Abstract
Revascularization of ischemic tissue is a highly desirable outcome in multiple diseases, including cardiovascular diseases and ischemic retinopathies. Oxidative stress and inflammation are both known to play a role in suppressing reparative angiogenesis in ischemic disease models including oxygen-induced retinopathy (OIR), but the regulatory molecules governing these pathophysiologic processes in retinal ischemia are largely unknown. Nrf2 is a major stress-response transcription factor that has been implicated in regulating ischemic angiogenesis in the retina and other tissue beds. Using Nrf2-deficient mice, we investigated the effects of Nrf2 in regulating revascularization and modulating the retinal tissue milieu during ischemia. Strikingly, Nrf2’s beneficial effect on reparative angiogenesis only became manifested in the later phase of ischemia in OIR, from postnatal day 14 (P14) to P17. This was temporally associated with a reduction in both oxidative stress and inflammatory mediators in wild-type compared to Nrf2−/− mice. Nrf2−/− retinas exhibited an increase in VEGF but also induction of anti-angiogenic Dll4-Notch signaling. NADPH oxidase (NOX), and especially NOX2, is a major pathogenic molecule and a particularly important contributor to oxidative stress in multiple retinal disease processes. Nrf2−/− mice exhibited a significant exacerbation of NOX2 induction in OIR that manifested in the later phases of ischemia. Pharmacologic inhibition of NADPH oxidase abrogated the adverse effect of Nrf2 deficiency on reparative angiogenesis. Taken together, this suggests that Nrf2 is an important regulator of the retinal milieu during tissue ischemia, and that the Nrf2/NOX2 balance may play a critical role in determining the fate of ischemic revascularization.
Keywords: angiogenesis, ischemia, Nrf2, NADPH oxidase, inflammation, oxidative stress
Introduction
Ischemia due to vascular dysfunction and vessel loss leads to a variety of pathological alterations in ischemic nervous tissue in multiple disease settings including stroke and ischemic retinopathies [1]. Prompt restoration of blood supply is a fundamental theme for the preservation of neuronal function and prevention from irreversible injury in ischemic nervous tissue [2, 3]. Ischemic retinopathies are major causes of blindness and are characterized by two stages, initial vascular degeneration and subsequent ischemia-driven pathologic neovascularization. Physiologic revascularization is highly desirable in ischemic retinopathies, both in diminishing retinal tissue damage and in curtailing the retinal ischemia that drives maladaptive pathologic neovascularization [2]. Therapies that promote this revascularization would be extremely beneficial for these disease conditions [4, 5]. It is therefore important to gain an improved understanding of the mechanisms and molecular determinants that promote or impede reparative angiogenesis in ischemic retinopathies.
Oxidative stress plays a critical role in the regulation of revascularization. Reactive oxygen species can have both a positive and negative effect on angiogenesis, depending on factors including tissue context, degree, and duration of oxidative stress. A low and transient level of reactive oxygen species (ROS) is known to promote angiogenesis in multiple tissues, including the retina [6, 7]. On the other hand, excessive oxidative stress has a deleterious effect on revascularization in ischemic retinopathies, including retinopathy of prematurity and the well-characterized oxygen-induced retinopathy (OIR) model [8]. Exogenous administration of antioxidants, including superoxide dismutase [9] or pharmacologic antioxidants [10] significantly improve revascularization of ischemic retina, with amelioration of retinal avascularity in oxygen-induced retinopathy. Among the effects of oxidative stress is the instigation of inflammation, including inflammatory cytokine production, which can in turn promote further reactive oxygen species production via a positive feedback loop. Indeed, inflammatory cytokines including TNF-α [11, 12] and IL-1β [13] suppress revascularization in ischemic retinopathy.
The superoxide generating enzyme NADPH oxidase (NOX) has received extensive attention as a major source of oxidative stress, and excessive NOX activity is implicated in multiple vascular disease processes [14]. NADPH oxidases have been implicated in multiple retinal diseases, including retinopathy of prematurity and diabetic retinopathy [7, 15-19]. Of the NOX isoforms, NOX2 has received considerable attention in relation to ischemic retinopathies [7]. NOX2 has been directly implicated in retinal superoxide induction in oxygen-induced retinopathy [18]. NOX2 expression is induced in multiple retinal conditions, including oxygen-induced retinopathy [18], ischemia-reperfusion [20], and diabetic retinopathy [17]. Genetic ablation of NOX2 results in attenuation of retinal and retinal vascular pathology in inflammatory retinal disease [17], retinal ischemia-reperfusion [20], diabetic retinopathy [21], and oxygen-induced retinopathy [22].
In treating ischemic retinopathies, it would be of great benefit to modulate critical pathogenic mediators in the retinal milieu, including reactive oxygen species and NADPH oxidase. However, the key regulators of the retinal milieu in the setting of ischemia are largely unknown. NF-E2-related factor 2 (Nrf2) is a cytoprotective transcription factor that plays an important role in the regulation of stress-induced antioxidant and anti-inflammatory responses [23]. We have previously demonstrated that Nrf2 has a protective role in several ischemic retinal diseases, including ischemia-reperfusion [24-26], diabetic retinopathy [27], and oxygen-induced retinopathy [28]. Additionally, we found that Nrf2 regulates retinal vascularization in both development and disease settings. In development, Nrf2 promotes vascular sprouting in a Dll4/Notch-dependent fashion [29]. Importantly, Nrf2 promotes revascularization in oxygen-induced retinopathy [28]. We are therefore interested in gaining further insights into the mechanisms by which Nrf2 promotes vascular regeneration in the ischemic retina, and particularly in its modulation of the retinal milieu.
Here we report that Nrf2 plays a key role in precise modulation of oxidative stress and inflammation, which helps establish a more favorable environment for revascularization in oxygen-induced retinopathy. Nrf2 activation protects retina from exacerbation of both ROS and inflammatory cytokines during the ischemic phase of OIR. Importantly, Nrf2 protects against retinal NOX2 induction, and modulation of NADPH oxidase and NOX2 appears to be a critical mechanism by which Nrf2 promotes revascularization in OIR. Our findings define an antagonistic relationship between Nrf2 and NOX2 in the retina. This suggests a therapeutic strategy for ischemic retinopathy by modulation of ROS, NOX2, and inflammation through regulation of Nrf2.
Results
Nrf2 deletion inhibits vessel regrowth and induces pathological neovascularization.
In order to gain insights into Nrf2’s role in regulating vascular regeneration in ischemic neuronal tissue, we used the mouse model of oxygen-induced retinopathy (OIR). Mice are exposed to 75% oxygen from postnatal day 7 (P7) to P12, and returned to room air [30, 31]. Exposure to hyperoxia from P7-P12 results in vaso-obliteration of the central retina. Upon return to room air at P12, the central ischemic retina becomes hypoxic. From P12 to P17, there is some degree of revascularization of this central ischemic retina. However, delayed or incomplete vascular recovery of the retina proper results in prolonged retinal ischemia, which in turn exacerbates the production of pro-angiogenic growth factors that drive maladaptive pathologic pre-retinal neovascularization (pathological NV) from P14 to P17 (Fig. 1A). This ischemia-driven pathologic NV is reminiscent of human ischemic retinopathies including retinopathy of prematurity and proliferative diabetic retinopathy [31]. To determine the importance of Nrf2 in ischemic retina, we compared wild-type (Nrf2+/+) and Nrf2-deficient mice (Nrf2−/−) with respect to vascular changes in OIR [28]. The Nrf2−/− mice have a non-functional Nrf2 based on the absence of the b-ZIP region in exon 5 [32]. Loss of the wild-type functional Nrf2 in the Nrf2−/− retinas in OIR was confirmed by real-time PCR assay (Supplemental Fig. 1). We previously found that Nrf2−/− mice exhibits a remarkably higher extent of avascular retina at P17 compared to wild type mice in OIR, reflecting a significantly lower degree of revascularization in the Nrf2−/− mice [28]. We were therefore interested in gaining further insights into the time-course of Nrf2’s beneficial effect on revascularization, by performing analyses at P12, P14, and P17. There was no appreciable difference in avascular area between Nrf2+/+ and Nrf2−/− retinas either immediately upon return to room air at postnatal day 12 (P12) or after two days of ischemia at P14 (Fig. 1B and C). In striking contrast, at P17 there was a dramatic increase in avascular area, accompanied by a considerable increase (about 5-fold) in pathologic pre-retinal neovascularization in Nrf2−/− retinas compared with wild-type (Fig. 1B-D). The results indicate that Nrf2’s beneficial effects in enhancing revascularization are manifested between P14 and P17.
Fig. 1.
Genetic ablation of Nrf2 impedes vascular regrowth and increases pathological neovascularization in oxygen-induced retinopathy (OIR). (A) Diagram of OIR. Mice are placed in a hyperoxia chamber containing 75% O2 at post natal day 7 (P7) and return to room air at P12. The retinal blood vessels are obliterated in hyperoxia (vaso-obliteration), and vascular regeneration occurs from P12 to P12 when retinas becomes relatively hypoxic. Retinal hypoxia induces vascular regeneration including regrowth of normal retinal vessels and pathologic pre-retinal neovascularization (pathological NV). The pathological NV is evident at P17. (B) Retinal flatmounts of Nrf2+/+ and Nrf2−/− mice at P12, P14 and P17 of OIR. Blood vessels were visualized by GS lectin staining. Avascular retina was outlined by white lines. No significant difference in avascular area between Nrf2+/+ and Nrf2−/− retinas was observed at P12 or P14 (C), while a dramatic increase in avascular area and area of neovascular tufts (arrowheads) was found in Nrf2−/− retinas compared with Nrf2+/+ at P17 (D). n = 8-14. Scale bar, 400 μm. Data are presented as mean ± SEM. *P< 0.05; **P< 0.01; NS, not significant. (E) Quantitative RT-PCR analysis shows that the Nrf2 target gene NQO1 was upregulated in Nrf2+/+, but not in Nrf2−/− retinas from P12 to P17 of OIR. Values represent fold change relative to P12 (0 h). n = 5. Data are presented as mean ± SEM. *P< 0.05; ** P< 0.01 compared with P12-0h; NS, not significant compared with P12-0h. #P< 0.05; ##P< 0.01 between wild-type and Nrf2−/−.
We next investigated Nrf2 activation by assessing expression of the Nrf2 target gene, NQO1, from P12 to P17. NQO1 mRNA increased in wild-type retina as early as 2 hours (P12 + 2 hours) after onset of the ischemic/hypoxic phase (Fig.1D).This increase in NQO1 mRNA was sustained through P17 (Fig.1D). In contrast to wild-type, no induction of NQO1 mRNA was observed in Nrf2−/− retinas except P14 at which there was a slight increase in NQO1 mRNA compared with P12 -0h (immediately after hyperoxia). NQO1 mRNA levels in Nrf2−/− retinas were consistently lower than in wild-type at all time-points. These results confirm activation of Nrf2, reflected by Nrf2 target gene activation in wild-type but not Nrf2−/− retinas, throughout the entire ischemic phase of OIR.
Nrf2-deficient mice exhibit greater GSH depletion and exaggerated increase in superoxide correlating with delayed revascularization in OIR.
Given the pivotal role of Nrf2 in modulating intracellular ROS, we next assessed levels of reduced glutathione (GSH) and superoxide in retinas subjected to OIR. A dramatic decrease in GSH level was observed in Nrf2+/+ and Nrf2−/− retinas at P12-2h (2 hours after hyperoxia) of OIR compared with Nrf2+/+ retinas at P12-0h (immediately after hyperoxia). GSH levels increased in both Nrf2+/+ and Nrf2−/− retinas after P12-2h. In striking contrast, a marked increase in GSH level was found in Nrf2+/+ but not Nrf2−/− retinas at OIR P15 and P17, resulting in a significantly higher level of GSH content in Nrf2+/+ retinas as compared with Nrf2−/− retinas at OIR P15 and P17 (Fig. 2A).
Fig. 2.

Nrf2-deficient mice exhibit greater decrease in GSH and accentuated increase in superoxide correlating with suppression of reparative angiogenesis in OIR. (A) Change in GSH levels in revascularization stage of OIR. A dramatic decrease in GSH level was observed in Nrf2+/+ and Nrf2−/− retinas at P12-2h (2 hours after hyperoxia) of OIR compared with Nrf2+/+ retinas at P12-0h (immediately after hyperoxia). GSH levels increased in both Nrf2+/+ and Nrf2−/− retinas after P12-2h.. A marked increase in GSH level was shown in Nrf2+/+ but not Nrf2−/− retinas at OIR P15 and P17, resulting in a significantly higher level of GSH content in Nrf2+/+ retinas as compared with Nrf2−/− retinas at OIR P15 and P17 (highlighted by dashed box). Data are expressed as percentage of GSH change relative to Nrf2+/+ at OIR P12-0 h. n = 4. (B) Retinal superoxide levels were determined by biochemical assay using lucigenin at P15 of normoxia or OIR. In Nrf2+/+ mice, retinas subjected to OIR exhibit an increased level of superoxide as compared to retinas in normoxia. Compared with Nrf2+/+ retina, a slight increase in superoxide was shown in Nrf2−/− retina in normoxia and a greater increase was observed in OIR. Values represent fold change relative to Nrf2+/+ in normoxia (Norm). n = 8. Data are presented as mean ± SEM. * P< 0.05; ** P< 0.01.
In light of the contrast in GSH levels between wild-type and Nrf2−/− retinas at OIR P15, we next investigated retinal superoxide by biochemical assay using lucigenin at P15. In Nrf2+/+ mice, retinas subjected to OIR produced significantly more superoxide as compared to retinas in normoxia, as expected (Fig. 2B). Nrf2−/− retinas exhibited a slight but significant increase in superoxide compared to wild-type under normoxic conditions, and a greater increase in superoxide compared to wild-type in OIR (Fig. 2B). In contrast, there was no significant increase in superoxide in Nrf2−/− retinas compared to wild-type at the early OIR P12-2h (2 hours after hyperoxia) time-point (Supplemental Fig. 2). These superoxide results, together with the GSH findings above, indicate a significant exacerbation of oxidative stress in Nrf2−/− retinas between OIR P14 to P17, correlating with the time-frame of delayed vascular regrowth in OIR.
Nrf2 suppresses the induction of inflammatory regulators of revascularization in OIR.
Inflammatory cytokines are known to be induced in angiogenic eye diseases in humans and play a critical role in modulating retinal revascularization in animal models of ischemic retinopathy, including OIR [11-13]. We have previously found that Nrf2 is an important regulator of inflammation in other retinal disease conditions, including ischemia-reperfusion injury [24, 25] and diabetic retinopathy [27]. To investigate the effect of Nrf2 on inflammatory gene expression in OIR, we assessed the mRNA levels of two major inflammatory cytokines implicated in OIR, TNF-α [11, 12] and IL-1β [13], in wild-type and Nrf2−/− retinas. No appreciable change in TNF-α or IL-1β mRNA levels was observed between wild-type and Nrf2−/− retinas at P15 of normoxia. In contrast, a significant accentuation in the induction of these cytokines in Nrf2−/− retinas compared with wild-type at P15 of OIR (Fig.3A and B). In parallel with its RNA levels, protein levels of TNF–α were increased in Nrf2−/− retinas compared with wild-type at the same time-point (Supplemental Fig. 3A). The results suggest that Nrf2 activation in wild-type retina suppresses the induction of inflammatory mediators in OIR. The role of Nrf2 in inflammation was further confirmed by pharmacologic activation of Nrf2 in OIR. Intravitreal injection of 24 nM CDDO-Im, a potent activator of Nrf2 which we have previously used for both retinal and retinal cell culture studies [25, 26, 28], at OIR P12 and P14 dramatically inhibited the mRNA expression of TNF-α and IL-1β (Supplemental Fig. 3B). Nrf2 activation by CDDO-Im in this OIR experiment has been validated in our previous study [28].
Fig. 3.

Nrf2 suppresses the induced inflammatory mediators in ischemic retinas in OIR. (A and B) Deletion of Nrf2 accentuates mRNA expression of inflammatory mediators in ischemic retinas in OIR. Real-time PCR analysis demonstrates that retinal TNF-α (A) and IL-1β (B) were induced in Nrf2+/+ mice subjected to OIR compared with normoxia at P15 and an accentuated mRNA level was observed in Nrf2−/− retinas in OIR. Values represent fold change relative to wild-type in normoxia (Norm). n = 5. Data are presented as mean ± SEM. * P< 0.05; ** P< 0.01.
As a complementary approach to these in vivo findings, we performed studies using a human Müller glial cell culture system. We focused on Müller cells since they have been implicated in the regulation of OIR [33-35], express Nrf2 [27, 28], and are an important source of inflammatory cytokines in the retina [33]. We found that CDDO-Im treatment suppressed inflammatory induction of multiple cytokines, including IL-1β, MCP-1, and ICAM-1 (Supplemental Fig. 3C-E). This suggests that regulation of inflammatory cytokine production in Müller cells by Nrf2 could contribute to the improvement in revascularization in wild-type compared to Nrf2 knockout mice.
Loss of Nrf2 increases Dll4/Notch signaling in ischemic retina in OIR.
Nrf2 has been suggested to regulate angiogenesis in part via VEGF in multiple ischemic tissues in systemic settings, in which inhibition of Nrf2 is associated with reduced VEGF expression [36, 37]. In oxygen-induced retinopathy, VEGF protein in Nrf2−/− retinas was higher than wild-type at P15 (Fig 4A). We previously found that Nrf2 promotes vascular sprouting and branching by regulating Dll4/Notch signaling in the context of developmental angiogenesis in the retina [29]. In oxygen-induced retinopathy, Dll4 and Notch target genes (Hes1 and Hey1) were upregulated in Nrf2−/− retinas as compared to wild-type at P15 (Fig. 4B-D). The relationship between Nrf2 and Dll4/Notch signaling was similar to that observed in retinal developmental angiogenesis. This suggests that Dll4/Notch signaling is similarly involved in Nrf2 regulation of revascularization in OIR.
Fig. 4.

Loss of Nrf2 increases VEGF and Dll4/Notch signaling in ischemic retinas in OIR. (A) Retinal VEGF protein level in Nrf2−/− retina at P15 of OIR is higher than Nrf2+/+. (B-D) Quantitative RT-PCR analysis shows that mRNA levels of Dll4 (B) and Notch target genes, Hes1 (C) and Hey1 (D), increase in Nrf2−/− retinas at P15 of OIR. n = 5. Data are presented as mean ± SEM. * P< 0.05; ** P< 0.01.
Deletion of Nrf2 increases mRNA expression of NOX2 in ischemic retina in OIR.
Our results above indicate that Nrf2 exerts a protective role in modulating the retinal milieu with respect to oxidative stress and inflammation during the phase of vascular regeneration in OIR. We examined Nrf2’s effect on the retinal milieu with respect to an especially important pathogenic molecule and source of ROS in OIR, the superoxide-generating enzyme NADPH oxidase. We compared gene expression of 4 major NADPH oxidases (NOX1-NOX4) in retina in wild-type and Nrf2−/− mice in normoxia or after being subjected to OIR at P15 (Fig. 5A-D). Among them, NOX2 mRNA in Nrf2−/− retinas was slightly increased in normoxia while being markedly increased in OIR as compared to wild-type (Fig. 5B), consistent with the superoxide levels shown in Fig. 2B. Aside from NOX2, p47phox, a key cytosolic subunit of NOX2 complex, was also upregulated in Nrf2−/− retinas as compared with wild-type in OIR (Fig. 5E). There was no evidence for upregulation of the other NOX’s in Nrf2−/− retinas relative to wild-type in OIR. The results suggest a role of Nrf2 in regulating NOX2 expression in OIR.
Fig. 5.

Deletion of Nrf2 increases mRNA expression of NOX2 in ischemic retina in OIR. Quantitative RT-PCR analysis of NOX1, NOX2, NOX3, NOX4 and p47phox mRNAs in normoxia (Norm) and OIR. There was no increase in NOX1, NOX3, or NOX4 mRNAs in Nrf2−/− retinas at P15 of OIR, whereas NOX2 and p47phox mRNAs were significantly increased in Nrf2−/− retinas at P15 of OIR. NOX2 mRNA expression pattern in Nrf2+/+ and Nrf2−/− retina at normoxia and OIR was consistent with superoxide level in Fig. 2B. n = 5. Data are presented as mean ± SEM. * P< 0.05; ** P< 0.01; NS, not significant.
Deletion of Nrf2 increases NOX2 protein in ischemic retina in OIR.
We further investigated the regulation of NOX2 by Nrf2, comparing the regulation of NOX2 protein levels during oxygen-induced retinopathy from P12 to P17 in wild-type and Nrf2−/− retinas. In wild-type retinas, NOX2 protein was induced at P13 to P15 compared with P12-0 h (Fig. 6A and B). By P17, NOX2 protein levels in wild-type had returned to baseline. Nrf2−/− retinas in constrast exhibited an immediate increase in NOX2 protein levels at P12-2h that was sustained throughout the ischemic phase to P17, as compared with P12-0h. Strikingly, NOX2 protein levels in Nrf2−/− retinas at P14, P15 and P17 were markedly higher compared to wild-type at the same time-points (Fig. 6A and B). Consistent with NOX2 protein levels, there was a strong increase in NADPH oxidase activity in Nrf2−/− retinas as compared with Nrf2+/+ at OIR P15 (Fig. 6C). The temporal pattern of NOX2 protein expression in wild-type and Nrf2−/− in OIR, in which NOX2 is markedly higher in Nrf2−/− in the later stages of ischemia, is consistent with the observed differences in oxidative stress between wild-type and Nrf2−/− (Fig. 2). The results suggest that the excessive oxidative stress in Nrf2−/− retina may be directly attributable to increased NOX2. The role of Nrf2 in the regulation of NOX2 was further demonstrated by a study of pharmacologic activation of Nrf2 in OIR, in which intravitreal injection of 24 nM CDDO-Im significantly inhibited NOX2 mRNA expression (Supplemental Fig. 4). We next examined localization of NOX2 protein in ischemic retinas during OIR in wild-type and Nrf2−/− mice at P15, the time-point at which NOX2 protein was highest in Nrf2−/− retina. NOX2 was highly expressed in retinal vessels and also present in non-vascular tissues adjacent to vascular endothelial cells in both wild-type and Nrf2−/− mice. Greater expression of NOX2 protein was observed both in blood vessels (including pre-retinal neo-vessels) and the surrounding cells in the retina, including ganglion cell layer, in Nrf2−/− retina (Fig. 6D).
Fig. 6.
Deletion of Nrf2 increases NOX2 expression in the later phase of ischemia in OIR. (A and B) Immunoblot analysis of NOX2 in retinas subjected to OIR. NOX2 protein increases in Nrf2+/+ retina from P13-P15, and Nrf2−/− retina from P12-2 h to P17, as compared to P12-0 h. The higher levels of NOX2 protein were observed in Nrf2−/− retinas from P14 to P17 (highlighted by dashed box) compared to Nrf2+/+, peaking at P15. β-actin was used as a loading control. n = 3. (C) NADPH oxidase activity in Nrf2+/+ retina was about 2.5 fold higher than Nrf2−/− retina. NADPH oxidase activity was assessed by lucigenin-enhanced chemiluminescence. Data are expressed as U/sec/mg protein (U, light unit; sec, second). n = 6. (D) Retinal cryosection staining at P15 of OIR. NOX2 was highly expressed in retinal vessels (arrowheads). In Nrf2−/− retinas, greater expression of NOX2 was observed in retinal blood vessels and pre-retinal neo-vessels (arrowhead in the inset for Nrf2−/−), as well as surrounding cells in the retina, including the ganglion cell layer (GCL; arrows) in Nrf2−/− retina. INL, inner nuclear layer; ONL, outer nuclear layer. Scale bar, 20 μm. Data are presented as mean ± SEM. * P< 0.05.
Increase in avascular retina and pathologic neovascularization is abrogated by administration of apocynin in Nrf2 deficient mice.
We next determined whether upregulation of NOX2 is a critical mechanism for the suppression of revascularization in OIR by Nrf2 deficiency. For this purpose, we examined the effect of the NADPH oxidase inhibitor, apocynin, on revascularization. Administration of apocynin at 5 mg/kg or 10 mg/kg daily from P14 to P16 led to a significant decrease in both avascular retinal area and neovascular tufts in Nrf2−/− retinas at P17 of OIR (Fig. 7). The extent of avascular area and area of tufts in apocynin-treated Nrf2−/− retinas were comparable to that in Nrf2+/+ retinas (Fig. 7B and C). These results, together with the Nrf2 regulation of NOX2 detailed above, indicate that the compromised revascularization in Nrf2−/− retinas is the consequence of increased NADPH oxidase activity, presumably derived from NOX2.
Fig. 7.
Increase in avascular retina and pathologic neovascularization is abrogated by administration of apocynin in Nrf2-deficient mice. (A) Retinal flatmounts show that intraperitoneal injection of apocynin at 5 mg/kg or 10 mg/kg daily from P14 to P16 leads to a significant decrease in avascular area (B) and neovascular tufts (arrowheads) (C) in Nrf2−/− retinas at P17 of OIR, which are comparable to that in Nrf2+/+ retinas. n = 8. Scale bar, 400 μm. Data are presented as mean ± SEM. *P< 0.05; ** P< 0.01 compared with Nrf2+/+ Vehicle; NS, not significant compared with Nrf2+/+ Vehicle. #P< 0.05; ##P< 0.01 compared with Nrf2−/− Vehicle.
Discussion
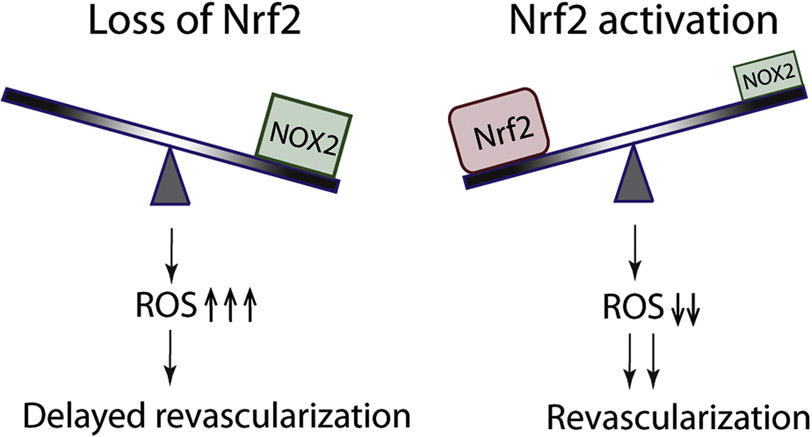
Physiologic revascularization is a highly desirable event in ischemic conditions of the CNS and retina. Although ischemic retinal tissue is known to produce pro-angiogenic factors that might promote reparative angiogenesis, the overall retinal environment is not optimally conducive to reparative angiogenesis, in large part due to excessive ROS [8-10] and inflammation [11-13]. The ability to modulate these important influences would be highly beneficial. However, the important molecules regulating ROS and inflammation in the ischemic retina remain to be better defined. We have previously found that Nrf2 plays an important role in promoting reparative angiogenesis in ischemic retina [28]. Given Nrf2’s major function as a regulator of ROS and its effects in modulating inflammation, we were therefore interested in determining the ability of Nrf2 to modify these critical factors in the ischemic retina. In the current study, we found that Nrf2 plays a significant role in modulating the retinal milieu with respect to both ROS and inflammatory cytokines, providing a more favorable environment for reparative angiogenesis in the setting of ischemia. A particularly important aspect of Nrf2’s favorable effect on ROS is its ability to counteract excessive NOX2 expression, which appears to be a key mechanism by which Nrf2 promotes revascularization of the ischemic retina. Together our results suggest that the balance between Nrf2 and NOX2 may be a critical determinant of the propensity of the ischemic retina toward revascularization (Fig. 8).
Fig. 8.
Schematic of Nrf2 and NOX2 balance in reparative angiogenesis in the retina. Superoxide generating NADPH oxidase is a major source of oxidative stress in the retina, and NOX2 is particularly important in retinal diseases. Deficiency of Nrf2 leads to unchecked NOX2 activity resulting in excessive oxidative stress and suppression of reparative angiogenesis. Nrf2 activation counteracts NOX2, allowing appropriate control of oxidative stress, thereby promoting reparative angiogenesis in the retina.
An interesting and striking aspect of the current study is the time course of Nrf2’s beneficial effect. While Nrf2-deficient mice exhibited much less revascularization of the central ischemic retina at P17 compared to wild-type, the beneficial effect of Nrf2 on revascularization was not yet apparent at P14 (Figure 1C). Although the effect on revascularization was delayed, Nrf2 activation itself was already evident very early on (as early as 2 hours after the hyperoxic phase) and was sustained throughout the ischemic phase of OIR. In addition, expression of the Nrf2 target gene NQO1 reached a peak by P14. After P14, the degree of reparative angiogenesis between wild-type and Nrf2-deficient mice decidedly diverged, with much greater revascularization in the wild-type at P17. This was associated with much less pathologic neovascularization in the wild-type at P17. These results strongly suggest that the critical molecular events underlying Nrf2’s salutary effects in wild-type retinas occurred between P14 and P17, serving to enhance physiologic vessel regrowth, reduce retinal ischemia, and suppress pathological neovascularization.
In light of the time-course of Nrf2’s effects on revascularization, we were highly interested in its corresponding effects on ROS and inflammatory cytokines. In OIR, wild-type and Nrf2 knockout retinas exhibited a similar reduction of the antioxidant GSH from P12 to P14, suggesting a comparable degree of oxidative stress between wild-type and Nrf2 knockout during this time-frame. This corresponded with an equal extent of revascularization in wild-type and Nrf2 deficient retina at P14, indicating a minimal impact of Nrf2 on vascular regrowth at this early stage of ischemia. However, Nrf2 deficient retinas exhibited conspicuous reduction in GSH compared to wild-type at P15 of OIR, which corresponded with an exacerbation of induction of both superoxide and inflammatory cytokines in the Nrf2 knockout at P15 of OIR. The excessive levels of ROS and inflammatory cytokines and the prolonged duration of oxidative stress in the Nrf2 knockout correlated with the suppression of revascularization in these mice from P14 to P17. These results indicate that the modulation of ROS and inflammatory cytokine levels in the retina are an important basis for its creation of a favorable environment to promote reparative angiogenesis. Nrf2 therefore represents an attractive therapeutic target for modulating both oxidative stress and inflammation in OIR and ischemic retinopathies.
We previously demonstrated that Nrf2 promotes angiogenic sprouting and vascularization by suppression of Dll4/Notch signaling during vascular development in the retina [29]. A direct role of Dll4 in regulating revascularization in OIR has previously been reported [38]. In the current study, we therefore inquired whether Nrf2 might regulate Dll4/Notch signaling in retina in the setting of disease. In OIR, Nrf2-deficient mice exhibited both increased Dll4 expression and Notch signaling in the retina. This suggests that Nrf2 modulation of Dll4/Notch signaling might contribute to its regulation of revascularization in OIR. In addition to Dll4/Notch signaling, we also investigated VEGF, known to be a key regulator of angiogenesis in OIR [31]. We observed higher VEGF protein levels in Nrf2 knockout retinas at P15. It is therefore unlikely that modulation of VEGF contributes to Nrf2’s beneficial effect on revascularization.
Since Nrf2 exhibits prominent effects in modulating ROS, we investigated whether it specifically regulates the superoxide-producing NADPH oxidase, given the major role of NOX both in vascular disease and retinal disease processes [7, 15-19]. Our study indicates that NADPH oxidase is indeed an important mediator of Nrf2’s effects. Specifically, we found that RNA expression of NOX2, but not the other NOX isoforms, is accentuated in Nrf2-deficient retinas compared to wild-type. This increase in NOX2 RNA expression is associated with increase in NOX2 protein levels in Nrf2-deficient retinas compared to wild-type beginning from P14 in OIR and extending to P17. This time-course displayed a similar pattern to the reduction in GSH levels, which diverged between Nrf2-deficient and wild-type retinas beginning around P15. NOX2 is therefore likely to be a major source of the excessive ROS in Nrf2 KO retina in the later stages of ischemia in OIR. Our studies demonstrating that administration of the NADPH oxidase inhibitor apocynin from P14 to P16 abrogates the suppression of revascularization in Nrf2-deficient mice, together with our studies of retinal NOX expression and enzymatic activity, indicate that modulation of NOX2 is a critical basis for Nrf2’s favorable effects on reparative angiogenesis. Notably, a previous study found that administration of apocynin from P12 through P17 suppressed both pathological neovascularization and physiologic revascularization in wild-type mice in OIR [18]. We speculate that low levels of NOX2 may be beneficial for reparative angiogenesis, but that excessive NOX2 is detrimental, therefore highlighting the important role of Nrf2 in maintaining a homeostatic retinal environment.
As an approach for NADPH oxidase inhibition, we used apocynin because of its excellent inhibitory effects on NOX activity and its successful use for inhibition of retinal NOX in multiple studies [18, 19]. The ability of apocynin treatment to abrogate the inhibitory effects of Nrf2 deficiency is consistent with the notion that upregulation of NOX2 accounts for the suppression of revascularization in Nrf2-deficient mice. It is recognized, however, that apocynin is not a specific inhibitor of NOX2. Therefore, more in-depth studies will be required in the future to definitively implicate NOX2.
Our study indicates that Nrf2 has an important counter-balancing effect to NADPH oxidase and NOX2 in the retina and that this balance plays a critical role in determining the redox status of the retina during retinal ischemia. Interestingly, evidence has emerged recently indicating that Nrf2 negatively regulates NADPH oxidase and particularly NOX2 in other settings. Nrf2-deficient macrophages display enhanced induction of NADPH oxidase in LPS-induced pulmonary inflammation [39]. Nrf2-deficient mice exhibit upregulation of NOX mRNA expression in brain tissue [40] and exacerbated induction of NOX2 protein levels in the brain in a traumatic brain injury model [41]. From a functional standpoint, modulation of NOX2 has been implicated in the protective effect of Nrf2 against LPS-induced sepsis [39]. In the current study, we find that regulation of NOX2 and NADPH oxidase is a major mechanism by which Nrf2 modulates the retinal environment, including suppression of excessive ROS, creating conditions more favorable toward reparative angiogenesis. Therapeutic targeting of Nrf2 may therefore represent a promising strategy of modulating NOX2 and ROS, thereby promoting reparative angiogenesis in ischemic retinopathies and possibly other cardiovascular conditions.
Materials and Methods
Animals.
The wild-type (Nrf2+/+) and Nrf2−/− mice have been published previously [28, 42]. The animals were maintained on an AIN-76A diet and water ad libitum and housed at a temperature range of 20–23°C under 12:12-hour light-dark cycles. Mice were used in accordance with protocols approved by the Institutional Animal Care and Use Committee of The Johns Hopkins University School of Medicine.
Oxygen-induced retinopathy.
The mouse OIR model was described previously [30, 43-45]. Pups were exposed to 75% oxygen for 5 days from postnatal day 7 (P7) to P12. At P12, P14, or P17, retinas were harvested for analysis of avascular area and/or neovascularization (neovascular tufts) area. Staining and quantification were performed as previously described [45, 46]. Briefly, retinal vessels were visualized by incubation overnight with Alexa-conjugated isolectin GS-IB4 from Griffonia simplicifolia (GS lectin) (Life Technologies). Avascular area and tufts were quantified by comparing the number of pixels in the area of vaso-obliteration or neovascular tufts with the total number of pixels in the retina. Quantitation was performed in a masked fashion. Mice with body weight lower than 6 g (P17) were excluded from analysis [45].
GSH.
The reduced glutathione (GSH) concentration was assessed using Glutathione Assay Kit (BioVision). Briefly, retinas were homogenized in Glutathione assay buffer and the homogenate was subjected to measurement according to the manufacturer’s instructions. GSH concentration was calculated based on a standard curve and normalized by protein concentration.
Superoxide anion assay.
Superoxide anion in retinas were quantified by lucigenin assay as previously described [23] with minor modifications. Briefly, fresh retinas were cut in half and placed in 0.2 mL Krebs/HEPES buffer and incubated in the dark at 37 °C under 5% CO2 for 10 min. Lucigenin (Sigma-Aldrich) was added to a final concentration of 0.5 mM and plates were incubated for 5 min at room temperature. Photon emission was measured over 10 seconds by a luminometer (BMG Labtech). Repeated measurements were made over a 30 min period for 15 measurements. After averaging the measurements and subtracting reaction blank measurements, the values were normalized by the protein concentration for each retina.
CDDO-Im injection.
Wild-type mice subjected to OIR were intravitreally injected with 1 μL 24 nM of CDDO-Im [kindly provided by Michael B. Sporn, Dartmouth School of Medicine, dissolved in 10% (v/v) DMSO, 10% (v/v) cremophor-EL, 80% (v/v) PBS] or vehicle at OIR P12 and P14. For each mouse, one eye was injected with CDDO-Im, and the contralateral eye was injected with vehicle. Results were normalized to vehicle-injected eye.
Western blot.
Retina samples were isolated, snap-frozen in liquid nitrogen, and stored in −80°C. Retinas were ultrasonically homogenized in RIPA buffer (Sigma-Aldrich) containing protease inhibitor cocktail (Sigma- Aldrich). Western blot was performed as previously described [46]. Protein were separated by SDS-PAGE, and transferred to Hybond ECL nitrocellulose membrane (GE Healthcare). The membranes were then blocked and probed with monoclonal anti-gp91[phox] (BD Transduction Laboratories) and monoclonal anti-β-actin (Cell Signaling Technology). HRP-conjugated secondary antibodies were detected using SuperSignal West Pico or Femto chemiluminescent substrates (Thermo Scientific). Band intensity was quantitated using Image J.
Quantitative RT-PCR.
Total RNA from retina was isolated using the RNeasy Mini Kit (Qiagen), then treated with DNase I (Qiagen) as previously described [25, 29]. Single-stranded cDNA was synthesized using oligo (dT)12-18 primer ( Life Technologies) and MMLV Reverse Transcriptase (Life Technologies). Real-time PCR was performed using SYBR Premix Ex Taq (Takara) with Stratagene Mx3005P qPCR system (Agilent Technologies). Cyclophilin A was used for normalization. Each cDNA sample was run in duplicate.
NADPH oxidase activity.
NADPH oxidase activity was assessed by lucigenin-enhanced chemiluminescence as described previously [47] with minor modifications. Briefly, three retinas were pooled and homogenized in 750 μL of Krebs/HEPES buffer containing 1/100 (v/v) protease inhibitor cocktail (Sigma-Aldrich). Tissue homogenates were added into a LUMITRAC 96-well plate (Greiner) (180 μL/well) and incubated at 37°C for 20 min. Apocynin (1.5 mM), a NADPH oxidase inhibitor, was added into one well of each homogenate as a negative control. NADPH (100 μM) (Sigma–Aldrich) and dark-adapted lucigenin (5 μM) (Sigma–Aldrich) were then added to homogenates and incubated at room temperature for 15 min. Chemiluminescence was measured over 10 seconds by a CLARIOstar microplate reader (BMG Labtech) and repeated measurements were made over a 30 min period for 10 measurements. Chemiluminescence was normalized by the protein concentration.
Cryosection immunofluorescence staining.
Eyeballs were enucleated and directly embedded in optimal cutting temperature (OCT) medium (Sakura Finetek) at −80°C. Staining of cryosections was performed as previously described [46]. Briefly, sections (8 μm) were cut and fixed for 10 minutes with 4% PFA. After blocking with 5% normal serum, sections were incubated overnight with monoclonal anti-gp91[phox] (BD Transduction Laboratories) and GS lectin (Life Technologies) diluted with PBS plus 0.1% Triton X-100 at 4°C. Secondary detection was performed with Alexa-conjugated secondary antibodies (Life Technologies). Stained samples were covered with Vectashield mounting medium containing 4.6-diamidino-2-phenylindole (DAPI) (Vector labs) and visualized using a Laser Scanning Microscope 710 (Zeiss).
ELISA.
The protein concentrations of VEGF and TNF-α were assessed using Mouse VEGF DuoSet ELISA kit (R&D Systems) and Mouse TNF-α DuoSet ELISA kit (R&D Systems) as previously described [48]. Briefly, retinas were ultrasonically homogenized in 0.1% Triton X-100 in PBS containing a cocktail of protease inhibitors (Life Technologies). The samples were cleared by centrifugation, and supernatants were subjected to measurement according to the manufacturer’s instructions. Each sample was measured in duplicate. Protein concentration was calculated based on a standard curve and normalized by protein concentration.
Apocynin intervention.
For inhibition of NADPH oxidase, mice subjected to OIR were intraperitoneally injected daily with apocynin (Sigma-Aldrich) at 5 mg/kg, 10 mg/kg or vehicle from P14 to P16. Apocynin was dissolved in hot balanced salt solution (BSS) (Alcon) to prepare a stock solution (4 mg/mL).
Cell culture.
The human Müller cell line MIO-M1 was maintained in DMEM with 10% (v/v) FBS. For Nrf2 activation experiments, cells were pretreated with CDDO-Im for 18 hours, followed by stimulation of inflammation using 1 ng/μL of Lipopolysaccharide (LPS) (Sigma-Aldrich). After 24 hours, cell lysates were prepared using RLT buffer provided in RNeasy mini kit (Qiagen).
Statistical Analysis.
Results were expressed as mean ± SEM. Data were analyzed by Student’s t-test (two groups’ comparison) and one-way ANOVA (three or more groups’ comparison). P values less than 0.05 were considered statistically significant.
Supplementary Material
Highlights.
Nrf2-deficient mice exhibit delayed revascularization temporally associated with an increase in both oxidative stress and inflammatory mediators
Nrf2-deficiency leads to increased VEGF and induction of anti-angiogenic Dll4-Notch signaling in ischemic retina
Nrf2-deficient mice exhibit increase in NADPH oxidase 2 (NOX2) which is correlated with GSH depletion during retinal ischemia
Pharmacologic inhibition of NADPH oxidase in retinal ischemia improves reparative angiogenesis and suppresses pathologic neovascularization in Nrf2-deficient mice
ACKNOWLEDGMENTS.
We thank Shyam Biswal and Rajesh Thimmulappa for their helpful discussions, and Ruth Caldwell and Mohamed Al-Shabrawey for assistance regarding NOX2 immunostaining of retinas. This work was supported by research grants from the National Institutes of Health (EY022383 and EY022683; to E.J.D.) and Core Grant P30EY001765, Imaging and Microscopy Core Module.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Osborne NN; Casson RJ; Wood JP; Chidlow G; Graham M; Melena J Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Prog Retin Eye Res 23:91–147; 2004. [DOI] [PubMed] [Google Scholar]
- [2].Sapieha P Eyeing central neurons in vascular growth and reparative angiogenesis. Blood 120:2182–2194; 2012. [DOI] [PubMed] [Google Scholar]
- [3].Moskowitz MA; Lo EH; Iadecola C The science of stroke: mechanisms in search of treatments. Neuron 67:181–198; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hartnett ME The effects of oxygen stresses on the development of features of severe retinopathy of prematurity: knowledge from the 50/10 OIR model. Doc Ophthalmol 120:25–39; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Smith LE Through the eyes of a child: understanding retinopathy through ROP the Friedenwald lecture. Investigative ophthalmology & visual science 49:5177–5182; 2008. [DOI] [PubMed] [Google Scholar]
- [6].Kim YW; Byzova TV Oxidative stress in angiogenesis and vascular disease. Blood 123:625–631; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wilkinson-Berka JL; Rana I; Armani R; Agrotis A Reactive oxygen species, Nox and angiotensin II in angiogenesis: implications for retinopathy. Clinical science 124:597–615; 2013. [DOI] [PubMed] [Google Scholar]
- [8].Sapieha P; Joyal JS; Rivera JC; Kermorvant-Duchemin E; Sennlaub F; Hardy P; Lachapelle P; Chemtob S Retinopathy of prematurity: understanding ischemic retinal vasculopathies at an extreme of life. The Journal of clinical investigation 120:3022–3032; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Niesman MR; Johnson KA; Penn JS Therapeutic effect of liposomal superoxide dismutase in an animal model of retinopathy of prematurity. Neurochemical research 22:597–605; 1997. [DOI] [PubMed] [Google Scholar]
- [10].Penn JS; Tolman BL; Bullard LE Effect of a water-soluble vitamin E analog, trolox C, on retinal vascular development in an animal model of retinopathy of prematurity. Free radical biology & medicine 22:977–984; 1997. [DOI] [PubMed] [Google Scholar]
- [11].Gardiner TA; Gibson DS; de Gooyer TE; de la Cruz VF; McDonald DM; Stitt AW Inhibition of tumor necrosis factor-alpha improves physiological angiogenesis and reduces pathological neovascularization in ischemic retinopathy. Am J Pathol 166:637–644; 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Connor KM; SanGiovanni JP; Lofqvist C; Aderman CM; Chen J; Higuchi A; Hong S; Pravda EA; Majchrzak S; Carper D; Hellstrom A; Kang JX; Chew EY; Salem N Jr.; Serhan CN; Smith LE Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nature medicine 13:868–873; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rivera JC; Sitaras N; Noueihed B; Hamel D; Madaan A; Zhou T; Honore JC; Quiniou C; Joyal JS; Hardy P; Sennlaub F; Lubell W; Chemtob S Microglia and interleukin-1beta in ischemic retinopathy elicit microvascular degeneration through neuronal semaphorin-3A. Arteriosclerosis, thrombosis, and vascular biology 33:1881–1891; 2013. [DOI] [PubMed] [Google Scholar]
- [14].Manea SA; Constantin A; Manda G; Sasson S; Manea A Regulation of Nox enzymes expression in vascular pathophysiology: Focusing on transcription factors and epigenetic mechanisms. Redox biology 5:358–366; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Saito Y; Geisen P; Uppal A; Hartnett ME Inhibition of NAD(P)H oxidase reduces apoptosis and avascular retina in an animal model of retinopathy of prematurity. Molecular vision 13:840–853; 2007. [PMC free article] [PubMed] [Google Scholar]
- [16].Saito Y; Uppal A; Byfield G; Budd S; Hartnett ME Activated NAD(P)H oxidase from supplemental oxygen induces neovascularization independent of VEGF in retinopathy of prematurity model. Investigative ophthalmology & visual science 49:1591–1598; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Al-Shabrawey M; Bartoli M; El-Remessy AB; Ma G; Matragoon S; Lemtalsi T; Caldwell RW; Caldwell RB Role of NADPH oxidase and Stat3 in statin-mediated protection against diabetic retinopathy. Investigative ophthalmology & visual science 49:3231–3238; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Al-Shabrawey M; Bartoli M; El-Remessy AB; Platt DH; Matragoon S; Behzadian MA; Caldwell RW; Caldwell RB Inhibition of NAD(P)H oxidase activity blocks vascular endothelial growth factor overexpression and neovascularization during ischemic retinopathy. Am J Pathol 167:599–607; 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Al-Shabrawey M; Rojas M; Sanders T; Behzadian A; El-Remessy A; Bartoli M; Parpia AK; Liou G; Caldwell RB Role of NADPH oxidase in retinal vascular inflammation. Investigative ophthalmology & visual science 49:3239–3244; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yokota H; Narayanan SP; Zhang W; Liu H; Rojas M; Xu Z; Lemtalsi T; Nagaoka T; Yoshida A; Brooks SE; Caldwell RW; Caldwell RB Neuroprotection from retinal ischemia/reperfusion injury by NOX2 NADPH oxidase deletion. Investigative ophthalmology & visual science 52:8123–8131; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rojas M; Zhang W; Xu Z; Lemtalsi T; Chandler P; Toque HA; Caldwell RW; Caldwell RB Requirement of NOX2 expression in both retina and bone marrow for diabetes-induced retinal vascular injury. PloS one 8:e84357; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chan EC; van Wijngaarden P; Liu GS; Jiang F; Peshavariya H; Dusting GJ Involvement of Nox2 NADPH oxidase in retinal neovascularization. Investigative ophthalmology & visual science 54:7061–7067; 2013. [DOI] [PubMed] [Google Scholar]
- [23].Kensler TW; Wakabayashi N; Biswal S Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol 47:89–116; 2007. [DOI] [PubMed] [Google Scholar]
- [24].Cho H; Hartsock MJ; Xu Z; He M; Duh EJ Monomethyl fumarate promotes Nrf2-dependent neuroprotection in retinal ischemia-reperfusion. Journal of neuroinflammation 12:239; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wei Y; Gong J; Yoshida T; Eberhart CG; Xu Z; Kombairaju P; Sporn MB; Handa JT; Duh EJ Nrf2 has a protective role against neuronal and capillary degeneration in retinal ischemia-reperfusion injury. Free radical biology & medicine 51:216–224; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Xu Z; Cho H; Hartsock M; Mitchell KL; Gong J; Wu L; Wei Y; Wang S; Thimmulappa RK; Sporn MB; Biswal S; Welsbie DS; Duh EJ Neuroprotective role of Nrf2 for retinal ganglion cells in ischemia-reperfusion. Journal of neurochemistry; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Xu Z; Wei Y; Gong J; Cho H; Park JK; Sung ER; Huang H; Wu L; Eberhart C; Handa JT; Du Y; Kern TS; Thimmulappa R; Barber AJ; Biswal S; Duh EJ NRF2 plays a protective role in diabetic retinopathy in mice. Diabetologia 57:204–213; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wei Y; Gong J; Xu Z; Thimmulappa RK; Mitchell KL; Welsbie DS; Biswal S; Duh EJ Nrf2 in ischemic neurons promotes retinal vascular regeneration through regulation of semaphorin 6A. Proceedings of the National Academy of Sciences of the United States of America 112:E6927–6936; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wei Y; Gong J; Thimmulappa RK; Kosmider B; Biswal S; Duh EJ Nrf2 acts cell-autonomously in endothelium to regulate tip cell formation and vascular branching. Proceedings of the National Academy of Sciences of the United States of America 110:E3910–3918; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Smith LE; Wesolowski E; McLellan A; Kostyk SK; D'Amato R; Sullivan R; D'Amore PA Oxygen-induced retinopathy in the mouse. Investigative ophthalmology & visual science 35:101–111.; 1994. [PubMed] [Google Scholar]
- [31].Stahl A; Connor KM; Sapieha P; Chen J; Dennison RJ; Krah NM; Seaward MR; Willett KL; Aderman CM; Guerin KI; Hua J; Lofqvist C; Hellstrom A; Smith LE The mouse retina as an angiogenesis model. Investigative ophthalmology & visual science 51:2813–2826; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Itoh K; Chiba T; Takahashi S; Ishii T; Igarashi K; Katoh Y; Oyake T; Hayashi N; Satoh K; Hatayama I; Yamamoto M; Nabeshima Y An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochemical and biophysical research communications 236:313–322; 1997. [DOI] [PubMed] [Google Scholar]
- [33].Bai Y; Ma JX; Guo J; Wang J; Zhu M; Chen Y; Le YZ Muller cell-derived VEGF is a significant contributor to retinal neovascularization. J Pathol 219:446–454; 2009. [DOI] [PubMed] [Google Scholar]
- [34].Lin M; Chen Y; Jin J; Hu Y; Zhou KK; Zhu M; Le YZ; Ge J; Johnson RS; Ma JX Ischaemia-induced retinal neovascularisation and diabetic retinopathy in mice with conditional knockout of hypoxia-inducible factor-1 in retinal Muller cells. Diabetologia 54:1554–1566; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Zhou KK; Benyajati S; Le Y; Cheng R; Zhang W; Ma JX Interruption of Wnt signaling in Muller cells ameliorates ischemia-induced retinal neovascularization. PloS one 9:e108454; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ji X; Wang H; Zhu J; Zhu L; Pan H; Li W; Zhou Y; Cong Z; Yan F; Chen S Knockdown of Nrf2 suppresses glioblastoma angiogenesis by inhibiting hypoxia-induced activation of HIF-1alpha. International journal of cancer. Journal international du cancer 135:574–584; 2014. [DOI] [PubMed] [Google Scholar]
- [37].Kim TH; Hur EG; Kang SJ; Kim JA; Thapa D; Lee YM; Ku SK; Jung Y; Kwak MK NRF2 blockade suppresses colon tumor angiogenesis by inhibiting hypoxia-induced activation of HIF-1alpha. Cancer research 71:2260–2275; 2011. [DOI] [PubMed] [Google Scholar]
- [38].Lobov IB; Renard RA; Papadopoulos N; Gale NW; Thurston G; Yancopoulos GD; Wiegand SJ Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proceedings of the National Academy of Sciences of the United States of America 104:3219–3224; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kong X; Thimmulappa R; Kombairaju P; Biswal S NADPH oxidase-dependent reactive oxygen species mediate amplified TLR4 signaling and sepsis-induced mortality in Nrf2-deficient mice. J Immunol 185:569–577; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kovac S; Angelova PR; Holmstrom KM; Zhang Y; Dinkova-Kostova AT; Abramov AY Nrf2 regulates ROS production by mitochondria and NADPH oxidase. Biochimica et biophysica acta 1850:794–801; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lu XY; Wang HD; Xu JG; Ding K; Li T Deletion of Nrf2 Exacerbates Oxidative Stress After Traumatic Brain Injury in Mice. Cellular and molecular neurobiology 35:713–721; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rangasamy T; Guo J; Mitzner WA; Roman J; Singh A; Fryer AD; Yamamoto M; Kensler TW; Tuder RM; Georas SN; Biswal S Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J Exp Med 202:47–59; 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Binet F; Mawambo G; Sitaras N; Tetreault N; Lapalme E; Favret S; Cerani A; Leboeuf D; Tremblay S; Rezende F; Juan AM; Stahl A; Joyal JS; Milot E; Kaufman RJ; Guimond M; Kennedy TE; Sapieha P Neuronal ER stress impedes myeloid-cell-induced vascular regeneration through IRE1alpha degradation of netrin-1. Cell metabolism 17:353–371; 2013. [DOI] [PubMed] [Google Scholar]
- [44].Dejda A; Mawambo G; Cerani A; Miloudi K; Shao Z; Daudelin JF; Boulet S; Oubaha M; Beaudoin F; Akla N; Henriques S; Menard C; Stahl A; Delisle JS; Rezende FA; Labrecque N; Sapieha P Neuropilin-1 mediates myeloid cell chemoattraction and influences retinal neuroimmune crosstalk. The Journal of clinical investigation 124:4807–4822; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Connor KM; Krah NM; Dennison RJ; Aderman CM; Chen J; Guerin KI; Sapieha P; Stahl A; Willett KL; Smith LE Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis. Nat Protoc 4:1565–1573; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Xu Z; Gong J; Maiti D; Vong L; Wu L; Schwarz JJ; Duh EJ MEF2C ablation in endothelial cells reduces retinal vessel loss and suppresses pathologic retinal neovascularization in oxygen-induced retinopathy. Am J Pathol 180:2548–2560; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Murdoch CE; Alom-Ruiz SP; Wang M; Zhang M; Walker S; Yu B; Brewer A; Shah AM Role of endothelial Nox2 NADPH oxidase in angiotensin II-induced hypertension and vasomotor dysfunction. Basic research in cardiology 106:527–538; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Huang H; Gandhi JK; Zhong X; Wei Y; Gong J; Duh EJ; Vinores SA TNFalpha is required for late BRB breakdown in diabetic retinopathy, and its inhibition prevents leukostasis and protects vessels and neurons from apoptosis. Investigative ophthalmology & visual science 52:1336–1344; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.