Supplemental Digital Content is available in the text.
Keywords: age-specific regimen, dexmedetomidine, intensive care, mechanical ventilation, pediatric patients, sedation
OBJECTIVES:
To demonstrate the efficacy, safety, and pharmacokinetics of dexmedetomidine as a potential sedative for pediatric surgery patients in the ICU.
DESIGN:
Phase 3, multicenter, open-label study.
SETTING:
This study included 61 patients at 13 tertiary hospitals in Japan.
PATIENTS:
Pediatric patients (≥ 45 wk corrected gestational age to < 17 yr) undergoing intensive care treatment with mechanical ventilation requiring greater than 6 hours estimated duration of sedation following elective cardiac surgery.
INTERVENTIONS:
Dexmedetomidine was IV administered without a loading dose at age-specific dose regimens 0.2–1.4 (< 6 yr) and 0.2–1.0 µg/kg/hr (≥ 6 yr). The primary endpoint was the percentage of patients who did not require a rescue sedative (midazolam) infusion during mechanical ventilation or for the first 24 hours of a greater than 24 hours ventilation following the commencement of dexmedetomidine administration.
MEASUREMENTS AND MAIN RESULTS:
Overall, 47 of the 61 patients (77.0%) did not require rescue midazolam. Adverse events were reported in 53 patients (86.9%). Frequently observed adverse events were hypotension (47.5%), bradycardia (31.1%), and respiratory depression (26.2%). Most of these adverse events were mild, a few moderate, and none severe. Although serious adverse events occurred in four patients, including one cardiac tamponade resulting in the withdrawal of dexmedetomidine, none of the adverse events resulted in mortality or were directly related to dexmedetomidine. The plasma dexmedetomidine concentration generally reached the target concentration of 0.3–1.25 ng/mL at 1–2 hours prior to completion of administration or immediately prior to the commencement of tapering.
CONCLUSIONS:
The age-specific dose regimens of dexmedetomidine without an initial loading dose achieved an adequate sedation level during mechanical ventilation and caused no clinically significant adverse events in the intensive care pediatric patients. These effects were achieved within the therapeutic range of dexmedetomidine plasma concentration and were accompanied by minimal effects on hemodynamics and respiration.
The establishment of ideal sedation and analgesia has become the cornerstone of achieving favorable outcomes for patients in intensive care. Although benzodiazepines (midazolam) and opioids (morphine and fentanyl) are the most commonly used conventional sedatives and analgesics in the PICU (1, 2), prolonged use of these drugs may induce drug tolerance, dependency, and withdrawal symptoms, including delirium (3).
Dexmedetomidine is a potent and highly selective central α2-adrenergic receptor agonist, which can maintain the spontaneous breathing of a patient while providing easily arousable sedation (4, 5). Dexmedetomidine also provides a weak analgesic effect (5). Studies have shown that patients administered dexmedetomidine experience a significantly shorter duration of mechanical ventilation and less postoperative delirium compared with benzodiazepines and propofol in adult intensive care patients (6–8). Furthermore, administration of dexmedetomidine provides nearly spontaneous sleep that is arousable (9, 10) and maintains an anxiety- and pain-free state when sufficient sedation is achieved with continuous administration, which suppresses the development of postoperative delirium (11). Therefore, dexmedetomidine is a favorable sedative that can be administered during weaning from mechanical ventilation and following extubation. It has also been reported that dexmedetomidine produces a neuroprotective effect in juvenile animal models of isoflurane-induced neuroapoptosis (12, 13).
Dexmedetomidine is already approved for clinical use in adults for intensive care patients and surgical/procedural sedation globally; thus, the efficacy and safety of this drug are well established (6, 14, 15). However, dexmedetomidine had not been approved for use in pediatric patients in countries despite efficacy in younger patients and high medical demand for another sedative for use in children (16, 17). Recent approval in Japan allows dexmedetomidine to now be administered to pediatric patients, based on the results of clinical trials involving pediatric patients undergoing intensive care treatment, including mechanical ventilation following elective surgery or during medical management. This study evaluates the results of patients undergoing elective surgery to determine the efficacy, safety, and pharmacokinetics of age-specific doses of dexmedetomidine in Japan.
MATERIALS AND METHODS
Study Overview and Ethics
This phase 3, multicenter, single-arm, open-label clinical study was conducted concurrently at 13 tertiary hospitals in Japan between July 2016 and May 2017. The Institutional Review Board at each hospital reviewed and approved the final protocol, amendments, and informed consent documentation. Written informed consent was obtained from each patient or their guardian prior to commencement of this study. The study was registered prior to patient enrollment at ClinicalTrials.gov (NCT02757625, Date of registration: May 2, 2016). The principal investigators and institutions are listed in the Acknowledgments section. This study was sponsored by Pfizer Japan Inc., Tokyo Japan, and Maruishi Pharmaceutical Co., Ltd., Osaka, Japan.
Study Design
The time course of clinical events and drug administration are shown in Supplemental Fig. 1 (Supplemental Digital Content 1, http://links.lww.com/PCC/B717). The study cohort consisted of pediatric patients (≥ 45 wk corrected gestational age [CGA] to < 17 yr) whose preoperative physical statuses were graded as class I–III based on the ASA guidelines (18), undergoing intensive care treatments, including mechanical ventilation (> 6 hr estimated duration of sedation) immediately following elective (scheduled nonurgent) surgery. Patients with neurologic disease, significant hemodynamic disorders, liver dysfunction, or severe infection were excluded from the study (Appendix A, Supplemental Digital Content 2, http://links.lww.com/PCC/B718, which summarizes the inclusion and exclusion criteria).
Dexmedetomidine Administration
Patients were admitted to an ICU with shared nursing staff, and baseline values, including sedation levels, were assessed, followed by commencement of the dexmedetomidine infusion. The sedation level was assessed with the State Behavioral Scale (SBS) score (19). A loading dose of dexmedetomidine was not permitted, and the exact infusion dose was determined based on the baseline body weight. The lowest starting dose for dexmedetomidine infusion was 0.2 µg/kg/hr. The infusion rate was gradually adjusted within 0.2–1.4 µg/kg/hr for patients less than 6 years old and 0.2–1.0 µg/kg/hr for patients greater than or equal to 6 years old to achieve the target sedative depth, which SBS score ranged from –2 to 0 during mechanical ventilation and –1 to 0 following extubation. If the investigator decided that the patient had not yet reached an adequate sedative state, the investigator increased the rate of the dexmedetomidine infusion by 0.1 µg/kg/hr (1.67 ng/kg/min) for at least 3 minutes per increase. For example, if the investigator increased the rate by a total of 0.4 µg/kg/hr, then the investigator kept the same rate for at least 12 minutes. The SBS score was assessed at 10, 20, and 30 minutes and 1, 2, and 4 hours and then every 4 hours following the initiation of dexmedetomidine infusion. When the infusion rate was changed, the level was assessed within 10 minutes prior to changing the infusion rate of the study drug and at 3–30 minutes after changing the rate. At the time of extubation, the SBS score was assessed 10 minutes prior to extubation and at 5, 15, and 30 minutes and 1, 2, 4, and 12 hours after extubation. The maximum maintenance doses were set based on the dexmedetomidine weight-adjusted clearance data from four previous pediatric studies (DEX-09-08, Children’s Hospital of Philadelphia, DEX-08-01, and DEX-11-01) (20, 21). The predicted steady state plasma dexmedetomidine concentrations in this pediatric study population were similar to the steady state plasma drug concentration achieved with the approved maintenance dose for adults (0.2–0.7 μg/kg/hr).
Since postoperative agitation and delirium are frequently observed during extubation and following this procedure (22), dexmedetomidine infusion was continued as necessary after extubation in the absence of severe adverse events (AEs). Cases that required a prolonged dexmedetomidine infusion greater than 24 hours were gradually weaned off of this drug over 4 hours or longer to prevent withdrawal symptoms. At the completion of dexmedetomidine infusion, the SBS score was assessed at the end of dosing and also after 1, 2, and 4 hours.
During surgery, all the usual drugs were allowed, including those for sedation (midazolam), agitation (phenobarbital), analgesia (fentanyl), and anesthesia (inhaled anesthetics). However, during and until 24 hours following dexmedetomidine administration, it was prohibited in this study to administer the following drugs other than dexmedetomidine as study drug and midazolam as rescue drug: sedatives/analgesics midazolam, continuous infusion of muscle relaxants, or other adrenergic α2-receptor agonists such as clonidine. Fentanyl was also prohibited; however, fentanyl could be used continuously for pain management without a dose change as a rule even after the start of dexmedetomidine, if fentanyl was continuously infused prior to the dexmedetomidine commencement, and discontinuation of the infusion of fentanyl was considered likely to disadvantage the patients. Nonsteroidal anti-inflammatory analgesic drugs, such as paracetamol (acetaminophen) for pain control and fever and aspirin for the antiplatelet therapy, were also allowed for use after extubation.
If additional sedation was required to achieve the target sedative depth even under the maximum dose of dexmedetomidine infusion, the investigator could add midazolam as a rescue sedative at a dose of 0.05–0.2 mg/kg IV over a period of 2–3 minutes. However, if patients required rescue midazolam at greater than or equal to five doses per hour at the maximum dose of dexmedetomidine infusion, then those were discontinued from the study. An additional bolus administration of fentanyl (1–2 µg/kg) was also allowed as a rescue analgesic for pain management at the investigator’s discretion.
Efficacy of Dexmedetomidine
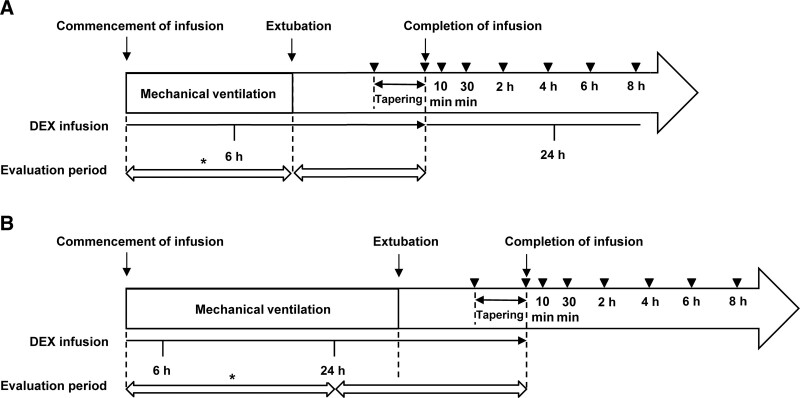
The primary endpoint was the percentage of patients who did not require a rescue sedative (midazolam) during mechanical ventilation or for 24 hours if mechanical ventilation lasted greater than 24 hours following the commencement of dexmedetomidine administration (“the efficacy percentage”). The secondary endpoints were the percentage of patients who did not require a rescue analgesic (fentanyl) during the same period as the primary endpoint and the percentage of the patients requiring no rescue midazolam or fentanyl from 24 hours to extubation in the patients who received greater than 24 hours mechanical ventilation. Additional outcomes were the percentage of the patients requiring no rescue midazolam or fentanyl during dexmedetomidine administration after extubation (Fig. 1).
Figure 1.
Timeline for evaluation of drug efficacy. A, Condition in which mechanical ventilation was completed within 24 hr after the commencement of infusion, and (B) condition in which mechanical ventilation lasted for longer than 24 hr after the commencement of infusion. Arrowheads represent times of blood sampling for pharmacokinetic measurements. The asterisk indicates the period of efficacy evaluation for the primary endpoint. DEX = dexmedetomidine.
Measurement of the Plasma Concentration of Dexmedetomidine and Pharmacokinetic Analysis
Blood samples (0.5 mL) were collected, and the plasma dexmedetomidine concentration was analyzed using a validated high-performance liquid chromatography-tandem mass spectroscopy method. Sampling points were 1–2 hours and taken immediately prior to the completion of administration, then at 10 and 30 minutes and 2, 4, and 6–8 hours after the completion. When dexmedetomidine was administered at greater than 24 hours and tapered to discontinuation, blood samples were collected exactly at the commencement of tapering, and at 0, 10, and 30 minutes and 2, 4, and 6–8 hours after completion of administration (Fig. 1). Plasma samples were prepared, frozen, and sent to inVentiv Health Clinique, Inc. (Québec, Canada) for analysis. Population pharmacokinetic (PopPK) analysis was performed to evaluate the pharmacokinetic of dexmedetomidine using the Cognigen PopPK model used by the previous dexmedetomidine pediatric studies in the United States (DEX-09-08, CHOP Study, DEX-08-01, and DEX-11-01) (20, 21).
Safety Evaluation
To evaluate the safety of dexmedetomidine, treatment-emergent AEs (TEAEs) and other abnormal variations in the laboratory tests were collected. Of note, postoperative surgical site symptoms and operation-related abnormal clinical tests were not classified as TEAEs. The details are presented in Appendix B (Supplemental Digital Content 3, http://links.lww.com/PCC/B719). TEAEs that occurred during and 24 hours after dexmedetomidine administration and all serious AEs (SAEs) that occurred from the time of informed consent to 28 days after dexmedetomidine administration were required to be reported regardless of their relationship to the pharmacologic effects of dexmedetomidine. Any clinically significant symptoms related to surgery were also reported as TEAEs. Protocol-specified TEAEs included hypotension below the reference limit of systolic blood pressure (SBP) (SBP < 70 mm Hg for age ≥ 45 wk CGA to < 1 yr; SBP < 70 + [2 × age in yr] mm Hg for age ≥ 1 yr to < 10 yr; SBP < 90 mm Hg for age ≥ 10 yr to < 17 yr) (23), bradycardia below the percentile limit (≤ 10th percentile of heart rate [HR] for healthy children) (24), and respiratory depression below the percentile limit (≤ 10th percentile of respiratory rate [RR] for healthy children) (24). AEs after dexmedetomidine discontinuation were defined as AEs that newly occurred or those that worsened following the conclusion of dexmedetomidine administration.
The severity of TEAE was graded as “mild” that does not interfere with subject’s usual function, “moderate” that interferes to some extent with subject’s usual function, or “severe” that interferes significantly with subject’s usual function.
Statistical Analysis
The sample size for the primary efficacy analysis was determined using the following information: the expected efficacy percentage of dexmedetomidine was assumed to be 60%, and an efficacy threshold value was defined to be 40% based on information from the previous dexmedetomidine studies for U.S. Food and Drug Administration applications on children (DEX-08-05 [25]) and adults (W97-245 [26, 27], W97-246 [28], and J-DEX-99-001 [29]). Assuming the 60% efficacy percentage, 48 patients would provide greater than or equal to 80% power to detect efficacy compared with the threshold value of 40% via the two-sided binomial test with a 5% significance level. Thus, the required number of patients for efficacy analysis was estimated to be 60, including possible dropouts. To examine the effects of age, the patient population was to include at least eight infants (≥ 45 wk CGA to < 12 mo), 16 toddlers (≥ 12 to < 24 mo), 16 preschoolers (≥ 2 to < 6 yr), and eight schoolchildren (≥ 6 to < 17 yr). The elective surgery patients who received dexmedetomidine were evaluated as efficacy and safety sets in this article. The efficacy percentage and 95% CI were calculated for the efficacy analysis set. The lower limit of the 95% CI based on the Agresti-Coull method was compared with the threshold value. All elective surgery patients who received dexmedetomidine for greater than 6 hours and whose plasma concentrations were measured more than once were included in a pharmacokinetic analysis set. At least eight patients were required in each age subset to estimate the primary pharmacokinetic variables.
All statistics were performed using commercially available software (SAS Version 9.2; SAS Institute, Cary, NC).
RESULTS
Patients
A total of 63 patients were registered in this study. Sixty-one of these patients received dexmedetomidine following elective surgery for the efficacy and safety of dexmedetomidine; the remaining two patients without surgery were excluded from analysis in this article. Dexmedetomidine administration was discontinued in two male preschoolers of the 61 patients due to SAE and the investigator’s judgment. Furthermore, one preschooler was excluded from laboratory evaluations due to missing data following dexmedetomidine commencement.
The demographic characteristics of the patients in each age subset are shown in Supplemental Table 1 (Supplemental Digital Content 4, http://links.lww.com/PCC/B720). Many patients (53/61 patients) exhibited an SBS score of –3 at the start of dexmedetomidine infusion. The mean (sd) of dexmedetomidine infusion time was 21.09 (13.10) hours from the start to the end and that included 6.91 (5.11) hours of ventilation. Two infants (3.3%) received dexmedetomidine for longer than 24 hours. The mean (sd) of the exposure dose was 0.726 (0.278) µg/kg/hr.
Surgeries were all heart surgeries, and the major types were ventricular septal defect closure (49.2%) or atrial septal defect closure (21.3%). During surgery, midazolam for sedation and fentanyl and acetaminophen for pain control were commonly used. Following surgery, fentanyl was continuously used in most of the patients (91.8%). Concomitant use of acetaminophen was allowed by the protocol, and it was used for pain control or fever reduction after extubation (31.1%), including two cases (3.3%) of protocol deviation (administration before extubation).
Efficacy
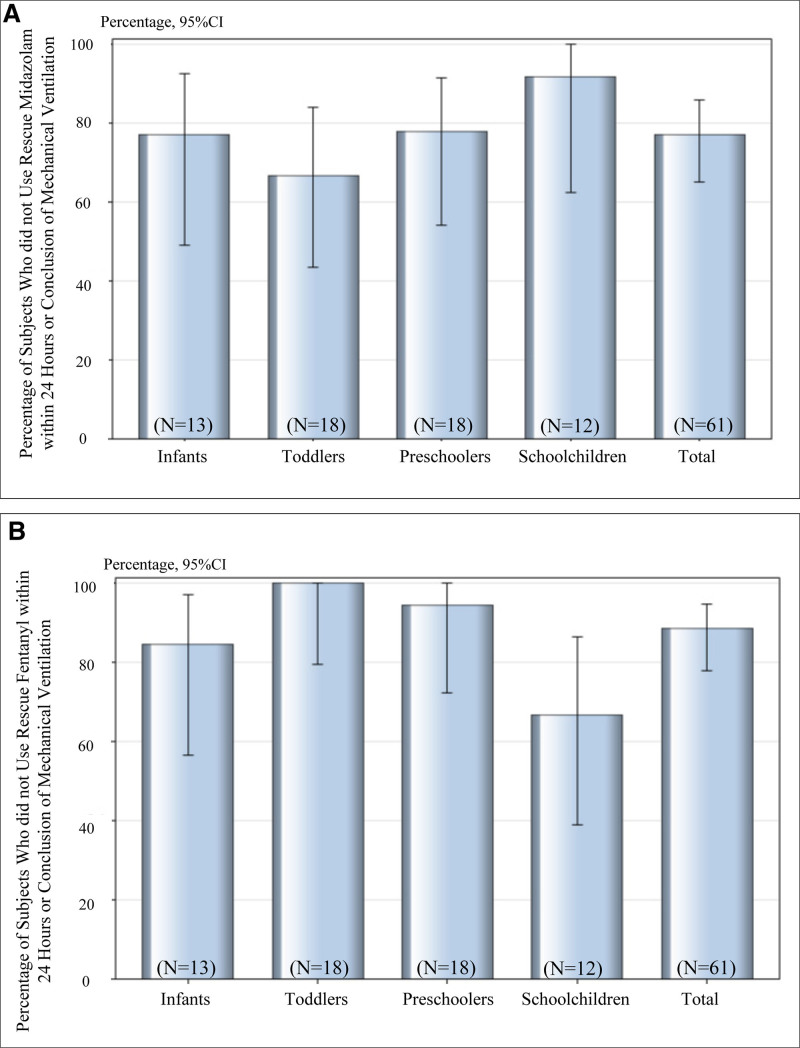
The primary analysis indicated that 47 of the 61 patients (77.0%, the efficacy percentage) did not receive rescue midazolam during mechanical ventilation (or for 24 hr) if ventilation was continued for greater than 24 hours mechanical ventilation following the commencement of dexmedetomidine administration (Fig. 2A). The lower limit of 95% CI for the efficacy percentage was greater than the prespecified threshold value of 40%. The median SBS score at extubation was –1. The mean percentage (sd) of time within the target sedation range (SBS score –2 to 0) was 55.11% (29.32) of the 61 patients. Each age subset exhibited the lower limit of the 95% CI greater than 40%, and the lowest limit for toddlers was 43.6%. A post hoc exploratory analysis was performed for the dichotomous variable of rescue midazolam use; however, no difference was found for potential risk factors (Supplemental Table 2, Supplemental Digital Content 5, http://links.lww.com/PCC/B721).
Figure 2.
Percentage of subjects who did not receive rescue midazolam (A) or fentanyl (B) during mechanical ventilation during the study period. Error bars indicate 95% CIs.
Two patients required dexmedetomidine infusion more than 24 hours because of long mechanical ventilation. However, none of these patients received rescue midazolam after 24 hours infusion of dexmedetomidine until weaning from the ventilator. A total of 60 patients continued to receive dexmedetomidine infusion following extubation regardless of the infusion time, and 55 patients (91.7%) did not receive rescue midazolam during the period from extubation to the completion of dexmedetomidine administration. These results indicated that dexmedetomidine was effective as a sedative in pediatric patients.
The secondary endpoint results related to rescue fentanyl showed that 54 of the 61 patients (88.5%) did not require rescue analgesic fentanyl during the same specified period as the primary endpoint (Fig. 2B). All the patients who received rescue fentanyl were those who continued infusion of fentanyl following their surgery, and in the subsets by age, more patients in the schoolchildren subset received rescue fentanyl. Dexmedetomidine was administered for greater than 24 hours in two patients under mechanical ventilation. Neither of the two patients who were administered dexmedetomidine for greater than 24 hours under mechanical ventilation had received rescue fentanyl after 24 hours infusion of dexmedetomidine until weaning from the ventilator. Furthermore, of the 60 patients who continued to receive dexmedetomidine infusion from extubation to the end of dexmedetomidine infusion, 59 (98.3%) did not receive rescue fentanyl.
Plasma Concentration of Dexmedetomidine and Pharmacokinetic Analysis
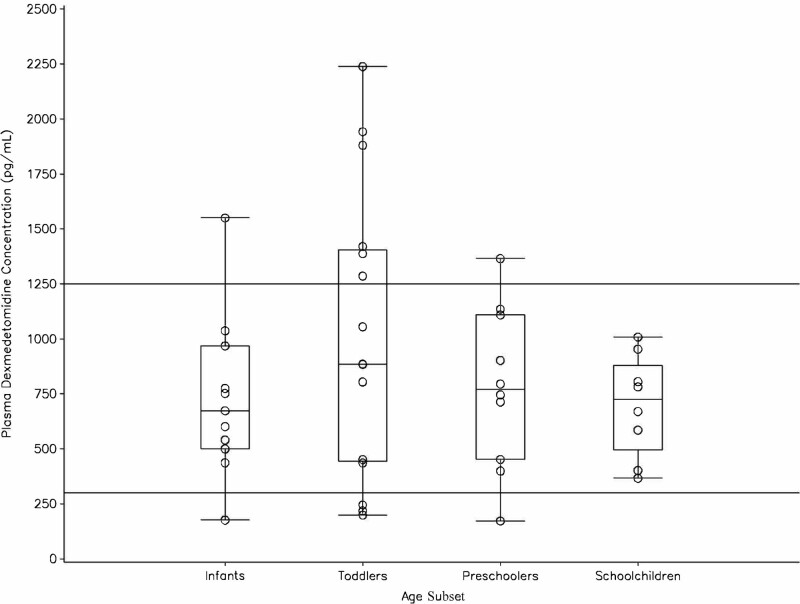
Although the plasma dexmedetomidine concentrations in each age subset varied over a wide range, overall, they reached the target concentration range of 0.3–1.25 ng/mL derived from the pharmacokinetic data in adults (30) at 1–2 hours prior to the completion of administration or before the commencement of tapering (Fig. 3). Thereafter, no significant differences in the plasma dexmedetomidine concentrations were observed at any of the sampling points among the age subsets (data not shown).
Figure 3.
Plasma dexmedetomidine concentrations 1–2 hr before the completion of dexmedetomidine administration or before the commencement of tapering. Circles represent individual values. The box plot represents the median and 25%/75% quartiles with whiskers to the largest or lowest point within 1.5 times the interquartile range. The lines drawn at 300 and 1,250 pg/mL indicate the therapeutic range of plasma dexmedetomidine concentration derived from the previous dose-ranging study in adults (30).
Based on the PopPK analysis, the mean estimated body weight-adjusted clearance was 1.15, 0.99, and 1.07 L/hr/kg in the three younger age subsets of infants, toddlers, and preschoolers, respectively. The value in schoolchildren was 0.83 L/hr/kg, which was lower than that found in the other age subsets. A similar trend was observed in the body weight-adjusted volume of distribution at the steady state (2.52, 2.26, and 2.21 L/kg vs 1.79 L/kg, respectively). These variables appeared to decrease with increasing age and to approach the values expected in adults (Table 1).
TABLE 1.
Estimated Clearance and Volume Distribution in Japanese Pediatric Patients by Age Subset Based on Population Pharmacokinetic Analysis
| Variables, Mean (sd) | Infants(n = 11) | Toddlers(n = 16) | Preschoolers(n = 11) | School Children(n = 8) |
|---|---|---|---|---|
| Body weight-adjusted clearance (L/hr/kg) | 1.15 (0.29) | 0.99 (0.26) | 1.07 (0.19) | 0.83 (0.12) |
| Body weight-adjusted volume of distribution at steady state (L/kg) | 2.52 (0.40) | 2.26 (0.34) | 2.21 (0.25) | 1.79 (0.24) |
Safety
A total of 124 TEAEs occurred in 53 of the 61 patients (86.9%); however, none of the TEAEs were clinically significant in severity. Common TEAEs were only the protocol-specified TEAEs hypotension (47.5%), bradycardia (31.1%), and respiratory depression (26.2%) (Supplemental Table 3, Supplemental Digital Content 6, http://links.lww.com/PCC/B722). Dexmedetomidine-related TEAEs were observed in 14 patients (23.0%) and included hypotension (4.9%), bradycardia (11.5%), and respiratory depression (3.3%). Most of these dexmedetomidine-related TEAEs were mild, a few moderate, and none severe. Among these cases, hypotension required additional medication, and one case of respiratory depression required a dose reduction of fentanyl. Notably, these TEAEs disappeared immediately following completion of the dexmedetomidine infusion. The most common features of AEs after dexmedetomidine discontinuation were vomiting (11.1%), nausea (7.9%), and bradycardia (6.3%). However, no delirium was observed during the study period.
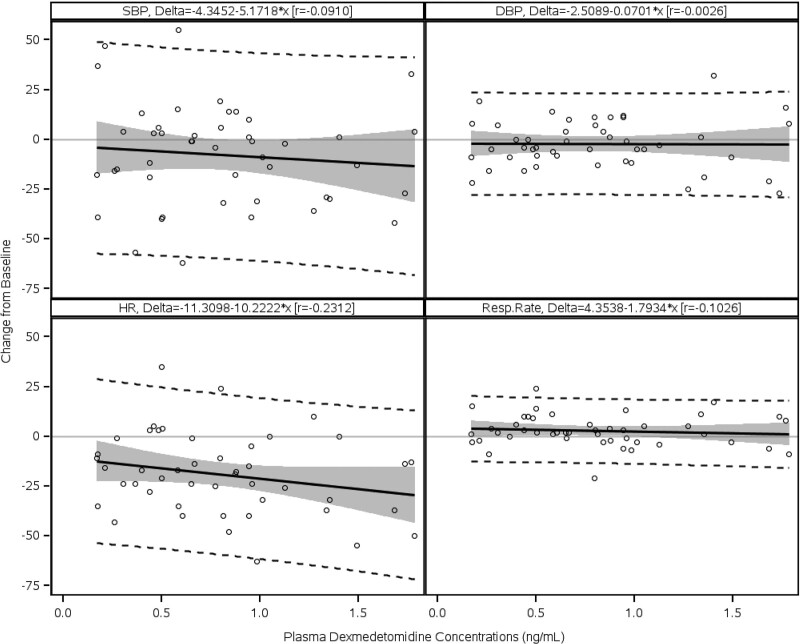
Four SAEs occurred in four patients; however, none were related to dexmedetomidine. Among the SAEs, cardiac tamponade in one preschooler led to discontinuation from the study for this patient. The other SAEs cardiac tamponade, pericardial effusion, and postoperative wound infection (one each) occurred following completion of dexmedetomidine administration. No deaths occurred. The relationship between the plasma dexmedetomidine concentration and percent change from the baseline in SBP, diastolic blood pressure (DBP), HR, and RR at discontinuation were evaluated using simple linear regression analysis (Fig. 4). Although no obvious changes in DBP and RR were observed within the wide range of dexmedetomidine plasma concentrations, the SBP and HR appeared to decrease as the dexmedetomidine concentration increased.
Figure 4.
Percentage changes from baseline in blood pressure, heart rate (HR), and respiratory rate (Resp. Rate) at various plasma dexmedetomidine concentrations at discontinuation of infusion. Circles represent observed individual data. The black solid line in each graph represents regression between each vital sign and the plasma concentration of dexmedetomidine. The shaded area in each graph represents the predicted 95% CI of regression. The two dashed lines in each graph represent the 95% upper and lower prediction limits. DBP = diastolic blood pressure, r = correlation coefficient, SBP = systolic blood pressure.
There were no abnormalities found in the laboratory variables, vital signs, or the electrocardiogram within the infusion rate of dexmedetomidine used in this study.
DISCUSSION
The purpose of this study was to break away from off-label use and obtain approval. The main objectives were to show that dexmedetomidine as a single agent could be used in pediatric patients as an effective and safe sedative in intensive care and to present a recommended protocol for individual age group. Therefore, it was not significant to show noninferiority compared with midazolam, and we decided to examine the usefulness of dexmedetomidine in a single-arm, open study. Following consultations with the Japanese regulatory authorities, we prepared a new protocol of drug dosing, which reflected their ideas and concerns.
There were three major findings of this study. First, dexmedetomidine alone achieved adequate sedation levels that did not require rescue midazolam during and after mechanical ventilation in the pediatric intensive care patients. Second, the pharmacokinetic data demonstrated that the dose settings of dexmedetomidine that we introduced achieved the targeted therapeutic range of plasma concentration in the pediatric population as reported in adult patients. Third, although dexmedetomidine caused some TEAEs, such as bradycardia and hypotension due to its innate pharmacologic effects, severe TEAEs or withdrawal symptoms, such as delirium, did not occur throughout this study.
Accordingly, 77.0% of the pediatric patients did not require rescue midazolam, and the efficacy percentage for the entire trial (and each age subset) was higher than the threshold value of 40% at a 95% lower confidence limit. The sedation level quickly reached to adequate levels after commencement of dexmedetomidine infusion partly because most of the patients had an SBS score of –3 at baseline, probably the anesthetics and sedatives used in surgery remained immediately after ICU admission. Taken together, with the assumed efficacy percentage of the placebo (around 20%) derived from previous clinical studies (25–29), dexmedetomidine itself was at least in part, considered an effective sedative, achieving adequate sedation in pediatric patients under intensive care after elective surgery. Furthermore, efficacy was maintained during mechanical ventilation and after weaning in most of the patients. This feature was a plausible aspect in favor of dexmedetomidine over midazolam, which should generally be discontinued during the ventilation weaning period due to the potential respiratory depression, and the discontinuation of midazolam often causes sudden agitation (31).
In this study, we introduced two different dose settings of dexmedetomidine infusion via age subset and allowed titration to achieve adequate sedative levels without an initial loading dose that sometimes cause acute hemodynamic deterioration. Possible remaining effects of anesthetics and sedatives used in surgery may help avoid the use of the initial loading dose. As a fact, this protocol enabled us to achieve both effective sedation and a plasma concentration of dexmedetomidine within the therapeutic range of 0.3–1.25 ng/mL (derived from a previous dose-ranging study showing a minimal effective plasma concentration of 0.3 ng/mL [20] and the pharmacokinetic data in adult patients [30]) in all age subsets. Therefore, titration of the dexmedetomidine infusion rate by precise evaluation of the sedation level could achieve efficacy. Based on PopPK analysis, the mean estimated weight-adjusted clearance and weight-adjusted volume of distribution at the steady state were higher in the three youngest age subsets (infants, toddlers, and preschoolers) than in schoolchildren, which supported a study regimen of higher doses in younger age subsets.
In addition, although the data used in the analysis were limited to the end of the infusion, the results have shown that there was no significant correlation between hemodynamic and respiratory variables and the plasma concentration of dexmedetomidine in this study. The relationship had not been clearly shown in previous pediatric studies either (20, 21). With the age-specific regimens we used in this pediatric study, modest decreases in SBP and HR were the two most frequent TEAEs as acknowledged in adult populations (28, 32). These hemodynamic changes were acceptable because of the basic pharmacologic α2-adrenergic receptor agonist action of dexmedetomidine. These negative impacts of dexmedetomidine on the hemodynamics did not lead to discontinuation or dose reduction in this study. Taken together, the overall safety profile on the hemodynamics in the pediatric population in the postsurgery ICU management appeared to be comparable with that of adults. Respiratory depression was another frequently reported AE in this study, although without any worsening of the blood-gas analysis. This probably occurred due to one of the following two reasons: the protocol-specific criteria for respiratory depression as described in RR were strict for the postoperative patients; any decrease in RR from baseline observed during continuous monitoring of vital signs, even if it occurred once, was reported as an AE, and RR-setting of the mechanical ventilator settings was also counted as RR. In fact, no patients required reintubation after weaning from the mechanical ventilation, even with dexmedetomidine administration.
There were several limitations to this study that should be addressed. First, this study did not have a placebo control group, thus making any definitive conclusions difficult. However, our findings supported that dexmedetomidine was a plausible sedative that reduces uses of previous sedatives, such as benzodiazepines and opiates that often cause various suffering AEs (33). Second, the small sample size was inadequate to draw definite conclusions regarding safety; however, the safety profile of this study was equivalent to that of previous adult studies with larger sample sizes. Third, the plasma concentrations of dexmedetomidine during hemodynamic and respiratory events were not measured. Therefore, an association between the two could not be drawn. Last, the study population was limited to postsurgical patients. Therefore, we could not show whether our dexmedetomidine protocol was also effective for PICU patients with other different complex backgrounds, such as encephalopathy, respiratory disorder, and sepsis. However, at least, in part, dexmedetomidine could be one of the plausible sedatives and used in a combination with other sedatives even in pediatric patients other than postsurgical cases.
CONCLUSIONS
The age-specific dose regimens of dexmedetomidine without an initial loading dose could achieve an adequate sedation level during mechanical ventilation without clinically significant TEAEs in the pediatric patients under intensive care after elective surgery. The therapeutic range of the plasma dexmedetomidine concentration achieved with this protocol may minimalize the effects on hemodynamics and respiration.
ACKNOWLEDGMENTS
We thank the following principal investigators of this study and their respective institutions in Japan: Yoshiyasu Egawa, MD (Shikoku Medical Center for Children and Adults, Kagawa); Tatsuo Iwasaki, MD (Okayama University Hospital, Okayama); Shiori Kawasaki, MD (Juntendo University Hospital, Tokyo); Takayuki Kunisawa, MD (Asahikawa Medical University Hospital, Hokkaido); Hiroshi Kurosawa, MD (Hyogo Prefectural Kobe Children’s Hospital, Hyogo); Keiichiro Mizuno, MD (Fukuoka Children’s Hospital, Fukuoka; did not assign patients); Masaki Osaki, MD (Shizuoka Children’s Hospital, Shizuoka); Naoki Shimizu, MD (Tokyo Metropolitan Children’s Medical Center, Tokyo); Naoyuki Taga, MD (Jichi Medical University Hospital, Tochigi); Muneyuki Takeuchi, MD (Osaka Women’s and Children’s Hospital, Osaka); Atsushi Ujiro, MD and Hideki Shimaoka, MD (Osaka City General Hospital, Osaka); Osamu Umegaki, MD (Osaka Medical College Hospital, Osaka); and Masaaki Yamagishi, MD (University Hospital, Kyoto Prefectural University of Medicine, Kyoto). Upon request and subject to certain criteria, conditions, and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual deidentified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines and medical devices 1) for indications that have been approved in the United States and/or European Union or 2) in programs that have been terminated (i.e., development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The deidentified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/pccmjournal).
Drs. Takeuchi, Nemoto, and Suzuki have received honoraria as medical advisers of this study both from Pfizer Japan Inc., Tokyo, Japan, and Maruishi Pharmaceutical Co., Ltd., Osaka, Japan. Mr. Takahashi is a Maruishi employee. Ms. Takenaka, Ms. Takata, and Dr. Kobayashi are Pfizer employees. Ms. Takenaka and Dr. Kobayashi received funding from Pfizer Japan. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Rhoney DH, Murry KR: National survey on the use of sedatives and neuromuscular blocking agents in the pediatric intensive care unit. Pediatr Crit Care Med. 2002; 3:129–133 [DOI] [PubMed] [Google Scholar]
- 2.Twite MD, Rashid A, Zuk J, et al. : Sedation, analgesia, and neuromuscular blockade in the pediatric intensive care unit: Survey of fellowship training programs. Pediatr Crit Care Med. 2004; 5:521–532 [DOI] [PubMed] [Google Scholar]
- 3.Tobias JD: Tolerance, withdrawal, and physical dependency after long-term sedation and analgesia of children in the pediatric intensive care unit. Crit Care Med. 2000; 28:2122–2132 [DOI] [PubMed] [Google Scholar]
- 4.Hsu YW, Cortinez LI, Robertson KM, et al. : Dexmedetomidine pharmacodynamics: Part I: Crossover comparison of the respiratory effects of dexmedetomidine and remifentanil in healthy volunteers. Anesthesiology. 2004; 101:1066–1076 [DOI] [PubMed] [Google Scholar]
- 5.Hall JE, Uhrich TD, Barney JA, et al. : Sedative, amnestic, and analgesic properties of small-dose dexmedetomidine infusions. Anesth Analg. 2000; 90:699–705 [DOI] [PubMed] [Google Scholar]
- 6.Riker RR, Shehabi Y, Bokesch PM, et al. ; SEDCOM (Safety and Efficacy of Dexmedetomidine Compared With Midazolam) Study Group: Dexmedetomidine vs midazolam for sedation of critically ill patients: A randomized trial. JAMA. 2009; 301:489–499 [DOI] [PubMed] [Google Scholar]
- 7.Pandharipande PP, Pun BT, Herr DL, et al. : Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: The MENDS randomized controlled trial. JAMA. 2007; 298:2644–2653 [DOI] [PubMed] [Google Scholar]
- 8.Liu X, Xie G, Zhang K, et al. : Dexmedetomidine vs propofol sedation reduces delirium in patients after cardiac surgery: A meta-analysis with trial sequential analysis of randomized controlled trials. J Crit Care. 2017; 38:190–196 [DOI] [PubMed] [Google Scholar]
- 9.Nelson LE, Lu J, Guo T, et al. : The alpha2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology. 2003; 98:428–436 [DOI] [PubMed] [Google Scholar]
- 10.Huupponen E, Maksimow A, Lapinlampi P, et al. : Electroencephalogram spindle activity during dexmedetomidine sedation and physiological sleep. Acta Anaesthesiol Scand. 2008; 52:289–294 [DOI] [PubMed] [Google Scholar]
- 11.Nunes SL, Forsberg S, Blomqvist H, et al. : Effect of sedation regimen on weaning from mechanical ventilation in the intensive care unit. Clin Drug Investig. 2018; 38:535–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanders RD, Sun P, Patel S, et al. : Dexmedetomidine provides cortical neuroprotection: Impact on anaesthetic-induced neuroapoptosis in the rat developing brain. Acta Anaesthesiol Scand. 2010; 54:710–716 [DOI] [PubMed] [Google Scholar]
- 13.Sanders RD, Xu J, Shu Y, et al. : Dexmedetomidine attenuates isoflurane-induced neurocognitive impairment in neonatal rats. Anesthesiology. 2009; 110:1077–1085 [DOI] [PubMed] [Google Scholar]
- 14.Ozaki M, Takeda J, Tanaka K, et al. : Safety and efficacy of dexmedetomidine for long-term sedation in critically ill patients. J Anesth. 2014; 28:38–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jakob SM, Ruokonen E, Grounds RM, et al. ; Dexmedetomidine for Long-Term Sedation Investigators: Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation: Two randomized controlled trials. JAMA. 2012; 307:1151–1160 [DOI] [PubMed] [Google Scholar]
- 16.Buck ML: Dexmedetomidine use in pediatric intensive care and procedural sedation. J Pediatr Pharmacol Ther. 2010; 15:17–29 [PMC free article] [PubMed] [Google Scholar]
- 17.Mahmoud M, Mason KP: Dexmedetomidine: Review, update, and future considerations of paediatric perioperative and periprocedural applications and limitations. Br J Anaesth. 2015; 115:171–182 [DOI] [PubMed] [Google Scholar]
- 18.American Society of Anesthesiologists: ASA Physical Status Classification System, 2020. Available at: https://www.asahq.org/resources/clinical-information/asa-physical-status-classification-system. Accessed December 24, 2020
- 19.Curley MA, Harris SK, Fraser KA, et al. : State behavioral scale: A sedation assessment instrument for infants and young children supported on mechanical ventilation. Pediatr Crit Care Med. 2006; 7:107–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.US Food and Drug Administration, Center for Drug Evaluation and Research: Clinical Pharmacology Review: N21-038 S021 & S022; Dexmedetomidine Clinpharm BPCA (Reference ID: 3313647), 2013. Available at: https://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DevelopmentResources/UCM362466.pdf. Accessed December 24, 2020
- 21.Chrysostomou C, Schulman SR, Herrera Castellanos M, et al. : A phase II/III, multicenter, safety, efficacy, and pharmacokinetic study of dexmedetomidine in preterm and term neonates. J Pediatr. 2014; 164:276–82. e1 [DOI] [PubMed] [Google Scholar]
- 22.Mason KP: Paediatric emergence delirium: A comprehensive review and interpretation of the literature. Br J Anaesth. 2017; 118:335–343 [DOI] [PubMed] [Google Scholar]
- 23.Haque IU, Zaritsky AL: Analysis of the evidence for the lower limit of systolic and mean arterial pressure in children. Pediatr Crit Care Med. 2007; 8:138–144 [DOI] [PubMed] [Google Scholar]
- 24.Fleming S, Thompson M, Stevens R, et al. : Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: A systematic review of observational studies. Lancet. 2011; 377:1011–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.US Food and Drug Administration, Center for Drug Evaluation and Research: Statistical Review and Evaluation. Application Numbers: 021-038 S012 & 022, Dexmedetomidine-FDA (Reference ID: 3312516), 2013. Available at: https://www.fda.gov/media/86355/download. Accessed December 24, 2020
- 26.US Food and Drug Administration, Center for Drug Evaluation and Research: Medical Review(s): Part 1. 1999. Application No.: 21-038. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/99/21-038_Precedex.cfm. Accessed December 24, 2020
- 27.US Food and Drug Administration, Center for Drug Evaluation and Research: Drug Approval Package: Precedex (Dexmedetomidine Hydrochloride). Application No.: 21-038. Medical Review(s): Part 3, 1999. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/99/21-038_Precedex_medr_P3.pdf. Accessed December 24, 2020
- 28.Martin E, Ramsay G, Mantz J, et al. : The role of the alpha2-adrenoceptor agonist dexmedetomidine in postsurgical sedation in the intensive care unit. J Intensive Care Med. 2003; 18:29–41 [DOI] [PubMed] [Google Scholar]
- 29.The Pharmaceuticals and Medical Devices Agency: Summary Technical Documentation of Precedex [in Japanese]. Available at: http://www.pmda.go.jp/drugs/2004/P200400001/10015900_21600AMY00007_X118_1.pdf. Accessed December 24, 2020
- 30.Hospira, Inc.: Dexmedetomidine Hydrochloride Injection Label, 2013. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021038s021lbl.pdf. Accessed December 24, 2020
- 31.Pfizer Canada Inc: Midazolam Injection USP [Product Monograph], 2017. Available at: https://www.pfizer.ca/sites/g/files/g10050796/f/201712/2017.10.25_Midazolam_PM_PF_E_210318.pdf. Accessed December 24, 2020
- 32.Mason KP, Zurakowski D, Zgleszewski SE, et al. : High dose dexmedetomidine as the sole sedative for pediatric MRI. Paediatr Anaesth. 2008; 18:403–411 [DOI] [PubMed] [Google Scholar]
- 33.Czaja AS, Zimmerman JJ: The use of dexmedetomidine in critically ill children. Pediatr Crit Care Med. 2009; 10:381–386 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.