ABSTRACT
Neohesperidin (NH) was reported to regulate osteoclastic differentiation, while LncRNA SNHG1 could inhibit osteogenic differentiation of bone marrow stromal cells (BMSCs). In this study, we aimed to explore whether SNHG1-mediated osteogenic differentiation could be regulated by NH. Osteonecrosis and adjacent tissues, as well as normal bone marrow samples were gathered. BMSCs were isolated from normal bone marrow samples by Ficoll density gradient and identified by flow cytometry. Histopathological changes of tissues were detected by hematoxylin-eosin staining. After the treatment with NH or transfection, cell viability, osteogenic differentiation, and the activity of alkaline phosphatase (ALP) in BMSCs were detected by MTT, alizarin red staining, and microplate method, respectively. The histone modification and expressions of SNHG1 and osteogenic marker genes in tissues or BMSCs were detected by q-PCR and Chromatin Immunoprecipitation (ChIp). SNHG1 was highly expressed in osteonecrosis tissues, and typical signs of empty lacunae appeared in the necrotic tissues zone. NH increased viability and osteogenic differentiation of BMSCs, activity of ALP, and expressions of RUNX2, OCN and ALP. NH decreased both SNHG1 expression and H3K4me3 (activating histone modification) occupancies and increased H3K27me3 (inhibiting histone modification) occupancies of SNHG1. Furthermore, siSNHG1 enhanced osteogenic differentiation of BMSCs and expressions of RUNX2, OCN and ALP, while SNHG1 overexpression did the opposite and reversed the effects of NH on the osteogenic differentiation of BMSCs. In a word, NH promotes the osteogenic differentiation of human BMSCs by inhibiting the histone modifications of lncRNA SNHG1.
KEYWORDS: Neohesperidin, SNHG1, bone marrow stromal cells, osteogenesis, osteonecrosis of the femoral head
Introduction
Osteonecrosis of the femoral head (ONFH) is a common refractory disease, which is characterized by the death of osteocytes and bone marrow components, and the internal structure changes of the femoral head, contributing to inevitable collapse [1]. Statistics showed that there are 500,000–7500,000 patients diagnosed with ONFH in China, with an increase of 10,000–20,000 cases every year [2]. In addition, steroid-induced ONFH (SONFH) has the highest incidence in the nontraumatic types of ONFH, with major occurrence in young and middle-aged patients [3]. Most SONFH patients must undergo total hip arthroplasty [4,5], which imposes a huge psychological and financial burden on their families and society. At present, the exact mechanism of SONFH development is still unclear, but the defect of bone marrow stromal cells (BMSCs) is closely associated with ONFH [6].
BMSCs are characterized by their ability to self-renew and differentiate into osteoblasts and adipocytes, as well as endothelial chondrocytes [7,8]. Therefore, BMSCs, also known as ideal seed cells for treating a series of human bone disease, have a crucial effect on normal bone metabolism [9,10]. In addition, the decreased activity and the changes of MSCs biological behaviors have been proved to be related to nontraumatic ONFH [6]. Both the proliferation and differentiation abilities of BMSCs in SONFH patients are changed [11,12]. Based on these findings, the strategy to enhance the proliferation and osteogenesis of BMSCs may be a potential method for the treatment of SONFH.
Neohesperidin (NH), a compound extracted from citrus fruits, is a kind of flavanone glycoside with a strong bitter taste [13]. Endowed with numerous beneficial pharmacological actions, NH brings new therapy strategies to various disorders [14]. For instance, Lee et al. reported NH could significantly inhibit HCl/ethanol-induced gastric lesions and might be useful for the protection of gastritis [15]; Du et al. found that NH possessed the inhibitory effect on gram-negative and gram-positive bacteria [16]; and Gong et al. proved that NH could suppress the development of colorectal cancer through changing the intestinal flora [14]. What’s more, research also put forward that NH was capable of inhibiting osteoclast differentiation and bone resorption [17]. However, the effect of NH on the osteogenesis of BMSCs is still largely unknown and needs to be explored.
Long non-coding RNAs (lncRNAs) are RNAs over 200 nt in length that have no protein-coding role, the functions of which are widely explored in the last decade [18]. LncRNAs have been proposed to carry out diverse functions including cis or trans transcriptional regulation, organization of nuclear domains, and regulation of proteins or RNA molecules [19]. LncRNA dysregulation has been observed in various diseases, such as cancer, cardiovascular diseases, Alzheimer’s disease and osteoarthritis [20–25]. As a well-characterized oncogenic lncRNA, SNHG1 has recently been reported to be able to inhibit osteogenic differentiation of BMSCs [26,27]. However, whether SNHG1-mediated osteogenic differentiation could be regulated by NH has not been investigated.
Therefore, based on the previous research and findings, we speculated that NH has an effect on the osteogenesis of BMSCs via regulating lncRNA SNHG1. Then we explored this effect through a series of experiments.
Methods
Sample collection and ethics statement
All osteonecrosis tissue (ONT group) and adjacent osteonecrosis tissue (ANT group) were gathered from seven patients diagnosed with SONFH at the Department of Orthopedic in Shanghai Changzheng Hospital between December 2018 and March 2019. Normal bone marrow samples were also gathered from the posterior iliac crests of three consenting healthy adult volunteers. Written informed consent was obtained from all patients and volunteers. The use of samples were approved by the Ethics Committee of Shanghai Changzheng Hospital (Z20190408G).
Hematoxylin-eosin (HE) staining
After we obtained the tissues from the patients, paraffin (S25190, Yuanye, Shanghai, China) was used to embed these tissues. Paraffin-embedded tissues were fixed on the microtome (RM2235, Leica, Solms, Germany), with each being cut into 4 μm thick slices. Then the slices were fixed on a glass slide (80,302–3101-16-P4, ShiTai, Jiangsu, China) followed by deparaffinization. Next, tissue slices were firstly stained with hematoxylin (B25380, Yuanye) for 10 min and then with eosin (G1100, Solarbio, Beijing, China) for 1 min at room temperature. Finally, the detected indexes were observed and documented by a phase-contrast optical microscope (Axio Lab.A1 pol; Leica, Solms, Germany).
Isolation and culture of BMSCs
BMSCs were isolated from the normal femoral tissue using a Ficoll density gradient [2,28]. In brief, after filtering the bone marrow samples through a 100 mm filter (251600506SC, Merck Millipore, Darmstadt, Germany), the samples were mixed with Hank’s buffer (C0218, Beyotime, Shanghai, China) and then lymphoprep (07851, Stemcell, Canada) was gently layered. The samples were then centrifuged for 30 min at 20°C (400 ×g). BMSCs located at the interface between the bone marrow sample and the lymphoprep were collected and further cultured in Dulbecco’s modified Eagle’s medium (DMEM) (C11995500BT, Gibco, MA, USA) containing 10% fetal bovine serum (FBS) (10,437,010, Gibco), 10 U/mL penicillin (DEPENE01, Demeditec, Germany), and 10 mg/mL streptomycin (DESTRE02, Germany) at 37°C in a humid atmosphere with 5% CO2. For morphological observation, cells were observed under a phase-contrast optical microscope (Axio Lab.A1 pol, Leica, Solms, Germany).
Phenotype identification of BMSCs
Cells isolated from bone marrow samples were used for phenotype identification. After culture to reach confluence, the cells were harvested and re-suspended in fluorescence-activated cell sorting (FACS) buffer (MB-089-0500, Dickinson Biosciences, Philadelphia, USA) and incubated with primary unconjugated antibodies as follows: CD29 (MAB17781, R&D System, MA, USA), CD90 (ab123511, Abcam, CA, USA), and CD34 (ab8536, Abcam, CA, USA) for 25 min at 20°C. The cells were then washed and incubated with secondary antibody: phycoerythrin-conjugated goat anti-mouse immunoglobulin G antibody (ab6785, Abcam, CA, USA) for 15 min. The cells were subsequently washed and analyzed using flow cytometry on a FACS Calibur (Becton Dickinson, Oxford, UK). Data were analyzed using FCS Express software 3.0 (Dickinson Biosciences).
Transfection
Small interfering RNA directed against SNHG1 (siSNHG1; siB141118105920-1-5), negative control for siSNHG1 (siNC; lnc3N0000001-1-5), and SNHG1 overexpression plasmids were obtained from RIBOBIO (Guangzhou, China). Before transfection, the BMSCs were plated into 6-well plates, with each well containing 1.0 × 106 cells and 2 ml DMEM complete medium. After growing overnight until the cell confluence reached 80%, the siSNHG1 and SNHG1 plasmids were transfected into the cells with the help of 3 μl lipofectamine 2000 (11,668–019, Invitrogen, MA, USA). After being transfected for 48 h, the cells were collected for later use.
NH treatment
NH, with molecular weight of 610.5606 and a purity of over 98%, was obtained from Yuanye (13,241–33-3, Shangai, China). NH was dissolved in osteogenic induction medium (including 10−8 mol/L dexamethasone, 50 mM L-ascorbicacid-2-phosphate, and 10−2 mol/L β-glycerophosphate) (wr2008, Bio-tool, Beijing, China). BMSCs were seeded into 6-well plates at a density of 1.0 × 106 cells/well in 2 ml DMEM complete medium. After the cells reached 60%-70% confluence, they were treated with different doses of NH (12.5, 25, 50, and 100 μmol/L) and continued to grow for 24 h. Then the cell samples were used in the follow-up experiments.
MTT assays
MTT (B7777, APExBIO, Houston, USA) was used to test cell viability. After BMSCs were treated with different doses of NH, cells were laid into 96-well plates at a density of 1.0 × 104 cells/well in 100 μl complete medium. After growth for 24 h, the cells were incubated by MTT reagent (0.5 mg/ml) for 4 h. Then, the MTT solution was replaced by 100 μl DMSO (ST038, Beyotime) and added to each well. Finally, the absorbance of each well was detected at 570 nm by a microplate reader (InfiniteM200 PRO, Tecan Austria GmbH, Austria).
Detection of alkaline phosphatase (ALP) activity
ALP detection kit (G5610, Solarbio, Beijing, China) was used to detect the activity of ALP in BMSCs. After being treated with different doses of NH, BMSCs were collected and lysed by RIPA lysis buffer (P0013B, Beyotime). And cell lysates were collected and added into 55 μl to each well of a 96-well plate. Then 50 μl ALP buffer was added into each well and incubated for 5 min at 37 °C. Next, 50 μl ALP coloration buffer was added into each well and incubated for 15 min at 37 °C. Finally, the absorbance of each well was detected at 510 nm by a microplate reader (InfiniteM200 PRO, Tecan Austria GmbH, Austria).
Alizarin red staining
Alizarin red staining was performed by the Alizarin red S staining kit (0223, ScienCell, CA, USA). After being treated with NH or transfected with siSNHG1, BMSCs were collected and washed with PBS twice. Then the cells were fixed with 97% ethanol (E111992-12X, Aladdin, Shanghai, China) for 10 min. Finally, the fixed cells were incubated with Alizarin red staining solution for 30 min at 37 °C and observed under a phase-contrast optical microscope (Axio Lab.A1 pol, Leica, Solms, Germany).
RNA extraction and q-PCR
mRNA was extracted from the clinic tissues and BMSCs transfected with siSNHG1 through TRIzol reagent (15,596, Invitrogen, MA, USA). In brief, the tissues and cells were lysed by TRIzol and collected into a new 1.5 ml centrifugal tube (615,001, Nest, Wuxi, China). The chloroform (C805334, Macklin, shanghai, China) was added into a tube and centrifuged for 20 min (14,000 × g). The supernatant was collected and added with an equal volume of isopropanol (H822173, Macklin), and centrifuged for 5 min (14,000 ×g). mRNA sediment was diluted using RNase-free H2O. Then, the PrimeScript RT kit (RR037A, Takara, Dalian, China) was used to reverse transcribe RNA into cDNA according to the reference instructions. Finally, gene expression was tested by q-PCR assay using Verso 1-step RT-qPCR Kit (A15300, Thermo Scientific, MA, USA) in ABI7500 Fast Real-Time PCR System (Applied Biosystems, CA, USA), with the condition of q-PCR as follows: at 95 °C for 30 s, 60 °C for 30 s, and 45 cycles at 60 °C for 30 s. RNA relative quantity was quantified by 2−ΔΔCT method. All primer sequences are shown in Table 1.
Table 1.
Q-PCR primers
| Target gene | Forward primers, 5ʹ-3’ | Reverse primers, 5ʹ-3’ |
|---|---|---|
| LncSNHG1 | TCTCTAAAGCCCAAGAGGA | ACCTTATTTGCTCAGACCTG |
| RUNX2 | TGGTTACTGTCATGGCGGGTA | TCTCAGATCGTTGAACCTTGCTA |
| OCN | CACTCCTCGCCCTATTGGC | CCCTCCTGCTTGGACACAAAG |
| ALP | GAGATGTTGTCCTGACACTTGTG | AGGCTTCCTCCTTGTTGGGT |
| GAPDH | GGAGCGAGATCCCTCCAAAAT | GGCTGTTGTCATACTTCTCATGG |
Chromatin immunoprecipitation (ChIP) assay
Cross-linking and ChIP were conducted as described previously [29]. Briefly, all of the subsequent steps were performed at 0–4 °C, and all of the buffers contained 0.1 mM EDTA (15,576,028, Gibco), 0.5 mM EGTA (S30018, Yuanye), 1 mM dithiothreitol (15,508,013, Gibco), and protease inhibitors (27,588,800, Aks mics, Shanghai, China). After being treated with different doses of NH, BMSCs were washed with phosphate-buffered saline (PBS) (C0221A, Beyotime) and lysed. After centrifugation, the pellet was re-suspended in 10 ml of 10 mM pH 8.0 Tris-HCl (10,812,846,001, ROCHE, Barcelona, Switzerland) and 200 mM NaCl (A610476-0005, Sangon Biotech, Shanghai, China); rotated for 10 min; and centrifuged at 15,000 × g for 10 min. The chromatin pellet was re-suspended in 1 ml of 50 mM pH 7.9 Tris-HCl and 5 mM CaCl2 (20–305, Merck Millipore) and digested with 500 units of micrococcal nuclease (LN101-02, TransGen Biotech, Shanghai, China) at 37 °C for 10 min. For ChIP reactions, the samples (1 ml) were incubated with the antibodies against H3K4me3 (ab8580, Abcam), H3K27me3 (ab6002, Abcam), or control rabbit IgG (ab172730, Abcam) overnight. Finally, 3 μl ChIP DNA was purified and quantified using q-PCR.
Western blot assays
RIPA lysis buffer (P0013B, Beyotime) was applied to isolate total protein from the BMSCs, while a BCA assay kit (23,250, Pierce, MA, USA) was used to detect the total protein concentrations. Finally, total protein (30 µg) was separated in each lane on 10% SDS-PAGE gels (P0052A, Beyotime), and electro-blotted and transferred to NC membranes (HTS112M, Millipore). Then all membranes were blocked by a 5% concentration of skimmed milk for 2 h at normal atmospheric temperature, followed by incubation with primary antibodies at 4°C overnight: RUNX2 (1:1000, 57 kDa, ab76956, Abcam), OCN (1:1000, 12 kDa, ab93876, Abcam), ALP (1:1000, 39 kDa, ab83259, Abcam) and GAPDH (1:1000, 36kD, ab181602, Abcam). Next day, rabbit secondary antibody (1:5000, ab205718, Abcam) or mouse secondary antibody (1:5000, ab205719, Abcam) was incubated with the membranes for 1 h at room temperature. Finally, SuperSignal West Pico Chemiluminescent Substrate (34,078, Thermo Scientific, MA, USA) was used to incubate the membranes for detecting the signal. Image Lab™ Software (version 3.0) was used for densitometric analysis and quantification of the Western blot data (Bio-Rad Laboratories Inc., Hercules, CA, USA).
Statistical analysis
All statistical analyses were performed using GraphPad Prism 8.0 (GraphPad, USA). Student’s t-test or one-way ANOVA was applied to analyze the differences among diverse experiment groups. Dunnett’s was used as post-hoc tests. Mean ± standard deviation indicated the Statistical Data. All experiments were conducted three times. P < 0.05 was considered as statistically significant.
Results
SNHG1 was high-expressed in osteonecrosis tissue of patients with SONFH
A comparative analysis of SNHG1 expression level was conducted between osteonecrosis tissue and adjacent osteonecrosis tissue. The result revealed that SNHG1 expression was obviously higher in ONT group compared with that in ANT group (P < 0.001) (Figure 1a). Then HE staining was further conducted to observe the pathological changes of these tissues. As shown in Figure 1b, a typical sign of empty lacunae was observed in the necrotic zone. These results exhibited that SNHG1 was high-expressed in osteonecrosis tissue of patients with SONFH. We, therefore, speculated that SNHG1 might play an important role in the occurrence or development of SONFH.
Figure 1.

SNHG1 was high-expressed in osteonecrosis tissue of patients with SONFH. (a). SNHG1 expression in clinic osteonecrosis tissue and adjacent osteonecrosis tissue of SONFH patients was analyzed by q-PCR. (b). HE staining was conducted to observe the pathological changes of clinic tissues. All experiments were conducted in triplicate. (^^^P < 0.001, vs. ANT). (SONFH: steroi-induced osteonecrosis of the femoral head, HE: Hematoxylin-eosin, ANT: adjacent osteonecrosis tissue group)
BMSCs was isolated and identified whose viability was increased by NH
In order to investigate the effect of NH on SONFH, BMSCs were isolated from the normal femoral tissue, and expanded and passaged 3–5 times. On day 7 of primary culture, BMSCs had attached and exhibited spindle and flat shapes, while passage 2 BMSCs reached 90% confluence on day 3, similar to a shoal of fish (Figure 2a). These characteristics are consistent with previous studies [2,30]. As shown in Figure 2b, passage 2 cells were detected using flow cytometry. Notably, 92.59% of cells were CD29-positive and 97.91% of cells were CD90-positive, whereas 0% of cells were CD34-negative. Thus, most of the isolated and purified cells expressed standard markers of BMSCs. Then BMSCs were treated with or without different concentrations of NH to explore the effect of NH on BMSCs viability. As shown in Figure 2c, the results of MTT assay revealed that NH significantly increased the cell viability at different time points at the dose of 50 μmol/L as compared with control group (P < 0.01). Considering that 12.5, 25, and 100 μmol/L of NH had no effect on the BMSCs viability, and only the 50 μmol/L of NH could increase the viability of BMSCs, the doses of 12.5 μmol/L and 50 μmol/L were chosen for subsequent experiments.
Figure 2.

BMSCs was isolated and identified whose viability was increased by NH. (a). Cell morphology of primary and passage 2 BMSCs. (b). The levels of the biomarkers in BMSCs were detected by flow cytometry. (c). Cell viability of BMSCs after treated with NH (12.5, 25, 50, 100 μmol/L) was detected by MTT assays. All experiments were conducted in triplicate. (^^P < 0.01, vs. Control). (NH: Neohesperidin, BMSCs: bone marrow mesenchymal stem cells)
NH enhanced BMSCs osteogenic differentiation
We further investigated the effect of NH on BMSCs osteogenic differentiation. As shown in Figure 3a, on the day 2, 3, 7 and 14 of NH treatment and culture, NH significantly increased the ALP activity of BMSCs both at the doses of 12.5 μmol/L and 50 μmol/L (P < 0.05, P < 0.01, or P < 0.001, respectively). Then Alizarin red staining was used (Figure 3b) with the results exhibiting that NH remarkably induced the calcium nodules formation of BMSCs on day 14. Based on current findings, we further detected the expressions of osteogenic marker genes (Runx2, OCN, and ALP) in BMSCs by q-PCR. It can be noted in Figure 3c, NH markedly increased the expressions of Runx2 and OCN of BMSCs both at the doses of 12.5 μmol/L and 50 μmol/L (P < 0.05, or P < 0.001, respectively), and increased the expression of ALP of BMSCs at the dose of 50 μmol/L (P < 0.001). All these results demonstrated that NH enhanced BMSCs osteogenic differentiation.
Figure 3.
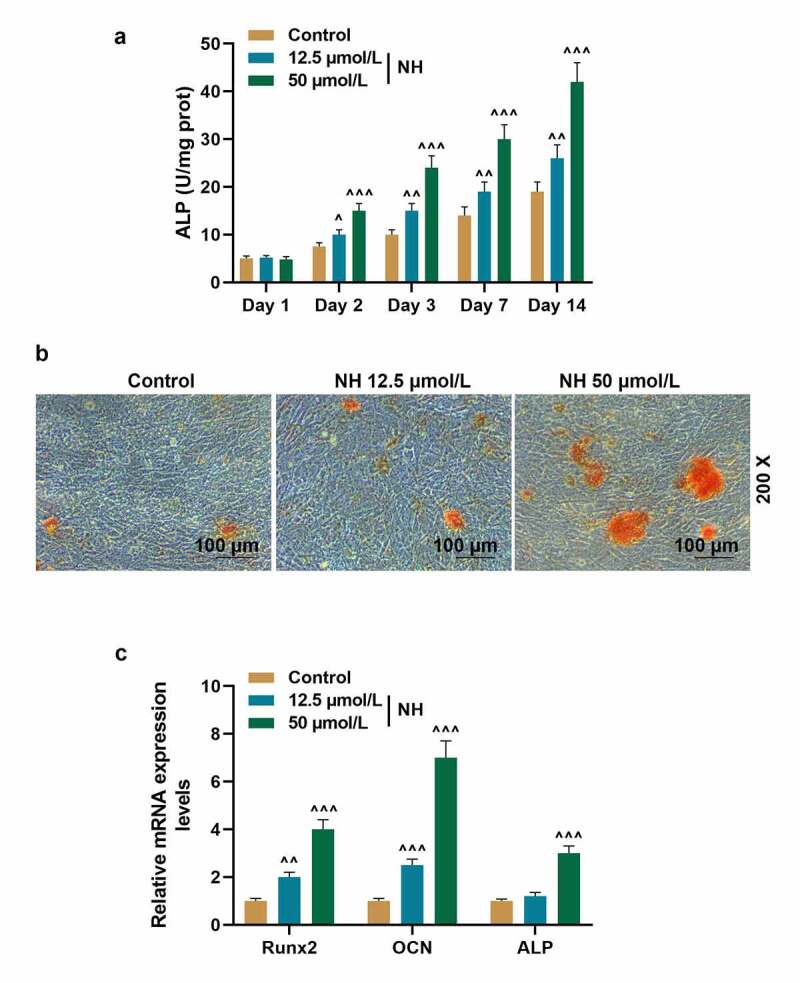
NH enhanced BMSCs osteogenic differentiation. (a). ALP activity of BMSCs after treated with NH (12.5 and 50 μmol/L) was detected through microplate method. (b). The formation of calcium nodules of BMSCs after treated with NH (12.5 and 50 μmol/L) was detected by Alizarin red staining. (c). The expressions of Runx2, OCN and ALP in BMSCs after treated with NH (12.5 and 50 μmol/L) were detected by q-PCR. GAPDH was used as an internal control. All experiments were conducted in triplicate. (^P < 0.05, ^^P < 0.01, ^^^P < 0.001, vs. Control). (NH: Neohesperidin, BMSCs: bone marrow mesenchymal stem cells)
NH inhibited the expression and histone modification of SNHG1 in BMSCs
We explored, at least in part, the underlying mechanism through which NH enhanced osteogenesis of BMSCs and wondered whether SNHG1 was involved in mediating the effect of NH on osteogenesis of BMSCs. As shown in Figure 4a, NH significantly down-regulated the expression of SNHG1 in BMSCs both at the doses of 12.5 μmol/L and 50 μmol/L (P < 0.001, respectively). Mechanistically, we checked the H3K4me3 (activating histone modification) and H3K27me3 (inhibiting histone modification) occupancies in different regions of the SNHG1’s promoter, as the histone methylations are associated with target gene expression in stem cells to regulate cell fate [31]. The ChIP assay exhibited that 50 μmol/L of NH decreased the occupancies of H3K4me3 but increased the occupancies of H3K27me3 (Figure 4b). These results indicated that NH inhibited the expression and histone modification of SNHG1 in BMSCs.
Figure 4.
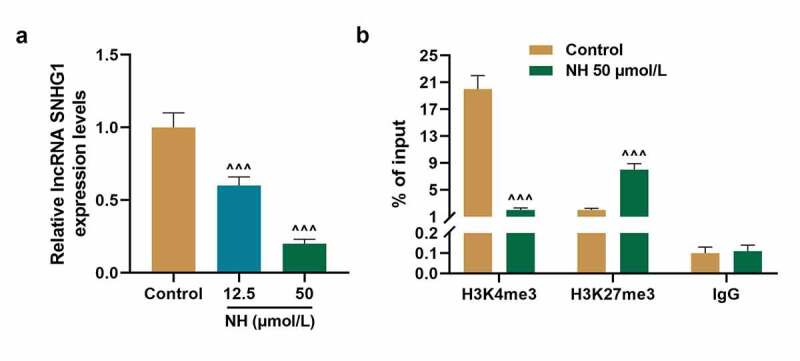
NH inhibited the expression and histone modification of SNHG1 in BMSCs. (a). The expression of SNHG1 in BMSCs after treated with NH (12.5 and 50 μmol/L) was detected by q-PCR. GAPDH was used as an internal control. (b). The occupancies of H3K4me3 and H3K27me3 on SNHG1 promoter in BMSCs after treated with 50 μmol/L NH were detected by ChIP assay. All experiments were conducted in triplicate. (^^^P < 0.001, vs. Control). (NH: Neohesperidin, BMSCs: bone marrow mesenchymal stem cells, ChIP: chromatin immunoprecipitation)
SNHG1 silencing enhanced BMSCs osteogenic differentiation
As shown in Figure 5a, we found that SNHG1 expression was significantly decreased on day 3 and day 7 after BMSCs were cultured in osteogenic induction medium (P < 0.001, respectively). Therefore, siSNHG1 was transfected into BMSCs (Figure 5b) to confirm the effect of SNHG1 on osteogenesis of BMSCs. Then Alizarin red staining was used (Figure 5c) with the results exhibiting that siSNHG1 notably induced the calcium nodules formation of BMSCs. As per current findings, we further detected the expressions of osteogenic marker genes (Runx2, OCN, and ALP) in BMSCs by q-PCR. Figure 6a-c depicted that on day 0 after BMSCs were cultured in osteogenic induction medium, siSNHG1 exerted no impact on the expressions of Runx2, OCN, and ALP both at transcription and translation levels. However, on day 7 after BMSCs were cultured in osteogenic induction medium (Figure 6d-f), siSNHG1 significantly up-regulated the expressions of Runx2, OCN, and ALP both at transcription and translation levels (P < 0.001, respectively). Collectively, these results revealed that SNHG1 silencing enhanced BMSCs osteogenic differentiation.
Figure 5.
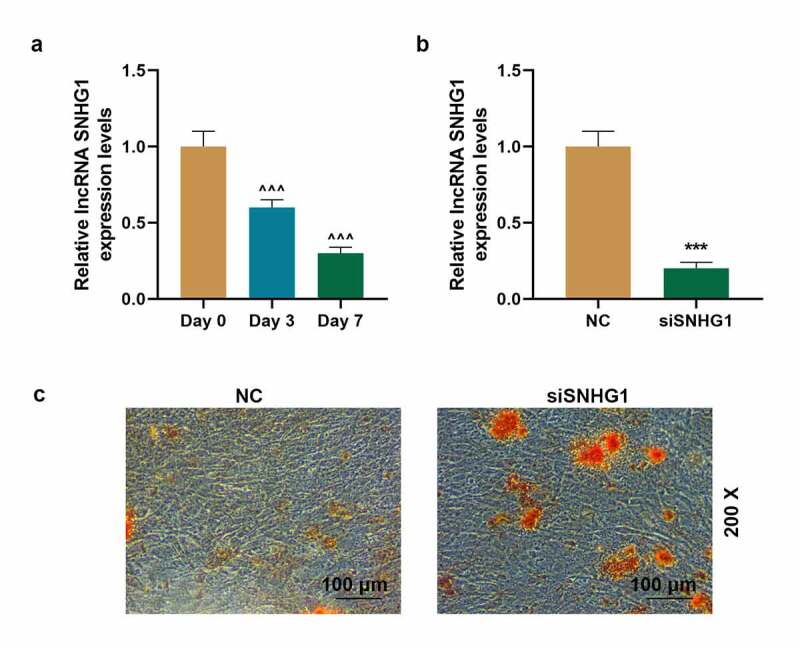
SNHG1 silencing enhanced BMSCs osteogenic differentiation. (a). The expression of SNHG1 in BMSCs after cultured in osteogenic induction medium was detected by q-PCR. GAPDH was used as an internal control. (b). The transfection efficiency of siSNHG1 in BMSCs was detected by q-PCR. GAPDH was used as an internal control. (c). The formation of calcium nodules of BMSCs after transfected with siSNHG1 was detected by Alizarin red staining. All experiments were conducted in triplicate. (^^^P < 0.001, vs. Day 0; ***P < 0.001, vs. NC). (BMSCs: bone marrow mesenchymal stem cells, NC: negative control)
Figure 6.
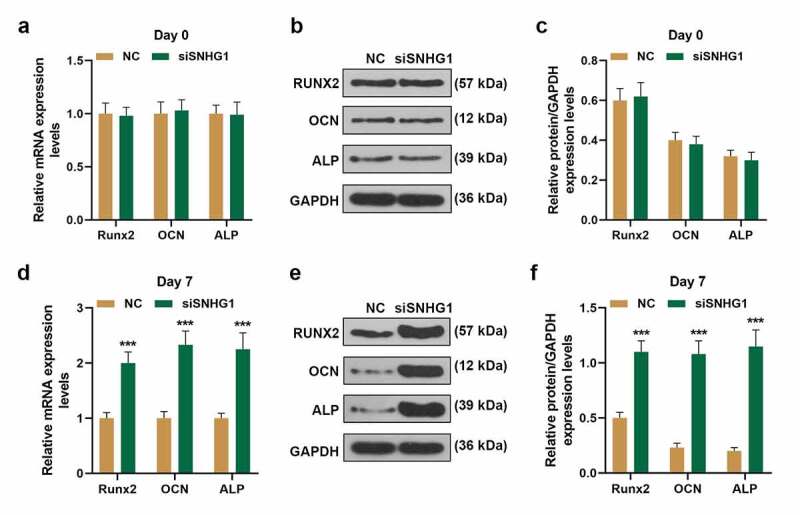
SNHG1 silencing enhanced the expressions of Runx2, OCN, and ALP in BMSCs. (a, d). The expressions of Runx2, OCN and ALP in BMSCs transfected with siSNHG1 after incubated in osteogenic induction medium were detected by q-PCR. (B-C, E-F). The expressions of Runx2, OCN and ALP in BMSCs transfected with siSNHG1 after incubated in osteogenic induction medium were detected by western blot. GAPDH was used as an internal control. All experiments were conducted in triplicate. (***P < 0.001, vs. NC). (BMSCs: bone marrow mesenchymal stem cells, NC: negative control)
SNHG1 overexpression inhibited BMSCs osteogenic differentiation
To further verify that the promotion of NH in the osteogenic differentiation of BMSCs was modulated by down-regulating SNHG1, the BMSCs were transfected with SNHG1 overexpression plasmids and further cultured with 50 μm/ml NH. As shown in Figure 7a, the expression of SNHG1 in BMSCs was up-regulated by SNHG1 overexpression plasmids (P < 0.001) but was down-regulated by NH (P < 0.01). Besides, the effect of NH on SNHG1 expression was reversed by SNHG1 overexpression. The ALP level was also detected. As illustrated in Figure 7b, on the day 2, 3, 7, 14 of NH treatment and culture, the ALP level of BMSCs was decreased by SNHG1 (P < 0.05) but increased by NH (P < 0.001). Similarly, the effect of NH on the level of ALP was reversed by SNHG1 overexpression. Then Alizarin red staining was used (Figure 7c) with results depicting that NH significantly induced while SNHG1 overexpression inhibited the calcium nodules formation of BMSCs on day 14. The effect of NH was also overturned by SNHG1 overexpression. Finally, the expressions of osteogenic marker genes (Runx2, OCN, and ALP) in BMSCs were analyzed. As exhibited in Figure 7d-F, the gene and protein levels of Runx2, OCN, and ALP of BMSCs were up-regulated by NH but were down-regulated by SNHG1 overexpression. In addition, SNHG1 overexpression further overturned the effect of NH on the expression of these markers. All these results revealed that NH down-regulated the expression of SNHG1 to enhance BMSCs osteogenic differentiation.
Figure 7.
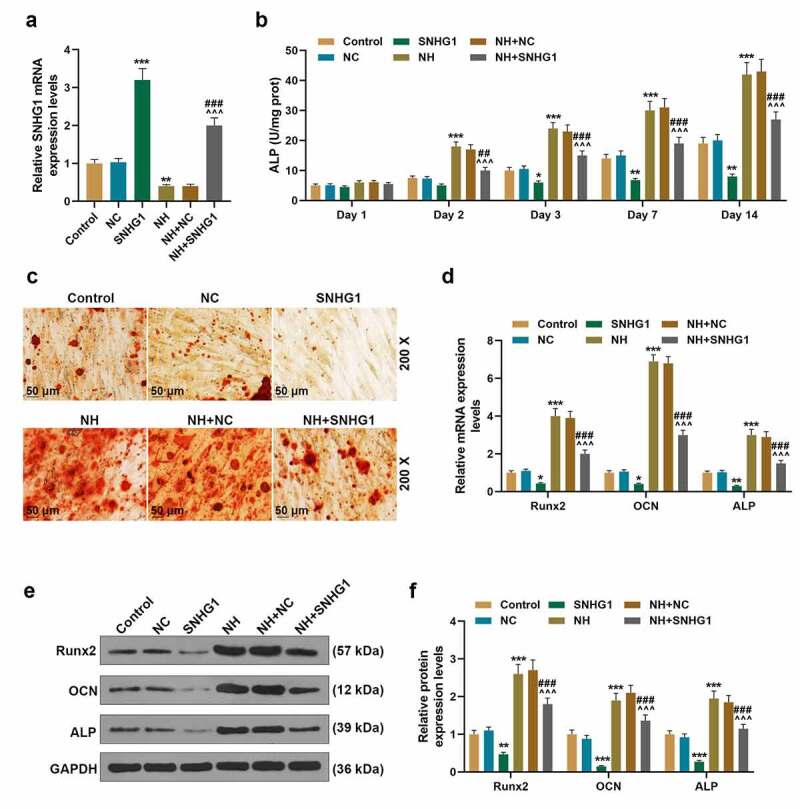
SNHG1 overexpression inhibited BMSCs osteogenic differentiation. (a). The expression of SNHG1 in BMSCs after being transfected with SNHG1 overexpression plasmids or treated with NH was detected by q-PCR. GAPDH was used as an internal control. (b). The level of ALP of BMSCs after being overexpressed with SNHG1 or treated with NH was detected through microplate method. (c). The formation of calcium nodules of BMSCs after being overexpressed with SNHG1 or treated with NH was detected by Alizarin red staining. (d). The expressions of Runx2, OCN and ALP in BMSCs after being overexpressed with SNHG1 or treated with NH were detected by q-PCR. GAPDH was used as an internal control. (e-f). The expressions of Runx2, OCN and ALP in BMSCs after being overexpressed with SNHG1 or treated with NH were detected by Western blot. GAPDH was used as an internal control. All experiments were conducted in triplicate. (*P < 0.05, **P < 0.01, ***P < 0.001, vs. NC; ^P < 0.05, ^^P < 0.01, ^^^P < 0.001, vs. NH+NC; #P < 0.05, ##P < 0.01, ###P < 0.001, vs. SNHG1). (BMSCs: bone marrow mesenchymal stem cells, NC: negative control)
Discussion
The defect of BMSCs is related to ONFH, therefore, the strategy of promoting the osteogenesis of BMSCs may be a promising method for treating SONFH [6]. In this study, we purposed to investigate the effects of NH and lncRNA SNHG1 on the osteogenesis of BMSCs. We figured out that SNHG1 was high-expressed in osteonecrosis tissues of SONFH patients. NH not only increased the viability and differentiation of BMSCs but also up-regulated the expressions of osteogenic marker genes and down-regulated SNHG1 expression. Additionally, NH decreased H3K4me3 occupancies but increased H3K27me3 occupancies of SNHG1. However, siSNHG1 enhanced the differentiation of BMSCs and the expressions of osteogenic marker genes. Our findings in this study revealed that NH could promote the osteogenic differentiation of human BMSCs by inhibiting the histone modification of lncRNA SNHG1.
BMSCs have become a research hotspot in the progression of ONFH and SONFH owing to its multiple differentiation potential [27]. Accumulating studies have put forward that the progression of ONFH is closely related to the decrease in the number of BMSCs and the decline in the ability to proliferate and differentiate [12]. SONFH osteopathy lesion areas can exhibit excessive accumulation of fat and necrotic empty lacunae, accompanied by a decrease in osteogenic markers [32,33]. In this study, we also found the necrotic zone in osteonecrosis showed a typical sign of empty lacunae. Changes in the characteristics of BMSCs lead to the destruction of bone tissue, especially the proliferation and differentiation of BMSCs [34]. Therefore, the strategy of promoting the proliferation and osteogenesis of BMSCs may be a prospective method for treating SONFH. In the light of a recent systematic review, Chinese herbal medicine as an adjuvant therapy might improve the curative efficacy of SONFH [35].
NH, known as a natural flavanone glycoside, has been widely used as herbal medicine in China due to its numerous beneficial pharmacological actions [13,14]. In addition to anti-inflammatory, neuro-protective and cardiovascular protective effects, research has also reported that NH could inhibit osteoclast differentiation [17,36], which encouraged us to further investigate the effect of NH on the osteogenesis of BMSCs. Although a pharmacokinetics study has not been conducted in humans, NH within 100 μmol/L had no cytotoxicity on BMSCs, while 50 μmol/L NH was found significantly increased the viability of BMSCs in this study. Therefore, the dose of 50 μmol/L was chosen for subsequent experiments. Considering that ALP is an early-stage marker of osteogenesis which enhances mineralization during the osteogenesis [37], we first detected the activity of ALP in BMSCs treated with NH and found that NH actively increased the ALP in BMSCs. This result was further confirmed by the Alizarin red staining as the NH induced the formation of calcium nodules of BMSCs. Besides, ALP, Runx2 and OC Nare are also the important markers during osteogenesis [2]. Runx2 is a major regulator expressed early in osteogenesis and functions at the intersection of many other osteogenesis-related pathways [38]. OCN, a non-collagenous protein found in bone and dentine, also expressed early in osteogenesis with its expression being further enhanced by the expression of Runx2 [39]. Therefore, the expression levels of these osteogenesis markers (ALP, Runx2, OCN) can help evaluate osteogenic differentiation [39]. Therefore, we then detected the expressions of ALP, Runx2 and OCN in BMSCs treated with NH. The results showed that the expressions of these proteins were all up-regulated by NH, proving that NH was capable of enhancing osteogenic differentiation.
The next step we did was to clarify, at least in part, the effect mechanism of NH on osteogenic differentiation. The alterations of cell characteristics can be caused by abnormal gene expression. Moreover, increasing researches have been conducted on ncRNAs, especially mRNAs were performed to identify how they regulate BMSCs behaviors [40–42]. Other ncRNAs, such as lncRNAs, were investigated in the regulation of orthopedic disorders, such as osteoarthritis and osteoporosis [43,44], whereas little attention was paid to the effect of lncRNAs on BMSCs and SONFH. Among these less studied lncRNAs, SNHG1, which was high-expressed in osteoporosis, was recently reported to inhibit osteogenic differentiation of BMSCs [27]. Consistent with this research, we also found that the expression of SNHG1 was high in osteonecrosis tissues of SONFH patients. Therefore, we then explored whether SNHG1 mediated osteogenic differentiation had relationship with the effect of NH on osteogenic differentiation. To our delight, we figured out that NH not only decreased the expression of SNHG1 in BMSCs but also inhibited the histone modification of SNHG1. Thus, we further confirmed the effect of SNHG1 on osteogenic differentiation. In line with the effect of NH on osteogenic differentiation, the results revealed that siSNHG1 enhanced the differentiation of BMSCs and the expressions of osteogenic marker genes, while SNHG1 overexpression did the opposite and further reversed the effect of NH on the osteogenic differentiation of BMSCs. All these results exhibited that the effect of NH on promoting the osteogenic differentiation of human BMSCs was mediated through inhibiting the histone modification of SNHG1. The role of some lncRNAs in SONFH has been reported in studies, such as lncRNA RP11-154D6 [45], NORAD [46] and HOTAIR [47]. The RNA-seq assay was used to analyze differential genes in diseases [48–50]. In future studies, RNA-seq experiment can be used to analyze the differential genes of lncRNAs in SONFH, and explore the link between the lncRNAs with targets/pathways in SONFH.
In conclusion, this research indicates that NH can promote the osteogenic differentiation of human BMSCs by inhibiting the histone modification of lncRNA SNHG1.
Funding Statement
No funding was received.
Disclosure of Conflict-of-Interest
The authors declare no conflicts of interest.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- [1].Cohen-Rosenblum A, Cui Q.. Osteonecrosis of the femoral head. Orthop Clin North Am. 2019 Apr;50(2): 139–149. PubMed PMID: 30850073; eng. [DOI] [PubMed] [Google Scholar]
- [2].Chen XJ, Shen YS, He MC, et al. Polydatin promotes the osteogenic differentiation of human bone mesenchymal stem cells by activating the BMP2-Wnt/beta-catenin signaling pathway. Biomed Pharmacother. 2019 Apr;112:108746. PubMed PMID: 30970530. [DOI] [PubMed] [Google Scholar]
- [3].Zhao DW, Yu M, Hu K, et al. Prevalence of nontraumatic osteonecrosis of the femoral head and its associated risk factors in the Chinese population: results from a nationally representative survey. Chin Med J (Engl). 2015 Nov 5;128(21):2843–2850. PubMed PMID: 26521779; PubMed Central PMCID: PMCPMC4756878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fu W, Liu B, Wang B, et al. Early diagnosis and treatment of steroid-induced osteonecrosis of the femoral head. Int Orthop. 2019 May;43(5):1083–1087. PubMed PMID: 29876626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Baig SA, Baig MN. Osteonecrosis of the femoral head: etiology, investigations, and management. Cureus. 2018 Aug 21;10(8): e3171. PubMed PMID: 30357068; PubMed Central PMCID: PMCPMC6197539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lee JS, Lee JS, Roh HL, et al. Alterations in the differentiation ability of mesenchymal stem cells in patients with nontraumatic osteonecrosis of the femoral head: comparative analysis according to the risk factor. J Orthop Res. 2006 Apr;24(4):604–609. PubMed PMID: 16514658. [DOI] [PubMed] [Google Scholar]
- [7].Ruiz M, Cosenza S, Maumus M, et al. Therapeutic application of mesenchymal stem cells in osteoarthritis. Expert Opin Biol Ther. 2016;16(1):33–42. PubMed PMID: 26413975. [DOI] [PubMed] [Google Scholar]
- [8].Kong L, Zheng LZ, Qin L, et al. Role of mesenchymal stem cells in osteoarthritis treatment. J Orthop Translat. 2017 Apr;9:89–103. PubMed PMID: 29662803; PubMed Central PMCID: PMCPMC5822967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yim RL, Lee JT, Bow CH, et al. A systematic review of the safety and efficacy of mesenchymal stem cells for disc degeneration: insights and future directions for regenerative therapeutics. Stem Cells Dev. 2014 Nov 1;23(21):2553–2567. PubMed PMID: 25050446; PubMed Central PMCID: PMCPMC4201280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Liu Y, Wu J, Zhu Y, et al. Therapeutic application of mesenchymal stem cells in bone and joint diseases. Clin Exp Med. 2014 Feb;14(1):13–24. PubMed PMID: 23124706. [DOI] [PubMed] [Google Scholar]
- [11].Sun ZB, Wang JW, Xiao H, et al. Icariin may benefit the mesenchymal stem cells of patients with steroid-associated osteonecrosis by ABCB1-promoter demethylation: a preliminary study. Osteoporos Int. 2015 Jan;26(1):187–197. PubMed PMID: 25112719; eng. [DOI] [PubMed] [Google Scholar]
- [12].Houdek MT, Wyles CC, Packard BD, et al. Decreased osteogenic activity of mesenchymal stem cells in patients with corticosteroid-induced osteonecrosis of the femoral head. J Arthroplasty. 2016 Apr;31(4):893–898. PubMed PMID: 26404846. [DOI] [PubMed] [Google Scholar]
- [13].Zhang J, Zhu X, Luo F, et al. Separation and purification of neohesperidin from the albedo of citrus reticulata cv. suavissima by combination of macroporous resin and high-speed counter-current chromatography. J Sep Sci. 2012 Jan;35(1):128–136. PubMed PMID: 22102373. [DOI] [PubMed] [Google Scholar]
- [14].Gong Y, Dong R, Gao X, et al. Neohesperidin prevents colorectal tumorigenesis by altering the gut microbiota. Pharmacol Res. 2019 Oct;148:104460. PubMed PMID: 31560944. [DOI] [PubMed] [Google Scholar]
- [15].Lee JH, Lee SH, Kim YS, et al. Protective effects of neohesperidin and poncirin isolated from the fruits of poncirus trifoliata on potential gastric disease. Phytother Res. 2009 Dec;23(12):1748–1753. PubMed PMID: 19367677. [DOI] [PubMed] [Google Scholar]
- [16].Du L, Jiang Z, Xu L, et al. Microfluidic reactor for lipase-catalyzed regioselective synthesis of neohesperidin ester derivatives and their antimicrobial activity research. Carbohydr Res. 2018 Jan 2;455:32–38. PubMed PMID: 29161612; eng. [DOI] [PubMed] [Google Scholar]
- [17].Tan Z, Cheng J, Liu Q, et al. Neohesperidin suppresses osteoclast differentiation, bone resorption and ovariectomised-induced osteoporosis in mice. Mol Cell Endocrinol. 2017 Jan 5;439:369–378. PubMed PMID: 27664516. [DOI] [PubMed] [Google Scholar]
- [18].Tsagakis I, Douka K, Birds I, et al. Long non-coding RNAs in development and disease: conservation to mechanisms. J Pathol. 2020 Apr;250(5):480–495. PubMed PMID: 32100288; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kopp F, JT M. Functional classification and experimental dissection of long noncoding RNAs. Cell. 2018 Jan 25;172(3): 393–407. PubMed PMID: 29373828; PubMed Central PMCID: PMCPMC5978744. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Uchida S, Dimmeler S. Long noncoding RNAs in cardiovascular diseases. Circ Res. 2015 Feb 13;116(4): 737–750. PubMed PMID: 25677520; eng. [DOI] [PubMed] [Google Scholar]
- [21].Zhou M, Zhao H, Wang X, et al. Analysis of long noncoding RNAs highlights region-specific altered expression patterns and diagnostic roles in alzheimer’s disease. Brief Bioinform. 2019 Mar 25;20(2):598–608. PubMed PMID: 29672663; eng. [DOI] [PubMed] [Google Scholar]
- [22].Huang Y. The novel regulatory role of lncRNA-miRNA-mRNA axis in cardiovascular diseases. J Cell Mol Med. 2018 Dec;22(12): 5768–5775. PubMed PMID: 30188595; PubMed Central PMCID: PMCPMC6237607. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Shi X, Sun M, Liu H, et al. Long non-coding RNAs: a new frontier in the study of human diseases. Cancer Lett. 2013 Oct 10;339(2):159–166. PubMed PMID: 23791884; eng. [DOI] [PubMed] [Google Scholar]
- [24].Cen X, Huang XQ, Sun WT, et al. Long noncoding RNAs: a new regulatory code in osteoarthritis. Am J Transl Res. 2017;9(11):4747–4755. PubMed PMID: 29218077; PubMed Central PMCID: PMCPMC5714763. eng. [PMC free article] [PubMed] [Google Scholar]
- [25].Donato L, Scimone C, Alibrandi S, et al. Transcriptome analyses of lncRNAs in A2E-stressed retinal epithelial cells unveil advanced links between metabolic impairments related to oxidative stress and retinitis pigmentosa. Antioxidants (Basel). 2020 Apr 15;9(4). DOI: 10.3390/antiox9040318. PubMed PMID: 32326576; PubMed Central PMCID: PMCPMC7222347. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Thin KZ, Tu JC, Raveendran S, et al. Long non-coding SNHG1 in cancer. Clin Chim Acta. 2019 Jul;494:38–47. PubMed PMID: 30849309. [DOI] [PubMed] [Google Scholar]
- [27].Jiang Y, Wu W, Jiao G, et al. LncRNA SNHG1 modulates p38 MAPK pathway through nedd4 and thus inhibits osteogenic differentiation of bone marrow mesenchymal stem cells. Life Sci. 2019 Jul 1;228:208–214. PubMed PMID: 31055087. [DOI] [PubMed] [Google Scholar]
- [28].Fan JJ, Cao LG, Wu T, et al. The dose-effect of icariin on the proliferation and osteogenic differentiation of human bone mesenchymal stem cells. Molecules. 2011 Dec 6;16(12):10123–10133. PubMed PMID: 22146373; PubMed Central PMCID: PMCPMC6264195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Fang B, Wang D, Zheng J, et al. Involvement of tumor necrosis factor alpha in steroid-associated osteonecrosis of the femoral head: friend or foe?. Stem Cell Res Ther. 2019 Jan 3;10(1):5. PubMed PMID: 30606261; PubMed Central PMCID: PMCPMC6318982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhai L, Sun N, Zhang B, et al. Effects of focused extracorporeal shock waves on bone marrow mesenchymal stem cells in patients with avascular necrosis of the femoral head. Ultrasound Med Biol. 2016 Mar;42(3):753–762. PubMed PMID: 26674675. [DOI] [PubMed] [Google Scholar]
- [31].Fang B, Li Y, Chen C, et al. Huo xue tong luo capsule ameliorates osteonecrosis of femoral head through inhibiting lncRNA-miat. J Ethnopharmacol. 2019 Jun 28;238:111862. PubMed PMID: 30970282. [DOI] [PubMed] [Google Scholar]
- [32].Ma XL, Liu ZP, Ma JX, et al. Dynamic expression of runx2, osterix and AJ18 in the femoral head of steroid-induced osteonecrosis in rats. Orthop Surg. 2010 Nov;2(4):278–284. PubMed PMID: 22009963; PubMed Central PMCID: PMCPMC6583573. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wang W, Liu L, Dang X, et al. The effect of core decompression on local expression of BMP-2, PPAR-gamma and bone regeneration in the steroid-induced femoral head osteonecrosis. BMC Musculoskelet Disord. 2012 Aug 9;13(1):142. PubMed PMID: 22876776; PubMed Central PMCID: PMCPMC3461435. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Nuttall ME, Gimble JM. Is there a therapeutic opportunity to either prevent or treat osteopenic disorders by inhibiting marrow adipogenesis?. Bone. 2000 Aug;27(2): 177–184. PubMed PMID: 10913909; eng. [DOI] [PubMed] [Google Scholar]
- [35].Zhang Q, Yang F, Chen Y, et al. Chinese herbal medicine formulas as adjuvant therapy for osteonecrosis of the femoral head: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2018 Sep;97(36):e12196. PubMed PMID: 30200126; PubMed Central PMCID: PMCPMC6133442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Benavente-Garcia O, Castillo J. Update on uses and properties of citrus flavonoids: new findings in anticancer, cardiovascular, and anti-inflammatory activity. J Agric Food Chem. 2008 Aug 13;56(15): 6185–6205. PubMed PMID: 18593176. [DOI] [PubMed] [Google Scholar]
- [37].Jiang T, Ge S, Shim YH, et al. Bone morphogenetic protein is required for fibroblast growth factor 2-dependent later-stage osteoblastic differentiation in cranial suture cells. Int J Clin Exp Pathol. 2015;8(3):2946–2954. PubMed PMID: 26045803; PubMed Central PMCID: PMCPMC4440112. eng. [PMC free article] [PubMed] [Google Scholar]
- [38].Luu HH, Song WX, Luo X, et al. Distinct roles of bone morphogenetic proteins in osteogenic differentiation of mesenchymal stem cells. J Orthop Res. 2007 May;25(5):665–677. PubMed PMID: 17290432. [DOI] [PubMed] [Google Scholar]
- [39].Martins CM, de Azevedo Queiroz IO, Ervolino E, et al. RUNX-2, OPN and OCN expression induced by grey and white mineral trioxide aggregate in normal and hypertensive rats. Int Endod J. 2018 Jun;51(6):641–648. PubMed PMID: 29143348. [DOI] [PubMed] [Google Scholar]
- [40].Bing W, Pang X, Qu Q, et al. Simvastatin improves the homing of BMSCs via the PI3K/AKT/miR-9 pathway. J Cell Mol Med. 2016 May;20(5):949–961. PubMed PMID: 26871266; PubMed Central PMCID: PMCPMC4831354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Li Y, Yang F, Gao M, et al. miR-149-3p regulates the switch between adipogenic and osteogenic differentiation of BMSCs by targeting FTO. Mol Ther Nucleic Acids. 2019 Sep 6;17:590–600. PubMed PMID: 31382190; PubMed Central PMCID: PMCPMC6690430. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wen T, Wang L, Sun XJ, et al. Sevoflurane preconditioning promotes activation of resident CSCs by transplanted BMSCs via miR-210 in a rat model for myocardial infarction. Oncotarget. 2017 Dec 29;8(70):114637–114647. PubMed PMID: 29383108; PubMed Central PMCID: PMCPMC5777720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Dang X, Lian L, Wu D, et al. The diagnostic value and pathogenetic role of lncRNA-ATB in patients with osteoarthritis. Cell Mol Biol Lett. 2018;23(1):55. PubMed PMID: 30505322; PubMed Central PMCID: PMCPMC6258155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Huang S, Zhu X, Xiao D, et al. LncRNA SNHG1 was down-regulated after menopause and participates in postmenopausal osteoporosis. Biosci Rep. 2019 Nov 29;39(11). DOI: 10.1042/bsr20190445. PubMed PMID: 31693735; PubMed Central PMCID: PMCPMC6851504. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Xiang S, Li Z, Weng X, et al. The role of lncRNA RP11-154D6 in steroid-induced osteonecrosis of the femoral head through BMSC regulation. J Cell Biochem. 2019 Oct;120(10):18435–18445. PubMed PMID: 31190361; eng. [DOI] [PubMed] [Google Scholar]
- [46].Fu D, Yang S, Lu J, et al. LncRNA NORAD promotes bone marrow stem cell differentiation and proliferation by targeting miR-26a-5p in steroid-induced osteonecrosis of the femoral head. Stem Cell Res Ther. 2021 Jan 7;12(1):18. PubMed PMID: 33413642; PubMed Central PMCID: PMCPMC7792292. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Yuan S, Zhang C, Zhu Y, et al. Neohesperidin ameliorates steroid-induced osteonecrosis of the femoral head by inhibiting the histone modification of lncRNA HOTAIR. Drug Des Devel Ther. 2020;14:5419–5430. PubMed PMID: 33324039; PubMed Central PMCID: PMCPMC7733036. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Scimone C, Alibrandi S, Scalinci SZ, et al. Expression of pro-angiogenic markers is enhanced by blue light in human RPE cells. Antioxidants (Basel). 2020 Nov 20;9(11). DOI: 10.3390/antiox9111154. PubMed PMID: 33233546; PubMed Central PMCID: PMCPMC7699675. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Donato L, Scimone C, Alibrandi S, et al. Possible A2E mutagenic effects on RPE mitochondrial DNA from innovative RNA-seq bioinformatics pipeline. Antioxidants (Basel). 2020 Nov 20;9(11). DOI: 10.3390/antiox9111158. PubMed PMID: 33233726; PubMed Central PMCID: PMCPMC7699917. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Cheng X, Yang YL, Li WH, et al. Cerebral ischemia-reperfusion aggravated cerebral infarction injury and possible differential genes identified by RNA-seq in rats. Brain Res Bull. 2020 Mar;156:33–42. PubMed PMID: 31877338; eng. [DOI] [PubMed] [Google Scholar]


