Anthracycline-based chemotherapy (Anth-bC) is used to treat adjuvant breast cancer and is a component of the curative treatment for lymphoma, leukemia, and sarcoma. Although anthracyclines are associated with decreased cancer recurrence and higher effective cure rates, administration of anthracyclines is associated with the future occurrence of cardiac events, including left ventricular (LV) dysfunction and heart failure. Mechanistically, anthracyclines promote myocellular injury and endothelial dysfunction through increasing oxidative and nitrosative stress as well as through combining with and inactivating topoisomerase II-beta.
Cardiac magnetic resonance (CMR) has been recently used to identify individuals who may experience LV dysfunction as a result of receiving Anth-bC therapy. In both children and adults undergoing CMR examinations, decrements in LV ejection fraction and myocardial mass, as well as increases in myocardial T1 measurements and assessments of extracellular volume fraction (ECVF) have been identified (1–5). These latter increases in LV ECVF are somewhat interesting because they may point to a potentially new mechanism of LV dysfunction after receipt of anthracyclines, mainly promotion of myocardial interstitial fibrosis, which has been identified in other forms of chronic LV dysfunction.
However, it is important to recognize that the calculation of ECVF actually represents a ratio of the volume of tissue within the myocardial interstitial space relative to the total volume of myocardium (myocytes, interstitial space, vascular tissue, and so forth). As such, in patients receiving Anth-bC therapy, which promotes myocellular injury and death, an increase in CMR-derived ECVF measurements may result from an increase in the interstitial space (thereby elevating the numerator of the ECVF fraction) or a decrease in size or number of myocytes (resulting in a reduction in myocardial volume that would decrease the denominator of the ECVF) (Figure 1). Identifying which process is operative after receipt of Anth-bC therapy or other potentially cardiotoxic chemotherapeutic regimens is very important because it may lead to differences in therapeutic interventions designed to preserve LV function.
FIGURE 1. Factors Influencing Myocardial ECV Fraction Calculation.
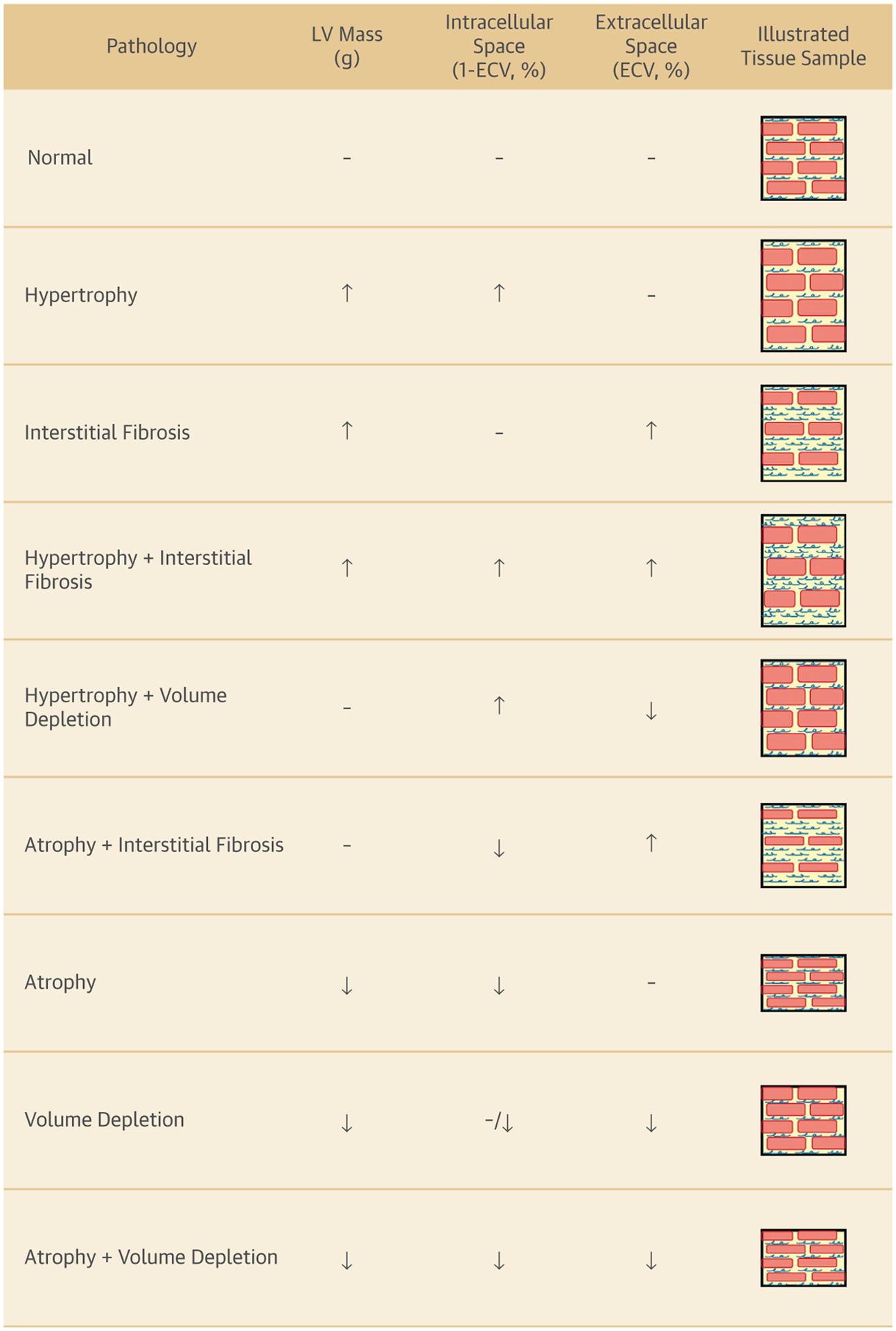
Potential underlying causes of changes in LV mass and remodeling of the intracellular and extracellular volume fractions due to changes in cardiomyocyte sizes, volume status, and collagen deposition resulting in myocardial fibrosis. ↑ = increased; ↓ = decreased; – = no change; red bars = cardiomyocytes; blue squiggles = interstitial fibrosis; ECV = extracellular volume; LV = left ventricular.
To this end, in this issue of iJACC, de Souza et al. (6) performed a unique series of experiments in patients receiving Anth-bC and used properties of the clearance of gadolinium to try and understand whether Anth-bC-associated ECVF increases were related to a decrease in myocellular size, an increase in the interstitial space, or a combination. To accomplish this, the study adapted a technique previously used to identify myocellular hypertrophy in the setting of hypertension. The study followed 27 women with breast cancer who were scheduled to receive Anth-bC and obtained CMR measurements of LV ejection fraction, ECVF, LV cardiomyocyte mass, and intracellular water lifetime measurements (τic), a marker of cardiomyocyte size.
T1 values represented whole-tissue longitudinal magnetization recovery, whereas τic assumed a 2-compartment model of transcytolemmal water exchange where gadolinium was restricted to 1 compartment (the interstitial space), while water could exchange freely across the cell membrane (7). Administration of gadolinium contrast constrains the rate of magnetization relaxation in the intracellular space due to water exchange, and thus, τic may be modeled. Prior work has demonstrated that τic is proportional to the volume-to-surface ratio of the intracellular compartment, which reduces to the diameter of the cardiomyocytes. To measure τic, serial T1 mapping was performed pre-contrast infusion and again 5 to 7 times after administration of gadolinium contrast. The relaxivity of the myocardium (R1 = 1/T1) is then fitted as a function of blood relaxivity with the 2-compartment model developed by Landis et al. (7) to determine τic.
This approach allowed interrogation of both the numerator and the denominator to understand whether remodeling was occurring due to interstitial changes or to changes in cardiomyocyte size and was not limited by compartment shape assumptions (whether ellipsoidal or cylindrical). Although seminal work has been performed that demonstrated τic increases with cardiomyocyte hypertrophy, no animal correlate has been studied for cardiac atrophy, and future work may need to be performed to refine this model. Independent of cardiac atrophy, cancer patients undergoing chemotherapy treatment may suffer from volume depletion (nausea, emesis) or weight loss that may confound these findings. End-diastolic diameter and volume did not change during the study, suggesting that considerations of volume depletion (8) did not occur; however, LV mass was indexed to both body surface area and end-diastolic volume without reports of changes in body weight. Although effects are likely minor, the potential for loss of body weight may be a lingering confounder in the cardiomyocytes atrophy result. Additional limitations of this study surround the use of gadolinium contrast to measure τic in patients who may be monitored serially. Also, it is unknown how impaired renal clearance would affect the sensitivity of τic to measure cardiomyocytes size given the fact that the model is predicated on differences in relaxivity due to gadolinium clearance.
As with other studies, the authors found that LV ejection fraction declined and that myocardial ECVF increased over the course of the study. In addition, cardiac troponin subtype T concentrations increased after the administration of anthracyclines. The investigators concluded that the decreases in LV mass may result from cardiomyocyte atrophy as opposed to an increase in interstitial fibrosis. This raises the possibility that decrement in myocyte mass may be as important or equally important to increases in the interstitial space related to accelerated interstitial fibrosis. Of note, we found it particularly interesting that τic values greater than the median were associated with LV dysfunction, following treatment. We wonder whether this may be an effect of the systemic inflammatory state with cancer that we have observed at baseline in other T1 mapping studies of cancer patients (4).
In summary, the administration of treatments for cancer may be associated with development of LV dysfunction, loss of myocardial volume, and increments in myocardial ECVF. Results of this study indicate that myocardial ECVF increases in association with a decrement in myocardial volume, as well as a decrement in LV ejection fraction after receipt of anthracycline-based chemotherapy for breast cancer. This increase in ECVF is not necessarily due to an expansion of the interstitial space. In part, expansion may also be related to a decrement in myocellular volume and mass. This raises important issues for patients being examined for identification of cardiac toxicity upon receipt of anthracycline-based chemotherapy and for other medical conditions that may require them to undergo CMR and experience an increase in ECVF. One needs to consider whether this expansion is related to a change in the numerator, the denominator, or both.
Acknowledgments
Supported by U.S. National Institutes of Health grants R01CA167821, R01CA199167, and R01HL118740 and a Thrive initiative research grant from the International Life Sciences Institute Health and Environmental Sciences Institute. Both authors have reported that they have no relationships with industry relevant to the contents of this paper to disclose.
Footnotes
Editorials published in JACC: Cardiovascular Imaging reflect the views of the authors and do not necessarily represent the views of iJACC or the American College of Cardiology.
REFERENCES
- 1.Tham EB, Haykowsky MJ, Chow K, et al. Diffuse myocardial fibrosis by T1-mapping in children with subclinical anthracycline cardiotoxicity: relationship to exercise capacity, cumulative dose and remodeling. J Cardiovasc Magn Reson 2013;15:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neilan TG, Coelho OR, Shah RV, et al. Myocardial extracellular volume by cardiac magnetic resonance imaging in patients treated with anthracycline-based chemotherapy. Am J Cardiol 2013;111:717–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jordan JH, Castellino SM, Meléndez GC, et al. Left ventricular mass change after anthracycline chemotherapy. Circ Heart Fail 2018;11:e004560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jordan JH, Vasu S, Morgan TM, et al. Anthracycline-associated T1 mapping characteristics are elevated independent of the presence of cardiovascular comorbidities in cancer survivors. Circ Cardiovasc Imaging 2016;9:e004325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toro-Salazar OH, Gillan E, O’Loughlin MT, et al. Occult cardiotoxicity in childhood cancer survivors exposed to anthracycline therapy. Circ Cardiovasc Imaging 2013;6:873–80. [DOI] [PubMed] [Google Scholar]
- 6.de Souza TF, Silva TQAC, Osorio Costa F, et al. Anthracycline therapy is associated with cardiomyocyte atrophy and preclinical manifestations of heart disease. J Am Coll Cardiol Img 2018;11:1045–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landis CS, Li X, Telang FW, et al. Equilibrium transcytolemmal water-exchange kinetics in skeletal muscle in vivo. Magn Reson Med 1999;42:467–78. [DOI] [PubMed] [Google Scholar]
- 8.Jordan JH, Melendez G, Suerken C, D’Agostino R, Hundley W. Decreases in left ventricular mass and not left ventricular ejection fraction are associated with heart failure symptoms in cancer patients six months after potentially cardiotoxic chemotherapy. J Am Coll Cardiol 2017;69:1410. [Google Scholar]


