Abstract
Two related kinases, IκB kinase α (IKKα) and IKKβ, phosphorylate the IκB proteins, leading to their degradation and the subsequent activation of gene expression by NF-κB. IKKβ has a much higher level of kinase activity for the IκB proteins than does IKKα and is more critical than IKKα in modulating tumor necrosis factor alpha activation of the NF-κB pathway. These results indicate an important role for IKKβ in activating the NF-κB pathway but leave open the question of the role of IKKα in regulating this pathway. In the current study, we demonstrate that IKKα directly phosphorylates IKKβ. Moreover, IKKα either directly or indirectly enhances IKKβ kinase activity for IκBα. Finally, transfection studies to analyze NF-κB-directed gene expression suggest that IKKα is upstream of IKKβ in activating the NF-κB pathway. These results indicate that IKKα, in addition to its previously described ability to phosphorylate IκBα, can increase the ability of IKKβ to phosphorylate IκBα.
The NF-κB proteins are a family of transcription factors that activate a variety of cellular genes involved in control of the inflammatory response and in regulating cellular growth (2, 3). NF-κB is sequestered in the cytoplasm of most cells, where it is bound to a family of inhibitory proteins known as IκB (2–4). A variety of extracellular stimuli, including tumor necrosis factor alpha (TNF-α), lipopolysaccharide, and interleukin-1 (IL-1), lead to the activation of signal transduction pathways that result in the phosphorylation of two amino-terminal serine residues in the IκB proteins (1, 5–7, 12, 32, 33). The IκB proteins are then ubiquitinated on amino-terminal lysine residues via interaction with β-TrCP (29, 31, 34, 37). After the formation of the ubiquitin-ligase complex, IκB is degraded by the 26S proteasome (8, 9).
Two related kinases that phosphorylate amino-terminal serine residues 32 and 36 in IκBα and 19 and 23 in IκBβ have been described (13, 22, 27, 35, 40). These IκB kinases are components of a 700-kDa kinase complex whose activity is markedly increased by treatment of cells with activators of the NF-κB pathway, such as TNF-α and IL-1 (8, 9, 15, 28). Other components of this complex include NEMO or IκB kinase γ (IKKγ), which is required for in vivo activation of IKK kinase activity (23, 28, 36), and IKAP, which may function as a scaffold protein (10). IKKα and IKKβ have a high degree of sequence homology and similar structural domains, including a conserved kinase domain in addition to leucine zipper and helix-loop-helix domains (13, 22, 27, 35, 40). The leucine zipper domain of these kinases facilitates their ability to homodimerize and heterodimerize (13, 22, 27, 35, 40). Although these kinases have a number of similarities, IKKβ has a 20- to 50-fold-higher level of kinase activity for IκB than does IKKα (16, 22, 24, 38, 39, 41).
TNF-α activation of the NF-κB pathway is mediated by multiple adapter proteins which lead to activation of NF-κB-inducing kinase (NIK) (21), which is capable of directly phosphorylating IKKα in its activation loop at serine residue 176 (20). However, other upstream kinases have also been demonstrated to activate the NF-κB pathway. For example, mitogen-activated protein/extracellular signal-regulated kinase 1 (MEKK1) can activate both IKKα and IKKβ kinase activity (15, 16, 24, 38, 39). Other upstream kinases, such as TAK1, MEKK2, and MEKK3, can also directly or indirectly lead to activation of the IκB kinases (26, 42). These results suggest that multiple signal transduction pathways can likely modulate IKK function.
Recent data suggest that TNF-α- and IL-1-mediated increases in the phosphorylation of IKKβ and potentially IKKα may be important in the regulation of their kinase activity (11). Both IKKα and IKKβ contain a canonical MAP kinase kinase activation loop motif with the sequence Ser-X-X-X-Ser that has similarities to domains found in other MAP kinases (13, 22, 27, 35, 40). Phosphorylation of two closely spaced serine residues in this domain, at positions 176 and 180 in IKKα and positions 177 and 181 in IKKβ, has been shown to be important for IKK kinase activity (22). For example, mutation of these serine residues to alanine in both IKKα and IKKβ can inactivate their ability to phosphorylate IκB. Moreover, replacement of these serine residues with glutamates results in the generation of proteins that have constitutively active IKK kinase activity (22). However, a recent study indicates that the serine residues in the activation loop of IKKβ but not IKKα are critical for modulating IKK kinase activity (11). The reason for the discrepancy between these studies remains unclear. NIK (20) and MEKK1 (16) can phosphorylate serine residues in the activation loop of the IKK proteins, although it is possible that autophosphorylation of these residues by the IKK proteins themselves may also provide a mechanism for activating IKK kinase activity.
Recent gene disruption studies of the murine IKK genes indicate their importance in mammalian development (14, 18, 19, 30). For example, disruption of the murine IKKα genes results in animals that die shortly after they are born (14, 18, 30). These mice have a number of developmental abnormalities, including those of the axial skeleton, limbs, and skin. In two studies, mice lacking IKKα are not impaired for activation of the NF-κB pathway or IκB degradation following treatment with inflammatory cytokines (14, 30). However, another study indicates that mice lacking IKKα are somewhat defective in activating the NF-κB pathway (18). In contrast, mice lacking IKKβ die as embryos due to extensive liver damage from uncontrolled apoptosis (19). Moreover, in these mice there are marked defects in activation of the NF-κB pathway by proinflammatory cytokines such as TNF-α (19). These results indicate that IKKβ appears to be more critical than IKKα in activating the NF-κB pathway.
In the current study, we address the role of IKKα in activating the NF-κB pathway. We demonstrate that IKKα directly phosphorylates IKKβ. Furthermore, we demonstrate that IKKα either directly or indirectly increases the ability of IKKβ to phosphorylate IκBα. Finally, transfection studies suggest that IKKα is upstream of IKKβ in mediating activation of NF-κB-directed gene expression. These results suggest that IKKα may modulate IKKβ kinase activity to regulate the NF-κB pathway.
MATERIALS AND METHODS
DNA constructs.
The cDNAs for wild-type IKKα and the IKKα mutants K44M (K/M), S176A/S180A (SS/AA), and K44M ΔHLH, in which amino acids 560 to 744 in the carboxy terminus of IKKα have been deleted, contain amino-terminal influenza virus hemagglutinin sequences and were cloned downstream of the cytomegalovirus (CMV) promoter in pCMV5. The cDNAs for wild-type IKKβ and the IKKβ mutants K44M (K/M) and S177A/S181A (SS/AA) contain amino-terminal Flag sequences and were cloned downstream of the CMV promoter in pCMV5 (22). The cDNAs for wild-type NIK and the dominant negative NIK mutant K429A/K430A (KK/AA) were cloned downstream of the CMV promoter in pCMV5 and contained an amino-terminal Myc tag (38). The cDNAs for wild-type MEKK1 and dominant negative MEKK1 mutant D1369A (D/A) contain a carboxy-terminal influenza virus hemagglutinin epitope (38) and were also cloned downstream of the CMV promoter in pCMV5.
Wild-type and mutant IKKα and IKKβ cDNAs tagged with six histidines or with influenza virus hemagglutinin were each cloned into the baculovirus expression vector pAcHLT, and recombinant baculoviruses were generated by cotransfection with the Baculo Gold DNA and transfer vectors (PharMingen). The recombinant baculoviruses were used to infect Sf9 cells at a multiplicity of infection of 5 to express the different IKK proteins. The baculovirus-produced IKK proteins were purified by nickel-agarose chromatography and then immunoprecipitated with the 12CA5 monoclonal antibody. These recombinant IKK proteins were assayed in in vitro kinase assays as described below.
Transfections.
COS cells were maintained in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum, and transfections were performed with Fugene 6 (Boehringer). COS cells were transfected with DNA concentrations ranging from 0.10 to 1.0 μg of either wild-type or kinase-defective Flag epitope-tagged IKKβ constructs or wild-type or kinase-defective influenza virus hemagglutinin-tagged IKKα cDNAs (38). The wild-type or dominant negative NIK and MEKK1 mutant constructs have been described previously (38). For assays of NF-κB-directed gene expression, a human immunodeficiency virus type 1 (HIV-1) long terminal repeat (LTR)-luciferase construct was transfected into COS cells in the presence of the indicated wild-type or mutant IKKα and IKKβ constructs, and luciferase activity was assayed 30 h posttransfection (38). A CMV–β-galactosidase plasmid was also incorporated into each transfection.
[32P]orthophosphate labeling of IKK proteins.
COS cells were maintained in DMEM with 10% fetal bovine serum and transfected with either the indicated IKKα or IKKβ cDNAs or either wild-type or mutant NIK or MEKK1 constructs. Before labeling the cells, the culture medium was changed to serum-free and either phosphate-free or methionine-free DMEM. Either [32P]orthophosphate (50 μCi/ml) or [35S]methionine (50 μCi/ml) was then added to the cells and incubated for 3 h. TNF-α (20 ng/μl) was added for 5 to 7 min before harvesting the cells. The cells were washed three times with cold phosphate-buffered saline, and the cell pellets were lysed on ice for 15 min in PD buffer (500 mM NaCl, 50 mM Tris-HCl [pH 8.0], 0.5% NP-40, 1 mM sodium orthovanadate, 1 mM NaF, 0.5 mM β-glycerophosphate, and protease inhibitors).
Immunoprecipitation and kinase assays.
Cell lysates from either [32P]orthophosphate- and [35S]methionine-labeled cells or nonlabeled cells were incubated with 50 μl of 12CA5 supernatant or 500 ng of anti-Flag M2 antibody for 2 h on ice. To assay endogenous IKK labeling with either [32P]orthophosphate or [35S]methionine, 50 μg of the cellular lysate was immunoprecipitated with rabbit polyclonal antibody directed against IKKα (Santa Cruz). Then 20 μl of protein A-agarose was added to each of the immunoprecipitates and incubated for 1 h at 4°C. The immunoprecipitates were washed twice with 10 volumes of 50 mM Tris-HCl (pH 8.0)–100 mM NaCl–protease inhibitor, and protein loading buffer was added prior to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and autoradiography.
For kinase assays, immunoprecipitates from cellular lysates (50 μg) were incubated in kinase reaction buffer containing 10 μCi of [γ-32P]ATP, 1 mM ATP, 5 mM MgCl2, 1 mM dithiothreitol, 100 mM NaCl, and 50 mM Tris-HCl (pH 8.0) at 30°C for 15 min (38). The substrates in these kinase reactions were either glutathione-S-transferase (GST)-IκBα (2 μg), wild type (amino acids 1 to 54) or mutant (S32/S36→A32/A36), or baculovirus-produced polyhistidine- and Flag-tagged IKKα or IKKβ proteins (500 ng). These proteins were produced by baculovirus expression, purified by nickel-agarose chromatography, and then subjected to chromatography on a Q-Sepharose column. Proteins were quantitated and analyzed following SDS-PAGE, silver staining, and Western blot analysis with anti-Flag M2 monoclonal antibody (38).
Chromatography of IKK proteins.
COS cells (108) were cotransfected with epitope-tagged expression vectors containing either IKKα K/M and IKKβ or IKKα and IKKβ. Cells were harvested by centrifugation for 10 min at 2,000 rpm (Beckman bench-top centrifuge, CH3.7 rotor). Pelleted cells were washed twice in cold phosphate-buffered saline and resuspended in 5 volumes of buffer A (10 mM HEPES [pH 7.9], 1.5 mm MgCl2, 10 mm KCl, 0.5 mm dithiothreitol) supplemented with phosphatase inhibitors (50 mm NaF, 50 mm glycerophosphate, 0.125 μM okadaic acid, and 1 mM sodium orthovanadate) and proteinase inhibitors (Roche Molecular Biochemicals). After incubation for 15 min on ice, the cells were lysed with 40 strokes of a Kontes all-glass Dounce homogenizer (B-type pestle). The nuclei were pelleted by centrifugation at 2,000 rpm. The supernatant was mixed with 0.11 volume of buffer B (0.3 M HEPES [pH 7.9], 0.03 M MgCl2) and then centrifuged for 60 min at 100,000 × g. The supernatant was dialyzed for 5 to 8 h against 20 volumes of buffer D (20 mM HEPES [pH 7.9], 0.1 M KCl, 0.5 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, 20% glycerol, 0.2 mM EDTA).
Equal amounts of proteins (2.5 mg) were fractionated on a Superdex 200 column (Amersham Pharmacia Biotech). Protein markers (Sigma) used for the column include bovine thyroglobulin (669 kDa), horse spleen apoferritin (443 kDa), β-amylase (200 kDA), bovine serum albumin (66 kDa), carbonic anhydrase (29 kDa), and cytochrome c (12.5 kDa). The column fractions were immunoprecipitated with either the M2 or 12CA5 monoclonal antibody, and in vitro kinase assays were performed as indicated. Western blot analysis of the column fractions with these monoclonal antibodies was also performed.
RESULTS
TNF-α induces endogenous IKK phosphorylation.
Stimulation of IKKβ kinase activity correlates with increases in its phosphorylation (11). To further analyze the role of phosphorylation in IKK function, we first tested the ability of TNF-α treatment or transfection of MEKK1 and NIK constructs to induce phosphorylation of endogenous IKK. COS cells were labeled with either [32P]orthophosphate (Fig. 1A, top panel) or [35S]methionine (Fig. 1A, middle panel) for 3 h prior to harvest. The IKK proteins were then immunoprecipitated with a polyclonal antibody directed against IKKα that immunoprecipitates the IKKα-IKKβ heterodimer (38).
FIG. 1.
Activators of the NF-κB pathway increase IKK phosphorylation. (A) COS cells were either untreated (lane 1), treated with TNF-α (20 ng/ml) for 5 to 7 min prior to harvest (lane 2), or transfected with 2 μg of expression vectors containing wild-type NIK or MEKK1 (lanes 3 and 4) or dominant negative mutants of NIK and MEKK1 (lanes 5 and 6). Cells were labeled with either [32P]orthophosphate (top panel) or [35S]methionine (middle panel) for 3 h prior to harvesting the cells. Immunoprecipitation was performed with IKKα polyclonal antibody (Santa Cruz) followed by SDS-PAGE and autoradiography. Extracts were also analyzed for IKK expression in Western blot analysis with IKKα antibody (lower panel). (B) COS cells were not transfected (lane 1) or transfected with 1 μg of the influenza virus hemagglutinin-tagged IKKα (lanes 2 and 3) or 0.2 μg of the Flag-tagged IKKβ (lanes 4 and 5) cDNAs in either the absence (lanes 2 and 4) or presence (lanes 3 and 5) of TNF-α. Cells were labeled with [32P]orthophosphate for 3 h prior to harvest, and TNF-α treatment was performed for 7 min prior to harvest. Immunoprecipitation was performed with either 12CA5 (lanes 1, 2, and 3, top panel) or the M2 (Flag) (lanes 4 and 5, top panel) monoclonal antibody using 50 μg of the cell lysate, followed by SDS-PAGE and autoradiography. Western blot analysis of the transfected IKK cDNAs was performed with 12CA5 (lanes 2 and 3, lower panel) or the M2 (lanes 4 and 5, lower panel) monoclonal antibody.
TNF-α treatment of COS cells stimulated the phosphorylation of the IKK proteins (Fig. 1A, lanes 1 and 2, top panel). Transfection of either of two kinases, NIK and MEKK1, that have been demonstrated to increase the IKK kinase activity (15, 16, 20, 24, 25) also increased IKK phosphorylation (Fig. 1A, lanes 3 and 4, top panel). In contrast, transfection of dominant negative mutants of MEKK1 and NIK did not significantly alter endogenous IKK phosphorylation (Fig. 1A, lanes 5 and 6, top panel). Similar quantities of [35S]methionine-labeled extracts prepared from TNF-α-treated or wild-type or mutant NIK- or MEKK1-transfected COS cells did not demonstrate differences in the levels of the [35S]methionine-labeled IKK proteins (Fig. 1A, lanes 1 to 6, middle panel). Western blot analysis confirmed the presence of similar amounts of IKKα in each of these extracts (Fig. 1A, lanes 1 to 6, lower panel).
We also determined whether TNF-α increased the phosphorylation of influenza virus hemagglutinin-tagged IKKα or Flag-tagged IKKβ cDNAs following transfection of each of these constructs into COS cells. The 32P-labeled IKKα and IKKβ proteins were immunoprecipitated with the 12CA5 and Flag monoclonal antibodies, respectively. The phosphorylation of both IKKα and IKKβ was increased following TNF-α treatment (Fig. 1B, lanes 2 to 5, top panel). There were similar amounts of the IKKα proteins in both untreated and TNF-α-treated extracts (Fig. 1B, lanes 2 to 5, lower panel). These results indicate that activators of the NF-κB pathway such as TNF-α increase the phosphorylation of both the IKKα and IKKβ proteins.
TNF-α induces phosphorylation of IKKα and IKKβ.
To address the mechanism by which activators of the NF-κB pathway increase the phosphorylation of IKKα and IKKβ, epitope-tagged cDNAs encoding each of these proteins were transfected into COS cells (Fig. 2). [32P]orthophosphate labeling of the transfected COS cells was performed in either the presence or absence of TNF-α. Dominant negative mutants of either IKKβ, IKKα, MEKK1, or NIK were included in these transfection assays as indicated.
FIG. 2.
IKKβ phosphorylation is inhibited by dominant negative IKKα mutants. (A and C) COS cells were transfected with HA-tagged IKKα or Flag-tagged IKKβ cDNAs alone (lanes 1 and 2) or in the presence of similar amounts of IKKα, IKKβ, NIK, or MEKK1 dominant negative mutants as indicated (lanes 3 to 6). The transfected cells were labeled with [32P]orthophosphate (top panel) or [35S]methionine (lower panel) for 3 h and either untreated (lane 1) or treated with TNF-α (20 ng/ml) for 5 to 7 min (lanes 2 to 6). Cell lysates were prepared, and 50 μg of this lysate was incubated with the 12CA5 antibody to immunoprecipitate the IKKα protein or with the Flag antibody M2 to immunoprecipitate the IKKβ protein. The immunoprecipitates were subjected to SDS-PAGE, and autoradiography was performed. (B and D) COS cells were transfected with the kinase-defective IKKα or IKKβ mutant SS/AA (lanes 1 and 2) or K/M (lanes 3 and 4). The cells were labeled with either [32P]orthophosphate (top panel) or [35S]methionine (lower panel) for 3 h prior to harvest in the absence (lanes 1 and 3) or presence of TNF-α for 5 to 7 min (lanes 2 and 4). The cell lysates were immunoprecipitated and subjected to SDS-PAGE.
TNF-α treatment of COS cells strongly induced the phosphorylation of IKKα (Fig. 2A, lanes 1 and 2). TNF-α induction of IKKα phosphorylation was not inhibited by cotransfection of either of two IKKβ dominant negative mutants (Fig. 2A, lanes 3 and 4) or a dominant negative MEKK1 mutant (Fig. 2A, lane 5). In contrast, TNF-α-induced IKKα phosphorylation was blocked by a dominant negative NIK mutant (Fig. 2B, lane 6). TNF-α treatment did not increase the phosphorylation of an IKKα mutant in the activation loop motif (Fig. 2B, lanes 1 and 2) or a catalytically inactive IKKα mutant (Fig. 2B, lanes 3 and 4).
COS cells were next transfected with an epitope-tagged IKKβ cDNA in either the presence or absence of TNF-α and labeled with either [32P]orthophosphate or [35S]methionine. TNF-α induced the phosphorylation of IKKβ (Fig. 2C, lanes 1 and 2). Cotransfection of dominant negative IKKα mutants decreased TNF-α-induced phosphorylation of IKKβ (Fig. 2C, lanes 3 and 4). A dominant negative NIK mutant also decreased IKKβ phosphorylation, while a dominant negative MEKK1 mutant did not decrease and in fact slightly increased IKKβ phosphorylation (Fig. 2C, lanes 5 and 6). There was no significant change in the level of [35S]methionine-labeled IKKβ proteins (Fig. 2C, lower panel). TNF-α treatment did not induce phosphorylation of an IKKβ mutant in the two serine residues in its activation loop (Fig. 2D, lanes 1 and 2) or of a catalytically inactive IKKβ mutant (Fig. 2D, lanes 3 and 4). These results are consistent with a role for IKKα in potentially modulating the phosphorylation state of IKKβ.
IKKα induces phosphorylation of IKKβ.
To determine whether IKKα may potentially be involved in either directly or indirectly stimulating the phosphorylation of IKKβ, we assayed the ability of IKKα to modulate the phosphorylation of IKKβ. COS cells were transfected with an epitope-tagged IKKβ cDNA either alone or in the presence of wild-type IKKα, a constitutively active IKKα construct, or two dominant negative IKKα mutants. The COS cells were labeled with either [32P]orthophosphate or [35S]methionine, and the Flag epitope-tagged IKKβ protein was immunoprecipitated with the M2 monoclonal antibody.
Both wild-type and constitutively active IKKα constructs increased the phosphorylation of IKKβ (Fig. 3A, lanes 1 to 3). In contrast, there was little or no increase in IKKβ phosphorylation with either of two IKKα mutants, IKKα SS/AA or IKKα K/M (Fig. 3A, lanes 4 and 5). In vivo labeling of the IKKβ proteins with [35S]methionine demonstrated that IKKα expression did not alter the level of the [35S]methionine-labeled IKKβ proteins (Fig. 3A, lower panel). Similar results from three independent experiments indicate that IKKα can either directly or indirectly modulate the level of IKKβ phosphorylation.
FIG. 3.
IKKα increases IKKβ phosphorylation. (A) COS cells were transfected with a Flag-tagged wild-type (WT) IKKβ cDNA construct (0.5 μg) alone (lane 1) or in the presence of 0.5 μg of influenza virus hemagglutinin-tagged wild-type IKKα (lane 2), a constitutively active IKKα construct (lane 3), or the mutant IKKα construct SS/AA or K/M (lanes 4 and 5), as indicated above the top panel. (B) COS cells were transfected with an influenza virus hemagglutinin-tagged wild-type (WT) IKKα cDNA construct (0.5 μg) alone (lane 1) or in the presence of 0.5 μg of Flag-tagged wild-type IKKβ (lane 2), a constitutively active IKKα construct (lane 3), or the mutant IKKα construct SS/AA or K/M (lanes 4 and 5), as indicated above the top panel. In both panels A and B, the cells were labeled with either [32P]orthophosphate (top panel) or [35S]methionine (lower panel) for 3 h prior to cell harvest. The cell lysates (50 μg) were immunoprecipitated with the (A) anti-Flag M2 monoclonal antibody to immunoprecipitate the epitope-tagged IKKβ proteins or (B) the 12CA5 monoclonal antibody to immunoprecipitate the epitope-tagged IKKα proteins. The immunoprecipitates were then subjected to SDS-PAGE, and autoradiography was performed.
To address whether IKKβ could increase IKKα phosphorylation, COS cells were transfected with an influenza virus hemagglutinin-tagged IKKα construct either alone or in the presence of different IKKβ constructs. COS cells were again labeled with either [32P]orthophosphate or [35S]methionine, and the influenza virus hemagglutinin-tagged IKKα protein was immunoprecipitated with the 12CA5 monoclonal antibody. Neither the wild-type nor the constitutively active IKKβ constructs altered the amount of IKKα phosphorylation (Fig. 3B, lanes 1 to 3). The dominant negative IKKβ mutants IKKβ SS/AA and IKKβ K/M also did not alter the phosphorylation of IKKα (Fig. 3B, lanes 4 and 5). In vivo labeling of the IKKα proteins with [35S]methionine demonstrated similar amounts of the IKKα proteins (Fig. 3B, lanes 1 to 5, lower panel). These results suggest that IKKβ does not markedly alter IKKα phosphorylation.
IKKα increases IKKβ phosphorylation in a high-molecular-weight IKK complex.
It was important to address whether IKKα could stimulate IKKβ kinase activity when these kinases were part of a high-molecular-weight IKK complex (8, 9, 15, 28). To address this point, COS cells were cotransfected with expression vectors containing wild-type IKKβ and either wild-type IKKα or a catalytically defective IKKα K/M mutant. The IKKα constructs were tagged with the influenza virus hemagglutinin epitope, while IKKβ was tagged with the Flag epitope. Cytoplasmic extracts were prepared at 30 h posttransfection and subjected to chromatography on a Superdex 200 column to isolate the high-molecular-weight IKK complex.
Fractions from the Superdex 200 column were immunoprecipitated with the anti-Flag M2 monoclonal antibody, and in vitro kinase assays were performed. IKKβ phosphorylation was present at low levels in column fractions migrating between 400 and 600 kDa in the presence of the IKKα K/M protein (Fig. 4A, top panel). There was no detectable IKKα K/M autophosphorylation in these column fractions.
FIG. 4.
IKKα phosphorylation of IKKβ in the IKK complex. COS cells were transfected with expression vectors encoding (A) hemagglutinin-tagged IKKα K/M and Flag-tagged IKKβ or (B) hemagglutinin-tagged wild-type IKKα and Flag-tagged IKKβ. Cytoplasmic extracts were prepared at 30 h posttransfection and fractionated on a Superdex 200 column. Column fractions 7 to 14 were immunoprecipitated with the M2 monoclonal antibody, and in vitro kinase assays of these fractions were performed and analyzed by SDS-PAGE and autoradiography (top panel). The positions of the epitope-tagged IKKα and IKKβ proteins transfected individually into COS cells are indicated in the last two lanes of panel B. Western blot analysis was performed with the 12CA5 monoclonal antibody to detect IKKα K/M and wild-type IKKα or with the M2 monoclonal antibody to detect IKKβ (lower two panels in A and B). The column fractions and the molecular mass markers, which indicate the positions of the fractions eluted from the Superdex 200 column, are indicated at the bottoms and tops of the figures, respectively. (C) Column fraction 9 from the Superdex 200 column analyzed in panels A and B was immunoprecipitated with the 12CA5 antibody to isolate either IKKα K/M or wild-type IKKα followed by in vitro kinase assays, SDS-PAGE, and autoradiography.
In contrast, the column fractions containing both wild-type IKKα and IKKβ showed markedly enhanced phosphorylation of both IKKβ and IKKα (Fig. 4B, top panel). The positions of the phosphorylated wild-type IKKα and IKKβ proteins which were transfected alone and immunoprecipitated followed by in vitro kinase assays are also shown (Fig. 4B, top panel). Western blot analysis indicated that there was similar expression of the IKKα and IKKβ proteins in these Superdex 200 fractions (Fig. 4A and B, lower panels). Finally, we determined whether immunoprecipitation of either IKKα K/M or IKKα present in column fraction 9 with the 12CA5 monoclonal antibody also demonstrated differences in IKKβ phosphorylation (Fig. 4C). This analysis demonstrated that the presence of wild-type IKKα but not IKKα K/M was associated with enhanced IKKβ phosphorylation. Immunoprecipitation of these column fractions followed by Western blot analysis indicated that the epitope-tagged IKKα and IKKα K/M proteins both strongly associated with the epitope-tagged IKKβ protein (data not shown). No IKKβ phosphorylation was noted when the catalytically defective IKK mutants IKKα K/M and IKKβ K/M were analyzed following cotransfection and Superdex 200 fractionation (data not shown). These results indicate that IKKα is associated with enhanced IKKβ phosphorylation when these kinases are present as heterodimers in a high-molecular-weight IKK complex.
IKKα stimulates IKKβ kinase activity.
Next we investigated whether IKKα-mediated increases in IKKβ phosphorylation correlate with its ability to stimulate IKKβ kinase activity. First, an epitope-tagged IKKβ cDNA was transfected into COS cells, the cells were either untreated or treated with TNF-α, and IKKβ kinase activity was assayed. Next, dominant negative mutants of either IKKα, NIK, or MEKK1 were cotransfected with IKKβ in the presence of TNF-α to determine their role in regulating IKKβ kinase activity. Finally, we assayed the ability of wild-type and constitutively active IKKα proteins to stimulate IKKβ kinase activity. The Flag-tagged IKKβ protein in each of these transfections was immunoprecipitated with the M2 monoclonal antibody and assayed for its ability to phosphorylate the amino terminus of IκBα (amino acids 1 to 54).
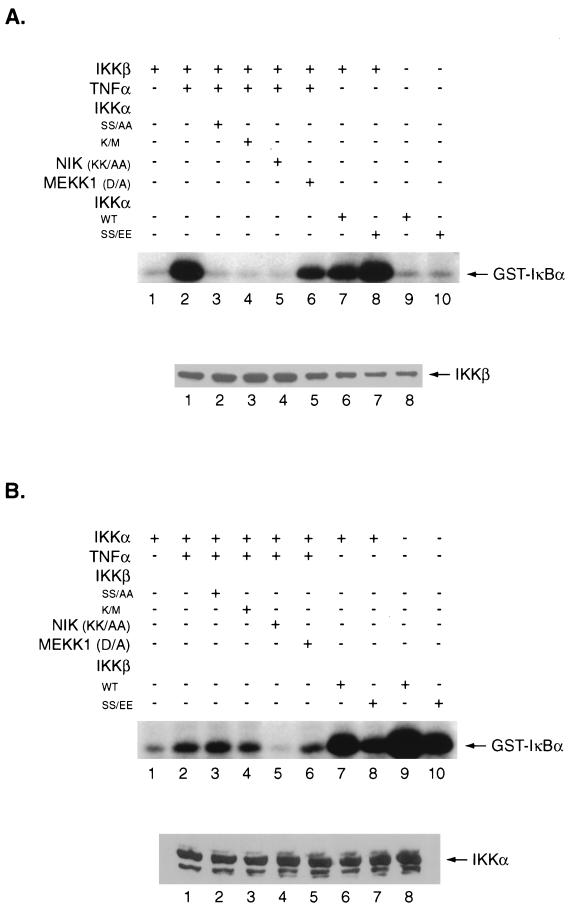
TNF-α treatment markedly increased IKKβ kinase activity for the GST-IκBα substrate (Fig. 5A, lanes 1 and 2). The TNF-α-mediated increase in IKKβ kinase activity was blocked by two dominant negative IKKα mutants (Fig. 5A, lanes 3 and 4) and a dominant negative NIK mutant (Fig. 5A, lane 5) but not a dominant negative MEKK1 mutant (Fig. 5A, lane 6). Next we assayed the ability of IKKα to stimulate IKKβ kinase activity. Transfection of wild-type IKKα markedly stimulated the ability of IKKβ to phosphorylate GST-IκBα (Fig. 5A, lanes 1 and 7). A constitutively active IKKα construct also markedly stimulated IKKβ kinase activity for the GST-IκBα substrate (Fig. 5A, lanes 1 and 8). IKKα mutants K/M and SS/AA did not stimulate IKKβ kinase activity, and the immunoprecipitated IKKβ did not phosphorylate a GST-IκB construct mutant at serine residues 32 and 36 (data not shown). When the wild-type and the constitutively active IKKα constructs were transfected alone and immunoprecipitated with the 12CA5 antibody, they had very low kinase activity with the GST-IκBα substrate (Fig. 5A, lanes 9 and 10). Western blot analysis demonstrated that there was little change in the level of the epitope-tagged IKKβ proteins in either the presence or absence of IKKα (Fig. 5A, lower panel). These results suggested that IKKα can either directly or indirectly modulate IKKβ kinase activity and that TNF-α induction of IKKβ kinase activity may be mediated in part through effects on IKKα.
FIG. 5.
IKKα stimulates IKKβ kinase activity. (A) COS cells were transfected with a Flag-tagged wild-type (WT) IKKβ cDNA construct (0.1 μg) (lanes 1 to 8) in the absence (lane 1) or presence of TNF-α (lanes 2 to 6). Either 0.3 μg of the dominant negative mutants IKKα SS/AA and IKKα K/M (lanes 3 and 4), NIK KK/AA (lane 5), or MEKK1 D/A (lane 6) or 0.3 μg of the wild-type or constitutively active IKKα constructs (lanes 7 and 8) was cotransfected with the wild-type IKKβ construct. Either wild-type IKKα or the constitutively active IKKα construct was also transfected alone (lanes 9 and 10). (B) COS cells were transfected with an influenza virus hemagglutinin-tagged wild-type (WT) IKKα construct (1 μg) (lanes 1 to 8) in the absence (lane 1) or presence of TNF-α (lanes 2 to 6). Dominant negative mutants (1 μg), including IKKβ SS/AA and K/M (lanes 3 and 4), NIK KK/AA (lane 5), and MEKK1 D/A (lane 6), or 1 μg of either wild-type IKKβ (lane 7) or a constitutively active IKKβ construct (lane 8) were cotransfected with the wild-type IKKα as indicated. Wild-type IKKβ (lane 9) and a constitutively active IKKβ construct (lane 10) were also transfected alone. Cell lysates (50 μg) were immunoprecipitated with (A) anti-Flag M2 antibody to immunoprecipitate IKKβ protein (lanes 1 to 8) or (B) 12CA5 antibody to immunoprecipitate the IKKα protein (lanes 1 to 8). In lanes 9 and 10, the 12CA5 antibody was used to immunoprecipitate IKKα and the M2 antibody was used to immunoprecipitate IKKβ. Kinase assays were performed with a GST-IκBα (amino acids 1 to 54) substrate, and the reaction mixtures were subjected to SDS-PAGE and autoradiography (top panel). Cell lysates from these immunoprecipitates were also analyzed by Western blot analysis with the M2 or 12CA5 antibody to quantitate the epitope-tagged IKKβ and IKKα proteins (lanes 1 to 8) (lower panel).
We next performed a similar analysis to address whether IKKβ could increase the ability of IKKα to phosphorylate the GST-IκBα substrate. First, we demonstrated that TNF-α treatment of COS cells transfected with IKKα resulted in increased IKKα kinase activity for the GST-IκBα substrate (Fig. 5B, lanes 1 and 2). TNF-α induction of IKKα kinase activity was not decreased by cotransfection of either of two dominant negative IKKβ mutants (Fig. 5B, lanes 3 and 4). However, a dominant negative NIK mutant, but not a dominant negative MEKK1 mutant, inhibited TNF-α stimulation of IKKα kinase activity (Fig. 5B, lanes 5 and 6). These results suggested that dominant negative IKKβ mutants did not block TNF-α-mediated increases in IKKα kinase activity.
To determine the role of IKKβ in modulating IKKα kinase activity, either wild-type IKKβ or the constitutively active IKKβ construct was cotransfected with IKKα. Immunoprecipitation of the epitope-tagged IKKα proteins resulted in increased IKK kinase activity for the GST-IκBα substrate (Fig. 5B, lanes 7 and 8). However, transfection of either the wild-type or the constitutively active IKKβ constructs alone, followed by immunoprecipitation with the M2 monoclonal antibody, demonstrated a level of kinase activity similar to that seen when both IKKα and IKKβ were cotransfected (Fig. 5B, lanes 9 and 10). The immunoprecipitated IKKα and IKKβ proteins did not phosphorylate a GST-IκBα protein mutant at serine residues 32 and 36 (data not shown). Immunoprecipitation followed by Western blot analysis indicated that IKKβ, which has a much higher level of kinase activity than does IKKα, coimmunoprecipitated with IKKα, resulting in enhanced phosphorylation of IκBα (data not shown). These results are consistent with the inability of IKKβ to directly stimulate IKKα kinase activity.
In vitro phosphorylation of IKKβ by IKKα.
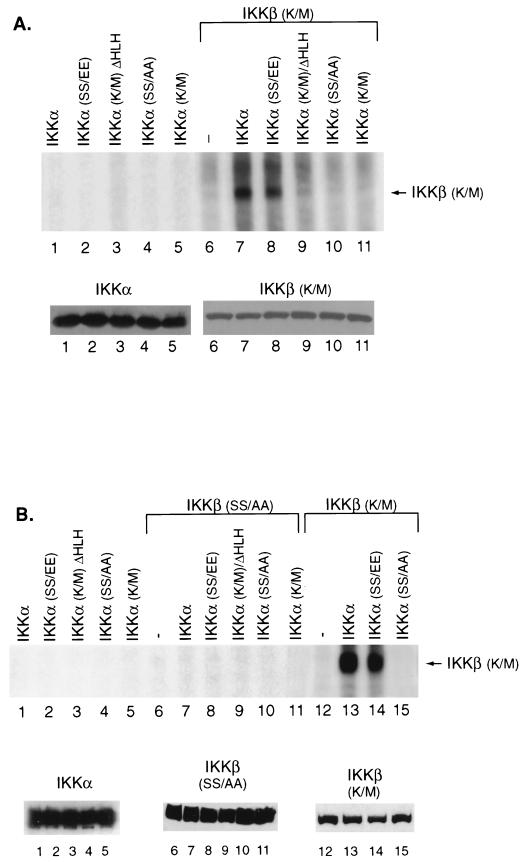
To address whether IKKα could directly phosphorylate IKKβ, we used an in vitro kinase assay in which the ability of wild-type or mutant IKKα proteins to phosphorylate IKKβ was analyzed. Epitope-tagged wild-type and mutant IKKα proteins, produced following transfection of COS cells, were immunoprecipitated. These epitope-tagged IKKα proteins were used because they exhibit little autophosphorylation in the in vitro kinase assays. In contrast, the baculovirus-produced IKKα proteins are autophosphorylated and thus make analysis of the effects of IKKα on IKKβ phosphorylation more difficult to interpret (data not shown). The immunoprecipitated IKKα proteins were assayed for their ability to phosphorylate a catalytically defective Flag-tagged IKKβ K/M protein which was purified following baculovirus expression. This substrate was used because baculovirus-produced wild-type IKKβ exhibited high levels of autophosphorylation which obscured IKKα-mediated effects on this substrate.
The immunoprecipitated IKKα proteins had little kinase activity when assayed in in vitro kinase assays without the addition of substrate (Fig. 6A, lanes 1 to 5, top panel). Western blot analysis demonstrated that equivalent amounts of these proteins were used in the kinase assay (Fig. 6A, lower panel). The baculovirus-produced IKKβ K/M substrate itself exhibited a low level of kinase activity (Fig. 6A, lane 6). Kinase assays were then performed with the IKKβ K/M substrate and each of the different immunoprecipitated IKKα proteins. The 32P-labeled IKKβ K/M substrate that was generated in the kinase assays was immunoprecipitated with the M2 monoclonal antibody and analyzed following SDS-PAGE and autoradiography. Both the wild-type and the constitutively active IKKα proteins phosphorylated the IKKβ K/M substrate (Fig. 6A, lanes 7 and 8). In contrast, there was no significant phosphorylation of IKKβ (K/M) by the kinase-deficient IKKα mutants, including IKKα K/M ΔHLH, IKKα K/M, and IKKα SS/AA (Fig. 6A, lanes 9 to 11). Equal amounts of the IKKβ K/M substrate were present in each of these kinase reactions as determined by Western blot analysis of portions of each kinase assay (Fig. 6A, lanes 6 to 11, lower panel).
FIG. 6.
In vitro phosphorylation of IKKβ by IKKα. (A) COS cells were transfected with the indicated influenza virus hemagglutinin-tagged IKKα constructs. Cellular extracts (50 μg) were immunoprecipitated with 12CA5 antibody for wild-type IKKα (lanes 1 and 7), a constitutively active IKKα construct (lanes 2 and 8), or the kinase-defective IKKα mutants K/M ΔHLH (lanes 3 and 9), SS/AA (lanes 4 and 10), and K/M (lanes 5 and 11). Kinase assays were performed in either the absence of substrate (lanes 1 to 5), with only the baculovirus-produced purified IKKβ K/M substrate (500 ng) (lane 6), or in the presence of the different IKKα proteins and the IKKβ K/M substrate (lanes 7 to 11) (top panel). Following kinase assays, the supernatant was isolated by centrifugation, and the 32P-labeled IKKβ K/M substrate was immunoprecipitated with the anti-Flag M2 monoclonal antibody and analyzed by SDS-PAGE and autoradiography. Western blot analysis of the influenza virus hemagglutinin-tagged IKKα immunoprecipitates (lanes 1 to 5) used in these assays or a portion of the immunoprecipitated Flag-tagged IKKβ K/M substrate from each of the kinase assays was analyzed (lanes 6 to 11) (lower panel). (B) The different IKKα proteins used in panel A were used in kinase assays in the absence of substrate (lanes 1 to 5) or in the presence of 500 ng of baculovirus-produced IKKβ SS/AA (lanes 7 to 11) or IKKβ K/M (lanes 13 to 15) (top panel). Western blot analysis of the different IKKα proteins from these assays was done with 12CA5 antibody (lanes 1 to 5) or the baculovirus-produced IKKβ SS/AA (lanes 6 to 11) or IKKβ K/M proteins was done with the M2 monoclonal antibody (lower panel).
We also determined whether the IKKα proteins (Fig. 6B, lanes 1 to 5) could phosphorylate a baculovirus-produced IKKβ SS/AA protein in which alanines were substituted for the serine residues at positions 177 and 181 in the IKKβ activation loop (Fig. 6B, lanes 6 to 10). There was no IKKα-mediated phosphorylation of this protein, although both the wild-type and constitutively active IKKα proteins used in this experiment could phosphorylate the baculovirus-produced IKKβ K/M protein (Fig. 6B, lanes 13 and 14). There were equal quantities of the different IKKα proteins used in these assays (Fig. 6B, lanes 1 to 5, lower panel) and equal quantities of the baculovirus-produced IKKβ SS/AA and IKKβ K/M substrates in these assays (Fig. 6B, lanes 6 to 15, lower panel). These results suggest that IKKα likely phosphorylates the activation loop of IKKβ.
IKKβ does not phosphorylate IKKα in vitro.
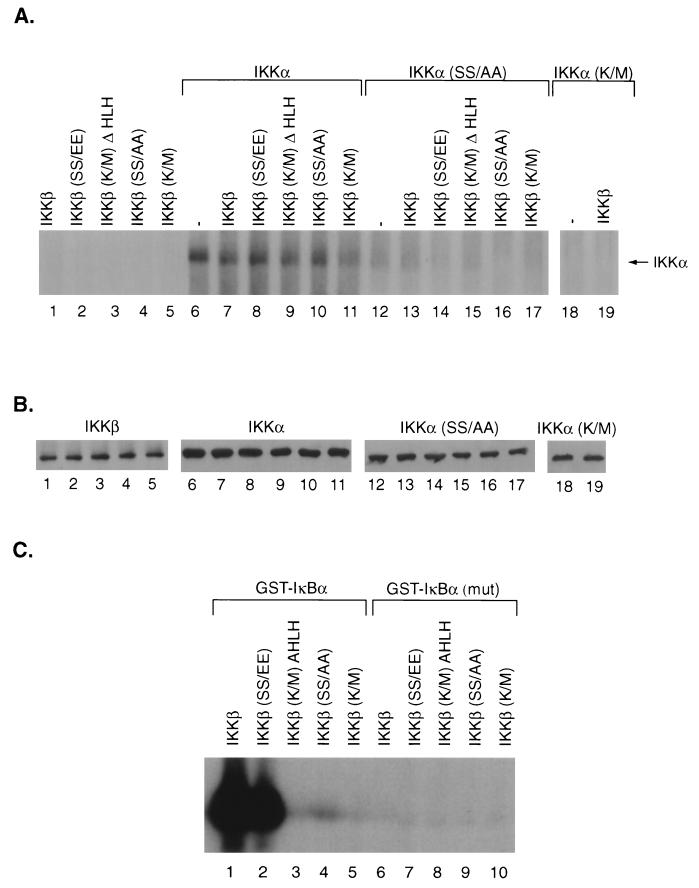
It was important to determine whether IKKβ could phosphorylate an IKKα substrate in in vitro kinase assays. Wild-type or mutant IKKβ proteins produced following transfection of COS cells were assayed for their ability to phosphorylate baculovirus-produced wild-type IKKα or the IKKα mutants SS/AA and K/M (Fig. 7A). The immunoprecipitated IKKβ proteins did not result in background phosphorylation (Fig. 7A, lanes 1 to 5), while the baculovirus-produced IKKα protein exhibited a low level of autophosphorylation (Fig. 7A, lane 6). IKKα phosphorylation was not stimulated by the addition of wild-type, constitutively active, or mutant IKKβ constructs (Fig. 7A, lanes 7 to 11). The IKKβ proteins also did not increase the phosphorylation of the baculovirus-produced IKKα SS/AA (Fig. 7A, lanes 12 to 17) or IKKα K/M (Fig. 7A, lanes 18 and 19) substrates. Western blot analysis indicated that there were equivalent amounts of IKKβ (Fig. 7B, lanes 1 to 5) and wild-type and mutant IKKα (Fig. 7B, lanes 6 to 19) substrates used in these kinase assays.
FIG. 7.
IKKβ does not phosphorylate IKKα in vitro. (A) COS cells were transfected with the indicated Flag-tagged IKKβ constructs. The extracts (50 μg) were immunoprecipitated with M2 monoclonal antibody for wild-type IKKβ (lanes 1 and 7), a constitutively active IKKβ construct (lanes 2 and 8), or the kinase-defective IKKβ mutants K/M ΔHLH (lanes 3 and 9), SS/AA (lanes 4 and 10), and K/M (lanes 5 and 11). Kinase assays were performed in either the absence of substrate (lanes 1 to 5), with 500 ng of the baculovirus-produced purified IKKα substrate alone (lane 6), the IKKα SS/AA substrate alone (lane 12), or the different IKKβ proteins and either the IKKα (lanes 7 to 11), the IKKα SS/AA (lanes 13 to 17), or the IKKα K/M (lanes 18 and 19) substrate. Following kinase assays, the supernatant was isolated by centrifugation, and the 32P-labeled IKKα and IKKα SS/AA substrates were immunoprecipitated with the 12CA5 monoclonal antibody and analyzed by SDS-PAGE and autoradiography. (B) Western blot analysis was performed on a portion of the Flag-tagged IKKβ immunoprecipitates (lanes 1 to 5) or a portion of the immunoprecipitated influenza virus hemagglutinin-tagged IKKα (lanes 6 to 11), IKKα SS/AA (lanes 12 to 17), or IKKα K/M (lanes 18 and 19) substrate from each of the kinase assays using the epitope-specific monoclonal antibodies. (C) The different immunoprecipitated IKKβ proteins used in panel A were used in kinase assays with GST-IκBα (amino acids 1 to 54) or mutant GST-IκBα, in which serine residues 32 and 36 were changed to alanine. Following SDS-PAGE, autoradiography was performed.
Since the IKKβ proteins did not enhance the in vitro phosphorylation of IKKα, it was important to address whether these IKKβ proteins exhibited kinase activity with an IκBα substrate. Each of the IKKβ proteins used in part A were tested for their ability to phosphorylate GST fusion proteins containing the amino-terminal 54 amino acids of IκBα or a mutant IκBα protein in which serine residues 32 and 36 were changed to alanine. Wild-type and constitutively active IKKβ proteins strongly phosphorylated wild-type GST-IκBα (Fig. 7C, lanes 1 and 2), while the mutant IKKβ proteins did not significantly phosphorylate this substrate (Fig. 7C, lanes 3 to 5). The IKKβ proteins did not phosphorylate the GST-IκBα protein mutant at serine residues 32 and 36 (Fig. 7C, lanes 6 to 10). These results indicate that although IKKβ did not phosphorylate IKKα, it strongly phosphorylated the IκBα substrate.
In vivo analysis of constitutively active IKK proteins.
Finally, we addressed whether our results suggesting a role for IKKα in modulating IKKβ phosphorylation and kinase activity could be correlated with in vivo studies regarding IKK activation of an NF-κB reporter construct. In these studies, TNF-α was not used to stimulate the activity of the transfected IKKα and IKKβ cDNAs because this cytokine itself strongly activates NF-κB reporter constructs (24, 38). Instead, we tested the ability of dominant negative IKKα and IKKβ mutants to alter the ability of constitutively active IKKα and IKKβ constructs to activate gene expression of an NF-κB reporter construct.
An HIV-1 LTR-luciferase reporter construct which contains two NF-κB binding sites was transfected into COS cells with either a constitutively active IKKα or IKKβ construct (Fig. 8). In addition, either of two dominant negative IKKα or IKKβ mutants was also cotransfected. Thus, the ability of the dominant negative IKKα and IKKβ mutants to prevent IKK activation of an NF-κB reporter construct could be assayed. Both of the constitutively active IKK constructs, IKKα SS/EE and IKKβ SS/EE, activated gene expression from the HIV-1 LTR-luciferase reporter (Fig. 8). Neither of these constitutively active IKK constructs stimulated gene expression from an HIV-1 LTR-luciferase reporter construct with mutated NF-κB binding sites (data not shown). Cotransfection of either of the two dominant negative IKKβ constructs prevented IKKα SS/EE activation of the NF-κB reporter construct (Fig. 8). This result may be explained by the fact that the IKKα SS/EE protein formed heterodimers with the IKKβ dominant negative mutants and thus was not able to phosphorylate endogenous IKKβ or endogenous IκBα.
FIG. 8.
IKKβ dominant negative mutants inhibit NF-κB activation by a constitutively active IKKα construct. An HIV-1 LTR-luciferase construct (10 ng) was transfected into COS cells either alone (—), with a constitutively active IKKα SS/EE construct (0.5 μg) (lane 2), or with 0.25 μg of either IKKβ SS/AA or IKKβ K/M. The HIV-1 LTR-luciferase construct was also transfected with IKKβ SS/EE alone (0.3 μg) or together with 0.5 μg of the IKKα K/M or SS/AA dominant negative mutant. Cells were harvested at 30 h posttransfection, and luciferase activity was quantitated and normalized by using a CMV–β-galactosidase control plasmid. The results are the means of three independent experiments.
In contrast, neither of the dominant negative IKKα mutants was able to significantly inhibit IKKβ SS/EE activation of the NF-κB reporter construct (Fig. 8). Since IKKβ SS/EE does not require phosphorylation by IKKα for stimulation of its kinase activation, the dominant negative IKKα constructs would not be expected to alter IKKβ SS/EE activation of the NF-κB reporter. These transfection studies provide indirect evidence that IKKα may modulate IKKβ activation of the NF-κB pathway.
DISCUSSION
In this study, we present several lines of evidence that IKKα can modulate IKKβ function. First, we demonstrate that dominant negative IKKα mutants prevent TNF-α-induced phosphorylation of IKKβ. Second, we show that wild-type and constitutively active IKKα proteins stimulate IKKβ phosphorylation both in transfection assays and following isolation of high-molecular-weight IKK complexes. Third, our data indicate that IKKα stimulates IKKβ kinase activity for the IκBα substrate. Finally, we demonstrate that IKKα can phosphorylate IKKβ in in vitro kinase assays. These results suggest that IKKα likely modulates IKKβ function.
Our studies utilized transient-expression assays to analyze IKKα function. Thus, we cannot rule out that these results might not entirely reflect those obtained with IKKα and IKKβ are present in the high-molecular-weight IKK complex. However, we did demonstrate that the presence of IKKα and IKKβ in a complex migrating between 400 and 700 kDa correlates with increases in IKKβ phosphorylation. Although the size of this IKK complex is less than the 700 to 900 kDa of an IKK complex that has been described before (8, 9, 15, 28), it is likely that the IKK complex generated from transfection of IKKα and IKKβ expression vectors lacks sufficient quantities of proteins like NEMO (23, 28, 36) or IKAP (10) that are components of the endogenous IKK complex. Overexpression of IKK proteins in transfection assays likely also accounts for the fact that wild-type IKKα and the constitutively active IKKα mutant have similar abilities to stimulate IKKβ phosphorylation and kinase activity. When low concentrations of these plasmids are transfected into COS cells, the constitutively active IKKα mutant has a greater ability to stimulate IKKβ phosphorylation and kinase activity for IκBα than does wild-type IKKα (unpublished observations). However, when larger quantities of IKKα and the constitutively active IKKα mutant are transfected, these constructs have a similar ability to stimulate IKKβ phosphorylation and kinase activity. Thus, it is important to note that several of the conclusions reached in this study are based on the results of transfection assays with IKKα and IKKβ.
A recent study examined the patterns of phosphorylation of the IKKα and IKKβ proteins in response to different activators of the NF-κB pathway, including TNF-α, IL-1, and NIK (11). In agreement with this study, we find that TNF-α treatment of cells markedly stimulates both IKKα and IKKβ phosphorylation. However, catalytically inactive and activation loop mutants of IKKα and IKKβ exhibit decreased in vivo phosphorylation in response to TNF-α. These data suggest that at least a portion of IKKα and IKKβ phosphorylation in response to TNF-α treatment likely results from autophosphorylation of these kinases. In contrast to the results of this latter study, which indicate that mutations in the IKKα activation loop do not alter IKKα phosphorylation of IκBα, our data and several previous studies indicate that such mutants exhibit defective kinase activity (20, 22). Thus, we suggest that phosphorylation of IKKα is critical for enhancing its ability to phosphorylate both IκBα and IKKβ.
IKKβ appears to be the dominant kinase required for activating NF-κB, based on its higher level of activity for IκBα compared with IKKα (17, 22, 24, 35, 38, 39) and the failure to activate the NF-κB pathway when this gene is disrupted in mice (18). IKKα, in addition to NIK (20) and MEKK1 (16), may also be involved in activating IKKβ kinase activity. The fact that multiple kinases can activate IKKβ kinase activity may explain the somewhat different results seen in IKKα knock-out mice. For example, two such studies found TNF-α induction of the NF-κB pathway to be intact (14, 19, 30), while another study found defects in activating the NF-κB pathway (18). Additional studies will be required to further define the specific roles of NIK, MEKK1, NEMO, and IKKα in regulating IKKβ kinase activity.
In summary, IKKα may potentially have multiple effects on regulating the NF-κB pathway. First, it can phosphorylate IκBα and IκBβ to result in their ubiquitination and subsequent degradation by the proteasome. In addition, our data suggest that IKKα can phosphorylate IKKβ. The physiologic relevance of IKKα in each of these processes will need to be better elucidated by both in vivo studies and reconstituted in vitro assay systems to more clearly determine the role of this kinase in regulating the NF-κB pathway.
ACKNOWLEDGMENTS
We thank Sharon Johnson and Stephanie Guyer for preparation of the manuscript and figures, respectively.
This work was supported by grants from the NIH and the Veterans Administration.
REFERENCES
- 1.Alkalay I, Yaron A, Hatzubai A, Orian A, Ciechanover A, Ben-Neriah Y. Stimulation-dependent I kappa B alpha phosphorylation marks the NF-kappa B inhibitor for degradation via the ubiquitin-proteasome pathway. Proc Natl Acad Sci USA. 1995;92:10599–10603. doi: 10.1073/pnas.92.23.10599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baeuerle P A, Baltimore D. NF-κB: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 3.Baldwin A S. The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–681. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 4.Beg A A, Finco T S, Nantermet P V, Baldwin A S J. Tumor necrosis factor and interleukin-1 lead to phosphorylation and loss of IκBα: a mechanism for NF-κB activation. Mol Cell Biol. 1993;13:3301–3310. doi: 10.1128/mcb.13.6.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beg A A, Ruben S M, Scheinman R I, Haskill S, Rosen C A, Baldwin A S., Jr IκB interacts with the nuclear localization sequences of the subunits of NF-κB: a mechanism for cytoplasmic retention. Genes Dev. 1992;6:1899–1913. doi: 10.1101/gad.6.10.1899. [DOI] [PubMed] [Google Scholar]
- 6.Brockman J A, Scherer D C, MsKinsey T A, Hall S M, Qi X, Lee W Y, Ballard D W. Coupling of a signal response domain in IκBα to multiple pathways for NF-κB activation. Mol Cell Biol. 1995;15:2809–2818. doi: 10.1128/mcb.15.5.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown K, Gerstberger S, Carlson L, Fransozo G, Siebenlist U. Control of IκBα proteolysis by site-specific, signal-induced phosphorylation. Science. 1995;267:1485–1488. doi: 10.1126/science.7878466. [DOI] [PubMed] [Google Scholar]
- 8.Chen Z, Hagler J, Palombella V J, Melandri F, Scherer D, Ballard D, Maniatis T. Signal-induced site-specific phosphorylation targets IκBα to the ubiquitin-proteasome pathway. Genes Dev. 1995;9:1586–1597. doi: 10.1101/gad.9.13.1586. [DOI] [PubMed] [Google Scholar]
- 9.Chen Z J, Parent L, Maniatis T. Site-specific phosphorylation of IκBα by a novel ubiquitination-dependent protein kinase activity. Cell. 1996;84:853–862. doi: 10.1016/s0092-8674(00)81064-8. [DOI] [PubMed] [Google Scholar]
- 10.Cohen L, Henzel W J, Baeuerle P A. IKAP is a scaffold protein of the IkappaB kinase complex. Nature. 1998;395:292–296. doi: 10.1038/26254. [DOI] [PubMed] [Google Scholar]
- 11.Delhase M, Hayakawa M, Chen Y, Karin M. Positive and negative regulation of IkappaB kinase activity through IKKbeta subunit phosphorylation. Science. 1999;284:309–313. doi: 10.1126/science.284.5412.309. [DOI] [PubMed] [Google Scholar]
- 12.DiDonato J A, Mercurio F, Karin M. Phosphorylation of IκBα precedes but is not sufficient for its dissociation from NF-κB. Mol Cell Biol. 1995;15:1302–1311. doi: 10.1128/mcb.15.3.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DiDonato J A, Hayakawa M, Rothwarf D M, Zandi E, Karin M. A cytokine-responsive IκB kinase that activates the transcription factor NF-κB. Nature. 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 14.Hu Y, Baud V, Delhase M, Zhang P, Deerinck T, Ellisman M, Johnson R, Karin M. Abnormal morphogenesis but intact IKK activation in mice lacking the IKKalpha subunit of IkappaB kinase. Science. 1999;284:316–320. doi: 10.1126/science.284.5412.316. [DOI] [PubMed] [Google Scholar]
- 15.Lee F S, Hagler J, Chen Z J, Maniatis T. Activation of the IκBα kinase complex by MEKK1, a kinase of the JNK pathway. Cell. 1997;88:213–222. doi: 10.1016/s0092-8674(00)81842-5. [DOI] [PubMed] [Google Scholar]
- 16.Lee F S, Peters R T, Dang L C, Maniatis T. MEKK1 activates both IkappaB kinase alpha and IkappaB kinase beta. Proc Natl Acad Sci USA. 1998;95:9319–9324. doi: 10.1073/pnas.95.16.9319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li N, Karin M. Ionizing radiation and short wavelength UV activate NF-kappaB through two distinct mechanisms. Proc Natl Acad Sci USA. 1998;95:13012–13017. doi: 10.1073/pnas.95.22.13012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Q, Lu Q, Hwang J Y, Buscher D, Lee K F, Izpisua-Belmonte J C, Verma I M. IKK1-deficient mice exhibit abnormal development of skin and skeleton. Genes Dev. 1999;13:1322–1328. doi: 10.1101/gad.13.10.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Q, Van Antwerp D, Mercurio F, Lee K F, Verma I M. Severe liver degeneration in mice lacking the IkappaB kinase 2 gene. Science. 1999;284:321–325. doi: 10.1126/science.284.5412.321. [DOI] [PubMed] [Google Scholar]
- 20.Ling L, Cao Z, Goeddel D V. NF-kappaB-inducing kinase activates IKK-alpha by phosphorylation of Ser-176. Proc Natl Acad Sci USA. 1998;95:3792–3797. doi: 10.1073/pnas.95.7.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malinin N L, Boldin M P, Kovalenko A V, Wallach D. MAP3K-related kinase involved in NF-κB induction by TNF, CD95 and IL-1. Nature. 1997;385:540–548. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- 22.Mercurio F, Zhu H, Murray B W, Shevchenko A, Bennett B L, Li J, Young D B, Barbosa M, Mann M. IKK-1 and IKK-2: cytokine-activated IκB kinases essential for NF-κB activation. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 23.Mercurio F, Murray B W, Shevchenko A, Bennett B L, Young D B, Li J W, Pascual G, Motiwala A, Zhu H, Mann M, Manning A M. IκB kinase (IKK)-associated protein 1, a common component of the heterogeneous IKK complex. Mol Cell Biol. 1999;19:1526–1538. doi: 10.1128/mcb.19.2.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakano H, Shindo M, Sakon S, Nishinaka S, Mihara M, Yagita H, Okumura K. Differential regulation of IkappaB kinase alpha and beta by two upstream kinases, NF-kappaB-inducing kinase and mitogen-activated protein kinase/ERK kinase kinase-1. Proc Natl Acad Sci USA. 1998;95:3537–3542. doi: 10.1073/pnas.95.7.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nemoto S, DiDonato J A, Lin A. Coordinate regulation of IκB kinases by mitogen-activated protein kinase kinase kinase 1 and NF-κB-inducing kinase. Mol Cell Biol. 1998;18:7336–7343. doi: 10.1128/mcb.18.12.7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ninomiya-Tsuji J, Kishimoto K, Hiyama A, Inoue J, Cao Z, Matsumoto K. The kinase TAK1 can activate the NIK-I kappaB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature. 1999;398:252–256. doi: 10.1038/18465. [DOI] [PubMed] [Google Scholar]
- 27.Regnier C H, Song H Y, Gao X, Goeddel D V, Cao Z, Rothe M. Identification and characterization of an IκB kinase. Cell. 1997;90:373–383. doi: 10.1016/s0092-8674(00)80344-x. [DOI] [PubMed] [Google Scholar]
- 28.Rothwarf D M, Zandi E, Natoli G, Karin M. IKK-gamma is an essential regulatory subunit of the IkappaB kinase complex. Nature. 1998;395:297–300. doi: 10.1038/26261. [DOI] [PubMed] [Google Scholar]
- 29.Spencer E, Jiang J, Chen Z J. Signal-induced ubiquitination of IκBα by the F-box protein, Slimb/B-TrCP. Genes Dev. 1999;13:284–294. doi: 10.1101/gad.13.3.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takeda K, Takeuchi O, Tsujimura T, Itami S, Adachi O, Kawai T, Sanjo H, Yoshikawa K, Terada N, Akira S. Limb and skin abnormalities in mice lacking IKKα. Science. 1999;284:313–316. doi: 10.1126/science.284.5412.313. [DOI] [PubMed] [Google Scholar]
- 31.Tan P, Fuchs S Y, Chen A, Wu K, Gomez C, Ronai Z, Pan Z Q. Recruitment of a ROC1-CUL1 ubiquitin ligase by Skp1 and HOS to catalyze the ubiquitination of IκBα. Mol Cell. 1999;3:527–533. doi: 10.1016/s1097-2765(00)80481-5. [DOI] [PubMed] [Google Scholar]
- 32.Traenckner E B M, Pahl H L, Henkel T, Schmidt K N, Wilk S, Baeuerle P A. Phosphorylation of human IκBα on serines 32 and 36 controls IκBα proteolysis and NF-κB activation in response to diverse stimuli. EMBO J. 1995;14:2876–2883. doi: 10.1002/j.1460-2075.1995.tb07287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whiteside S T, Ernst M K, LeBail O, Laurent-Winter C, Rice N, Israel A. N- and C-terminal sequences control degradation of MAD3/IκBα in response to inducers of NF-κB activity. Mol Cell Biol. 1995;15:5339–5345. doi: 10.1128/mcb.15.10.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winston J T, Strack P, Beer-Romero P, Chu C, Elledge S J, Harper J W. The SCF-βTRCP ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IκBβ and β-catenin and stimulates IκBα ubiquitination in vitro. Genes Dev. 1999;3:270–283. doi: 10.1101/gad.13.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woronicz J D, Gao X, Cao Z, Rothe M, Goeddel D V. IκB kinase-β: NF-κB activation and complex formation with IκB kinase-α and NIK. Science. 1997;278:866–869. doi: 10.1126/science.278.5339.866. [DOI] [PubMed] [Google Scholar]
- 36.Yamaoka S, Courtois G, Bessia C, Whiteside S T, Weil R, Agou F, Kirk H E, Kay R J, Israel A. Complementation cloning of NEMO, a component of the IkappaB kinase complex essential for NF-kappaB activation. Cell. 1998;93:1231–1240. doi: 10.1016/s0092-8674(00)81466-x. [DOI] [PubMed] [Google Scholar]
- 37.Yaron A, Hatzubai A, Davis M, Lavon I, Amit S, Manning A M, Andersen J S, Mann M, Mercurio F, Ben-Neriah Y. Identification of the receptor component of the IκBα-ubiquitin ligase. Nature. 1998;396:590–594. doi: 10.1038/25159. [DOI] [PubMed] [Google Scholar]
- 38.Yin M-J, Christerson L B, Yamamoto Y, Kwak Y-T, Xu S, Mercurio F, Barbosa M, Cobb M H, Gaynor R B. HTLV-I Tax protein binds to MEKK1 to stimulate IκB kinase activity and NF-κB activation. Cell. 1998;93:875–884. doi: 10.1016/s0092-8674(00)81447-6. [DOI] [PubMed] [Google Scholar]
- 39.Yin M-J, Yamamoto Y, Gaynor R B. The anti-inflammatory agents aspirin and salicylate inhibit the activity of IκB kinase-β. Nature. 1998;396:77–80. doi: 10.1038/23948. [DOI] [PubMed] [Google Scholar]
- 40.Zandi E, Rothwarf D M, Delhase M, Hayakawa M, Karin M. The IκB kinase complex (IKK) contains two kinase subunits, IKKα and IKKβ, necessary for IκB phosphorylation and NF-κB activation. Cell. 1997;91:243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]
- 41.Zandi E, Chen Y, Karin M. Direct phosphorylation of IkappaB by IKKalpha and IKKbeta: discrimination between free and NF-kappaB-bound substrate. Science. 1998;281:1360–1363. doi: 10.1126/science.281.5381.1360. [DOI] [PubMed] [Google Scholar]
- 42.Zhao Q, Lee F S. Mitogen-activated protein kinase/ERK kinase kinases 2 and 3 activate nuclear factor-kappaB through IkappaB kinase-alpha and IkappaB kinase-beta. J Biol Chem. 1999;274:8355–8358. doi: 10.1074/jbc.274.13.8355. [DOI] [PubMed] [Google Scholar]