Abstract
The lack of access to safe and effective antimicrobials for human populations is a threat to global health security and a contributor to the emergence and spread of antimicrobial resistance (AMR). The increasingly common shortages of antimicrobials are an additional threat to the emergence of AMR. While the threat of such drug shortages is most acutely experienced in low-income and middle-income settings, their consequences impact the quality and effectiveness of antimicrobials worldwide. Furthermore, there is a need for robustly conducted studies examining the impact of these increasingly prevalent shortages on patient outcomes and on the emergence and spread of AMR. In this review, we have mapped common drivers for antimicrobial shortages and propose strategies for rethinking the regulation, supply and pricing of antimicrobials to secure their sustainable access across diverse healthcare systems and to help minimise the unintended consequences of weak and ineffective supply chains. Greater government involvement in antimicrobial manufacture and supply is essential to ensure no one is left behind. Dedicated demand systems need to be developed for antimicrobials which take into consideration evolving AMR patterns, burden of diseases, pandemic events and supply and demand issues and facilitate implementation of strategies to address them. Interventions, ranging from advocacy and forecasting to public–private collaborations, new economic models and international consortia working across countries and supply chains, will help assure access to safe and effective antimicrobials to all populations around the globe and ensure that shortages no longer contribute to AMR.
Keywords: health services research, health policy, infections, diseases, disorders, injuries
Summary box.
Shortages of antimicrobials are an increasingly common occurrence worldwide. Such shortages are a threat to the emergence and spread of antimicrobial resistance, and more importantly a threat to access to effective therapies.
While the drivers for shortages vary, the consequences impact all countries. Globally, there is no consensus in policy or strategy for forecasting or managing such shortages.
Mapping the most common drivers for antimicrobial drug shortages, we propose potential strategies for rethinking the regulation, supply and pricing of antimicrobials to secure their sustainable access across diverse healthcare systems and help minimise the unintended consequences of weak and ineffective supply chains.
Identifying a global solution with regional focus to the prevailing antimicrobial supply issues will contribute to global antimicrobial security and support more equitable access to antimicrobial therapies for populations in different countries.
Introduction
Sustainable availability and supply of antimicrobials is under constant threat both locally and globally. Antimicrobial shortages are increasingly hindering timely access to effective therapies. The lack of access to antimicrobials is a driver of excess mortality, particularly in low-income and middle-income countries (LMICs).1 The list of affected antimicrobials includes penicillin, cefazolin,2 3 clofazimine,4 dapsone,5 rifabutin,6 piperacillin–tazobactam, ceftolozane–tazobactam7 and cloxacillin.8 The consequences of these shortages are not well documented in literature. Besides a direct effect on healthcare outcomes, shortages of antimicrobials pose an additional public health threat. Resorting to alternative antimicrobials due to the unavailability of the most appropriate agent (eg, as in the case of piperacillin–tazobactam and cefazolin) may lead to compromised outcomes including suboptimal use of antibiotics.9 10 The more recent shortages and their cause and consequences are summarised in table 1. The economic consequences of such shortages are immense, with a WHO report estimating the economic cost of the shortage of just one antimicrobial to be €20–30 million.11 Though not mentioned in the report, the economic loss could be a result of many factors including using higher cost antibiotics, relying on suboptimal therapeutic options leading to more complications and creation of market opportunities for substandard and falsified alternatives.12
Table 1.
Summarising some of the key antimicrobial shortages worldwide in the last 10 years and the causes and consequences of these shortages
| Drug shortage | Timeline | Regions affected* | Cause | Consequences |
| Amoxicillin70 | 2018–2021 | Canada, India | Suspension by manufacturer, disruption in supply of API due to pandemic, possible increase interest in investment in amoxicillin-clavulanic acid. | For community acquired infections, one may be compelled to use amoxicillin-clavulanic acid which is a broad-spectrum antimicrobial. The shortage of the paediatric formulation is particularly important. |
| Amphotericin B71 | 2021 | India | Surge in demand as a first line treatment for mucormycosis. | Using alternative agents with no or sparse clinical trial evidence of efficacy, for example, posaconazole. Potential for treatment failure and increased morbidity and mortality. |
| Cefazolin3 | 2016 onwards | Japan, India | Decreased production, increased demand, price caps. | Cefazolin is a major drug recommended for surgical prophylaxis and for treatment of staphylococcal infections. Lack of its availability may compel use of alternatives which may not be as effective and could have broader spectrum, adversely affecting AMR. |
| Ceftolozane–tazobactam7 | 2021 onwards | European Union, Canada | Issues with compliance of good manufacturing practices. | Required for treatment of complicated intra-abdominal, urinary tract and other hospital acquired infections. May necessitate use of carbapenems. |
| Cloxacillin8 72 | 2017 onwards | USA, Europe, Canada, India | Disruption of manufacturing due to declining market returns. | Cloxacillin is an important drug for methicillin susceptible staphylococcal aureus infection, compels use of alternative agents such as amoxicillin-clavulanic acid or vancomycin leading to worse outcomes. |
| Cotrimoxazole73 74 | 2018 onwards | Africa, Australia | Suspension of production by manufacturing companies. | Cotrimoxazole is an important drug for community acquired infections and some hospital acquired infections. It is also an important drug for prophylaxis and treatment of opportunistic infections in immunocompromised states such as HIV infections. |
| Penicillin G75 | 2015– ongoing | 39 countries | Only four companies produce the active pharmaceutical ingredient and due to lack of profitability production levels were kept low. Fragmented production process, including lack of regulation and quality standards in factories producing raw materials. | Restricts treatment options for syphilis and rheumatic heart disease—conditions disproportionately affecting low-income and middle-income countries. Increased risk of maternal to foetal transmission of syphilis. Increased costs of therapy as substitute therapies more expensive. |
| Piperacillin–tazobactam13 | 2017 | Worldwide | Explosion at the sole factory in China supplying the active pharmaceutical ingredient. | Use of more broad-spectrum agents such as carbapenems. Increased use of cephalosporins with increasing risk of c-difficile infection. |
| Dapsone5 | 2017 | Korea | Shortage of raw material and rising costs. | Compromises the management of both leprosy and several other dermatological conditions for which dapsone is often used. |
| TB medication shortages76–78 | 2011 | UK, South Africa, India | Manufacture and supply chain issues for various drugs including ethionamide, clofazimine, streptomycin, rifabutin, protionamide, capreomycin. | Interruptions to TB therapy regimens including for MDR-TB therapy. Using unlicensed, variable-strength and unlicensed suspensions for paediatric dosing. |
*The mentioned countries/regions are highlighted because they have reported shortages
AMR, antimicrobial resistance; API, active pharmaceutical ingredients; MDR-TB, multidrug-resistant tuberculosis; TB, tuberculosis.
Selecting suboptimal antibiotics can result in drug-resistant infections and contribute to the burden of antimicrobial resistance (AMR).13AMR is recognised as a global public health challenge with an estimated annual mortality of 700 000 attributed to AMR.14 AMR is an impediment to achieving the Sustainable Development Goals; insufficient action could push a further 24 million people into extreme poverty by 2030.15
Data on the availability of medicines are patchy and limited. The WHO has conducted a survey of availability of selected generic medicines, for which there are data from only 30 countries.16 The data for most of the countries are from one survey on the availability of a specific list of medicines in the country using a sample of medicine dispensing units. The available data report that the median percent availability (calculated from medicine dispensing outlets in at least four geographic or administrative areas) of most drugs seldom reach the global target of 80%.16 The limited data, which are available on the extent and consequence of such shortages,17 18 indicate that antimicrobial shortages are an increasingly global problem with many high-income countries (HICs) also affected.19 20
Reasons for antimicrobial shortages
Across the development, distribution and use pathway, shortages can be attributed to: (a) inefficient and fragmented supply chains issues, (b) poorly functioning systems of financing and drug pricing environment and (c) issues related to policy and regulatory processes. Within these broadly defined categories, a more specific classification of the causes of shortages includes the following: (1) a lack of commercial incentive and market; (2) unavailable or inefficient forecasting systems; (3) single source or gaps in active pharmaceutical ingredients (APIs) and other essential materials; (4) limited quality manufacturing of source materials; (5) complex and fragmented procurement of products; (6) regulatory challenges and (7) inadequate constitutive recognition of access to essential medicines (figure 1).21 22 All these reasons contribute to commercial non-viability, discouraging pharmaceutical companies from investing in manufacture and supply of antimicrobials. The causes of shortages are often interrelated and lead to adverse unintended consequences. For example, the growing prevalence of betalactamase-producing Gram-negative bacteria has increased the availability of combination beta-lactamase inhibitors. Many of these combination agents lack a robust evidence-base for their use, and they are often used inappropriately, especially where single agent preparations are not available. Practitioners in India, for instance, have difficulty in getting amoxicillin, while amoxicillin-clavulanic acid is easily available.
Figure 1.
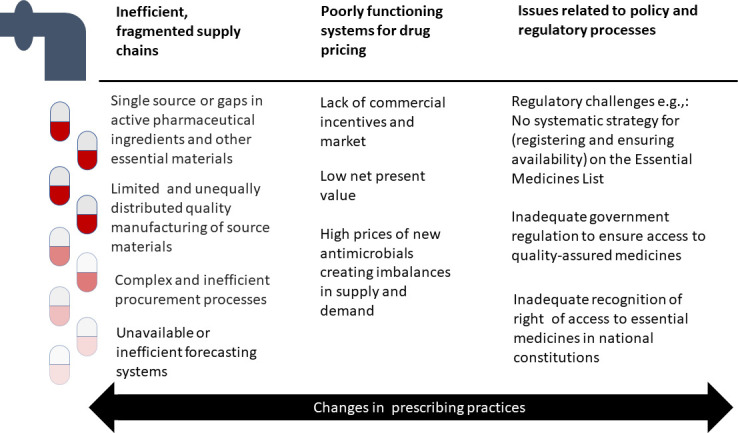
The reasons for antimicrobial shortages across the development, distribution and use pathway.
In the following sections, we discuss some of the key drivers for antimicrobial shortages which are impacting therapeutic and prophylactic approaches to infection and may be exacerbating the threat of AMR.
Inefficient and fragmented supply chains compounded by weak forecasting systems
Global antimicrobial supply chains are inefficient and fragmented. Such fragmentation of supply systems hinders the ability to forecast and mitigate against shortages. Some segments of the antimicrobial supply chain may have multiple stakeholders and companies with vested interests, while other segments of the supply chain may have very few players and thus may be highly concentrated.23 India and China are the major suppliers of API for antimicrobials, while manufacturers are more widely distributed.24 A disruption at any point in the API supply chain can bring the entire process to a halt. In 2016, a fire in the manufacturing unit of the API for piperacillin–tazobactam disrupted its global supply.11 17 The fragility of global supply chains is exacerbated by weak forecasting systems. Although the importance of forecasting systems in maintaining supply chains for antibiotics has been highlighted,25 the demand forecasting systems continue to be inaccurate.26 More importantly, in developing countries, scarcely available and poorly functioning forecasting systems contribute to the problem of disrupted supply chains.27 Additionally, the absence of pooled procurement mechanisms can both assure demand, and proactively represent the interests of end users to stabilise production and supply.28 Pooled institutional procurement for antimicrobials is either absent or underdeveloped, except for the global drug facility (GDF), which is hosted by the Stop TB Partnership, and which purchases antimicrobials for TB and distributes them to at least 130 countries, including HICs, for example, Australia.29 However, there are circumstances when even a pooled procurement facility such as GDF cannot guarantee timely availability of medicines.
Strategic partnerships and alliances, of which there are many in global health for different diseases (such as leprosy, river blindness or leishmaniasis) are also vulnerable to fragile supply chains. However, even for such partnerships and alliances, if a company stops manufacturing or supplying the drugs critical to a partnership or alliance, citing, for example, market-related issues, then the recipients in a partnership suffer. One example is clofazimine, a drug used for the treatment of multidrug resistant TB, originally provided by Abbott. Subsequently, Abbott India informed the National Pharmaceutical Pricing Authority (NPPA) that the company was unable to continue with production of clofazimine citing nonavailability of raw materials. In this instance, active intervention from the drug regulators was needed to rectify the situation.30
Poorly functioning system of financing and drug pricing environment
Both patented and off-patent antimicrobials are affected by the financing and drug pricing environment. For pharmaceutical companies, the opportunity cost of investing in antimicrobials and the net present value compared with other therapeutic areas such as cancer and chronic cardiovascular disorders discourages commercial research and development investments.31 This has created an environment where innovation and technology and early translational research in the field of antimicrobials frequently springs from public sector funding, philanthropic agencies and institutions, either through direct investments in companies and trials or through indirect funding of product development partnerships such as the TB alliance. If successful throughout the initial discovery phase, investigational compounds and other medical technologies are ‘handed over’ or ‘spun out’ to private enterprises whose efforts in further development, marketing and distribution are driven by market considerations.32
While significant public and philanthropic funding drives the drug development cycle, manufacturing and a company’s decision to maintain adequate supply, often depends on the product price. Low prices of antimicrobials can be a direct cause of a shortage. For example, in India, the availability of certain antimicrobials was negatively affected when antimicrobials listed on the national essential medicines list (EML) were placed under the ambit of the Drugs and Price Control Order (DPCO) 2013, capping the maximum market price for drugs in the national EML.33 EMLs, first produced by the WHO, are intended to be a minimum list of medicines that satisfy basic requirements of a healthcare system.34 Since the first EML issued by WHO, countries have developed national EMLs reflecting the healthcare needs of their populations. The purpose of the DPCO was to cap the maximum market price for drugs on EML and ensure essential medicines remain accessible to all. However, an unintended consequence was that the manufacture and availability of certain drugs were threatened as pharmaceutical companies possibly determined profitability was inadequate to sustain manufacturing.2
Benzylpenicillin, a first-choice treatment for prevention of mother to child transmission of syphilis as well as the management of rheumatic heart disease, is an example of an antimicrobial that though medically essential, is not a financially viable product. The manufacture of the injectable form requires sterile conditions, which means investment in manufacturing infrastructure. However, the finished product has a very low market price (US$ 0.11–0.2 per unit dose, depending on the dosage). Worldwide, only four companies manufacture the API for penicillin, and due to low profit margins, manufacturing is now downsized to a minimum.35
In contrast to the conditions of financing and the drug pricing environment, intellectual property laws and regulations that create market monopolies can suppress demand when prices are too high. The price structure of patented new agents that come to market often prohibit their use in LMICs. Companies frequently do not make such new products available in LMICs due to an anticipated lack of financial return.36 Whether decreased initial availability impacts antimicrobial shortages needs to be studied.
Issues related to policy and regulatory processes
It is important to also reflect on the central role that governments have in addressing the problem of shortages. Ensuring availability and access to essential services and essential goods remain the responsibility of governments and should be required under a country’s constitution or equivalent. And yet, while the provision of healthcare services is regarded as an ‘essential service’, there is no systematic tracking of whether and how countries ensure access to essential medicines.37 This is important because sustained availability of essential medicines is integral to access to antimicrobials, at least those which are included on the EML. Until 2016, only 22 National Constitutions across the globe recognised governments’ responsibility towards protecting the right to access and/or availability and quality of medicines.37 A constitutional mandate to uphold a right to access to medicines would require governments to regulate, forecast and ensure that supply chain management does not undermine access to affordable, quality-assured antimicrobials and establish mechanisms for accountability for antimicrobial shortages.
Another problem is that shortages create a void in the market. Antibiotic demand does not diminish in times of antibiotic shortages. The gap in the market is at risk of being exploited by substandard, counterfeit and falsified medicines. In countries where legislation around quality assurance of antibiotics is not enforced or strictly regulated, the infiltration of such products into the market is made easier. Such a situation may be particularly prevalent in pluralistic health systems where the population supplement their healthcare needs through out-of-pocket expenses and choose between private and public care.38
Strategies and tactics to address antimicrobial shortages
Addressing shortages will require different strategies to both anticipate and respond to this on-going challenge. In the section below, we present the key strategies together with enabling tactics to foster appropriate responses to acute and chronic shortages.
Organised, coordinated, and strengthened forecasting
Economically viable strategies should be explored for addressing existing challenges in manufacture and supply. An economically viable strategy requires accurate, dedicated and trustworthy forecasting which itself is based on collaboration between public sector organisations, governments and civil society.39 Systems for collating sales and purchase data of antimicrobials, future requirements of antimicrobials and available buffer stocks, are lacking in several regions. Beyond establishing these systems, substantial training is required to ensure such a system is viable and sustainable. Effective forecasting requires strategies for procurement and for measurement. Developing procurement partnerships across health systems, countries and regions will enable greater leverage to exploit opportunities for optimising acquisition of antimicrobial agents.
Building forecasting capacity, particularly in LMICs requires an understanding of the market dynamics, both formal and informal and the drivers for antimicrobial use and demand.26 40 Improved data gathering, managing and sharing is essential in benchmarking for medication use, budgetary planning, as well as tracking infectious diseases epidemiology and AMR. We propose that dedicated demand forecasting and preparedness systems for antimicrobials are developed, building on existing surveillance systems and investing in developing sustainable surveillance where it is lacking. Cooperation and appropriate data sharing are essential with and across existing platforms like WHO’s Global Antimicrobial Resistance Surveillance System (GLASS), World Organisation for Animal Health (OIE) surveillance network for AMR in animals and from data on antimicrobial sales. Capacity needs to be built for surveillance across private organisations. Any issue related to AMR, including that shortage of essential antimicrobials would necessarily need to be planned within a ‘One Health’ framework at the outset or in a staged manner. The planning and implementation for running such a system is daunting and would require intersectoral cooperation and commitment and support of national governments. Therefore, political commitment is critical to the strategy of forecasting which should also incorporate pandemic preparedness. Important challenges such as international power disequilibrium overshadowing the interests of LMICs, regional politics and conflict zones, all would need to be simultaneously addressed with added safeguards.
Subscription models
While demand for antimicrobials has grown, the number of actors involved in manufacturing and distribution has decreased. Several AMR stakeholders have highlighted this issue and suggested options such as fixed yearly payment for guaranteed supply, incentives to maintain or expand production and tenders that ensure allocation to several suppliers to sustain a healthy market.21 The ‘Netflix model’ adopted in Australia to procure Direct Acting Antivirals (DAA) to treat Hepatitis C is an example of such a model, although its use was tied more closely to assuring affordability as opposed to safeguarding supply.41 The Australian model was built on a form of delinkage, or decoupling funding of or payment for research and development from the end-product price. To do so, the government paid a lump-sum that estimated total demand for DAAs for patients with Hepatitis C infection. In AMR, delinkage can focus on delinking payment from sales revenue (volume and prices) to discourage inappropriate use and assure affordability.42 Such a model could be deployed to assure adequate supply based on forecasted need of an antibiotic over a defined period, while ensuring a manufacturer’s sales revenue is tied neither to the price paid nor the volumes sold.43
More recently, countries like the UK and Sweden have started to explore alternative payment models for both new and older antibiotics. Such models need to be explored and piloted by LMICs.43
Voluntary licensing
Voluntary licensing refers to the practice by ‘patent holding pharmaceutical corporations (licensors) to set out the terms under which a generic version of a patented medicine can enter the market from alternative suppliers (licensees)’.44 This practice can have a positive public health impact and enable greater access to generic medicines in LMICs if patent holders issue appropriate voluntary licenses, with technology transfer (where appropriate). One example of a voluntary license contributing to public health objectives is the National Program on Control and Prevention of viral hepatitis in India, which facilitated mechanisms for health systems within each State to procure affordable, generic versions of DAAs for Hepatitis C (which otherwise are patented), providing it free of charge to patients in government-funded clinics. These medicines were manufactured under voluntary licenses.45 The production of DAAs under voluntary license in India provided greater access to these medicines in other LMICs through inclusion in the territory of the voluntary license or because the patent holder had not sought patent protection for those DAAs in certain countries. By contrast, the unwillingness of patent holders for new anti-TB medicines bedaquiline and delaminid to negotiate voluntary licenses with generics manufacturers or entities such as the Medicines Patent Pool has translated into on-going challenges with affordability, availability and uptake of these medicines to treat drug-resistant TB.46 These drugs are made available in certain parts of the world through their compassionate use programme or conditional access for programmatic management for multidrug resistant TB. While availability of drugs through compassionate use programmes ensures availability of medicines, dependency on the manufacturer continues to remain. Another case in point are the few voluntary licenses for COVID-19 vaccines and therapeutics.47 These examples highlight that while voluntary licensing can be a useful tool to ensure early access to antimicrobials in countries with a high burden of infectious diseases, it is essential that such licenses have appropriate terms and conditions and are backed by strategies for quality assurance. Appropriate voluntary licenses can be assured through contractual obligations introduced by public and philanthropic funders of research and development, or by governments (that are excluded from or negatively impacted by voluntary licenses for antimicrobials) exercising public health safeguards enshrined in global trade agreements.
Facilitating regulatory processes
Strategies targeted at improving regulatory approval processes could be instrumental in making new therapeutics available without a significant lag period between first introduction to market and subsequent product launches in different countries. Collaborative regulatory approval processes, supported by WHO such as the prequalification programme and the collaborative registration system have been instrumental in ensuring timely access, positively impacting public health and cost savings.48 Other approaches such as deployment of emergency use authorisation (EUA) and compassionate use, can also facilitate early availability of new antimicrobials. EUA has enabled companies to supply their products to countries during the current pandemic and facilitated access to drugs in certain areas of India to address multidrug resistant TB.49 The process of WHO prequalification programme can be strengthened to enable quicker access to finished pharmaceutical products and API particularly in LMICs. Certification of quality for vaccines by WHO under its EUA procedure has been instrumental in accelerating access to quality vaccines to large parts of the world. However, EUA should primarily be seen as a short-term tool prior to introduction of harmonised and collaborative regulatory processes and should not undermine requirements for companies to conduct full clinical trials and pharmacovigilance to ensure safety and efficacy.
Importantly, the need for inclusion of access to essential medicines either independently or within the framework of right to health in national constitutions needs considerable deliberation. A good starting point would be to make a repository of existing national legislations and corresponding enabling regulations and policies about access to essential medicines including essential antimicrobials, as has been previously suggested.50 51 A model legislation with provisions for modification in accordance with legal frameworks in which individual countries function would be useful. International cooperation to encourage adoption of such legislation in all countries would be required.
Local drug manufacturing
The local manufacture of drugs, often viewed as an exceptional measure to expand access,22 has gained traction as a means to assure supply and access to medicines in response to the shortages linked to the COVID-19 pandemic.52 Government-funded local or regional manufacturing hubs have been mooted and more recently piloted for mRNA COVID-19 vaccine manufacture. A portfolio of strategic antimicrobials at risk of shortages could be targeted for manufacturing and supply through such regional hubs. Furthermore, these regional hubs could be developed to facilitate the prioritisation and manufacturing of API, where needed. Expanding local and regional production hubs to manufacture and supply APIs, could shift the current dominance of API production from China53 to more globally dispersed market hubs.54 This would prevent monopolisation of manufacturing capacities and could also introduce competitive pricing of API, which would be beneficial in reducing cost of goods. The production hub model, however, may not be the solution for sustainable access to less profitable antimicrobials whose profit margins may be a disincentive to large-scale manufacture. Resilient models which are not vulnerable to supply chain disruptions could be more useful in achieving global health and antimicrobial security.42 Important elements of such models include balanced geographic distribution of production units, multiple players at each step of manufacturing and systems to facilitate forecasting. While HICs have already started addressing the issue in a more organised manner,55 there is little evidence of deliberate and strategic planning within or across LMICs.56
Such strategies need to be facilitated through tactical engagement and collaboration with key stakeholders including policy makers, civil society and the public and private sector. Three key tactics are needed: (i) establishing international consortia; (ii) public–private partnerships for antimicrobial manufacture and (iii) building capacity for public sector manufacture of antimicrobials.
Establishing international consortia to forecast, manage and respond to the antimicrobial supply and availability issues
One means to address the growing problems of shortages of antimicrobials would be to create global and regional consortia with the participation of different stakeholders. This can be backed by an international legal framework to address AMR as has been previously suggested.57 Figure 2 outlines what such a consortium might look like. The legitimacy of such an international consortium would be grounded in the right to healthcare and the progressive integration of access to medicines within the framework of constitutions of nations. Successful collaboration with different stakeholders within the consortium may be able to influence international regulations and trade rules, facilitating more effective and sustainable movement of essential pharmaceutical components. Yet even if international consortia intend to encourage collaboration among governments and between the public and private sector on an equitable basis, such entities need to avoid replicating or exacerbating existing imbalances and need to allow individual governments, the flexibility to address supply and access challenges independently when required. Importantly, governance structures need to be balanced between Northern and Southern countries (with a role for civil society and independent experts), via transparent decision-making and reporting to facilitate accountability and by encouraging financial contributions by all participating governments according to ability to pay.
Figure 2.
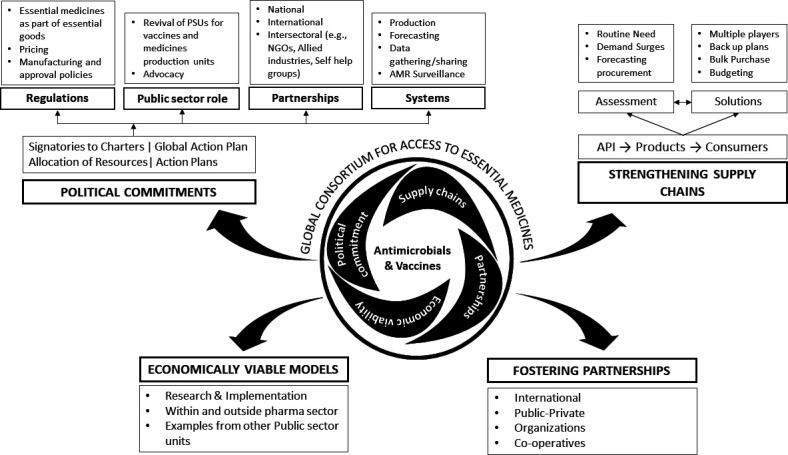
A proposed consortium for assuring access to antimicrobials and vaccines. API, active pharmaceutical ingredients; NGOs, non-governmental organisations; PSU, Public Sector Undertakings.
Public–private partnerships for antimicrobial manufacture
Production and supply of medicines can be located exclusively within the public or private sector. We discuss several ways in which public sector manufacture of medicines can be deployed and strengthened. Yet such public sector production may not be adequate for all medicines and vaccines of public health importance, especially as some governments may not wish to assume responsibility for public sector manufacture. Partnering with or regulating the private sector, with appropriate regulation, can help the public sector fill gaps or address other unmet needs.
One reason public sector production may not be sufficient is that manufacturing can be a complex endeavour that may be best accomplished through collaboration between the public and private sectors. Public–private collaboration in the pharmaceutical field has already achieved significant outcomes on an accelerated timetable, such as the development and manufacture of new COVID-19 vaccines and therapeutics, although such outcomes may not always be equitable.58
The manufacture and timely supply of antimicrobials may involve utilising a mix of approaches including through public production, oversight of the private sector or through public–private partnerships. Ultimately, it can only be successful if governments are responsible for assuring supply.
Different models from other sectors should be evaluated for learning, for example, partnerships which encourage innovation while successfully working with government and research organisations. A good learning, for example, would be that of manufacturing capacities for space programmes which require great deal of sophistication and complexity and successful programme modelled on public–private collaboration exists.59
Dedicated facilities for production of antimicrobials and/or vaccines facing shortages or named in EMLs
An internet search for public sector units (units primarily funded by government with or without support from other international organisation) producing finished pharmaceutical products of antimicrobials indicates that a substantial percentage are situated in India.60 Some key examples are Hindustan Antibiotics,61 Rajasthan Drugs and Pharmaceuticals, Jaipur,62 Bengal Chemicals and Pharmaceuticals, Kolkata,63 Indian Drugs and Pharmaceuticals64 and the Central Research Institute, Kasauli.65 Examples from across the globe include Institute Pasteur Tunis,66 Drug Technology Institute, Fiocruz67 and Staatens Serum Institute.68 Fiocruz, which is affiliated with the Oswaldo Cruz foundation in Brazil, contributes to nearly 40% of the Brazilian government’s drug procurement. Such public sector units both manufacture small molecules, and are important facilities for vaccine, sera and antibody production. The major Chinese companies now dominating international API manufacturing originated as publicly owned units in the 1950s. In recent years, many of these companies are operating as private entities.53 These institutes have existed for several decades and have benefited from investments and contributions by intergovernmental agencies such as WHO and UNICEF (Hindustan Antibiotics Limited). The infrastructure and trained manpower of such manufacturing units can augment production and assure the availability of antimicrobials. We suggest that building on such existing investments and infrastructure, a dialogue should be initiated to strengthen the capacities of existing units, invest in more units, improve the efficiency of the workforce and ensure a constant supply of medicines, especially for conditions of public health importance. Importantly, such units can be useful for manufacturing of important drugs requiring only small volumes and drugs that need dedicated facilities like beta-lactams.
Such dedicated facilities do not have to reside solely in the public sector. Collaboration with private manufacturers and innovator companies can be central to developing and operating such a facility, as well as for technology and knowledge transfer and ensuring second site production for low volume old and new antibiotics. Where the private sector no longer wishes to produce commercially non-viable products, such collaborations, with technology transfer to a public sector facility, can be a viable exit strategy for such products. A good example of such an initiative is that of CivicaRx in the USA which is dedicated to manufacturing and supplying low-cost generics and drugs on EML.69 Although it is a private enterprise, the company is partnering with governmental and regulatory agencies to enable the focus of the company.
Conclusion
Shortages of medicines, especially antimicrobials, are a threat to global health security and need to be treated with the same urgency as food and energy shortages. Not only do antimicrobial drug shortages affect access, but they also contribute to inappropriate antimicrobial use and drive AMR. To build robust systems capable of forecasting, mitigating against and responding to antimicrobial shortages, improved government collaboration and participation is essential. Sustainable preparedness against the threat of drug shortages and their unintended consequences, through policy and strategy will ensure that no one is left behind. Private sector, non-governmental organisations, community groups and societies also have an important role to play in meeting strategic goals and objectives tackling antimicrobial shortages worldwide. To ensure that antimicrobial shortages no longer pose a threat to the emergence of AMR and global health security, a multimodal strategy is needed, which includes interventions ranging from advocacy and forecasting to public–private collaborations, new economic models and the proposed international consortia. Only through global partnership and collaboration will we be able to address the issues that undermine antimicrobial supply and access.
Acknowledgments
EC, MM and AH acknowledge funding from the Economic and Social Research Council (ESRC) and the National Institute for Health Research ASPIRES project (Antibiotic use across Surgical Pathways: Investigating, Redesigning and Evaluating Systems) (https://www.imperial.ac.uk/arc/aspires/). ASPIRES aims to address antimicrobial resistance and improve clinical outcomes optimising antibiotic usage along surgical pathways. The support of ESRC as part of the Antimicrobial Cross Council initiative supported by the seven UK research councils, and the support of the Global Challenges Research Fund, is gratefully acknowledged. Chakrant Mothsara, PhD student in the Department of Pharmacology, Postgraduate Institute of Medical Education and Research prepared the first draft of
figure 2 with input from the coauthors.
Footnotes
Handling editor: Senjuti Saha
Twitter: @e_charani
Contributors: NS, EC and SM conceived of and drafted the paper. MB and RM moderated on the first draft. MB, RM, AKP, AH and MM contributed to framing of key points. All authors contributed to editing and finalising the paper. EC as the guarantor accepts full responsibility for the work and overall content.
Funding: EC was funded by the Academy of Medical Sciences Hamied Foundation UK-India AMR Visiting Professorship Award.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
All data relevant to the study are included in the article.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Mendelson M, Røttingen J-A, Gopinathan U, et al. Maximising access to achieve appropriate human antimicrobial use in low-income and middle-income countries. Lancet 2016;387:188–98. 10.1016/S0140-6736(15)00547-4 [DOI] [PubMed] [Google Scholar]
- 2.Kakkar AK, Shafiq N, Malhotra S. Ensuring access to 'access' antibiotics: an imminent consideration for sustainable antimicrobial stewardship in the developing world. Infect Dis 2019;51:395–8. 10.1080/23744235.2019.1574978 [DOI] [PubMed] [Google Scholar]
- 3.Kakkar AK, Shafiq N, Malhotra S. Cefazolin shortages in the developing world: the same, but different too. Clin Infect Dis 2021;72:1293–5. 10.1093/cid/ciaa847 [DOI] [PubMed] [Google Scholar]
- 4.Tuberculosis drug shortage alert - Telegraph India. Available: https://www.telegraphindia.com/health/tuberculosis-drug-shortage-alert/cid/1748324 [Accessed 6 May 2021].
- 5.2 orphan drugs find manufacturers in Korea. Korea biomedical review. Available: https://www.koreabiomed.com/news/articleView.html?idxno=1584 [Accessed 28 Jun 2021].
- 6.Scott JC, Shah N, Porco T, et al. Cost resulting from anti-tuberculosis drug shortages in the United States: a hypothetical cohort study. PLoS One 2015;10:e0134597. 10.1371/journal.pone.0134597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European Medicines Agency . Zerbaxa (ceftolozane / tazobactam) shortage, 2020. Available: www.ema.europa.eu/contact [Accessed 28 Jun 2021].
- 8.Srinivasaraghavan R, Dhandapany G. Non availability of cloxacillin, a deterrent for rational antimicrobial practice. Indian Pediatr 2016;53:1032–3. [PubMed] [Google Scholar]
- 9.Baraldi E. Causes of antibiotic shortages and the solutions to address them. revive, 2021. Available: https://revive.gardp.org/causes-of-antibiotic-shortages-and-the-solutions-to-address-them/ [Accessed 27 Apr 2021].
- 10.Edwards WH, Kaiser AB, Kernodle DS, et al. Cefuroxime versus cefazolin as prophylaxis in vascular surgery. J Vasc Surg 1992;15:35–42. 10.1067/mva.1992.33841 [DOI] [PubMed] [Google Scholar]
- 11.Norwegian Directorate of Health, Oslo, Norway . Meeting report: antibiotic shortages: magnitude, causes and possible solutions. Available: https://www.who.int/publications/i/item/meeting-report-antibiotic-shortages-magnitude-causes-and-possible-solutions [Accessed 27 Apr 2021].
- 12.Khumra S, Mahony AA, Devchand M, et al. Counting the cost of critical antibiotic shortages. J Antimicrob Chemother 2019;74:273–5. 10.1093/jac/dky410 [DOI] [PubMed] [Google Scholar]
- 13.Gross AE, Johannes RS, Gupta V, et al. The effect of a piperacillin/tazobactam shortage on antimicrobial prescribing and Clostridium difficile risk in 88 US medical centers. Clin Infect Dis 2017;65:613–8. 10.1093/cid/cix379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antimicrobial resistance. Available: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance [Accessed 2 Jun 2021].
- 15.General Assembly of the United Nations . High-Level interactive dialogue on antimicrobial resistance|. Available: https://www.un.org/pga/75/antimicrobial-resistance/ [Accessed 1 Jun 2021].
- 16.World Health Organisation . Median availability of selected generic medicines (%). the global health observatory. Available: https://www.who.int/data/gho/indicator-metadata-registry/imr-details/10 [Accessed 6 Mar 2021].
- 17.Beraud G. Shortages without frontiers: antimicrobial drug and vaccine shortages impact far beyond the individual! Front Med 2021;8:593712. 10.3389/fmed.2021.593712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pulcini C, Beovic B, Béraud G, et al. Ensuring universal access to old antibiotics: a critical but neglected priority. Clin Microbiol Infect 2017;23:590–2. 10.1016/j.cmi.2017.04.026 [DOI] [PubMed] [Google Scholar]
- 19.Drug shortages Summit, 2014. Available: https://www.pewtrusts.org/-/media/assets/2015/02/2014-drug-shortages-summit.pdf [Accessed 6 May 2021].
- 20.The Society of Hospital Pharmacists of Australia . Medicine shortages in Australia. A snapshot of shortages in Australian hospitals, 2017. Available: https://www.shpa.org.au/sites/default/files/uploaded-content/website-content/shpa_medicines_shortages_in_australia_report_june_2017.pdf [Accessed 27 May 2021].
- 21.Acosta A, Vanegas EP, Rovira J, et al. Medicine shortages: gaps between countries and global perspectives. Front Pharmacol 2019;10:763. 10.3389/fphar.2019.00763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beyer P, Balasegaram M, Chandy S. Ensuring sustainable access to effective antibiotics for everyone-everywhere. Available: https://www.reactgroup.org/wp-content/uploads/2021/03/ReAct-Report-Ensuring-sustainable-access-to-effective-antibiotics-for-everyone-everywhere-How-to-address-the-global-crisis-in-antibiotic-Research-and-Development-March-2021.pdf [Accessed 25 Mar 2021].
- 23.Frost I, Craig J, Joshi J, Center for Disease Dynamics, Economics & Policy . Access barriers to antibiotics. Washington, DC, 2019. https://cddep.org/wp-content/uploads/2019/04/AccessBarrierstoAntibiotics_CDDEP_FINAL.pdf [Google Scholar]
- 24.Etchegorry MG, Helenport JP, Pecoul B, et al. Availability and affordability of treatment for human African trypanosomiasis. Trop Med Int Health 2001;6:957–9. 10.1046/j.1365-3156.2001.00764.x [DOI] [PubMed] [Google Scholar]
- 25.Access to Medicine Foundation . Shortages, stockouts and scarcity: the issues facing the security of antibiotic supply and the role for pharmaceutical companies -. Available: https://accesstomedicinefoundation.org/publications/shortages-stockouts-and-scarcity-the-issues-facing-the-security-of-antibiotic-supply-and-the-role-for-pharmaceutical-companies [Accessed 28 April 2021].
- 26.Subramanian L. Effective demand forecasting in health supply chains: emerging trend, enablers, and blockers. Logistics 2021;5:12. 10.3390/logistics5010012 [DOI] [Google Scholar]
- 27.Yadav P. Health product supply chains in developing countries: diagnosis of the root causes of Underperformance and an agenda for reform. Health Syst Reform 2015;1:142–54. 10.4161/23288604.2014.968005 [DOI] [PubMed] [Google Scholar]
- 28.Dubois P, Lefouili Y, Straub S. Pooled procurement of drugs in low and middle income countries pooled procurement of drugs in low and middle income countries DC: center for global development. Available: https://www.cgdev.org/publication/pooled-procurement-drugs-low-and-middle-income-countries,www.cgdev.orgwww.cgdev.org-2019 [Accessed 05 Oct 2021].
- 29.Stop TB partnership. Available: http://www.stoptb.org/ [Accessed 27 Apr 2021].
- 30.The Indian Express . To offset supply shortages, NPPA may HIKE prices of some medicines | business news. Available: https://indianexpress.com/article/business/to-offset-supply-shortages-nppa-may-hike-prices-of-some-medicines-5835000/ [Accessed 06 Mar 2021].
- 31.Plackett B. Why big pharma has abandoned antibiotics. Nature 2020;586:S50–2. 10.1038/d41586-020-02884-3 [DOI] [Google Scholar]
- 32.Vincent Rajkumar S. The high cost of prescription drugs: causes and solutions. Blood Cancer J 2020;10:1–5. 10.1038/s41408-020-0338-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.The drugs (prices control) order, 2013. Available: https://www.nppaindia.nic.in/wp-content/uploads/2018/12/DPCO2013_03082016.pdf [Accessed 6 May 2021].
- 34.WHO model list of essential medicines - 21st list, 2019. Available: https://www.who.int/publications/i/item/WHOMVPEMPIAU2019.06 [Accessed 30 Aug 2021].
- 35.Jazeera A. Why is the world suffering from a penicillin shortage? | health. Available: https://www.aljazeera.com/features/2017/5/21/why-is-the-world-suffering-from-a-penicillin-shortage [Accessed 26 Jun 2021].
- 36.Rahman S, Lindahl O, Morel CM, et al. Market concentration of new antibiotic sales. J Antibiot 2021;74:421–3. 10.1038/s41429-021-00414-5 [DOI] [PubMed] [Google Scholar]
- 37.Katrina Perehudoff S, Toebes B, Hogerzeil H. Essential medicines in national constitutions: progress since 2008. Health Hum Rights 2016;18:141–56. [PMC free article] [PubMed] [Google Scholar]
- 38.Meessen B, Bigdeli M, Chheng K, et al. Composition of pluralistic health systems: how much can we learn from household surveys? an exploration in Cambodia. Health Policy Plan 2011;26 Suppl 1:30–44. 10.1093/heapol/czr026 [DOI] [PubMed] [Google Scholar]
- 39.Bhattacharya B, Lam F. Overcoming shortages of essential medicines: perspectives from industrial and systems engineering and public health practice. Transforming Global Health 2020:179–91. [Google Scholar]
- 40.Charani E, McKee M, Ahmad R, et al. Optimising antimicrobial use in humans - review of current evidence and an interdisciplinary consensus on key priorities for research. Lancet Reg Health Eur 2021;7:100161. 10.1016/j.lanepe.2021.100161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trusheim MR, Cassidy WM, Bach PB. Alternative state-level financing for hepatitis C treatment-The "Netflix Model". JAMA 2018;320:1977–8. 10.1001/jama.2018.15782 [DOI] [PubMed] [Google Scholar]
- 42.Roades T, Kroetsch A, Mcclellan M. Supporting resilient drug supply chains in the United States 2 Stephen Colvill. Available: https://healthpolicy.duke.edu/sites/default/files/2021-07/Supporting%20Resilient%20Drug%20Supply%20Chains%20in%20the%20United%20States%207.13.pdf [Accessed 30 Aug 2021].
- 43.Gotham D, Moja L, van der Heijden M, et al. Reimbursement models to tackle market failures for antimicrobials: approaches taken in France, Germany, Sweden, the United Kingdom, and the United States. Health Policy 2021;125:296–306. 10.1016/j.healthpol.2020.11.015 [DOI] [PubMed] [Google Scholar]
- 44.Voluntary licenses and access to medicines | Médecins sans Frontières access campaign. Available: https://msfaccess.org/sites/default/files/2020-10/IP_VoluntaryLicenses_summary-brief_Oct2020_ENG.pdf [Accessed 30 Aug 2021].
- 45.Correa CM, Velásquez G. Access to medicines: experiences with compulsory licenses and government use– the case of hepatitis C. research paper 85. South centre, 2019. Available: https://www.southcentre.int/wp-content/uploads/2019/04/RP85_Access-to-Medicines-Experiences-with-Compulsory-Licenses-and-Government-Use-The-Case-of-Hepatitis-C_EN.pdf [Accessed 7 Mar 2021].
- 46.Médecins sans Frontières access campaign. DR-TB drugs under the microscope, 6th edition, 2019. Available: https://msfaccess.org/sites/default/files/2019-10/IssueBrief_UTM_6th_Ed_FINAL_web.pdf [Accessed 5 Sep 2021].
- 47.The New York Times . Covid vaccines produced in Africa are being exported to Europe -. Available: https://www.nytimes.com/2021/08/16/business/johnson-johnson-vaccine-africa-exported-europe.html [Accessed 30 Aug 2021].
- 48.Impact assessment of who prequalification and systems supporting activities external assessment report on programmes in the Department of regulation of medicines and other health technologies. Available: https://www.who.int/medicines/news/2019/report_Impact-assessment_WHO-PQ-Reg-systems.pdf [Accessed 30 Aug 2021].
- 49.Udwadia ZF, Ganatra S, Mullerpattan JB. Compassionate use of bedaquiline in highly drug-resistant tuberculosis patients in Mumbai, India. Eur Respir J 2017;49:1601699. 10.1183/13993003.01699-2016 [DOI] [PubMed] [Google Scholar]
- 50.Perehudoff SK, Alexandrov NV, Hogerzeil HV. Legislating for universal access to medicines: a rights-based cross-national comparison of UHC laws in 16 countries. Health Policy Plan 2019;34:iii48–57. 10.1093/heapol/czy101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perehudoff K. Universal access to essential medicines as part of the right to health: a cross-national comparison of national laws, medicines policies, and health system indicators. Glob Health Action 2020;13:1699342. 10.1080/16549716.2019.1699342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prabhala A, Menghaney L. The world’s poorest countries are at India’s mercy for vaccines. It’s unsustainable | The Guardian. Available: https://www.theguardian.com/commentisfree/2021/apr/02/india-in-charge-of-developing-world-covid-vaccine-supply-unsustainable [Accessed 27 Apr 2021].
- 53.World Health Organization . China policies to promote local production of pharmaceutical products and protect public health. Geneva, 2017. https://www.who.int/phi/publications/2081China020517.pdf [Google Scholar]
- 54.Global API industry snapshot pharma demands and evolving markets. Available: www.cphi.com/europe [Accessed 1 Jun 2021].
- 55.Sardella A, De Bona P. Safeguarding the United States pharmaceutical supply chain and policy considerations to mitigate shortages of essential medicines. Available: https://wustl.app.box.com/s/wpqr6704f02eywivqsz7h1vfn8seb848 [Accessed 5 Sep 2021].
- 56.Tukamuhabwa B, Stevenson M, Busby J. Supply chain resilience in a developing country context: a case study on the interconnectedness of threats, strategies and outcomes. SCM 2017;22:486–505. 10.1108/SCM-02-2017-0059 [DOI] [Google Scholar]
- 57.Hoffman SJ, Outterson K, Røttingen J-A, et al. An international legal framework to address antimicrobial resistance. Bull World Health Organ 2015;93:66. 10.2471/BLT.15.152710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.National Academies of Sciences, Engineering, and Medicine . Public–private partnership responses to COVID-19 and future pandemics: proceedings of a workshop—in brief. Available: https://www.nap.edu/catalog/25999/public-private-partnership-responses-to-covid-19-and-future-pandemics [Accessed 28 Aug 2021].
- 59.Public-Private partnerships in space. Available: https://www.issnationallab.org/research-on-the-iss/public-private-partnerships-in-space/ [Accessed 01 Jun 2021].
- 60.Central public sector Enterprises | department of pharmaceuticals. Available: https://pharmaceuticals.gov.in/central-public-sector-enterprises [Accessed 08 Mar 2021].
- 61.Hindustan antibiotics limited | department of pharmaceuticals. Available: https://pharmaceuticals.gov.in/hindustan-antibiotics-limited# [Accessed 08 Mar 2021].
- 62.Rajasthan Drugs & Pharmaceuticals Limited | Department of Pharmaceuticals. Available: https://pharmaceuticals.gov.in/rajasthan-drugs-pharmaceuticals-limited [Accessed 08 Mar 2021].
- 63.Bengal Chemicals & Pharmaceuticals Limited | Department of Pharmaceuticals. Available: https://pharmaceuticals.gov.in/bengal-chemicals-pharmaceuticals-limited [Accessed 08 Mar 2021].
- 64.Indian Drugs & Pharmaceuticals Ltd. Available: http://www.idplindia.in/ [Accessed 8 Mar 2021].
- 65.Central research Institute. Available: https://crikasauli.nic.in/ [Accessed 08 Mar 2021].
- 66.Institut Pasteur de Tunis – home. Available: http://www.pasteur.tn/ [Accessed 30 Aug 2021].
- 67.Production and innovation - Fundação Oswaldo Cruz (Fiocruz): Ciência e tecnologia em saúde para a população brasileira. Available: https://portal.fiocruz.br/en/production-and-innovation [Accessed 28 Aug 2021].
- 68.Antibodies. Available: https://antibodies.ssi.dk/ [Accessed 29 Aug 2021].
- 69.What we do | Civica RX | combating drug shortages and affordability. Available: https://civicarx.org/about/ [Accessed 26 Jun 2020].
- 70.CBC News . From EpiPens to antibiotics, report finds Canada’s drug shortages could be getting worse |. Available: https://www.cbc.ca/news/health/canada-drug-shortage-report-1.4597574 [Accessed 31 Aug 2021].
- 71.The Wire Science . India faces amphotericin B shortage as ‘Black Fungus’ adds to COVID woes -. Available: https://science.thewire.in/health/india-faces-amphotericin-b-shortage-as-black-fungus-adds-to-covid-woes/ [Accessed 31 Aug 2021].
- 72.Beovic B, Béraud G, Carlet J. Ensuring access to old antibiotics. Available: https://www.who.int/selection_medicines/committees/expert/21/applications/ESCMID_WAAAR_ReAct_ISC_BSAC_SPILF-antibiotics.pdf?ua=1 [Accessed 06 Sep 2021].
- 73.The Hindu . Short supply of anti-fungal tablets causes worry. Available: https://www.thehindu.com/news/cities/bangalore/short-supply-of-anti-fungal-tablets-causes-worry/article24455314.ece [Accessed 01 Sep 2021].
- 74.Antibiotic shortages are putting Aboriginal kids at risk. Available: https://theconversation.com/antibiotic-shortages-are-putting-aboriginal-kids-at-risk-114355 [Accessed 31 Aug 2021].
- 75.WHO . | shortages of benzathine penicillin. How big is the problem? and why it matters. Available: https://www.who.int/reproductivehealth/shortages-benzathine-penicillin/en/ [Accessed 31 Aug 2021].
- 76.MedPage Today . Drug shortages hamper MDR-TB treatment. Available: https://www.medpagetoday.com/infectiousdisease/tuberculosis/36890 [Accessed 31 Aug 2021].
- 77.The Hindu . Crisis looms as country faces TB drugs stock-out -. Available: https://www.thehindu.com/news/national/crisis-looms-as-country-faces-tb-drugs-stockout/article4824396.ece [Accessed 01 Sep 2021].
- 78.Koomen LEM, Burger R, van Doorslaer EKA. Effects and determinants of tuberculosis drug stockouts in South Africa. BMC Health Serv Res 2019;19:1–10. 10.1186/s12913-019-3972-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data relevant to the study are included in the article.


