Abstract
Porocarcinoma is a rare tumor of the eccrine sweat glands that usually disseminates to the regional lymph nodes, but it can also develop distant metastasis. Case presentation: We report the case of a 67 year-old female patient who underwent wide surgical resection of a left cervical cutaneous tumor in a primary care center, for which the histology exam of the specimen was mixed basal cell and squamous cell carcinoma. She was referred to our hospital’s oncology clinic and histologic re-evaluation changed the diagnosis to eccrine porocarcinoma (EPC). Computer-tomography (CT) revealed cervical lymphadenopathies for which the patient underwent 4 cycles of chemotherapy, without regression. She subsequently underwent a left upper anterior jugular lymphadenectomy (group IIa) with all nodes being negative and, three months later, she developed a unique adenopathy under the parotid gland that was excised and confirmed to be metastatic. Postoperative external radiotherapy was administered with a good outcome on CT scan. Nine months after her last surgery, the patient did not show any sign of recurrence or distant metastasis. Conclusion: EPC is a challenge, both diagnostically and therapeutically. In the absence of consensus regarding the indications and extent of lymphadenectomy and adjuvant therapy, patients with EPC should be referred to an experienced multidisciplinary team in a tertiary center.
Keywords: eccrine porocarcinoma, skin cancer, sweat glands
Introduction
Tumors of the skin adnexa are rare, predominantly benign. Almost all of these benign tumor types have a malignant counterpart [1]. Malignant skin adnexal tumors are aggressive, metastasizing in regional lymph nodes or distant organs, or may have recurrence risk [2, 3], thus a clear histological diagnosis is extremely important.
Eccrine porocarcinoma (EPC) is a malignant tumor of the eccrine sweat glands, amounting for only 0.005% of skin cancers [4].
Clinical and histological diagnosis of this type is difficult, as is its yet unstandardized management.
Case report
A 67 year-old woman was referred to our hospital’s oncology clinic for the management of a skin-tumor in the left cervical area who underwent surgical resection twelve months before, at a primary care facility. The histology exam she provided described a mixed basal cell and squamous cell carcinoma.
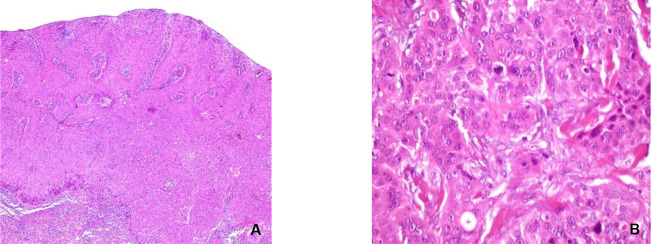
Histological re-evaluation of the paraffin bloc performed in our department of pathology revealed a tumor growth with focal ulcers on its surface, with a solid architecture, cell nests or thick trabecula, some emerging from superjacent epidermis and infiltrating the profound reticular dermis. The tumor was constituted of pleomorphic cells, with round-oval nuclei, some with prominent nucleolus, dispersed chromatin, amphophilic or eosinophilic cytoplasm, with evident cell limits. Small cystic structures were observed focally. Mitotic activity was high – 4-5 mitoses/high power field – and the tumor stroma was fibrous and in low quantity. A moderate lympho-plasmocytic inflammatory reaction was observed around the tumor. The profound dermal and hypodermal layer contained satellite tumor nodules with focal necrosis, away from the tumor invasion front (Figure 1). Immunohistochemistry staining of the tumor cells revealed diffuse positivity for p63, zonal positivity for epithelial membrane antigen (EMA) and the low molecular-weight cytokeratin CK7. Carcinoembryonic antigen (CEA) was present in rare tumor cells and the B-cell lymphoma-2 marker (Bcl-2) was positive in some of the tumor cells. Estrogen-receptor was weakly positive in rare neoplastic cells, while the tumor was negative for Gross cystic disease fluid protein 15 (GCDFP15). Immune-histo-chemical analysis is summarized in Table 1. The histology diagnostic was an eccrine porocarcinoma.
Fig. 1.
Eccrine porocarcinoma, histopathology. A: the tumor displays solid and trabecular architecture, with a large growth front (HE, X5); B: major cellular pleomorphism, high mytotic activity, microcysts (HE, X40)
Table 1.
Summary of immunohistochemical tests:
| Marker | Description |
|---|---|
| p63 | Diffusely positive in tumor cells |
| EMA | Zonally positive in tumor |
| CK7 | Zonally positive in tumor |
| CEA | Positive in rare tumor cells |
| Bcl-2 | Positive in some tumor cells |
| ER | Weakly positive in rare tumor cells |
| GCDFP15 | Negative |
EMA=epithelial membrane antigen, CK7=cytokeratin 7, CEA=Carcinoembryonic antigen, Bcl-2= B-cell lymphoma-2 marker, ER=estrogen receptor; GCDFP15= Gross cystic disease fluid protein 15
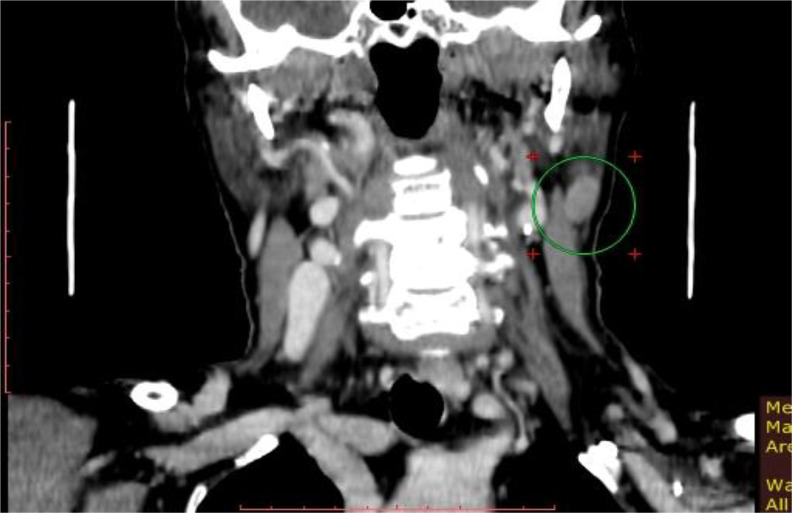
The patient was re-evaluated with thoraco-abdomino-pelvic CT-scan, which excluded secondary lesions. On the other hand, the cervical CT-scan revealed multiple left lateral cervical lymphadenopathies moderately enhancing after contrast and two other left jugular-carotid lymphadenopathies. After multidisciplinary discussion, 4 cycles of chemotherapy with platinum derivatives and taxanes were administered, after reviewing the literature [3, 5, 6]. However, cervical CT-scan performed two months later showed an important increase in contrast enhancement of one of the left lateral cervical lymphadenopathies, with stationary imaging aspects of the other previously described lymphadenopathies (Figure 2). Given this increased size of cervical lymph nodes, the patient was considered a non-responder to chemotherapy, and surgery was indicated. The patient underwent a wide resection of the previous operative-scar with left latero-cervical lymphadenectomy of the superior compartment (IIa). The cutaneous surgical specimen was tumor free and all of the eleven harvested lymph nodes were negative for metastasis on the histology exam.
Fig. 2.
Cervical CT-scan (coronal view). Left cervical adenopathy (in green circle)
Three months later, as part of the patient’s follow-up, a cervical soft-tissue echography revealed a left supero-lateral cervical hypoechogenic lesion, 9/12/9 mm in size, with Doppler signal present inside, well-defined in contact with the inferior pole of the parotid gland. Two other satellite nodules with similar characteristics were observed, one at the superior pole of the lesion and another at the inferior pole of the lesion, measuring 4 mm and 3.8 mm respectively. No lateral cervical lymphadenopathies were observed on either side. The right parotid gland was normal as well.
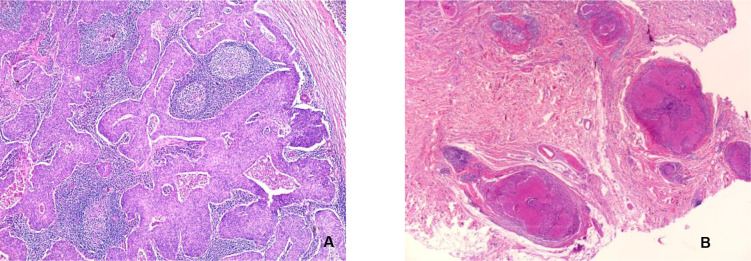
A positron emission tomography (PET) scan with fluoro-deoxy-glucose (FDG) performed 6 months after surgery described an intense metabolic activity of a left lateral cervical nodular lesion, 14/15/27 mm in size, in contact with the inferior pole of the left parotid gland and the anterior aspect of the left sternocleido-mastoid muscle. No pathological metabolic activity was observed on the lateral cervical nodes bilaterally and the rest of the body. The patient underwent surgical excision of the adenopathy under the parotid gland. Histological examination confirmed the malignant nature of the adenopathy, describing a nodule with extensive metastasis from a carcinoma with solid-type insular architecture, with necrosis in certain comedo-type tumor islets. Mitotic activity was evaluated at 7-8 mitosis / high-power field (Figure 3).
Fig. 3.
Metastatic lymphadenopathy. A: lymph node metastasis (HE, x5). B: satellite tumor lymph node (HE, x 2,5)
The patient underwent postoperative external radiotherapy 50 Gy/25 fractions for CTV-N-LR (low-risk nodal clinical target volume) - ipsilateral neck levels Ib, II, III, IV, V, VIII, 60 Gy/30 fractions for CTV-N-HR (high-risk nodal clinical target volume) - ipsilateral neck levels II, VIII. Cervical CT-scan after radiotherapy revealed no signs of tumor lymphadenopathies or distant metastasis which, according to the data from literature [7, 8], this would indicate a good long-term prognosis.
Discussions
Accurate histologic diagnosis of malignant cutaneous adnexal tumors is crucial. However, the rarity of these tumors makes the diagnosis a considerable challenge. The rate of misdiagnosis is around 37-50% cases analyzed by pathologists in primary care clinics [9, 10]. Tumor margins can be infiltrative or pushing, the former type being more prone to metastasis and local recurrence. In our case, the tumor margins were infiltrative after first operation that is why the patient underwent a larger resection of the scar.
Robson et al. [11] identify the following features of poor prognosis: more than 14 mitoses/high power field, lympho-vascular invasion and tumor depth > 7mm. In this case, the mitotic activity was 4-5 mitoses / high power field, without lympho-vascular invasion, this means a good prognosis. However, the profound dermal and hypodermal layer presented satellite tumoral nodules away from the tumor invasion front, which may increase the risk of developing distant metastasis.
Wide-surgical excision is curative in 70-80% of patients, while the mortality rate in case of metastatic lymphadenopathy is 67% [12].
Regional lymph node metastases are predominant among secondary lesions (57%) [13]. Porocarcinoma shows rates of lymph-node metastasis of around 20 percent [14]. However, whether loco-regional lymphadenectomy should always be performed at the time of the primary tumor excision is a matter of debate, some claiming it does not improve the outcome [6, 13].
When the diagnosis is certain, the sentinel lymph node biopsy may be performed, but there is not sufficient data to recommend its routine use [5, 14-16]. Regardless of location, the tendency is to perform lymphadenectomy only if there are clinical signs of regional lymph node involvement [17-20].
Barnes et al. [21] found in 69-case series that no malignant adnexal tumor metastasized to the regional lymph nodes before first developing local recurrence.
In our case, the decision for lymphadenectomy was taken given the high probability of metastasis in regional lymph nodes on the CT-scan performed 12 months after resection. Our patient developed a metastasis three months later, in an adjacent lymph node area but still has no cutaneous recurrence by the time of publishing this article.
Stam et al. [14] further recommend, according to melanoma guidelines, adjuvant radiotherapy if two or more lymph nodes are metastatic or in case of extracapsular rupture.
Cervical localization of EPC is rare, around 5% of all EPCs [22], those with lymph node involvement and lymph node dissection even less so. Lymph node cervical groups and subgroups are numerous, thus the extent of cervical lymph node dissection for EPC is prone to considerable variation. Also, EPCs could have a hazardous lymph node metastasis behavior, for example Gutermuth et al. [5] reported only left axillary node involvement for a similar left cervical EPC. Further studies should investigate upon the extent of lymphadenectomy that is required.
The response of skin adnexal tumors to chemotherapy and/or radiotherapy may vary considerably from patient to patient. Patients can survive disease-free for many years after recurrence [23, 24] or may conversely have a fulminant oncologic evolution from an apparently early-stage tumor, despite aggressive adjuvant treatment [25]. Our patient had a disease-free interval of 15 months after primary surgery of the skin lesion. By the time of publishing this article, the patient is disease-free 9 months after surgical excision of the lymph node metastasis.
Radiation and chemotherapy (e.g. cyclophosphamide, bleomycin, cisplatin, 5-FU) are not particularly effective and are generally employed for metastatic disease which has an overall survival of 5-24 months [26]. Barzi et al. [27] obtained good results with isotretinoin and interpheron-alpha.
Because of the low mitotic activity and negative thoraco-abdomino-pelvic CT-scan for distant metastasis after 9 months from the surgical excision of the nodule situated in the inferior pole of the parotid gland, we may say that the patient has a good prognosis.
Conclusion
Eccrine porocarcinoma is a rare and aggressive malignant skin adnexal tumor with particular diagnostic challenges. There is no consensus regarding the need for systematic regional lymphadenectomy and its extent. Further evidence is needed also to address the issue of adjuvant therapy strategy in case of metastatic and/or recurrent eccrine porocarcinoma. The authors’ opinion is that EPC would be best treated in a tertiary center by an experienced multidisciplinary team.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Alsaad KO, Obaidat NA, Ghazarian D. Skin adnexal neoplasms-part 1: an approach to tumours of the pilosebaceous unit. J Clin Pathol. 2007;60(2):129–144. doi: 10.1136/jcp.2006.040337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Obaidat NA, Alsaad KO, Ghazarian D. Skin adnexal neoplasms-part 2: an approach to tumours of cutaneous sweat glands. J Clin Pathol. 2007;60(2):145–159. doi: 10.1136/jcp.2006.041608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oyasiji T, Tan W, Kane J, et al. Malignant adnexal tumors of the skin: a single institution experience. World J Surg Oncol. 2018;16(1):99. doi: 10.1186/s12957-018-1401-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riera-Leal L, Guevara-Gutiérrez E, Barrientos-García JG, Madrigal-Kasem R, Briseño-Rodríguez G, Tlacuilo-Parra A. Eccrine porocarcinoma: epidemiologic and histopathologic characteristics. Int J Dermatol. 2015;54(5):580–586. doi: 10.1111/ijd.12714. [DOI] [PubMed] [Google Scholar]
- 5.Gutermuth J, Audring H, Voit C, Trefzer U, Haas N. Antitumour activity of paclitaxel and interferon-alpha in a case of metastatic eccrine porocarcinoma. J Eur Acad Dermatology Venereol. 2004;18(4):477–479. doi: 10.1111/j.1468-3083.2004.00949.x. [DOI] [PubMed] [Google Scholar]
- 6.De Iuliis F, Amoroso L, Taglieri L, et al. Chemotherapy of rare skin adnexal tumors: a review of literature. Anticancer Res. 2014;34(10):5263–5268. [PubMed] [Google Scholar]
- 7.Kurashige Y, Minemura T, Nagatani T. Eccrine porocarcinoma: clinical and pathological report of eight cases. Case Rep Dermatol. 2013;5:259–266. doi: 10.1159/000355606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prieto-Granada C, Castner N, Chen A, et al. Behavior of cutaneous adnexal malignancies: a single institution experience. Pathol Oncol Res. 2018 doi: 10.1007/s12253-018-0427-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luz M de A, Ogata DC, Montenegro MFG, Biasi LJ, Ribeiro LC. Eccrine porocarcinoma (malignant eccrine poroma): a series of eight challenging cases. Clinics (Sao Paulo) 2010;65(7):739–742. doi: 10.1590/S1807-59322010000700014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belin E, Ezzedine K, Stanislas S, et al. Factors in the surgical management of primary eccrine porocarcinoma: prognostic histological factors can guide the surgical procedure. Br J Dermatol. 2011;165(5):985–989. doi: 10.1111/j.1365-2133.2011.10486.x. [DOI] [PubMed] [Google Scholar]
- 11.Robson A, Greene J, Ansari N, et al. Eccrine porocarcinoma (malignant eccrine poroma): a clinicopathologic study of 69 cases. Am J Surg Pathol. 2001;25(6):710–720. doi: 10.1097/00000478-200106000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Marone U, Caracò C, Anniciello AM, et al. Metastatic eccrine porocarcinoma: report of a case and review of the literature. World J Surg Oncol. 2011;9:32. doi: 10.1186/1477-7819-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salih AM, Kakamad FH, Baba HO, et al. Porocarcinoma; presentation and management, a meta-analysis of 453 cases. Ann Med Surg. 2017;20:74–79. doi: 10.1016/j.amsu.2017.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stam H, van de Wiel BA, Klop WMC, et al. Skin adnexal carcinoma of the head and neck: a retrospective study in a tertiary referral center. Eur Arch Oto-Rhino-Laryngology. 2015;272(4):1001–1010. doi: 10.1007/s00405-014-3324-8. [DOI] [PubMed] [Google Scholar]
- 15.Shiohara J, Koga H, Uhara H, Takata M, Saida T. Eccrine porocarcinoma: Clinical and pathological studies of 12 cases. J Dermatol. 2007;34(8):516–522. doi: 10.1111/j.1346-8138.2007.00324.x. [DOI] [PubMed] [Google Scholar]
- 16.Sahn RE, Lang PG. Sentinel lymph node biopsy for high-risk nonmelanoma skin cancers. Dermatologic Surg. 2007;33(7):786–793. doi: 10.1111/j.1524-4725.2007.33171.x. [DOI] [PubMed] [Google Scholar]
- 17.Chang O, Elnawawi A, Rimpel B, Asarian A, Chaudhry N. Eccrine porocarcinoma of the lower extremity: A case report and review of literature. World J Surg Oncol. 2011;9:94. doi: 10.1186/1477-7819-9-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramasenderan N, Shahir H, Omar SZ. A synchronous incidence of eccrine porocarcinoma of the forearm and facial squamous cell carcinoma: A case report. Int J Surg Case Rep. 2018;42:116–120. doi: 10.1016/j.ijscr.2017.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walsh MS. A case of eccrine porocarcinoma. J R Soc Med. 1990;83(8):529–530. doi: 10.1177/014107689008300819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orella Jal, Penalba Av, Juan Ccs, Nadal Rv, Morrondo Jc, Alvarez Tt. Eccrine porocarcinoma. Dermatologic Surg. 1997;23(10):925–928. [PubMed] [Google Scholar]
- 21.Barnes M, Hestley A, Murray DR, Carlson GW, Parker D, Delman KA. The risk of lymph node involvement in malignant cutaneous adnexal tumors. Am Surg. 2014;80(3):270–274. [PubMed] [Google Scholar]
- 22.Urso C, Bondi R, Paglierani M, Salvadori A, Anichini C, Giannini A. Carcinomas of sweat glands: report of 60 cases. Arch Pathol Lab Med. 2001;125(4):498–505. doi: 10.5858/2001-125-0498-COSG. [DOI] [PubMed] [Google Scholar]
- 23.González-López MA, Vázquez-López F, Soler T, et al. Metastatic eccrine porocarcinoma: a 5.6-year follow-up study of a patient treated with a combined therapeutic protocol. Dermatol Surg. 2003;29(12):1227–1232. doi: 10.1111/j.1524-4725.2003.29393.x. [DOI] [PubMed] [Google Scholar]
- 24.Ponzetti A, Ribero S, Caliendo V, Spadi R, Macripò G, Lista P. Long-term survival after multidisciplinary management of a metastatic sarcomatoid porocarcinoma with repeated exeresis, radiotherapy, chemotherapy and cetuximab: case report and review of literature. G Ital Dermatol Venereol. 2017;152(1):66–70. doi: 10.23736/S0392-0488.16.04778-7. [DOI] [PubMed] [Google Scholar]
- 25.Fujimine-Sato A, Toyoshima M, Shigeta S, et al. Eccrine porocarcinoma of the vulva: a case report and review of the literature. J Med Case Rep. 2016;10(1):319. doi: 10.1186/s13256-016-1106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sawaya JL, Khachemoune A. Poroma: a review of eccrine, apocrine, and malignant forms. Int J Dermatol. 2014;53(9):1053–1061. doi: 10.1111/ijd.12448. [DOI] [PubMed] [Google Scholar]
- 27.Barzi AS, Ruggeri S, Recchia F, Bertoldi I. Malignant metastatic eccrine poroma. Proposal for a new therapeutic protocol. Dermatol Surg. 1997;23(4):267–272. [PubMed] [Google Scholar]