Abstract
Background
A substantial proportion of patients infected with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) develop severe/critical coronavirus disease 2019 (COVID‐19) characterized by acute respiratory distress syndrome (ARDS) with thrombosis.
Objectives
We tested the hypothesis that SARS‐CoV‐2‐‐induced upregulation of tissue factor (TF) expression may be responsible for thrombus formation in COVID‐19.
Methods
We compared autopsy lung tissues from 11 patients with COVID‐19‐‐associated ARDS with samples from 6 patients with ARDS from other causes (non‐COVID‐19 ARDS) and 11 normal control lungs.
Results
Dual RNA in situ hybridization for SARS‐CoV‐2 and TF identified sporadic clustered SARS‐CoV‐2 with prominent co‐localization of SARS‐CoV‐2 and TF RNA. TF expression was 2‐fold higher in COVID‐19 than in non‐COVID‐19 ARDS lungs (P = .017) and correlated with the intensity of SARS‐CoV‐2 staining (R2 = .36, P = .04). By immunofluorescence, TF protein expression was 2.1‐fold higher in COVID‐19 versus non‐COVID‐19 ARDS lungs (P = .0048) and 11‐fold (P < .001) higher than control lungs. Fibrin thrombi and thrombi positive for platelet factor 4 (PF4) were found in close proximity to regions expressing TF in COVID‐19 ARDS lung, and correlated with TF expression (fibrin, R2 = .52, P < .001; PF4, R2 = .59, P < .001).
Conclusions
These data suggest that upregulation of TF expression is associated with thrombus formation in COVID‐19 lungs and could be a key therapeutic target. Correlation of TF expression with SARS‐CoV‐2 in lungs of COVID‐19 patients also raises the possibility of direct TF induction by the virus.
Keywords: COVID‐19, fibrin, SARS‐CoV‐2, thrombosis, tissue factor
Essentials
-
•
The process by which severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection promotes coronavirus disease 19 (COVID‐19)‐‐related thrombosis is unknown.
-
•
We tested the hypothesis that SARS‐CoV‐2 induces upregulation of tissue factor, comparing lung tissues from patients with COVID‐19 acute respiratory distress syndrome (ARDS) with non‐COVID‐19 ARDS and normal controls.
-
•
SARS‐CoV‐2 was associated with upregulation of tissue factor expression in lungs of COVID‐19 patients.
-
•
Tissue factor‐‐mediated extrinsic coagulation pathway activation may be responsible for thrombi formation in COVID‐19
Alt-text: Unlabelled Box
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19), caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2),1 has initiated a worldwide pandemic. In severe cases, dysfunction of the lungs and other organs has been linked to systemic microvascular injury and thrombosis. These lesions occur in the context of a hypercoagulable state, with increased plasma fibrinogen and D‐dimer levels accompanied by persistent activation of the complement cascade and pro‐inflammatory cytokine release.2., 3., 4., 5., 6. Tissue factor (TF) may have a key role in this pro‐thrombotic state.7., 8. TF expression is usually negligible on the luminal surfaces of healthy, non‐inflamed endothelial cells (EC) and circulating blood cells,7 but can be induced by inflammatory cytokines; trauma; and many pathogens,9 including viruses.10 TF binds to factor VII, initiating the extrinsic coagulation cascade that generates factor Xa and thrombin.8 Thrombin activates platelets and converts fibrinogen to fibrin, required for blood clotting.11 It was recently reported that severe COVID‐19 with acute respiratory distress syndrome (ARDS) is associated with elevated TF activity in circulating extracellular vesicles.12 This is consistent with the observation that influenza A infection in mice is associated with induction of TF in the lung and activation of coagulation, and that TF expression in lung epithelial cells is essential to this process.13., 14. SARS‐CoV‐2 is known to directly infect endothelial cells, at least in engineered human cells.15 This is controversial, however, as EC usually express low levels of the primary SARS‐CoV‐2 receptor ACE2 and cofactor TMPRSS2, and primary human EC appear to be resistant to infection in vitro (Ahmetaj‐Shala B, et al. bioRxiv preprint doi.org/10.1101/2020.11.08.372581).
Several recent reviews have suggested a possible role for TF in the thrombosis of severe COVID‐19.7., 16., 17. To examine associations among SARS‐CoV‐2, TF, and pathologic thrombi in COVID‐19, we examined autopsy lung specimens from patients with critical COVID‐19 compared to ARDS from other causes and normal lungs.
2. METHODS
2.1. COVID‐19 and control cases
Eleven pulmonary autopsy specimens were obtained from COVID‐19 patients, 10 from Weill Cornell Medical Center in New York City and one previously reported case18 from Oklahoma (Table 1 ). Six non‐‐COVID‐19 ARDS samples from the Weill Cornell archive tissue bank were also examined. These samples represented the first consecutive cases collected by the Translational Research Program in Pathology and Pulmonary Critical Care Biobanks from which adequate numbers of unstained, paraffin‐embedded tissue slides were available. All COVID‐19 and non‐COVID‐19 ARDS patients were admitted to intensive care. Five of the 11 COVID‐19 patients were intubated for 5 to 29 days and four of the five non‐COVID‐19 patients were intubated for 2 to 25 days (Table 1). The non‐COVID‐19 ARDS cases were linked to aspiration pneumonia in one and bacterial sepsis in four, including one with a concomitant influenza infection (Table 1).
TABLE 1.
Demographics and clinical characteristics of patients with COVID‐19 ARDS and non‐COVID‐19 ARDS
| Patient code | Respiratory infection | Age | Sex | Comorbidities | Intubated (days) |
|---|---|---|---|---|---|
| ARDS‐201 | Aspiration pneumonia | 74 | F | CAD, DM2, HTN, breast cancer, dementia | 25 |
| ARDS‐3 | Bacterial pneumonia | 77 | M | CAD, DM1, PE, dementia | None |
| ARDS‐4 | Bacterial pneumonia | 39 | F | Sjogren’s syndrome | 3 |
| ARDS‐5 | Bacterial pneumonia | 34 | M | CAD, gastric cancer | 15 |
| ARDS‐203 | Bacterial pneumonia, Influenza | 47 | F | DM2, HTN, hypothyroidism | 4 |
| COVID‐10 | SARS‐CoV‐2 pneumonia |
56 | F | DM2, HTN, sickle cell anemia |
None |
| COVID‐11 | SARS‐CoV_2 pneumonia |
75 | M | HTN, lung cancer | 25 |
| COVID‐14 | SARS‐CoV‐2 pneumonia |
70 | M | Arteritis | None |
| COVID‐19 | SARS‐CoV‐2 pneumonia |
70 | M | Unknown | None |
| COVID‐20 | SARS‐CoV‐2 pneumonia |
30 | F | Diabetes, pulmonary valve atresia |
12 |
| COVID‐30 | SARS‐CoV‐2 pneumonia | 52 | M | CAD, HDK, DM2 | 29 |
| COVID‐7 | SARS‐CoV‐2 pneumonia | 69 | M | DM2, AML | None |
| COVID‐2 | SARS‐CoV‐2 pneumonia | 73 | M | Pre‐diabetes, COPD | 5 |
| COVID‐3 | SARS‐CoV‐2 pneumonia | 71 | M | Diabetes, HTN | None |
| COVID‐8 | SARS‐CoV‐2 pneumonia | 46 | M | DM2, HTN, asthma | 7 |
| COVID‐OK | SARS‐CoV‐2 Pneumonia, cardiac arrest |
77 | M | HTN, splenectomy | None |
Abbreviations: AML, acute myelogenous leukemia; ARDS, acute respiratory distress syndrome; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; COVID, coronavirus disease; DM1, type 1 diabetes mellitus; DM2, type 2 diabetes mellitus; HLD, hyperlipidemia; HTN, hypertension; PE, pulmonary embolism; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
Five normal lung samples were obtained as controls from individuals on mechanical ventilation collected before the COVID‐19 pandemic and designated as lung donors for allogeneic transplantation. Additional normal pulmonary tissue was derived from lobectomies of six lung cancer patients, obtained from the University of Oklahoma Health Sciences Center Pathology tissue repository. Fixed autopsy tissues were paraffin‐embedded and sectioned at 5 to 6 μm and stored at 4°C until studied.
2.2. RNA in situ hybridization with RNAscope technology
RNA in situ hybridization was performed with RNAscope technology on a Leica BondRX platform using RNAscope LS Multiplex Fluorescent Reagents (ACD, Cat#322800) following the manufacturer’s guidelines. Paraffin‐embedded lung tissue blocks were sectioned on slides and transferred to the Leica platform. Hybridization was performed by incubating the slides with target RNAscope probes (Table S1 in supporting information) following the manufacturer’s protocol. To detect positive‐sense SARS‐CoV‐2, the target probe was 2.5 LS Probe V‐nCoV2019‐S (ACD Cat#848568). To detect positive‐sense TF, the target probe was 2.5 LS Hs‐F3‐C2 Probe (Cat#407618‐C2). Fluorescence images were obtained with a Zeiss 710 or Nikon Eclipse 80i confocal microscopes and fluorescence image intensities were quantified by ImageJ software.
2.3. Histology, immunofluorescence staining, and image quantification
All lung tissues were fixed with formalin and embedded in paraffin. 5µ sections were stained with hematoxylin–eosin (H&E) for histology. For immunofluorescence and histochemical staining, deparaffinized and rehydrated slides underwent heat‐induced (97°C) epitope retrieval using Tris‐ethylenediaminetetraacetic acid buffer, pH 9.0 for 10 min. Slides were incubated overnight at 4°C with blocking buffer (1% bovine serum albumin + 0.3 M glycine in Tris‐NaCl‐Tween buffer) and then incubated again overnight at 4°C with primary antibodies (Table S1) at 1:250 dilution. Excess primary antibodies were washed in buffer (Tris‐NaCl‐Tween, pH 7.5) and samples were incubated with the respective species‐matched secondary antibodies tagged with Alexa Fluor 488 (green channel), Alexa Fluor 594 (red channel), or Alexa Flour 647 and Cy5 (far red channel) and incubated at room temperature for 2 h. Sections were washed and mounted in medium containing DAPI to stain the nuclei. Fluorescence images were obtained with a Zeiss 710 confocal or Nikon Eclipse 80i fluorescence microscope.
For quantification, individual pseudo‐color‐stained areas of the lung tissues at high resolution with 20x magnification were scanned/tiled using the Zen Blue program of a Zeiss 710 microscope. Because areas of immunostaining were heterogeneous, scanned/tiled images of whole lung sections were used to quantify staining intensity. Positively stained areas and total areas in immunofluorescence images were analyzed and quantified using ImageJ software (NIH) with a background setting threshold. The percentage of positives was calculated by dividing the immunostained areas by the total area, then multiplying by 100. The specificity of TF staining was confirmed by comparing adjacent lung sections from in situ hybridization and immunofluorescence staining (Figure S1 in supporting information). The analysis was done in a blinded fashion and each slide was labeled with a code; after staining, the code was broken.
2.4. Statistics
All data are expressed as means, with error bars representing standard deviation (SD). Pearson linear regression is used to test the correlation between TF and SARS‐CoV‐2 RNA, or fibrin, or platelet factor 4 (PF4). Absolute numeric values were used in correlation with a simple linear regression analysis. P‐value <.05 is considered statistically significant. Statistical analyses were performed using GraphPad Prism software.
3. RESULTS AND DISCUSSION
3.1. SARS‐CoV‐2 RNA level correlates with TF expression in COVID‐19 ARDS lungs
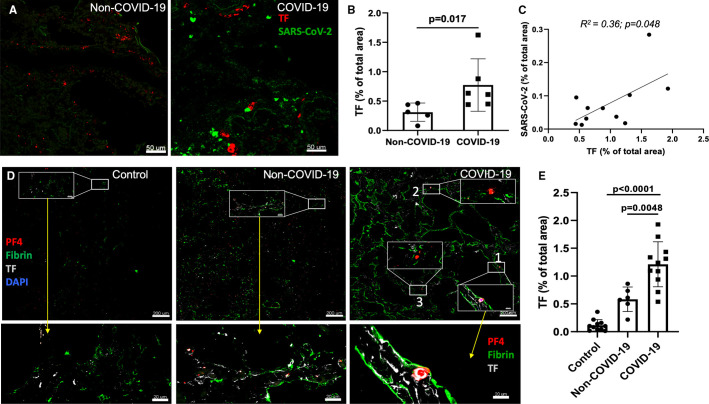
Dual RNA in situ hybridization with SARS‐CoV‐2 and TF RNA fluorescence probes in six COVID‐19 samples and immunofluorescence staining with an antibody specific for N‐protein of the virus in five COVID‐19 specimens showed viral and TF RNA and protein expression in COVID‐19 lungs. High‐resolution confocal immunofluorescence images of RNAscope and immunostaining slides identified both sporadic and clustered SARS‐CoV‐2, and about 20% TF expression co‐localized with the virus clusters (Figures 1A , and S1A,B). As measured by in situ hybridization using RNAscope imaging, TF expression was 2‐fold higher in COVID‐19 than in non‐COVID‐19 ARDS lungs (0.77% ± 0.44% vs. 0.31% ± 0.12%, P = .017; Figure 1B). TF expression correlated with SARS‐CoV‐2 in COVID‐19 lungs as measured by RNAscope (n = 6) and immunostaining (n = 5; R 2 = .36, P = .048; Figure 1C).
FIGURE 1.
In situ hybridization detecting severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) viral RNA and tissue factor (TF) RNA. A, Dual RNA in situ hybridization of SARS‐CoV‐2 (green color dots) and TF (red color dots) in coronavirus disease 19 (COVID‐19)‐acute respiratory distress syndrome (ARDS) and non‐‐COVID‐19 ARDS cases. B, Quantification of TF RNA expression from RNAscope (n = 6) using ImageJ program. C, Correlation of TF expression with SARS‐CoV‐2 expression (combined RNAscope [n = 6] and immunofluorescence ([n = 5]; R2 = .66; P < .01). Staining of TF mRNA and immunofluorescence produced similar results, so the combined TF mRNA expression from five COVID‐19 patients and TF staining from six COVID‐19 patients were shown. D, Immunofluorescence assessment of lungs tissues from COVID‐19‐ARDS cases identifies higher TF expression associated with fibrin‐ and platelet factor 4 (PF4)‐positive thrombi. Immunostaining for TF (white), fibrin (green), and PF4 (red) of COVID‐19‐ARDS and non‐COVID‐19 ARDS as well as pathologically normal control lung tissues. Inset with white box and bottom panels showing magnified vessels/area with fibrin‐ and/or platelet‐rich thrombi. E, Quantification of TF expression
3.2. TF expression is associated with fibrin‐ and PF4‐rich thrombi in COVID‐19 versus non‐COVID‐19 ARDS lungs and normal controls
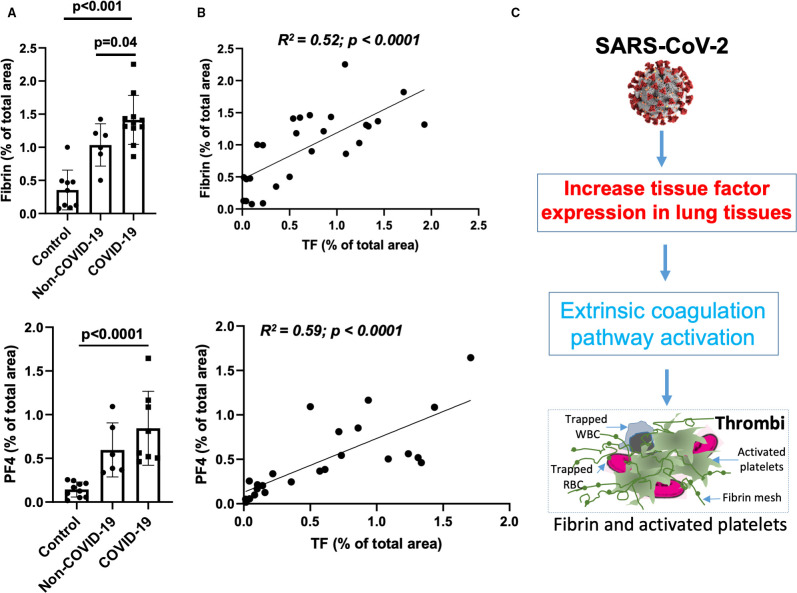
In COVID‐19 ARDS samples, multi‐color immunofluorescence staining demonstrated TF protein expression throughout lung tissue samples on both luminal and bronchoalveolar sides, including areas with many blood vessels that were filled with large‐ and micro‐thrombi positive for fibrin or PF4 or both. This was prominent compared to non‐COVID‐19 ARDS and normal control lung tissues (Figures 1D and S2 in supporting information). Quantification of immunofluorescent intensity of TF protein expression in the lungs of COVID‐19 cases were 2.1‐fold higher than non‐COVID‐19 ARDS lung tissues (1.21% ± 0.36% vs. 0.55% ± 0.23%, P = .0048), and 11‐fold higher vs. pathologically normal control lung tissues (0.11% ± 0.13%, P < .001; Figure 1E). Using multi‐color immunofluorescence staining, fibrin‐positive areas were more frequent and 1.5‐fold higher in COVID‐19 lungs than in non‐COVID‐19 ARDS lungs (1.4% ± 0.37% vs. 1.06% ± 0.34% of total area of images, P = .047) and 3‐fold higher than in normal controls (0.36 ± 0.29, P < .001; Figure 2A ). PF4‐positive areas were also prominent in COVID‐19 lung samples versus both non‐COVID‐19 ARDS and normal controls (0.85% ± 0.42% in COVID‐19 and 0.6% ± 0.34% in non‐COVID‐19 ARDS [P = .07] and 0.14% ± 0.1% in normal lung [P = .001]; Figure 2A). Both fibrin‐ and PF4‐positive areas (absolute values in arbitrary units) strongly correlated with absolute TF expression quantification values ([R 2 = .52, P < .0001, for correlation between fibrin and TF] and R 2 = .59, P < .0001 for correlation between PF4 and TF; Figure 2B).
FIGURE 2.
Tissue factor (TF) expression correlates with fibrin and platelet factor 4 (PF4) expression. A, Quantification of fibrin‐ and PF4‐positive thrombi images from confocal immunofluorescent in coronavirus disease 19 (COVID‐19)‐acute respiratory distress syndrome (ARDS; n = 11) and non‐‐COVID‐19 ARDS (n = 6) and pathologically normal control (n = 11) lungs tissues using Imaris and ImageJ software. B, Linear correlations of TF with fibrin (R2 = .52; P < .002) and TF with PF4 (R2 = .63; P = .001). C, Schematic diagram depicts potential mechanism by which severe acute respiratory syndrome coronavirus 2 infection in the lungs can increase TF expression, which might cause thrombi formation, by initiating fibrin formation and platelet activation
Both fibrin‐ and PF4‐positive thrombi were found in close proximity to TF‐expressing areas (Figure 1D and S2). In high‐magnification images of COVID‐19 lungs, we also observed a few cells with nuclei (DAPI‐positive) inside thrombi that were positive for TF, which were leukocytes trapped inside the thrombi (not shown). Because lung epithelial cells can express high levels of TF, and EC can express TF under disease conditions, we identified epithelial cells, stained with E‐cadherin, and EC, stained with von Willebrand factor (vWF). We found TF expression by alveolar epithelial cells in both control and COVID‐19 lungs, and an increase of TF expression in EC of COVID‐19 lungs compared to control lungs (Figures 1D, S2 and S3 in supporting information).
3.3. Pulmonary histopathology of COVID‐19 and non‐‐COVID‐19 ARDS patients, including characterization of CD61+ platelet thrombi
Histological findings showed characteristics of ARDS in both COVID‐19 and non‐‐COVID‐19 ARDS lung specimens. Histological changes included hyaline deposition and inflammatory cells, diffuse alveolar damage, and multifocal acute bronchopneumonia. These findings were indistinguishable pathologically between those with or without COVID‐19, and with or without ventilator exposure (Figure S4 in supporting information). Immunohistochemistry staining showed that CD61+ areas, presumably platelet‐filled (CD61+ platelet thrombi) blood vessels were higher in COVID‐19 versus non‐‐COVID‐19 ARDS samples (Figure S4).
In summary, significantly higher TF expression in COVID‐19 versus non‐‐COVID‐19 ARDS lungs was confirmed by two methods: a very sensitive in situ hybridization using RNAscope and immunostaining of TF protein with a specific antibody using confocal imaging. The juxtaposition of TF and SARS‐CoV‐2 expression by both methods suggests the possibility that de novo gene transcription and protein synthesis of TF is induced by SARS‐CoV‐2, although changes in mRNA stability cannot be excluded. It is plausible that TF has a key role in the acute lung failure of COVID‐19 by triggering extrinsic coagulation cascade‐induced thrombosis (Figure 2C). Higher levels of fibrin and activated platelets in PF4‐positive thrombi correlated to high TF expression in COVID‐19 lungs, indicating that TF may have initiated an extrinsic coagulation pathway. Both arterial and venous thrombi, as well as small vessel microangiopathy, have been observed in COVID‐19 lungs,3., 19. consistent with these findings.
COVID‐19 ARDS is known to differ clinically from non‐‐COVID‐19, primarily bacteria‐driven ARDS, being associated with a higher fatality rate (70–80% vs. 40% for non‐‐COVID‐19 ARDS) and a greater rate of clinically important thromboses in the lungs and other organs.20., 21. Our finding of elevated TF expression in non‐‐COVID‐19 ARDS lungs versus normal control lung also suggests a role for TF‐mediated thrombosis in ARDS apart from SARS‐CoV‐2 infection. The difference in severity of thrombosis in COVID‐19 ARDS versus non‐‐COVID‐19 ARDS may include not only the increase in TF expression documented here, but additional factors, including complement activation,3 or more prolonged exposure to pro‐inflammatory cytokines such as IL6.22 Dysregulation of interferon‐I responses23., 24. also contribute to the pathology.
Previous studies have connected aggregates of platelets and monocytes that express TF25 with fibrinogen, D‐dimer, and neutrophil extracellular traps (NETs) as likely contributing to thrombus formation by interaction with platelets26., 27. in COVID‐19. This suggests that monocytes and neutrophils, key components of the innate immune response to virus, might contribute to systemic thrombosis. A recent study showed that SARS‐CoV‐2 pseudovirus infection in monocyte‐derived cells increased TF activity,28 and another study found both TF and PAI‐1 activity higher in COVID‐19 patients than sepsis patients.29 Thus, TF activity and inhibition of fibrinolytic activity could contribute to COVID‐19‐‐related thrombosis.
A major limitation of our study is that it involved fixed autopsy samples from a relatively small number of heterogenous cases. This issue was mitigated by quantifying a large area of lung tissue in both COVID‐19 and non‐‐COVID‐19 samples without selection bias for only the thrombotic areas side by side using the same antibodies, RNA‐probes, and conditions, and the same confocal imaging. Additional studies are required to identify the mechanism by which SARS‐CoV‐2 is associated with TF induction, to determine whether this relates to a systemic cytokine surge and/or local TF expression by virus, and how it contributes to lung thrombi generation and ARDS pathophysiology.
In conclusion, our data showing increased TF expression in the lung tissues of COVID‐19 cases associated with fibrin‐ and PF4‐positive activated platelet‐rich thrombi indicate that the TF‐initiated coagulation pathway might be involved in the coagulopathy of progressive COVID‐19. Thus, inhibition of the TF‐mediated extrinsic pathway, or elements downstream of both the extrinsic or intrinsic pathway, such as inhibitors of factor Xa or thrombin, might improve thrombotic control in COVID‐19.
CONFLICTS OF INTEREST
The authors report no conflicts of interest.
AUTHOR CONTRIBUTIONS
SS performed the majority of the experiments, including histology and immunostaining, and also analyzed, quantified, and plotted the data. AB and SS collected the COVID‐19 autopsy samples, processed the tissue histology, and performed CD61 staining and analysis. KMF provided consultation and six control lung biopsy specimens, and assisted with RNAscope. JL and JTM provided consultation on data interpretation and edited the manuscript. JA conceived the idea, analyzed data interpretation, supervised the project, and wrote the manuscript.
ACKNOWLEDGMENTS
The work of the authors herein is supported by NHLBI grant # HL148123, HL123605 (JA) and grants GM114731; GM103639; CA225520, OCASCR, PHF, which supported various core facilities. The authors thank Drs. M. Jayaraman and S. Aravindan for performing RNAscope experiments in the pathology core at OUHSC; B. Flower and J. Crane for image analysis and quantification. The authors also thank M.K. Occhipinti and the Life Science Editors for editing the manuscript. We thank the Office of the Chief Medical Examiner, Oklahoma City, for their generous gift of one COVID‐19 autopsy lung tissue.
National Institute of General Medical SciencesGM103639GM114731
National Heart, Lung, and Blood InstituteHL123605HL148123
Footnotes
Manuscript handled by: Marcel Levi
Supporting Information
Supplementary Material
REFERENCES
- 1.Zhu N.A., Zhang D., Wang W., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Connors J.M., Levy J.H. COVID‐19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magro C., Mulvey J.J., Berlin D., et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID‐19 infection: a report of five cases. Transl Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klok F.A., Kruip M., van der Meer N.J.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levi M., Thachil J., Iba T., Levy J.H. Coagulation abnormalities and thrombosis in patients with COVID‐19. Lancet Haematol. 2020;7 doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DiNicolantonio J.J., McCarty M. Thrombotic complications of COVID‐19 may reflect an upregulation of endothelial tissue factor expression that is contingent on activation of endosomal NADPH oxidase. Open. Heart. 2020;7 doi: 10.1136/openhrt-2020-001337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grover S.P., Mackman N. Tissue factor: an essential mediator of hemostasis and trigger of thrombosis. Arterioscler Thromb Vasc Biol. 2018;38:709–725. doi: 10.1161/ATVBAHA.117.309846. [DOI] [PubMed] [Google Scholar]
- 9.Ahamed J., Niessen F., Kurokawa T., et al. Regulation of macrophage procoagulant responses by the tissue factor cytoplasmic domain in endotoxemia. Blood. 2007;109:5251–5259. doi: 10.1182/blood-2006-10-051334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Subramaniam S., Scharrer I. Procoagulant activity during viral infections. Front Biosci (Landmark Ed) 2018;23:1060–1081. doi: 10.2741/4633. [DOI] [PubMed] [Google Scholar]
- 11.Mackman N. The many faces of tissue factor. J Thromb Haemost. 2009;7(Suppl 1):136–139. doi: 10.1111/j.1538-7836.2009.03368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosell A., Havervall S., von Meijenfeldt F., et al. Patients with COVID‐19 have elevated levels of circulating extracellular vesicle tissue factor activity that is associated with severity and mortality. Arterioscler Thromb Vasc Biol. 2020;41(2):878–882. doi: 10.1161/ATVBAHA.120.315547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antoniak S., Tatsumi K., Hisada Y., et al. Tissue factor deficiency increases alveolar hemorrhage and death in influenza A virus‐infected mice. J Thromb Haemost. 2016;14:1238–1248. doi: 10.1111/jth.13307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mackman N. Regulation of the tissue factor gene. FASEB J. 1995;9:883–889. doi: 10.1096/fasebj.9.10.7615158. [DOI] [PubMed] [Google Scholar]
- 15.Monteil V., Kwon H., Prado P., et al. Inhibition of SARS‐CoV‐2 infections in engineered human tissues using clinical‐grade soluble human ACE2. Cell. 2020;181:905–913.e7. doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bautista‐Vargas M., Bonilla‐Abadia F., Canas C.A. Potential role for tissue factor in the pathogenesis of hypercoagulability associated with in COVID‐19. J Thromb Thrombolysis. 2020;50:479–483. doi: 10.1007/s11239-020-02172-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mackman N., Antoniak S., Wolberg A.S., Kasthuri R., Key N.S. Coagulation abnormalities and thrombosis in patients infected with SARS‐CoV‐2 and other pandemic viruses. Arterioscler Thromb Vasc Biol. 2020;40:2033–2044. doi: 10.1161/ATVBAHA.120.314514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barton L.M., Duval E.J., Stroberg E., Ghosh S., Mukhopadhyay S. COVID‐19 Autopsies, Oklahoma, USA. Am J Clin Pathol. 2020;153:725–733. doi: 10.1093/ajcp/aqaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ackermann M., Verleden S.E., Kuehnel M., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in covid‐19. N Engl J Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bussani R., Schneider E., Zentilin L., et al. Persistence of viral RNA, pneumocyte syncytia and thrombosis are hallmarks of advanced COVID‐19 pathology. EBioMedicine. 2020;61 doi: 10.1016/j.ebiom.2020.103104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Helms J., Tacquard C., Severac F., et al. High risk of thrombosis in patients with severe SARS‐CoV‐2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bester J., Pretorius E. Effects of IL‐1beta, IL‐6 and IL‐8 on erythrocytes, platelets and clot viscoelasticity. Sci Rep. 2016;6:32188. doi: 10.1038/srep32188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bastard P., Rosen L.B., Zhang Q., et al. Autoantibodies against type I IFNs in patients with life‐threatening COVID‐19. Science. 2020;370(6515) doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang X., Cheng X., Tang Y., et al. The role of type 1 interferons in coagulation induced by gram‐negative bacteria. Blood. 2020;135:1087–1100. doi: 10.1182/blood.2019002282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hottz E.D., Azevedo‐Quintanilha I.G., Palhinha L., et al. Platelet activation and platelet‐monocyte aggregates formation trigger tissue factor expression in severe COVID‐19 patients. Blood. 2020;136(11):1330–1341. doi: 10.1182/blood.2020007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barnes B.J., Adrover J.M., Baxter‐Stoltzfus A., et al. Targeting potential drivers of COVID‐19: Neutrophil extracellular traps. J Exp Med. 2020;217 doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Middleton E.A., He X.Y., Denorme F., et al. Neutrophil extracellular traps (NETs) contribute to immunothrombosis in COVID‐19 acute respiratory distress syndrome. Blood. 2020;136(10):1169–1179. doi: 10.1182/blood.2020007008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang J., Pendurthi U.R., Yi G., Rao L.V.M. SARS‐CoV‐2 infection induces the activation of tissue factor‐mediated coagulation by activation of acid sphingomyelinase. Blood. 2021 doi: 10.1182/blood.2021010685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campbell R.A., Hisada Y., Denorme F., et al. Comparison of the coagulopathies associated with COVID‐19 and sepsis. Res Pract Thromb Haemost. 2021;5 doi: 10.1002/rth2.12525. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material