ABSTRACT
Aims/Introduction
The relationship between glycated hemoglobin (HbA1c) and cardiovascular events in older adults was investigated using a Japanese administrative medical database.
Materials and Methods
Anonymized medical data on patients with diabetes mellitus aged ≥65 years for the period from January 2010 to December 2019 were extracted from the EBM Provider database. The primary end‐point was a composite of cardiovascular events, whereas the other end‐points included severe hypoglycemia and fracture. The association between cardiovascular events and HbA1c at the index date (i.e., approximately 10 months after initial diabetes mellitus diagnosis) was evaluated using the Cox proportional hazards model.
Results
Among the 3,186,751 patients in the database, 3,946 older adults with diabetes mellitus were eligible for inclusion and were subsequently grouped according to HbA1c quartiles at the index date. Cardiovascular events occurred in 142 patients. Patients with HbA1c in the highest quartile had significantly higher risk of hospitalization for cardiovascular disease than those with HbA1c in the lowest quartile (hazard ratio 1.948; 95% confidence interval 1.252–3.031, P = 0.003). However, the events risk was similar across subgroups with HbA1c <7.2%. The incidence of hypoglycemia and fracture was not significantly associated with the level of glycemic control.
Conclusions
Among older adults with diabetes mellitus, those with poor glycemic control were at higher risk for cardiovascular events compared with those with better glycemic control. However, strict glycemic control had no effect on cardiovascular risk in patients with HbA1c <7.2%.
Keywords: Cardiovascular events, Glycated hemoglobin, Older diabetes patients
We investigated the relationship between glycated hemoglobin and cardiovascular events in older adults with diabetes mellitus using administrative medical databases in Japan. Among approximately 4,000 older patients without a history of dementia, cancer or cardiovascular disease, those with glycated hemoglobin in the highest quartile had a significantly higher risk of hospitalization for cardiovascular disease than those with glycated hemoglobin in the lowest quartile. Age, sex, prescription of antihypertensive drugs and comorbidities were also associated with the risk of cardiovascular events.

INTRODUCTION
The number of older adults with diabetes mellitus has been increasing due to the overall aging of the Japanese population 1 . This is of particular concern given that older patients with diabetes mellitus are at a high risk for cardiovascular diseases 2 , 3 , 4 , 5 . Although hyperglycemia is a risk factor for macro‐ and microvascular complications, intensive glycemic control does not always provide cardiovascular benefits in older adults with diabetes mellitus 3 . To date, limited large‐scale studies have investigated the relationship between glycemic levels and the incidence of cardiovascular events. Accordingly, the study from the UK General Practice Research Database showed a U‐shaped association between glycated hemoglobin (HbA1c) level and mortality, with the lowest hazard ratio (HR) for death observed at an HbA1c level of approximately 7.5% in patients aged ≥50 years 6 . In contrast, the Japanese Elderly Intervention Trial (J‐EDIT) showed a J‐shaped relationship between glycemic levels and incidence of stroke after 6 years of follow up, with the lowest number of events having been observed at an HbA1c level of 7.3–7.9% in patients aged ≥65 years 7 .
The enrollment period in the J‐EDIT study was between March 2001 and February 2002 8 . However, the treatment approach for diabetes mellitus has greatly changed over the past decade, since the launch of new antidiabetic agents, including incretin‐related drugs and sodium–glucose cotransporter 2 inhibitors. In addition, management guidelines established by the Japan Diabetes Society/Japan Geriatric Society recommend that older patients with diabetes mellitus set individual treatment goals for glycemic control and classify their goals into categories I–III based on activities of daily life, cognitive function, risk of hypoglycemia and concomitant diseases 9 . Despite these changes in the treatment environment, collecting data on older patients with diabetes mellitus from randomized control trials aiming to assess the efficacy of the treatment has been difficult given that such studies often exclude older patients and those with diverse characteristics or multiple comorbidities 3 . Currently, anonymized real‐world epidemiological data can be obtained from the Japanese EBM Provider database 10 , and it is also possible to extract a dataset of populations with different backgrounds.
The present study aimed to clarify the relationship between HbA1c levels and the occurrence of cardiovascular events by extracting an older population dataset from the EBM Provider database 10 . Given that older adults with diabetes mellitus are at risk for developing hypoglycemia 11 , 12 , 13 that might trigger a fall and fractures 14 that subsequently impair quality of life 15 , 16 , the relationship between glycemic levels and severe hypoglycemia and fractures as secondary and exploratory end‐points were also investigated, respectively.
MATERIALS AND METHODS
Study design and data source
The present retrospective study utilized landmark analysis of data from the EBM Provider database (Medical Data Vision Co., Ltd, Tokyo Japan) 10 . The database consists of patient information, such as sex, age, disease data, prescriptions/medical procedure data and Diagnosis Procedure Combination records data. Laboratory data are collected by approximately 10% of the patients. This database contains anonymously processed electronic health record‐based data collected since April 2008 from approximately 300 acute care hospitals representing approximately 20% of all large hospitals across Japan 10 , and it has been used in a study for the assessment of cardiovascular events 17 .
The study period was set to the latest 10 years (i.e., from 1 January 2010 to 31 December 2019) to extract the dataset of patients with diabetes diagnosis codes (International Classification of Diseases Revision 10 Code: E11–14). In the preliminary examination of the time course of HbA1c, it was deemed that HbA1c levels reach a steady state 6 months after treatment initiation in the target population (Figure S1). While the J‐EDIT study set the landmark period of 1 year after the treatment, the index date used herein was defined as the day of HbA1c testing closest to 9 months within the 9‐month to 1‐year period after initial diagnosis of diabetes mellitus in the database. The baseline period was defined as that between the first visit and the index date.
Study population
The inclusion criteria were as follows: (i) patients with a first visit due to diabetes mellitus on January 2010 or later in the database; (ii) continuous physician‐supervised treatment; that is, at least two recorded visits to the study site for diabetes mellitus other than type 1 diabetes mellitus (International Classification of Diseases Revision 10 code E10) within 9 months from the initial visit; (iii) age ≥65 years at the index date; (iv) availability of medical practice data after the index date; and (v) HbA1c of ≥6.5% on the initial visit or prescription of an antidiabetic agent (Anatomical Therapeutic Chemical Classification code: A10C, insulin; A10H, sulfonylurea; A10J, biguanide; A10K, thiazolidinedione; A10L, α‐glucosidase inhibitor; A10M, glinide; A10N, dipeptidyl peptidase‐4 inhibitor; A10P, sodium–glucose cotransporter 2 inhibitor; A10S, glucagon‐like peptide‐1 receptor agonist) for diabetes mellitus at least once during the baseline period 18 .
We excluded vulnerable older patients who required more extensive care, as the glycemic control goal was set based on patient conditions, such as severe comorbidities 9 . The exclusion criteria were as follows: (i) records suggesting a definitive cancer or dementia diagnosis throughout the entire observation period; (ii) history of angina pectoris, heart failure, myocardial infarction, or stroke (except transient ischemic attacks); and (iii) documented medical procedures for coronary revascularization before the index date. The definitions of diseases and medical procedures are presented in Tables S1 and S2.
Outcomes
Patients were classified to four subgroups based on their HbA1c levels at the index date (groups 1–4 corresponding to the lowest and highest quartiles, respectively), after which the incidence of the primary, secondary and other end‐points were compared between the subgroups. The primary end‐point was a composite of hospitalization for stroke, angina pectoris, heart failure or myocardial infarction, as determined as a main diagnosis by the physician, 17 or for coronary revascularization documented as a medical procedure, noting the first event occurring after the index date. The definitions of components of the primary end‐point are shown in Table S1. Incidences of each component were evaluated in the same manner.
The secondary end‐point was the incidence of severe hypoglycemia, and the day of prescription of glucose (≥20%) injection was defined as the occurrence date in the month when hypoglycemia was definitely diagnosed based on any of the codes in International Classification of Diseases Revision 10 (Table S1). Incidences of fractures were evaluated as an exploratory end‐point, given that this database contained only a limited number of known risk factors for fracture. The day of onset of fracture was the first of treatment for fracture that can be confirmed on the database. The incidences of fractures were grouped in the thoracic vertebra, lumber vertebra, shoulder/upper arm, forearm and femur (Table S1). All‐cause death was defined as any death documented in the Diagnosis Procedure Combination records for the entire observable period after the index date. The date of death was defined as the date of discharge.
Statistical analysis
Demographic and baseline characteristics were summarized according to HbA1c categories at the index date. Categorical variables were expressed as numbers and percentages, whereas continuous variables were expressed as mean ± standard deviation. The number and incidence (per 1,000 person‐years) of each event were calculated according to HbA1c subgroups. A generalized Wilcoxon test was used to compare the incidence rate with group 1 with the lowest HbA1c quartile.
The multivariate Cox proportional hazards model was used to compare the risk of the primary, secondary and other end‐points between the HbA1c subgroups. The following confounding factors were included as covariates that might be associated with outcomes based on the Japanese Clinical Practice Guideline for Diabetes 9 . Accordingly, covariates for the primary end‐point included demographic parameters (age at index date and sex); baseline treatments/prescriptions of antihypertensive agents, antihyperlipidemic agents and antithrombotic agents/coronary vasodilator; comorbidities/history of retinopathy, neuropathy, chronic kidney disease (CKD) and atrial fibrillation; Charlson Comorbidity Index 19 ; and HbA1c on the initial visit for diabetes mellitus. Covariates for the secondary end‐point included age, baseline period, depression, liver disease, CKD, prescription of insulin/sulfonylureas (SUs)/glinides, number of drugs prescribed during the baseline period and HbA1c on the initial visit for diabetes mellitus. The definitions of diseases and drugs for confounding factors are shown in Table S1. The Charlson Comorbidity Index calculated the sum of the weighted scores for each of the chronic conditions 20 . Covariates for fractures included age, sex and HbA1c on the initial visit for diabetes mellitus. All analyses were carried out using SAS Version 9.4 (SAS Institute Inc., Cary NC, USA), with a P value of <0.05 showing statistical significance.
RESULTS
Identification of study population
Among the 3,186,751 patients diagnosed with diabetes mellitus in the database, 10,290 patients satisfied the eligibility criteria. Among such patients, 3,946 were eligible for the analysis after excluding those aged <65 years (Figure 1). Among the included patients, 1,227 were aged ≥75 years.
Figure 1.
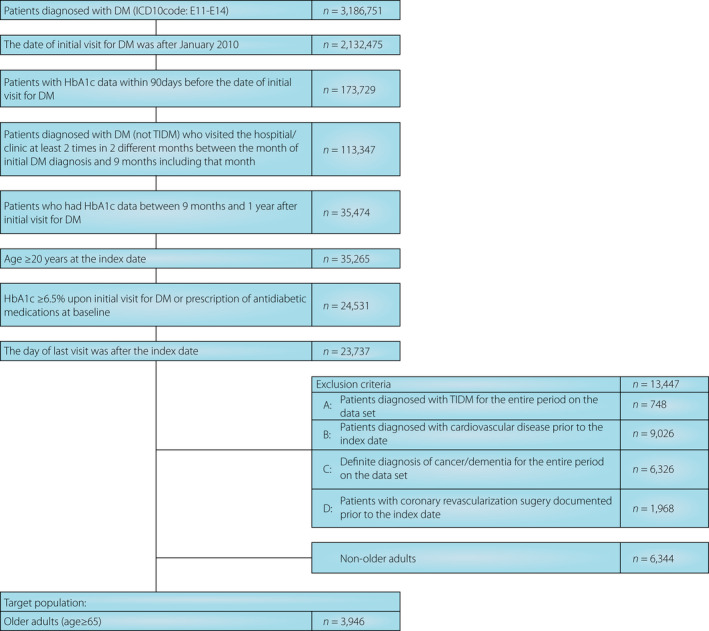
Patient selection flowchart. DM, diabetes mellitus; HbA1c, glycated hemoglobin; ICD10, International Classification of Diseases Revision 10; T1DM, type 1 diabetes mellitus.
Patient characteristics
The mean age of the study population was 72.2 years, with men accounting for 53.2% (Table 1). The mean baseline period in our population was 9.88 months, whereas the mean follow‐up period after the index date was 2.51 years. The median HbA1c was 7.30% at the initial visit and 6.60% at the index date. As HbA1c quartiles at the index date were 6.3, 6.6 and 7.2%, patients with HbA1c of <6.3%, 6.3 to <6.6%, 6.6% to <7.2% and ≥7.2% were classified into groups 1–4, respectively. The median HbA1c values of groups 1–4 were 6.0, 6.4, 6.8 and 7.7%, respectively. Although 30 patients received dialysis during the baseline period, HbA1c quartiles remained unchanged when their data were excluded.
Table 1.
Baseline characteristics of older patients with diabetes mellitus according to glycated hemoglobin quartiles at the index date
| Categories | All | Group 1 | Group 2 | Group 3 | Group 4 | P‐value § | |
|---|---|---|---|---|---|---|---|
| HbA1c range (%) | – | <6.3 | 6.3 to <6.6 | 6.6 to <7.2 | ≥7.2 | ||
| No. patients | n | 3,946 | 973 | 785 | 1,177 | 1,011 | |
| Follow‐up period (years) | Mean ± SD | 2.51 ± 1.88 | 2.60 ± 1.91 | 2.54 ± 1.90 | 2.55 ± 1.89 | 2.33 ± 1.82 | 0.007 |
| Age (years) | Mean ± SD | 72.2 ± 5.9 | 72.4 ± 5.9 | 71.9 ± 5.8 | 72.2 ± 6.0 | 72.2 ± 5.9 | 0.311 |
| Sex (male) | n (%) | 2098 (53.2) | 525 (54.0) | 396 (50.4) | 623 (52.9) | 554 (54.8) | 0.298 |
| HbA1c † (%) | Mean ± SD | 8.17 ± 2.19 | 7.79 ± 2.32 | 7.68 ± 1.81 | 7.98 ± 1.91 | 9.13 ± 2.35 | <0.001 |
| HbA1c at the index date (%) | Mean ± SD | 6.82 ± 0.94 | 5.91 ± 0.28 | 6.40 ± 0.08 | 6.82 ± 0.17 | 8.02 ± 0.98 | <0.001 |
| Antihypertensive agents | n (%) | 2120 (53.7) | 557 (57.2) | 425 (54.1) | 598 (50.8) | 540 (53.4) | 0.030 |
| Antihyperlipidemic agents | n (%) | 1565 (39.7) | 394 (40.5) | 308 (39.2) | 444 (37.7) | 419 (41.4) | 0.317 |
| Antithrombotic agents/coronary vasodilators | n (%) | 502 (12.7) | 150 (15.4) | 87 (11.1) | 131 (11.1) | 134 (13.3) | 0.011 |
| Insulin/sulfonylureas/glinides ‡ | n (%) | 1767 (44.8) | 422 (43.4) | 259 (33.0) | 451 (38.3) | 635 (62.8) | <0.001 |
The glycated hemoglobin (HbA1c) quartiles were 6.3, 6.6 and 7.2%.
HbA1c on initial visit for diabetes mellitus.
Antihyperglycemic agents with a high risk of hypoglycemia.
One‐way anova and χ2‐tests were used for comparison of mean values of continuous data (follow‐up period, age, HbA1c and HbA1c at the index date) and categorical variables (sex, antihypertensive agents, antihyperlipidemic agents, antithrombotic agents/coronary vasodilators and insulin/sulfonylureas/glinides prescriptions), respectively. SD, standard deviation.
Onset of events
A composite of cardiovascular events was observed in 142 patients, with group 4 having the highest incidence rates (24.5/1,000 person‐years; Table 2). Meanwhile, groups 1–3 had similar incidence rates. At the index date, the number of patients was highest with HbA1c 6.0 to <7.0%, followed by HbA1c 7.0–8.0% (Figure 2). The cardiovascular incident rate was gradually increased starting at approximately 7.0% of HbA1c (Figure 2). With group 1 as the reference, the HR was the highest and statistically significant in group 4 (HR 1.948, 95% confidence interval 1.252–3.031; P = 0.003), whereas groups 2 and 3 had a similar risk as group 1 (Table 3). Factors affecting the composite end‐point included age, sex, antihypertensive drug prescription and the Charlson Comorbidity Index score (Table 3). The Kaplan–Meier curve is shown in Figure S2.
Table 2.
Number and incidence rate of cardiovascular events in older patients with diabetes mellitus
| All | Group 1 | Group 2 | Group 3 | Group 4 | |
|---|---|---|---|---|---|
| HbA1c range (%) | – | <6.3 | 6.3 to <6.6 | 6.6 to <7.2 | ≥7.2 |
| No. patients | 3,946 | 973 | 785 | 1,177 | 1,011 |
| Composite of cardiovascular events | |||||
| No. patients | 142 | 33 | 23 | 30 | 56 |
| Incidence rate (per 1,000 person‐years) | 14.6 | 13.2 | 11.7 | 10.1 | 24.5 |
| P‐value † | – | – | 0.882 | 0.562 | 0.001 |
| Stroke | |||||
| No. patients | 54 | 12 | 11 | 14 | 17 |
| Incidence rate (per 1,000 person‐years) | 5.4 | 4.7 | 5.5 | 4.6 | 7.2 |
| P‐value † | – | – | 0.449 | 0.891 | 0.104 |
| Cardiac disease | |||||
| No. patients | 85 | 18 | 12 | 16 | 39 |
| Incidence rate (per 1,000 person‐years) | 8.7 | 7.2 | 6.0 | 5.3 | 16.9 |
| P‐value † | – | – | 0.672 | 0.682 | <0.001 |
| Coronary revascularization | |||||
| No. patients | 51 | 10 | 8 | 10 | 23 |
| Incidence rate (per 1,000 person‐years) | 5.1 | 3.9 | 4.0 | 3.3 | 9.8 |
| P‐value † | – | – | 0.971 | 0.712 | 0.011 |
| Severe hypoglycemia | |||||
| No. patients | 20 | 6 | 1 | 3 | 10 |
| Incidence rate (per 1,000 person‐years) | 2.0 | 2.3 | 0.5 | 1.0 | 4.2 |
| P‐value † | – | – | 0.078 | 0.170 | 0.149 |
| Fracture | |||||
| No. patients | 181 | 51 | 29 | 54 | 47 |
| Incidence rate (per 1,000 person‐years) | 18.7 | 20.6 | 14.7 | 18.3 | 20.4 |
| P‐value † | – | – | 0.471 | 0.439 | 0.775 |
HbA1c, glycated hemoglobin.
The generalized Wilcoxon test was used to compare the incidence rate with group 1.
Figure 2.
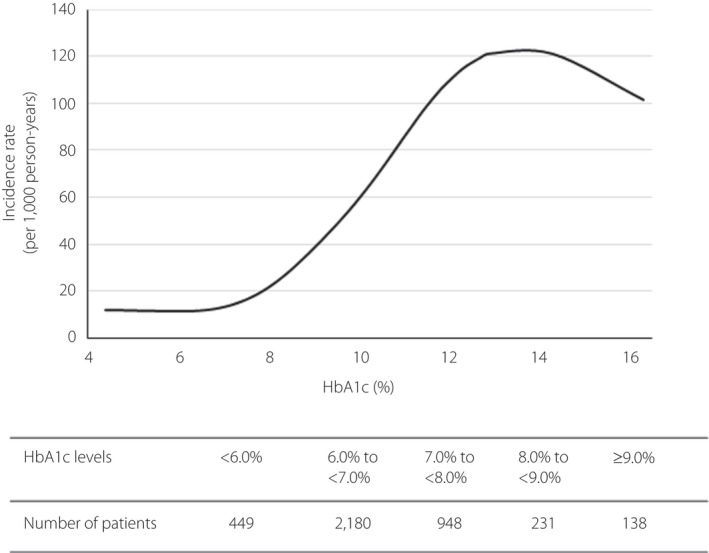
The relationship between glycated hemoglobin (HbA1c) and incidence rate of cardiovascular events. The unadjusted incidence rate (per 1,000 person‐years) was fitted using a cubic spline modeling.
Table 3.
Hazard ratios with 95% confidence intervals using multivariate Cox proportional hazards models for the primary outcome and cardiovascular components
| HR | 95% CI | P‐value | |
|---|---|---|---|
| Primary outcome | |||
| Group 1 | 1 | − | − |
| Group 2 (vs group 1) | 1.025 | 0.600–1.750 | 0.928 |
| Group 3 (vs group 1) | 0.907 | 0.549–1.497 | 0.702 |
| Group 4 (vs group 1) | 1.948 | 1.252–3.031 | 0.003 |
| Age | 1.041 | 1.013–1.070 | 0.004 |
| Sex, female (vs male) | 0.466 | 0.324–0.670 | <0.001 |
| Antihypertensive agents (vs without the agents) | 1.468 | 1.018–2.115 | 0.040 |
| Antihyperlipidemic agents (vs without the agents) | 0.939 | 0.662–1.331 | 0.724 |
| Antithrombotic agents/coronary vasodilators (vs without the agents) | 1.549 | 0.983–2.439 | 0.059 |
| CKD (vs without) | 1.376 | 0.962–1.969 | 0.080 |
| Diabetic retinopathy (vs without) | 1.212 | 0.779–1.884 | 0.393 |
| Diabetic neuropathy (vs without) | 0.652 | 0.353–1.203 | 0.171 |
| Atrial fibrillation (vs without) | 1.077 | 0.491–2.361 | 0.853 |
| CCI | 1.156 | 1.017–1.314 | 0.026 |
| HbA1c on the first visit day for DM | 1.058 | 0.981–1.142 | 0.145 |
| Stroke | Cardiac disease | Coronary revascularization | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P‐value | HR | 95% CI | P‐value | HR | 95% CI | P‐value | |
| Cardiovascular components | |||||||||
| Group 1 | 1 | − | − | 1 | − | − | 1 | − | − |
| Group 2 (vs group 1) | 1.339 | 0.588–3.050 | 0.487 | 0.985 | 0.473–2.053 | 0.969 | 1.231 | 0.483–3.136 | 0.664 |
| Group 3 (vs group 1) | 1.147 | 0.525–2.510 | 0.731 | 0.877 | 0.444–1.734 | 0.706 | 1.107 | 0.454–2.697 | 0.823 |
| Group 4 (vs group 1) | 1.564 | 0.733–3.337 | 0.247 | 2.503 | 1.409–4.448 | 0.002 | 2.649 | 1.234–5.686 | 0.012 |
| Age | 1.051 | 1.005–1.098 | 0.028 | 1.047 | 1.010–1.084 | 0.012 | 1.013 | 0.966–1.062 | 0.597 |
| Sex, female (vs male) | 0.499 | 0.278–0.895 | 0.020 | 0.495 | 0.311–0.786 | 0.003 | 0.348 | 0.181–0.671 | 0.002 |
| Antihypertensive agents (vs without the agents) | 1.164 | 0.655–2.068 | 0.605 | 1.892 | 1.159–3.088 | 0.011 | 1.332 | 0.729–2.432 | 0.351 |
| Antihyperlipidemic agents (vs without the agents) | 1.000 | 0.566–1.768 | 0.999 | 0.842 | 0.534–1.325 | 0.457 | 0.715 | 0.391–1.306 | 0.275 |
| Antithrombotic agents/coronary vasodilators (vs without the agents) | 1.266 | 0.588–2.727 | 0.546 | 1.440 | 0.788–2.633 | 0.236 | 1.627 | 0.759–3.487 | 0.210 |
| CKD (vs without) | 1.153 | 0.643–2.068 | 0.632 | 1.510 | 0.951–2.398 | 0.081 | 2.434 | 1.344–4.407 | 0.003 |
| Diabetic retinopathy (vs without) | 1.002 | 0.480–2.094 | 0.995 | 1.430 | 0.816–2.507 | 0.211 | 1.648 | 0.833–3.262 | 0.151 |
| Diabetic neuropathy (vs without) | 1.225 | 0.528–2.839 | 0.637 | 0.335 | 0.120–0.933 | 0.036 | 0.704 | 0.270–1.839 | 0.474 |
| Atrial fibrillation (vs without) | 0.746 | 0.165–3.382 | 0.705 | 1.464 | 0.572–3.750 | 0.426 | 0.362 | 0.046–2.834 | 0.333 |
| CCI | 1.165 | 0.949–1.431 | 0.145 | 1.119 | 0.946–1.324 | 0.190 | 1.103 | 0.887–1.373 | 0.378 |
| HbA1c on the first visit day for DM | 1.037 | 0.914–1.177 | 0.570 | 1.080 | 0.981–1.189 | 0.115 | 1.087 | 0.963–1.227 | 0.175 |
Primary outcome and cardiovascular components: For the multivariate Cox proportional hazards model, the following baseline parameters were included as covariates: glycated hemoglobin (HbA1c; groups 1–4) at the index date, age, sex, prescription of antihypertensive agents, prescription of antihyperlipidemic agents, prescription of antithrombotic agents/coronary vasodilators, chronic kidney disease, diabetic retinopathy, diabetic neuropathy, atrial fibrillation,; Charlson Comorbidity Index and HbA1c on the first visit day for diabetes mellitus.
CI, confidence interval; HR, hazard ratio; CKD, chronic kidney disease; CCI, Charlson Comorbidity Index.
Among the components of the primary end‐point, incidences of hospitalization for cardiac disease, stroke and coronary revascularization were highest in group 4, whereas no marked differences were noted between the three other subgroups (Table 2). The incidence of hospitalization for stroke was numerically high in group 4, although no significant difference was observed between all four subgroups (Table 2). Factors affecting the risk of hospitalization for stroke included age and sex. Group 4 had significantly higher incidences of hospitalization for both cardiac disease and coronary revascularization than group 1, with HRs of 2.503 (95% confidence interval 1.409–4.448) and 2.649 (95% confidence interval 1.234–5.686), respectively (Table 3). However, no significant difference in such risks were observed between groups 1–3.
Factors affecting hospitalization for cardiac diseases included age, sex and prescription of antihypertensive medication. Furthermore, the HR for cardiac diseases was significantly low with diabetic neuropathy, but tended to be high with CKD, albeit not significantly. Sex and CKD were also identified as significant factors affecting hospitalization for coronary revascularization in group 4, with the highest HR for CKD being 2.434.
Severe hypoglycemia, the secondary end‐point, occurred in just 20 participants, half of whom were in group 4 (Table 2). All these patients had a continuous prescription record of insulin/SUs/glinides. HRs for severe hypoglycemia were lower in groups 2 and 3, but tended to be higher in group 4 compared with group 1, although no significant differences in risks were observed between the subgroups (Table 4). HR for depression could not be calculated given that none of the patients with severe hypoglycemia were diagnosed with depression. Prescription of insulin/SUs/glinides was identified as a factor strongly affecting severe hypoglycemia (HR 4.209; P = 0.037).
Table 4.
Multivariate Cox proportional hazards model for severe hypoglycemia and fracture
| Severe hypoglycemia | HR | 95% CI | P‐value |
|---|---|---|---|
| Group 1 | 1 | − | − |
| Group 2 (vs group 1) | 0.259 | 0.031–2.169 | 0.213 |
| Group 3 (vs group 1) | 0.484 | 0.119–1.963 | 0.310 |
| Group 4 (vs group 1) | 1.403 | 0.488–4.036 | 0.530 |
| Age | 1.046 | 0.974–1.124 | 0.218 |
| Baseline period | 1.228 | 0.678–2.226 | 0.498 |
| Depression (vs without) | 0.000 | − | 0.988 |
| Liver disease (vs without) | 0.619 | 0.142–2.691 | 0.522 |
| CKD (vs without) | 1.487 | 0.590–3.747 | 0.400 |
| Insulin/sulfonylurea/glinide (vs without insulin/sulfonylurea/glinide) | 4.209 | 1.090–16.251 | 0.037 |
| No. drug types prescribed during baseline period | 1.012 | 0.980–1.046 | 0.461 |
| HbA1c on the first visit day for DM | 1.089 | 0.916–1.294 | 0.335 |
| Fractures | HR | 95% CI | P value |
|---|---|---|---|
| Group 1 | 1 | − | − |
| Group 2 (vs group 1) | 0.733 | 0.464–1.157 | 0.182 |
| Group 3 (vs group 1) | 0.925 | 0.630–1.358 | 0.691 |
| Group 4 (vs group 1) | 1.169 | 0.773–1.766 | 0.460 |
| Age | 1.071 | 1.048–1.095 | <0.001 |
| Sex, female (vs male) | 2.164 | 1.586–2.955 | <0.001 |
| HbA1c on the first visit day for DM | 0.919 | 0.847–0.997 | 0.043 |
Severe hypoglycemia: In the multivariate Cox proportional hazards model, the following baseline parameters were included as covariates: glycated hemoglobin (HbA1c; groups 1–4) at the index date, age, baseline period, depression, liver disease, CKD, prescription of insulin/sulfonylurea/glinide, number of drugs prescribed and HbA1c on the first visit day for diabetes mellitus (DM). Fracture: In the multivariate Cox proportional hazards model, the following baseline parameters were included as covariates: HbA1c (groups 1–4) at the index date, age, sex and HbA1c on the first visit day for DM.
CI, confidence interval; CKD, chronic kidney disease; HR, hazard ratio.
Fractures occurred in 181 patients with no obvious difference in incidence rates between the subgroups (Table 2). Hospitalization for fractures as a major cause was documented in 65 patients, but the event rates were also similar across the subgroups (data not shown). The number of patients by fracture site is shown in Table S4. The most common fracture was vertebral, including thoracic, lumbar and thoracolumbar, in 113 patients. A total of 33 patients had femur fractures, whereas 35 patients had arm fractures, including the shoulder/upper arm or forearm. Sex, age and HbA1c on the first visit day for diabetes mellitus were identified as factors associated with fractures. In particular, sex (female) was identified as a factor strongly affecting the incidence of fractures (Table 4).
After the index date, 64 all‐cause deaths were identified (Table S4). No significant difference was noted in the incidence rate between the groups.
DISCUSSION
The current study showed that the risk for cardiovascular events during the follow‐up period was significantly higher in group 4 (i.e., the highest quartile) compared with the other subgroups. Although the incidence of cardiovascular events was high in patients with HbA1c ≥7.2%, no significant differences had been noted between the three subgroups with an HbA1c of <7.2%. Previously, the J‐EDIT study 7 reported a J‐shaped relationship between the HbA1c level and the onset of stroke or cardiovascular events in the older adults, with the lowest incidence rates having been observed at HbA1c 7.3–7.9%, whereas the UK General Practice Research Database study 6 found a U‐shaped relationship, with the lowest HRs having been observed at HbA1c 7.5%. Although high blood glucose levels significantly increase the risk of diabetic complications and mortality 2 , strict glucose control has not always been appropriate for the prevention of cardiovascular events, with some reports raising questions regarding the risk–benefit balance of strict glycemic control 21 , 22 . The results of the present study suggest that poor glycemic control was associated with the risk of cardiovascular events, and that the stricter glycemic control was not associated with reduced risk in the older adults with diabetes mellitus.
Among the components of the primary end‐point, the risk of hospitalization for cardiac disease and coronary revascularization was approximately 2.5‐ and 2.6‐fold higher in group 4 than in the lower HbA1c subgroups, respectively. Although the risk of stroke was numerically higher in group 4, no significant difference between the HbA1c subgroups had been observed. Prescription of antihypertensive agents and CKD had been identified as factors affecting the risk of cardiac diseases and coronary artery revascularization, respectively. The aforementioned results are consistent with those presented in previous studies, suggesting that renal function is an independent predictor of the occurrence of myocardial infarction in the older population and that renal impairment further increases cardiovascular risk 23 . The HR for cardiac diseases was significantly low with diabetic neuropathy, although the reason for that was unclear. Given that older patients with neuropathy have impaired pain perception and often have no symptoms of cardiac diseases (e.g., silent myocardial ischemia) 24 , the lack of symptom recognition of cardiac diseases might explain the low HR value. Thus, the present study highlighted that glycemic control, hypertension and CKD were factors that critically affected the risk of cardiovascular diseases in older adults with diabetes mellitus.
Among the included patients, <1% developed severe hypoglycemia. Although the risk was numerically highest in group 4, no significant difference had been observed between the subgroups. The risk of severe hypoglycemia was associated with prescription of insulin, SUs or glinides at the index date. Studies have suggested that severe hypoglycemia might increase the risk of cardiovascular events 6 , 8 . Furthermore, no hypoglycemia was observed in the population with the lowest risk of stroke, whereas the J‐EDIT suggested the possible association between hypoglycemia and stroke 7 . The incidence of stroke was also numerically high in group 4, which had the highest incidence of severe hypoglycemia, although no significant difference in the risk was observed between the subgroups. Therefore, stroke might be associated with the incidence of hypoglycemia, but no definitive conclusions can be drawn from the present study.
In the J‐EDIT study carried out during the early 2000s, the initial screening criteria for HbA1c were ≥7.4% or ≥7.9%, and the median HbA1c level was 7.5% at the landmark time 7 . At that time, it could have been difficult for physicians to provide strict glycemic control to older patients while avoiding hypoglycemia 8 . Alternatively, the median HbA1c level was 6.6% in the present study, which was lower compared with that in the J‐EDIT, probably due to inclusion of patients with low HbA1c levels (HbA1c levels <7.0%) at the first visit. Therefore, the lower HbA1c level of the study compared with the previous study might be attributable to the inclusion of patients with adequate glycemic control, although it is possible that advances in diabetes therapy over the past decade, as well as setting of glycemic control goals for older patients based on the guideline after the J‐EDIT study, have improved the control of HbA1c levels.
No consistent evidence has suggested an association between glycemic control and the risk of fractures 14 , 25 , 26 . Accordingly, the current study showed no relationship between glycemic control levels and fractures. Apart from HbA1c levels, several risk factors have been reported to be associated with fractures, including decreased skeletal muscle mass, decreased visual acuity due to diabetic complications, falls due to neuropathy, bodyweight, family history and smoking 26 , 27 . Given that data regarding such factors were not included in the utilized database, our analysis of fractures could only be exploratory in nature.
The current study had some limitations worth noting. First, the utilized database does not collect information on patients with diabetes mellitus who visit general clinics or non‐Diagnosis Procedure Combination hospitals. As such, it is not possible to capture the outcome of events if patients are transported to a hospital other than the hospital where they visit regularly due to diabetes mellitus. However, given that this database contains data from acute care hospitals, including approximately 20% of major hospitals across Japan, the dataset can be deemed representative of the clinical practice involving older adults. Second, considering that the present study excludes vulnerable older adults with a history of cardiovascular diseases, dementia or cancer and a small proportion of older adults aged ≥75 years, the results cannot be extrapolated to include such patients. Third, there are some potential confounders in the present study. Bodyweight, blood pressure and smoking history are well‐known risk factors for CV events, but these factors are not completely described in the database and, thus, were not analyzed. Lipid parameters (such as low‐density lipoprotein cholesterol) and renal related parameter (such as estimated glomerular filtration rate) are also potential confounders, but these values were only seen in a limited number of patients. For these reasons, we used prescriptions or diagnosis records as alternatives to these parameters for risk assessment. Finally, the follow‐up period was shorter than that of previous studies. The results of the study warrant further investigation with a longer follow‐up period.
In conclusion, the current study showed that the risk of cardiovascular events is associated with poor glycemic control among older adults with diabetes mellitus. However, strict control in patients with HbA1c ≤7.2% is not associated with a reduced risk of cardiovascular diseases.
DISCLOSURE
KY has received consulting fees and/or speakers' bureau fees from Astellas Amgen BioPharma K.K., Astellas Pharma Inc., AstraZeneca K.K., Eli Lilly Japan K.K., Daiichi Sankyo Co., Ltd., Janssen Pharmaceutical K.K., Kowa Soyaku Co., Ltd., Kyowa Hakko Kirin Co., Ltd., MSD K.K., Mitsubishi Tanabe Pharma Corporation, Nippon Boehringer Ingelheim Co., Ltd., Novo Nordisk Pharma Ltd., Novartis Pharma K.K., Ono Pharmaceutical Co., Ltd., Pfizer Japan Inc., Sanofi K.K., Sumitomo Dainippon Pharma Co., Ltd., Taisho Toyama Pharmaceutical Co., Ltd. and Takeda Pharmaceutical Co., Ltd.; received research support from Taisho Pharmaceutical Co., Ltd. and Taisho Toyama Pharmaceutical Co., Ltd.; received scholarship grants from Astellas Pharma Inc., Bayer Yakuhin, Ltd., Eli Lilly Japan K.K., Daiichi Sankyo Co., Ltd., Kao Corporation, Kowa Soyaku Co., Ltd., Mitsubishi Tanabe Pharma Corporation, MSD K.K., Nippon Boehringer Ingelheim Co., Ltd., Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co., Ltd., Pfizer Japan Inc., Shionogi Pharma Co. Ltd., Sumitomo Dainippon Pharma Co., Ltd., Taisho Toyama Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd. and Teijin Pharma Limited; and belongs to endowed departments courses endowed by MSD K.K. RS has received consulting fees and/or speakers' bureau fees from Eli Lilly Japan K.K., MSD K.K., Novartis Pharma K.K., Ono Pharmaceutical Co., Ltd., Sanofi K.K., Sumitomo Dainippon Pharma Co., Ltd., Takeda Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation and Novo Nordisk Pharma Ltd.; received research support from MSD K.K.; and received scholarship grants from Daiichi Sankyo Co., Ltd. MG, HI, MI and AY are employees of Mitsubishi Tanabe Pharma Corporation, Japan.
Supporting information
Figure S1 | Time course of glycated hemoglobin in older patients with diabetes mellitus.
Figure S2 | Kaplan–Meier curves for the incidence of cardiovascular events.
Table S1 | Disease codes, medical procedures and medication prescriptions.
Table S2 | Disease codes for the exclusion criteria (malignant neoplasms and dementia).
Table S3 | Incidence rate of all‐cause death in older patients with diabetes mellitus.
Table S4 | Number of patients with fracture by site.
ACKNOWLEDGMENTS
The authors express their gratitude to the following co‐workers: Hiroaki Fujii (Mitsubishi Tanabe Pharma Corporation) for analytical support, Kosuke Iwasaki (Milliman Inc.) for support in preparing the study protocol and Akira Saito PhD (International Medical Translation Service, Inc.) for providing medical writing support funded by Mitsubishi Tanabe Pharma Corporation. This study was funded by Mitsubishi Tanabe Pharma Corporation. The study protocol was reviewed and approved by the Mitsubishi Tanabe Pharma Corporation Ethics Committee on 29 October 2019 (Review No.: KENRIN H‐19‐025).
J Diabetes Investig 2021; 11: 2036–2045
[Correction added on 11 June 2021, after first online publication: ORCIDs have been added for Koutaro Yokote and Hiroaki Iijima; Hiroaki Iijima's name has been corrected.]
REFERENCES
- 1. Charvat H, Goto A, Goto M, et al. Impact of population aging on trends in diabetes prevalence: a meta‐regression analysis of 160,000 Japanese adults. J Diabetes Investig 2015; 6: 533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care 2012; 35: 2650–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Halter JB, Musi N, McFarland Horne F, et al. Diabetes and cardiovascular disease in older adults: current status and future directions. Diabetes 2014; 63: 2578–2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang ES, Laiteerapong N, Liu JY, et al. Rates of complications and mortality in older patients with diabetes mellitus: the diabetes and aging study. JAMA Intern Med 2014; 174: 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lind M, Olsson M, Rosengren A, et al. The relationship between glycaemic control and heart failure in 83,021 patients with type 2 diabetes. Diabetologia 2012; 55: 2946–2953. [DOI] [PubMed] [Google Scholar]
- 6. Currie CJ, Peters JR, Tynan A, et al. Survival as a function of HbA(1c) in people with type 2 diabetes: a retrospective cohort study. Lancet 2010; 375: 481–489. [DOI] [PubMed] [Google Scholar]
- 7. Araki A, Iimuro S, Sakurai T, et al. Non‐high‐density lipoprotein cholesterol: an important predictor of stroke and diabetes‐related mortality in Japanese elderly diabetic patients. Geriatr Gerontol Int 2012; 12(Suppl 1): 18–28. [DOI] [PubMed] [Google Scholar]
- 8. Araki A, Iimuro S, Sakurai T, et al. Long‐term multiple risk factor interventions in Japanese elderly diabetic patients: the Japanese Elderly Diabetes Intervention Trial–study design, baseline characteristics and effects of intervention. Geriatr Gerontol Int 2012; 12(Suppl 1): 7–17. [DOI] [PubMed] [Google Scholar]
- 9. Haneda M, Noda M, Origasa H, et al. Japanese clinical practice guideline for diabetes 2016. Diabetol Int 2018; 9: 1–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Survey of Japanese databases in Japan available for clinical/pharmacoepidemiology. Available from: http://www.jspe.jp/mt‐static/FileUpload/files/JSPE_DB_TF_J.pdf Accessed February 22, 2021.
- 11. Geller AI, Shehab N, Lovegrove MC, et al. National estimates of insulin‐related hypoglycemia and errors leading to emergency department visits and hospitalizations. JAMA Intern Med 2014; 174: 678–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maggi S, Noale M, Pilotto A, et al. The METABOLIC Study: multidimensional assessment of health and functional status in older patients with type 2 diabetes taking oral antidiabetic treatment. Diabetes Metab 2013; 39: 236–243. [DOI] [PubMed] [Google Scholar]
- 13. Bremer JP, Jauch‐Chara K, Hallschmid M, et al. Hypoglycemia unawareness in older compared with middle‐aged patients with type 2 diabetes. Diabetes Care 2009; 32: 1513–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kachroo S, Kawabata H, Colilla S, et al. Association between hypoglycemia and fall‐related events in type 2 diabetes mellitus: analysis of a U.S. commercial database. J Manag Care Spec Pharm 2015; 21: 243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Laiteerapong N, Karter AJ, Liu JY, et al. Correlates of quality of life in older adults with diabetes: the diabetes & aging study. Diabetes Care 2011; 34: 1749–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vergara I, Vrotsou K, Orive M, et al. Wrist fractures and their impact in daily living functionality on elderly people: a prospective cohort study. BMC Geriatr 2016; 16: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kosiborod M, Lam CSP, Kohsaka S, et al. Cardiovascular events associated with SGLT‐2 inhibitors versus other glucose‐lowering drugs: the CVD‐REAL 2 study. J Am Coll Cardiol 2018; 71: 2628–2639. [DOI] [PubMed] [Google Scholar]
- 18. Fujihara K, Igarashi R, Yamamoto M, et al. Impact of glucose tolerance status on the development of coronary artery disease among working‐age men. Diabetes Metab 2017; 43: 261–264. [DOI] [PubMed] [Google Scholar]
- 19. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care 2005; 43: 1130–1139. [DOI] [PubMed] [Google Scholar]
- 20. Frenkel WJ, Jongerius EJ, Mandjes‐van Uitert MJ, et al. Validation of the Charlson Comorbidity Index in acutely hospitalized elderly adults: a prospective cohort study. J Am Geriatr Soc 2014; 62: 342–346. [DOI] [PubMed] [Google Scholar]
- 21. Huang ES, Liu JY, Moffet HH, et al. Glycemic control, complications, and death in older diabetic patients: the diabetes and aging study. Diabetes Care 2011; 34: 1329–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358: 2560–2572. [DOI] [PubMed] [Google Scholar]
- 23. Brugts JJ, Knetsch AM, Mattace‐Raso FUS, et al. Renal function and risk of myocardial infarction in an elderly population: the Rotterdam Study. Arch Intern Med 2005; 165: 2659–2665. [DOI] [PubMed] [Google Scholar]
- 24. Inoguchi T, Yamashita T, Umeda F, et al. High incidence of silent myocardial ischemia in elderly patients with non insulin‐dependent diabetes mellitus. Diabetes Res Clin Pract 2000; 47: 37–44. [DOI] [PubMed] [Google Scholar]
- 25. Puar TH, Khoo JJ, Cho LW, et al. Association between glycemic control and hip fracture. J Am Geriatr Soc 2012; 60: 1493–1497. [DOI] [PubMed] [Google Scholar]
- 26. Yau RK, Strotmeyer ES, Resnick HE, et al. Diabetes and risk of hospitalized fall injury among older adults. Diabetes Care 2013; 36: 3985–3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rubin KH, Friis‐Holmberg T, Hermann AP, et al. Risk assessment tools to identify women with increased risk of osteoporotic fracture: complexity or simplicity? A systematic review. J Bone Miner Res 2013; 28: 1701–1717. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 | Time course of glycated hemoglobin in older patients with diabetes mellitus.
Figure S2 | Kaplan–Meier curves for the incidence of cardiovascular events.
Table S1 | Disease codes, medical procedures and medication prescriptions.
Table S2 | Disease codes for the exclusion criteria (malignant neoplasms and dementia).
Table S3 | Incidence rate of all‐cause death in older patients with diabetes mellitus.
Table S4 | Number of patients with fracture by site.


