.5) | 0.77 |
| Man, n (%) | 2,421 (38.0) | 1,178 (36.9) | 1,243 (39.1) | 0.074 |
| High school or further education, n (%) | 1,876 (29.5) | 924 (29.0) | 952 (29.9) | 0.39 |
| Heritable risk factor | ||||
| Family history of diabetes, n (%) | 910 (14.3) | 423 (13.3) | 487 (15.3) | 0.019 |
| Genetic risk score | 34.5 (3.8) | 31.5 (2.4) | 37.6 (2.3) | <0.001 |
| Healthy lifestyle factor | ||||
| BMI (kg/m2) | 25.7 (3.7) | 25.9 (3.8) | 25.5 (3.7) | <0.001 |
| Fruit and vegetable intake (cup/day) | 5.0 (3.8) | 5.1 (3.9) | 4.9 (3.8) | 0.062 |
| Smoking, n (%) † | 1,728 (27.1) | 842 (26.4) | 886 (27.9) | 0.19 |
| Physical activity (MET‐min/week) | 2,126 (3,113) | 2,190 (3,229) | 2,061 (2,990) | 0.098 |
| Sleep (h/day) | 8.22 (1.57) | 8.24 (1.55) | 8.20 (1.60) | 0.24 |
| Fasting plasma glucose (mmol/L) | 6.2 (2.0) | 6.0 (1.8) | 6.3 (2.1) | <0.001 |
| OGTT‐2h plasma glucose (mmol/L) | 8.9 (4.3) | 8.6 (4.1) | 9.2 (4.5) | <0.001 |
| HbA1c (%) | 5.9 (1.1) | 5.8 (1.1) | 5.9 (1.2) | <0.001 |
| Waist circumstance (cm) | 83.5 (10.0) | 84.0 (10.0) | 83.1 (9.9) | <0.001 |
| Systolic blood pressure (mmHg) | 138.4 (20.0) | 138.3 (19.7) | 138.6 (20.3) | 0.54 |
| Diastolic blood pressure (mmHg) | 78.5 (10.1) | 78.7 (10.0) | 78.4 (10.1) | 0.17 |
| Lipid profile (mmol/L) | ||||
| Total cholesterol | 5.42 (1.00) | 5.42 (1.02) | 5.42 (0.99) | 0.77 |
| Triglycerides | 1.58 (1.10) | 1.59 (1.06) | 1.58 (1.13) | 0.58 |
| High‐density lipoprotein cholesterol | 1.29 (0.32) | 1.29 (0.32) | 1.29 (0.32) | 0.85 |
| Low‐density lipoprotein cholesterol | 3.17 (0.84) | 3.18 (0.84) | 3.17 (0.83) | 0.76 |
| Youyi community | ||||
| No. participants | 5,225 | 2,612 | 2,613 | |
| Age (years) | 64.4 (10.0) | 64.4 (9.9) | 64.5 (10.2) | 0.61 |
| Man, n (%) | 1,711 (32.8) | 812 (31.1) | 899 (34.4) | 0.011 |
| High school or further education, n (%) | 1,795 (34.4) | 865 (33.1) | 930 (35.6) | 0.28 |
| Heritable risk factor | ||||
| Family history of diabetes, n (%) | 856 (16.4) | 375 (14.4) | 491 (18.4) | <0.001 |
| Genetic risk score | 34.5 (4.0) | 31.4 (2.4) | 37.7 (2.4) | <0.001 |
| Healthy lifestyle factor | ||||
| BMI (kg/m2) | 24.8 (3.4) | 24.8 (3.5) | 24.7 (3.3) | 0.11 |
| Fruit and vegetable intake (cup/day) | 3.9 (1.5) | 4.0 (1.5) | 3.9 (1.5) | 0.27 |
| Smoking, n (%) † | 736 (14.1) | 356 (13.6) | 380 (14.5) | 0.34 |
| Physical activity (MET‐min/week) | 1,683 (2,221) | 1,646 (2,227) | 1,720 (2,215) | 0.23 |
| Sleep (h/day) | 7.86 (1.22) | 7.86 (1.21) | 7.85 (1.23) | 0.59 |
| Fasting plasma glucose (mmol/L) | 5.8 (1.4) | 5.7 (1.3) | 5.9 (1.5) | <0.001 |
| OGTT 2 h plasma glucose (mmol/L) | 8.8 (3.8) | 8.5 (3.6) | 9.2 (4.0) | <0.001 |
| HbA1c (%) | 6.2 (0.9) | 6.1 (0.9) | 6.2 (1.0) | <0.001 |
| Waist circumstance (cm) | 83.2 (9.2) | 83.3 (9.2) | 83.1 (9.2) | 0.60 |
| Systolic blood pressure (mmHg) | 135.4 (20.6) | 135.3 (20.6) | 135.6 (20.6) | 0.58 |
| Diastolic blood pressure (mmHg) | 75.9 (10.2) | 76.0 (10.4) | 75.9 (10.0) | 0.66 |
| Lipid profile (mmol/L) | ||||
| Total cholesterol | 4.36 (1.12) | 4.36 (1.09) | 4.36 (1.14) | 0.82 |
| Triglycerides | 1.50 (1.07) | 1.49 (0.99) | 1.51 (1.14) | 0.40 |
| High‐density lipoprotein cholesterol | 1.11 (0.32) | 1.11 (0.31) | 1.11 (0.32) | 0.83 |
| Low‐density lipoprotein cholesterol | 2.53 (0.82) | 2.53 (0.81) | 2.53 (0.83) | 0.83 |
Data are mean (standard deviation) or n (%).
BMI, body mass index; HbA1c, glycated hemoglobin; MET‐min/week, metabolic equivalent minutes per week; OGTT, oral glucose tolerance test.
Current smokers and those who quit smoking not more than 1 year.
Associations of FH of diabetes and the GRS with diabetes
Compared with participants with a low GRS, participants with a high GRS showed a higher risk of prevalent diabetes, with the OR for diabetes of 1.57 (95% CI 1.39–1.77) in the Songnan community, and 1.67 (95% CI 1.42–1.96) in the Youyi community (Table 2). A similar and greater association between FH of diabetes and diabetes was observed: the OR for diabetes associated with a FH was 2.78 (95% CI 2.37–3.26) in Songnan and 3.67 (95% CI 3.01–4.46) in Youyi. FH of diabetes showed a stronger impact on diabetes than a high GRS in both communities. For participants in Songnan, the AR% for diabetes was 24.4% attributable to a high GRS and 39.3% attributable to FH of diabetes. For participants in Youyi, a high GRS accounted for 35.2% and FH of diabetes accounted for 53.3% of the AR% for diabetes (Figure S2).
Table 2.
Associations of family history and the genetic risk score with diabetes in participants from two communities
| Heritable risk factor | Songnan community | Youyi community | ||||
|---|---|---|---|---|---|---|
| No. participants | OR (95% CI) | No. of participants | OR (95% CI) | |||
| Model 1 † | Model 2 ‡ | Model 1 † | Model 2 ‡ | |||
| Genetic risk score | ||||||
| Low | 3,191 | 1 (ref) | 1 (ref) | 2,612 | 1 (ref) | 1 (ref) |
| High | 3,180 | 1.46 (1.30−1.64) | 1.57 (1.39−1.77) | 2,613 | 1.64 (1.40−1.93) | 1.67 (1.42−1.96) |
| Family history | ||||||
| No | 5,461 | 1 (ref) | 1 (ref) | 4,369 | 1 (ref) | 1 (ref) |
| Yes | 910 | 2.65 (2.27−3.09) | 2.78 (2.37−3.26) | 856 | 3.57 (2.95−4.33) | 3.67 (3.01−4.46) |
CI, confidence interval; OR, odds ratio.
Model 1 was adjusted for age and sex.
Model 2 was adjusted for age, sex, education attainment (less than high school, high school or further education), waist circumstance, body mass index, hypertension (yes, no) and dyslipidemia (yes, no).
Associations of individual lifestyle factors and the healthy lifestyle score with diabetes
Of the five individual healthy lifestyle factors, BMI <24 kg/m2 (Songnan OR 0.57, 95% CI 0.50–0.65; Youyi OR 0.74, 95% CI 0.63–0.88) and fruit and vegetable intake ≥4.5 cups/day (Songnan OR 0.86, 95% CI 0.77–0.97; Youyi OR 0.50, 95% CI 0.41–0.60) were independently associated with a decreased risk of prevalent diabetes in both Songnan and Youyi communities (Table 3).
Table 3.
Associations of individual lifestyle factors and the healthy lifestyle score with diabetes in participants from two communities
| Lifestyle factor | Songnan community | Youyi community | ||||
|---|---|---|---|---|---|---|
| No. participants | OR (95% CI) | No. participants | OR (95% CI) | |||
| Model 1 † | Model 2 ‡ | Model 1 † | Model 2 ‡ | |||
| BMI (kg/m2) | ||||||
| ≥24 | 4,242 | 1 (ref) | 1 (ref) | 2,993 | 1 (ref) | 1 (ref) |
| <24 | 2,129 | 0.51 (0.44−0.58) | 0.57 (0.50−0.65) | 2,232 | 0.67 (0.57−0.79) | 0.74 (0.63−0.88) |
| Fruit and vegetable intake (cup/day) | ||||||
| <4.5 | 3,332 | 1 (ref) | 1 (ref) | 3,608 | 1 (ref) | 1 (ref) |
| ≥4.5 | 3,039 | 0.86 (0.77−0.97) | 0.86 (0.77−0.97) | 1,617 | 0.51 (0.42−0.61) | 0.50 (0.41−0.60) |
| Smoking | ||||||
| Current or quit ≤1 year | 1,728 | 1 (ref) | 1 (ref) | 736 | 1 (ref) | 1 (ref) |
| Never or quit >1 year | 4,643 | 1.06 (0.91−1.23) | 1.08 (0.93−1.25) | 4,489 | 0.86 (0.68−1.10) | 0.85 (0.67−1.08) |
| Physical activity, MET‐min/week | ||||||
| <600 | 1,867 | 1 (ref) | 1 (ref) | 1,644 | 1 (ref) | 1 (ref) |
| ≥600 | 4,504 | 0.96 (0.84−1.08) | 0.96 (0.84−1.09) | 3,581 | 1.08 (0.91−1.28) | 1.10 (0.93−1.31) |
| Sleep (h/day) | ||||||
| <6 or >8 | 3,029 | 1 (ref) | 1 (ref) | 1,780 | 1 (ref) | 1 (ref) |
| 6–8 | 3,342 | 0.95 (0.85−1.07) | 0.97 (0.86−1.09) | 3,445 | 0.87 (0.74−1.03) | 0.86 (0.73−1.02) |
| Healthy lifestyle score | ||||||
| Low | 2,485 | 1 (ref) | 1 (ref) | 1,697 | 1 (ref) | 1 (ref) |
| Intermediate | 2,321 | 0.80 (0.71−0.91) | 0.84 (0.74−0.96) | 1,935 | 0.75 (0.62−0.89) | 0.77 (0.64−0.93) |
| High | 1,565 | 0.62 (0.53−0.73) | 0.68 (0.58−0.80) | 1,593 | 0.52 (0.43−0.65) | 0.56 (0.45−0.68) |
BMI, body mass index; CI, confidence interval; MET‐min/week, metabolic equivalent minutes per week; OR, odds ratio.
Model 1 was adjusted for age and sex.
Model 2 was adjusted for age, sex, education attainment (less than high school, high school or further education), hypertension (yes, no), and dyslipidemia (yes, no).
When analyzed collectively, compared with participants with a low healthy lifestyle score, the ORs for diabetes were 0.84 (95% CI 0.74–0.96) among participants with an intermediate healthy lifestyle score and 0.68 (95% CI 0.58–0.80) among participants with a high healthy lifestyle score in the Songnan community, and the corresponding ORs for diabetes were 0.77 (95% CI 0.64–0.93) and 0.56 (95% CI 0.45–0.68) in the Youyi community.
Associations of heritable risk factors with diabetes by the healthy lifestyle score
We found consistent and non‐significant interactions of the healthy lifestyle score with the GRS (Songnan P for interaction = 0.70; Youyi P for interaction = 0.93) and FH of diabetes (Songnan P for interaction = 0.46; Youyi P for interaction = 0.30) in relation to prevalent diabetes in both the Songnan and Youyi communities (Table 4). In the combined communities, within each category of the healthy lifestyle score, the OR for diabetes associated with each additional 10 points of the GRS was 2.10 (95% CI 1.74–2.54) among participants with a low healthy lifestyle score, 2.03 (95% CI 1.66–2.47) among participants with an intermediate healthy lifestyle score and 2.28 (95% CI 1.73–2.99) among participants with a high healthy lifestyle score (P for interaction = 0.93). The OR for diabetes associated with a FH of diabetes was 2.90.
Table 4.
Associations between heritable risk factors and diabetes by the healthy lifestyle score in participants from two communities
| Community | Healthy lifestyle score | P for interaction | ||
|---|---|---|---|---|
| Low | Intermediate | High | ||
| Songnan | ||||
| Genetic risk score per 10‐point increment | ||||
| Model 1 † | 2.02 (1.60−2.56) | 1.78 (1.39−2.28) | 2.35 (1.67−3.32) | 0.67 |
| Model 2 ‡ | 2.05 (1.61−2.60) | 1.81 (1.41−2.32) | 2.39 (1.68−3.41) | 0.70 |
| Family history | ||||
| Model 1 † | 2.32 (1.82−2.96) | 2.92 (2.26−3.79) | 3.01 (2.18−4.16) | 0.52 |
| Model 2 ‡ | 2.36 (1.85−3.02) | 3.05 (2.34−3.97) | 3.06 (2.20−4.27) | 0.46 |
| Youyi | ||||
| Genetic risk score per 10‐point increment | ||||
| Model 1 † | 2.18 (1.59−2.99) | 2.43 (1.74−3.39) | 2.22 (1.44−3.41) | 0.98 |
| Model 2 ‡ | 2.21 (1.61−3.04) | 2.48 (1.78−3.48) | 2.11 (1.37−3.26) | 0.93 |
| Family history | ||||
| Model 1 † | 3.95 (2.91−5.37) | 3.33 (2.42−4.59) | 3.48 (2.32−5.21) | 0.22 |
| Model 2 ‡ | 4.01 (2.94−5.47) | 3.34 (2.42−4.61) | 3.75 (2.48−5.66) | 0.30 |
| Pooled results | ||||
| Genetic risk score per 10‐point increment | ||||
| Model 1 † | 2.08 (1.72−2.51) | 1.99 (1.63−2.42) | 2.30 (1.76−3.01) | 0.93 |
| Model 2 ‡ | 2.10 (1.74−2.54) | 2.03 (1.66−2.47) | 2.28 (1.73−2.99) | 0.93 |
| Family history | ||||
| Model 1 † | 2.85 (2.36−3.45) | 3.08 (2.52−3.77) | 3.19 (2.47−4.10) | 0.37 |
| Model 2 ‡ | 2.90 (2.39−3.52) | 3.17 (2.58−3.88) | 3.32 (2.56−4.29) | 0.41 |
Model 1 was adjusted for age and sex.
Model 2 was adjusted for age, sex, education attainment (less than high school, high school or further education), hypertension (yes, no) and dyslipidemia (yes, no).
(95% CI 2.39–3.52), 3.17 (95% CI 2.58–3.88) and 3.32 (95% CI 2.56–4.29) among participants with a low, intermediate and high healthy lifestyle score, respectively (P for interaction = 0.41).
Association of the healthy lifestyle score with diabetes by heritable risk factors
Across the Songnan and Youyi communities, a healthier lifestyle was associated with a lower prevalence of diabetes, regardless of the GRS levels and a FH of diabetes (Figure 1). In the combined communities, the ORs for diabetes associated with each additional healthy lifestyle factor were 0.83 (95% CI 0.77−0.89) among participants with a low GRS and 0.86 (95% CI 0.81−0.91) among participants with a high GRS, and were 0.85 (95% CI 0.80−0.89) and 0.83 (95% CI 0.75−0.92) among participants without and with a FH of diabetes, respectively.
Figure 1.
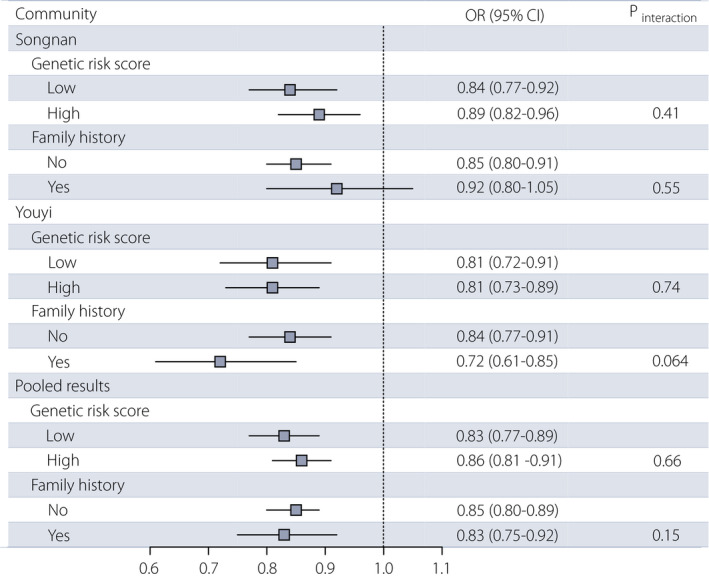
Association between the healthy lifestyle score and diabetes by heritable risk factors in two communities. Analyses were adjusted for age, sex, education attainment (less than high school, high school or further education), hypertension (yes, no) and dyslipidemia (yes, no). CI, confidence interval; OR, odds ratio.
The association between the healthy lifestyle score and diabetes was consistent according to the combined categories of the heritable risk factors in the Songnan and Youyi communities (Figure 2). In the combined communities, among participants with a low GRS, the ORs for diabetes associated with each additional healthy lifestyle factor were 0.84 (95% CI 0.77−0.90) for participants without a FH of diabetes, and 0.82 (95% CI 0.70−0.97) for participants with a FH. Among participants with a high GRS, the ORs for diabetes were 0.86 (95% CI 0.80−0.92) and 0.83 (95% CI 0.72−0.94) for participants without and with a FH of diabetes, respectively.
Figure 2.
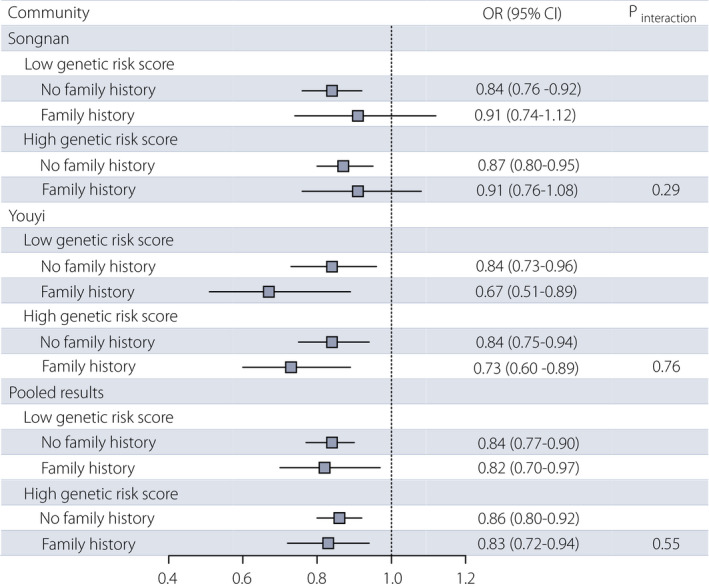
Association between the healthy lifestyle score and diabetes by the combined categories of heritable risk factors in two communities. Analyses were adjusted for age, sex, education attainment (less than high school, high school or further education), hypertension (yes, no) and dyslipidemia (yes, no). CI, confidence interval; OR, odds ratio.
DISCUSSION
In the present large, population‐based cohort study of 11,596 Chinese adults, both a higher genetic susceptibility and a FH of diabetes were positively associated with the risk of prevalent diabetes, with FH showing a greater impact on diabetes in terms of association strength and attributable risk. By contrast, a healthier lifestyle consisting of a normal BMI (<24 kg/m2), adequate fruit and vegetable intake (≥4.5 cups/day), never smoked or quit smoking >1 year prior, sufficient physical activity (≥600 MET‐min/week), and a sleep duration of ≥6 to ≤8 h/day was independently associated with a substantially lower risk of prevalent diabetes, regardless of different heritable risk categories classified by the GRS and FH of diabetes. We observed no statistically significant interaction effects between the heritable risk factors and lifestyle factors in relation to diabetes, suggesting that adhering to a healthy lifestyle might attenuate the risk of prevalent diabetes among the entire population.
The present findings supported two noteworthy conclusions. First, in this study, the GRS, FH of diabetes and lifestyle factors were independently associated with diabetes, which is well aligned with previous studies 2 , 5 , 22 . Notably, we found that a FH of diabetes showed a stronger impact on diabetes than the GRS, which highlights the necessity of taking into account both FH and the GRS when examining the interplay between heritable risk factors and lifestyle factors in relation to diabetes.
Second, we observed that a healthier lifestyle was associated with a lower risk of prevalent diabetes regardless of heritable risk backgrounds determined by individual and combined categories of the GRS and FH of diabetes. The present findings emphasize the importance of adhering to a healthy lifestyle in the prevention of diabetes among the entire population. Thus far, studies on the interaction between genetic variations and lifestyle factors on diabetes have shown inconsistent findings. Cohort studies and trials in European populations have reported interactions between genetic factors and lifestyle factors, such as diet and physical activity, on diabetes 7 , 23 . However, emerging evidence has suggested non‐significant interactions. In the European Prospective Investigation into Cancer (EPIC)‐InterAct study, no statistically significant interactions between variants of the GIPR, CAV2 and HFE gene and various dietary factors were observed 24 , 25 . In addition, most of the interaction findings have not yet been replicated across multiple study populations. So far, the interaction of TCF7L2 rs7903146 with whole grain food has been well replicated in more than one independent study 26 , 27 , 28 . However, given the complex pathogenesis of diabetes, individual variants can only explain very limited heritability 29 . Therefore, the ensemble of genetic variants to form a GRS might be more appropriate to investigate gene–lifestyle interactions. Cohort studies of American populations have suggested consistent interaction effects of healthy lifestyle factors including healthy dietary patterns and physical activity with genetic predisposition of obesity captured by GRSs on long‐term weight gain 30 , 31 . However, whether the beneficial effects persist for diabetes and among other populations remains unknown. Obesity is closely related to an increased risk of diabetes, but there are various differences between the etiology of diabetes and obesity, especially heritability. Consensus estimates of heritability for obesity and diabetes are approximately 70 and 35%, respectively 22 . Together with previous studies, the present study further added evidence to non‐significant interactions of heritable risk determined by both the GRS and FH of diabetes with lifestyle factors on diabetes among Chinese adults.
The precise mechanisms to explain our observations are not fully unveiled. It has been suggested that environmental exposures, such as diet and physical activity, could influence individual predisposition to diabetes through ligand binding efficiency, membrane channeling, deoxyribonucleic acid replication and repair or methylation 22 . Variations in the “intrinsic environment,’’ reflected in proteome, metabolome or microbiome profiles, might also modulate genome function, thus generating complex feedback loops of gene–environment interactions 22 . Previous studies suggested that certain genes associated with diabetes might encode nuclear receptors implicated in insulin signaling, adipogenesis and the matching of lipid storage provision to nutritional state, which could partly explain a gene–environment interaction on diabetes 22 . Thus, future experimental or multi‐omics studies are required to provide biological insight into gene–environment interactions in relation to diabetes.
Several strengths merit consideration. First, different from previous studies, we comprehensively evaluated the heritable risk of diabetes by adding FH of diabetes to the GRS. Given the considerable popularity of FH, we assumed a combination of the GRS and FH might obtain more comprehensive information about heritable risk of diabetes, and thus the findings could be more directly relevant to public health and clinical implications. Second, we comprehensively assessed a healthy lifestyle consisting of five risk factors based on a prior knowledge and public health recommendations. Third, our main results were highly consistent and were mutually validated in two communities, which strengthened the reliability of the findings. Fourth, genotyping was carried out with high‐quality control standards, which guaranteed the accuracy and reliability of our genetic data.
The present study had several limitations. First, due to the nature of a cross‐sectional study, potential bias, confounding and reverse causality might exist, which hindered efforts to determine a causal effect. For example, participants with diabetes might be more health‐conscious and adopt a healthier lifestyle. Second, the GRS based on the 34 common diabetes‐related variants in East Asian individuals could not capture the potential contribution of other variants, such as certain SNPs identified or validated among Han Chinese individuals 32 , 33 . However, these 34 SNPs have been identified or validated in East Asians, and almost all the diabetes‐associated SNPs identified or validated in Han Chinese were included in this study (in an identical or proxy way) except for C2CD4A/B rs1370176, GRK5 rs10886471, RASGRP1 rs7403531 and DUSP9 rs5945326 32 , 33 , 34 , 35 , suggesting a robust relationship with diabetes and representing a considerable heritability of diabetes 18 , 19 . Third, this analysis was restricted to Chinese adults aged ≥40, therefore caution should be taken to generalize the present findings to other ethnic or age groups.
In conclusion, in the present study of 11,596 middle‐aged and elderly Chinese adults, a healthier lifestyle was associated with a substantially lower prevalence of diabetes regardless of heritable risk categories determined by the GRS and FH of diabetes. This study provides new evidence of the importance of adhering to a healthy lifestyle in the prevention of diabetes among the entire population.
DISCLOSURE
The authors declare no conflict of interest.
Supporting information
Table S1 | Information and effect of each individual single‐nucleotide polymorphism associated with diabetes
Figure S1 | Flowchart of the study participants
Figure S2 | Attributable risk% for diabetes attributable to the genetic risk score and family history of diabetes in participants from two communities
ACKNOWLEDGMENTS
The authors thank all staff and study participants for their contributions. This work was funded by the National Natural Science Foundation of China (82022011, 81970706, 81941017, 81970728 and 81770842), the National Key R&D Program of China (2016YFC1305600, 2016YFC1305202, 2016YFC1304904, 2017YFC1310700 and 2018YFC1311800) and the Innovative Research Team of High‐level Local Universities in Shanghai.
J Diabetes Investig. 2021; 11: 2089–2098
REFERENCES
- 1. Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet 2017; 389: 2239–2251. [DOI] [PubMed] [Google Scholar]
- 2. Vujkovic M, Keaton JM, Lynch JA, et al. Discovery of 318 new risk loci for type 2 diabetes and related vascular outcomes among 1.4 million participants in a multi‐ancestry meta‐analysis. Nat Genet 2020; 52: 680–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Udler MS, McCarthy MI, Florez JC, et al. Genetic risk scores for diabetes diagnosis and precision medicine. Endocr Rev 2019; 40: 1500–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Merino J, Guasch‐Ferré M, Ellervik C, et al. Quality of dietary fat and genetic risk of type 2 diabetes: individual participant data meta‐analysis. BMJ 2019; 366: l4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ding M, Ahmad S, Qi LU, et al. Additive and multiplicative interactions between genetic risk score and family history and lifestyle in relation to risk of type 2 diabetes. Am J Epidemiol 2020; 189: 445–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Silverman‐Retana O, Hulman A, Nielsen J, et al. Effect of familial diabetes status and age at diagnosis on type 2 diabetes risk: a nation‐wide register‐based study from Denmark. Diabetologia 2020; 63: 934–943. [DOI] [PubMed] [Google Scholar]
- 7. InterAct Consortium . Investigation of gene‐diet interactions in the incretin system and risk of type 2 diabetes: the EPIC‐InterAct study. Diabetologia 2016; 59: 2613–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Klimentidis YC, Chen Z, Arora A, et al. Association of physical activity with lower type 2 diabetes incidence is weaker among individuals at high genetic risk. Diabetologia 2014; 57: 2530–2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li L, Wang J, Ping Z, et al. Interaction analysis of gene variants of TCF7L2 and body mass index and waist circumference on type 2 diabetes. Clin Nutr 2020; 39: 192–197. [DOI] [PubMed] [Google Scholar]
- 10. Ning G, Reaction Study Group . Risk evaluation of cAncers in Chinese diabeTic Individuals: a lONgitudinal (REACTION) study. J Diabetes 2012; 4: 172–173. [DOI] [PubMed] [Google Scholar]
- 11. Bi Y, Lu J, Wang W, et al. Cohort profile: risk evaluation of cancers in Chinese diabetic individuals: a longitudinal (REACTION) study. J Diabetes 2014; 6: 147–157. [DOI] [PubMed] [Google Scholar]
- 12. Lloyd‐Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation 2010; 121: 586–613. [DOI] [PubMed] [Google Scholar]
- 13. Cappuccio FP, Cooper D, D'Elia L, et al. Sleep duration predicts cardiovascular outcomes: a systematic review and meta‐analysis of prospective studies. Eur Heart J 2011; 32: 1484–1492. [DOI] [PubMed] [Google Scholar]
- 14. Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12‐country reliability and validity. Med Sci Sports Exerc 2003; 35: 1381–1395. [DOI] [PubMed] [Google Scholar]
- 15. Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc 1993; 25: 71–80. [DOI] [PubMed] [Google Scholar]
- 16. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults . Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 2001; 285: 2486–2497. [DOI] [PubMed] [Google Scholar]
- 17. Bi Y, Wang W, Xu M, et al. Diabetes genetic risk score modifies effect of bisphenol a exposure on deterioration in glucose metabolism. J Clin Endocrinol Metab 2016; 101: 143–150. [DOI] [PubMed] [Google Scholar]
- 18. Cho YS, Lee J‐Y, Park KS, et al. Genetics of type 2 diabetes in East Asian populations. Curr Diab Rep 2012; 12: 686–696. [DOI] [PubMed] [Google Scholar]
- 19. Kato N. Insights into the genetic basis of type 2 diabetes. J Diabetes Investig 2013; 4: 233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Palmer TM, Lawlor DA, Harbord RM, et al. Using multiple genetic variants as instrumental variables for modifiable risk factors. Stat Methods Med Res 2012; 21: 223–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care 2010; 33: S62–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Franks PW, McCarthy MI. Exposing the exposures responsible for type 2 diabetes and obesity. Science 2016; 354: 69–73. [DOI] [PubMed] [Google Scholar]
- 23. Kilpeläinen TO, Lakka TA, Laaksonen DE, et al. Interaction of single nucleotide polymorphisms in ADRB2, ADRB3, TNF, IL6, IGF1R, LIPC, LEPR, and GHRL with physical activity on the risk of type 2 diabetes mellitus and changes in characteristics of the metabolic syndrome: the finnish diabetes prevention study. Metabolism 2008; 57: 428–436. [DOI] [PubMed] [Google Scholar]
- 24. Li SX, Imamura F, Ye Z, et al. Interaction between genes and macronutrient intake on the risk of developing type 2 diabetes: systematic review and findings from European Prospective Investigation into Cancer (EPIC)‐InterAct. Am J Clin Nutr 2017; 106: 263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Meidtner K, Podmore C, Kröger J, et al. Interaction of dietary and genetic factors influencing body iron status and risk of type 2 diabetes within the EPIC‐InterAct study. Diabetes Care 2018; 41: 277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fisher E, Boeing H, Fritsche A, et al. Whole‐grain consumption and transcription factor‐7‐like 2 (TCF7L2) rs7903146: gene‐diet interaction in modulating type 2 diabetes risk. Br J Nutr 2009; 101: 478–481. [DOI] [PubMed] [Google Scholar]
- 27. Hindy G, Sonestedt E, Ericson U, et al. Role of TCF7L2 risk variant and dietary fibre intake on incident type 2 diabetes. Diabetologia 2012; 55: 2646–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cornelis MC, Qi LU, Kraft P, et al. TCF7L2, dietary carbohydrate, and risk of type 2 diabetes in US women. Am J Clin Nutr 2009; 89: 1256–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Weedon MN, McCarthy MI, Hitman G, et al. Combining information from common type 2 diabetes risk polymorphisms improves disease prediction. PLoS Med 2006; 3: e374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang T, Huang T, Heianza Y, et al. Genetic susceptibility, change in physical activity, and long‐term weight gain. Diabetes 2017; 66: 2704–2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang T, Heianza Y, Sun D, et al. Improving adherence to healthy dietary patterns, genetic risk, and long term weight gain: gene‐diet interaction analysis in two prospective cohort studies. BMJ 2018; 360: j5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cui B, Zhu X, Xu M, et al. A genome‐wide association study confirms previously reported loci for type 2 diabetes in Han Chinese. PLoS One 2011; 6: e22353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li H, Gan W, Lu L, et al. A genome‐wide association study identifies GRK5 and RASGRP1 as type 2 diabetes loci in Chinese Hans. Diabetes 2013; 62: 291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tsai F‐J, Yang C‐F, Chen C‐C, et al. A genome‐wide association study identifies susceptibility variants for type 2 diabetes in Han Chinese. PLoS Genet 2010; 6: e1000847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ma RCW, Hu C, Tam CH, et al. Genome‐wide association study in a Chinese population identifies a susceptibility locus for type 2 diabetes at 7q32 near PAX4. Diabetologia 2013; 56: 1291–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 | Information and effect of each individual single‐nucleotide polymorphism associated with diabetes
Figure S1 | Flowchart of the study participants
Figure S2 | Attributable risk% for diabetes attributable to the genetic risk score and family history of diabetes in participants from two communities


