Abstract
Background and Objectives:
The clinical presentation of gastric cancer varies between racial and ethnic groups. While historically studied as a monolithic population, the Hispanic ethnicity is comprised of heterogenous groups with considerable biologic, socioeconomic, and cultural variability; therefore, intra-group differences among Hispanic gastric cancer patients may have been overlooked in past research.
Methods:
We conducted a retrospective review of the National Cancer Database (NCDB) to compare Hispanic patients with gastric adenocarcinoma diagnosed between 2004–2015, by NCDB-reported location of patient ancestry.
Results:
We identified a cohort of 3811 patients. There were higher proportions of females, patients with early disease onset, and stage IV disease among patients of Mexican and South/Central American ancestry. Additionally, a significantly larger proportion of Mexican (15%) and South/Central American patients (11%) were diagnosed before age 40, in contrast to Cubans (2%), DR (6%), and PR (3%; p< 0.0001). Mexican ancestry was independently associated with a 34% increased rate of all-cause mortality at 5 years (HR 1.34; 95% CI 1.09–1.64).
Conclusions:
Significant clinical and epidemiological differences exist among Hispanic gastric cancer patients based on location of ancestry. Future data collection endeavors should strive to capture this granularity inherent to the Hispanic ethnicity.
Keywords: gastric cancer, Hispanic ethnicity
INTRODUCTION
Gastric cancer is the fifth most common cancer worldwide and the third-leading cause of cancer-related deaths, resulting in an estimated 783,000 deaths in 2018.[1] The incidence of this disease varies across the globe; notably, parts of South and Central America have some of the highest rates of new gastric cancer diagnoses and mortality in the world.[2] Similarly, there is heterogeneity in the clinical presentation of gastric cancer between different racial and ethnic populations in the United States.[3–5] Hispanic patients have a higher incidence of gastric cancer, are diagnosed at younger ages, present with more advanced disease, and have a higher proportion of diffuse-type cancers compared with non-Hispanic Whites.[6–9] Identifying ways to reduce cancer outcome disparity for Hispanic gastric cancer patients is a critical issue as the growth of the Hispanic population in the United States is projected to triple from 42 million in 2005 to 128 million in 2050.[10]
Historically, the Hispanic ethnicity has been studied as a monolithic group; however, the population is comprised of heterogenous groups with considerable biologic, socioeconomic, and cultural variability.[11] No previous study has compared the clinical and epidemiologic features of Hispanic gastric cancer patients by geographic location of ancestry; as such, significant differences between patients may have been overlooked. This possibility is highlighted by a recent study showing that Asian Americans, another group often studied as a single population, have significant differences in their gastric cancer pattern of presentation and mortality depending on their country of ancestry.[12]
In this study, we compared clinicopathologic variables of Hispanic gastric cancer patients treated in the United States based upon the location of their ancestry, as recorded in the National Cancer Database (NCDB). We hypothesized that differences in gastric cancer presentation exist among Hispanic patients of varying NCDB-reported locations of ancestry.
MATERIALS AND METHODS
Data Source and Study Population
The study was approved by the University of Texas Southwestern Medical Center institutional review board. We analyzed the NCDB participant use file to identify Hispanic patients 18 years of age and older diagnosed with gastric adenocarcinoma between 2004–2015. The NCDB is a registry that receives data from over 1500 Commission on Cancer-accredited programs in the United States, capturing approximately 70% of all incident cancer cases.
Our analytic group was categorized by the NCDB-designated Spanish origin variable. The five stated locations of patient ancestry classified by the Spanish origin variable were Mexican, Puerto Rican (PR), Cuban, South/Central American (excluding Brazilian), and Dominican Republican (DR). Patients with a “not otherwise specified” (NOS) Spanish/Hispanic/Latino origin or whose evidence of Hispanic/Spanish origin was based only on surname were excluded.
Outcome Variables/Covariates
The patients’ cancer stage at diagnosis was based on clinical TNM staging. For outcome reporting, the NCDB’s analytic stage variable was used, which represents the pathologic stage when available, and the clinical stage in patients for whom a pathologic stage is not reported. We collected data on surgical resection margins and adequate lymph node examination (≥15 nodes) for patients who underwent curative-intent gastrectomy, which was determined based on a site-specific code documenting the type of surgical procedure performed. Patients with missing clinical stage information were excluded.
Statistical Analysis
Chi-square tests were used to analyze categorical differences between groups. ANOVAs were used to compare differences in group means. All tests were two-sided and performed at the 5% significance level. Overall survival was estimated using the Kaplan-Meier method and compared using log-rank tests. A multivariable Cox regression model was used to assess the impact of Hispanic ancestry group membership on survival and included the following covariates in addition to the variable of interest: age at presentation, sex, Charlson-Deyo score, analytic stage group, receipt of any treatment, treatment facility type, surgical margins, and adequate lymph node examination. For all covariates in the model, the proportionality of hazards assumption was tested using suprenum tests and Pearson product-moment correlations between the scaled Schoenfeld residuals and log(time) for each covariate. The proportional hazards assumption was found to be satisfied. Statistical analysis was performed utilizing SAS 9.4 (SAS Institute, Cary, NC).
RESULTS
Study cohort
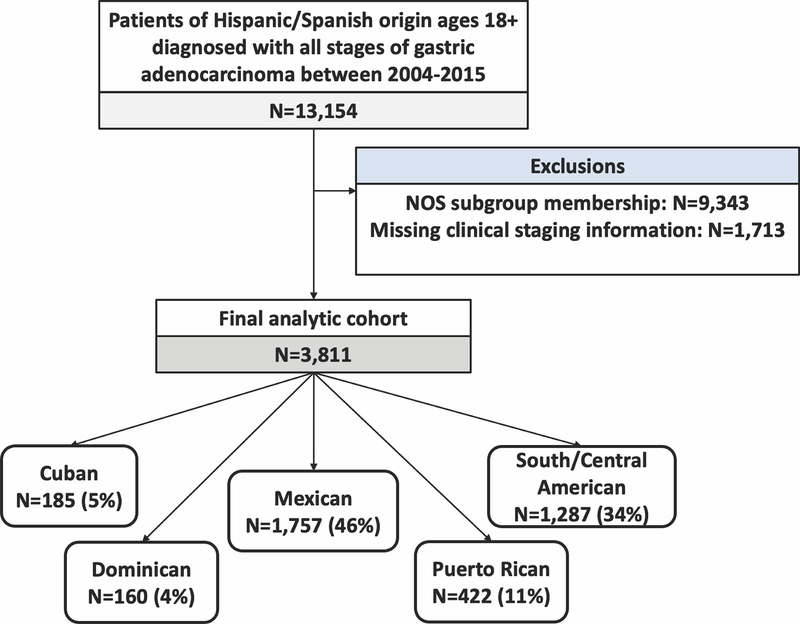
The study population is presented in Figure 1. After exclusions, our final analytic cohort consisted of 3811 patients. Mexican patients were the most common group (n = 1757; 46%), followed by South/Central American (1287; 34%), PR (422; 11%), Cuban (185; 5%), and DR patients (160; 4%). There were no missing data for demographic or socioeconomic variables with the exception of insurance status (missing in 3.2% of patients). The capture of data on surgery receipt/type was complete. Of patients who underwent gastrectomy, surgical margin data and lymph node examination data were missing in 5.0% and 8.7%, respectively. Data on treatment receipt were missing for 4.1% of our study cohort. Data were missing at random. Patients with missing covariate data were conditionally excluded from analyses utilizing the missing variables; however, these patients were included in analyses of all other covariates. A sensitivity analysis of the NOS cohort and study cohort was performed. This demonstrated a higher mean age at diagnosis in the NOS group (62.2 vs. 59.3 years; p < 0.001). Additionally, small differences in stage at presentation were noted between cohorts. In the NOS cohort, a higher proportion of patients presented with stage 1 disease (20% vs. 17%) and a smaller proportion of patients presented with stage 3 or 4 disease (66% vs. 70%; p < 0.01). No significant differences in gender distribution, insurance status, treatment receipt, surgical resection margin, or adequacy of lymph node harvest were observed (Supplemental Table 1).
Figure 1.
Patient cohort. Abbreviations: NOS, not otherwise specified Spanish/Hispanic/Latino origin.
Clinical Characteristics
Significant differences in gender distribution were identified. Mexican and South/Central American patients had significantly greater female predominance than other groups with male-to-female ratios of 1.4:1 and 1.3:1, respectively. In contrast, the proportion was 1.9:1 for Cuban patients, 2.0:1 for PR patients, and 1.6:1 for DR patients (p < 0.01; Table 1).
Table 1.
Clinical and socioeconomic characteristics by location of patient ancestry
| n (%) | |||||||
|---|---|---|---|---|---|---|---|
| Cuban (n = 185) | Dominican (n = 160) | Mexican (n = 1757) | Puerto Rican (n = 422) | South/Central American (n = 1287) | P Value | ||
| Age Group at Diagnosis | <40 | 4 (2) | 10 (6) | 258 (15) | 12 (3) | 147 (11) | <0.0001 |
| ≥40 | 181 (98) | 150 (94) | 1499 (85) | 410 (97) | 1140 (89) | ||
| Sex | Male | 121 (65) | 98 (61) | 1039 (59) | 280 (66) | 738 (57) | <0.01 |
| Female | 64 (35) | 62 (39) | 718 (41) | 142 (34) | 549 (43) | ||
| Charlson/Deyo Score | 0 | 134 (72) | 110 (69) | 1409 (80) | 251 (60) | 1007 (78) | <0.0001 |
| 1 | 37 (20) | 37 (23) | 267 (15) | 120 (28) | 221 (17) | ||
| ≥2 | 14 (8) | 13 (8) | 81 (5) | 51 (12) | 59 (5) | ||
| Insurance Status | Uninsured | 11 (6) | 6 (4) | 290 (17) | 15 (4) | 229 (18) | <0.0001 |
| Private | 48 (26) | 41 (26) | 461 (26) | 109 (26) | 351 (27) | ||
| Medicaid | 22 (12) | 50 (31) | 485 (28) | 66 (16) | 348 (27) | ||
| Medicare | 103 (56) | 61 (38) | 438 (25) | 66 (16) | 312 (24) | ||
| Other Government | 1 (0.5) | 0 (0) | 13 (0.7) | 3 (0.7) | 5 (0.4) | ||
| Median Income Quartile | <$38,000 | 56 (31) | 71 (44) | 425 (24) | 144 (34) | 229 (18) | <0.0001 |
| $38,000–$47,999 | 38 (21) | 27 (17) | 451 (26) | 79 (19) | 275 (22) | ||
| $48,000–$62,999 | 50 (27) | 34 (21) | 546 (31) | 111 (26) | 344 (27) | ||
| $63,000+ | 39 (21) | 28 (18) | 324 (19) | 87 (21) | 429 (34) | ||
| No High School Degree (in Quartiles Shown by Percentages) | ≥17.6% | 85 (47) | 89 (56) | 1066 (61) | 189 (45) | 575 (45) | <0.0001 |
| 10.9%-17.5% | 44 (24) | 31 (19) | 347 (20) | 120 (28) | 323 (25) | ||
| 6.3%-10.8% | 32 (18) | 29 (18) | 240 (14) | 79 (19) | 244 (19) | ||
| <6.3% | 22 (12) | 11 (7) | 95 (5) | 34 (8) | 135 (11) | ||
Significant differences in age at presentation were also identified. The median age at diagnosis was 58 years (IQR 45–69) for Mexican patients and 59 years (IQR 47–71) for South/Central American patients. In contrast, the median age at diagnosis for Cuban patients was 70 years (IQR 58–77), for DR patients it was 66 years (IQR 52–74), and for PR patients it was 67 years (IQR 57–74). A significant proportion of Mexican (15%) and South/Central American (11%) patients were diagnosed at a very young age, which we arbitrarily defined as younger than 40 years, compared to Cuban (2%), DR (6%), and PR patients (3%; p < 0.0001). The more elderly Cuban, DR, and PR patient populations had more comorbidities as evidenced by higher Charlson-Deyo scores.
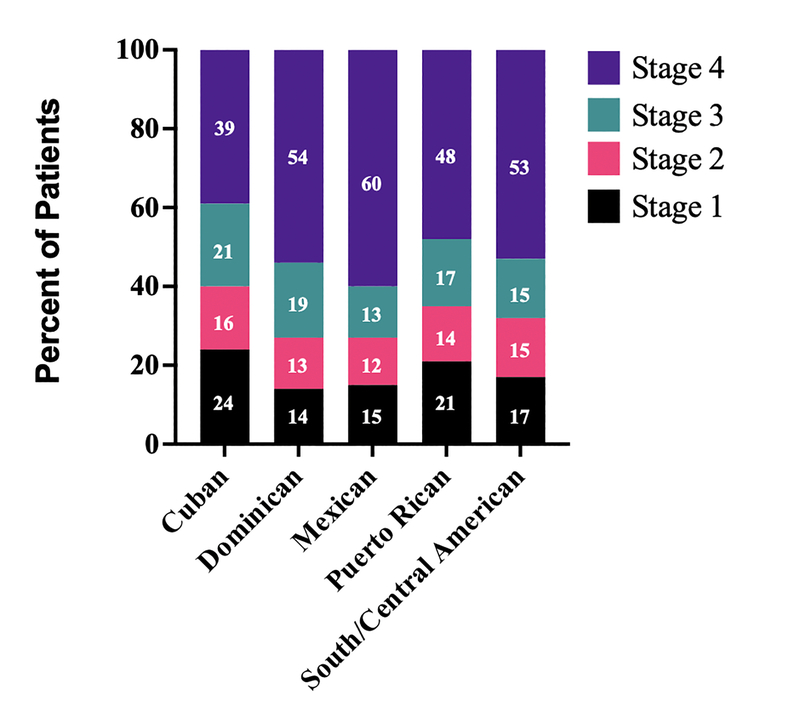
There were also significant differences in the clinical stage of disease between groups at diagnosis. Even though Mexicans and South/Central Americans were diagnosed younger, they also had a higher rate of stage IV disease. In all, 60% of Mexican, 53% of South/Central American and 54% of DR patients presented with stage IV disease, while only 39% of Cuban and 48% of PR patients had metastatic disease at diagnosis (p < 0.0001; Figure 2).
Figure 2.
Clinical stage at diagnosis by location of patient ancestry.
Treatment Patterns
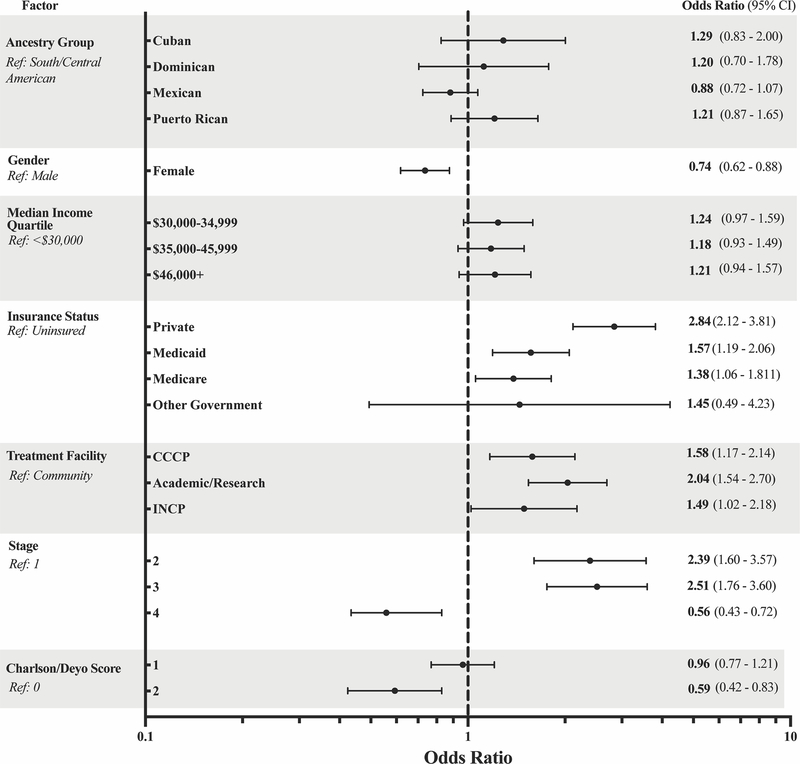
There were significant differences between groups in the percentage of patients receiving any treatment, which ranged from 74% for Mexican patients up to 83% for Cuban patients (p < 0.05; Table 2). However, after adjusting for covariates in a multivariable logistic regression model, ancestry group was not found to be significantly associated with treatment receipt. Covariates associated with an increased rate of treatment receipt included male gender, insured status, treatment at a noncommunity-based facility, and stage 2 or 3 disease. Factors associated with a decreased rate of treatment receipt were stage 4 disease and a Charlson-Deyo score of 2 (Figure 3).
Table 2.
Treatment characteristics by location of patient ancestry
| n (%) | |||||||
|---|---|---|---|---|---|---|---|
| Cuban (n = 185) | Dominican (n = 160) | Mexican (n = 1757) | Puerto Rican (n = 422) | South/Central American (n = 1287) | P Value | ||
| Treatment Facility Type | Community | 5 (3) | 16 (11) | 164 (11) | 44 (11) | 89 (8) | <0.0001 |
| Community Cancer Program | 59 (33) | 24 (16) | 469 (31) | 86 (21) | 258 (23) | ||
| Academic | 92 (51) | 100 (67) | 740 (49) | 245 (60) | 689 (60) | ||
| Integrated Network Cancer Program | 25 (14) | 10 (7) | 126 (8) | 35 (9) | 104 (9) | ||
| Received Any Treatment | No | 32 (17) | 30 (21) | 433 (26) | 80 (20) | 268 (22) | <0.05 |
| Yes | 151 (83) | 115 (79) | 1259 (74) | 323 (80) | 960 (78) | ||
| Surgical Resection Margin | R0 | 72 (91) | 55 (93) | 424 (85) | 146 (92) | 419 (91) | NS |
| R1 | 6 (8) | 4 (7) | 63 (13) | 10 (6) | 37 (8) | ||
| R2 | 1 (1) | 0 (0) | 11 (2) | 2 (1) | 6 (1) | ||
| Examination of ≥15 Lymph Nodes | No | 133 (75) | 115 (74) | 1403 (82) | 323 (79) | 971 (78) | <0.01 |
| Yes | 44 (25) | 41 (26) | 301 (18) | 84 (21) | 278 (22) | ||
Figure 3.
Forest plot of odds ratios associated with treatment receipt. Gender, insurance status, treatment facility, cancer stage, and Charlson/Deyo score were significantly associated with treatment receipt.
Ancestry group and median income quartile were not significantly associated with receipt of treatment.
Abbreviations: CCCP, Comprehensive Community Cancer Program; INCP, Integrated Network Cancer Program.
The majority of patients were treated at academic centers or integrated cancer network programs as opposed to the community setting (64% vs 36%; p < 0.0001; Table 2). Mexican patients had a significantly lower rate of adequate lymph node examination (18%) compared with Cuban (25%), DR (26%), PR (21%), and South/Central American patients (22%; p < 0.01). However, only 18%−26% of all patients received an adequate lymph node examination, defined as the evaluation of at least 15 nodes.[13] There was no significant difference in R0 resection rates, which ranged from 85%−93%. There was no difference in receipt of chemotherapy for stage IV patients.
Socioeconomic Status
Hispanic groups differed significantly with regard to socioeconomic status. Mexican (17%) and South/Central American patients (18%) were more likely to be uninsured as compared to the other three groups (4%−6%; p < 0.0001; Table 1). DR had the greatest percentage of patients living in census tracts with an average median income of <$38,000/year (44%). South/Central Americans had higher income than other groups, with 61% of patients in a census tract with a median income of at least $48,000/year (p < 0.0001). In all subgroups, 45% or more of patients lived in communities where ≥17.6% of individuals had no high school degree.
Survival
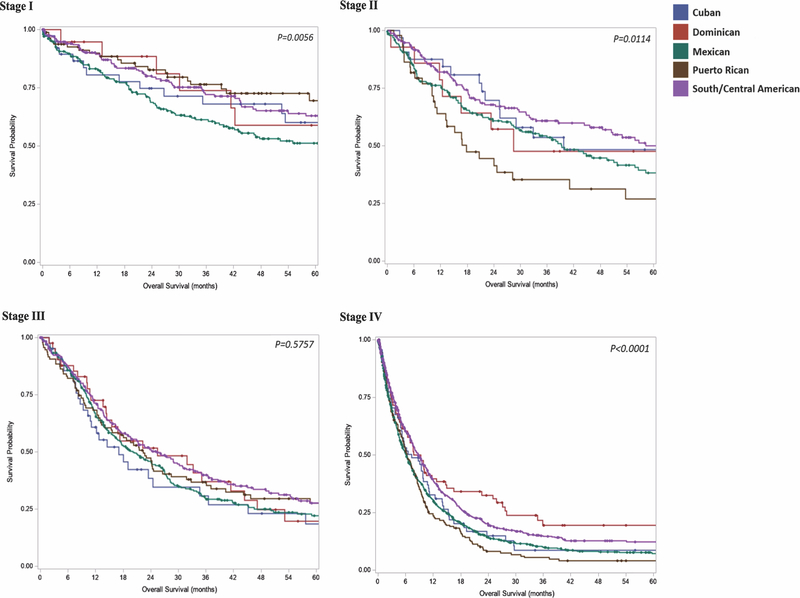
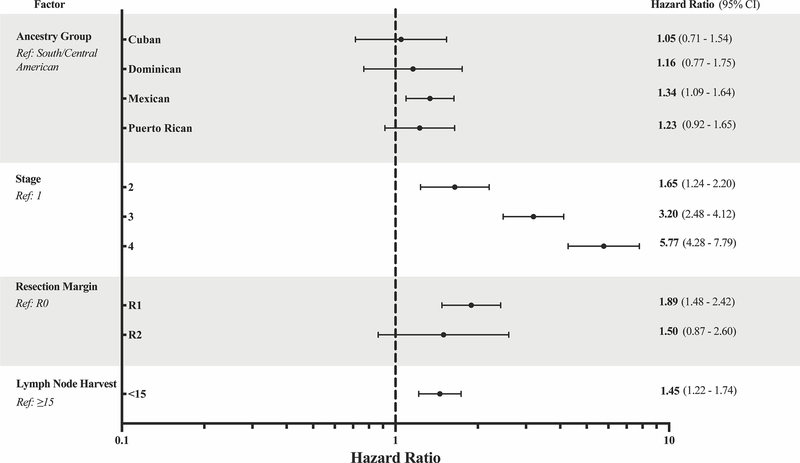
Unadjusted survival was different between subgroups (Figure 4). On multivariate analysis, Mexican ancestry was independently associated with a 34% increased rate of all-cause mortality at 5 years (HR 1.34; 95% CI 1.09–1.64). No significant difference was noted for all-cause mortality between Cuban, DR, PR, or South/Central American patients. Compared to patients who had at least 15 lymph nodes examined, those with inadequate lymph node harvest were 1.4 times more likely to be deceased at 5 years. Similarly, R1 resection was 1.9 times more likely than R0 resection to be associated with all-cause mortality at 5 years (Figure 5).
Figure 4.
Overall survival by stage and ancestry group. Cancer stage-specific Kaplan-Meier curves by Hispanic ancestry group. Abbreviations: DR, Dominican (Republic); PR, Puerto Rican
Stage 1. 5-year overall survival (percent surviving): Cuban 60.0%, DR 59%, Mexican 51%, PR 69.5%, South/Central American 63%
Stage 2. 5-year overall survival (percent surviving): Cuban 49.2%, DR 47.6%, Mexican 38.3%, PR 26.9%, South/Central American 50.1%
Stage 3. Median overall survival (months): Cuban 17.1, DR 25.5, Mexican 20.2, PR 22.4, South/Central American 25.2
Stage 4. Median overall survival (months): Cuban 7.4 months, DR 9.3 months, Mexican 6.1 months, PR 6.3 months, South/Central American 8.7 months.
Figure 5.
Forest plot of adjusted hazard ratios for all-cause mortality. Hispanic ancestry group, age group at diagnosis, gender, treatment facility type, cancer stage, Charlson-Deyo score, surgical margins, and lymph node harvest were entered as covariates for stepwise selection in the multivariable model. Only significant factors are shown
DISCUSSION
In this retrospective review of Hispanic gastric adenocarcinoma patients in the NCDB, we identified significant clinical and epidemiologic differences based on location of patient ancestry. While gastric cancer has a 2:1 male-to-female ratio worldwide,[1] in our study Mexican and South/Central American patients had significantly lower male-to-female ratios of 1.4:1 and 1.3:1, respectively. Additionally, Mexican and South/Central American patients were more likely to be diagnosed at a younger age than Cuban, PR, and DR patients. The differences in age were due, in part, to the percentage of Mexican and South/Central American patients diagnosed with gastric adenocarcinoma before age 40, which was 15% and 11%, respectively. This stands in dramatic contrast to the overall NCDB cohort, in which only 3% were diagnosed with gastric cancer before age 40 during that same time period. Additionally, despite their younger age of diagnosis, Mexican and South/Central American patients were more likely to present with stage IV disease. While Zhao et al. previously noted that Hispanic gastric cancer patients tended to be younger at the time of diagnosis than non-Hispanic patients, this is the first description of differences in clinical presentation among groups comprising the Hispanic ethnicity.[14]
The differences identified in patient presentation between ancestry groups suggest that there may be unmeasured factors which project beyond environmental exposure and social determinants of health. While socioeconomic differences were noted in our population, they do not explain the relative female predominance of Mexican and South/Central American patients. Moreover, the remarkably high proportion of Mexican and South/Central American patients diagnosed before age 40 raises the possibility that one of the unmeasured factors may be biologic differences between groups. While self-identified race and ethnicity encompass a complex interplay of social, cultural, and geographic factors that affect health outcomes,[15, 16] race and ethnicity are also associated with genetic ancestry. The Hispanic population is comprised of diverse populations with different admixtures of European, African, and Indigenous ancestry. [17] Notably, Conomos et al. demonstrated, in a genome-wide association study, that significant genetic diversity exists between Cubans, DR, PR, Mexican, and South/Central Americans.[18] The clinical relevance of the genomic differences that exist between Hispanic populations may be related to the differences in presentation we found in our analytic groups. Indeed, genetic ancestry has been associated with cancer-causing mutations in Hispanic patients; for example, Marker et al. showed that HER2 tumor positivity in Latina breast cancer patients is associated with Indigenous ancestry.[19] Similarly, Carrot-Zhang et al. found that the frequency of EGFR and KRAS mutations in Latino lung cancer patients is correlated with Indigenous ancestry.[20] Notably, we recently performed an integrated genomic analysis of samples from 83 Hispanic gastric cancer patients and compared them to predominantly Non-Hispanic White and Asian patients in The Cancer Genome Atlas. We found that Hispanic gastric cancer patients had a higher proportion of tumors that were of the genomically stable subtype and a high rate of CDH1 germline variants, which may explain the aggressiveness of their tumors.[21] Evaluated in conjunction with our findings that Mexican and South/Central American patients not only were more likely to present before age 40, but also were more likely to present with metastatic disease, a higher rate of CDH1 germline variants may be found in patients of Mexican and South/Central American ancestry.
Our report also highlights the critical need to study Hispanic patients at a more granular level. Conflating all Hispanic patients into one group, regardless of location of ancestry, may mask relevant clinical and epidemiological distinctions between groups. Additionally, the current five NCDB-designated Hispanic groups, while helpful, are overly broad in their combination of all patients of South and Central American ancestry into one category. This is of particular importance as discrete immigrant groups from South and Central American countries constitute rapidly growing segments of the US population.[22] As such, we advocate for increasing the granularity of data capture for Hispanic location of ancestry in national administrative cancer databases and other research endeavors, which we believe will improve our understanding of the distinctive clinicopathologic features of Hispanic patients with varying locations of ancestry.
Our study has several limitations inherent to the use of a large retrospective database. A significant number of patients had a designation of NOS Hispanic group membership and were not included in our study cohort. However, we found that the NOS cohort generally had similar characteristics as our analytic group. In comparing the NOS group with our study cohort, we found only small differences in age and cancer stage, and we found similar proportions in terms of gender, socioeconomic markers, receipt of any treatment, R0 resection rate, and rate of adequate lymph node examination (Supplemental Table 1). Thus, the NOS group appeared to be similar overall to our study cohort. Other limitations include the lack of granularity in the South/Central American group, which is comprised of patients of varied ancestry, as well as the membership classification of study groups being based on patient self-reports rather than genetic ancestry markers, and the small sample size of certain subgroups. Despite these limitations, the NCDB contains data from a large national set of heterogenous cancer programs, and our study provides an overview of the clinical differences among Hispanic patients of varying locations of ancestry.
CONCLUSION
There are significant clinical and epidemiologic differences between Hispanic gastric cancer patients based on location of ancestry. In particular, Mexican and South/Central American patients have a higher number of females, earlier disease onset, and present more often with stage IV disease. Additionally, Mexican and South/Central American patients are more frequently diagnosed before age 40. In the future, database managers should improve the capture of granularity that exists between groups that comprise the Hispanic population. Moreover, it is imperative to increase Hispanic patient representation in basic and clinical science research to improve our understanding of the unique features affecting gastric cancer patients with varying locations of ancestry.
Supplementary Material
Synopsis:
A review of 3811 Hispanic gastric cancer patients in the National Cancer Database showed that patients had differing presentation and outcomes depending on their location of ancestry. There were higher proportions of females, patients with early disease onset, and stage IV disease among patients of Mexican and South/Central American ancestry. Additionally, Mexican ancestry was independently associated with a 34% increased rate of all-cause mortality at 5 years.
Acknowledgements
The authors would like to thank Dave Primm of the UT Southwestern Department of Surgery for help in editing this article.
Funding: JDK holds a Physician-Scientist Institutional Award from the Burroughs Wellcome Fund. MRP is a Dedman Family Scholar in Clinical Care. SCW is a Disease-Oriented Clinical Scholar at UT Southwestern and is supported by the NCI/NIH (K08 CA222611).
Abbreviations:
- DR
Dominican (Republic)
- NCDB
National Cancer Database
- NOS
not otherwise specified Spanish/Hispanic/Latino origin
- PR
Puerto Rican
Footnotes
Disclosures: None
Data Availability:
The primary dataset (NCDB) is available publicly through the American College of Surgeons (https://www.facs.org/quality-programs/cancer/ncdb). The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Bray F, et al. , Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin, 2018. 68(6): p. 394–424. [DOI] [PubMed] [Google Scholar]
- 2.Sierra MS, et al. , Cancer patterns and trends in Central and South America. Cancer Epidemiol, 2016. 44 Suppl 1: p. S23–S42. [DOI] [PubMed] [Google Scholar]
- 3.Ikoma N, et al. , Racial disparities in preoperative chemotherapy use in gastric cancer patients in the United States: Analysis of the National Cancer Data Base, 2006–2014. Cancer, 2018. 124(5): p. 998–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klapheke AK, Carvajal-Carmona LG, and Cress RD, Racial/ethnic differences in survival among gastric cancer patients in california. Cancer Causes Control, 2019. 30(7): p. 687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yao Q, et al. , A Comprehensive Assessment of the Racial and Ethnic Disparities in the Incidence of Gastric Cancer in the United States, 1992–2014. Cancer Res Treat, 2019. 51(2): p. 519–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Refaie WB, et al. , The impact of ethnicity on the presentation and prognosis of patients with gastric adenocarcinoma. Results from the National Cancer Data Base. Cancer, 2008. 113(3): p. 461–9. [DOI] [PubMed] [Google Scholar]
- 7.Bautista MC, et al. , Significant Racial Disparities Exist in Noncardia Gastric Cancer Outcomes Among Kaiser Permanente’s Patient Population. Dig Dis Sci, 2015. 60(4): p. 984–95. [DOI] [PubMed] [Google Scholar]
- 8.Miller KD, et al. , Cancer Statistics for Hispanics/Latinos, 2018. CA Cancer J Clin, 2018. 68(6): p. 425–445. [DOI] [PubMed] [Google Scholar]
- 9.Yao JC, et al. , Clinicopathologic behavior of gastric adenocarcinoma in Hispanic patients: analysis of a single institution’s experience over 15 years. J Clin Oncol, 2005. 23(13): p. 3094–103. [DOI] [PubMed] [Google Scholar]
- 10.Passel JS, Cohn D, U.S. Population Projections: 2005–2050, Hispanic Trends. 2008, Pew Research Center. [Google Scholar]
- 11.Ramirez AG, et al. , The emerging Hispanic population: a foundation for cancer prevention and control. J Natl Cancer Inst Monogr, 1995(18): p. 1–9. [PubMed] [Google Scholar]
- 12.Huang RJ, et al. , One Size Does Not Fit All: Marked Heterogeneity in Incidence of and Survival from Gastric Cancer among Asian American Subgroups. Cancer Epidemiol Biomarkers Prev, 2020. [DOI] [PubMed] [Google Scholar]
- 13.Ajani JA, et al. , Gastric cancer, version 2.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw, 2013. 11(5): p. 531–46. [DOI] [PubMed] [Google Scholar]
- 14.Zhao B, et al. , Evaluation of treatment and outcomes for Hispanic patients with gastric cancer at Commission on Cancer-accredited centers in the United States. Journal of surgical oncology, 2019. 119(7): p. 941–947. [DOI] [PubMed] [Google Scholar]
- 15.Borrell LN, et al. , Race and Genetic Ancestry in Medicine — A Time for Reckoning with Racism. New England Journal of Medicine, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonham VL, Green ED, and Perez-Stable EJ, Examining How Race, Ethnicity, and Ancestry Data Are Used in Biomedical Research. JAMA, 2018. 320(15): p. 1533–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bryc K, et al. , Genome-wide patterns of population structure and admixture among Hispanic/Latino populations. Proceedings of the National Academy of Sciences, 2010. 107(Supplement 2): p. 8954–8961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conomos MP, et al. , Genetic diversity and association studies in US Hispanic/Latino populations: applications in the Hispanic Community Health Study/Study of Latinos. The American Journal of Human Genetics, 2016. 98(1): p. 165–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marker KM, et al. , Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer Is Associated with Indigenous American Ancestry in Latin American Women. Cancer Res, 2020. 80(9): p. 1893–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carrot-Zhang J, et al. , Genetic ancestry contributes to somatic mutations in lung cancers from admixed Latin American populations. Cancer Discovery, 2020: p. CD-20–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang SC, et al. , Hispanic/Latino patients with gastric adenocarcinoma have distinct molecular profiles including a high rate of germline CDH1 variants. Cancer research, 2020. 80(11): p. 2114–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tienda M and Sánchez SM, Latin American Immigration to the United States. Daedalus, 2013. 142(3): p. 48–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The primary dataset (NCDB) is available publicly through the American College of Surgeons (https://www.facs.org/quality-programs/cancer/ncdb). The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.