ABSTRACT
Plasma extracellular vesicles (EVs) have been reported to be a promising source of diagnostic and prognostic biomarkers in various cancers. However, further research in this area is needed due to the limitations of circulating extracellular vesicles detection methods. Using the Single Molecule array (SiMoa) technology, we developed two extracellular vesicle detection assays, CD9-CD63 and PD-L1-CD63, to determine circulating universal EVs and PD-L1 positive EVs, respectively. A total of 164 diffuse large B-cell lymphoma (DLBCL) patients were retrospectively included in this study. Compared with healthy volunteers (n = 25), elevated CD9-CD63 and PD-L1-CD63 signals were detected in the plasma of DLBCL patients (n = 164). High CD9-CD63 signals was associated with molecular subtype, extranodal site and treatment response in DLBCL. A high PD-L1-CD63 signal was also associated with certain clinical features, including extranodal site and treatment response. CD9-CD63 and PD-L1-CD63 signals were found to be important prognostic factors for both progression-free and overall survival. Furthermore, PD-L1-positive EVs were found in all patients, though PD-L1 protein expression was positive in only 35.4% (17/48) of tumor biopsies. No correlation was found between circulating PD-L1+ EVs and soluble PD-L1 (sPD-L1) levels. Our results show that plasma universal EV and PD-L1-positive EV levels are significantly elevated in DLBCL and might serve as biomarkers for predicting survival outcomes in DLBCL patients.
KEYWORDS: DLBCL, lymphoma, plasma, extracellular vesicles, PD-L1
Introduction
Extracellular vesicles (EVs) are 30–200-nm membrane-bound vesicles released by viable cells that mediate various pathologic processes involved in cancer development.1–3 Recent studies have found that thousands of coding and noncoding RNAs are enriched in lymphoma cell-derived EVs and that these RNAs may transfer genomic information between tumor cells and contribute to the pathogenesis of diffuse large B-cell lymphoma (DLBCL).4,5 Plasma EVs, as well as tumor cell-derived EVs, play an important role in tumor development and are promising biomarkers in cancer.6–8 Plasma exosomes have been reported to suppress lymphocyte functions and correlate with disease activity in head and neck cancer.6 However, as most studies have focused on extracellular vesicles isolated from the supernatants of cancer cells, little is known regarding the level of plasma extracellular vesicles in lymphoma patients.
Programmed death ligand 1 (PD-L1) was considered as an important immune checkpoint which could mediate immune escape in lymphoma.9,10 Our previous study showed that PD-L1 protein expression is significantly higher in non-germinal center B-cell-like (non-GCB) DLBCL than in GCB DLBCL and that high expression of PD-L1 protein is an adverse prognostic factor in DLBCL patients.10 Immune checkpoint inhibition, such as programmed death 1 (PD-1) inhibitors, is considered a promising therapeutic approach in B cell lymphoma.11 However, PD-1 inhibitors have limited efficacy in patients with relapsed/refractory DLBCL, with an objective response rate of less than 10%,12 indicating that the PD-L1/PD-1 pathway may not yet be fully understood. Recent research has demonstrated that PD-L1 is abundant on tumor-derived EVs and suppresses the cytotoxic function of CD8 + T cells.13,14 Several studies have also reported that circulating exosomal PD-L1 (exoPD-L1) is an important prognostic factor in various cancers, including head and neck cancer and melanoma.15–17 Exosome-based liquid biopsy is a promising noninvasive procedure because exosomes were relatively stable in body fluids, with higher sensitivity and specificity than conventional tumor biopsy.17 Nevertheless, the clinical significance of plasma exoPD-L1 in DLBCL remains unclear.
Single Molecule array (SiMoa) technology is a digital immunoassay used for the detection of proteins in a fully automated instrument. Our previous studies showed that SiMoa could accurately measure EVs over free proteins in the plasma with high sensitivity.7,15 Several tetraspanins, including CD9, CD63 and CD81 are enriched on EVs and are frequently used as EV markers,18 and recent studies have shown that CD63 is the most specific marker for exosomes.19,20 EVs bearing either CD81 or CD9 but not CD63 do not form in endosomes, whereas those bearing CD63 together with at least one other tetraspanin may correspond to endosome-derived exosomes.20 Therefore, we developed three detection assays CD9-CD63 (CD9/CD63 double-positive EVs), Epcam-CD63 (Epcam/CD63 double-positive EVs) and PD-L1-CD63 (PD-L1/CD63 double-positive EVs), to represent universal EVs, tumor-derived EVs and PD-L1+ EVs, respectively. The results of our studies showed that these three markers are independent prognostic factors for cancer patients.7,15
In this study, CD9-CD63 and PD-L1-CD63 were measured to reflect universal EVs and PD-L1+ EVs in the plasma of DLBCL patients. Furthermore, the clinicopathological relevance and diagnostic and prognostic utility of plasma universal and PD-L1+ EVs were analyzed.
Materials and methods
Patients
Data for 164 newly diagnosed DLBCL patients between 2013 and 2017 were obtained from the pathology database of Fudan University Shanghai Cancer Center. The inclusion criteria in this study included the following: pathologic diagnosis of DLBCL according to World Health Organization (WHO) classification,21 availability of plasma samples and complete clinical data including age, sex, tumor stage, B symptoms, serum lactate dehydrogenase (LDH), imaging examinations (CT or PET/CT) findings, chemotherapy regimens, treatment response and follow-up.
Peripheral blood samples were collected from DLBCL patients before treatment and centrifuged at 1,000 × g for 15 minutes. The plasma samples were stored in 2 mL aliquots at −80°C. This study was performed in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Fudan University Shanghai Cancer Center, and all the participants provided written informed consent.
Isolation of extracellular vesicles and peripheral blood mononuclear cell (PBMC) isolation from blood
Plasma samples (2 mL) were thawed on ice, and EVs were isolated using an exoRNeasy Plasma Midi Kit (Qiagen, #77044). PBMCs were extracted from the whole blood of DLBCL patients using density gradient medium (LymphoprepTM, STEMCELL Technologies, #07851).
EV characterization
The morphology of the isolated plasma EVs was examined by transmission electron microscopy (TEM) as described previously.22 The size of EVs was assessed using a Flow NanoAnalyzer (NanoFCM Inc., Xiamen, China). Exosomal markers such as TSG101, ALIX and CD63 were detected by western blot.
Detection of plasma universal EVs and PD-L1+ EVs by SiMoa
Universal EVs and PD-L1+ EVs in the plasma were detected by SiMoa with CD9-CD63 and PD-L1-CD63 assays as previously described.7,15 In brief, plasma samples were incubated for 45 min with microscopic beads that capture EVs by anti-PD-L1 or anti-CD9 antibodies. The beads were then incubated with biotin-labeled anti-CD63 monoclonal antibodies, which could bind to streptavidin-β-galactosidase (SBG). Resorufin β-D-galactopyranoside (RGP) was added before loading onto the disc array. The level of EVs is reflected by SiMoa signal, which is expressed in AEB as previously described.23 In brief, AEB is determined by the amount of wells containing both a bead and fluorescent signal (“on” well) relative to the total number of wells containing beads, using the digital methods based on the concentration of captured analyte.
Detection of lymphoma markers on plasma EVs
Lymphoma markers on plasma EVs were detected by flow cytometry, as described by Tian et al.24 In brief, plasma samples (1 mL) were processed via serial centrifugation using an Optima XE ultracentrifuge (Beckman Coulter, Milan, Italy) at 2000 × g for 15 min, 10,000 × g for 30 min, and 100,000 × g for 1 h. The vesicle pellet was washed with phosphate-buffered saline (PBS, Gibco, Life Technologies, USA) and centrifuged at 100,000 × g for 1 h. The pellet was then resuspended in PBS (50 μL), and FITC-conjugated mouse antihuman CD19 or CD20 antibody (10 μL) was added. The mixture was incubated at 37°C for 30 min and washed twice with PBS by ultracentrifugation at 100,000 × g for 20 min at 4°C. The pellet was resuspended in 100 μL of PBS for HSFCM analysis.
Detection of plasma soluble PD-L1 (sPD-L1)
In this study, sPD-L1 in plasma was detected using a commercial enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, # DB7H10) following the manufacturer’s instructions. Samples and standards were measured in duplicate.
Immunohistochemistry
Immunohistochemistry (IHC) was performed for 48 DLBCL patients with available formalin-fixed paraffin-embedded tissue samples. A primary antibody against PD-L1 (clone number 22 C3, Dako) was used with a Benchmark automated apparatus (Ventana Medical Systems). PD-L1 positivity was defined as complete circumferential or partial cell membrane staining of viable tumor cells. PD-L1 immunostaining of DLBCL tissue samples was evaluated by two pathologists (XC Wan and BH Yu).
Tumor response evaluation
The treatment response was assessed via computed tomography (CT)/magnetic resonance imaging (MRI) or positron emission tomography-computed tomography (PET/CT). Response to chemotherapy was evaluated according to response criteria for lymphoma,25 including complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD).
Statistical analysis
Overall survival (OS) was defined as the time from the date of diagnosis to the date of death or last follow-up. Progression-free survival (PFS) was defined as the time of diagnosis to disease progression, death or the date of last follow-up. The Kaplan-Meier method and log-rank test were used for survival analysis. Significant prognostic factors (P < .05) in univariate analysis were further analyzed by multivariate Cox regression analysis. The prognostic cutoff values for the CD9-CD63 and PD-L1-CD63 assays were determined by X-tile, and the optimal threshold for determining (PFS) was selected. The significance of group differences was assessed by unpaired two-tailed Student’s t tests. Correlations between variables were identified with Spearman correlation analysis. Statistical analysis was carried out with GraphPad Prism (version 5, GraphPad Software), and a P value less than 0.05 was considered statistically significant.
Results
Clinicopathologic characteristics of DLBCL patients
The clinicopathologic characteristics of the DLBCL patients in this study are summarized in Table 1. No differences were found in the ages and sexes of the healthy controls and DLBCL patients (Supplementary Table 1). The median age of healthy controls and DLBCL patients were 45 (range 21–65) and 51 (range 19–78), respectively. Over half of the patients in our cohort were male (n = 95, 57.9%) and had lymph node involvement (n = 85, 51.8%), early-stage disease (n = 117, 71.3%), a normal LDH level (n = 110, 69.2%) and a low International Prognostic Index (IPI) score of less than two (n = 82, 51.6%). B symptoms occurred in only a small proportion of patients (n = 39, 23.8%). The patients were classified based on immunohistochemistry results into germinal center B cell (GCB) (n = 54, 39.1%) and non-GCB (n = 84, 60.9%) subgroups. All patients in this cohort received 6–8 cycles of R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) chemotherapy. The median Ki-67 value was 80% (range, 30%-100%).
Table 1.
Clinicopathologic parameters of the DLBCL patients in this stud
| Variable | N (%) |
|---|---|
| Gender | |
| Male | 95(57.9) |
| Female | 69(42.1) |
| Age (years) | |
| > 60 | 39(23.8) |
| ≤ 60 | 125(76.2) |
| Primary sites | |
| Nodal | 85(51.8) |
| Extranodal | 79(48.2) |
| Stage | |
| I–II | 117(71.3) |
| III–IV | 47(28.7) |
| Mass | |
| Yes | 30(18.3) |
| No | 134(81.7) |
| B symptoms | |
| Yes | 39(23.8) |
| No | 125(76.2) |
| LDH level | |
| Elevated | 49(30.8) |
| Normal | 110(69.2) |
| Type | |
| GCB | 54(39.1) |
| Non-GCB | 84(60.9) |
| IPI | |
| 0–1 | 82(51.6) |
| ≥2 | 77(48.4) |
| Treatment response | |
| CR | 106(64.6) |
| Non-CR | 58(35.4) |
LDH, lactate dehydrogenase; GCB, Germinal center B-cell-like lymphoma;
IPI, International Prognostic Index; CR, Complete remission.
Treatment outcome of DLBCL patients
Of the 164 patients, 106 (64.6%) achieved CR after chemotherapy; 58 patients (35.6%) did not achieve CR, including 25 with a PR, 2 with SD and 11 with PD. The median follow-up time was 60 months (10–92 months), with 5-year progression-free survival (PFS) and overall survival (OS) rates of 73.1% and 78.4% for the entire cohort, respectively (Figure 1a-b).
Figure 1.
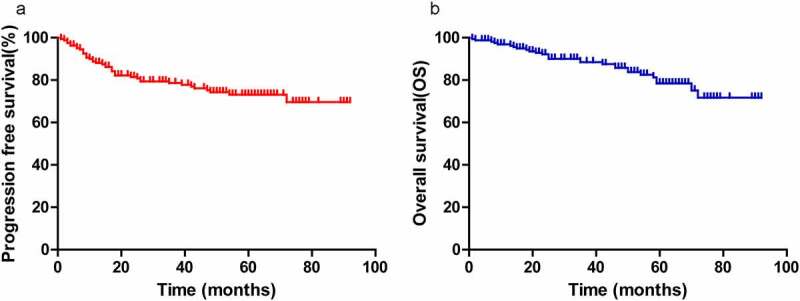
A, B, Progression-free survival (PFS) and overall survival (OS) for all patients (a, b)
Plasma universal EV level and its correlation with clinicopathological features in DLBCL
As shown in supplementary Figure 1, universal EVs and PD-L1+ EVs in plasma samples from 164 DLBCL patients and 25 healthy volunteers were detected by SiMoa with the CD9-CD63 assay and PD-L1-CD63 assay, respectively (A). The CD9-CD63 signal in DLBCL patients was significantly higher than that in healthy volunteers (P < .001) (Figure 2a). Receiver operating characteristic curve (ROC) analysis showed that the CD9-CD63 assay accurately differentiated DLBCL patients (n = 99) from healthy controls (area under the ROC curve (AUC) = 0.99) (Figure 2b). The association of CD9-CD63 signals with the clinicopathologic features of DLBCL patients was also investigated. As shown in Figure 2, a significant elevation in CD9-CD63 signal was observed in GCB-type DLBCL patients versus non-GCB patients (Figure 2c, p = .042). Regarding the association of CD9-CD63 signals and disease burden (including tumor mass>5 cm and number of extranodal involved sites), patients with ≥2 extranodal sites had higher levels of EVs in plasma than patients with <2 extranodal sites (Figure 2d, p = .0001). However, no significant difference in CD9-CD63 signal was observed between patients with and without tumor masses (Figure 2e, p = .722). In addition, patients who did not achieve CR had a higher EV level than those who did achieve CR (figure 2f, p = .019).
Figure 2.
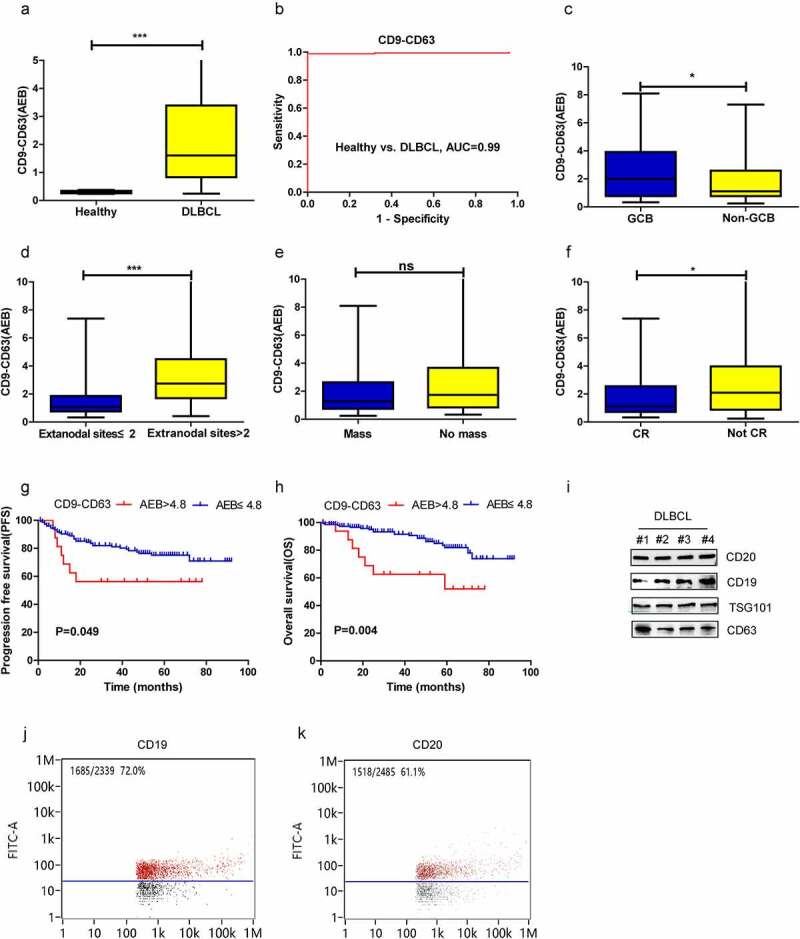
Diagnostic and prognostic value of CD9-CD63 signal detected by SiMoa in patients with DLBCL. (a) The CD9-CD63 signal in DLBCL and healthy subjects measured by SiMoa. (b) ROC analysis to evaluate the diagnostic power to differentiate DLBCL cases (n = 164) from the healthy controls (n = 25) with CD9-CD63 assay. (c-f) The association of CD9-CD63 signal with molecular subtype, the number of extranodal sites, tumor mass and CR status after treatment. (g, h) The progression-free survival (PFS) and overall survival (OS) in the high and low CD9-CD63 signal group. (i) CD19 and CD20 expression on the plasma EVs detected by western blots. (j-k) CD19 and CD20 expression on the plasma EVs observed by flow cytometry
Next, the prognostic significance of the plasma CD9-CD63 signal was explored. The prognostic cutoff value for the CD9-CD63 assays was determined by X-tile, and the patients in our study were classified into high CD9-CD63 signal group (AEB > 4.8, n = 17) and low CD9-CD63 signal group (AEB ≤ 4.8, n = 147) based on the optimal cutoff point (Supplementary figure 1B). Patients with a high CD9-CD63 signal had worse 5-year PFS (56.3% vs. 75.1%, P = .049) and OS (52.1% vs. 81.8%, P = .004) than those with a low CD9-CD63 signal (Figure 2, G-H). Furthermore, multivariate analysis revealed a high CD9-CD63 signal to be an independent prognostic factor affecting OS in DLBCL (Table 2).
Table 2.
Univariate and multivariate analysis of prognostic factors for survivals (by Cox regression)
| Clinical factor | Progression-free survival |
Overall survival |
||||||
|---|---|---|---|---|---|---|---|---|
| Univariate |
Multivariate |
Univariate |
Multivariate |
|||||
| P | HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | |
| CD9-CD63 | 0.049 | 0.450 (0.199–1.019) | 0.007 | 0.305 (0.130–0.720) | 0.000 | 0.130 (0.050–0.340) | ||
| PD-L1-CD63 | 0.007 | 0.341 (0.156–0.743) | 0.026 | 0.362 (0.152–0.680) | 0.003 | 0.277 (0.117–0.654) | ||
| Age(<60 vs >60) | 0.055 | 0.529 (0.276–1.013) | 0.109 | 0.521 (0.235–1.156) | ||||
| Gender (M vs F) | 0.562 | 1.201 (0.646–2.235) | 0.510 | 1.283 (0.661–2.693) | ||||
| B symptom (yes vs no) | 0.011 | 0.435 (0.229–0.827) | 0.047 | 0.505 (0.258–0.990) | 0.003 | 0.325 (0.154–0.689) | 0.017 | 0.385(0.175–0.845) |
| Serum LDH (<240 vs >240) | 0.832 | 0.927 (0.459–1.870) | 0.277 | 0.638 (0.284–1.435) | ||||
| Ki67 ≥ 80% | 0.493 | 1.243 (0.668–2.313) | 0.797 | 1.103 (0.523–2.324) | ||||
| Primary tumor site (nodal vs extranodal) | 0.869 | 1.053 (0.566–1.959) | 0.991 | 1.004 (0.478–2.110) | ||||
| Stage (I–II vs III–IV) | 0.015 | 2.189 (1.168–4.103) | 0.160 | 1.746 (0.804–3.796) | ||||
| Mass (yes vs no) | 0.973 | 1.014 (0.448–2.293) | 0.404 | 1.666 (0.502–5.522) | ||||
| IPI score (≥ 2 vs <2) | 0.020 | 0.461 (0.240–0.885) | 0.063 | 0.475 (0.217–1.041) | ||||
| Type (IHC) (GCB vs non-GCB) |
0.661 | 1.172 (0.576–2.384) | 0.517 | 1.352 (0.544–3.360) | ||||
| CR after treatment (yes vs no) | 0.000 | 7.537 (3.745–15.172) | 0.000 | 8.667 (1.72412.150) | 0.000 | 5.922 (2.667–13.147) | 0.000 | 7.495 (3.117–18.023) |
Phenotype characterization of EVs in the plasma
According to the above results, abundant EVs are present in the plasma of DLBCL patients, and surface markers related to B lymphoid origin (CD19 and CD20) were further evaluated by flow cytometry and western blotting. As depicted in Figure 2, CD19 and CD20 were detected on plasma EVs of four DLBCL patients (Figure 2i). High amounts of CD19+ and CD20+ EVs were also observed by flow cytometry, accounting for approximately 72.0% and 61.1% of all EVs, respectively (Figure 2j-k).
Detection of universal EVs and PD-L1 on EVs
Universal EVs in plasma were detected by the CD9-CD63 assay, as described previously,7 and the PD-L1 level on plasma EVs was assessed by SiMoa and EV kit-based extraction. EVs were isolated from the plasma of two DLBCL patients and two healthy volunteers using an exoRNeasy Plasma Midi Kit. As illustrated in Figure 3, based on TEM, EVs are oval membrane-bound vesicles with a diameter of 50–150 nm (Figure 3a). Flow NanoAnalyzer analysis revealed that EV particles are 50–200 nm in size, with a median diameter of 70 nm (Figure 3b). Western blot analysis confirmed enrichment of some biomarkers associated with extracellular vesicles, such as ALIX, TSG101 and CD63, in the EVs isolated (Figure 3c). As shown in Figure 3, the PD-L1-CD63 signal in DLBCL patients was significantly higher than that in healthy subjects, consistent with the western blot results (Figure 3d-e).
Figure 3.
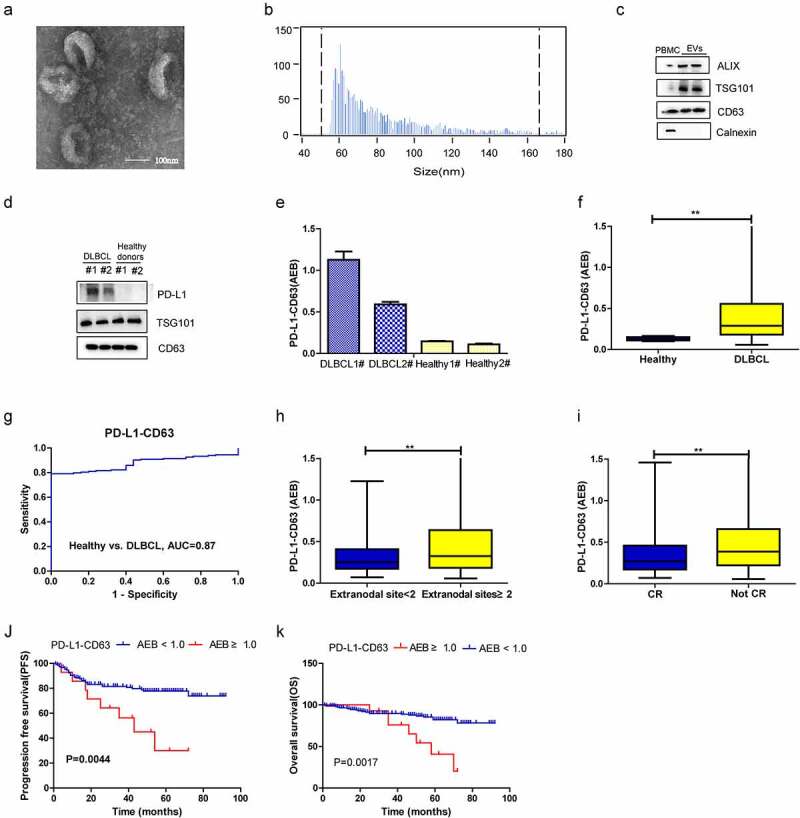
Diagnostic and prognostic value of PD-L1-CD63 signal measured by SiMoa in patients with DLBCL. (a-c). Characterization of isolated exosomes by transmission electron microscopy (TEM) (a), size distribution (b) and western blots (c). (d-e) Qualification of PD-L1+ EVs in DLBCL patients and healthy subjects by western blot and SiMoa. (f) The PD-L1-CD63 signal in DLBCL and healthy subjects measured by SiMoa. (g) ROC analysis to evaluate the diagnostic power to differentiate DLBCL cases (n = 164) from the healthy controls with PD-L1-CD63 assay. (h, i) The association of PD-L1-CD63 signal with the number of extranodal sites, tumor mass and CR status after treatment. (j, k) The progression-free survival (PFS) and overall survival (OS) in high and low PD-L1-CD63 signal group
Plasma PD-L1-positive EV level and its correlation with clinicopathological features in DLBCL
In addition to the CD9-CD63 assay, the PD-L1-CD63 assay was applied to the above patients and healthy volunteers, and we found that the PD-L1-CD63 assay signal was significantly higher in the former (figure 3f, p < .001). ROC analysis demonstrated that the PD-L1-CD63 assay was able to differentiate DLBCL patients (n = 164) from healthy controls (n = 25), though with a relatively lower sensitivity and specificity than the CD9-CD63 assay (Figure 3g, AUC = 0.87). Patients with ≥2 extranodal sites had a significantly higher PD-L1-CD63 signal than those with <2 extranodal sites (Figure 3h, p = .01), and the PD-L1-CD63 signal was associated with treatment response, whereby a lower signal was observed for patients who achieved CR (Figure 3i, p = .002).
In assessing the prognostic significance of the plasma PD-L1-CD63 signal, the patients in our study were subdivided into high PD-L1-CD63 signal group (AEB ≥ 1.0, n = 15) and low PD-L1-CD63 signal group (AEB < 1.0, n = 149) based on the optimal cutoff point determined by X-tile (Supplementary figure 1 C).the high PD-L1-CD63 signal group had worse 5-year PFS (30.0% vs. 77.8%, P = .004) and 5-year OS (40.7% vs. 82.2%, P = .002) than the low CD9-CD63 signal group (Figure 3, J-K). Moreover, high PD-L1-CD63 signal was an independent prognostic factor for PFS of DLBCL patients by multivariate Cox regression analysis (Table 2).
Expression of PD-L1 in circulating EVs or in plasma versus DLBCL tissues
Levels of plasma PD-L1+ EVs were compared with PD-L1 protein expression in tumor tissues; we also compared levels of plasma soluble PD-L1 with PD-L1+ EVs (Figure 4). Plasma PD-L1+ EVs were detected in all patients in our cohort (100%) (Figure 4b); however, only 35.4% (17/48) of tumor biopsies were PD-L1+ (Figure 4, A-B). No significant difference in PD-L1-CD63 signal was found between PD-L1 protein-positive and PD-L1 protein-negative groups (Figure 4c), and no significant correlation was observed between plasma sPD-L1 and PD-L1+ EVs (Spearman correlation at P = .62, r = 0.01; Figure 4d).
Figure 4.
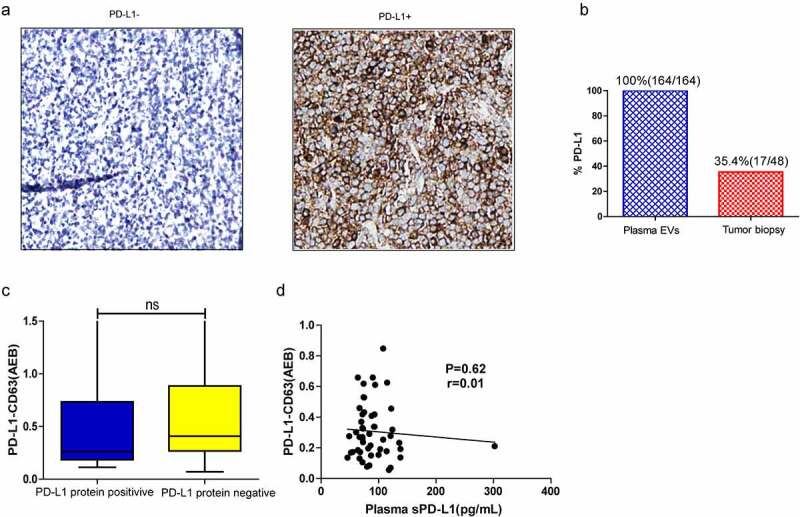
(a) Representative IHC image of PD-L1 negative (PD-L1−) or positive (PD-L1+) tumors (22C3 antibody). (b) Percentage of patients positive for PD-L1 in circulating EVs versus tumor tissues. (c) The PD-L1-CD63 signal between PD-L1 protein positive and negative group. (d) Correlation between the PD-L1-CD63 signaling and sPD-L1 level in the plasma; Spearman’s correlation at P = .19, r = 0.03
Discussion
Recently, EVs have emerged as a novel communication method between cells, and growing evidence has shown that they play an important role in various cancers, including colorectal cancer, melanoma and lymphoma.1,3,13 Tumor-derived EVs can mediate immune escape and drug resistance and promote tumor development.1 Lymphoma-derived EVs also act in multiple pathologic processes related to lymphoma pathogenesis, drug resistance and lymphoma spread.1 However, research to date has mainly focused on lymphoma cell-derived EVs, and the role of EVs in the blood remains largely unknown in DLBCL.
EVs are reported to be abundant in the plasma of cancer patients and can serve as an independent prognostic factor.6,7 Plasma EVs in head and neck cancer patients suppress the function of lymphocytes and are associated with disease activity, indicating that plasma EVs in cancer patients may play a key role in tumor development.6 Nonetheless, the sample sizes of previous studies were relatively small, as the purification of EVs is time-consuming and tedious. Our previous studies showed that the level of plasma EVs is markedly higher in colorectal cancer patients than in healthy volunteers and that the plasma EV level is an independent prognostic factor for colorectal cancer.7 In the present study, we demonstrate that a higher CD9-CD63 signal correlates with some adverse clinical features and poor prognosis in DLBCL, suggesting that abundant EVs present in plasma play a crucial role in lymphoma progression and therapeutic response. Elevated levels of exosomes in DLBCL patients compared with healthy volunteers suggest that plasma exosomes are involved in lymphoma development and might serve as diagnostic biomarkers for differentiating DLBCL patients from the healthy population.
Previous studies have demonstrated that malignancy-related marker antigens are expressed on cancer cell derived exosomes and circulating exosomes in hematological neoplastic disorders.26,27 Consistently, high amounts of CD19+ and CD20+ EVs were detected in the plasma of DLBCL in our study, indicating that most EVs are released from cancer cells in DLBCL. However, CD19+ EVs were not specific markers for DLBCL patients. A recent study showed that abundant CD19+ EVs could be produced by tumor B cells and the circulating CD19+ EVs in gastric and colon cancer patients were significantly higher than healthy controls and lower serum CD19+ EVs was a favorable factor.28 Antibody-based anticancer therapy is considered a promising treatment for hematological malignancies. Aung T et al reported that CD20 on the B-cell lymphoma cell-derived exosomes could bind therapeutic anti-CD20 antibodies, consume complement and protect lymphoma cells from antibody attack.29 Exosomal CD19 and CD20 might intercept immunotherapy in DLBCL and it may be an advantage to characterize the expression of B-cell surface antigens on the plasma exosomes.
The PD-1/PD-L1 pathway is an important tumor escape mechanism in lymphoma.9,30 We previously reported that PD-L1 mRNA and PD-L1 protein levels are significantly higher in DLBCL patient samples than in reactive lymphoid hyperplasia (RLH) samples and that a higher level of PD-L1 protein is associated with poor prognosis.30 Recent studies found that PD-L1 is present in the EV membrane and can mediate immune escape in cancer and promote tumor development.13,14 Circulating exosomal PD-L1 was demonstrated to be an important prognostic factor of cancer in subsequent studies.15,16 In our study, a high PD-L1-CD63 signal correlated with some adverse clinical parameters, including extranodal sites and treatment response. In addition, a high PD-L1-CD63 signal was associated with poor prognosis. The above results indicated that plasma PD-L1+ EVs may play a similar role as PD-L1 expressed on tumor cells and mediate immune escape to facilitate cancer development.
PD-L1 is reported to be positive in only approximately 20% of DLBCL patients, and high PD-L1 protein expression is associated with adverse prognosis.30 In our study, PDL-1+ EVs were detected in all DLBCL patients in the cohort; however, only 20% of tumor biopsies from patients were positive for PD-L1 expression. The abundance of circulating PD-L-1+ EVs may be explained by the fact that a large amount of PD-L-1+ EVs can be released by a single lymphoma cell and PD-L-1+ EVs originate from different cells, including macrophages and dendritic cells.31–33 As circulating exoPD-L1 levels are a predictor for anti-PD-1 therapy in cancer patients,13 it will be an advantage to qualify exoPD-L1 in the blood when PD-1 inhibitor was applied.
The correlation between PD-L1+ EVs and sPD-L1 levels in blood has been investigated in two studies,15,16 and consistent with the results, no correlation was observed between these two variables in DLBCL patients, which is possibly due to the different stabilities of exoPD-L1 and sPD-L1, and different cell sources, production and release between sPD-L1 and PD-L1+ exosomes.
In conclusion, the results of our study suggest that EVs and PD-L1+ EVs are abundant in the plasma of DLBCL patients and that these two markers are associated with some clinicopathologic parameters and can serve as diagnostic, predictive and prognostic biomarkers. Nevertheless, the potential roles of plasma EVs and PD-L1+ EVs in patients receiving immune checkpoint inhibition therapy need to be validated in the future.
Supplementary Material
Funding Statement
This study was supported by the National Natural Science Foundation of China (No. 81470353, 81870155, 81700195), Innovation Group Project of Shanghai Municipal Health Comission (2019CXJQ03), Shanghai Science and technology development fund (Shanghai Science and Technology Development Foundation 19MC1911000), Shanghai Municipal Key Clinical Specialty (shslczdzk01301), andInnovation Program of Shanghai Science and technology committee (20Z11900300), Clinical Research Plan of Shanghai Hospital Development Center (SHDC2020CR3046B).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Li J, Tian T, Zhou X.. The role of exosomal shuttle RNA (esRNA) in lymphoma. Crit Rev Oncol Hematol. 2019;137:27–9. doi: 10.1016/j.critrevonc.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 2.Feng Y, Zhong M, Zeng S, Wang L, Liu P, Xiao X, Liu Y. Exosome-derived miRNAs as predictive biomarkers for diffuse large B-cell lymphoma chemotherapy resistance. Epigenomics. 2019;11(1):35-51. doi: 10.2217/epi-2018-0123. [DOI] [PubMed] [Google Scholar]
- 3.He XF, Zhong XY, Hu ZJ, Zhao S, Wei P, Li D. An insight into small extracellular vesicles: their roles in colorectal cancer progression and potential clinical applications. Clin Transl Med. 2020;10(8). ARTN e249. doi: 10.1002/ctm2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rutherford SC, Fachel AA, Li S, Sawh S, Muley A, Ishii J, Saxena A, Dominguez PM, Caldas Lopes E, Agirre X, et al. Extracellular vesicles in DLBCL provide abundant clues to aberrant transcriptional programming and genomic alterations. Blood. 2018;132(7):e13–e23. doi: 10.1182/blood-2017-12-821843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Z, You L, Wang L, Huang X, Liu H, Wei JY, Zhu L, Qian W. Dual effect of DLBCL-derived EXOs in lymphoma to improve DC vaccine efficacy in vitro while favor tumorgenesis in vivo. J Exp Clin Cancer Res. 2018;37(1):190. doi: 10.1186/s13046-018-0863-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ludwig S, Floros T, Theodoraki M-N, Hong C-S, Jackson EK, Lang S, Whiteside TL. Suppression of lymphocyte functions by plasma exosomes correlates with disease activity in patients with head and neck cancer. Clin Cancer Res. 2017;23(16):4843–4854. doi: 10.1158/1078-0432.CCR-16-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei P, Wu F, Kang B, Sun X, Heskia F, Pachot A, Liang J, Li D. Plasma extracellular vesicles detected by single molecule array technology as a liquid biopsy for colorectal cancer. J Extracell Vesicles. 2020;9(1):1809765. doi: 10.1080/20013078.2020.1809765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu W, Hurley J, Roberts D, Chakrabortty SK, Enderle D, Noerholm M, Breakefield XO, Skog JK. Exosome-based liquid biopsies in cancer: opportunities and challenges. Ann Oncol. 2021;32(4):466–477. doi: 10.1016/j.annonc.2021.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kataoka K, Miyoshi H, Sakata S, Dobashi A, Couronné L, Kogure Y, Sato Y, Nishida K, Gion Y, Shiraishi Y, et al. Frequent structural variations involving programmed death ligands in Epstein-Barr virus-associated lymphomas. Leukemia. 2019;33(7):1687–1699. doi: 10.1038/s41375-019-0380-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun CB, Jia YJ, Wang WG, Bi R, Wu L, Bai Q, Zhou X. Integrative analysis of PD-L1 DNA status, mRNA status and protein status, and their clinicopathological correlation, in diffuse large B-cell lymphoma. Histopathology. 2019;74(4):618–628. doi: 10.1111/his.13765. [DOI] [PubMed] [Google Scholar]
- 11.Xu-Monette ZY, Zhou J, Young KH. PD-1 expression and clinical PD-1 blockade in B-cell lymphomas. Blood. 2018;131(1):68–83. doi: 10.1182/blood-2017-07-740993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ansell SM, Minnema MC, Johnson P, Timmerman JM, Armand P, Shipp MA, Rodig SJ, Ligon AH, Roemer MGM, Reddy N, et al. Nivolumab for relapsed/refractory diffuse large B-cell lymphoma in patients ineligible for or having failed autologous transplantation: a single-arm, phase II study. J Clin Oncol. 2019;37(6):481–489. doi: 10.1200/JCO.18.00766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, Yu Z, Yang J, Wang B, Sun H, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560(7718):382–386. doi: 10.1038/s41586-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poggio M, Hu T, Pai -C-C, Chu B, Belair CD, Chang A, Montabana E, Lang UE, Fu Q, Fong L, et al. Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell. 2019;177(2):414–427 e13. doi: 10.1016/j.cell.2019.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li JW, Wei P, Guo Y, Shi D, Yu BH, Su YF, Li XQ, Zhou XY. Clinical significance of circulating exosomal PD-L1 and soluble PD-L1 in extranodal NK/T-cell lymphoma, nasal-type. Am J Cancer Res. 2020;10(12):4498–4512. [PMC free article] [PubMed] [Google Scholar]
- 16.Theodoraki MN, Yerneni SS, Hoffmann TK, Gooding WE, Whiteside TL. Clinical significance of PD-L1+ exosomes in plasma of head and neck cancer patients. Clin Cancer Res. 2018;24(4):896–905. doi: 10.1158/1078-0432.CCR-17-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cordonnier M, Nardin C, Chanteloup G, Derangere V, Algros M-P, Arnould L, Garrido C, Aubin F, Gobbo J. Tracking the evolution of circulating exosomal-PD-L1 to monitor melanoma patients. J Extracell Vesicles. 2020;9(1):1710899. Artn 1710899. doi: 10.1080/20013078.2019.1710899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andreu Z, Yanez-Mo M. Tetraspanins in extracellular vesicle formation and function. Front Immunol. 2014;5:442. doi: 10.3389/fimmu.2014.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mathieu M, Nevo N, Jouve M, Valenzuela JI, Maurin M, Verweij FJ, Palmulli R, Lankar D, Dingli F, Loew D, et al. Specificities of exosome versus small ectosome secretion revealed by live intracellular tracking of CD63 and CD9. Nat Commun. 2021;12(1):4389. doi: 10.1038/s41467-021-24384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, Dingli F, Loew D, Tkach M, Théry C, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A. 2016;113(8):E968–77. doi: 10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011;117(19):5019–5032. doi: 10.1182/blood-2011-01-293050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;Chapter 3:Unit 3 22. doi: 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- 23.Rissin DM, Fournier DR, Piech T, Kan CW, Campbell TG, Song L, Chang L, Rivnak AJ, Patel PP, Provuncher GK, et al. Simultaneous detection of single molecules and singulated ensembles of molecules enables immunoassays with broad dynamic range. Anal Chem. 2011;83(6):2279–2285. doi: 10.1021/ac103161b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tian Y, Ma L, Gong M, Su G, Zhu S, Zhang W, Wang S, Li Z, Chen C, Li L, et al. Protein profiling and sizing of extracellular vesicles from colorectal cancer patients via flow cytometry. ACS Nano. 2018;12(1):671–680. doi: 10.1021/acsnano.7b07782. [DOI] [PubMed] [Google Scholar]
- 25.Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca E, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 26.Oksvold MP, Kullmann A, Forfang L, Kierulf B, Li M, Brech A, Vlassov AV, Smeland EB, Neurauter A, Pedersen KW, et al. Expression of B-cell surface antigens in subpopulations of exosomes released from B-cell lymphoma cells. Clin Ther. 2014;36(6):847–862 e1. doi: 10.1016/j.clinthera.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 27.Caivano A, Laurenzana I, De Luca L, La Rocca F, Simeon V, Trino S, D’Auria F, Traficante A, Maietti M, Izzo T, et al. High serum levels of extracellular vesicles expressing malignancy-related markers are released in patients with various types of hematological neoplastic disorders. Tumour Biol. 2015;36(12):9739–9752. doi: 10.1007/s13277-015-3741-3. [DOI] [PubMed] [Google Scholar]
- 28.Zhang F, Li R, Yang Y, Shi C, Shen Y, Lu C, Chen Y, Zhou W, Lin A, Yu L, et al. Specific decrease in B-cell-derived extracellular vesicles enhances post-chemotherapeutic CD8+ T cell responses. Immunity. 2019;50(3):738–750 e7. doi: 10.1016/j.immuni.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 29.Aung T, Chapuy B, Vogel D, Wenzel D, Oppermann M, Lahmann M, Weinhage T, Menck K, Hupfeld T, Koch R, et al. Exosomal evasion of humoral immunotherapy in aggressive B-cell lymphoma modulated by ATP-binding cassette transporter A3. Proc Natl Acad Sci U S A. 2011;108(37):15336–15341. doi: 10.1073/pnas.1102855108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun C, Jia Y, Wang W, Bi R, Wu L, Bai Q, Zhou X. Integrative analysis of PD-L1 DNA status, mRNA status and protein status, and their clinicopathological correlation, in diffuse large B-cell lymphoma. Histopathology. 2019;74(4):618–628. doi: 10.1111/his.13765. [DOI] [PubMed] [Google Scholar]
- 31.Lu C, Redd PS, Lee JR, Savage N, Liu K. The expression profiles and regulation of PD-L1 in tumor-induced myeloid-derived suppressor cells. Oncoimmunology. 2016;5(12):e1247135. doi: 10.1080/2162402X.2016.1247135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sabari JK, Leonardi GC, Shu CA, Umeton R, Montecalvo J, Ni A, Chen R, Dienstag J, Mrad C, Bergagnini I, et al. PD-L1 expression, tumor mutational burden, and response to immunotherapy in patients with MET exon 14 altered lung cancers. Ann Oncol. 2018;29(10):2085–2091. doi: 10.1093/annonc/mdy334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alsaab HO, Sau S, Alzhrani R, Tatiparti K, Bhise K, Kashaw SK, Iyer AK. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol. 2017;8:561. doi: 10.3389/fphar.2017.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


