Abstract
Intravascular large B-cell lymphoma (ILBCL) is a subtype of non-Hodgkin’s large B-cell lymphoma that is characterised by neoplastic lymphocyte proliferation within the lumen of small blood vessels, which may occur without an extracellular tumour mass or peripheral blood involvement. This report highlights some of the diagnostic issues for ILBCL, and how it can be approached. The two cases described below highlight two significantly different presentations, one with predominately neurological phenomena, and the other with fever of unknown origin for investigation. Both patients were managed with chemotherapy and intercalated intrathecal chemotherapy, with good clinical outcomes, without further evidence of clinical relapse. These cases along with a review of the literature highlight the key learning points in the difficulties in the diagnosis of this condition, and the appropriate use of random skin biopsy in patient suspected of having ILBCL, such as those with constitutional symptoms with otherwise negative malignancy screening, and unexplained neurological phenomena, especially if recurrent in nature.
Keywords: haematology (incl blood transfusion), medical education
Background
Intravascular large B-cell lymphoma (ILBCL) has an annual incidence of 0.5 per 1 million people1 with a median age of diagnosis of 60–70, and no sex predilection.2 Given its varied presentations, it can be found late in its course, and can be associated with a poor prognosis, with a progression-free survival and overall survival at 2 years of 56% and 66%, respectively, with the use of optimum therapy.3 This condition poses diagnostic challenges due to its heterogeneity in presentation, and therapeutic importance due to its typically poor prognosis, particularly when diagnosed late. These cases aim to build understanding of the varied presentations of this condition, and an approach to investigation and management to allow for earlier diagnosis.
Case presentation
Case 1
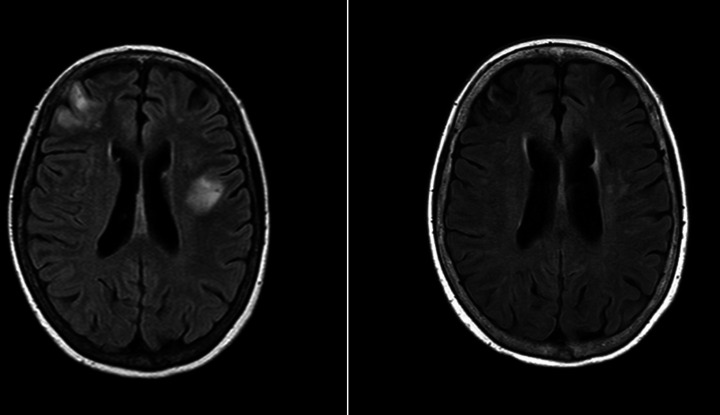
Here, we describe a case of a 71-year-old woman of Asian ethnicity who presented with an subacute neurological decline. The patient’s medical history was significant for bilateral deep vein thrombosis (DVT), type 2 diabetes mellitus, thyroid nodules and gastro-oesophageal reflux disease. Case 1 initially presented postbilateral below knee DVTs after a long-haul flight from the Philippines, which were managed with direct oral anticoagulants for 3 months. This was followed by presumed post-thrombotic syndrome, with violaceous cutaneous lesions on the upper thighs 1 year later. This was investigated with skin biopsy, with findings suspicious of IgG4-related disease, and blood tests finding polyclonal hypergammaglobulinaemia. The patient was subsequently managed with prednisolone and mycophenolate for presumed IgG4-related disease. The patient re-presented with two episodes of intracerebral haemorrhage 1 and 2 months post this presumptive diagnosis, with an initial MRI-brain demonstrated multifocal changes presumed to be related to cerebral amyloid angiopathy. The patient re-presented 1 month later with a subacute history of recurrent falls, progressive ataxia and dysarthria with associated constitutional symptoms over a 4-week period. An MRI brain on this admission demonstrated multifocal infarcts and focal cortical and subcortical haemorrhages (figure 1). At this time, she was noted to have anaemia, and thrombocytopaenia, with an elevated ferritin, negative fluorodeoxyglucose positron emission tomography (FDG-PET) scan, and bone marrow biopsy demonstrating mildly hypercellular marrow. The skin biopsy of the violaceous lesion on the thigh was repeated, with findings consistent with a diagnosis of intravascular B-cell lymphoma.
Figure 1.
MRI imaging for case 1, MRI brain (axial FLAIR) findings at onset of neurological illness with follow-up imaging over 1 year later demonstrating stable gliosis and encephalomalacia with no new lesions. FLAIR refers to fluid-attenuated inversion recovery MRI sequence.
Case 2
An 81-year-old Caucasian man presented to a rural hospital with a 4-week history of progressive constitutional symptoms of fatigue and night sweats, with an associated cough. Previously functionally independent, the patient had a significant reduction in functional mobility, requiring assistance with personal activities of daily living. During this time, the patient had been trialled on broad spectrum antibiotics and subsequent brief trial of 50 mg prednisolone for possible autoimmune or inflammatory condition with nil response, and extensive infectious screening, including for tuberculosis and malaria, and autoimmune screening had been completed with no cause found. A CT chest/abdomen/pelvis and PET imaging were completed, with mild paratracheal lymphadenopathy and splenomegaly that was not PET avid. This in context of significantly elevated LDH and anaemia led to the initial bone marrow biopsy. The initial bone marrow biopsy demonstrated a normocellular marrow with mild plasmacytosis and rare abnormal large B-cells, and a concomitant serum protein electrophoresis demonstrated an increased in IgG heavy chains without light chains, which at that time was thought insufficient for a diagnosis of lymphoma or heavy chain myeloma. The patient was then transferred to a tertiary hospital for further evaluation and reviewed by multiple teams including haematology, infectious diseases, neurology and rheumatology. During the admission, the patient continued to have fevers, and progressive weakness, becoming fully dependent in activities of daily living. A repeat bone marrow biopsy was completed, and subsequent random skin biopsies from the chest and left thigh were taken given the possibility of intravascular lymphoma given the bone marrow findings of low burden large B-cell Lymphoma and unremarkable PET scan. These procedures determined the presence of ILBCL.
Investigations
The pertinent investigations for both case 1 and 2 are summarised in table 1. The baseline blood tests demonstrate an anaemia and thrombocytopenia in both patients. In conjunction with the significantly elevated lactate dehydrogenase, this was concerning for a haematological malignancy. The PET imaging is a useful tool in diagnosis of lymphoma in its ability to localise involved lymph nodes or extranodal sites that may be amenable to biopsy. However, in both of these cases, the PET imaging and bone marrow biopsies were fairly unremarkable, which is consistent with the variability of PET imaging and bone marrow biopsy results in this condition.4–6 The skin biopsy was the final test in both cases leading to the diagnosis. However, it should be noted that case 1 had a prior biopsy demonstrating polyclonal plasma cell infiltrate consistent with cutaneous plasmacytosis or IgG4-related disease, and one of the biopsies for case 2 was a negative biopsy, which could be explained by the depth of the biopsy.7 8 The pathognomonic findings in both cases that led to the diagnosis was the presence of atypical large lymphoid cells within the lumen of blood vessels in the cutaneous tissue.
Table 1.
Summary of investigations for case 1 and 2
| Case 1 | Case 2 | |
| Haemoglobin | 66 g/L (115–155 g/L) | 80 x g/L (120–170 g/L) |
| White cell count | 9.8×109 /L (4.0–12.0 ×109 /L) | 8.5×109 /L (4.0–12.0×109 /L) |
| Platelets | 136×109 /L (150–400×109 /L) | 102×109 /L (150–400×109 /L) |
| Lactate dehydrogenase | 896 U/L (120–250 U/L) | 2109 U/L (120–250 U/L) |
| C reactive protein | 78.9 mg/L (<5 mg/L) | 167 mg/L (<5 mg/L) |
| Erythrocyte sedimentation rate | 78 mm/hour (1–12 mm/hour) | 105 mm/hour (1–12 mm/hour) |
| Albumin | 22 g/L (35–50 g/L) | 14 g/L (35–50 g/L) |
| Vasculitis screen | Unremarkable | Negative |
| Lumbar puncture | Elevated protein, normal glucose and cell counts within normal range. Cytology unremarkable | Normal protein with elevated glucose, unremarkable cell counts and cytology demonstrating scattered lymphocytes without malignant cells |
| FDG-PET imaging (Fluorodeoxyglucose Positron Emission Tomography) | Reactive changes only, otherwise unremarkable | Reactive changes only, otherwise unremarkable |
| Biopsy-bone | A mildly hypercellular and reactive marrow, with no morphological or immunophenotypic involvement with lymphoma | A mildly hypocellular marrow with a low burden of involvement with large B-cell lymphoma. A plasmacytosis with lack of light chain expression in approximately 40% of plasma cells is of uncertain significance. |
| Biopsy-skin | Vessel lumina contain large atypical cells with small to moderate amounts of amophilic cytoplasm with irregular outlines. Atypical cells positive for CD20, CD79a and MUM-1. | Atypical enlarged lymphoid cells within the sampled blood vessels. Cells positive for CD20, PAX-5, MUM-1 and BCL-6. |
| Other (relevant to each case) | Antiphospholipid screen-negative | Myositis screen-negative |
| Thrombophilia screen-negative | Neuromuscular Antibodyscreen-negative | |
| MRI brain-several foci of diffusion restriction consistent with acute and subacute ischaemic changes in subcortical white matter of posterior right frontal lobe, with smaller foci in the left precentral gyrus, left frontal lobe and right frontal lobe. Major intracranial arteries patent with no large vessel vascular lesion identified on MRI TOF (Time of Flight). | Nerve conduction studies/electromyography-no evidence of large fibre peripheral neuropathy, myositis, myopathy or neuromuscular junction disorder |
The blood and cerebrospinal fluid results are those taken closest to the time of biopsy. Sex specific reference ranges for blood tests in brackets. The FDG-PET imaging results are summarised from the scans taken prior to diagnosis (see detailed results in online supplemental table 1).
FDG, Fluorodeoxyglucose; PET, positron emission tomography; TOF, Time of Flight.
bcr-2021-244069supp001.pdf (248.5KB, pdf)
Differential diagnosis
Case 1
Case 1 had a complex presentation that spanned over several months of recurrent neurological presentations with both haemorrhagic and ischaemic infarcts in the setting of a new rash. The initial differentials for the violaceous rash, prior to the onset of neurological presentations, was of a post-thrombotic syndrome given the recent history of lower limb DVT. However, biopsies of the thigh lesions were consistent with cutaneous plasmacytosis or IgG4 disease. By the time of the neurological presentations, an infectious screen was negative, and central embolic source was excluded with a negative transoesophageal echocardiogram and no evidence of atrial fibrillation on telemetry. An alternative diagnosis such as cerebral amyloid angiopathy and progressive multifocal leukoencephalopathy were investigated with MRI brain, and cerebrospinal fluid at time of diagnosis was non-inflammatory alongside a negative autoimmune screen, making an autoimmune encephalopathy less likely. In association with this, multiple other findings were appearing with progressive anaemia and thrombocytopenia with a polyclonal hypergammaglobinaemia was thought to be more in keeping with a haematological malignancy, and so PET and bone marrow investigations were completed. Given that a diagnosis had so far not been made, a new biopsy was completed of the thigh lesions to reinvestigate this prior diagnosis of IgG4-related disease, leading to findings consistent with ILBCL.
Case 2
For case 2, the differentials included an infectious aetiology, autoimmune pathology, myositis or myopathy, or haematological malignancy. Initially, case 2 was investigated and managed as an infectious aetiology, with multiple blood cultures, serology, and imaging modalities completed such as CT and PET CT without a cause found. An autoimmune cause was investigated in tandem, with PET imaging, temporal artery biopsy and rheumatological serological tests which all returned as within normal range. Given the symptoms of generalised weakness and fatigue, nerve conduction and electromyographic studies were completed alongside myositis panels to exclude peripheral neuropathy, myositis, myopathy or neuromuscular junction disorder. Without a clear aetiology discovered, a repeat bone marrow biopsy and skin biopsy was completed. The repeat bone marrow biopsy (approximately 1 month post the initial bone marrow) demonstrated a hypocellular marrow with rare small foci of large B-cells strongly positive for CD20, PAX5 and weakly for CD79a and CD19. The findings of this bone marrow biopsy led to the completion of skin biopsies which demonstrated the presence of ILBCL.
Treatment
Both cases initially underwent six 21-day cycles of R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone). However, case 2 had a dose reduced schedule (R-mini-CHOP) due to functional status. For both cases, this was followed by two additional cycles of rituximab monotherapy. Additionally, both cases received central nervous system (CNS) directed therapy with two cycles of intercalated high dose methotrexate. Due to patient age and comorbidities, high-dose chemotherapy with autologous stem cell transplantation rescue was not offered.
Outcome and follow-up
Given the inconclusive results of PET imaging to determine complete metabolic response (CMR) in these patients, the response to therapy was based off alternative measures. For case 1, serial MRI brain scans were completed demonstrating stable gliotic changes and no new lesions. Alongside this, the resolution of skin lesions and improvement in symptoms was monitored. For case 2, an improvement in functional state and symptoms were primarily used, with an associated improvement in anaemia and reduction in LDH to normal levels. Post-treatment PET imaging was also completed, but this was effectively unchanged from the pretreatment scan. After cycle 2 of R-CHOP, case 1 presented with neutropenic fevers with reduced conscious state with an aetiology of cryptococcal meningitis diagnosed on lumbar puncture. This was treated with induction and maintenance antifungal treatment for the duration of the patient’s chemotherapy course. Both cases are receiving follow-up every 3 months to monitor for relapse, which is ultimately based off a combination of patient symptoms and signs alongside basic investigations such as full blood examination and LDH measurement. The utility of subsequent skin biopsies to determine and follow response is uncertain and so was not performed, however may pose an area for future research in these patients.
Discussion
The two cases described demonstrate the multitude of issues associated with the diagnosis and investigation of ILBCL. The patient in case 1 presented with a cutaneous manifestation and recurrent neurological sequelae, without constitutional symptoms. In contrast, case 2 presented with progressive constitutional symptoms, mild lymphadenopathy and splenomegaly, and mildly abnormal bone marrow, without cutaneous or neurological involvement.
The heterogeneity in presentations and rarity of ILBCL lends itself to be a very difficult condition to diagnose.9 The frequencies of different presentations are summarised in table 2.
Table 2.
Frequency of presenting symptoms, signs, and investigations of ILBCL from varying cohorts
| Cohort and date | Murase et al5 | Shimada et al3 4 | Matsue et al2 | Ferreri et al16 | Ferreri et al6 |
| Year | 2007 | 2008–09 | 2019 | 2004 | 2007 |
| Population no | 96 | 106 | 42 | 38 | 50 |
| Median age | 67 | 67 | 73 | 70 | 68 |
| Male (%) | 52 | 56 | 52.4 | 45 | 46 |
| Symptoms (%) | |||||
| Constitutional (Fever, night sweats, weight loss, fatigue) | 76 | 84 | 98 | 55 | 22–42 |
| Neurological (dementia, cerebrovascular events, peripheral neuropathy) | 27 | 25 | 45 | 34 | 42 |
| Respiratory (dyspnoea, hypoxaemia / hypoxia, pulmonary involvement) | 34 | 33 | 80 (hypoxia only) | 3 | 18 |
| Signs (%) | |||||
| Cutaneous (nodules, plaques, macules, telangiectasia, oedema, pain) | 15 | 17 | 14 | 39 | 38 |
| Spleen | 67 | 65 | 81 | 26 | 26 |
| Liver | 55 | 48 | – | 26 | 26 |
| Lymph node involvement | 11 | – | – | 11 | 8 |
| Investigations (%) | |||||
| Bone marrow (haemophagocytosis, atypical lymphoid cells, histiocytosis) | 75 | 59 | 60 | 32 | 30 |
| Haemoglobin <110–120 g/L | 66 | 68 | 95 | 63 (<120) | 66 |
| White cells count <4.0×109/L | 27 | 27 | 24 | 22 | |
| Platelets <100–150×109/L | 58 | 58 | 69 | 29 (<150) | 32 |
| Albumin <30–36 g/L | 47 | 61 | 86 | 18 (<36) | – |
| Elevated Lactate Dehydrogenase (greater than upper limit of normal) | 93 | 98 | 100 (median—934 units/L) | 86 | 85 |
| Elevated Soluble Interluekin-2 Receptor (>5000 U/mL) | 56 | 66 | 69 (median—6886 units/mL) | – | – |
| Blood film: atypical lymphoid cells | 24 | 34 | 14 | 5 | 4 |
ILBCL, intravascular large B-cell lymphoma.
The presentation of significant constitutional symptoms with negative malignancy screening (particularly with negative PET and bone marrow aspirate) could herald the presence of ILBCL, such as with case 2, with fever of unknown origin as a presenting issue.10 While PET imaging can be useful in screening for evidence of malignancy and for guiding biopsy acquisition, in the case of ILBCL, the studies are variable. One review found PET imaging useful in two out of seven cases,4 whereas another study found bone marrow involvement on PET in 76.5% of cases.2 Aside from the small sample sizes, this heterogeneity between studies may be accounted for by the variability in bone marrow involvement in ILBCL, with a finding of tumour burden in 30%–75%.5 6 A proposed algorithm for the diagnostic workup for ILBCL is included in figure 2, with reference to the more common presenting issues of constitutional symptoms, neurological involvement, and cutaneous symptoms, and the roles of various investigations.
Figure 2.
Proposed algorithm for ILBCL diagnostic workup. For patients with a suggestive clinical picture, such as constitutional symptoms or unexplained neurological phenomena, suggested baseline investigations are included. These findings both clinically and through imaging may help determine a possible site of organ biopsy, and blood and bone marrow investigation may demonstrate supportive features for the diagnosis. The determination of biopsy site is also demonstrated, with specific organ or lesion biopsy suggested, and random skin biopsy utilised as an alternative if nil organ involvement determined on initial investigations. ILBCL, intravascular large B-cell lymphoma; FDG-PET, Fluorodeoxyglucose positron emission tomography; LDH, Lactate dehydrogenase; SIL, Soluble Interleukin; Hb, Haemoglobin; WCC, White Cell Count; CNS, Central Nervous System.
The presenting features can vary widely with the two characterised variants,9 and the rarity of this condition, means that clinicians must have a high index of suspicion for ILBCL prior to its work up. This is particularly important because biopsy is critical for diagnosis, and may rely on random skin biopsy, or invasive organ biopsy such as a biopsy of the brain, spleen, lung or adrenal glands.4 Aside from the risks associated with more invasive biopsies,1 skin biopsies themselves are also associated with bleeding complications, even leading to death in one case report,11 however, this has not been demonstrated in larger studies.8 In the case of ILBCL, as demonstrated by case 1, patients may have multiple skin and invasive biopsies before a diagnosis can be made. As yet there is no formalised algorithm to aid in the diagnosis of ILBCL, a proposed algorithm is included in figure 2. If a random skin biopsy is chosen for investigation of ILBCL, the recommended location is adipose containing tissues, particularly the abdominal curtain, thigh, and upper arm, with a sensitivity and specificity of 77.8% and 98.7%, respectively, in one trial.7 An incisional biopsy is suggested over a punch biopsy due to the variable depth of ILBCL lesions from 2 to 11 mm, and the punch biopsy approximate depth samples is 4–5 mm which may increase false negative results.8 The number of biopsies to improve the diagnostic yield is between one and three sites, which in one trial was determined based of the soluble interleukin 2 receptor level, with a value less than 3000 units/mL associated with lower rates of tumour burden, and so three sites were recommended in these patients.1
An important consideration is the high rate of central nervous system (CNS) involvement at initial presentation which indicates the consideration of ILBCL in the diagnostic workup of recurrent or unexplained neurological phenomena,12–14 as in case 1. This can often be misdiagnosed initially as other mimicking other conditions such as cerebral amyloid angiopathy,15 as demonstrated in case 1. Further, in the absence of other potential sites, a presentation with neurological symptoms with a high index of suspicion for ILBCL, could potentially warrant a brain biopsy for diagnosis.1 CNS involvement is associated with higher mortality in patients with ILBCL, with a median survival of 18 months.12 In one study, the rate of CNS involvement at diagnosis was deemed to be 25%,12 with a further 25% CNS involvement during the course of the disease. It should be noted however that the rates of neurological symptoms range in frequency, from 34% to 76% depending on the cohort.16 17 In patients without CNS involvement at diagnosis, there is a risk of CNS recurrence at 3 years of 25%, and a 25% risk of recurrence within the first year for patients with CNS involvement at diagnosis.12
In regard to treatment of ILBCL, the mainstay of treatment is systemic therapy and CNS directed therapy. As such, ILBCL is typically treated similarly to diffuse large B-cell lymphoma. Anthracycline-based chemotherapy forms the backbone of systemic therapy,18 with use of rituximab (ie, R-CHOP), which has been associated with an improved complete response and progression-free survival.3 Given the high rates of CNS involvement, patients with ILBCL should be considered for CNS direct therapy or prophylaxis with either intrathecal chemotherapy, systemic high-dose chemotherapy, or targeted radiotherapy depending on involvement.12 Given the paucity of long-term data on ILBCL, the prognostication has been made on 18–24 months follow-up. Rituximab containing anthracycline based regimens have demonstrated a higher complete response rate compared with chemotherapy alone (82% vs 51%), with a progression-free survival and overall survival at 2 years of 56% and 66% for patients on rituximab containing regimens vs 27% and 46% with chemotherapy alone.3 As such, early diagnosis to begin treatment is critical to improve outcomes.3 6 19
Learning points.
The diagnosis of intravascular large B-cell lymphoma (ILBCL) is difficult due to the heterogeneity of presentations and often inconclusive results of screening investigations.
ILBCL should be in the differential diagnosis of patients with unexplained neurological phenomena, suspected malignancies and fever of unknown origin, particularly in the presence of constitutional symptoms, elevated LDH values, peripheral blood or bone marrow abnormalities.
The diagnosis is made by demonstrating abnormal large B-cells in the lumen of blood vessels on tissue biopsy, either from a specific involved organ or cutaneous lesion, or random skin biopsy.
If random skin biopsy is chosen, the current evidence supports 1–3 incisional biopsies of the abdomen, thigh or upper arm.
Further areas of study for ILBCL include comparison studies of incisional biopsy versus punch biopsy for diagnosis, and the formulation and subsequent validation studies for an algorithm-based approach to guide the diagnostic workup for this condition to better allow for earlier identification, and therefore earlier treatment of ILBCL.
Footnotes
Twitter: @neuroimaging
Contributors: JLB: primary author of article. OS: editing of work, took part in literature review and writing of discussion. AP: supervisor, haematologist primarily caring after the two included patients, editor of work and assistance with creation of discussion.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s)
References
- 1.Yamada E, Ishikawa E, Watanabe R, et al. Random skin biopsies before brain biopsy for intravascular large B-cell lymphoma. World Neurosurg 2019;121:e364–9. 10.1016/j.wneu.2018.09.110 [DOI] [PubMed] [Google Scholar]
- 2.Matsue K, Abe Y, Narita K, et al. Diagnosis of intravascular large B cell lymphoma: novel insights into clinicopathological features from 42 patients at a single institution over 20 years. Br J Haematol 2019;187:328–36. 10.1111/bjh.16081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shimada K, Matsue K, Yamamoto K, et al. Retrospective analysis of intravascular large B-cell lymphoma treated with rituximab-containing chemotherapy as reported by the IVL Study group in Japan. J Clin Oncol 2008;26:3189–95. 10.1200/JCO.2007.15.4278 [DOI] [PubMed] [Google Scholar]
- 4.Shimada K, Kinoshita T, Naoe T, et al. Presentation and management of intravascular large B-cell lymphoma. Lancet Oncol 2009;10:895–902. 10.1016/S1470-2045(09)70140-8 [DOI] [PubMed] [Google Scholar]
- 5.Murase T, Yamaguchi M, Suzuki R, et al. Intravascular large B-cell lymphoma (IVLBCL): a clinicopathologic study of 96 cases with special reference to the immunophenotypic heterogeneity of CD5. Blood 2007;109:478–85. 10.1182/blood-2006-01-021253 [DOI] [PubMed] [Google Scholar]
- 6.Ferreri AJM, Dognini GP, Campo E, et al. Variations in clinical presentation, frequency of hemophagocytosis and clinical behavior of intravascular lymphoma diagnosed in different geographical regions. Haematologica 2007;92:486–92. 10.3324/haematol.10829 [DOI] [PubMed] [Google Scholar]
- 7.Matsue K, Abe Y, Kitadate A, et al. Sensitivity and specificity of incisional random skin biopsy for diagnosis of intravascular large B-cell lymphoma. Blood 2019;133:1257–9. 10.1182/blood-2018-11-887570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enzan N, Kitadate A, Tanaka A, et al. Incisional random skin biopsy, not punch biopsy, is an appropriate method for diagnosis of intravascular large B-cell lymphoma: a clinicopathological study of 25 patients. Br J Dermatol 2019;181:200–1. 10.1111/bjd.17603 [DOI] [PubMed] [Google Scholar]
- 9.Ponzoni M, Campo E, Nakamura S. Intravascular large B-cell lymphoma: a chameleon with multiple faces and many masks. Blood 2018;132:1561–7. 10.1182/blood-2017-04-737445 [DOI] [PubMed] [Google Scholar]
- 10.Gill S, Melosky B, Haley L, et al. Use of random skin biopsy to diagnose intravascular lymphoma presenting as fever of unknown origin. Am J Med 2003;114:56–8. 10.1016/S0002-9343(02)01378-5 [DOI] [PubMed] [Google Scholar]
- 11.Maekawa T, Komine M, Murata S, et al. Random skin biopsy of patients with intravascular large B-cell lymphoma associated with thrombocytopenia and coagulation abnormalities: proposal of a modified biopsy method. J Dermatol 2015;42:318–21. 10.1111/1346-8138.12756 [DOI] [PubMed] [Google Scholar]
- 12.Shimada K, Murase T, Matsue K, et al. Central nervous system involvement in intravascular large B-cell lymphoma: a retrospective analysis of 109 patients. Cancer Sci 2010;101:1480–6. 10.1111/j.1349-7006.2010.01555.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giannini C, Dogan A, Salomão DR. Cns lymphoma: a practical diagnostic approach. J Neuropathol Exp Neurol 2014;73:478–94. 10.1097/NEN.0000000000000076 [DOI] [PubMed] [Google Scholar]
- 14.Lozsadi DA, Wieshmann U, Enevoldson TP. Neurological presentation of intravascular lymphoma: report of two cases and discussion of diagnostic challenges. Eur J Neurol 2005;12:710–4. 10.1111/j.1468-1331.2005.01054.x [DOI] [PubMed] [Google Scholar]
- 15.Leclercq L, Mechtouff L, Hermier M, et al. Intravascular large B-cell lymphoma mimicking cerebral amyloid angiopathy-related inflammation. Rev Neurol 2018;174:265–6. 10.1016/j.neurol.2017.06.023 [DOI] [PubMed] [Google Scholar]
- 16.Ferreri AJM, Campo E, Seymour JF, et al. Intravascular lymphoma: clinical presentation, natural history, management and prognostic factors in a series of 38 cases, with special emphasis on the 'cutaneous variant'. Br J Haematol 2004;127:173–83. 10.1111/j.1365-2141.2004.05177.x [DOI] [PubMed] [Google Scholar]
- 17.Brunet V, Marouan S, Routy J-P, et al. Retrospective study of intravascular large B-cell lymphoma cases diagnosed in Quebec: a retrospective study of 29 case reports. Medicine 2017;96:e5985. 10.1097/MD.0000000000005985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferreri AJM, Campo E, Ambrosetti A, et al. Anthracycline-based chemotherapy as primary treatment for intravascular lymphoma. Ann Oncol 2004;15:1215–21. 10.1093/annonc/mdh274 [DOI] [PubMed] [Google Scholar]
- 19.Ponzoni M, Ferreri AJM, Campo E, et al. Definition, diagnosis, and management of intravascular large B-cell lymphoma: proposals and perspectives from an international consensus meeting. J Clin Oncol 2007;25:3168–73. 10.1200/JCO.2006.08.2313 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bcr-2021-244069supp001.pdf (248.5KB, pdf)