ABSTRACT
We intended to investigate the underlying mechanism of action of long noncoding RNA (lncRNA) HOX transcript antisense RNA (HOTAIR) in colorectal cancer (CRC) progression, especially in tumor cell stemness. For that purpose, different assays were performed such as real-time PCR and western blotting to determine the expression of target genes. Cell stemness was determined by sphere formation assay, flow cytometry assay, and the analysis of stemness‐related markers. The interplay among target genes was evaluated using bioinformatics analyses, luciferase reporter and biotin-labeled RNA pull down assays. We found that HOTAIR was highly expressed and predicted poor prognosis survival in CRC. Downregulation of HOTAIR repressed tumor malignant behaviors and cancer stemness. Mechanistically, HOTAIR facilitated the expression of the microRNA (miR)-211-5p target gene fms-like tyrosine kinase-1 (FLT-1), thereby modulating cancer stem cell (CSC) properties in CRC. We conclude that HOTAIR/miR-211-5p/FLT-1 axis contributes to CRC cancer stemness.
KEYWORDS: Colorectal cancer, FLT-1, HOTAIR
Introduction
Colorectal cancer (CRC) accounts for 10% of global cancer incidence [1,2]. Due to the influence of lifestyle and other factors, CRC is the second leading cause of cancer death in developed countries [3]. At present, the alternative treatment methods for CRC include surgical operation, endoscopy, chemotherapy and radiotherapy; however, the therapeutic efficacy is not optimistic [4]. Cancer stem cells (CSCs) are a rare type of cells with pluripotent differentiation potentials and self-renewal abilities in malignant tumor tissues [5]. To our knowledge, CSCs are considered the leading cause for tumor metastasis and recurrence [6].
Long noncoding RNAs (lncRNAs) refer to a class of RNA transcripts that are longer than 200 nucleotides without protein-coding potentials [7]. They are recognized as crucial regulators of malignant tumors by regulating cell proliferation, differentiation, apoptosis, and survival [8], and the maintenance of CSCs [9]. HOX transcript antisense RNA (HOTAIR) has been identified as a carcinogenic lncRNA in diverse malignancies. For example, the lncRNA HOTAIR can promote the occurrence and progression of breast cancer [10], oral squamous cell carcinoma [11] and ovarian cancer [12]. In addition, HOTAIR contributes to the stemness of colorectal cancer stem cells (CCSCs) [13]. However, the molecular mechanism by which HOTAIR regulates CCSCs remain unclear. In this work, we found that HOTAIR was highly expressed in the CRC tissues. Also, several CRC cell lines and especially CD133+CD44+ CSCs showed higher expression of HOTAIR. Consequently, CRC cells (HCT116 and LoVo) with HOTAIR inhibition were established to examine its function. Results showed HOTAIR silencing impaired the stemness of CSCs and inhibited cancer cell migration and invasion. Further, miR-211-5p was demonstrated to be the target of HOTAIR and subsequently regulated the expression of FLT-1. Overall, identification of HOTAIR in CSCs could be a novel diagnostic marker for CRC as well as provided a new insight into CRC treatment, prognosis and next-step translational investigations.
Materials and methods
Patients
Paired tumor and adjacent normal tissues were collected from 50 CRC patients undergoing surgical excision from January 2013 to March 2015 after signed informed consent. Overall survival was used as a prognostic indicator. This study was approved by the Ethics Committee of The First Affiliated Hospital of Jinzhou Medical University.
Cell lines
Human HCT116, SW480, LoVo, and HT29 cell lines and normal colon epithelial NCM460 cells, purchased from the National Collection of Authenticated Cell Cultures (Shanghai, China) were all maintained in RPMI‑1640 media containing 10% fetal bovine serum at 37°C with 5% CO2.
Transfection
The lentivirus vector containing short hairpin RNA targeted HOTAIR (sense, 5′‐GATCCCCGAACGGGAGTACAGAGAGATTCAAGAGATCTCTCTGTACTCCCGTTCTTTTTGGAAA‐3′; antisense, 5′‐AGCTT TTCCAAAAAGAACGGGAGTACAGAGAGATCTCTTGAATCTCTCTGTACTCCCGTTCGGG‐3′) or scramble sequences (sense, 5′‐GATCCCCTTCTCCGAACGTGTCACGTTTCAAGAGAACGTGACACGTTCGGAGATTTTTGGAAA‐3′; antisense, 5′‐AGCTTTTCCAAAATTCTCCGAACGTGTCACGTTCTCTTGAAACGTGACACGTTCGGAGAAGGG‐3′) were amplified and packaged by GeneChem (Shanghai, China). Using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA), lentivirus plasmids were transfected into HEK293T cells, and the supernatant was collected 48 h after transfection. HCT116 and LoVo cells were incubated with the liquid supernatant for lentivirus infection. Meanwhile, miR-211-5p mimic, inhibitor, FLT-1 overexpression vector, or the corresponding negative controls were transfected into cells. Transfection efficiency was analyzed at 48 h posttransfection.
Real‐time PCR
Total RNA was isolated from tissues or cultured cells using TRIzol reagent (Invitrogen), and added for cDNA synthesis using PrimeScript™ 1st Strand cDNA Synthesis Kit (Takara Biomedical Technology, Beijing, China). The 2−ΔΔCt method was applied to calculate the expression of HOTAIR, miR-211-5p, and FLT-1, using U6 and GAPDH as normalizing controls. The primers were shown in Table 1.
Table 1.
Primers for qRT‐PCR
| Forward (5′–3′) | Reverse (5′–3′) | |
|---|---|---|
| HOTAIR | CAGTGGGGAACTCTGACTCG | GTGCCTGGTGCTCTCTTACC |
| miR-211-5p | ACACTCCAGCTGGGCAAGTAGCATCAACTA | TGGTGTCGTGGAGTCG |
| FLT-1 | AGCAGGTGCTTGAAACCGTAG | GTCGCAGGTAACCCATCTTTT |
| U6 | CTCGCTTCGGCAGCACATATACT | CGCTTCACGAATTTGCGTGT |
| GAPDH | ACAACTTTGGTATCGTGGAAGG | GCCATCACGCCACAGTTTC |
Immunoblotting
Proteins isolated from cultured cells were loaded on 12% electrophoresis gels and prepared for electroblotting. The primary antibodies were used: rabbit monoclonal anti-FLT-1 (#64,094), anti-Nanog (#4903), Sox2 (#3579), Oct4 (#2890) and anti-GAPDH (#5154), purchased from Cell Signaling Technology (Shanghai, China). Bound signals were visualized after incubation with IgG secondary antibody (#7074).
Cell proliferation assays
After 48 h transfection, the number of surviving HCT116 and LoVo cells was assessed after adding the commercial CCK-8 kit (Beyotime Biotechnology, Shanghai, China). Meanwhile, as described in other articles [14,15], 500 cells were incubated for 14 days at 6-well plates, and the colony numbers were afterward counted after fixation and staining.
Transwell migration and invasion assays
Based on the published reports [16,17], HCT116 and LoVo cells were loaded onto the Matrigel-coated or -uncoated upper chamber, while the culture medium containing serum was added to the lower chamber. After incubation for 24 h, the filters were removed. Thereafter, the migrated or invaded cells were photographed and counted under microscopy (Olympus Corporation, Tokyo, Japan).
Spheroid formation assay
The capability of self-renewal was assessed by spheroid formation assay in HCT116 and LoVo cells. Cells (5 × 103 cells/well) were grown in 6-well ultralow attachment plates. The spheroid numbers were analyzed using microscopy after 14 days.
Flow cytometry
CD133+CD44+ and CD133−CD44− subpopulations were sorted from HCT116 and LoVo cells by magnetic activated cell sorting (MACS) method. Briefly, the single-cell suspension (2 × 104 cells) was labeled on ice with PE-conjugated anti-CD133 (ab252128) and FITC-conjugated anti-CD44 (ab27285) antibodies (Abcam, Shanghai, China) for 30 min. The CD133+CD44+ and CD133−CD44− cells were obtained by using flow cytometer (Beckman Coulter, Brea, CA, USA).
Luciferase reporter assay
Luciferase assays were performed in HCT116 and LoVo cells following the co-transfection with HOTAIR or FLT-1-WT or -MUT reporter plasmids and miR-211-5p mimic or miR-NC.
RNA pull-down assay
The biotin-modified miR-211-5p or scramble sequences were incubated with the cell lysates of HCT116 and LoVo cells at room temperature for 1 h. The co-precipitated RNAs were isolated with streptavidin-labeled magnetic beads, and then subjected to real-time PCR.
Xenograft experiment
Female BALB/c nude mice subcutaneously received LoVo cells (1 × 105) transfected with sh-HOTAIR or sh-NC (6 mice per group). After injection, the tumor size was measured every 7 days. After 28 days, the tumor samples were processed for hematoxylin and eosin (H&E) staining as previously described [18,19].
Statistical analysis
Data were expressed as mean ± standard deviation (SD), and the differences were assessed by t test or ANOVA. P < 0.05 was considered to be statistically significant difference.
Results
HOTAIR is upregulated in CRC, and indicates poor prognosis
Compared to the normal tissues, HOTAIR expression was significantly increased in CRC tissues, (Figure 1(a)). Then, high HOTAIR expression was associated with poor overall survival (Figure 1(b)). It was found that HOTAIR was also upregulated in CRC cell lines (Figure 1(c)). We further selected HCT116 and LoVo cells for the isolation of CD133+CD44+ and CD133−CD44− subpopulations, the hallmarks of CCSCs, and observed a higher expression of HOTAIR in CD133+CD44+ cells than that in CD133−CD44− cells (Figure 1(d)). These results indicated CD133+CD44+ cells occupied the capacity of stem-like cells and HOTAIR might be closely associated with CSC stemness.
Figure 1.
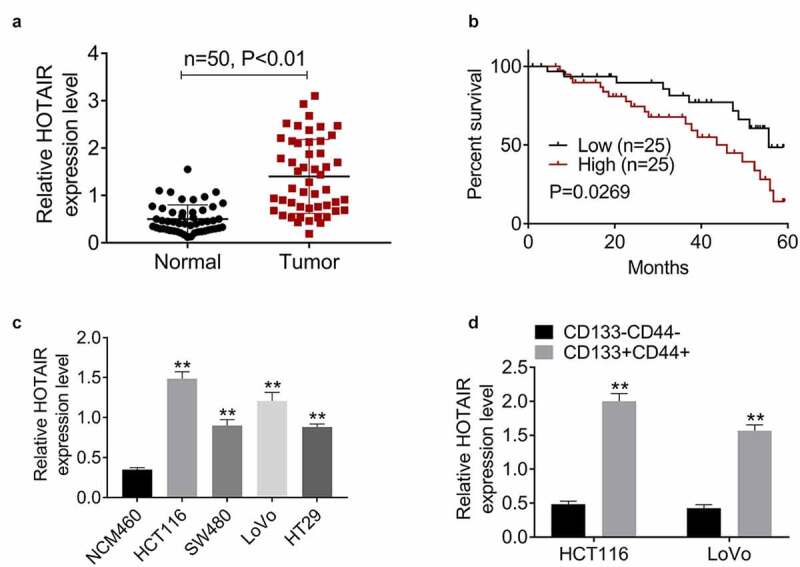
HOTAIR is upregulated in CCSCs, and indicates poor prognosis. (a) The expression levels of HOTAIR were significantly increased in tumor (CRC) tissues compared to normal (control) tissues. (b) Patients with high HOTAIR expression had overall survival than patients with low levels of HOTAIR. (c) The expression levels of HOTAIR were significantly increased in CRC cell lines (HCT116, SW480, LoVo, and HT29) compared to normal colon mucosal epithelial cell line NCM460. (d) The expression levels of HOTAIR were significantly increased in CD133+CD44+ HCT116 and LoVo cells than that in CD133−CD44− cells
Data represented mean ± SD of three independent experiments. **P < 0.01.
HOTAIR silencing inhibits stemness properties and tumorigenicity in CRC
The shRNA-mediated silencing of HOTAIR was performed in HCT116 and LoVo cells (Figure 2(a)). After the suppression of HOTAIR, the cell viability (Figure 2(b)) and colony formation (Figure 2(c)) were observably inhibited. Moreover, the downregulation of HOTAIR markedly reduced cell migration and invasion (Figure 2(d)). The effect of HOTAIR on the characteristics of CSCs was further explored. Sphere formation assay indicated that HOTAIR inhibition led to the reduction of tumor spheres number (Figure 2(e)). As evidenced by flow cytometric analysis, the percentage of CD133+CD44+ cells was memorably decreased upon HOTAIR deficiency (Figure 2(f)). Furthermore, the protein levels of stemness‐related markers (Nanog, Sox2, and Oct4) were signally decreased after HOTAIR depletion (Figure 2(g)). We also analyzed the effects of HOTAIR knockdown on in vivo tumorigenicity via the establishment of tumor xenografts in nude mice. The knockdown of HOTAIR reduced the volume of the tumors (Figure 2(h-i)) and aggravated tumor tissue necrosis (Figure 2(j)) in comparison with the sh-NC group. These results indicated that HOTAIR is critical for the maintenance of CCSC properties.
Figure 2.
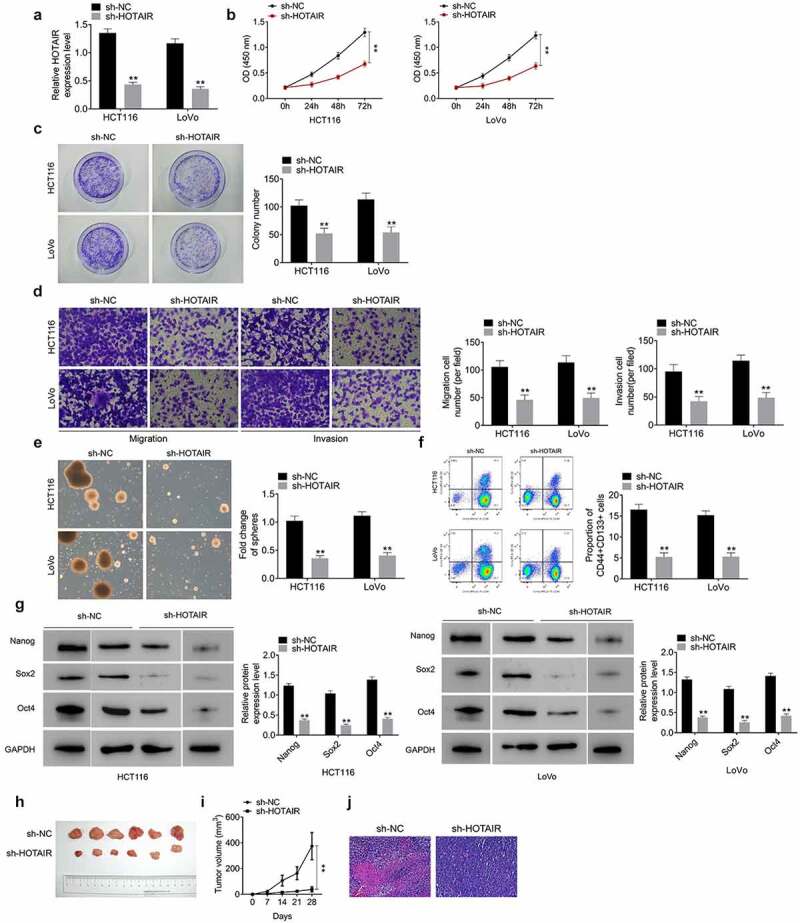
HOTAIR knockdown inhibits the proliferation, migration, invasion, and stemness properties of CRC cells and suppresses the tumorigenicity. (a) Silencing HOTAIR declined HOTAIR levels in HCT116 and LoVo cells. Silencing HOTAIR meaningfully declined cell viability (b), colony formation (c), migration and invasion (d), tumor spheres number (e), the percentage of CD133+CD44+ cells (f) and the protein levels of stemness‐related markers (Nanog, Sox2, and Oct4) (g). At the same time, the knockdown of HOTAIR also reduced the volume of the tumors (h-i) but aggravated tumor tissue necrosis (j)
Data represented mean ± SD of three independent experiments. **P < 0.01.
HOTAIR interacts with miR-211-5p
It was predicted potential binding sites between miR-211-5p and HOTAIR (Figure 3(a)). Dual luciferase reporter assay in HCT116 and LoVo cells showed that miR-211-5p mimic dramatically decreased the relative luciferase activity of HOTAIR-WT, while exhibiting no influence on HOTAIR-Mut (Figure 3(b)). In addition, biotin pull-down assay confirmed the interaction of HOTAIR and miR-211-5p in HCT116 and LoVo cells (Figure 3(c))). Furthermore, miR-211-5p expression was upregulated by HOTAIR knockdown (Figure 3(d)). These data demonstrated the direct binding relationship between HOTAIR and miR-211-5p.
Figure 3.
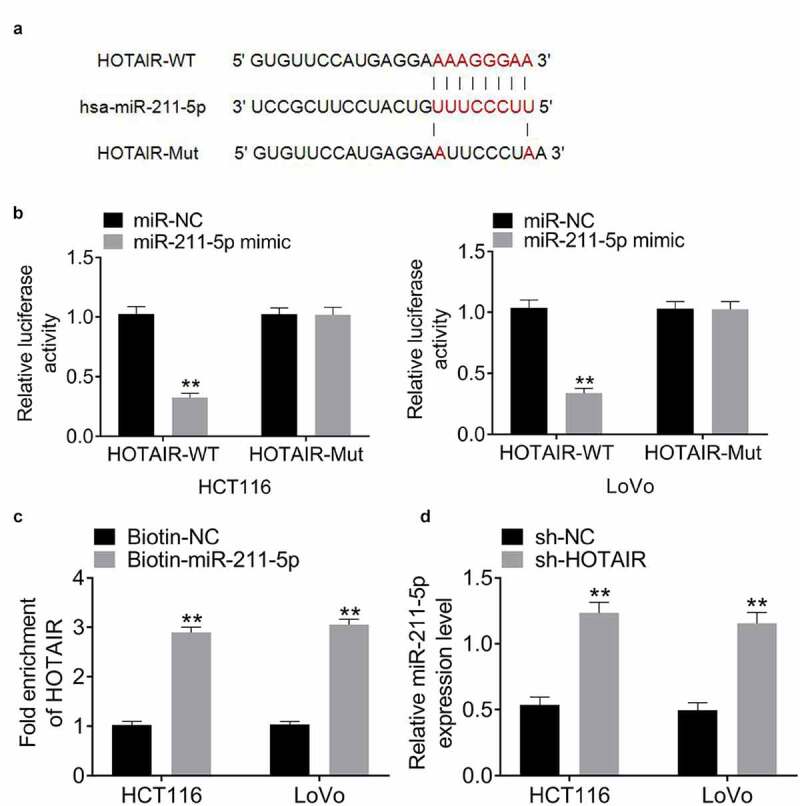
HOTAIR interacts with miR-211-5p in CRC cells. (a) There existed a direct relationship between miR-211-5p and HOTAIR. (b) Dual luciferase reporter assay displayed that the luciferase activity in HOTAIR-wt + miR-211-5p mimic group was relatively weaker compared with that in HOTAIR-wt +miR-NC group. (c) Biotin pull-down assay confirmed the interaction of HOTAIR and miR-211-5p in HCT116 and LoVo cells. (d) The expression of miR-211-5p was upregulated in HCT116 and LoVo cells after HOTAIR knockdown
Data represented mean ± SD of three independent experiments. **P < 0.01.
MiR-211-5p overexpression impairs the stemness of CRC cells
The transfection of miR-211-5p mimic led to the upregulation of miR-211-5p in HCT116 and LoVo cells (Figure 4(a)). The inhibition abilities of miR-211-5p overexpression were also determined on cell proliferation (Figure 4(b-c)), migration and invasion (Figure 4(d)), respectively. The ectopic expression of miR-211-5p led to a remarkable decrease in spheroid formation (Figure 4(e)), the proportion of CD133+CD44+ cells (Figure 4(f)) and the expression of Nanog, Sox2, and Oct4 (Figure 4(g)). These results indicated that miR-211-5p influenced cell stemness characteristics of CRC cells.
Figure 4.
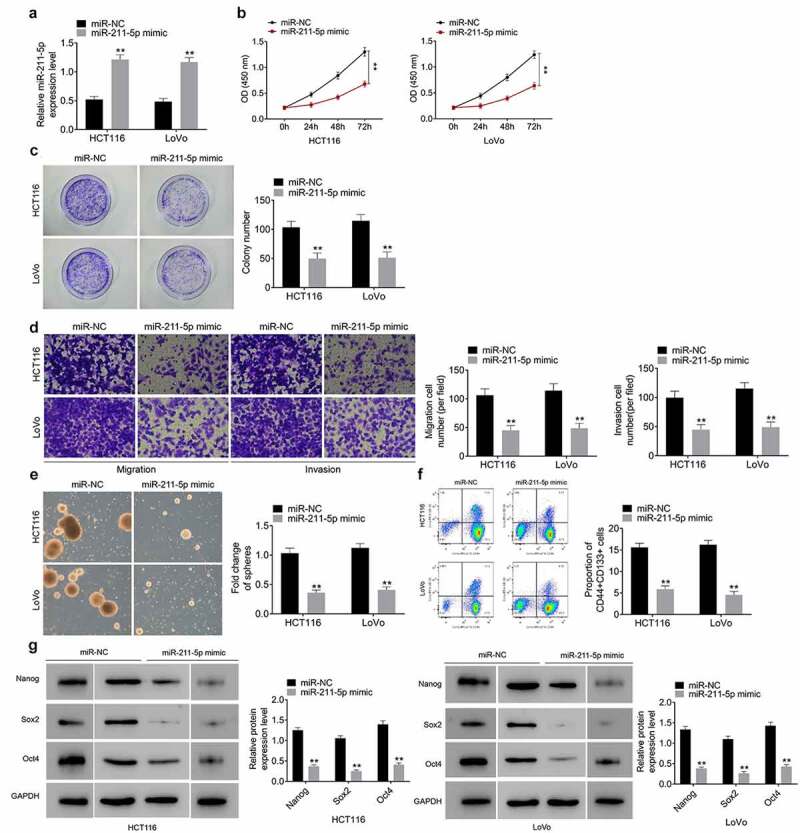
MiR-211-5p overexpression impairs the stemness of CRC cells. (a) Overexpression of miR-211-5p meaningfully strengthened miR-211-5p mRNA levels in HCT116 and LoVo cells. Overexpression of miR-211-5p meaningfully declined cell viability (b), colony formation (c), migration and invasion (d), tumor spheres number (e), the percentage of CD133+CD44+ cells (f) and the protein levels of stemness‐related markers (Nanog, Sox2, and Oct4) (g)
Data represented mean ± SD of three independent experiments. **P < 0.01.
HOTAIR sponges miR-211-5p to upregulate the expression of FLT-1 in CRC cells
TargetScan (http://www.targetscan.org) showed the putative binding sites between miR-211-5p and 3ʹUTR of FLT-1 (Figure 5(a)). Luciferase assay confirmed the binding association of miR-211-5p and FLT-1 (Figure 5(b)). As represented in Figure 5(c), the transfection of miR-211-5p inhibitor resulted in the interference of miR-211-5p in HCT116 and LoVo cells. Additionally, FLT-1 was reduced with HOTAIR knockdown; however, the downregulation of FLT-1 expression induced by HOTAIR silencing was attenuated by miR-211-5p inhibitor (Figure 5(d–e)). Thus, these results suggested that HOTAIR plays a role as a molecular sponge for miR-211-5p to regulate the expression of FLT-1.
Figure 5.
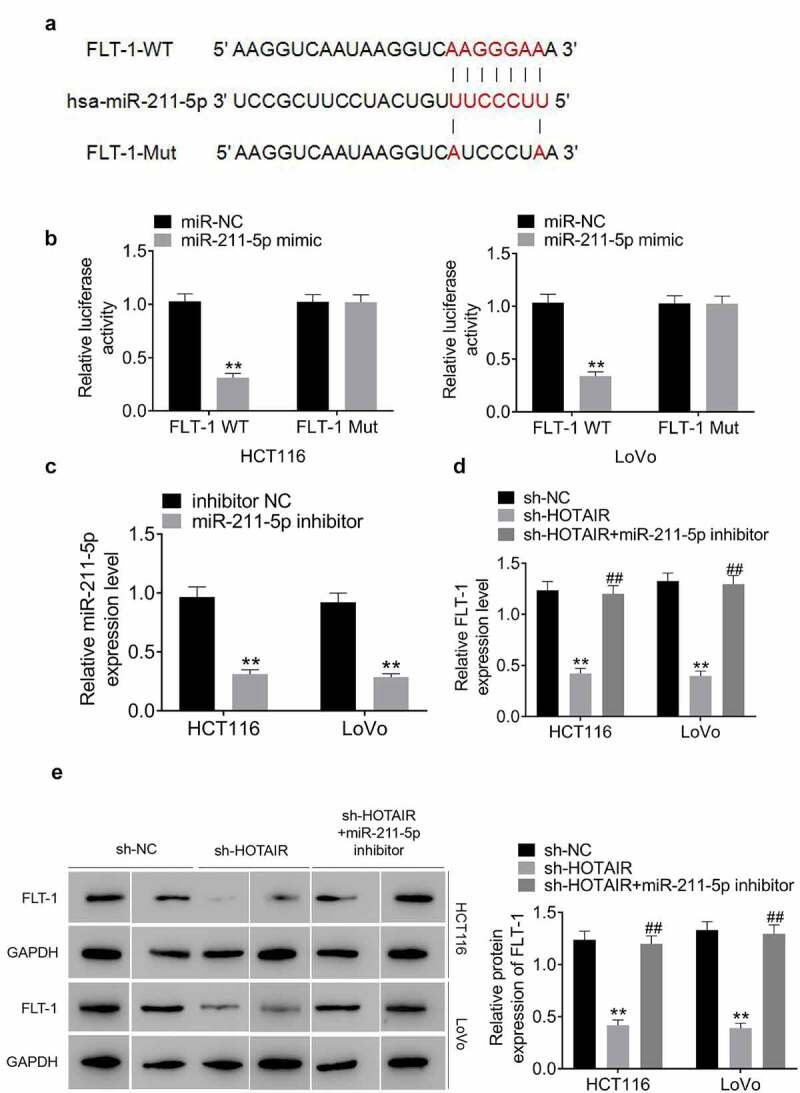
HOTAIR sponges miR-211-5p to upregulate the expression of FLT-1 in CRC cells. (a) There existed a direct relationship between miR-211-5p and FLT-1 3ʹ-UTR. (b) Dual luciferase reporter assay denoted that the luciferase activity in FLT-1-wt + miR-211-5p mimic group was lower than that in FLT-1-wt +miR-NC group. (c) miR-211-5p expression was silenced in HCT116 and LoVo cells following transfection with miR-211-5p inhibitor. The mRNA (d) and protein (e) expression of FLT-1 were downregulated in HCT116 and LoVo cells following transfection with sh-HOTAIR, and these effects were attenuated miR-211-5p inhibitor
Data represented mean ± SD of three independent experiments. **;##P < 0.01.
HOTAIR/miR‑211‑5p/FLT-1 axis is critical for the maintenance of CCSC properties
The overexpression of FLT-1 was achieved by transfecting cells with FLT-1 overexpression plasmid (Figure 6(a)). Functional analysis suggested that the inhibition of miR-211-5p or restoration of FLT-1 expression attenuated the suppressive effects of HOTAIR knockdown on cell proliferation (Figure 6(b,c)), migration and invasion (Figure 6(d)), sphere formation (Figure 6(e)), the percentage of CD133+CD44+ cells (Figure 6(f)), and the expression of Nanog, Sox2, and Oct4 (Figure 6(g)). Taken together, these findings demonstrated the involvement of miR-125b downregulation or FLT-1 overrxpression in role of HOTAIR in promoting CRC cells, particularly, in the migration, invasion and stemness of cancer cells.
Figure 6.
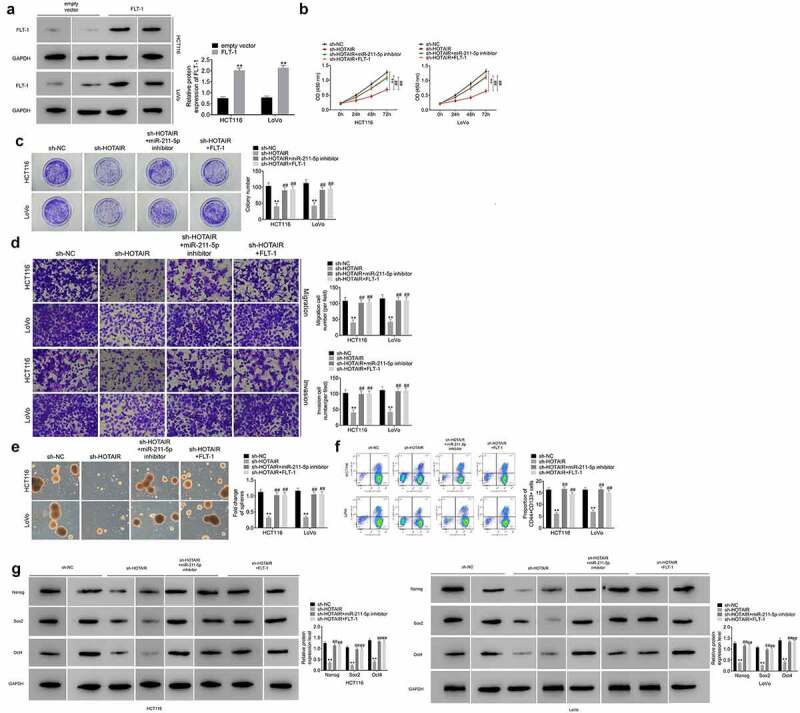
HOTAIR/miR‑211‑5p/FLT-1 axis is critical for the maintenance of CCSC properties. (a) Overexpression of FLT-1 meaningfully strengthened FLT-1 protein levels in HCT116 and LoVo cells. The malignant phenotypes including cell viability (b), colony formation (c), migration and invasion (d) of HCT116 and LoVo cells transfected with sh-HOTAIR were rescued by miR-211-5p downregulation or FLT-1 overexpression. MiR-211-5p downregulation or FLT-1 overexpression reversed sh-HOTAIR-induced inhibition of the capacity of HCT116 and LoVo cells self-renewal (e), the population of CD133+CD44+ cells (f), the protein levels of stemness‐related markers (Nanog, Sox2, and Oct4) (g)
Data represented mean ± SD of three independent experiments. **;##P < 0.01.
Discussion
There is mounting evidence that lncRNAs regulate CSC features in CRC progression. For example, Tang reported that MALAT1 promotes cancer cell stemness characteristics and accelerates tumor progression targeting miR-20b-5p/Oct4 axis in CRC [20]. Another study showed that lncRNA BCAR4 maintains cancer cell stemness in CRC by sponging miR-665, which targets STAT3 [21]. Recent studies revealed that HOTAIR acts as an oncogene in CRC by affecting apoptosis, invasion, and migration as well as chemoresistance [22–24], and exogenous silencing of HOTAIR suppresses the stemness and self-renewal of CCSCs [13]. However, the underlying mechanism of HOTAIR dysregulation in CCSCs is still largely unknown. Herein, consistent with the previous reports [25,26], high HOTAIR expression was observed in CRC tissues and cells, and predicted poor survival. HOTAIR knockdown led to tumor-inhibitory effects in CRC cells and suppressed tumor growth in BALB/c nude mice. The specific surface markers, such as CD133, CD44, Nanog, Sox2, and Oct4, have been commonly employed to identify CSCs [27]. The expression level of HOTAIR is found to be elevated in CD133+CD44+ CSCs. The exogenetic inhibition of HOTAIR suppressed spheroid-forming ability and the percentage of CD133+CD44+ cells and downregulated these stem factors (Nanog, Sox2, and Oct4), indicating the inhibition effects of HOTAIR knockdown on CCSC stemness properties.
Based on the competing endogenous RNA (ceRNA) hypothesis, which sustains that transcripts with shared miRNA binding sites compete for post-transcriptional control [28], the downstream targets of HOTAIR were further elucidated, and we confirmed that miR-211-5p was negatively regulated by HOTAIR. Indeed, miR-211-5p was previously reported to inhibit the progression of various cancer types [29–31]. FLT-1, also known as vascular endothelial growth factor receptor-1 (VEGFR-1) is a core regulator of tumor angiogenesis [32]. The tumor-promoting role of FLT-1 in CRC has been reported in multiple studies [33,34]. Our previous studies proved that FLT-1 was highly expressed in patients with stage III CRC and affected the characteristics of CSCs [35]. Our findings showed that the functional role of HOTAIR was mediated by regulating FLT-1 via sponging miR-211-5p.
In summary, our data supported the findings that HOTAIR was implicated in the regulation of CSC characteristics through acting as a ceRNA to regulate miR-211-5p/FLT-1 signaling axis in CRC cells.
Funding Statement
This work was supported by Science and Technology Research Project of Liaoning Provincial Education Department (JYTJCZR2020059).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
References
- [1].Matsumura T, Sugimachi K, Iinuma H, et al. Exosomal microRNA in serum is a novel biomarker of recurrence in human colorectal cancer. Br J Cancer. 2015;113:275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Brenner H, Kloor M, Pox CP.. Colorectal cancer. Lancet. 2014;383:1490–1502. [DOI] [PubMed] [Google Scholar]
- [3].Greenwald B. A review of the American cancer society’s 2015 colorectal cancer screening recommendations. Gastroenterol Nurs Off J Soc Gastroenterol Nurs Assoc. 2015;38:230–234. [DOI] [PubMed] [Google Scholar]
- [4].Gunawardene A, Desmond B, Shekouh A, et al. Disease recurrence following surgery for colorectal cancer: five-year follow-up. N Z Med J. 2018;131:51–58. [PubMed] [Google Scholar]
- [5].Nassar D, Blanpain C. Cancer stem cells: basic concepts and therapeutic implications. Annu Rev Pathol. 2016;11:47–76. [DOI] [PubMed] [Google Scholar]
- [6].Kusoglu A, Biray Avci C. Cancer stem cells: a brief review of the current status. Gene. 2019;681:80–85. [DOI] [PubMed] [Google Scholar]
- [7].Jarroux J, Morillon A, Pinskaya M. History, discovery, and classification of lncRNAs. Adv Exp Med Biol. 2017;1008:1–46. [DOI] [PubMed] [Google Scholar]
- [8].Rivera-Barahona A, Pérez B, Richard E, et al. Role of miRNAs in human disease and inborn errors of metabolism. J Inherit Metab Dis. 2017;40:471–480. [DOI] [PubMed] [Google Scholar]
- [9].Chen S, Zhu J, Wang F, et al. LncRNAs and their role in cancer stem cells. Oncotarget. 2017;8:110685–110692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mozdarani H, Ezzatizadeh V, Rahbar Parvaneh R. The emerging role of the long non-coding RNA HOTAIR in breast cancer development and treatment. J Transl Med. 2020;18:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tao D, Zhang Z, Liu X, et al. LncRNA HOTAIR promotes the invasion and metastasis of oral squamous cell carcinoma through metastasis-associated gene 2. Mol Carcinog. 2020;59:353–364. [DOI] [PubMed] [Google Scholar]
- [12].Zhang Y, Ai H, Fan X, et al. Knockdown of long non-coding RNA HOTAIR reverses cisplatin resistance of ovarian cancer cells through inhibiting miR-138-5p-regulated EZH2 and SIRT1. Biol Res. 2020;53:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dou J, Ni Y, He X, et al. Decreasing lncRNA HOTAIR expression inhibits human colorectal cancer stem cells. Am J Transl Res. 2016;8:98–108. [PMC free article] [PubMed] [Google Scholar]
- [14].Shang T, Zhou X, Chen W. LINC01123 promotes progression of colorectal cancer via miR-625-5p/LASP1 axis. Cancer Biother Radiopharm. 2020. DOI: 10.1089/cbr.2020.3740 [DOI] [PubMed] [Google Scholar]
- [15].Huang F, Zheng C, Huang L, et al. USP18 directly regulates Snail1 protein through ubiquitination pathway in colorectal cancer. Cancer Cell Int. 2020;20:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sun SQ, Ren LJ, Liu J, et al. Sevoflurane inhibits migration and invasion of colorectal cancer cells by regulating microRNA-34a/ADAM10 axis. Neoplasma. 2019;66:887–895. [DOI] [PubMed] [Google Scholar]
- [17].Zhu G, Cheng Z, Huang Y, et al. MyD88 mediates colorectal cancer cell proliferation, migration and invasion via NFkappaB/AP1 signaling pathway. Int J Mol Med. 2020;45:131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lu ML, Zhang Y, Li J, et al. MicroRNA-124 inhibits colorectal cancer cell proliferation and suppresses tumor growth by interacting with PLCB1 and regulating Wnt/beta-catenin signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23:121–136. [DOI] [PubMed] [Google Scholar]
- [19].Zhan W, Liao X, Chen Z, et al. LINC00858 promotes colorectal cancer by sponging miR-4766-5p to regulate PAK2. Cell Biol Toxicol. 2020;36:333–347. [DOI] [PubMed] [Google Scholar]
- [20].Tang D, Yang Z, Long F, et al. Long noncoding RNA MALAT1 mediates stem cell-like properties in human colorectal cancer cells by regulating miR-20b-5p/Oct4 axis. J Cell Physiol. 2019;234:20816–20828. [DOI] [PubMed] [Google Scholar]
- [21].Ouyang S, Zhou X, Chen Z, et al. LncRNA BCAR4, targeting to miR-665/STAT3 signaling, maintains cancer stem cells stemness and promotes tumorigenicity in colorectal cancer. Cancer Cell Int. 2019;19:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Huang KB, Zhang SP, Zhu YJ, et al. Hotair mediates tumorigenesis through recruiting EZH2 in colorectal cancer. J Cell Biochem. 2019;120:6071–6077. [DOI] [PubMed] [Google Scholar]
- [23].Lin K, Jiang H, Zhang LL, et al. Down-regulated LncRNA-HOTAIR suppressed colorectal cancer cell proliferation, invasion, and migration by mediating p21. Dig Dis Sci. 2018;63:2320–2331. [DOI] [PubMed] [Google Scholar]
- [24].Xiao Z, Qu Z, Chen Z, et al. LncRNA HOTAIR is a prognostic biomarker for the proliferation and chemoresistance of colorectal cancer via MiR-203a-3p-mediated Wnt/ss-catenin signaling pathway. Cell Physiol Biochem. 2018;46:1275–1285. [DOI] [PubMed] [Google Scholar]
- [25].Liu B, Liu Q, Pan S, et al. The HOTAIR/miR-214/ST6GAL1 crosstalk modulates colorectal cancer procession through mediating sialylated c-Met via JAK2/STAT3 cascade. J Exp Clin Cancer Res. 2019;38:455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Peng CL, Zhao XJ, Wei CC, et al. LncRNA HOTAIR promotes colon cancer development by down-regulating miRNA-34a. Eur Rev Med Pharmacol Sci. 2019;23:5752–5761. [DOI] [PubMed] [Google Scholar]
- [27].Dotse E, Bian Y. Isolation of colorectal cancer stem-like cells. Cytotechnology. 2016;68:609–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Thomson DW, Dinger ME. Endogenous microRNA sponges: evidence and controversy. Nat Rev Genet. 2016;17:272–283. [DOI] [PubMed] [Google Scholar]
- [29].Wang L, Shen YF, Shi ZM, et al. Overexpression miR-211-5p hinders the proliferation, migration, and invasion of thyroid tumor cells by downregulating SOX11. J Clin Lab Anal. 2018;32:e22293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Quan J, Pan X, He T, et al. Tumor suppressor miR-211-5p is associated with cellular migration, proliferation and apoptosis in renal cell carcinoma. Exp Ther Med. 2018;15:4019–4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zhang S, Ma H, Zhang D, et al. LncRNA KCNQ1OT1 regulates proliferation and cisplatin resistance in tongue cancer via miR-211-5p mediated Ezrin/Fak/Src signaling. Cell Death Dis. 2018;9:742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Atzori MG, Tentori L, Ruffini F, et al. The anti-vascular endothelial growth factor receptor-1 monoclonal antibody D16F7 inhibits glioma growth and angiogenesis in vivo. J Pharmacol Exp Ther. 2018;364:77–86. [DOI] [PubMed] [Google Scholar]
- [33].Nagano H, Tomida C, Yamagishi N, et al. VEGFR-1 regulates EGF-R to promote proliferation in colon cancer cells. Int J Mol Sci. 2019;20:5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zygon J, Szajewski M, Kruszewski WJ, et al. VEGF, Flt-1, and microvessel density in primary tumors as predictive factors of colorectal cancer prognosis. Mol Clin Oncol. 2017;6:243–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Huang Y, Huang Y, Liu D, et al. Flt-1-positive cells are cancer-stem like cells in colorectal carcinoma. Oncotarget. 2017;8:76375–76384. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


