Abstract
Aims/Introduction
Maternal hyperglycemia leads to adverse pregnancy outcomes, and also subsequently affects both mothers and their offspring in later life. The prevalence of type 2 diabetes mellitus is increasing worldwide, and gestational diabetes mellitus (GDM) is also believed to be increasing. More precise nationwide and up‐to‐date data on GDM are required.
Materials and Methods
A population‐based retrospective cohort study was carried out with the Birth Certificate Application database and linked to the National Health Insurance Research Database to explore trends in the annual crude prevalence of GDM in all women who gave birth between 1 January 2004 and 31 December 2015 in Taiwan and their pregnancy outcomes. The registry is considered complete, reliable and accurate.
Results
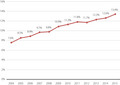
A total of 2,468,793 births from 2,430,307 pregnancies were reported between 1 January 2004 and 31 December 2015. Finally, 2,053,305 pregnancies were included for further analysis. The annual prevalence of GDM increased by 1.8‐fold during the 12 years from 2004 to 2015, with a significant continuous increasing trend (from 7.6% to 13.4%, P < 0.001). The annual prevalence of GDM significantly increased in each age group (all trends P < 0.001), particularly for women with maternal ages of 31 years and older. Urbanization level, geographic risk factors and seasonal variations were also noted.
Conclusion
The annual prevalence of GDM increased by 1.8‐fold in the 12‐year period from 2004 to 2015 in Taiwan, with a significant continuous increasing trend (from 7.6% to 13.4%, P < 0.001).
Keywords: Gestational diabetes mellitus, National Health Insurance Research Database, Prevalence
The annual prevalence of gestational diabetes mellitus increased by 1.8‐fold in the 12‐year period from 2004 to 2015 in Taiwan, with a significant continuous increasing trend (from 7.6% to 13.4%, P < 0.001).
Introduction
Gestational diabetes mellitus (GDM) is defined as the onset or first recognition of glucose intolerance in the second or third trimester of pregnancy with no overt diabetes before gestation 1 . Exposure to maternal hyperglycemia at 24–28 weeks of gestation and onward has a continuum of adverse short‐ and long‐term outcomes in mothers and offspring 2 . The Hyperglycemia and Adverse Pregnancy Outcome study, a multinational prospective cohort study of >23,000 pregnant women, showed that maternal hyperglycemia led to adverse pregnancy outcomes, such as increased birthweight, primary cesarean delivery, clinical neonatal hypoglycemia and increased cord blood serum C‐peptide level (fetal hyperinsulinemia). Maternal hyperglycemia has significant associations with premature delivery (before 37 weeks of gestation), shoulder dystocia or birth injury, a need for intensive neonatal care, hyperbilirubinemia and pre‐eclampsia 3 . This condition is a global public health concern, and the International Association of the Diabetes and Pregnancy Study Groups recommended that a more sensitive one‐step strategy be used. It increased the prevalence of GDM twofold to threefold 4 . Worldwide, the prevalence of GDM varies widely (from 1% to 28%) depending on population characteristics (e.g., maternal age, socioeconomic status, race or ethnicity, or body composition), screening methods and diagnostic criteria 5 , 6 , 7 , 8 , 9 , 10 . In Taiwan, the prevalence of adult diabetes increased by 74% from almost 1 million in 2005 to 1.73 million in 2015. The prevalence of GDM is also believed to be increasing, but little relevant nationwide public data are available. The aim of the present study was to examine the trend in the annual crude prevalence of GDM and associated risk factors in Taiwan, and to reflect the scale of the major public health problem that GDM represents.
Materials and Methods
Research ethics approval
All data used in the present study were anonymous without identifiable personal information, and were available through formal application to the Health and Welfare Data Science Center at the Ministry of Health and Welfare, Taiwan. As such, no informed consent was required to analyze the claims data. The protocol of this study was approved by the institutional review board of Kuang Tien General Hospital (date of approval 17 October 2018, approval no. KTGH 10733).
Study design and data source
We carried out a retrospective population‐based cohort study by using the Birth Certificate Application database (BCA) and the National Health Insurance Research Database to explore trends in the annual crude prevalence of GDM among women who gave birth in Taiwan between 1 January 2004 and 31 December 2015, and pregnancy outcome trends. Every citizen of Taiwan has an identification number, and this number is used to link registries. Legally, all births in Taiwan must be registered within 7 days of delivery. Almost 99% of pregnant Taiwanese women receive free prenatal care, including at least 10 prenatal care visits during pregnancy that are covered by Taiwan’s National Health Insurance. A validation study of the BCA database showed a low percentage of missing information (1.6%), and high validity and reliability 11 . The National Health Insurance Research Database is administered by the government and contains health data for 99.99% of the 23 million residents of Taiwan. The registry is considered to be complete, reliable and accurate 12 .
Study population
We identified all women aged 15–45 years who had singleton deliveries and whose birth weeks were between 20 and 43 weeks between 1 January 2004 and 31 December 2015 in Taiwan. In addition to eliminating potentially confounding factors, we excluded mothers with pre‐existing diabetes (International Classification of Diseases, Ninth Revision, Clinical Modification code: 250.x).
Outcome measurement
We calculated the GDM prevalence and implemented further classification according to age group, urbanization level and geographic region. For numerators, we identified all pregnant women with a GDM diagnosis (International Classification of Diseases, Ninth Revision, Clinical Modification code: 648.0 or 648.8) at clinics during pregnancy, and the denominator was all deliveries in the respective year. Pregnancy outcomes included preterm delivery (<35 weeks or <37 weeks), low birthweight (<2,500 g), macrosomia (≥4,000 g), fetal death and an Apgar score of <7 at 5 min; all of such outcomes were recorded in the BCA.
Statistical analysis
The associations between GDM and age group, season, urbanization level, and geographic region were established using the χ2‐test. The trends of annual GDM prevalence and adverse pregnancy outcomes over time were evaluated using the Cochran–Armitage test. We considered a P‐value of <0.05 to denote statistical significance. All data were merged and analyzed using SAS 9.4 (SAS Institute, Cary, NC, USA). Age was identified at conception and classified in 5‐year ranges from 15 to 45 years. Season was also identified at conception. The months of March to May were defined as spring, June to August was defined as summer, September to November was defined as autumn, and December to February was defined as winter. The level of urbanization and geographical differences observed were on the basis of the mother’s residence. Urbanization level was determined using the standards established by the National Health Insurance Research Institute of Taiwan. All city districts and townships in Taiwan were classified into seven urbanization levels (1, most urbanized; 7, least urbanized) on the basis of population density (people/km2), proportion of residents with higher education, the population of elderly people and agricultural workers, and the number of physicians per 100,000 people in each area 13 . Geographic regions were defined by the Council for Economic Planning and Development, Executive Yuan (Northern, Central, Southern, Eastern regions and Outer islands).
Results
A total of 2,468,793 births of 2,430,307 pregnancies were reported between 1 January 2004 and 31 December 2015. Pregnancy records for multifetal births (n = 37,991), deliveries before week 20 or after week 43 (n = 601), mothers with missing age data (n = 9), mothers with an age at conception of <15 or >45 years (n = 1,942) and foreigners (n = 222,985) were excluded. In addition, to eliminate potential confounding factors, pregnant women with pre‐existing diabetes were excluded (n = 113,474; Figure 1). Finally, 2,053,305 pregnancies were included for further analysis.
Figure 1.
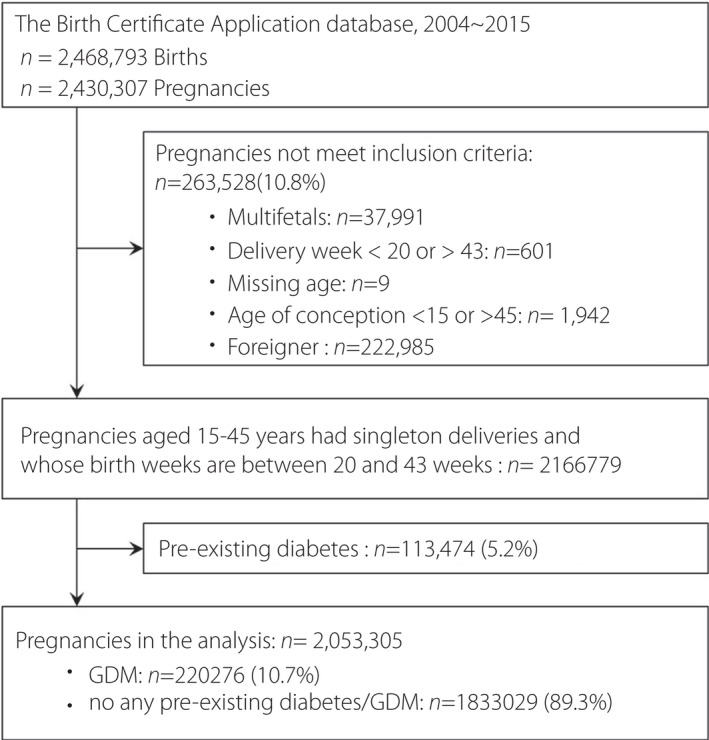
Flow chart of enrolled pregnancies. A total of 2,468,793 births of 2,430,307 pregnancies were reported between 1 January 2004 and 31 December 2015. Pregnancy records for multifetal births (n = 37,991), deliveries before week 20 or after week 43 (n = 601), mothers with missing age data (n = 9), mothers with an age at conception of <15 or >45 years (n = 1,942) and foreigners (n = 222,985) were excluded.
The annual GDM prevalence increased by 1.8‐fold from 2004 to 2015, with a significant continuous increasing trend (from 7.6% to 13.4%, P < 0.001; Figure 2). Table 1 shows the demographics of pregnant women by GDM status in Taiwan. The age at conception, the season of pregnancy and the mother’s place of residence were all significantly related to the prevalence of GDM (all P < 0.001). The older the conception age was, the higher the rate of GDM was. Women who conceived in winter had the highest GDM rate (11.2% vs 10.5–10.9%). For the mother’s place of residence, in terms of urbanization, the highest GDM rates occurred among women living in level 2 and level 5 areas (12.5% and 12.0%). Level 3 and level 4 areas had the lowest GDM prevalence (8.9% and 9.4%). In terms of geographic region, women living in outlying islands had the lowest GDM prevalence (4.8%), and the highest prevalence rates were in the east and the south (21.1%, 19.1%).
Figure 2.
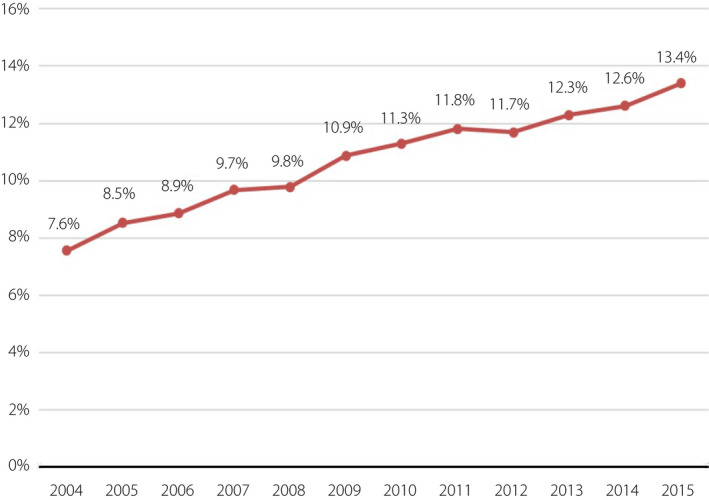
Gestational diabetes mellitus prevalence over the years. The annual gestational diabetes mellitus prevalence increased by 1.8‐fold from 2004 to 2015, with a significant continuous increasing trend.
Table 1.
Demographics of pregnant women by gestational diabetes mellitus status in Taiwan from 2004 to 2015.
| Variable | n | GDM | Non‐GDM | ||
|---|---|---|---|---|---|
| Total | 2,053,305 | 220,276 | (10.7) | 1,833,029 | (89.3) |
| Age at conception (years) | |||||
| 15–20 | 90,400 | 5,005 | (5.5) | 85,395 | (94.5) |
| 21–25 | 352,401 | 26,631 | (7.6) | 325,770 | (92.4) |
| 26–30 | 797,665 | 81,411 | (10.2) | 716,254 | (89.8) |
| 31–35 | 630,903 | 80,428 | (12.8) | 550,475 | (87.3) |
| 36–40 | 166,903 | 24,404 | (14.6) | 142,499 | (85.4) |
| 41–45 | 15,033 | 2,397 | (15.9) | 12,636 | (84.1) |
| Season of conception | |||||
| Spring | 521,446 | 56,581 | (10.9) | 464,865 | (89.2) |
| Summer | 485,610 | 49,815 | (10.3) | 435,795 | (89.7) |
| Autumn | 492,068 | 51,743 | (10.5) | 440,325 | (89.5) |
| Winter | 554,181 | 62,137 | (11.2) | 492,044 | (88.8) |
| Urbanization | |||||
| 1 Highly urbanized | 453,034 | 48,740 | (10.8) | 404,294 | (89.2) |
| 2 Moderately urbanized | 647,271 | 81,121 | (12.5) | 566,150 | (87.5) |
| 3 Newly urbanized | 482,702 | 42,942 | (8.9) | 439,760 | (91.1) |
| 4 General township | 288,711 | 27,079 | (9.4) | 261,632 | (90.6) |
| 5 Aged township | 28,124 | 3,384 | (12.0) | 24,740 | (88.0) |
| 6 Agricultural township | 66,357 | 7,435 | (11.2) | 58,922 | (88.8) |
| 7 Remote township | 80,879 | 9,358 | (11.6) | 71,521 | (88.4) |
| Geographic region | |||||
| North | 972,055 | 80,485 | (8.3) | 891,570 | (91.7) |
| Central | 527,171 | 34,857 | (6.6) | 492,314 | (93.4) |
| South | 500,624 | 95,716 | (19.1) | 404,908 | (80.9) |
| East | 40,868 | 8,605 | (21.1) | 32,263 | (78.9) |
| Outlying islands | 12,550 | 607 | (4.8) | 11,943 | (95.2) |
All P‐values <0.001.
GDM, gestational diabetes mellitus.
Age‐stratified analysis showed that the annual prevalence of GDM significantly increased in each age group (all trends P < 0.001; Figure 3). Urbanization‐stratified analysis showed that the annual prevalence of GDM significantly increased in each level (all trends P < 0.001; Figure 4). The GDM rate for level 5 areas increased significantly faster than for other levels. Level 3 areas had the lowest rate of GDM and the slowest annual growth. Geographic region‐stratified analysis showed that the annual prevalence of GDM significantly increased in each region (all trends P < 0.001; Figure 5). The east region had the highest rate of GDM and fastest yearly growth. Outlying islands had the lowest annual GDM rate, and the north region had the slowest annual growth.
Figure 3.
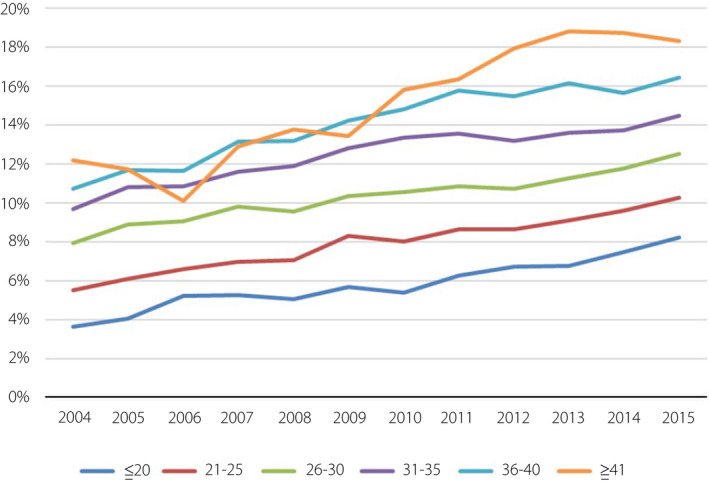
Gestational diabetes mellitus trends of pregnant women according to age group. Age‐stratified analysis showed that the annual prevalence of gestational diabetes mellitus significantly increased in each age group.
Figure 4.
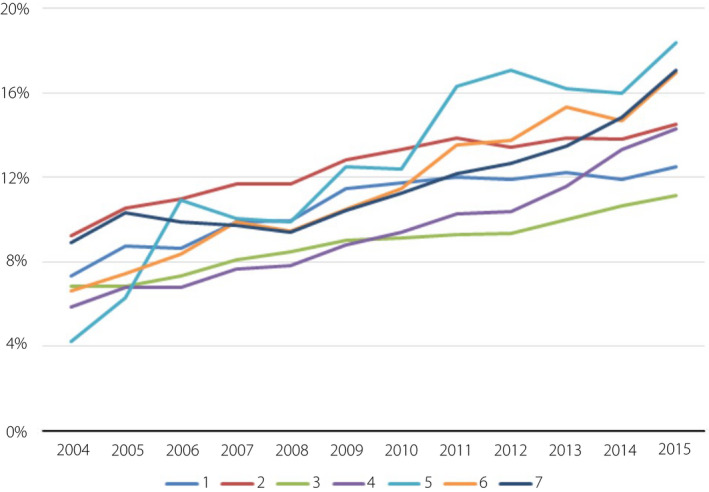
Gestational diabetes mellitus trends of pregnant women according to urbanization level. Urbanization‐stratified analysis showed that the annual prevalence of gestational diabetes mellitus significantly increased in each level. Urbanization level was determined using the standards established by the National Health Insurance Research Institute of Taiwan. All city districts and townships in Taiwan were classified into seven urbanization levels (1, most urbanized; 7, least urbanized) on the basis of population density (people/km2), proportion of residents with higher education, the population of elderly people and agricultural workers, and the number of physicians per 100,000 people in each area.
Figure 5.
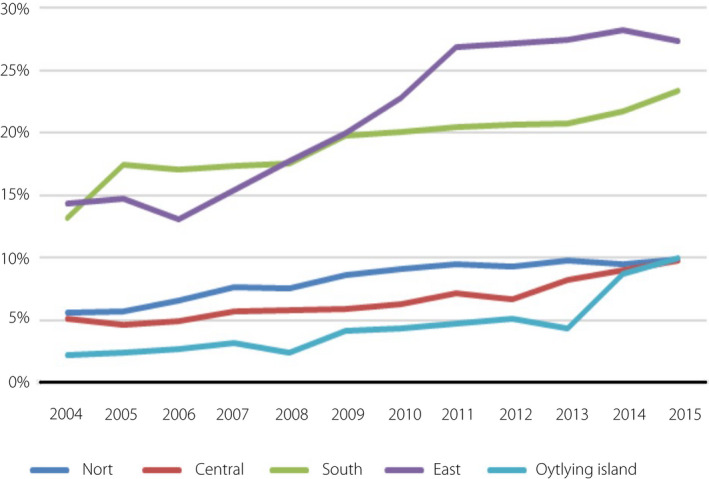
Gestational diabetes mellitus trends of pregnant women according to geographic region. Geographic region‐stratified analysis showed that the annual prevalence of gestational diabetes mellitus significantly increased in each region. Geographic regions were defined by the Council for Economic Planning and Development, Executive Yuan (Northern, Central, Southern, Eastern regions and Outer islands).
Table 2 shows the trends of pregnancy adverse outcomes for pregnant Taiwanese women from 2004 to 2015. The prevalence of premature delivery (<35 weeks and <37 weeks), low birthweight (<2,500 g) and fetal death tend to increase year by year (all trends P < 0.001). However, the downward trend of fetal macrosomia decreased from 2.26% to 1.22% (trend P < 0.001). Apgar score <7 at 5 min had no yearly change.
Table 2.
Trends of pregnancy adverse outcomes for pregnant Taiwanese women from 2004 to 2015
| Birth year | Preterm (<35 week) | Preterm (<37 week) | Low birthweight (<2500 g) | Macrosomia (≥4000 g) | Fetal death | Apgar score at 5 min of <7 |
|---|---|---|---|---|---|---|
| 2004 | 2.77% | 8.31% | 5.98% | 2.26% | 0.93% | 1.46% |
| 2005 | 2.71% | 8.01% | 6.00% | 2.00% | 0.95% | 1.46% |
| 2006 | 2.67% | 7.89% | 5.90% | 2.04% | 0.97% | 1.44% |
| 2007 | 2.67% | 7.88% | 5.97% | 1.85% | 0.98% | 1.41% |
| 2008 | 2.82% | 8.25% | 6.17% | 1.75% | 1.01% | 1.42% |
| 2009 | 2.71% | 7.91% | 6.21% | 1.65% | 1.01% | 1.40% |
| 2010 | 2.93% | 8.33% | 6.51% | 1.55% | 1.08% | 1.52% |
| 2011 | 2.92% | 8.06% | 6.38% | 1.55% | 1.09% | 1.48% |
| 2012 | 2.69% | 7.77% | 6.43% | 1.49% | 0.97% | 1.33% |
| 2013 | 2.90% | 7.90% | 6.69% | 1.30% | 1.08% | 1.46% |
| 2014 | 2.90% | 7.80% | 6.61% | 1.26% | 1.09% | 1.48% |
| 2015 | 2.96% | 8.05% | 6.94% | 1.22% | 1.10% | 1.50% |
| Trend P | <0.0001 | 0.0002 | <0.0001 | <0.0001 | <0.0001 | 0.438 |
Discussion
Diabetes mellitus is a global concern, currently affecting 8.8% of the world’s adult population. The estimated prevalence of adults with type 2 diabetes mellitus, including undiagnosed diabetes mellitus, is predicted to increase worldwide, with the largest increase to occur in certain territories 14 . Diabetes mellitus is in the top 10 causes of non‐communicable death, and it places a burden on healthcare systems, damages economic productivity, causes premature non‐communicable death and burdens households.
Maternal hyperglycemia without treatment can lead to adverse perinatal outcomes. The most frequent adverse events include increased risk of birth trauma, and higher incidence of cesarean sections, macrosomia, episodes of neonatal hypoglycemia and respiratory distress syndrome and/or prematurity, all of which increase the risk of perinatal death. Children of mothers with GDM have a higher risk of obesity and metabolic syndrome in adulthood 15 . Mothers with GDM are also at an increased risk of developing type 2 diabetes mellitus and metabolic syndrome in later life, as well as pre‐eclampsia in subsequent pregnancies 9 , 16 , 17 . This vicious cycle must be slowed. In the present study, we found that the annual prevalence of GDM increased by 1.8‐fold from 2004 to 2015, with a significant continuous increasing trend (from 7.6% to 13.4%, P < 0.001; Figure 2a).
A diagnosis of GDM can be made using one of two strategies: (i) the one‐step 75‐g oral glucose tolerance test derived from the International Association of the Diabetes and Pregnancy Study Groups criteria 3 ; and (ii) the older two‐step approach with a 50‐g (non‐fasting) screen followed by a 100‐g oral glucose tolerance test for those who screen positive, according to the National Diabetes Data Group threshold or Carpenter and Coustan criteria 18 , 19 . In Taiwan, both strategies are accepted, and the one‐step method is becoming increasingly common. The one‐step strategy has been anticipated to significantly increase GDM incidence (from 5–6% to 15–20%), primarily because only one abnormal value, not two, has become sufficient for diagnosis 20 , 21 , 22 . An increase in the prevalence of GDM generates an increased workload for obstetricians, endocrinologists and dietitian services in hospitals, maternal and familial stress (regarding medicalization), and healthcare system costs. That reflects that the GDM prevalence rate from earlier studies of 3.50–8.14 per 100 (2001–2011) has increased (prevalence of 7.6% in 2004 to 13.4% in 2015) 23 , 24 , 25 , 26 , 27 , 28 , 29 .
Advanced maternal age is a well‐known risk factor for GDM 28 , 29 . We found that the proportion of pregnancies among older women has increased annually. Older reproductive age is speculated to be a major factor in the annually increasing rate of GDM. Women pregnant in winter show the highest prevalence of GDM, and the GDM screening time is approximately 24–28 weeks into pregnancy (6–7.5 months after pregnancy). GDM was mostly diagnosed in the summer, which is consistent with the literature: summer screening yields higher GDM diagnosis rates 30 , 31 . In recent systematic review 32 showed there is mounting evidence that ambient temperature and season during pregnancy is associated with an increased risk of developing GDM and adverse glycemic outcomes. It was explained by the hypothesis of brown adipose tissue activation, which induces weight change, altered metabolic function and insulin sensitivity. Early recognition and intervention of maternal hyperglycemia significantly reduces fetal macrosomia. Most cases can be controlled well with lifestyle modifications alone 10 , 25 , 33 .
Regarding urbanization and geographic region parameters, all items show increasing trends. Different development of urbanization results in different GDM prevalence. Overall, all categories are increasing at different growth rates. Urbanized cities above level 3 showed slower growth rates, and cities below level 4 showed relatively rapid growth between 2004 and 2015. Some studies reported that women living in urban areas had higher GDM prevalence compared with women in rural areas 34 , 35 , 36 . The reason was explained by obesity and an aged population. However, no studies investigate the effect on GDM growth rate. The present result showed the GDM rate for level 5 areas increased significantly faster than for other levels. Level 3 areas had the lowest rate of GDM and the slowest annual growth. Level 5 areas (aged township) and level 3 areas (new developed) showed the proportion of people aged >65 years to be the highest and lowest, respectively, among the clusters 13 .
Regarding geographic regions, the overall trends of GDM in the eastern and southern regions were higher than that of other regions. In addition, the eastern region had a rapid upward trend of GDM prevalence than other regions in 2006–2011. It is related to the geographical environment, climate, medical resources, lifestyle, physical activity habits and living habits. A recent systematic review found consistent evidence of a seasonal effect on GDM risk, with a higher prevalence of GDM and higher pregnancy glucose levels in summer months 32 . Preston et al. found suggestive evidence of an association between higher temperature and elevated glucose levels from GDM screening tests. This might be one of the reasons for the higher prevalence of GDM in southern Taiwan. The effect factor is worth more precise further exploration.
Other parameters showing increasing trends were preterm delivery (both <35 weeks and <37 weeks), low birthweight (<2,500 g) and fetal death; trends of Apgar scores of <7 at 5 min did not increase 37 . An ongoing epidemic of obesity and diabetes exists globally, and this has led to higher rates of type 2 diabetes among women of reproductive age, with an increase in the number of pregnant women with undiagnosed prediabetes or type 2 diabetes during early pregnancy. However, a universal preconception and first trimester screening is hampered by a lack of data and consensus on appropriate diagnostic thresholds and outcomes 38 , 39 . Only those with risk factors are tested for undiagnosed prediabetes and diabetes at the first prenatal visit using standard diagnostic criteria.
GDM is increasing in prevalence worldwide and in Taiwan (since 2004 to 2015), where the rate increased from 7.6% to 13.4%, P < 0.001 during a 12‐year period (1.8‐fold). The prevalence has increased for all reproductive age groups, but more significantly so for ages ≥30 years. The risk factors include: older maternal age at conception, certain seasons of conception, urbanization level and geographic variations (ethnic group, access to health facilities, eating habits). Effects on adverse pregnancy outcomes included a reduction in fetal macrosomia (potentially due to the introduction and adoption of a more sensitive one‐step method and early intervention) 10 , but little effect was noted in other outcomes (low birthweight, preterm delivery, fetal death).
GDM has emerged as a global public health burden, as well as a local concern. Randomized controlled trials are difficult to carry out. Screening, diagnosis and treatment of hyperglycemia during pregnancy should be carried out as early as possible to prevent adverse pregnancy outcomes 17 . Screening and management of GDM should be incorporated as a universal routine antenatal service. Hyperglycemic surveillance should also be carried out for all pregnancies at the first prenatal visit to exclude prediabetes and diabetes. Postpartum long‐term follow up of mothers with GDM and their offspring is required for effective lifestyle changes, early detection and cost‐effective treatment for metabolic syndrome, obesity, prediabetes and diabetes 4 , 9 , 37 , 40 , 41 , 42 . Coordinated multisectoral public health policies are required to end the vicious cycle of GDM, and its subsequent effects on both mothers with GDM and their offspring 43 .
However, like any other study, the present study had some limitations. Our study was a retrospective cohort observational study. Body mass index (BMI) is a well‐known factor for diabetes and GDM. Although the BCA and NHRID cover almost 99% of pregnant Taiwanese women, the database still has some restrictions about BMI, parity, socioeconomic status of the family and maternal education level. Multiple pregnancies are also excluded from the present study. The prevalence of obesity is rising globally due to physical inactivity and consumption of an unhealthy diet 44 . In Taiwan, the Health Promotion Administration warns that the number of Taiwanese considered being overweight, or with a BMI >24, has grown from 32.7% between 1993 and 1996 to a shockingly high 45.4% between 2013 and 2016, making the Taiwanese population the most overweight in Asia 45 . The rising trend of obesity and overweight globally and in Taiwan might be the cause of the increase in GDM prevalence. Some large population‐based studies 29 , 39 , 40 including BMI data showed BMI is an important risk factor for GDM. However, the average BMI of GDM patients in the Asian population 29 or Asian subgroup 5 was relatively low. The increasing trend of GDM in the Asian population could not be related to the relatively stable trend of their BMI 29 , which requires further careful interpretation regarding BMI cut‐off 5 for GDM for Asian women.
DISCLOSURE
The authors declare no conflict of interest.
Acknowledgments
This study is based on data from the Health and Welfare Data Science Center (HWDC), Ministry of Health and Welfare, Taiwan. The interpretation and conclusions contained herein do not represent those of the HWDC. This study was supported by Kuang Tien General Hospital (KTGH) Research Funding (KTGH106‐1, KTGH107‐2).
J Diabetes Investig. 2021; 11: 2080–2088
Contributor Information
Shih‐Ting Tseng, Email: d97841006@ntu.edu.tw, Email: tsn4830@gmail.com.
Yuan‐Horng Yan, Email: d97841006@ntu.edu.tw, Email: tsn4830@gmail.com.
References
- 1. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus, Diabetes Care. 1997; 20: 1183–1197. [DOI] [PubMed] [Google Scholar]
- 2. Metzger BE, Lowe LP, Dyer AR, et al. HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008; 358: 1991–2002. [DOI] [PubMed] [Google Scholar]
- 3. International Association of Diabetes and Pregnancy Study Groups Consensus Panel , Metzger BE, Gabbe SG, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010; 33: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brown FM, Wyckoff J. Application of one‐step IADPSG versus two‐step diagnostic criteria for gestational diabetes in the real world: impact on health services, clinical care, and outcomes. Curr Diab Rep 2017; 17: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hedderson M, Ehrlich S, Sridhar S, et al. Racial/ethnic disparities in the prevalence of gestational diabetes mellitus by BMI. Diabetes Care 2012; 35: 1492–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dabelea D, Snell‐Bergeon JK, Hartsfield CL, et al. Increasing prevalence of gestational diabetes mellitus (GDM) over time and by birth cohort. Diabetes Care 2005; 28: 579–584. [DOI] [PubMed] [Google Scholar]
- 7. Ferrara A. Increasing prevalence of gestational diabetes mellitus. A public health perspective. Diabetes Care 2007; 30: S141–S146. [DOI] [PubMed] [Google Scholar]
- 8. Nguyen CL, Pham NM, Binns CW, et al. Prevalence of gestational diabetes mellitus in Eastern and Southeastern Asia: a systematic review and meta‐analysis. Hindawi Journal of Diabetes Research 2018; 2018: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhu Y, Zhang C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: a global perspective. Curr Diab Rep 2016; 16: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ovesen PG, Fuglsang J, Andersen MB, et al. Temporal Trends in Gestational Diabetes Prevalence, Treatment, and Outcomes at Aarhus University Hospital, Skejby. between 2004 and 2016. Hindawi Journal of Diabetes Research 2018; 2018: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lin CM, Lee PC, Teng SW, et al. Validation of the Taiwan birth registry using obstetric records. J Formosan Med Assoc 2004; 103: 297–301. [PubMed] [Google Scholar]
- 12. Hsieh CY, Su CC, Shao SC, et al. Taiwan's National Health Insurance Research Database: past and future. Clin Epidemiol 2019; 11: 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu CY, Hung YT, Chuang YL, et al. Incorporating developmental stratification of Taiwan townships into sampling design of large‐scale health interview survey. J Health Management 2006; 4: 1–22. [Google Scholar]
- 14. Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas. Diabetes Res Clin Pract 2019; 157: 107843. [DOI] [PubMed] [Google Scholar]
- 15. Wang J, Wang L, Liu H, et al. Maternal gestational diabetes and different indicators of childhood obesity: a large study. Endocrine Connections 2018; 7: 1464–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang C, Olsen SF, Hinkle SN, et al. Diabetes & Women’s Health (DWH) Study: an observational study of long term health consequences of gestational diabetes, their determinants and underlying mechanisms in the USA and Denmark. BMJ Open 2019; 9: e025517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chivese T, Norris SA, Levitt NS. Progression to type 2 diabetes mellitus and associated risk factors after hyperglycemia first detected in pregnancy: a cross‐sectional study in Cape Town, South Africa. PLoS Medicine 2019; 16: e1002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol 1982; 144: 768–773. [DOI] [PubMed] [Google Scholar]
- 19. National Diabetes Data Group . Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979; 28: 1039–1057. [DOI] [PubMed] [Google Scholar]
- 20. Ricart W, López J, Mozas J, et al. Potential impact of American Diabetes Association (2000) criteria for diagnosis of gestational diabetes mellitus in Spain. Diabetologia 2005; 48: 1135–1141. [DOI] [PubMed] [Google Scholar]
- 21. Ferrara A, Hedderson MM, Quesenberry CP, et al. Prevalence of gestational diabetes mellitus detected by the national diabetes data group or the carpenter and coustan plasma glucose thresholds. Diabetes Care 2002; 25: 1625–1630. [DOI] [PubMed] [Google Scholar]
- 22. Bing Y, Yu Y, Lin M, et al. High, but stable, trend in the prevalence of gestational diabetes mellitus: a population based study in Xiamen, China. J Diabetes Investig 2019; 10: 1358–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yan B, Yu Y, Lin M, et al. Pregnancy outcomes of Taiwanese women with gestational diabetes mellitus: a comparison of Carpenter‐Coustan and National Diabetes Data Group Criteria. J Women's Health 2010; 19: 935–939. [DOI] [PubMed] [Google Scholar]
- 24. Hung TH, Hsieh TT. The effects of implementing the International Association of Diabetes and Pregnancy Study Groups Criteria for diagnosing gestational diabetes on maternal and neonatal outcomes. PLoS One 2015; 10: article e0122261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lin CH, Wen SF, Wu YH, et al. Using the 100‐g oral glucose tolerance test to predict fetal and maternal outcomes in women with gestational diabetes mellitus. Chang Gung Med J 2009; 32: 283–289. [PubMed] [Google Scholar]
- 26. Chuang CM, Lin IF, Horng HC, et al. The impact of gestational diabetes mellitus on postpartum urinary incontinence: a longitudinal cohort study on singleton pregnancies. BJOG Int J Obstet Gynaecol 2012; 119: 1334–1343. [DOI] [PubMed] [Google Scholar]
- 27. Wang P, Lu MC, Yu CW, et al. Influence of food intake on the predictive value of the gestational diabetes mellitus screening test. Obstet Gynecol 2013; 121: 750–758. [DOI] [PubMed] [Google Scholar]
- 28. Kuo C‐H, Chen S‐C, Fang C‐T, et al. Screening gestational diabetes mellitus: the role of maternal age. PLoS One 2017; 12: e0173049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cho GJ, Kim LY, Sung YN, et al. Secular trends of gestational diabetes mellitus and changes in its risk factors. PLoS One 2015; 10: e0136017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moses RG, Wong VC, Lambert K, et al. Seasonal changes in the prevalence of gestational diabetes mellitus. Diabetes Care 2016; 39: 1218–1221. [DOI] [PubMed] [Google Scholar]
- 31. Wang P, Wu CS, Li CY, et al. Seasonality of gestational diabetes mellitus and maternal blood glucose levels: evidence from Taiwan. Medicine 2020; 99: e22684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Preston EV, Eberle C, Brown FM, et al. Climate factors and gestational diabetes mellitus risk–a systematic review. Environ Health 2020; 19: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Filardi T, Panimolle F, Crescioli C, et al. Gestational diabetes mellitus: the impact of carbohydrate quality in diet. Nutrients 2019; 11: 1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zargar AH, Sheikh MI, Bashir MI, et al. Prevalence of gestational diabetes mellitus in Kashmiri women from the Indian subcontinent. Diabetes Res Clin Pract 2004; 66: 139–145. [DOI] [PubMed] [Google Scholar]
- 35. Seshiah V, Balaji V, Balaji MS, et al. Prevalence of gestational diabetes mellitus in South India (Tamil Nadu): a community based study. JAPI 2008; 56: 329–333. [PubMed] [Google Scholar]
- 36. Mwanri AW, Kinabo J, Ramaiya K, et al. Prevalence of gestational diabetes mellitus in urban and rural Tanzania. Diabetes Res Clin Pract 2014; 103: 71–78. [DOI] [PubMed] [Google Scholar]
- 37. Fadl HE, Simmons D. Trends in diabetes in pregnancy in Sweden 1998–2012. BMJ Open Diabetes Research and Care 2016; 4: e000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rani PR, Begum J. Screening and diagnosis of gestational diabetes mellitus, where do we stand. J Clin Diagn Res 2016; 10: QE01–QE04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Harreiter J, Simmons D, Desoye G, et al. IADPSG and WHO 2013 gestational diabetes mellitus criteria identify obese women with marked insulin resistance in early pregnancy. Diabetes Care 2016; 39: e90–e92. [DOI] [PubMed] [Google Scholar]
- 40. Behboudi‐Gandevani S, Amiri M, Yarandi RB, et al. The impact of diagnostic criteria for gestational diabetes on its prevalence: a systematic review and meta‐analysis. Diabetol Metab Syndr 2019; 11: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shang M, Lin L. IADPSG criteria for diagnosing gestational diabetes mellitus and predicting adverse pregnancy outcomes. J Perinatol 2014; 34: 100–104. [DOI] [PubMed] [Google Scholar]
- 42. Sirimarco MP, Guerra HM, Lisboa EG, et al. Diagnostic protocol for gestational diabetes mellitus (GDM) (IADPSG/ADA, 2011): influence on the occurrence of GDM and mild gestational hyperglycemia (MGH) and on the perinatal outcomes. Diabetol Metab Syndr 2017; 9: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang M, Zhou Y, Zhong J, et al. Current guidelines on the management of gestational diabetes mellitus: a content analysis and appraisal. BMC Pregnancy Childbirth 2019; 19: 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wong MC, Huang J, Wang J, et al. Global, regional and time‐trend prevalence of central obesity: a systematic review and meta‐analysis of 13.2 million subjects. Eur J Epidemiol 2020; 35: 673–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kao Z. Taiwan remains most overweight Asian country despite increase in exercise. Taiwan News. Retrieved from: https://www.taiwannews.com.tw/en/news/3728344 (2019, June 20)


