Introduction
Vessel wall MRI (VWI) is an advanced MRI application that has progressively shown value in cerebrovascular disease. VWI has been applied to characterization of carotid atherosclerotic disease for more than 25 years1–4, but in more recent years, the application of extracranial vessel wall MRI (EVWMR) has expanded to include traumatic cerebrovascular arterial injuries5,6 and large artery vasculitis7. In addition, the technique has shown promise in differentiation8, characterization9–11 and detection10–16 of intracranial vasculopathies. While most studies to date have focused on adult vascular diseases, these techniques have also shown promise in the evaluation of pediatric intracranial17,18 and extracranial vasculopathies19. We will discuss the existing literature on applications of VWI on pediatric intracranial and extracranial vasculopathies and technical considerations as they apply to pediatric patients.
Intracranial Vessel Wall MRI
IVWMR Techniques
There are several technical considerations for intracranial vessel wall MRI (IVWMR). First, intracranial arteries are small, tortuous structures with thin walls; the normal middle cerebral artery wall measures 0.2-0.3mm20, necessitating high-resolution. Second, the vessel wall is surrounded by flowing blood and cerebrospinal fluid (CSF). Slow flow artifacts can mimic vessel wall pathology21 and insufficient CSF suppression can mimic pathology. Blood suppression21–23 and CSF suppression methods24 are required to characterize the wall features reliably. Third, due to resolution requirements, scan times are long (7-10 minutes per sequence), and protocol times can be limiting. Lastly, due to the various vasculopathies to consider intracranially, multi-contrast imaging (pre- and post-contrast, T1/T2 weighting) is important. Therefore, the implementation in a clinical setting is often challenging, especially for pediatric patients when anesthesia is required.
While earlier studies used 2D imaging methods, over the past decade 3D-IVWMR became popular because of high isotropic resolution, large coverage and high scan efficiency, which led to increased reliability/reproducibility, better pathology detection and characterization and less reliance on the radiologist for imaging targeting. The most widely used 3D sequence is turbo-spin-echo with variable refocusing flip angle train (VFA), termed VISTA (Philips Healthcare; Best, the Netherlands), SPACE (Siemens Healthineers, Erlangen, Germany), or Cube (GE Healthcare, Waukesha, WI, USA). The variable flip angle train modulates the signal evaluation and allows a long echo train (up to 40-60) without significant image blurring25, while the traditional fast-spin-echo sequences often have an echo train of 10-20. The long echo train means acquiring more k-space lines per repetition time (TR), therefore, the scanning efficiency is greatly improved. 3D-IVWMR covering the whole brain with 0.5-0.6mm isotropic resolution can be acquired within 7-10 minutes25, while 2D-IVWMR commonly has slice thickness of 2mm with 0.4-0.5mm in plane resolution that requires 36-45 seconds per slice8,9. The actual resolution of VFA sequences depends on the flip angle train design which determines the point-spread function and image sharpness25. Due to the high-resolution used in 3D-IVWMR, to achieve sufficient signal-to-noise, 3T is optimal.
A major consideration for 3D-IVWMR is blood and CSF suppression, considering volume acquisitions generate flow artifacts. The VFA train has inherent blood suppression effect due to intra-voxel phase dispersion9. In the case of slow or turbulent flow, additional blood suppression techniques including Motion-Sensitized Driven-Equilibrium (MSDE)23 and DANTE24 can be used (Figure 1). At 3T, anti-drive-equilibrium26 can be applied to suppress CSF without increasing scan time, and can help limit CSF flow artifact.
Figure 1.

Accelerated sagittal T1-SPACE (A) shows flow artifact (arrow) in the right V3 vertebral artifact. Post-contrast T1-SPACE (B) shows a similar flow artifact (arrow). DANTE flow-suppressed T1-SPACE post-contrast (C) shows suppression of the luminal flow artifact.
Acceleration techniques such as compressed sensing have shown the ability to accelerate imaging without loss in image quality27. In IVWMR applications, compressed sensing28 and wave-controlled aliasing in parallel imaging (WAVE-CAIPI)29 can reduce scan times by 40-65% while maintaining comparable image quality with reference scans. Artificial intelligence applications, through using a reference standard, can also improve image quality from more accelerated acquisitions30. With further imaging acceleration, reduced anesthesia time for pediatric patients can be achieved, and with sufficient acceleration, potentially reduce the need for MRI anesthesia.
IVWMR in pediatric intracranial vasculopathies
In adult and pediatric inflammatory vasculopathies, IVWMR has shown promise in vasculopathy detection8,31 and differentiation32,33 relative to other vascular diseases. The development of new techniques for inflammatory vasculopathies are especially important considering the limitations of current angiographic techniques; DSA has shown sensitivities that can be as low as 50%, and specificity in the range of 30% for inflammatory vasculopathies34–36. The limitations of luminal imaging, including DSA, in the evaluation of inflammatory vasculopathies, is the limited differential ability between various vascular diseases, as well as the limited detectability of small artery angiitis. IVWMR typically can depict small artery angiitis due to arterial wall inflammation spilling over and involving perivascular tissues, resulting in much more extensive inflammatory changes and enhancement, while luminal imaging cannot accurately depict the absence or irregularity of tiny perforator branches35,37.
Inflammatory vasculopathies are an important consideration in pediatric patients with strokes. Secondary CNS vasculitis can develop in the setting of systemic vasculitis resultant from numerous diseases, including systemic lupus erythematosus, infectious, mitochondrial abnormalities, malignancies, radiation, and toxic/drug exposures38. Childhood primary angiitis of the CNS was originally considered to be a rare disease, representing 2-6% of all pediatric arteriopathies39, however, it has been shown to be an important cause for pediatric strokes40. Similar to adult primary angiitis, pediatric angiitis can affect medium or small arteries, however, the specificity of angiographic techniques for inflammatory vasculopathies for children is likely higher than it is for adults, due to the absence of atherosclerosis and reversible cerebral vasoconstriction syndrome in pediatric populations. Two disease entities, specifically post-varicella angiopathy and transient cerebral arteriopathy do, however, overlap in imaging appearances with vasculitis. In addition, angiographic techniques are limited in detecting small artery vasculitides, and these cases frequently require brain biopsy for diagnosis. IVWMR has the potential to limit the need for highly invasive biopsies in this subset of vasculitis.
Dlamini et al17 evaluated 26 pediatric patients with acute ischemic stroke, and found that IVWMR appearances strongly correlated with specific types of vasculopathies, including transient cerebral arteriopathy, primary angiitis, dissection, cardioembolic stroke and dissecting aneurysm. 35% showed arterial wall enhancement, while 59% (10/17) of those that did not show enhancement had abnormal MRA appearance, and included steno-occlusive (n=8), dissection (n=1) and pseudo-aneurysm (n=1) diagnoses.
The typical imaging appearance of an inflammatory vasculopathy, specifically primary or secondary angiitis of the CNS, is circumferential, homogeneous, intense arterial wall enhancement that may or may not be multifocal on T1-weighted post-contrast IVWMR (Figure 2)8. In pediatric patients, however, circumferential wall enhancement is not exclusive to inflammatory vasculopathies, as this can also be seen in the setting of subarachnoid hemorrhage or vasospasm41. Inclusion of T2-weighted imaging can help better differentiate vasculopathies, as well inflammatory vasculopathy typically has wall thickening that is isointense to gray matter, while vasospasm or subarachnoid hemorrhage-related wall changes typically show little to no wall thickening on T2-weighted imaging8,33,41.
Figure 2.

18-year-old with history of Henoch-Schoenlein purpura and cerebral vasculitis presenting with vision changes, parasthesias and dysphasia. Axial contrast-enhanced MRA (A) shows mild bilateral ACA luminal narrowing with enhancing perivascular halo (arrows). Sagittal pre- (B) and sagittal (C) and axial (D) post-contrast T1-SPACE show multifocal bilateral ACA wall enhancement (arrows), representing recurrent vasculitis.
Focal cerebral arteriopathy (FCA) is a vasculopathy involving one side of the anterior circulation or a single large intracranial anterior circulation artery, and is a frequent cause of pediatric stroke42. Focal cerebral arteriopathy can further be classified into: 1) inflammatory, 2) post-traumatic dissection and 3) not otherwise specified sub-types. In the evaluation of 16 patients with FCA, Stence et al18 found that strong arterial wall enhancement was significantly associated with progressive arteriopathy (p=.008). In the assessment of 9 inflammatory FCA patients, Perez et al42 found that there was no correlation between the presence of arterial wall enhancement and infarct size, outcomes, and arteriopathy severity. These studies are limited by small sample sizes, and further investigation is necessary.
IVWMR has also been applied to sickle cell disease (SCD)43. In the evaluation of 69 adult and pediatric SCD patients, arterial walls were significantly thicker than normal controls (n=38) (1.07±.19mm vs. .97±.07mm, p<.001), and wall thickness was higher in SCD patients receiving chronic transfusions (p=.013). There was also a trend (p<.1) for wall thickness correlating with decreasing hematocrit and increasing circulating white cells.
IVWMR in Intracranial Aneurysms
There has been extensive investigation evaluating the value of IVWMR in intracranial aneurysms. Studies have shown that aneurysmal wall enhancement correlates with inflammation in the aneurysm wall on histology44. Aneurysm wall enhancement has also shown significant association with increased scores of aneurysm vulnerability45 and subsequent aneurysm growth46. Aneurysm wall enhancement and quantitative intensity of enhancement is associated with symptomatic aneurysms47. In the evaluation of 341 unruptured intracranial aneurysms with IVWMR, 93 of which were symptomatic, symptomatic aneurysms more frequently showed circumferential wall enhancement (66.7% vs. 17.3%, p<.001) and quantitative wall enhancement (p<.001), and both were independent factors associated with symptoms. The combined wall imaging characteristics had a sensitivity of 95.7% and specificity of 73.4% for symptomatic aneurysms. Wall enhancement is also seen with pseudoaneurysms and dissecting pseudoaneurysms, likely secondary to wall inflammatory changes48. The wall enhancement can extend beyond the region of luminal abnormality, likely indicating further extent of wall injury49.
In patients with aneurysmal SAH, an increased likelihood of IVWMR wall enhancement relative to those without aneurysm rupture (29.9% vs. 7.2%; OR 5.5, 95% CI 2.2-13.7) has been shown and a strong ability to predict subsequent vasospasm development when controlling for grade of hemorrhage (OR 3.9; 95% CI 1.7-9.4)41.
Moyamoya Vasculopathy
Moyamoya vasculopathy represents heterogeneous disease processes that lead to steno-occlusive changes involving the carotid terminus, proximal M1 MCA and/or proximal A1 ACA. Moyamoya disease (MMD) represents an idiopathic steno-occlusive process, though genetic predisposition has been indicated50. This is supported by the fact that the proportion of patients with afflicted first-degree relatives in Japan is 10% and in the US 6%51. MMD develops bilaterally. MMD has bimodal distribution, with peaks at 5 years and 40 years old, and represents the most common cerebrovascular disease in Japanese children (3 per 100,000)51. Moyamoya syndrome develops secondarily in neurofibromatosis-1, Down’s syndrome, SCD, radiation and other disorders51, and is more likely to appear unilaterally.
IVWMR has been able to better characterize MMD than luminal techniques. IVWMR improved the diagnostic accuracy of differentiating MMD from inflammatory Moyamoya syndrome compared to luminal imaging alone, with accuracy improvement from 31.6% to 86.8% (p<.001)32. MMD typically shows minimal or no wall post-contrast T1-weighted enhancement, and no wall thickening, which differs from inflammatory Moyamoya, which shows circumferential, intense wall enhancement and wall thickening. When MMD does show wall enhancement, there is no wall thickening on T2-SPACE (Figure 3), while inflammatory Moyamoya shows wall thickening.
Figure 3.
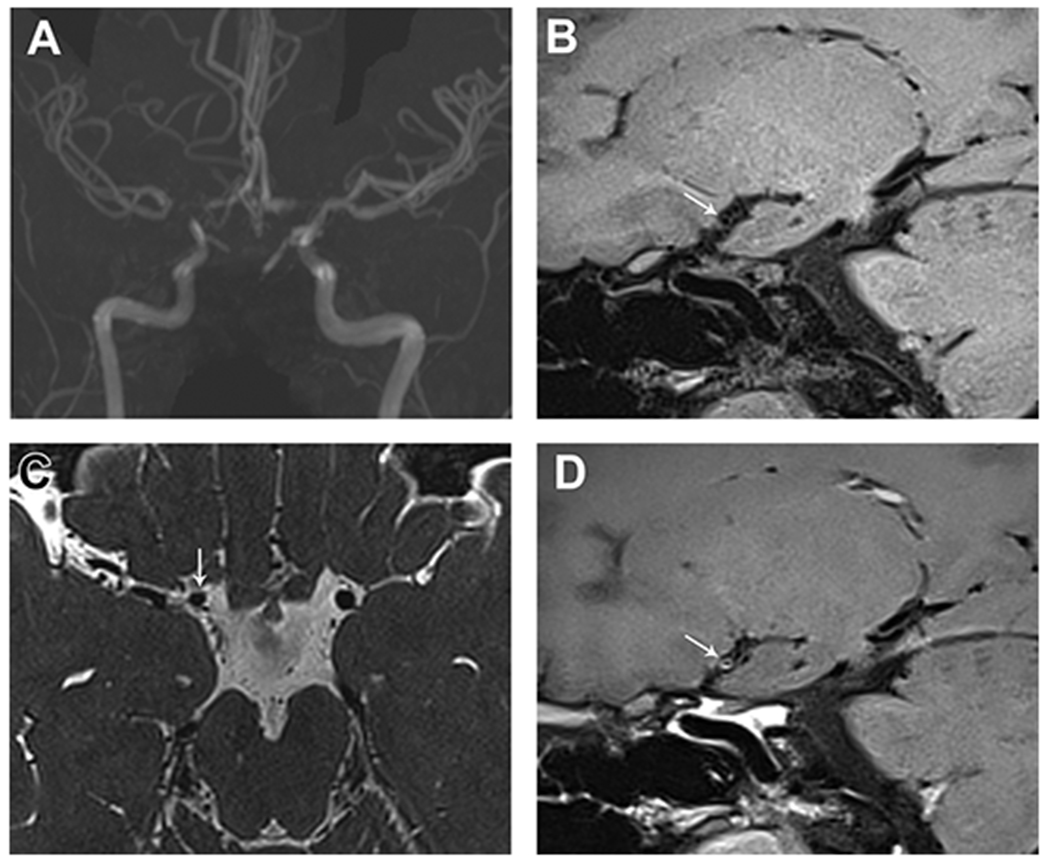
17-year-old female with history of polycystic kidney disease and progressive headaches. On MIP MRA (A), there is right greater than left steno-occlusive disease affecting the carotid termini, proximal ACA and MCA. On sagittal T1-SPACE pre- (B) and post-contrast (C) images, there is mild circumferential wall enhancement of the right carotid terminus just proximal to the occlusion (arrow). Axial T2-SPACE (D) shows no wall thickening of the affected segment (arrow), supporting the diagnosis of Moyamoya disease.
Extracranial Vessel Wall MRI
Techniques for EVWMR
Extracranial arteries, specifically the extracranial carotid arteries, origins of the great arteries and aorta, are large structures that generally have a straight course, making EVWMR less challenging than IVWMR. EVWMR initially relied on 2D techniques, however, these techniques are limited for pathology characterization and detection due to thicker slices and limited coverage especially when imaging the aorta or the length of the carotid arteries. During the past decade, clinical and research practice has moved towards 3D-EVWMR which provides higher resolution, extended coverage and shorter scan times52,53. These techniques use VFA or gradient echo sequences for image acquisition with MSDE or DANTE preparation pulses to improve blood suppression. These techniques can achieve high spatial resolution (.6 mm3) in 2-4-minute scan times.
Acceleration techniques such as compressed sensing54 and motion correction methods55 have been implemented as well to reduce the scan time and reduce the impact of patient motion. These applications may prove to be very useful for pediatric imaging, due to the need for fast, motion-resistant imaging.
As the carotid bifurcation is close to the skin, the use of dedicated surface coils can significantly improve image quality, however, this can create barriers to wider use in clinical settings due to limited availability. A recent study demonstrated that 3D-EVWMR using standard neurovascular coils achieved good agreement with EVWMR using carotid coils for carotid plaque evaluation56. Further optimization of EVWMR protocols for standard neurovascular coils may improve clinical adoption.
EVWMR in Large Artery Inflammatory Vasculopathies
Vasculitis is a group of conditions characterized by inflammation of arterial walls and they are commonly classified by the size of the affected vessels. Imaging is most helpful for large vessel vasculitis with Takayasu arteritis (TA) being the most frequently encountered in children. Due to the nonspecific clinical presentation of large vessel vasculitis in early disease and the lack of specific laboratory tests for diagnosis, diagnosis can be delayed by 10 to 15.5 months in pediatric patients which may lead to secondary organ damage before treatment can be started57. Imaging has been increasingly used to facilitate earlier diagnosis because of this. Traditionally, luminal imaging such as CTA and DSA has been predominantly used for evaluation of vessel stenosis or occlusion. However, with the advent of VWI, there has been a progressive paradigm shift from luminal imaging to EVWMR for detection of vessel wall inflammation 49,58,59. EVWMR is particularly advantageous in the pediatric patient population due to avoidance of ionizing radiation. Furthermore, EVWMR may be more sensitive in early disease before any significant luminal narrowing develops58, when wall changes may still be reversible. Once stenosis or occlusion is detected on luminal imaging, vascular changes are irreversible due to fibrosis and scarring in the arterial wall. Large artery vasculitis frequently shows circumferential artery wall enhancement, wall thickening (Figure 4) and wall T2-signal abnormality on EVWMR. In addition to earlier diagnosis, EVWMR can also be used for disease activity monitoring when the acute-phase markers are normal as luminal imaging does not track disease activity well, whether fixed stenoses have developed or not60 (Figure 5).
Figure 4.

18-year-old female with Takayasu arteritis who presented with elevated inflammatory markers including C-reactive protein of 67 mg/L and erythrocyte sedimentation rate of 31 mm/hour, raising concern for disease recurrence. Contrast-enhanced MIP MRA (A) demonstrated significant stenosis of the innominate artery (white arrow) and the right subclavian artery origin (red arrow), and occlusion of the right common carotid artery at its origin (blue arrow). On axial VWI (B), there is circumferential thick wall enhancement of the right common carotid artery (white arrows), with involvement of the other proximal great arteries (not shown).
Figure 5.

19-year-old woman 1 year after onset of generalized fatigue and extremity pain. She was initially diagnosed with late onset Still’s disease, however, on coronal post-contrast VWI (A) there was circumferential enhancement and wall thickening of the aortic arch and descending thoracic aorta, more than expected for a 20-year-old, presumed to represent large artery vasculitis. Immunomodulation therapy was initiated which led to improvement in some symptoms. VWI was used to track disease activity and on follow-up VWI 2 years later (B), enhancement and wall thickening decreased, coinciding with improved symptoms and lab values.
TA is the third most common vasculitis and the most common large vessel vasculitis in pediatric populations, although it is rare in children younger than 16 years61. TA is more common in female patients and Asian populations. The disease classically involves the aorta and its major branches (Figure 4). Chronic inflammation of the vessel wall may lead to stenosis and rarely thrombosis and aneurysm if left untreated.
As the availability and familiarity with VWI continue to increase, its impact on diagnosis and management of large vessel vasculitis will also likely increase. Specifically, EVWMR may facilitate earlier diagnosis which could help prevent end organ damage and provide more accurate assessment of disease activity which could allow for better titration of medications or even discontinuation of medication in quiescent disease. Finally, lack of ionizing radiation makes VWI particularly advantageous in the pediatric patient population.
VWI in Cranio-cervical Dissections
Cranio-cervical artery dissection (CAD) can occur either spontaneously or as a result of non-penetrating trauma, known as blunt cerebrovascular injury (BCVI). They are both relatively rare with 2.6 per 100,000 incidence of spontaneous CAD and 0.33% incidence of BCVI in pediatric patients after blunt trauma5,62. Although rare, they are clinically significant due to the risk of stroke if untreated63,64. Spontaneous CAD is increasingly recognized as a leading cause of stroke, particularly in young patients, accounting for up to 20% of stroke5. The risk of BCVI-related stroke in children is 37.4% with a mortality risk of 12.7%62.
On VWI, intramural hematoma is seen in 87-100% and eccentric wall enhancement in 67% of dissection cases49. Intramural hematoma signal intensity will depend on the age of the injury, with most injuries showing bright T1-weighted signal, typically in the subacute phase. Enhancement is likely secondary to inflammation, non-flowing blood and/or vasa vasorum injury.
Although CTA has largely supplanted DSA for diagnosis due to availability, cost and non-invasiveness, detection of low-grade injuries can be challenging on CTA. In one series, 10% of carotid and 20% of vertebral artery injuries were overlooked on CTA, all of which were low-grade65. EVWMR may be more accurate compared to CTA because of its ability to not only demonstrate luminal irregularity, but also the associated intramural hematomas (Figure 6). Inter-observer agreement in low-grade injuries on CTA can be suboptimal, with Kappa values ranging from 0.35-0.5266. In contrast, EVWMR has been shown to be more accurate in identifying and grading BCVI compared to CTA, particularly in low-grade injuries6. Furthermore, the false positive rate of EVWMR is also lower compared to CTA (0.03 versus 0.35) because subtle vessel contour irregularity representing artifact or vasospasm is less likely to be misclassified as BCVI (Figure 7). Given the risk of hemorrhagic events associated with antithrombotic therapy, which is the mainstay of treatment for CAD, EVWMR can be helpful in confirming arterial dissection before initiating such therapy in equivocal cases, particularly in patients at higher risk for intracranial or systemic hemorrhage. This can also reduce the number of costly hospital admissions, follow-up patient visits and serial imaging studies. Finally, intraluminal contrast enhancement on EVWMR is associated with acute ischemic strokes in patients with CAD. Therefore, EVWMR may be useful in identifying and stratifying patients at higher risk of stroke during the latent period.
Figure 6.

Right cervical internal carotid artery (ICA) blunt cerebrovascular injury after motor vehicle accident. Right cervical ICA pseudoaneurysm and dissection (red arrows) on MIP CTA (A) and MIP MRA (B). T1-SPACE (C) demonstrates associated intramural hematomas (blue arrows).
Figure 7.

Status post 25-foot fall. Coronal CTA (A) was interpreted as a Denver grade 1 injury of the left cervical internal carotid artery due to subtle luminal irregularity (red arrows). Subsequent T1-SPACE (B) demonstrates no abnormality (red arrows) in the questioned segment, ruling out injury. In retrospect, the luminal irregularity was due to streak artifact from adjacent mandibular metallic hardware.
Conclusion
VWI has the potential to improve diagnostic accuracy for pediatric vasculopathies, for both intracranial and extracranial vascular beds, specifically as it relates to detection of arterial wall inflammation and differentiation of arterial diseases. Ongoing work determining the impact of VWI and its ability to predict outcomes and stroke events is still needed to better establish the role of VWI in clinical diagnostic algorithms.
Key Points:
VWI can better differentiate intracranial and extracranial vasculopathies than luminal imaging alone
VWI can detect inflammatory changes in large artery vasculitis, earlier than luminal imaging, potentially preventing chronic steno-occlusive changes with earlier treatment
VWI is more accurate in evaluating low-grade vascular injury than CTA, and as a second-line imaging tool, could potentially reduce unneeded anti-platelet therapy and additional imaging and clinic visits
Synopsis:
Vessel wall MRI (VWI) is a technique that has progressively gained traction in clinical diagnostic applications for evaluation of intracranial and extracranial vasculopathies, with increasing use in pediatric populations. The technique has shown promise in detection, differentiation and characterization of both inflammatory and non-inflammatory vasculopathies. In the current article, we discuss optimal techniques for intracranial and extracranial VWI, as well as applications and value for pediatric vascular disease evaluation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Drs. Mossa-Basha, Zhu and Wu have no financial disclosures related to this work.
Contributor Information
Mahmud Mossa-Basha, Department of Radiology, University of Washington, Seattle, WA; Address: 1959 NE Pacific St, Seattle, WA 98195.
Chengcheng Zhu, Department of Radiology, University of Washington, Seattle, WA; Address: 325 9th Ave, Seattle, WA 98104.
Lei Wu, Department of Radiology, University of Washington, Seattle, WA; Address: 1660 S Columbian Way, Seattle, WA 98108.
References
- 1.de Havenon A, Tirschwell D, Majersik JJ, et al. Carotid intraplaque hemorrhage on vessel wall MRI does not correlate with TCD emboli monitoring in patients with recently symptomatic carotid atherosclerosis. Neuroradiol J. 2017;30(5):486–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mossa-Basha M, Wasserman BA. Low-Grade Carotid Stenosis: Implications of MR Imaging. Neuroimaging Clin N Am. 2016;26(1):129–145. [DOI] [PubMed] [Google Scholar]
- 3.Saba L, Mossa-Basha M, Abbott A, et al. Multinational Survey of Current Practice from Imaging to Treatment of Atherosclerotic Carotid Stenosis. Cerebrovasc Dis. 2021:1–13. [DOI] [PubMed] [Google Scholar]
- 4.Saba L, Yuan C, Hatsukami TS, et al. Carotid Artery Wall Imaging: Perspective and Guidelines from the ASNR Vessel Wall Imaging Study Group and Expert Consensus Recommendations of the American Society of Neuroradiology. AJNR Am J Neuroradiol. 2018;39(2):E9–e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rutman AM, Vranic JE, Mossa-Basha M. Imaging and Management of Blunt Cerebrovascular Injury. Radiographics. 2018;38(2):542–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vranic JE, Huynh TJ, Fata P, et al. The ability of magnetic resonance black blood vessel wall imaging to evaluate blunt cerebrovascular injury following acute trauma. J Neuroradiol. 2020;47(3):210–215. [DOI] [PubMed] [Google Scholar]
- 7.Bley TA, Uhl M, Carew J, et al. Diagnostic value of high-resolution MR imaging in giant cell arteritis. AJNR Am J Neuroradiol. 2007;28(9):1722–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mossa-Basha M, Hwang WD, De Havenon A, et al. Multicontrast high-resolution vessel wall magnetic resonance imaging and its value in differentiating intracranial vasculopathic processes. Stroke. 2015;46(6):1567–1573. [DOI] [PubMed] [Google Scholar]
- 9.Alexander MD, Yuan C, Rutman A, et al. High-resolution intracranial vessel wall imaging: imaging beyond the lumen. J Neurol Neurosurg Psychiatry. 2016;87(6):589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Havenon A, Mossa-Basha M, Shah L, et al. High-resolution vessel wall MRI for the evaluation of intracranial atherosclerotic disease. Neuroradiology. 2017;59(12):1193–1202. [DOI] [PubMed] [Google Scholar]
- 11.Mossa-Basha M, Alexander M, Gaddikeri S, Yuan C, Gandhi D. Vessel wall imaging for intracranial vascular disease evaluation. J Neurointerv Surg. 2016;8(11):1154–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brinjikji W, Mossa-Basha M, Huston J, Rabinstein AA, Lanzino G, Lehman VT. Intracranial vessel wall imaging for evaluation of steno-occlusive diseases and intracranial aneurysms. J Neuroradiol. 2017;44(2):123–134. [DOI] [PubMed] [Google Scholar]
- 13.de Havenon A, Yuan C, Tirschwell D, et al. Nonstenotic Culprit Plaque: The Utility of High-Resolution Vessel Wall MRI of Intracranial Vessels after Ischemic Stroke. Case Rep Radiol. 2015;2015:356582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mossa-Basha M, Watase H, Sun J, et al. Inter-rater and scan-rescan reproducibility of the detection of intracranial atherosclerosis on contrast-enhanced 3D vessel wall MRI. Br J Radiol. 2019;92(1097):20180973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarikaya B, Colip C, Hwang WD, et al. Comparison of time-of-flight MR angiography and intracranial vessel wall MRI for luminal measurements relative to CT angiography. Br J Radiol. 2021;94(1118):20200743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tian X, Tian B, Shi Z, et al. Assessment of Intracranial Atherosclerotic Plaques Using 3D Black-Blood MRI: Comparison With 3D Time-of-Flight MRA and DSA. J Magn Reson Imaging. 2021;53(2):469–478. [DOI] [PubMed] [Google Scholar]
- 17.Dlamini N, Yau I, Muthusami P, et al. Arterial Wall Imaging in Pediatric Stroke. Stroke. 2018;49(4):891–898. [DOI] [PubMed] [Google Scholar]
- 18.Stence NV, Pabst LL, Hollatz AL, et al. Predicting Progression of Intracranial Arteriopathies in Childhood Stroke With Vessel Wall Imaging. Stroke. 2017;48(8):2274–2277. [DOI] [PubMed] [Google Scholar]
- 19.Mossa-Basha M, Alexander M, Maki J, Cohen W, Hippe D, Yuan C, Huhdanpa H, Saam Tobias. Exploring Other Vascular Dimensions: Comparison of 3-Dimensional and 2-Dimensional Vessel Wall Imaging Techniques for the Assessment of Large Artery Vasculopathies. Paper presented at: 24th Annual International Society of Magnetic Resonance in Medicine Meeting; May 7-13, 2016, 2016; Singapore. [Google Scholar]
- 20.Jain KK. SOME OBSERVATIONS ON THE ANATOMY OF THE MIDDLE CEREBRAL ARTERY. Can J Surg. 1964;7:134–139. [PubMed] [Google Scholar]
- 21.Kalsoum E, Chabernaud Negrier A, Tuilier T, et al. Blood Flow Mimicking Aneurysmal Wall Enhancement: A Diagnostic Pitfall of Vessel Wall MRI Using the Postcontrast 3D Turbo Spin-Echo MR Imaging Sequence. AJNR Am J Neuroradiol. 2018;39(6):1065–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balu N, Zhou Z, Hippe DS, Hatsukami T, Mossa-Basha M, Yuan C. Accelerated multi-contrast high isotropic resolution 3D intracranial vessel wall MRI using a tailored k-space undersampling and partially parallel reconstruction strategy. Magma. 2019;32(3):343–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu C, Graves MJ, Yuan J, Sadat U, Gillard JH, Patterson AJ. Optimization of improved motion-sensitized driven-equilibrium (iMSDE) blood suppression for carotid artery wall imaging. J Cardiovasc Magn Reson. 2014;16(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J, Helle M, Zhou Z, Bornert P, Hatsukami TS, Yuan C. Joint blood and cerebrospinal fluid suppression for intracranial vessel wall MRI. Magn Reson Med. 2016;75(2):831–838. [DOI] [PubMed] [Google Scholar]
- 25.Zhu C, Haraldsson H, Tian B, et al. High resolution imaging of the intracranial vessel wall at 3 and 7 T using 3D fast spin echo MRI. Magma. 2016;29(3):559–570. [DOI] [PubMed] [Google Scholar]
- 26.Yang H, Zhang X, Qin Q, Liu L, Wasserman BA, Qiao Y. Improved cerebrospinal fluid suppression for intracranial vessel wall MRI. J Magn Reson Imaging. 2016;44(3):665–672. [DOI] [PubMed] [Google Scholar]
- 27.Vranic JE, Cross NM, Wang Y, Hippe DS, de Weerdt E, Mossa-Basha M. Compressed Sensing-Sensitivity Encoding (CS-SENSE) Accelerated Brain Imaging: Reduced Scan Time without Reduced Image Quality. AJNR Am J Neuroradiol. 2019;40(1):92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu C, Tian B, Chen L, et al. Accelerated whole brain intracranial vessel wall imaging using black blood fast spin echo with compressed sensing (CS-SPACE). Magma. 2018;31(3):457–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conklin J, Longo MGF, Cauley SF, et al. Validation of Highly Accelerated Wave-CAIPI SWI Compared with Conventional SWI and T2*-Weighted Gradient Recalled-Echo for Routine Clinical Brain MRI at 3T. AJNR Am J Neuroradiol. 2019;40(12):2073–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mardani M, Gong E, Cheng JY, et al. Deep Generative Adversarial Neural Networks for Compressive Sensing MRI. IEEE Trans Med Imaging. 2019;38(1):167–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Havenon ACL, Park M, Mossa-Basha M. Intracranial vessel wall MRI: a review of current indications and future applications. Neurovascular Imaging. 2016;2(10). [Google Scholar]
- 32.Mossa-Basha M, de Havenon A, Becker KJ, et al. Added Value of Vessel Wall Magnetic Resonance Imaging in the Differentiation of Moyamoya Vasculopathies in a Non-Asian Cohort. Stroke. 2016;47(7):1782–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mossa-Basha M, Shibata DK, Hallam DK, et al. Added Value of Vessel Wall Magnetic Resonance Imaging for Differentiation of Nonocclusive Intracranial Vasculopathies. Stroke. 2017;48(11):3026–3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Birnbaum J, Hellmann DB. Primary angiitis of the central nervous system. Arch Neurol. 2009;66(6):704–709. [DOI] [PubMed] [Google Scholar]
- 35.Hajj-Ali RA, Singhal AB, Benseler S, Molloy E, Calabrese LH. Primary angiitis of the CNS. Lancet Neurol. 2011;10(6):561–572. [DOI] [PubMed] [Google Scholar]
- 36.MacLaren K, Gillespie J, Shrestha S, Neary D, Ballardie FW. Primary angiitis of the central nervous system: emerging variants. Qjm. 2005;98(9):643–654. [DOI] [PubMed] [Google Scholar]
- 37.Campi A, Benndorf G, Filippi M, Reganati P, Martinelli V, Terreni MR. Primary angiitis of the central nervous system: serial MRI of brain and spinal cord. Neuroradiology. 2001;43(8):599–607. [DOI] [PubMed] [Google Scholar]
- 38.Elbers J, Benseler SM. Central nervous system vasculitis in children. Curr Opin Rheumatol. 2008;20(1):47–54. [DOI] [PubMed] [Google Scholar]
- 39.Friedman NR. Small vessel childhood primary angiitis of the CNS: first steps toward a standardised treatment regimen. Lancet Neurol. 2010;9(11):1042–1044. [DOI] [PubMed] [Google Scholar]
- 40.Benseler S, Schneider R. Central nervous system vasculitis in children. Curr Opin Rheumatol. 2004;16(1):43–50. [DOI] [PubMed] [Google Scholar]
- 41.Mossa-Basha M, Huynh TJ, Hippe DS, Fata P, Morton RP, Levitt MR. Vessel wall MRI characteristics of endovascularly treated aneurysms: association with angiographic vasospasm. J Neurosurg. 2018;131(3):859–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perez FA, Oesch G, Amlie-Lefond CM. MRI Vessel Wall Enhancement and Other Imaging Biomarkers in Pediatric Focal Cerebral Arteriopathy-Inflammatory Subtype. Stroke. 2020;51(3):853–859. [DOI] [PubMed] [Google Scholar]
- 43.Yuan S, Jordan LC, Davis LT, et al. A cross-sectional, case-control study of intracranial arterial wall thickness and complete blood count measures in sickle cell disease. Br J Haematol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shimonaga K, Matsushige T, Ishii D, et al. Clinicopathological Insights From Vessel Wall Imaging of Unruptured Intracranial Aneurysms. Stroke. 2018;49(10):2516–2519. [DOI] [PubMed] [Google Scholar]
- 45.Hartman JB, Watase H, Sun J, et al. Intracranial aneurysms at higher clinical risk for rupture demonstrate increased wall enhancement and thinning on multicontrast 3D vessel wall MRI. Br J Radiol. 2019;92(1096):20180950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gariel F, Ben Hassen W, Boulouis G, et al. Increased Wall Enhancement During Follow-Up as a Predictor of Subsequent Aneurysmal Growth. Stroke. 2020;51(6):1868–1872. [DOI] [PubMed] [Google Scholar]
- 47.Fu Q, Wang Y, Zhang Y, et al. Qualitative and Quantitative Wall Enhancement on Magnetic Resonance Imaging Is Associated With Symptoms of Unruptured Intracranial Aneurysms. Stroke. 2021;52(1):213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lehman VT, Brinjikji W, Mossa-Basha M, et al. Conventional and high-resolution vessel wall MRI of intracranial aneurysms: current concepts and new horizons. J Neurosurg. 2018;128(4):969–981. [DOI] [PubMed] [Google Scholar]
- 49.Young CC, Bonow RH, Barros G, Mossa-Basha M, Kim LJ, Levitt MR. Magnetic resonance vessel wall imaging in cerebrovascular diseases. Neurosurg Focus. 2019;47(6):E4. [DOI] [PubMed] [Google Scholar]
- 50.Akagawa H, Mukawa M, Nariai T, et al. Novel and recurrent RNF213 variants in Japanese pediatric patients with moyamoya disease. Hum Genome Var. 2018;5:17060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scott RM, Smith ER. Moyamoya disease and moyamoya syndrome. N Engl J Med. 2009;360(12):1226–1237. [DOI] [PubMed] [Google Scholar]
- 52.Balu N, Yarnykh VL, Chu B, Wang J, Hatsukami T, Yuan C. Carotid plaque assessment using fast 3D isotropic resolution black-blood MRI. Magn Reson Med. 2011;65(3):627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu C, Sadat U, Patterson AJ, Teng Z, Gillard JH, Graves MJ. 3D high-resolution contrast enhanced MRI of carotid atheroma--a technical update. Magn Reson Imaging. 2014;32(5):594–597. [DOI] [PubMed] [Google Scholar]
- 54.Jia S, Zhang L, Ren L, et al. Joint intracranial and carotid vessel wall imaging in 5 minutes using compressed sensing accelerated DANTE-SPACE. Eur Radiol. 2020;30(1):119–127. [DOI] [PubMed] [Google Scholar]
- 55.Dyverfeldt P, Deshpande VS, Kober T, Krueger G, Saloner D. Reduction of motion artifacts in carotid MRI using free-induction decay navigators. J Magn Reson Imaging. 2014;40(1):214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brinjikji W, DeMarco JK, Shih R, et al. Diagnostic accuracy of a clinical carotid plaque MR protocol using a neurovascular coil compared to a surface coil protocol. J Magn Reson Imaging. 2018;48(5):1264–1272. [DOI] [PubMed] [Google Scholar]
- 57.Abularrage CJ, Slidell MB, Sidawy AN, Kreishman P, Amdur RL, Arora S. Quality of life of patients with Takayasu’s arteritis. J Vasc Surg. 2008;47(1):131–136; discussion 136-137. [DOI] [PubMed] [Google Scholar]
- 58.Choe YH, Han BK, Koh EM, Kim DK, Do YS, Lee WR. Takayasu’s arteritis: assessment of disease activity with contrast-enhanced MR imaging. AJR Am J Roentgenol. 2000;175(2):505–511. [DOI] [PubMed] [Google Scholar]
- 59.Mandell DM, Mossa-Basha M, Qiao Y, et al. Intracranial Vessel Wall MRI: Principles and Expert Consensus Recommendations of the American Society of Neuroradiology. AJNR Am J Neuroradiol. 2017;38(2):218–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun Y, Ma L, Ji Z, et al. Value of whole-body contrast-enhanced magnetic resonance angiography with vessel wall imaging in quantitative assessment of disease activity and follow-up examination in Takayasu’s arteritis. Clin Rheumatol. 2016;35(3):685–693. [DOI] [PubMed] [Google Scholar]
- 61.Soliman M, Laxer R, Manson D, Yeung R, Doria AS. Imaging of systemic vasculitis in childhood. Pediatr Radiol. 2015;45(8):1110–1125. [DOI] [PubMed] [Google Scholar]
- 62.Harris DA, Sorte DE, Lam SK, Carlson AP. Blunt cerebrovascular injury in pediatric trauma: a national database study. J Neurosurg Pediatr. 2019:1–10. [DOI] [PubMed] [Google Scholar]
- 63.Bonow RH, Witt CE, Mosher BP, et al. Transcranial Doppler Microemboli Monitoring for Stroke Risk Stratification in Blunt Cerebrovascular Injury. Crit Care Med. 2017;45(10):e1011–e1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu L, Christensen D, Call L, et al. Natural History of Blunt Cerebrovascular Injury: Experience Over a 10-year Period at a Level I Trauma Center. Radiology. 2020;297(2):428–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bub LD, Hollingworth W, Jarvik JG, Hallam DK. Screening for blunt cerebrovascular injury: evaluating the accuracy of multidetector computed tomographic angiography. J Trauma. 2005;59(3):691–697. [PubMed] [Google Scholar]
- 66.Roberts DJ, Chaubey VP, Zygun DA, et al. Diagnostic accuracy of computed tomographic angiography for blunt cerebrovascular injury detection in trauma patients: a systematic review and meta-analysis. Ann Surg. 2013;257(4):621–632. [DOI] [PubMed] [Google Scholar]


