Abstract
Background
ChAdOx1-vectored vaccine candidates against several pathogens have been developed and tested in clinical trials and ChAdOx1 nCoV-19 has now been licensed for emergency use for COVID-19. We assessed the safety and immunogenicity of the ChAdOx1 MERS vaccine in a phase 1b trial in healthy Middle Eastern adults.
Method
MERS002 is an open-label, non-randomised, dose-escalation, phase 1b trial. Healthy Middle Eastern adults aged 18–50 years were included in the study. ChAdOx1 MERS was administered as a single intramuscular injection into the deltoid muscle of the non-dominant arm at three different dose groups: 5·0 × 109 viral particles in a low-dose group, 2·5 × 1010 viral particles in an intermediate-dose group, and 5·0 × 1010 viral particles in a high-dose group. The primary objective was to assess the safety and tolerability of ChAdOx1 MERS, measured by the occurrence of solicited and unsolicited adverse events after vaccination for up to 28 days and occurrence of serious adverse events up to 6 months. The study is registered with ClinicalTrials.gov, NCT04170829.
Findings
Between Dec 17, 2019, and June 1, 2020, 24 participants were enrolled (six to the low-dose, nine to the intermediate-dose, and nine to the high-dose group) and received a dose; 23 were available for follow-up at 6 months. The one dose of ChAdOx1 MERS vaccine was well tolerated with no serious adverse event reported during the 6 months of follow-up. Most adverse events were mild (67, 74%) and moderate (17, 19%). Six (7%) severe adverse events were reported by two participants in the intermediate-dose group (two feverish, two headache, one joint pain, and one muscle pain). Pain at the injection site was the most common local and overall adverse event, reported by 15 (63%) of the 24 participants. The most common systemic adverse event was headache, reported by 14 (58%), followed by muscle pain reported by 13 (54%). The vaccine induced both antibody and T cell immune responses in all volunteers; antibodies peaked at day 28 and T cell responses peaked at day 14; and continued until the end of follow-up at 6 months.
Interpretation
The acceptable safety and immunogenicity data from this phase 1b trial of ChAdOx1 MERS vaccine candidate in Healthy Middle Eastern adults, combined with previous safety and immunogenicity data from a trial in the UK, support selecting the ChAdOx1 MERS vaccine for advancement into phase 2 clinical evaluation.
Funding
UK Department of Health and Social Care, using UK Aid funding, managed by the UK National Institute for Health Research; and King Abdullah International Medical Research Center.
Introduction
MERS is a zoonotic disease caused by a coronavirus, leading to a respiratory infection in humans with manifestations ranging from asymptomatic to severe pneumonia and death. MERS-CoV is also known to cause severe acute respiratory illness in humans.1, 2 It was first identified in Saudi Arabia in 2012 and has since spread to around 27 countries.1, 3 Cases of sporadic zoonotic infections in the community, secondary household transmission, and clusters of outbreaks in health-care settings are well documented.4, 5 MERS-CoV is an enveloped, single-stranded, RNA virus that belongs to betacoronavirus genus of the Coronaviridae family. This family is known for evolving into a broader tropism and for their ability to cause an infection in mammalian and avian hosts.3 The virus spike protein facilitates entry into host cells through a mammalian cell receptor, DPP-4, which is variably expressed in dromedary camels (Camelus dromedarius) and humans.6 This expression would partly explain interspecies pathogenesis and transmissibility of MERS-CoV from camels to humans in areas with high camel–human interactions, such as Africa and the Arabian Peninsula.7, 8
Since the emergence of MERS-CoV in 2012, Saudi Arabia has been the main endemic country with the highest reported cases of this virus. By July 31, 2021, a total of 2578 laboratory-confirmed cases were reported globally to the WHO, including 2178 cases reported in Saudi Arabia.9 The case fatality rate of MERS is considered high: 34·3% globally and 37·1% in Saudi Arabia.9 Dromedary camels are the confirmed source of animal-to-human transmission in at-risk regions and 54% of primary human cases have reported direct or indirect camel contact.10 Although camel workers are not usually symptomatic and their viral infection is only detected retrospectively by serology testing, 50% of camel workers in one study in Saudi Arabia were found to be seropositive.11 Despite local and international efforts by health authorities, MERS-CoV will continue to be a significant public health issue in affected and at-risk countries in the absence of an antiviral or a vaccine with a proven efficacy.12, 13 ChAdOx1, a chimpanzee adenovirus vaccine vector, has been developed and used for vaccine candidates against several infections. Its targets include malaria, influenza, Ebola, and most recently, the ChAdOx1 nCoV-19 vaccine, which was licensed for emergency use on Feb 15, 2021 by WHO for COVID-19. ChAdOx1 was well tolerated and immunogenic in all previous clinical trials.14, 15, 16 The ChAdOx1 based vaccine for COVID-19 has proved efficacious in several phase 3 clinical trials17 and effective against COVID-19 in real-world postdistribution studies.18 A ChAdOx1 vector expressing full-length spike from MERS-CoV was constructed (ChAdOx1 MERS) and evaluated in mouse, non-human primates, and camel studies. The vector induced significant humoral and cellular immune response; with complete protection in mice or high level protection in non-human primates and camels.19, 20, 21, 22 In a phase 1 clinical trial in healthy people in the UK, ChAdOx1 MERS (MERS001 trial) was well tolerated with no serious adverse events and induced immune responses.23 The study showed that single dose regimens were safe and immunogenic. Here, we have worked on setting up infrastructure and conducted the first-ever vaccine, phase 1, clinical trial in Saudi Arabia. This trial is aimed at assessing the safety and immunogenicity of the ChAdOx1 MERS vaccine candidate in healthy Middle Eastern adults in Saudi Arabia as a phase 1b clinical trial in this main endemic country.
Research in context.
Evidence before this study
At this time, no vaccine or specific treatment is licensed for MERS. Several vaccine candidates are under development, including the ChAdOx1 vector, which was studied in a phase 1 trial. We searched PubMed for articles published since database inception up to June 4, 2021. With no language restrictions applied, we used the search terms: “Chadox”, “chadox1”, “MERS vaccine”, “MERS vaccine safety”, and “chadox1 vaccine”. We examined studies that investigated the safety of ChAdOx1 vectored vaccines as a primary outcome. A clinical study that took place in the UK found that the vaccine was safe and well tolerated and humoral and cellular MERS-CoV-specific immune responses were induced in most participants. A large-scale analysis of the ChAdOx1 nCoV-19 vectored vaccine, with 23 848 participants enrolled in the UK, Brazil, and South Africa, showed an acceptable safety profile. Another phase 1 trial in healthy adults is evaluating anti-MERS-CoV modified vaccinia virus Ankara-based vaccine candidate that expresses the MERS-CoV spike glycoprotein. Vaccination with modified vaccinia virus Ankara MERS-S had a favourable safety profile without serious or severe adverse events, and provides evidence of humoral and cellular immunogenicity in humans. In an earlier phase 1 clinical trial for a MERS-CoV DNA vaccine candidate, the GLS-5300 MERS-CoV vaccine was well tolerated with no vaccine-associated serious adverse events. Immune responses were dose independent, detected in more than 85% of participants after two doses of vaccination, and durable through 1 year of follow-up.
Added value of this study
This study is a phase 1b trial and the first clinical study of the ChAdOx1 MERS vaccine enrolling exclusively adults from the Middle East, where MERS-CoV is endemic. The vaccine investigated in this trial, which took place in Saudi Arabia, was safe and well tolerated. In addition, this study maintains the safety profile of the ChAdOx1 vector, which for SARS-CoV-2, has gained emergency use authorisation in several jurisdictions. The vaccine induced antibody and T cell immune responses in all participants; and continued until the end of follow-up. Unlike MERS001 in the UK, the high-dose group showed higher concentrations of antibodies compared with the lower-dose groups, which might indicate that the vaccine can induce different levels of immune responses in different populations.
Implications of all the available evidence
As a phase 1b trial, this study provided information on reactogenicity and immunogenicity of the clinical use of ChAdOx1 MERS in an endemic area. The outcome of this study supports clinical progress into a phase 2 study. A larger number of healthy adults, health-care workers, and people occupationally exposed to camels, mainly in the Middle East, can be recruited to further establish the safety and immunogenicity of a vaccine that can be used to prevent MERS outbreaks.
Methods
Study design and participants
MERS002 is an open-label, non-randomised, dose-escalation, extension for a first-in-human, single centre, phase 1b clinical trial, conducted at Kind Abdulaziz Medical City, Ministry of National Guard Health Affairs, Riyadh, Saudi Arabia. Our study followed a first-in-human study that was done in the UK23 and shared similarities in design and procedures. Volunteers were recruited through flyers and advertisements distributed in public areas in Riyadh. Healthy Middle Eastern people aged 18–50 with negative prevaccination tests for HIV antibodies, HBsAg, and hepatitis C antibodies were eligible for enrolment. Women must have had negative blood pregnancy tests during screening and immediately before vaccination to be eligible for enrolment. The patient's medical history was reviewed by the principal investigator in addition to reviewing clinical and laboratory findings from the urinalysis and blood tests at screening according to the study protocol (appendix).
Written informed consent was obtained from all potential volunteers before study-specific procedures were performed. The study was approved by Institutional Review Board and ethical committee at King Abdullah International Medical Research Centre (CT18/004/R); and the Saudi Drug and Food Authority (SCTR 18121302). An independent Data and Safety Monitoring Board provided safety review and supervision.
Procedures
The ChAdOx1 MERS vaccine candidate, manufactured by the Clinical Biomanufacturing Facility at the University of Oxford (Oxford, UK), was administered as a single intramuscular injection into the deltoid muscle of the non-dominant arm in three groups of patients: 5·0 × 109 viral particles (low-dose group), 2·5 × 1010 viral particles (intermediate-dose group), and 5·0 × 1010 viral particles (high-dose group). A staggered approach was used for enrolling volunteers. The first three volunteers of each group were admitted and observed in-unit, as defined in the protocol, for 48 h after vaccination. The Data and Safety Monitoring Board conducted a safety review after vaccination of the first three volunteers in group 1 before dose-escalation and enrolment of the rest of the group's patients. A similar safety procedure was done for the first three volunteers in groups 2 and 3 before the enrolment of the remaining volunteers. The remaining volunteers in each group were observed for at least 1 h after the vaccination and did not require in-unit observation. Full details can be found in the trial protocol in the appendix. Each volunteer had a follow-up visit at day 2, 7, 14, 28, 56, and 182 (6 months), in which clinical assessment was conducted and blood samples were taken for safety as well as for immunogenicity testing. Blood samples were also taken before vaccination, day 0, as a safety and immunological baseline. Volunteers were given thermometers, measuring tapes, and paper-based diaries to record any adverse events during the 28 days’ follow-up period. For the secondary outcome of immunogenicity testing, follow-up was completed at day 56 and 182.
Solicited adverse events (defined in protocol) include local events (eg, pain at the injection site, redness, swelling, warmth, and itchiness) and systemic (eg, fever [temperature higher than 37·5°C], feverishness [reported by participant], arthralgia, myalgia, fatigue, headache, nausea, and malaise) that occur within 7 days after vaccination. Unsolicited adverse events were adverse events other than the foreseeable adverse events occurring within the first 7 days, or any adverse events occurring after the first 7 days after vaccination. Unsolicited events occurring in the 28 days after vaccination were recorded and serious adverse events occurring within 6 months after vaccination were monitored closely and documented. When there is no specific consideration applied, general criteria for grading local and systemic solicited and unsolicited adverse events were as follows: mild, defined as no limitation in usual activity; moderate, defined as mild to moderate limitation in usual activity; and severe, defined as limitation in usual activity and medications were required. Unsolicited adverse events were reviewed for causality assessment by an independent infectious diseases physician and those deemed possibly, probably, or definitely related to the vaccine were reported. Laboratory values were assessed and graded in accordance to the US Food and Drug Administration Guidance for Industry document.24 The Medical Dictionary for Regulatory Activities does not support the Arabic language as of time of publication. All unsolicited adverse events reported by volunteers were translated first to English using the WHO unified medical dictionary and based on their description during the visits, then coded in accordance with Medical Dictionary for Regulatory Activities.
Immunogenicity testing
To evaluate the concentration of anti-MERS-CoV spike IgG, a standardised in-house indirect ELISA was set up as follows. Nunc MaxiSorp 96-well plates (Life Technologies, Paisley, UK) were coated with 1 μg/mL of full-length recombinant clamp MERS spike protein (supplied by Keith Chappell, School of Chemistry and Molecular Biosciences, University of Queensland, Brisbane, QLD, Australia) in phosphate buffered saline and incubated at 4°C for 18 h overnight. The coated plates were washed six times with phosphate-buffered saline-Tween and then blocked with casein for 1 h at room temperature. Serum samples diluted to fall within the linear range of the curve (typically 1/500 in casein) were then added to individual wells, in duplicates, of the plates. The plates were incubated at room temperature for 2 h followed by plate washing, as described previously. The plates were then incubated at room temperature for 1 h with a secondary antibody, alkaline phosphatase-conjugated goat anti-human IgG (γ-chain specific). After a final wash, plates were developed by adding 4-nitrophenyl phosphate in diethanolamine substrate buffer (Fisher Scientific UK, Loughborough, UK). The standard curve used on each plate was derived from a pool of volunteers’ serum samples containing high-titre anti-MERS spike IgG. Endpoint titre determined by ELISA was used to identify the volunteer samples with the highest anti-MERS IgG titres after vaccination. A 1/100 dilution of the standard pool was used in a two-fold serial dilution to produce ten standard points that were assigned arbitrary ELISA units. The optical density values of the standard points were fitted to a four-parameter hyperbolic curve against the arbitrary ELISA units using GEN5 software (version 3.04; BioTek Instruments, Winooski, VT, USA), and the parameters estimated from the standard curve were used to convert absorbance values of individual test samples into ELISA units. Each ELISA plate consists of the samples and internal positive control (1/800 dilution of the standard pool, corresponding to standard 4) in triplicates, ten standard points in duplicates, and four blank wells. The optical density reading of the plates at 405 nm was done with an ELx808 microplate reader (BioTek Instruments). The limit of detection was set as 200 EU.
To evaluate the concentration of neutralising antibodies for MERS-CoV, MERS pseudotyped viral particles (MERSpp) were produced and titrated using Huh7·5 cells, as described elsewhere.25, 26 Serum samples from the trial patients were prepared in a three-fold serial dilution starting from 1:20 and tested for neutralising antibodies in duplicate. A standard concentration of MERSpp (equivalent to 200 000 relative luminescence units) and Huh7·5 cells (10 000 cells) were added to each well. Cells only and cells with MERSpp only (both without serum) were included in quadruplicate as controls to determine 100% and 0% neutralisation activity, respectively. Following 48 h of incubation, supernatants were removed and cells were lysed; the assay was then developed by Bright-Glo Luciferase Assay System (Promega, WI, USA) and luciferase activity was measured using a luminometer. 50% of inhibitory concentration neutralisation titres were calculated for each serum sample using GraphPad Prism.
To evaluate the cell-mediated immunity, interferon-γ-linked enzyme linked immunospot (ELISpot) assays were done with fresh peripheral blood mononuclear cells (PBMCs) to determine responses to the MERS-CoV spike vaccine antigen. The method is described elsewhere27 with the following exceptions. PBMCs were separated from whole blood within 4 h of venipuncture. 275 synthetic peptides (15mers overlapping by ten amino acids) spanning the entire vaccine insert (MERS-CoV spike antigen), including the tissue plasminogen activator leader sequence, were used to stimulate PBMCs. Peptides were pooled into 13 pools for the MERS-CoV spike protein, containing 18 or 21 peptides, plus a single pool of five peptides for the tissue plasminogen activator leader. Peptide sequences and pooling have been summarised in the appendix of our previous work.23 Data were analysed according to a quality control standard operational procedure. The lower limit of detection for the assay was 56 spot forming cells for summed responses to the 13 MERS-CoV spike peptide pools. Data are presented as spot forming cells per million PBMCs.
Outcome
The primary outcome was to assess the safety and tolerability of ChAdOx1 MERS by the occurrence of solicited local reactogenicity signs and symptoms for 7 days following the vaccination; occurrence of solicited systemic reactogenicity signs and symptoms for 7 days following the vaccination; occurrence of unsolicited adverse events for 28 days following the vaccination; change from baseline for safety laboratory measures; and occurrence of serious adverse events during the whole study duration of 6 months. The secondary outcomes were cellular and humoral immunogenicity of ChAdOx1 MERS as measured by ELISA to quantify antibodies to MERS spike protein antigen and ex-vivo ELISpot responses to MERS spike protein antigen from baseline to 6 months.
Statistical analysis
For this descriptive phase 1b study, the sample size was not determined based on statistical power calculations because of its small number of participants. Safety endpoints are described as frequencies with their respective percentages alongside their 95% CIs. Immunology data were tested for normal distribution using the D’Agostino-Pearson omnibus normality test. Data were analysed with non-parametric measures. Kruskal-Wallis with Dunn's multiple comparison post-test was used to compare across timepoints or groups. p values of less than 0·05 were considered significant. For ELISpot data, values are spot forming cells per million PBMCs. Statistical analysis of safety and immunogenicity data was done with GraphPad Prism (version 8.01 for Windows).
The study is registered with ClinicalTrials.gov, NCT04170829.
Role of the funding source
The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Results
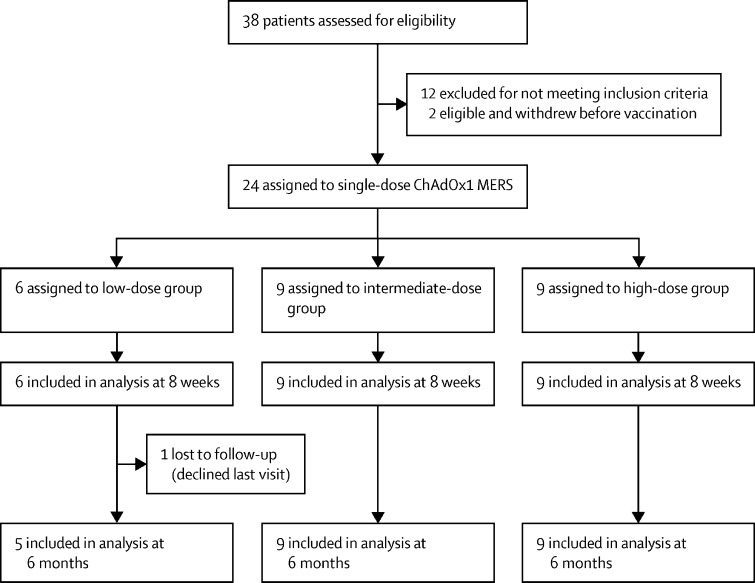
Between Dec 17, 2019, and June 1, 2020, 24 volunteers (15 men and nine women), aged 21–46 years, were enrolled (table 1 ). The group was divided into three dose groups: low-dose group (n=6), intermediate-dose group (n=9), and high-dose group (n=9; figure 1 ). All participants received a single dose of ChAdOx1 MERS according to their dose-group allocation. 23 participants were available for all follow-up visits; one participant was not able to attend the blood draw visit at the 6 months follow-up.
Table 1.
Characteristics of the trial volunteers
| All (n=24) | Low-dose group (n=6) | Intermediate-dose group (n=9) | High-dose group (n=9) | ||
|---|---|---|---|---|---|
| Age, years | 30 (21–46) | 28 (21–36) | 33 (22–46) | 27 (23–34) | |
| Sex | |||||
| Male | 15 (63%) | 5 (83%) | 5 (56%) | 5 (56%) | |
| Female | 9 (38%) | 1 (17%) | 4 (44%) | 4 (44%) | |
Data are median (range) or n (%).
Figure 1.
Trial profile
The vaccine showed an acceptable safety profile with no serious adverse event reported in any of the groups during the 6 months of follow-up. A total of 90 local and systemic solicited adverse events were reported during the 7 days after vaccination. Most of these solicited adverse events were mild (67; 74%) in all three groups and moderate (17, 19%) in the intermediate-dose and high-dose groups. Six severe adverse events were reported (7%) by two volunteers in the intermediate-dose group. 86 (96%) of the reported adverse events had an onset within 72 h after vaccination (23 [26%] at day 0, 57 [63%] at day 1, and six [7%] at day 2); all adverse events were self-limiting and completely resolved by the second follow-up at day 7.
Pain at the site of injection was the most common local adverse event reported by 15 (63%) of the 24 volunteers. The most common systemic adverse event was headache, reported by 14 (58%) and muscle pain reported by 13 (54%) participants. Two participants in the intermediate-dose group reported six severe adverse events following the vaccine (two feverish events and two headache events, one joint pain, and one muscle pain [table 2 ]). All participants who had onset of symptoms had them on the day following vaccination (day 1), lasted for 24 h, and resolved by the first follow-up visit (day 2), except with one participant in which headache persisted for 48 h. A total of 28 unsolicited adverse events were reported. In a causality evaluation, eight (29%) of these were deemed at least possibly related to the vaccine (six mild, one moderate, and one severe). One unsolicited adverse event persisted for more than 3 days in which a participant had paresthesia started on day 4 and was resolved by the next visit. Localised trunk erythema was observed on a participant not associated with other symptoms and disappeared gradually over a few days. A full list of unsolicited adverse events is shown in the appendix (p 2). A total of seven laboratory abnormalities were deemed related to the vaccine (appendix p 4) with neutropenia being the most common (n=3; 43%). Low lymphocyte count was noted in one patient only 2 days after vaccination, which was mild and reverted to the normal range on the day 7 visit. One volunteer developed mild leukocytosis, which started on day 7 and was resolved by day 28. All laboratory abnormalities were resolved by the fourth visit after vaccination (day 28).
Table 2.
Local and systemic solicited adverse events
|
Low-dose group (n=6) |
Intermediate-dose group (n=9) |
High-dose group (n=9) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | Mild | Moderate | Severe | Mild | Moderate | Severe | |
| Any symptom | 4 (67%) | 0 | 0 | 4 (44%) | 3 (33%) | 2 (22%) | 5 (56%) | 4 (44%) | 0 |
| Any local symptom | 3 (50%) | 0 | 0 | 8 (89%) | 1 (11%) | 0 | 7 (78%) | 0 | 0 |
| Pain | 3 (50%) | 0 | 0 | 7 (78%) | 1 (11%) | 0 | 4 (44%) | 0 | 0 |
| Erythema | 0 | 0 | 0 | 2 (22%) | 0 | 0 | 3 (33%) | 0 | 0 |
| Warmth | 0 | 0 | 0 | 2 (22%) | 1 (11%) | 0 | 3 (33%) | 0 | 0 |
| Pruritis | 0 | 0 | 0 | 1 (11%) | 0 | 0 | 0 | 0 | 0 |
| Any systemic symptom | 3 (50%) | 0 | 0 | 4 (44%) | 3 (33%) | 2 (22%) | 4 (44%) | 4 (44%) | 0 |
| Fever | 0 | 0 | 0 | 2 (22%) | 1 (11%) | 0 | 0 | 3 (33%) | 0 |
| Feverishness | 0 | 0 | 0 | 1 (11%) | 0 | 2 (22%) | 4 (44%) | 1 (11%) | 0 |
| Arthralgia | 0 | 0 | 0 | 2 (22%) | 1 (11%) | 1 (11%) | 2 (22%) | 0 | 0 |
| Myalgia | 2 (33%) | 0 | 0 | 4 (44%) | 1 (11%) | 1 (11%) | 4 (44%) | 1 (11%) | 0 |
| Fatigue | 0 | 0 | 0 | 1 (11%) | 3 (33%) | 0 | 5 (56%) | 0 | 0 |
| Headache | 0 | 0 | 0 | 6 (67%) | 1 (11%) | 2 (22%) | 4 (44%) | 1 (11%) | 0 |
| Nausea | 1 (17%) | 0 | 0 | 0 | 1 (11%) | 0 | 0 | 0 | 0 |
| Malaise | 1 (17%) | 0 | 0 | 1 (11%) | 1 (11%) | 0 | 2 (22%) | 0 | 0 |
Data are n (%). Allocated as the highest reported adverse event.
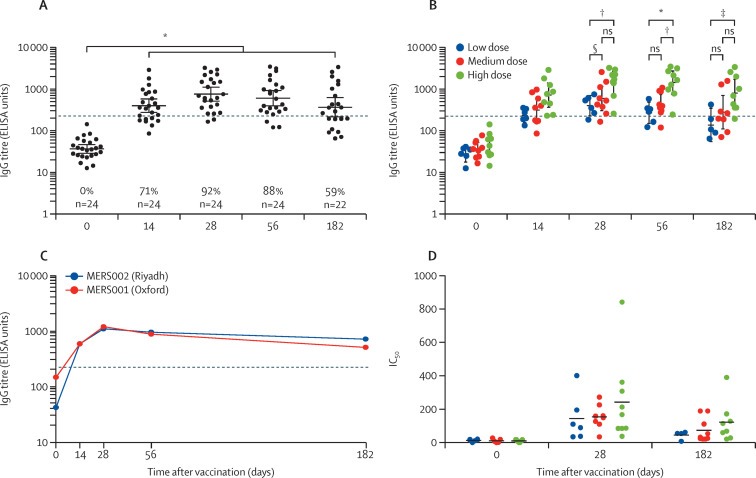
The single dose of ChAdOx1 MERS induced antibody responses in all volunteers across all groups, higher than the baseline levels. Antibodies were evident at day 14, peaked at day 28 after vaccination, and continued to the end of the trial follow-up, at 6 months. Seroconversion occurred after vaccination in 71% (17 of 24) of patients by day 14, 92% (22 of 24) by day 28, 88% (21 of 24) by day 56, and 59% (13 of 22) by day 182. The concentration of induced antibodies at the peak (day 28) was a geometric mean of 36 ELISA units at day 0 and 753 ELISA units at day 28 (95% CI 511–1110; p<0·0001), when data from all dose groups were combined (figure 2 A). The level at the last follow-up (day 182) was 365 ELISA units (95% CI 215–619, p<0·0001, day 0 vs day 182). Antibody responses in the high-dose group were the highest. IgG titres increased significantly at the peak response (day 28) as compared with the baseline; this finding was in line with the increasing dose. There were significant differences in anti-MERS spike IgG between the high-dose group at day 28, 56, and 182 as compared with the low-dose and medium-dose groups (figure 2B). No significant differences were reported in the IgG titres between the dose groups before the peak, at day 14. No anti-MERS IgG responses were detected at baseline in any of the 24 volunteers. Comparing these IgG titres to the serum IgG titres from the MERS001 trial23 in the UK showed that participants in the current study induced similar responses (figure 2C). Neutralisation assay based on pseudotyped lentiviral particles (MERSpp) was used to evaluate the neutralising antibodies in the 24 participants. All volunteers in the three dose groups showed significant neutralising antibody titres at day 28 after vaccination as compared with baseline. No significant differences were observed between the dose groups at day 28 (figure 2D).
Figure 2.
Antibody responses in patients vaccinated with a single dose regimen of ChAdOx1 MERS
IgG titres from all vaccinated patients (A) or from each dose group (B) for 6 months after vaccination, presented as ELISA units, with a cutoff 200 for seropositivity. IgG titres of 24 vaccinated patients in the Riyadh MERS002 trial (the current study) and Oxford MERS001 trial (C). Neutralising antibody titres are shown as IC50 for day 28 (D). Data from different dose groups are shown in different colours. Percentages shown on graphs indicate seropositivity percent. IC50=50% inhibitory concentration. ns=not-significant. Dotted lines represent the seropositivity. p value is indicated by *0·0001, †0·001, ‡0·05, §0·01; as tested by Kruskal-Wallis test and Dunn's multiple comparisons test.
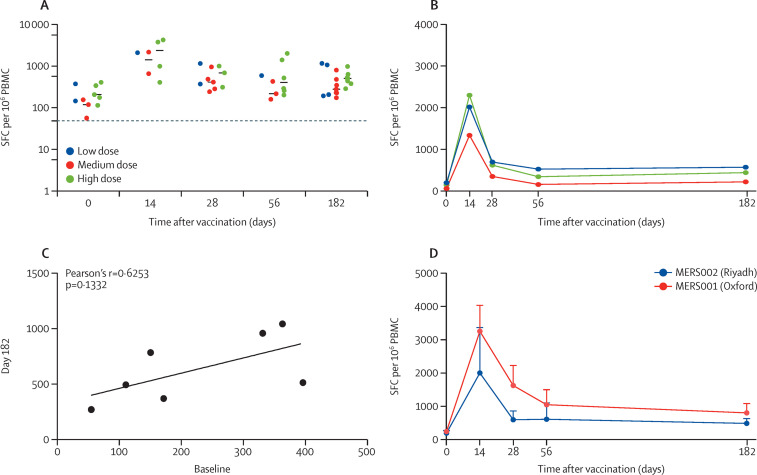
Cellular immunogenicity to ChAdOx1 MERS was assessed by ex-vivo ELISpot. The interferon-γ-secreting T cell responses peaked at day 14 after vaccination for all groups. At day 14, the response in the low-dose group was 2043 spot forming cells for the only sample available for analysis whereas spot forming cells at medium dose showed a median of 1374 and at high-dose showed a median of 2322 (figure 3 A). Responses at day 0 had medians of 252 for low-dose, 116 for intermediate-dose, and 201 for high-dose groups. Data from all volunteers across dose-groups were pooled since there were no significant differences in the T cell responses between the groups at any of the sampling timepoints. A significant increase in T cell responses was observed as compared with baseline at all timepoints, especially at day 14 and 28 after vaccination (figure 3A, B). The T cell responses at 6 months after vaccination showed median values of 620 for low-dose, 271 for intermediate-dose, and 490 for high-dose groups. These responses did not show a good correlation with the baseline responses, indicating long-term responses (figure 3C). Data from 37 (48%) of 76 ELISPot plates, assaying the trial samples, were removed from the final analysis because negative controls were above the predefined quality control parameters (56 spot forming cells) or the positive control failed. However, the available data for T cell response were not different than the T cell responses from the MERS001 trial in the UK (figure 3D).23
Figure 3.
T cell responses in patients vaccinated with a single-dose regimen of ChAdOx1 MERS
T cell responses measured by IFN-γ producing cells from all vaccinated individuals (A) or from each dose group (B) for 6 months post-vaccination, presented as SFC per 106 PBMCs. Correlation of paired T cell responses at day 0 versus day 182 (6 months) is presented (C) as analysed using Pearson's r test. T cell responses of patients in the Riyadh MERS002 trial (the current study) and the Oxford MERS001 trial are shown (D). Dotted lines represent the lowest detectable value by the assay. PBMCs=peripheral blood mononuclear cells. SFC=spot forming cells.
Discussion
In this study (MERS002), we evaluated ChAdOx1 MERS vaccine candidate in a phase 1b clinical trial in healthy Middle Eastern adults in the main endemic country of MERS-CoV, Saudi Arabia. The vaccine administered as a single dose was safe and well tolerated in all three dose groups. Our findings support the earlier phase 1a trial (MERS001) in the UK.23 Unlike the phase 1a study, higher reactogenicity was observed at the medium dose of 2·5 × 1010 virus particles, with feeling feverish, headaches, joint, and muscle pain in two participants. No severe adverse events in the high-dose group and no serious adverse reactions occurred in any of the participants.
In clinical trials of the recently approved ChAdOx1 vaccine against SARS-CoV-2, fatigue and headache were the most reported systemic reactions; and injection-site pain and tenderness were the most common solicited local adverse events.28, 29 These adverse events occurred mainly in the intermediate-dose and high-dose groups in our study. The boost vaccination with ChAdOx1 nCoV-19 resulted in fewer adverse events than did the prime vaccination, which might positively reflect the future design and development of homologous prime-boost ChAdOx1 MERS and other ChAdOx1-vectored vaccines.28, 29 Most of the recorded adverse events were mild or moderate in severity, and were all self-limiting. Heterologous prime-boost vaccination regimens have been evaluated in clinical trials for various vaccine candidates, most notably by use of DNA, adenoviral, and modified vaccinia Ankara poxviral vectors. These heterologous prime-boost vaccination regimens have resulted in an increased magnitude of immune responses.30, 31, 32, 33 For COVID-19 vaccines, a phase 2 trial evaluated a heterologous vaccination regimen with ChAdOx1 nCoV-19-prime and BNT162b2 boost, in 8–12 weeks intervals, showed a 14 day robust humoral and cellular immune response stronger than that from ChAdOx1 nCoV-19 prime-boost vaccination. No serious adverse events were reported.34 Other studies have also reported that a heterologous prime-boost vaccination regimen induced stronger, or similar, immunogenicity than did homologous regimens.35 MERS-CoV vaccine candidates could be considered for testing in heterologous prime-boost vaccination strategies with close observation of the possibility of higher adverse reaction rates than homologous prime-boost vaccination.
The adverse events reported in the present trial are not distinguished from other ChAdOx1 vectored vaccines. These adverse events are also similar for the newly developed ChAdOx1 nCoV-19 vaccine for COVID-19 and for other closely related simian adenovirus-based vaccines such as ChAdOx2, ChAd3, and ChAd63 vectored vaccines expressing different antigens.14, 15, 16, 17, 18, 19
Recent reports of rare development of immune thrombotic thrombocytopenia mediated by platelet-activating antibodies against PF4, which clinically mimics autoimmune heparin-induced thrombocytopenia, were associated with ChAdOx1 nCoV-19 vaccination.36 Such a reaction was not observed in our cohort; however, the sample size of this phase 1b was not powered to detect a rare event. Other MERS-CoV vaccine candidates were tested in clinical trials for GLS-5300 MERS-CoV DNA and Modified Vaccinia virus Ankara-based vaccine. Both vaccine candidates showed favourable safety profiles without serious adverse events. Similar to our findings, headaches, fatigue, and malaise were the most common systemic symptoms for the DNA and Modified Vaccinia virus Ankara-based vaccines.37, 38 These vaccines also induced strong antibody and T cell responses; and these trials had established the basis for selecting vaccine dose and boosting regimens to be further evaluated in phase 2 trials.
Immune responses induced by a single dose of ChAdOx1 MERS were evident at all three doses. Antibody responses were induced to a significantly higher concentration than at baseline, peaking at 4 weeks after vaccination, and plateauing until the end of follow-up at 6 months. Seroconversion occurred in almost all (92%) people who were vaccinated at day 28 after vaccination. The high-dose group showed a significantly higher concentration of antibodies from day 28 onwards. This differs from the UK-based MERS001 trial in which all vaccine doses induced similar levels of antibody responses;23 this observation was made because both MERS001 and MERS002 ELISAs were performed to the same protocol in the same laboratory. Although phase 1 trials are not designed primarily for immunogenicity evaluation, this interesting difference between these studies indicates that the vaccine might induce different levels of immune responses in different populations. Most responders showed antibody concentrations between 200 ELISA units and 1000 ELISA units at day 28. In addition, neutralising antibodies at day 28 were significantly higher than at baseline; supporting the data from the MERS001 trial. There were no significant differences between dose groups in neutralising antibody concentrations; consistent with the MERS001 data derived using a different neutralisation assay. As in the UK study, this study shows that the MERS-CoV-specific neutralising antibody response was low in some recipients suggesting that a second vaccination is warranted. A two-dose regimen has been used to vaccinate humans with ChAdOx1 nCoV-19, to increase the levels and durability of the SARS-CoV-2 specific immune responses. This strategy could be extended to ChAdOx1 MERS vaccine evaluation.17
T cell responses, specific to the ChAdOx1 MERS, were induced in all volunteers across dose groups, mostly at 1000–2000 spots per million cells at the peak, at day 14 after vaccination. Like previous data (MERS001),23 T cell responses peaked at day 14 with similar and overlapping levels across all dose groups. These concentrations of interferon-γ producing cells observed in the recently approved ChAdOx1 nCoV-19 vaccine showed a median of 856 spot-forming cells per million PBMCs (IQR 493–1802) at day 14 in a phase 1/2 clinical trial, analysing 43 of the 543 vaccinated patients.13, 14 This COVID-19 vaccine trial showed a drop by 50% in the level of these interferon-γ producing cells over the 6 months’ follow-up time, which is also observed in our study. Overall, ChAdOx1 MERS induced strong immune responses that peaked at day 14 for cellular immunogenicity and at day 28 for humoral immunogenicity and were similar to responses induced by ChAdOx1 nCoV-19. There is no confirmed correlate of protection for MERS or COVID-19, but the COVID-19 ChAdOx1 nCoV-19 vaccine has shown efficacy of 60–90% in phase 3 clinical trials.17 In addition, the ChAdOx1 nCoV-19 has been globally distributed and shown 80% effectiveness in real-world data studies, in people older than 70 years.18 Given the similarity between MERS-CoV and SARS-CoV-2, ChAdOx1 MERS could be a potential vaccine candidate for further clinical development.
Limitations of this study include the small sample size and the open-label, non-randomised, and uncontrolled trial design. Although T cell responses were analysed in only 51% of the samples of this study, the data are not different from the MERS001 trial, which analysed more samples for T cell immunogenicity. Long-term safety and immunogenicity might be warranted to have a better understanding of its safety and longevity. Nevertheless, as a phase 1b trial, this study provided information on reactogenicity and immunogenicity of the clinical use of ChAdOx1 MERS in an endemic area.
In conclusion, ChAdOx1 MERS was safe and well tolerated and able to elicit both humoral and cellular responses against MERS-CoV. The outcome of this phase 1b clinical trial supports clinical progress into phase 2 trials. These should include a large number of healthy adults, including adults older than 50 years, health-care workers, those occupationally exposed to or in close contact with camels, and other people at high risk to continue the assessment of the safety and immunogenicity of the ChAdOx1 MERS vaccine, which could be given as a single or two dose administration.
Data sharing
The study protocol is available with this publication as part of the appendix. Individual participant data are available on request addressed to the corresponding author, and after approval of a proposal, can be shared within a secure online platform. A data-sharing agreement will be needed.
Declaration of interests
SCG is a named inventor on a patent related to ChAdOx1 MERS. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
Funding was provided from the UK Department of Health and Social Care, using UK Aid funding, managed by the UK National Institute for Health Research. The King Abdullah International Medical Research Center financially supported the operational cost of this trial. Additionally, we thank King Abdulaziz Medical City in Riyadh, Ministry of National Guard Health Affairs (Riyadh, Saudi Arabia) for supporting the conduct of the trial. We thank Bandar Alknawy (Chief Executive Officer of Ministry of National Guard Health Affairs) who has been giving this trial special attention and support. We thank the Data and Safety Monitoring Board members for their input and critical review of the data throughout the study. HHB participated in this project during her tenure at Pediatric Infectious Diseases at King Saud bin Abdulaziz University for Health Science, Riyadh, Saudi Arabia, before joining WHO. The views expressed are those of the authors and not necessarily those of the Department of Health and Social Care.
Contributors
MBo, NKA, SCG, and HHB designed the study. HAA, MAlj, AJ, SA, RA, HAlh, NA, AAlo, PF, FRL, and MBo contributed to study design, protocol development, implementation management, and safety data monitoring. MAlj, KA, MAla, NKA, and MBo contributed to regulatory oversight and project management. NKA, HAA, MWA, AAlm, MBi, KE, JA, SB, and SCG conducted immunogenicity testing, laboratory testing, and validation. SA, HAlh, HAA, and MBo conducted safety data analysis and interpretation. MBo and NKA wrote the manuscript. All authors contributed to the reviewing and editing of the report and approved the final version. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.WHO . World Health Organization; Geneva: 2020. WHO Middle East respiratory syndrome coronavirus (MERS-CoV) [Google Scholar]
- 2.Killerby ME, Biggs HM, Midgley CM, Gerber SI, Watson JT. Middle East respiratory syndrome coronavirus transmission. Emerg Infect Dis. 2020;26:191–198. doi: 10.3201/eid2602.190697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 4.Alraddadi BM, Watson JT, Almarashi A, et al. Risk factors for primary Middle East respiratory syndrome coronavirus illness in humans, Saudi Arabia, 2014. Emerg Infect Dis. 2016;22:49–55. doi: 10.3201/eid2201.151340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan JF, Lau SK, Woo PC. The emerging novel Middle East respiratory syndrome coronavirus: the “knowns” and “unknowns”. J Formos Med Assoc. 2013;112:372–381. doi: 10.1016/j.jfma.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mou H, Raj VS, van Kuppeveld FJ, Rottier PJ, Haagmans BL, Bosch BJ. The receptor binding domain of the new Middle East respiratory syndrome coronavirus maps to a 231-residue region in the spike protein that efficiently elicits neutralizing antibodies. J Virol. 2013;87:9379–9383. doi: 10.1128/JVI.01277-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adney DR, van Doremalen N, Brown VR, et al. Replication and shedding of MERS-CoV in upper respiratory tract of inoculated dromedary camels. Emerg Infect Dis. 2014;20:1999–2005. doi: 10.3201/eid2012.141280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohd HA, Al-Tawfiq JA, Memish ZA. Middle East respiratory syndrome coronavirus (MERS-CoV) origin and animal reservoir. Virol J. 2016;13:87. doi: 10.1186/s12985-016-0544-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO Middle East respiratory syndrome coronavirus (MERS-CoV) - WHO disease outbreak news. Aug 17, 2021. https://www.who.int/emergencies/disease-outbreak-news/item/2021-DON333
- 10.Conzade R, Grant R, Malik MR, et al. Reported direct and indirect contact with dromedary camels among laboratory-confirmed MERS-CoV cases. Viruses. 2018;10:e425. doi: 10.3390/v10080425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alshukairi AN, Zheng J, Zhao J, et al. High prevalence of MERS-CoV infection in camel workers in Saudi Arabia. MBio. 2018;9:e01985–e02018. doi: 10.1128/mBio.01985-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Y, Jiang S, Du L. Prospects for a MERS-CoV spike vaccine. Expert Rev Vaccines. 2018;17:677–686. doi: 10.1080/14760584.2018.1506702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alharbi NK. Vaccines against Middle East respiratory syndrome coronavirus for humans and camels. Rev Med Virol. 2017;27 doi: 10.1002/rmv.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilbert SC, Warimwe GM. Rapid development of vaccines against emerging pathogens: The replication-deficient simian adenovirus platform technology. Vaccine. 2017;35(35 Pt A):4461–4464. doi: 10.1016/j.vaccine.2017.04.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antrobus RD, Coughlan L, Berthoud TK, et al. Clinical assessment of a novel recombinant simian adenovirus ChAdOx1 as a vectored vaccine expressing conserved influenza A antigens. Mol Ther. 2014;22:668–674. doi: 10.1038/mt.2013.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dicks MD, Spencer AJ, Edwards NJ, et al. A novel chimpanzee adenovirus vector with low human seroprevalence: improved systems for vector derivation and comparative immunogenicity. PLoS One. 2012;7 doi: 10.1371/journal.pone.0040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernal JL, Andrews N, Gower C, et al. Early effectiveness of COVID-19 vaccination with BNT162b2 mRNA vaccine and ChAdOx1 adenovirus vector vaccine on symptomatic disease, hospitalisations and mortality in older adults in England. medRxiv. 2021 doi: 10.1101/2021.03.01.21252652. published online March 2. [DOI] [Google Scholar]
- 19.Alharbi NK, Qasim I, Almasoud A, et al. Humoral immunogenicity and efficacy of a single dose of ChAdOx1 MERS vaccine candidate in dromedary camels. Sci Rep. 2019;9 doi: 10.1038/s41598-019-52730-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alharbi NK, Padron-Regalado E, Thompson CP, et al. ChAdOx1 and MVA based vaccine candidates against MERS-CoV elicit neutralising antibodies and cellular immune responses in mice. 2017;35:3780–3788. doi: 10.1016/j.vaccine.2017.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munster VJ, Wells D, Lambe T, et al. Protective efficacy of a novel simian adenovirus vaccine against lethal MERS-CoV challenge in a transgenic human DPP4 mouse model. NPJ Vaccines. 2017;2:28. doi: 10.1038/s41541-017-0029-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Doremalen N, Haddock E, Feldmann F, et al. A single dose of ChAdOx1 MERS provides protective immunity in rhesus macaques. Sci Adv. 2020;6 doi: 10.1126/sciadv.aba8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Folegatti PM, Bittaye M, Flaxman A, et al. Safety and immunogenicity of a candidate Middle East respiratory syndrome coronavirus viral-vectored vaccine: a dose-escalation, open-label, non-randomised, uncontrolled, phase 1 trial. Lancet Infect Dis. 2020;20:816–826. doi: 10.1016/S1473-3099(20)30160-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Food and Drug Administration Guidance for Industry: toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. https://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/ucm091977.pdf [DOI] [PubMed]
- 25.Almasaud A, Alharbi NK, Hashem AM. In: Methods in molecular biology. Vijay R, editor. Humana; New York, NY: 2020. Generation of MERS-CoV pseudotyped viral particles for the evaluation of neutralizing antibodies in mammalian sera. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grehan K, Ferrara F, Temperton N. An optimised method for the production of MERS-CoV spike expressing viral pseudotypes. MethodsX. 2015;2:379–384. doi: 10.1016/j.mex.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Folegatti PM, Bellamy D, Flaxman A, et al. Safety and immunogenicity of the heterosubtypic influenza A vaccine MVA-NP+M1 manufactured on the AGE1.CR.pIX avian cell line. Vaccines (Basel) 2019;7:e33. doi: 10.3390/vaccines7010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramasamy MN, Minassian AM, Ewer KJ, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396:1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gilbert SC, Moorthy VS, Andrews L, et al. Synergistic DNA-MVA prime-boost vaccination regimes for malaria and tuberculosis. Vaccine. 2006;24:4554–4561. doi: 10.1016/j.vaccine.2005.08.048. [DOI] [PubMed] [Google Scholar]
- 31.Kardani K, Bolhassani A, Shahbazi S. Prime-boost vaccine strategy against viral infections: mechanisms and benefits. Vaccine. 2016;34:413–423. doi: 10.1016/j.vaccine.2015.11.062. [DOI] [PubMed] [Google Scholar]
- 32.Lu S. Heterologous prime-boost vaccination. Curr Opin Immunol. 2009;21:346–351. doi: 10.1016/j.coi.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hill AV, Reyes-Sandoval A, O'Hara G, et al. Prime-boost vectored malaria vaccines: progress and prospects. Hum Vaccin. 2010;6:78–83. doi: 10.4161/hv.6.1.10116. [DOI] [PubMed] [Google Scholar]
- 34.Borobia AM, Carcas AJ, Pérez-Olmeda M, et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet. 2021;398:121–130. doi: 10.1016/S0140-6736(21)01420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hillus D, Schwarz T, Tober-Lau P, et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1-nCoV19 and BNT162b2: a prospective cohort study. medRxiv. 2021 doi: 10.1016/S2213-2600(21)00357-X. published online Jan 1. 2021.05.19.21257334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N Engl J Med. 2021;384:2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Modjarrad K, Roberts CC, Mills KT, et al. Safety and immunogenicity of an anti-Middle East respiratory syndrome coronavirus DNA vaccine: a phase 1, open-label, single-arm, dose-escalation trial. Lancet Infect Dis. 2019;19:1013–1022. doi: 10.1016/S1473-3099(19)30266-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koch T, Dahlke C, Fathi A, et al. Safety and immunogenicity of a modified vaccinia virus Ankara vector vaccine candidate for Middle East respiratory syndrome: an open-label, phase 1 trial. Lancet Infect Dis. 2020;20:827–838. doi: 10.1016/S1473-3099(20)30248-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The study protocol is available with this publication as part of the appendix. Individual participant data are available on request addressed to the corresponding author, and after approval of a proposal, can be shared within a secure online platform. A data-sharing agreement will be needed.