Abstract
Aims/Introduction
To compare the association of hypertension plus hyperuricemia with four insulin resistance surrogates, including glucose and triglycerides (TyG index), TyG index with body mass index (TyG‐BMI), the ratio of triglycerides divided by high‐density lipoprotein cholesterol (TG/HDL‐C) and metabolic score for insulin resistance (METS‐IR).
Materials and Methods
Data from a cross‐sectional epidemiological study enrolling a representative population sample aged ≥65 years were used to calculate the four indexes. The association with hypertension plus hyperuricemia and insulin resistance surrogates was examined with multivariate logistic regression and receiver operating characteristic.
Results
A total of 4,352 participants were included, including 93 (2.1%) patients with hyperuricemia alone, 2,875 (66.1%) with hypertension alone and 587 (13.5%) with hypertension plus hyperuricemia. Mutivariate logistic regression showed that TyG index, TyG‐BMI, TG/HDL‐C and METS‐IR were all significantly correlated with hyperuricemia, hypertension and hypertension plus hyperuricemia. Compared with the lowest quartile, the odds ratios (OR) of the highest quartile of the four indicators for hypertension plus hyperuricemia were TyG index: OR 6.39 (95% confidence interval [CI] 4.17–9.78); TyG‐BMI: OR 8.54 (95% CI 5.58–13.09); TG/HDL‐C: OR 7.21 (95% CI 4.72–11.01); METS‐IR: OR 9.30 (95% CI 6.00–14.43), respectively. TyG‐BMI and METS‐IR had moderate discriminative abilities for hypertension plus hyperuricemia and the AUC values were 0.72 (95% CI 0.70–0.74) and 0.73 (95% CI 0.70–0.75).
Conclusions
The present study suggested that TyG index, TyG‐BMI, TG/HDL‐C and METS‐IR had a significant correlation with hypertension plus hyperuricemia, and TyG‐BMI and METS‐IR had discriminative abilities for hypertension plus hyperuricemia.
Keywords: Hypertension, Hyperuricemia, Insulin resistance surrogates
Our study suggested that glucose and triglycerides (TyG index), TyG index with body mass index, the ratio of triglycerides divided by high‐density lipoprotein cholesterol and metabolic score for insulin resistance had a significant correlation with hypertension plus hyperuricemia, and TyG index with body mass index and metabolic score for insulin resistance had discriminative abilities for hypertension plus hyperuricemia.

Introduction
As a global public health problem, hypertension (HTN) is one of the critical risk factors affecting the morbidity and mortality from cardiovascular diseases (CVD) 1 , 2 . The prevalence of HTN has increased over the past four decades, even with the widespread use of antihypertensive drugs, especially in low‐ and middle‐income countries 3 . In China, the prevalence of HTN was approximately 27.9% in the general population and approximately 55.7% in the elderly population in 2012–2015 4 . Uric acid (UA) is produced in the liver from purine compounds ingested and broken down by the body. Elevated serum UA was found in 13.7% of the general population and 16.9% of the elderly population in China 5 , whereas hyperuricemia (HUA) affects approximately 25–40% of individuals with HTN 6 . HTN and HUA are major features of the metabolic syndrome, and they are important risk factors for CVD. Previous studies consistently showed that hypertensive patients with hyperuricemia (HTN‐HUA) had a higher CVD risk than hypertensive patients with normal serum UA levels 7 , 8 , 9 .
Insulin resistance (IR) is a systemic disorder that affects many organs and insulin‐regulated pathways, and has a critical and central role in the development of many CVD risk factors 10 , 11 , 12 . It has been well recognized that IR plays a vital role in the pathogenesis of HTN and HUA 13 , 14 , 15 . Evaluations of IR, including the homeostatic model assessment for IR and the quantitative insulin sensitivity check index, require insulin measurements or invasive methods, which are not suitable for large epidemiological studies. Therefore, as in previous epidemiological studies, non‐insulin‐based fasting IR indicators, namely IR surrogates, were selected to evaluate individual IR levels, including the production of glucose and triglycerides (TyG index) 16 , TyG index with body mass index (TyG‐BMI) 17 , the ratio of triglycerides divided by HDL‐C (TG/HDL‐C) 18 and metabolic score for insulin resistance (METS‐IR) 19 .
To our knowledge, although some studies have investigated the association of IR surrogates with HTN or HUA 20 , 21 , this still needs to be confirmed in large population samples, especially in the context of a high prevalence of HTN and HUA in the elderly population. Furthermore, few studies have comprehensively compared the predictive ability of different IR surrogates for patients with HTN‐HUA. The Shanghai Elderly Cardiovascular Health Study (SHECHS) 22 , 23 was carried out to recruit elderly residents aged ≥65 years in Shanghai to provide current and reliable data for investigating the association with the four IR surrogates and HTN‐HUA, and finding an optimal predictor of HTN‐HUA.
Materials and methods
Study population
The present study was carried out within the framework of The Shanghai Elderly Cardiovascular Health Study (SHECHS), which is a longitudinal, population‐based community study of non‐institutionalized elderly people. The study population included 4,753 elderly residents of the Shanghai community, China, who participated in a comprehensive health checkup in Shanghai Gaohang District in 2017. The participants included in the study were permanent residents of the Gaohang community with Shanghai Social security cards aged ≥65 years. Furthermore, the exclusion criteria of the present study included patients with advanced cancer, people unable to participate in community physical examinations and pregnant women. Finally, 4,352 (401 participants aged <65 were excluded) participants were enrolled in our final analysis.
The institutional review board of Shanghai East Hospital affiliated Tongji Medical School approved the study protocol (approval number: 2017‐010). The date on which the approval was granted was 13 April 2017. All studies were carried out following relevant guidelines and regulations and written informed consent was obtained from each participant before data collection.
Data collection
Participants' information includes, but is not limited to, age, sex, height, weight, smoking status, drinking status, physical activity, medical history (HTN, diabetes, dyslipidemia, HUA), use of medications known to influence insulin (antihypertensive agents: angiotensin‐converting enzyme inhibitors, angiotensin‐receptor blockers, thiazide and thiazide‐like diuretics, beta‐blockers; lipid‐lowering drugs: statins, ezetimibe; hypoglycemic agents: sulfonylureas, meglitinides, biguanides, insulin analogs and others), laboratory indicators (total cholesterol, high‐density lipoprotein cholesterol [HDL‐C], low‐density lipoprotein cholesterol [LDL‐C], triglycerides [TG], fasting plasma glucose [FPG], UA). Two seated blood pressure measurements using a mercury sphygmomanometer after at least 5 min of quiet rest were obtained by trained and certified staff who followed a standard protocol, with the averages of two measurements used for the analysis. Fasting plasma glucose was measured using the glucose oxidase method. Blood lipids were measured using ultracentrifugation. Serum creatinine was measured using the alkaline picric acid method.
Study definitions
BMI was calculated as bodyweight in kilograms divided by the square of the body height in meters (kg/m2). Current smoker was defined as smoking at least one cigarette per day at the time of the survey. Alcohol consumption was defined as anyone who consumed alcohol once a day or more. The amount of physical activity was determined by a questionnaire. Physical activity was considered active if at least 4 days of exercise or recreational activities were carried out per week and >30 min per day 23 . Estimated glomerular filtration rate (eGFR) was calculated by using the Modified Diet in Renal Disease equation where eGFR (mL/min/1.73 m2) = 186 × Scr (mg/dL) – 1.154 × age (years) – 0.203 × 0.742 (if female) × 1.233 (if Chinese) 24 .
HTN was defined as an average of two measurements of systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg, or current use of antihypertensive agents, or the participants reported a history of HTN 22 , 25 , 26 . Well‐controlled blood pressure was defined as an average of two measurements of <140/90 mmHg (<150/90 mmHg for patients aged ≥80 years). Poor‐controlled blood pressure was defined as an average of two measurements of ≥140/90 mmHg (≥150/90 mmHg for patients aged ≥80 years) 27 . HUA diagnosis was made with serum UA ≥420 μmol/L for men and UA ≥360 μmol/L for premenopausal women 21 , 28 , 29 . Diabetes was defined as FPG ≥7.0 mmol/L, or current use of insulin or oral antidiabetic agents, or the participants reported history of diabetes 30 . IR surrogates included TyG index, TyG‐BMI, TG/HDL‐C and METS‐IR. These were calculated using the following formulas: TyG = ln [TG (mg/dL) × FPG (mg/dL) / 2]; TyG‐BMI: = TyG index × BMI. TG/HDL‐C = TG (mg/dL) / HDL‐C (mg/dL); METS‐IR = {ln [2 × FPG (mg/dL) + TG (mg/dL)] × BMI (kg/m2) / ln [HDL‐C (mg/dL)]}.
Statistical analysis
Statistical analyses were carried out using IBM SPSS statistics 22 software (SPSS Inc., Chicago, IL, USA) and MedCalc 16.8 (Ostend, Belgium). All continuous variables are presented as the mean (standard deviation), and categorical variables are presented as numbers (percentages). Analysis of variance (anova) was used to compare the means of baseline characteristics of the study participants, and the least significance difference was used for pairwise comparisons. Categorical variables were analyzed by the χ2‐test, and Bonferroni adjustment was applied for pairwise comparisons in which a Bonferroni‐adjusted P‐value <0.05/3 or 0.017 was considered to be statistically significant. Multivariate logistic regression analyses were used to explore the association between the four IR surrogates and HUA, HTN and HTN‐HUA. Receiver operating characteristic curve analyses and the area under the curve (AUC) were then used to assess the ability of TG/HDL‐C, TyG, TyG‐BMI and METS‐IR to discriminate HUA, HTN and HTN‐HUA. The change in AUC was tested by the DeLong test. To avoid the interference caused by drugs, we carried out a sensitivity analysis by excluding those who used antihypertensive drugs, hypoglycemic drugs and lipid‐lowering drugs. All P‐values were two‐sided, and P < 0.05 was considered statistically significant.
Results
Basic characteristics
The baseline characteristics of the study participants are summarized in Table 1. In the present study, a total of 4,352 participants were included, including 3,462 (79.55%) participants with HTN and 680 (15.63%) participants with HUA. The prevalence of HTN‐HUA in the hypertensive population was 17.04%. One‐way anova showed that the differences in total cholesterol and LDL‐C levels among the four groups were not statistically significant (all P > 0.05). Compared with the control group, the age, BMI, TG, UA, systolic blood pressure and diastolic blood pressure levels of the HTN‐HUA or HTN group were higher than those in the control group. The difference was statistically significant (P < 0.05). However, there were no significant differences in BMI, eGFR, systolic blood pressure and diastolic blood pressure levels between the HUA and control groups. Notably, the difference in FPG levels was only found in comparing the HTN group and the control group. The TyG, TG/HDL‐C and METS‐IR of the HUA group, the HTN group and the HTN‐HUA group were higher than those in the control group, and the difference was statistically significant (P < 0.05). Figure 1 showed the TyG index, TyG‐BMI, TG/HDL‐C and METS‐IR values in different groups. TyG, TG/HDL‐C and METS‐IR in the HTN‐HUA group were significantly higher than in the other three groups.
Table 1.
General information and clinical characteristics of subjects
| C | HUA | HTN | HTN‐HUA | |
|---|---|---|---|---|
| n = 797 | n = 93 | n = 2875 | n = 587 | |
| Age (years) | 71.4 ± 6.67a | 73.23 ± 6.77b,c | 72.64 ± 6.31c | 74.11 ± 7.94b |
| Sex (M/F) | 358/439a | 66/27b,c | 1,171/1,704c | 362/225b |
| BMI (kg/m2) | 23.41 ± 3.31a | 23.98 ± 2.95a | 24.81 ± 3.12b | 25.75 ± 3.86c |
| Smoking (%) | 167 (21.0%)a | 32 (34.4%)b | 525 (18.3%)a | 154 (26.2%)b |
| Drinking (%) | 97 (12.2%)a | 22 (23.7%)b | 427 (14.9%)a | 132 (22.55%)b |
| Physical activity (%) | 628 (91.7%) | 78 (94.0%) | 2,207 (90.2%) | 467 (91.7%) |
| TC (mol/L) | 5.05 ± 0.91a | 4.91 ± 0.94a | 4.97 ± 1.38a | 4.90 ± 1.01b |
| TG (mol/L) | 1.49 ± 0.80a | 1.78 ± 1.08b | 1.62 ± 0.94b | 1.7 ± 1.21b |
| LDL‐C (mol/L) | 3.32 ± 0.81a | 3.28 ± 0.83a | 3.27 ± 0.91a | 3.28 ± 1.24a |
| HDL‐C (mol/L) | 1.53 ± 0.43a | 1.38 ± 0.37b | 1.44 ± 0.38b | 1.28 ± 0.35c |
| FPG (mol/L) | 5.61 ± 1.54a | 5.60 ± 1.45a | 6.08 ± 1.85b | 5.78 ± 1.28a |
| eGFR (mL/min/1.73 m2) | 0.60 ± 0.11a | 0.50 ± 011b | 0.59 ± 0.13c | 0.49 ± 0.13b |
| UA (µmol/L) | 297.35 ± 59.92a | 464.25 ± 39.41b | 307.69 ± 60.41c | 475.95 ± 57.51b |
| SBP (mmHg) | 122.21 ± 11.06a | 124.37 ± 10.27a | 147.75 ± 18.55b | 147.58 ± 19.36b |
| DBP (mmHg) | 75.13 ± 8.05a | 74.99 ± 9.95a | 81.92 ± 10.53b | 80.96 ± 11.57b |
| TyG | 8.66 ± 0.54a | 8.82 ± 0.57b | 8.82 ± 0.56b | 8.96 ± 0.57c |
| TyG‐BMI | 203.28 ± 34.08a | 212.11 ± 33.27b | 219.16 ± 35.18b | 230.73 ± 37.87c |
| TG/HDL‐C | 2.66 ± 2.66a | 3.60 ± 3.96b | 2.97 ± 2.54c | 4.07 ± 3.65b |
| METS‐IR | 33.85 ± 6.82a | 35.97 ± 6.86b | 36.83 ± 6.83b | 39.60 ± 7.29c |
| Medical history | ||||
| Diabetes (%) | 150 (11.0%)a | 21 (1.5%)a,b | 1,008 (73.7%)b | 189 (13.8%)b |
| Dyslipidemia (%) | 255 (14.9%)a | 36 (2.1%)a,b,c | 1,140 (66.4%)c | 285 (13.3%)b |
| Medication history | ||||
| Antihypertensive (%) | 0a | 0a | 415 (80.4%)b | 101 (19.6%)b |
| Lipid‐lowering drugs (%) | 39 (10.6%)a | 3 (0.8%)a,b | 272 (74.1%)b | 53 (14.4%)b |
| Antidiabetic (%) | 76 (12.0%)a | 13 (2.0%)a,b | 478 (75.3%)b | 68 (10.7%)a |
Summary of the clinical characteristics and laboratory results of the control group ( C group, the participants who had neither hypertension [HTN] nor hyperuricemia [HUA]), HUA group (HUA patients without HTN), HTN group (HTN patients without HUA) and HTN‐HUA group (patients with HUA and HTN). Data presented as the mean ± standard deviation, or percentages number (%). For the multiple comparisons, Bonferroni adjustment and least significance difference test were used following the χ2‐test and one‐way anova. The same superscript letters indicate no significant difference between any two groups. Body mass index (BMI) was calculated as bodyweight in kilograms divided by the square of the body height in meters. Estimated glomerular filtration rate (eGFR) was calculated by using the Modified Diet in Renal Disease equation, where eGFR (mL/min/1.73 m2) = 186 × Scr (mg/dL) – 1.154 × age (years) − 0.203 × 0.742 (if female) × 1.233 (if Chinese). DBP, diastolic blood pressure; FPG, fasting plasma glucose; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol level; METS‐IR, metabolic score for insulin resistance; SBP, systolic blood pressure; TC, total cholesterol levels; TG, triglyceride levels; TG/HDL‐C, the ratio of triglycerides divided by high‐density lipoprotein cholesterol; TyG, triglyceride and glucose index; TyG‐BMI, triglyceride and glucose index with body mass index.
Figure 1.
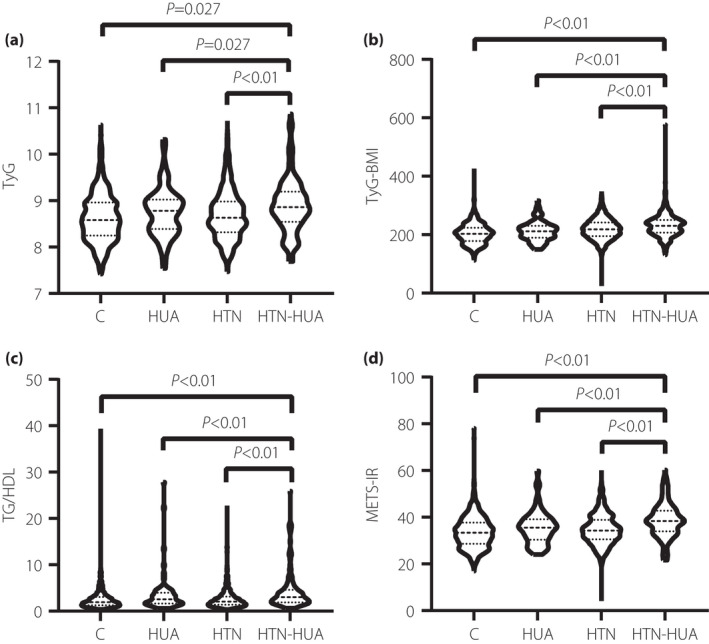
Glucose and triglycerides index (TyG index), TyG index with body mass index (TyG‐BMI), the ratio of triglycerides divided by high‐density lipoprotein cholesterol (TG/HDL‐C) and metabolic score for insulin resistance (METS‐IR) values in different groups. (a) The mean values of TyG in in the control group (C group), hyperuricemia (HUA) group, hypertension (HTN) group and hypertensive patients with hyperuricemia (HTN‐HUA) group were 8.66, 8.82, 8.82 and 8.96, respectively. (b) The mean values of TyG‐BMI in the C group, HUA group, HTN group and HTN‐HUA group were 203.28, 212.11, 219.16 and 230.73, respectively. (c) The mean values of TG/HDL‐C in the C group, HUA group, HTN group and HTN‐HUA group were 2.66, 3.60, 2.97 and 4.07, respectively. (d) The mean values of METS‐IR in the C group, HUA group, HTN group and HTN‐HUA group were 33.85, 35.97, 36.83 and 39.60, respectively. All the values in the HTN‐HUA group were significantly higher than in the other three groups (P < 0.05).
Association between four IR surrogates and risks of HUA, HTN and HTN‐HUA
The multivariable analysis for the association between four IR surrogates and risks of HUA, HTN and HTN‐HUA were shown in Table 2, in which we showed odds ratios (ORs) and 95% confidence intervals (95% CI) for the highest versus the lowest quartile. In model 1, TyG index, TyG‐BMI, TG/HDL‐C and METS‐IR were all significantly correlated with HUA, HTN and HTN‐HUA. The results remained highly consistent after adjusting for sex, age, educational status, smoking, alcohol consumption and physical activity (model 2). All variables in model 2 plus total cholesterol, LDL‐C and eGFR (model 3), all indexes remained significantly associated with HTN and HTN‐HUA (P < 0.05), but only TG/HDL was associated with HUA (P < 0.05). In the three groups, all the IR surrogates had the highest ORs for HTN‐HUA, the ORs of the highest quartile of the four indicators for hypertension plus hyperuricemia were TyG index: OR 6.39 (95% CI 4.17–9.78); TyG‐BMI: OR 8.54 (95% CI 5.58–13.09); TG/HDL‐C: OR 7.21 (95% CI 4.72–11.01); METS‐IR: OR 9.30 (95% CI 6.00–14.43), respectively. Given the interference caused by the use of drugs, we excluded those participants who used antihypertensive drugs, hypoglycemic drugs and lipid‐lowering drugs to carry out sensitivity analysis (Table S1). A similar pattern of associations was seen with all the indicators correlating with HTN and HTN‐HUA, and TG/HDL‐C correlating with HUA (P < 0.05). METS‐IR had the highest OR value (8.05) for HTN‐HUA.
Table 2.
Odds ratios and 95% confidence intervals for highest versus the lowest quartiles in multivariatelogistic regressions predicting presence of hyperuricemia, hypertension and hypertension plus hyperuricemia
| HUA | HTN | HTN‐HUA | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| By TyG index quartile | ||||||
| Model 1 | 1.67 (0.87–3.21) | 0.12 | 1.97 (1.57–2.48) | <0.01 | 3.94 (2.86–5.44) | <0.01 |
| Model 2 | 2.01 (0.97–4.19) | 0.06 | 2.08 (1.62–2.69) | <0.01 | 4.88 (3.33–7.07) | <0.01 |
| Model 3 | 1.50 (0.57–3.91) | 0.41 | 2.26 (1.74–2.93) | <0.01 | 6.39 (4.17–9.78) | <0.01 |
| By TyG‐BMI index quartile | ||||||
| Model 1 | 1.69 (0.87–3.31) | 0.12 | 3.50 (2.74–4.48) | <0.01 | 8.79 (6.24–12.38) | <0.01 |
| Model 2 | 1.77 (0.85–3.71) | 0.13 | 3.47 (2.65–4.56) | <0.01 | 8.32 (5.63–12.30) | <0.01 |
| Model 3 | 1.41 (0.57–3.48) | 0.46 | 3.56 (2.70–4.70) | <0.01 | 8.54 (5.58–13.09) | <0.01 |
| By TG/HDL‐C quartile | ||||||
| Model 1 | 2.88 (1.56–5.32) | <0.01 | 1.77 (1.41–2.23) | <0.01 | 6.14 (4.40–8.58) | <0.01 |
| Model 2 | 3.27 (1.62–6.62) | <0.01 | 1.98 (1.54–2.56) | <0.01 | 7.02 (4.76–10.33) | <0.01 |
| Model 3 | 3.68 (1.11–12.21) | 0.03 | 2.07 (1.60–2.69) | <0.01 | 7.21 (4.72–11.01) | <0.01 |
| By METS‐IR quartile | ||||||
| Model 1 | 2.08 (1.09–3.98) | 0.03 | 3.07 (2.41–3.91) | <0.01 | 9.13 (6.45–12.94) | <0.01 |
| Model 2 | 1.91 (0.91–3.98) | 0.09 | 3.28 (2.50–4.30) | <0.01 | 8.73 (5.88–12.95) | <0.01 |
| Model 3 | 1.07 (0.37–3.14) | 0.90 | 3.22 (2.45–4.24) | <0.01 | 9.30 (6.00–14.43) | <0.01 |
Model 1: unadjusted; model 2: adjusted for sex, age, education status, smoking, drinking and physical activity; model 3: adjusted for all variables in model 2 and total cholesterol, low‐density lipoprotein cholesterol and estimated glomerular filtration rate.
CI, confidence interval; HTN, hypertension; HTN‐HUA, hypertension plus hyperuricemia; HUA, hyperuricemia; METS‐IR, metabolic score for insulin resistance; OR, odds ratio; TG/HDL‐C, the ratio of triglycerides divided by high‐density lipoprotein cholesterol; TyG, triglyceride and glucose index; TyG‐BMI, triglyceride and glucose index with body mass index.
In addition, we divided the hypertensive population (n = 3462) into hyperuricemia group (HTN with HUA) and non‐hyperuricemia group (HTN without HUA), and explored the relationship between four IR surrogates and poor‐controlled blood pressure in the two groups (Table S2). Unfortunately, there were no positive results (P > 0.05).
AUCs and cut‐off values of TyG index, TyG‐BMI, TG/HDL‐C and METS‐IR for prediction of HTN‐HUA
The AUC values of the TyG index, TyG‐BMI, TG/HDL‐C and METS‐IR to discriminate HUA, HTN and HTN‐HUA are shown in Table 3. TyG‐BMI and METS‐IR had a significant discriminative ability for HTN‐HUA, and the AUC values were 0.72 (95% CI 0.70–0.74) and 0.73 (95% CI 0.70–0.75), respectively. The cut‐off value of TyG‐BMI to discriminate the patients with HTN‐HUA was 212.12, and the cut‐off value of METS‐IR was 37.27. The DeLong test was used to judge the difference between the four indexes in the prediction ability of HTN‐HUA, which showed that the difference of AUC between TyG‐BMI and METS‐IR was not statistically significant (P > 0.05). Also, we listed AUCs and cut‐off values of four indicators in predicting HTN‐HUA stratified by sex (Table S3) and having diabetes or not (Table S4).
Table 3.
Areas under the curve and cut‐off values of triglyceride and glucose index, triglyceride and glucose index with body mass index, the ratio of triglycerides divided by high‐density lipoprotein cholesterol, metabolic score for prediction of hypertension plus hyperuricemia
| Variable | HUA | HTN | HUA‐HTN | |||
|---|---|---|---|---|---|---|
| AUC (95% CI) | Cut‐off value | AUC (95% CI) | Cut‐off value | AUC (95% CI) | Cut‐off value | |
| TyGa | 0.58 (0.52–0.64) | 8.717 | 0.58 (0.56–0.60) | 8.38 | 0.65 (0.62–0.68) | 8.74 |
| TyG‐BMIb | 0.57 (0.51–0.63) | 186.22 | 0.63 (0.61–0.65) | 215.44 | 0.72 (0.70–0.74) | 212.12 |
| TG/HDLc | 0.60 (0.54–0.66) | 3.018 | 0.56 (0.54–0.59) | 1.81 | 0.68 (0.66–0.71) | 2.28 |
| METS‐IRb | 0.59 (0.53–0.65) | 35.08 | 0.63 (0.60–0.65) | 30.19 | 0.73 (0.70–0.75) | 37.27 |
DeLong test for the multiple comparisons, and the same superscript letters indicate no significant difference between any two indexes.
AUC, area under the curve; CI, confidence interval; HTN, hypertension; HTN‐HUA, hypertension plus hyperuricemia; HUA, hyperuricemia; METS‐IR, metabolic score for insulin resistance; TG/HDL‐C, the ratio of triglycerides divided by high‐density lipoprotein cholesterol; TyG, triglyceride and glucose index; TyG‐BMI, triglyceride and glucose index with body mass index.
Discussion
In the present study, the prevalence of HTN was 79.55%, and the patients with HTN‐HUA accounted for 16.96% in the hypertensive population. Numerous epidemiological studies have consistently confirmed that high serum uric acid increased the risk of cardiovascular events in hypertensive patients. For example, Alderman et al. 7 reported that HUA was independently and specifically associated with cardiovascular events in hypertensive patients based on a prospective study with 7,978 moderate‐to‐severe hypertensive patients. In a prospective cohort study, Cho et al. 31 reported that HUA’s presence increased the risk of uncontrolled HTN in people without metabolic syndrome. Viazzi et al. 32 studied 425 patients with essential HTN, and found that the incidence and degree of target organ damage in the hyperuricemia group were significantly higher than those with standard UA. The potential mechanisms might be multifactorial. High UA levels led to endothelial dysfunction, smooth muscle proliferation, inflammation and oxidative stress, resulting in stiff arteries and blood pressure elevation 33 . In particular, hyperuricemia was associated with left ventricular mass index and elevated serum UA could predict larger cardiac size in people with hypertension 34 .
When we explored the relationship between IR and HTN‐HUA, we used IR surrogates, which can be calculated according to the biochemical indexes of human body, and had the advantages of simplicity, convenience and economy. The present study found that the four easily measurable surrogate indexes of IR were significantly associated with the presence of HAU, HTN and HTN‐HAU. Among the four selected IR indicators, TG/HDL‐C contained pivotal components of hyperlipidemia, TyG combined FPG and lipid profile, whereas TyG‐BMI and METS‐IR included not only a lipid index and FPG, but also an obesity index: BMI. It is well established that dyslipidemia, including elevated TG, elevated LDL‐C and low HDL‐C, were independently associated with HTN and HUA 35 , 36 , 37 , 38 . In the present study, we found that only HDL was correlated with HUA, which might be explained by the combination of FPG with the other three indicators. FPG and UA are in an inverted U‐shaped relationship 39 , 40 . When FPG rises to a certain threshold, elevated urinary glucose levels lead to competitive inhibition of UA reabsorption and increased UA excretion 21 . We also found that TyG‐BMI and METS‐IR had a larger odds ratio for HTN than TyG and TG/HDL‐C, which was similar to the result of Bala et al. 20 , explained by the fact that the calculation of these two indicators depends on BMI. BMI as a predictor of hypertension was well proven, and it influenced blood pressure through a variety of mechanisms including insulin resistance. Overweight/obesity can cause significant insulin resistance, accompanied by a corresponding increase in the prevalence of hypertension, and weight control can significantly lower BP 41 , 42 .
Notably, the present study found that all four indexes had a more significant correlation with HTN‐HUA risk than that with HUA or HTN alone, which suggested more significant IR in patients with HTN‐HUA. We might suggest that the primary mechanism associated with HTN‐HUA and IR was that HTN could lead to the decrease of renal blood flow, which could also lead to the increase of urate reabsorption. Insulin promotes sodium reabsorption, while promoting the reabsorption of UA in renal tubules, resulting in water and sodium retention, and increased blood pressure, making HUA coexist with HTN 43 . Furthermore, TyG‐BMI and METS‐IR, which were more strongly associated with HTN, were also more strongly associated with HTN‐HUA. Similarly, TyG‐BMI and METS‐IR had a significant discriminative ability for HTN‐HUA.
The present study was the first large cross‐sectional study in an elderly population to examine the relationship between these four non‐insulin‐based indicators of IR with HTN‐HUA, HTN and HUA. To eliminate the effect of drug use on the results, we carried out a sensitivity analysis by excluding those people with drug use from the total population. However, there were several limitations in this study. First, this was a cross‐sectional study, which limited the inference of causality of our results. Second, the participants were all elderly, which prevented our results from being extrapolated in the general population. Third, we did not directly measure insulin indicators in the study population, so we could not calculate indicators of IR, such as homeostatic model assessment for IR, nor could we further compare those IR surrogates with direct markers of IR.
In conclusion, the present study suggested that TyG index, TyG‐BMI, TG/HDL‐C and METS‐IR had a more significant correlation with HTN‐HUA risk than that with HUA or HTN alone, and TyG‐BMI and METS‐IR had significant discriminative abilities for HTN‐HUA. The practical clinical significance of these findings was that the four obtainable and cost‐effective IR surrogates, especially TyG‐BMI and METS‐IR, could be potential monitors in hypertension with hyperuricemia management, and help develop prevention and intervention strategies against IR‐driven comorbidities of HTN‐HUA.
Disclosure
The authors declare no conflict of interest
Supporting information
Table S1 | Odds ratios and 95% confidence intervals for highest versus the lowest quartiles predicting the presence of hyperuricemia, hypertension and hypertensive patients with hyperuricemia in the participants without drugs.
Table S2 | Odds ratios and 95% confidence intervals for the highest versus the lowest quartiles in binary logistic regressions predicting the presence of insufficient control of blood pressure.
Table S3 | Areas under the curve and cut‐off values of four indicators in predicting hypertensive patients with hyperuricemia stratified by sex.
Table S4 | Areas under the curve and cut‐off values of four indicators in predicting hypertensive patients with hyperuricemia stratified by having diabetes or not.
Acknowledgments
The authors thank the participants for supporting the SHECHS study, the technicians for performing blood sample analysis, and the general practitioners for helping with data collection.
This study was supported by The Top‐level Clinical Discipline Project of Shanghai Pudong (PWYgf 2018‐05) and The Science and Technology Commission of Shanghai Municipality (17431906600).
J Diabetes Investig 2021; 11: 2046–2053
References
- 1. Kearney PM, Whelton M, Reynolds K, et al. Global burden of hypertension: analysis of worldwide data. Lancet (London, England) 2005; 365: 217–223. [DOI] [PubMed] [Google Scholar]
- 2. Fitzmaurice C, Abate D, Abbasi N, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability‐adjusted life‐years for 29 cancer groups, 1990 to 2017: a systematic analysis for the global burden of disease study. JAMA Oncol 2019; 5: 1749–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol 2020; 16: 223–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang Z, Chen Z, Zhang L, et al. Status of hypertension in China: results from the China hypertension survey, 2012–2015. Circulation 2018; 137: 2344–2356. [DOI] [PubMed] [Google Scholar]
- 5. Qiu L, Cheng X‐Q, Wu J, et al. Prevalence of hyperuricemia and its related risk factors in healthy adults from Northern and Northeastern Chinese provinces. BMC Public Health 2013;13:664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gois PHF, Souza ERdM. Pharmacotherapy for hyperuricemia in hypertensive patients. Cochrane Database Syst Rev 2017;4:CD008652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alderman MH, Cohen H, Madhavan S, et al. Serum uric acid and cardiovascular events in successfully treated hypertensive patients. Hypertension 1999; 34: 144–150. [DOI] [PubMed] [Google Scholar]
- 8. Verdecchia P, Schillaci G, Reboldi GP, et al. Relation between serum uric acid and risk of cardiovascular disease in essential hypertension. Hypertension 2000; 36: 1072–1078. [DOI] [PubMed] [Google Scholar]
- 9. Fang J, Alderman MH. Serum uric acid and cardiovascular mortality. JAMA 2000; 283: 2404–2410. [DOI] [PubMed] [Google Scholar]
- 10. Ormazabal V, Nair S, Elfeky O, et al. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol 2018; 17: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol 2014; 10: 293–302. [DOI] [PubMed] [Google Scholar]
- 12. Artunc F, Schleicher E, Weigert C, et al. The impact of insulin resistance on the kidney and vasculature. Nat Rev Nephrol 2016; 12: 721–737. [DOI] [PubMed] [Google Scholar]
- 13. Dawson J, Wyss A. Chicken or the egg? Hyperuricemia, insulin resistance, and hypertension. Hypertension 2017; 70: 698–699. [DOI] [PubMed] [Google Scholar]
- 14. Zhou MS, Wang A, Yu H. Link between insulin resistance and hypertension: what is the evidence from evolutionary biology? Diabetol Metab Syndrome. 2014; 6: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mancusi C, Izzo R, di Gioia G, et al. Insulin resistance the hinge between hypertension and type 2 diabetes. High Blood Press Cardiovasc Prev 2020; 27: 515–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guerrero‐Romero F, Simental‐Mendía LE, González‐Ortiz M, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic‐hyperinsulinemic clamp. J Clin Endocrinol Metab 2010; 95: 3347–3351. [DOI] [PubMed] [Google Scholar]
- 17. Er L‐K, Wu S, Chou H‐H, et al. Triglyceride glucose‐body mass index is a simple and clinically useful surrogate marker for insulin resistance in nondiabetic individuals. PLoS One 2016; 11: e0149731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McLaughlin T, Reaven G, Abbasi F, et al. Is there a simple way to identify insulin‐resistant individuals at increased risk of cardiovascular disease? Am J Cardiol 2005; 96: 399–404. [DOI] [PubMed] [Google Scholar]
- 19. Bello‐Chavolla OY, Almeda‐Valdes P, Gomez‐Velasco D, et al. METS‐IR, a novel score to evaluate insulin sensitivity, is predictive of visceral adiposity and incident type 2 diabetes. Eur J Endocrinol 2018; 178: 533–544. [DOI] [PubMed] [Google Scholar]
- 20. Bala C, Gheorghe‐Fronea O, Pop D, et al. The association between six surrogate insulin resistance indexes and hypertension: a population‐based study. Metab Syndrome Related Disord 2019; 17: 328–333. [DOI] [PubMed] [Google Scholar]
- 21. Liu XZ, Xu X, Zhu JQ, et al. Association between three non‐insulin‐based indexes of insulin resistance and hyperuricemia. Clin Rheumatol 2019; 38: 3227–3233. [DOI] [PubMed] [Google Scholar]
- 22. Peng S, Shen T, Liu J, et al. Uncontrolled hypertension increases with age in an older community‐dwelling Chinese population in Shanghai. Aging Dis 2017; 8: 558–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fan H, Li X, Zheng L, et al. Abdominal obesity is strongly associated with Cardiovascular Disease and its Risk Factors in Elderly and very Elderly Community‐dwelling Chinese. Sci Rep. 2016; 6: 21521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ma Y‐C, Zuo L, Chen J‐H, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol 2006; 17: 2937–2944. [DOI] [PubMed] [Google Scholar]
- 25. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018; 36: 1953–2041. [DOI] [PubMed] [Google Scholar]
- 26. Lu J, Lu Y, Wang X, et al. Prevalence, awareness, treatment, and control of hypertension in China: data from 1·7 million adults in a population‐based screening study (China PEACE Million Persons Project). Lancet 2017; 390: 2549–2558. [DOI] [PubMed] [Google Scholar]
- 27. Wang X, Wang F, Chen M, et al. Twenty‐four‐hour systolic blood pressure variability and renal function decline in elderly male hypertensive patients with well‐controlled blood pressure. Clin Interv Aging 2018; 13: 533–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li HH, Huang S, Liu XZ, et al. Applying the China‐PAR risk algorithm to assess 10‐year atherosclerotic cardiovascular disease risk in populations receiving routine physical examinations in eastern China. Biomed Environ Sci 2019; 32: 87–95. [DOI] [PubMed] [Google Scholar]
- 29. Johnson RJ, Bakris GL, Borghi C, et al. Hyperuricemia, acute and chronic kidney disease, hypertension, and cardiovascular disease: report of a scientific workshop organized by the national kidney foundation. Am J Kidney Dis 2018; 71: 851–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC Guidelines on diabetes, pre‐diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 2020; 41: 255–323. [DOI] [PubMed] [Google Scholar]
- 31. Cho J, Kim C, Kang DR, et al. Hyperuricemia and uncontrolled hypertension in treated hypertensive patients: K‐MetS Study. Med (Baltimore) 2016; 95: e4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Viazzi F, Parodi D, Leoncini G, et al. Serum uric acid and target organ damage in primary hypertension. Hypertension 2005; 45: 991–996. [DOI] [PubMed] [Google Scholar]
- 33. Zeng F, Huang R, Lu Y, et al. Association of anti‐hyperuricemia treatment and prevalent cardiovascular disease in hypertensive patients. Arch Med Sci 2020; 16: 545–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Visco V, Pascale AV, Virtuoso N, et al. Serum uric acid and left ventricular mass in essential hypertension. Front Cardiovasc Med 2020; 7: 570000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Laaksonen DE, Niskanen L, Nyyssönen K, et al. Dyslipidaemia as a predictor of hypertension in middle‐aged men. Eur Heart J 2008; 29: 2561–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Halperin RO, Sesso HD, Ma J, et al. Dyslipidemia and the risk of incident hypertension in men. Hypertension 2006; 47: 45–50. [DOI] [PubMed] [Google Scholar]
- 37. Keenan T, Blaha MJ, Nasir K, et al. Relation of uric acid to serum levels of high‐sensitivity C‐reactive protein, triglycerides, and high‐density lipoprotein cholesterol and to hepatic steatosis. Am J Cardiol 2012; 110: 1787–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stelmach MJ, Wasilewska N, Wicklund‐Liland LI, et al. Blood lipid profile and BMI‐Z‐score in adolescents with hyperuricemia. Irish J Med Sci 2015; 184: 463–468. [DOI] [PubMed] [Google Scholar]
- 39. Nan H, Dong Y, Gao W, et al. Diabetes associated with a low serum uric acid level in a general Chinese population. Diab Res Clin Pract 2007; 76: 68–74. [DOI] [PubMed] [Google Scholar]
- 40. Li H, Zha X, Zhu Y, et al. An invert U‐shaped curve: relationship between fasting plasma glucose and serum uric acid concentration in a large health check‐up population in China. Medicine (Baltimore) 2016; 95: e3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nurdiantami Y, Watanabe K, Tanaka E, et al. Association of general and central obesity with hypertension. Clin Nutri (Edinburgh, Scotland) 2018; 37: 1259–1263. [DOI] [PubMed] [Google Scholar]
- 42. Tyson CC, Appel LJ, Vollmer WM, et al. Impact of 5‐year weight change on blood pressure: results from the Weight Loss Maintenance trial. J Clin Hypertens (Greenwich, Conn) 2013; 15: 458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Imazu M, Yamamoto H, Toyofuku M, et al. Hyperinsulinemia for the development of hypertension: data from the Hawaii‐Los Angeles‐Hiroshima Study. Hypertens Res 2001; 24: 531–536. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 | Odds ratios and 95% confidence intervals for highest versus the lowest quartiles predicting the presence of hyperuricemia, hypertension and hypertensive patients with hyperuricemia in the participants without drugs.
Table S2 | Odds ratios and 95% confidence intervals for the highest versus the lowest quartiles in binary logistic regressions predicting the presence of insufficient control of blood pressure.
Table S3 | Areas under the curve and cut‐off values of four indicators in predicting hypertensive patients with hyperuricemia stratified by sex.
Table S4 | Areas under the curve and cut‐off values of four indicators in predicting hypertensive patients with hyperuricemia stratified by having diabetes or not.


