Summary
Chronic stress is a crucial public issue that occurs when a person is repetitively stimulated by various stressors. Previous researches have reported that chronic stress induces spermatogenesis dysfunction in the reproductive system, but its molecular mechanisms remain unclear. The nectin protein family, including nectin-1 to nectin-4, is Ca2+-independent immunoglobulin-like cell adhesion molecules, that are widely expressed in the hippocampus, testicular tissue, epithelial cells and other sites. Nectin-3 contributes to the sperm development at the late stage, and the abnormal expression of nectin-3 impairs spermatogenesis. Some recent studies have demonstrated that stress induces a decrease in nectin-3 expression in the hippocampus via corticotropin-releasing hormone (CRH) to corticotropin-releasing hormone receptor 1 (CRHR1) pathway. Here, we tested whether chronic stress also caused a reduction in nectin-3 expression in the testis. We established a chronic social defeat stress paradigm, which provides naturalistic and complex chronic stress in male C57BL/6 mice. After 25 days of chronic social defeat stress, the mice showed weight loss, thymic atrophy and some other typical symptoms of chronic stress (e.g. anxiety-like behavior and social avoidance behavior). We found gonad atrophy, testicular histological structure changes and semen quality reductions in the stressed mice. The stressed male mice significantly spent more time to impregnate the female mice than the control male mice. Moreover, nectin-3 protein levels in stressed mice were significantly decreased in the testes compared with those in control mice. In addition, we found that the CRHR1 expression level was increased in the testes of stressed mice. Therefore, we demonstrated a decreased level of nectin-3 expression and an increase in CRHR1 expression in the testis after exposure to chronic stress, which may provide a potential therapeutic target for the spermatogenesis dysfunction induced by chronic stress.
Keywords: Chronic social stress, Nectin-3, Spermatogenesis dysfunction, CRH and CRHR1
Introduction
Chronic stress is a crucial public issue that occurs when a person suffers uninterrupted stimulation from various stressors (Chrousos 2009). Chronic stress results in psychological and biological changes, and it affects endocrine response systems, immune responses, physiological processes and even behaviors (Chrousos and Gold 1992, Charmandari et al. 2005, Liu et al. 2015, Nicolaides et al. 2015). Many somatic and mental disorders are considered to be caused by chronic stress, including depression (Schmidt et al. 2010). In the reproductive system, chronic stress mainly leads to a decrease in semen quality and thus spermatogenesis dysfunction (Fenster et al. 1997, Janevic et al. 2014). However, the molecular mechanisms of chronic stress-induced reproductive dysfunction remain largely unknown.
Nectin-3 is a Ca2+-independent immunoglobulin-like cell adhesion molecule and widely expressed in the hippocampus, testicular tissue, epithelial cells and other sites (Irie et al. 2004, Kopera et al. 2010). Nectin-3 is localized at the spermatid side of Sertoli–spermatid junctions in the testis and plays a very important role in male reproductive function (Huang and Lui 2016). Nectin-3−/− mice display male-specific infertility due to functionally impaired spermatogenesis (Inagaki et al. 2006). Under chronic stress, the overactivated hypothalamus-pituitary-gonadal (HPG) axis and hypothalamus-pituitary-adrenal (HPA) axis lead to the release of different kinds of neuroendocrine hormones in the body (Chrousos 2009, Kalantaridou et al. 2010, Nicolaides et al. 2015). Among these hormones, corticotropin-releasing hormone (CRH) has destructive effects on the body and tissues (Kalantaridou et al. 2010). Recent researches have proved that nectin-3 expression levels in the hippocampus are decreased via CRH to corticotropin-releasing hormone receptor 1 (CRHR1) signaling pathway, resulting in stress-induced cognitive deficits (Wang et al. 2011, Wang et al. 2013). These findings indicate that nectin-3 is closely related to the stress reaction.
Here, we tested the possible effects of chronic stress on nectin-3 expression in the testis. We observed that, using the chronic social defeat stress paradigm, chronic stress impaired both social behaviors and spermatogenesis function. In addition, we found a significant decrease in nectin-3 expression level and an increase in CRHR1 expression level in testis.
Methods
Animals
Two kinds of male mice (C57BL/6 mice aged 2–3 months, and FVB/N mice aged 5 months) were used in all experiments. The mice were purchased from the Laboratory Animal Center at the Third Military Medical University. Before all experiments, C57BL/6 mice were socially housed and FVB/N mice were individually housed under a 12-h light/dark cycle. The animals were housed at a constant temperature (22±1 °C) with free access to food and water. All C57BL/6 mice were randomly divided into the following two groups: stressed mice and control mice. The Third Military Medical University Animal Care and Use Committee approved all experimental procedures, which were performed in accordance with institutional animal welfare guidelines.
Chronic social defeat paradigm
The chronic social defeat paradigm was performed according to previous reports (Golden et al. 2011, Berton et al. 2006), with minor modifications (Figure 1A). Adult male FVB/N mice were used as aggressive resident mice. Their aggressive behavior was trained in 3 sessions, and every session lasted 10 min with the C57BL/6 male mice. FVB/N mice with attack latencies of more than 5 min in the last training session were excluded from the experiments. The chronic social defeat stress procedure was performed between 3 PM and 6 PM and lasted for 25 days. Each stressed male C57BL/6 mouse was introduced into the home cage of a new aggressive resident FVB/N mouse each day. Then, the resident FVB/N mice rapidly recognized and attacked the C57BL/6 mice. After the aggressive encounter for 10 min, the stressed male C57BL/6 mice and FVB/N resident mice were separated by a holed plastic partition. The mice were only allowed to smell and hear each other without physical contact for the next 24 h. Control mice underwent similar handling without using FVB/N mice.
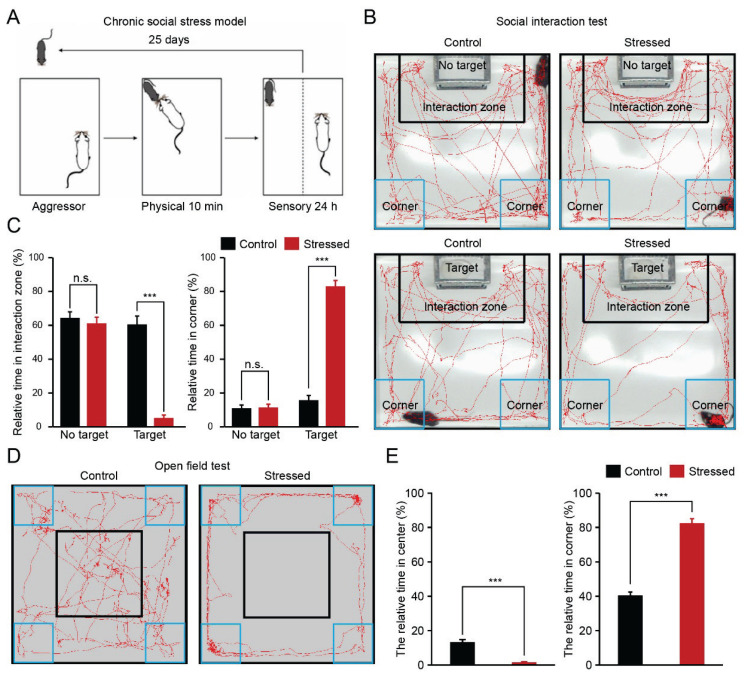
Fig. 1.
Chronic stress induced behavior alterations (A) A schematic showing the chronic social defeat stress paradigm. (B) Representative examples of the exploration traces of different groups revealed by the social interaction test. (C) Comparison of the relative time in the interaction/corner zone of different groups (n=15 mice for each group, *** p <0.001, ns, no significant difference). (D) Representative examples of the exploration traces of different groups revealed by the open field test. (E) Comparison of the relative time in the center/corner zone of different groups (n=15 mice for each group, *** p <0.001).
Open Field Test (OFT)
The OFT is a typical behavior test to assess anxiety performance. The locomotor activity of each mouse was evaluated by OFT after a 25-day social defeat procedure. The open field arena is a white opaque wooden chamber (42 × 42 × 42 cm3) divided into 16 identical sectors. The chamber was subdivided into peripheral and central sectors. The animals were placed into the center of the chamber and allowed to explore for 5 min. The open field arena was thoroughly cleaned with 70 % ethanol among each test. The behavior of each mouse was recorded by the camera placed above the chamber. The time that spent in the center area and corner area were analyzed automatically by the custom-written tracking software written in MATLAB.
Social interaction test
The social interaction test was conducted in the open field arena as previously described (Golden et al. 2011, Berton et al. 2006). In contrast, there was an iron-propped cage (10 × 6 × 10 cm3, self-restraint) placed in the middle of one wall of the chamber. The experiments were divided into two sections. First, C57BL/6 mice were placed in the center of the chamber and then allowed to explore the open field arena for 2.5 min. Then, the mouse was returned to its home cage for 1 min. After a FVB/N mouse was placed in the iron-propped cage, the same C57BL/6 mouse was placed in the chamber and allowed to explore the open field arena for 2.5 min again. All experimental procedures were recorded by the camera placed above the arena. The time spent in the interaction zone and corner zone were analyzed automatically by the custom-written tracking software written in MATLAB. Defeated mice that spent less time in the interaction zone were thought “susceptible” to chronic social defeat stress (Golden et al. 2011, Berton et al. 2006, Wang et al. 2017a), and were included in the following experiments.
Immunohistochemistry
The testes obtained from each mouse were fixed in the Bouin’s solution for 24 h. Coronal 5 μm thick testis sections were cut with a freezing microtome (Leica). Then, these testis sections were subjected to three 10 min washes with phosphate buffer saline (PBS) and permeabilized using PBST (1 % Triton X-100 in PBS) for 40 min. The blocking solution (10 % donkey serum, 0.2 % PBST) was applied for 2 h at the room temperature. All slices were incubated with primary antibodies (rabbit anti-nectin-3, 1:200, Abcam; goat anti-CRHR1, 1:200, Abcam) overnight at 4 °C, followed by secondary antibodies for 2 h at the room temperature. The secondary antibodies used were donkey anti-rabbit IgG conjugated with Alexa Fluor 568 (1:200, Invitrogen) and donkey anti-goat IgG conjugated with Alexa Fluor 594 (1:200, Invitrogen). For DAPI staining, the testis slices were incubated with DAPI (1:1000, Sigma) for 10 min. Thereafter, these slices were mounted onto coverslips and imaged. A scanning confocal microscope (TCS SP5, Leica) and LAS AF software (Leica) were used to obtain images.
Hematoxylin-eosin (HE) staining
All testes were collected as described above. Coronal testis sections, 5 μm thick, were obtained by using a freezing microtome (Leica). Each testis slice underwent 5 min of immersion in hematoxylin solution. The slices were transferred to 1 % acid alcohol for 30 s. After rinsing with 95 % alcohol, the testis sections were stained with eosin solution for 3 min and dehydrated in a graded series of ethanol. Finally, these sections were cleared in xylene solutions and mounted onto coverslips. All images were examined under the bright field illumination by use of a light microscope (Olympus).
Sperm count
The epididymal sperm count was conducted as previously described (Parhizkar et al. 2013). The epididymal fluid was collected by mincing the cauda epididymis in normal saline (0.9 % NaCl in distilled water) at 37 °C. A 10-μl aliquot of diluted cauda epididymal fluid was placed in a Neubauer hemocytometer, and the total number of sperms was counted under bright field illumination using a light microscope (Olympus). The sperm morphology was assessed by the preparation of a smear and the application of HE staining. Abnormal sperms were detected mainly on head and tail based on previous criteria (Ilgin 2017).
Western blotting
The testis was lysed in radioimmunoprecipitation assay (RIPA) buffer plus protease and phosphatase inhibitor. Total protein concentrations were determined by using the BCA method. Equal amounts of total proteins (25–50 μg/lane) were first separated by SDS-PAGE gels and then transferred to polyvinylidene fluoride membranes (PVDF, 0.45 μm, Millipore) using electroblotting. The PVDF membranes were blocked for 2 h with 5 % skim milk powder dissolved in TBST (0.1 %). The membranes were incubated with primary antibodies (rabbit anti-nectin-3 antibody, 1:200, Abcam; goat anti-CRHR1 antibody, 1:200, Abcam; mouse anti-GAPDH antibody, 1:1000, Beyotime) overnight at 4 °C. After incubation with secondary antibodies for 2 h, the protein bands were visualized using an enhanced chemiluminescence system and quantified by densitometry (Quantity One 4.2, Bio-Rad). The secondary antibodies used were horseradish peroxidase-conjugated goat anti-rabbit antibody (1:2000, Beyotime), rabbit anti-goat antibody (1:2000, Beyotime) and goat anti-mouse secondary antibody (1:2000, Beyotime).
Statistical analysis
All data are represented as the means±sem. Comparisons of two independent groups were performed using unpaired Student’s t-test or Wilcoxon rank-sum test. Statistical analyses were performed in MATLAB (MathWorks). Statistical significance was set at p<0.05.
Results
Chronic social defeat stress induced anxiety and depression-like behaviors in male mice
To evaluate whether the chronic social defeat stress paradigm was established successfully, we performed two kinds of behavior tests after 25 days of chronic social defeat stress (Fig. 1A). By use of a social interaction test (Fig. 1B), we tested social-avoidance behavior (depression-like phenotype). No significant differences were found in the relative time spent in the interaction zone or corner zone under nonsocial conditions (no aggressor in the target zone, “no target”) between stressed and control mice (Fig. 1C, interaction zone: 61.17±3.64 % vs 64.41±3.52 %; corner zone: 11.54±1.92 % vs 11.07±1.86 %). However, under social conditions (aggressor present in target zone, “target”), stressed mice spent less time in the interaction zone and more time in the corner zone compared with control mice (Fig. 1C, interaction zone: 5.27±1.74 % vs 60.61±4.87 %; corner zone: 83.06±3.37 % vs 15.73±2.80 %). Then, the open field test was used to assess the anxiety performance (Fig. 1D). Control mice spent more time in the center area of the open field, whereas stressed mice mainly stayed in the corner area of the open field (Figs 1D–E, stressed mice vs control mice; center: 1.46±0.45 % vs 13.12±1.50 %; corner: 82.58±2.57 % vs 40.46±2.01 %). Together, these results suggest that chronic social defeat stress induced anxiety and depression-like behaviors.
Chronic social defeat stress impaired the spermatogenesis function of male mice
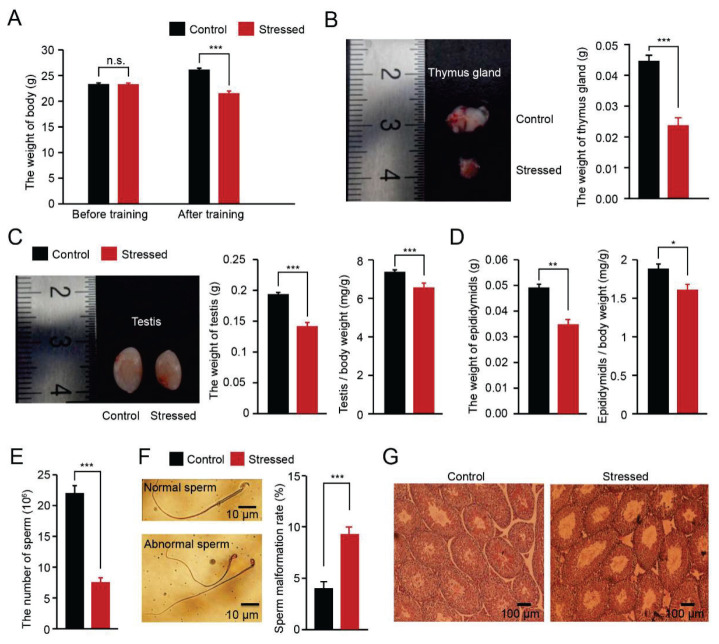
After 25 days of chronic social defeat stress, the stressed mice showed other typical symptoms of depression, such as weight loss and thymus atrophy (Fig. 2A–B, body weight: 23.31±0.23 g vs 23.37±0.20 g, 21.55±0.42 g vs 26.16±0.27 g; thymus gland weight: 0.024±0.002 g vs 0.045±0.001 g). The weight of the testis and epididymis were reduced in the stressed mice compared with the control mice (Fig. 2C–D, testis: 0.14±0.01 g vs 0.19±0.003 g; epididymis: 0.03±0.002 g vs 0.05±0.001 g). To avoid the interference of body weight, we also calculated the organ coefficient of the testis and epididymis (Fig. 2C–D, testis: 0.66±0.02 % g vs 0.74±0.01 %; epididymis: 0.16±0.01 % g vs 0.19±0.01 %). We further detected the semen quality and histological changes in testis sections from each group. The number of epididymal sperm was significantly decreased, and the rate of sperm deformation in morphology was higher in stressed mice than in control mice (Fig. 2E–F, 7.52±0.76 vs 22.06±1.21; 9±0.7 % vs 4±0.6 %). The histological analysis showed that the basement membranes of the seminiferous tubules were thinner in the stressed mice than in the control mice (Fig. 2G). To examine the effects of chronic stress on male mice fertility, each male mouse in each group was housed with two adult female mice. We found the stressed male mice significantly spent more time to impregnate the female micethan control male mice (Table 1). These data indicate that chronic social defeat stress impaired semen quality and spermatogenesis function.
Fig. 2.
Chronic social stress impaired the spermatogenesis function of male mice (A) Comparison of the body weights of different groups before and after the chronic stress paradigm (n=15 mice for each group, *** p<0.001; n.s., no significant difference). (B) Representative images of the thymus glands of different groups and comparison of the thymus gland weight of different groups (n=15 mice for each group, *** p<0.001). (C–D) Comparison of the testis and cauda epididymis of different groups. Representative images of the testes of different groups. Comparison of the organ coefficient of testis and cauda epididymis of different groups (n=15 mice for each group, ** p<0.01; * p<0.05). (E) Comparison of the sperm counts of different groups (n=15 mice for each group, *** p<0.001). (F) Comparison of the sperm abnormalities in different groups (n=8 mice for each group, *** p<0.001). (G) Representative examples of HE stained testis sections.
Table 1.
Chronic stress impaired the fertility of male mice
| Group | Number of female mice | Pregnant females | ||
|---|---|---|---|---|
| n | % | Time (month) | ||
| Control | 8 | 8 | 100 | 1 |
| Stressed | 8 | 0 | 0 | 1*** |
| 1 | 12.5 | 2 | ||
| 8 | 100 | 3 | ||
Four male mice of each group were used for this experiment. Within one-month observation time window, the control male mice impregnated all the female mice, while the stressed male mice impregnated 0 female mice.
p<0.001, Wilcoxon rank-sum test.
Chronic social defeat stress reduced nectin-3 protein expression and elevated CRHR1 expression
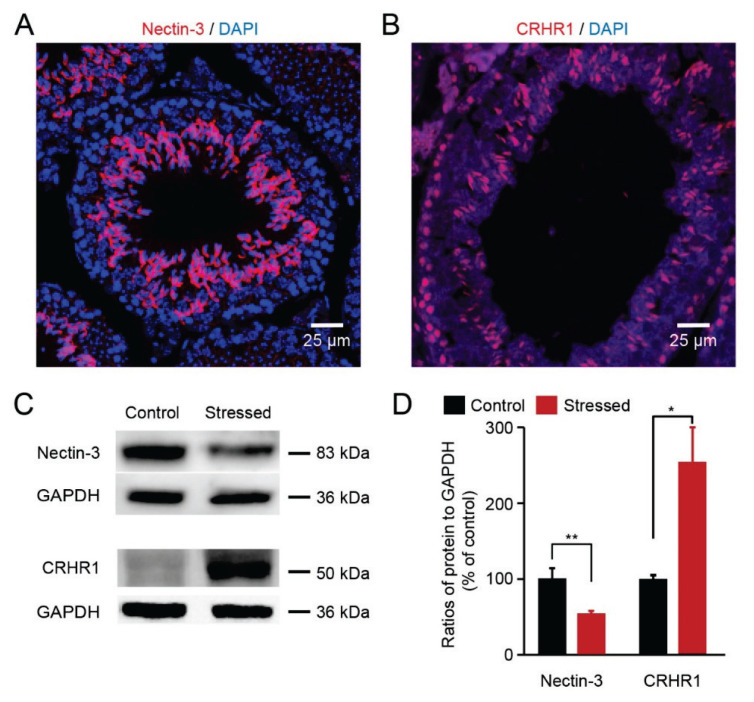
Next, we focused on the location of each protein (nectin-3 and CRHR1) in the testis. Immunohistochemical analyses of testis sections showed that nectin-3 was localized at the membrane of the spermatid head and CRHR1 was located in spermatocytes (Fig. 3A–B). Then, we investigated the protein expression of nectin-3 and CRHR1 in the testis. The nectin-3 protein levels were significantly decreased in the stressed mice compared with the control mice (Fig. 3C–D, 53.9 %±3.0 % vs 100 %±13.2 %). However, CRHR1 protein levels were increased in the stressed mice, compared with the control mice (Fig. 3C–D, 255.1 %±44.9 % vs 100 %±5.0 %). Thus, our results demonstrate that chronic social defeat stress induced the reduction of nectin-3 protein levels and the elevation of CRHR1 protein levels.
Fig. 3.
Chronic stress reduced nectin-3 protein expression and elevated CRHR1 expression (A) Immunofluorescence staining showed that nectin-3 staining colocalized with DAPI on the testis sections of each group. (B) Immunofluorescence staining showed that CRHR1 staining colocalized with DAPI on the testis sections. (C) Visualization of the protein bands of nectin-3 and CRHR1. (D) Quantitative analysis showed that nectin-3 protein levels were decreased and CRHR1 expression levels were increased in the testes of stressed mice (n=4 mice for each group, ** p <0.01, * p <0.05).
Discussion
In our present research, we studied the effects of depression on spermatogenesis function in male mice. All experiments were based on a previously established animal model of depression using the chronic social defeat stress paradigm. Our results showed that the stressed male mice displayed behavioral abnormalities, including social avoidance behavior and anxiety behavior. These mice also exhibited weight loss, thymus atrophy, gonad atrophy and spermatogenesis dysfunction. They were unable to impregnate the female mice temporarily within one month of observation time window. Furthermore, chronic social defeat stress induced a reduction in nectin-3 protein levels and an elevation in CRHR1 protein levels. Several reports have suggested that chronic stress impairs cell adhesion (including nectin-1 and nectin-3) in the brain (Wang et al. 2011, Wang et al. 2013, Wang et al. 2017b). Taken together, the expression levels of the nectin protein family are significantly affected by chronic stress.
It is a critical challenge to develop a reliable animal model to study the effect of depression on spermatogenesis function. Many studies have reported various kinds of chronic stress models, including restraint stress, foot-shock stress, chronic mild stress, chronic unpredictable stress and chronic social defeat stress (Nestler and Hyman 2010, Wang et al. 2012, Saki et al. 2009). We finally chose the naturalistic and complex chronic social defeat stress paradigm because of its unique advantage of having the ability to persistently activate the HPA axis through social confrontations (Golden et al. 2011). In this model, the stressed mice suffer from repeated social defeat and continuous psychological stress from sensory interactions with the aggressor each day (Golden et al. 2011). Chronic social defeat stress in rodents reliably induces robust depression-like performance marked by anxiety and social-avoidance behaviors (Berton et al. 2006, Rygula et al. 2006, Wang et al. 2017a). Our results also showed these depression-like phenotypes in the stressed mice, which revealed the feasibility and reliability of chronic social defeat stress to study depression.
Chronic stress is a risk factor for the development of male reproductive dysfunction. Several clinical reports have shown that stress reduces the count and motility of human sperm (Gollenberg et al. 2010, Fenster et al. 1997). Moreover, some kinds of chronic stress model studies in animals also revealed that chronic stress can cause a significant decline in sperm motility and count, fertilization capacity and organic damage in male germ cells (Saki et al. 2009, Wang et al. 2017a, Ribeiro et al. 2018). It has been reported that the basement membranes of the seminiferous tubules in stressed rats were thinner and had a low density, with enhanced apoptosis in germ cells after 5 weeks of chronic unpredictable mild stress exposure (Hou et al. 2014). Chronic immobilization stress leads to delayed testicular maturation in male rats (Almeida et al. 2000). The testis and germ cells mature are closely related to gonadotropins and sex hormones. Some studies have shown that chronic stress blocks the secretion of gonadotropins or testosterone mainly through CRH (Ghizzoni et al. 1997). CRH and its receptors have been identified in the testis (Tao et al. 2007), where CRH exerts autocrine inhibitory actions on testosterone biosynthesis (Fabbri et al. 1990). We also found that CRHR1 is located in spermatocytes and that CRHR1 expression is increased by chronic stress.
Nectins are Ca2+-independent, Ig-like transmembrane proteins that are wildly found in the brain and testicular tissue (Irie et al. 2004, Kopera et al. 2010). The mechanism of chronic stress-induced male spermatogenesis function could be highly correlated with the changes in the expressions of Nectin-3 and CRHR1 in testis. The previous study has shown the elevated CRH–CRHR1 signaling mediates the effects of stress-induced cognitive deficits by reducing nectin-3 protein level in the CA3 region of the hippocampus (Wang et al. 2013). Recent study has also confirmed that nectin-3 in the dentate granule region of the hippocampus is a target of stress and CRH-CRHR1 pathway (Wang et al. 2011, Wang et al. 2013, Wang et al. 2017b). Therefore, nectin-3 plays as a downstream molecule of CRHR1 and is implicated in stress-induced cognitive deficits and synaptic abnormalities in the brain (Wang et al. 2013). The intratesticular nectin protein family mainly contains nectin-2 (in Sertoli cells) and nectin-3 (on the membrane of the spermatid head) (Kopera et al. 2010). These proteins interact with each other and underlie the Sertoli cell-spermatid junctions to conduce to the sperm development at the late stage (Huang and Lui 2016). In addition, previous studies have found that, in testis, the abnormal expression of nectins (including nectin-3) impairs spermatogenesis (Inagaki et al. 2006, Toyama et al. 2008). In our study, we found that the chronic social defeat stress induced the reduction of nectin-3 protein levels and the elevation of CRHR1 protein levels in the stressed mice. Therefore, based on our results, we suggest that the reduction of nectin-3 protein level may be caused by the elevated CRH–CRHR1 expression in the testis, which in turn impairs spermatogenesis and fertility of the stress male mice. We still need to do some further investigation.
In summary, our work demonstrates that chronic social defeat stress induces anxiety and depression-like behaviors, decreases the weight of the testis and epididymis, reduces sperm count and elevates sperm abnormalities in male mice. Interestingly, the testicular protein levels of nectin-3 and CRHR1 are significantly disrupted in the stressed mice. Our results may provide novel insight into the unraveling molecular mechanisms responsible for stress-induced spermatogenesis dysfunction.
Footnotes
Conflict of interest
There is no conflict of interest.
References
- ALMEIDA SA, PETENUSCI SO, FRANCI JA, ROSA E SILVA AA, CARVALHO TL. Chronic immobilization-induced stress increases plasma testosterone and delays testicular maturation in pubertal rats. Andrologia. 2000;32:7–11. doi: 10.1111/j.1439-0272.2000.tb02858.x. [DOI] [PubMed] [Google Scholar]
- BERTON O, McCLUNG CA, DILEONE RJ, KRISHNAN V, RENTHAL W, RUSSO SJ, GRAHAM D, TSANKOVA NM, BOLANOS CA, RIOS M, MONTEGGIA LM, SELF DW, NESTLER EJ. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- CHARMANDARI E, TSIGOS C, CHROUSOS G. Endocrinology of the stress response. Annu Rev Physiol. 2005;67:259–284. doi: 10.1146/annurev.physiol.67.040403.120816. [DOI] [PubMed] [Google Scholar]
- CHROUSOS GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5:374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- CHROUSOS GP, GOLD PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–1252. doi: 10.1001/jama.267.9.1244. [DOI] [PubMed] [Google Scholar]
- FABBRI A, TINAJERO JC, DUFAU ML. Corticotropin-releasing factor is produced by rat Leydig cells and has a major local antireproductive role in the testis. Endocrinology. 1990;127:1541–1543. doi: 10.1210/endo-127-3-1541. [DOI] [PubMed] [Google Scholar]
- FENSTER L, KATZ DF, WYROBEK AJ, PIEPER C, REMPEL DM, OMAN D, SWAN SH. Effects of psychological stress on human semen quality. J Androl. 1997;18:194–202. doi: 10.1097/00005392-199811000-00100. [DOI] [PubMed] [Google Scholar]
- GHIZZONI L, MASTORAKOS G, VOTTERO A, BARRECA A, FURLINI M, CESARONE A, FERRARI B, CHROUSOS GP, BERNASCONI S. Corticotropin-releasing hormone (CRH) inhibits steroid biosynthesis by cultured human granulosa-lutein cells in a CRH and interleukin-1 receptor-mediated fashion. Endocrinology. 1997;138:4806–4811. doi: 10.1210/endo.138.11.5474. [DOI] [PubMed] [Google Scholar]
- GOLDEN SA, COVINGTON HE, 3RD, BERTON O, RUSSO SJ. A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 2011;6:1183–1191. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLLENBERG AL, LIU F, BRAZIL C, DROBNIS EZ, GUZICK D, OVERSTREET JW, REDMON JB, SPARKS A, WANG C, SWAN SH. Semen quality in fertile men in relation to psychosocial stress. Fertil Steril. 2010;93:1104–1111. doi: 10.1016/j.fertnstert.2008.12.018. [DOI] [PubMed] [Google Scholar]
- HOU G, XIONG W, WANG M, CHEN X, YUAN TF. Chronic stress influences sexual motivation and causes damage to testicular cells in male rats. J Sex Med. 2014;11:653–663. doi: 10.1111/jsm.12416. [DOI] [PubMed] [Google Scholar]
- HUANG K, LUI WY. Nectins and nectin-like molecules (Necls): Recent findings and their role and regulation in spermatogenesis. Semin Cell Dev Biol. 2016;59:54–61. doi: 10.1016/j.semcdb.2016.01.034. [DOI] [PubMed] [Google Scholar]
- ILGIN S, KILIC G, BAYSAL M, KILIC V, KORKUT B, UCARCAN S, ATLI O. Citalopram induces reproductive toxicity in male rats. Birth Defects Res. 2017;109:475–485. doi: 10.1002/bdr2.1010. [DOI] [PubMed] [Google Scholar]
- INAGAKI M, IRIE K, ISHIZAKI H, TANAKA-OKAMOTO M, MIYOSHI J, TAKAI Y. Role of cell adhesion molecule nectin-3 in spermatid development. Genes Cells. 2006;11:1125–1132. doi: 10.1111/j.1365-2443.2006.01006.x. [DOI] [PubMed] [Google Scholar]
- IRIE K, SHIMIZU K, SAKISAKA T, IKEDA W, TAKAI Y. Roles and modes of action of nectins in cell-cell adhesion. Semin Cell Dev Biol. 2004;15:643–656. doi: 10.1016/S1084-9521(04)00088-6. [DOI] [PubMed] [Google Scholar]
- JANEVIC T, KAHN LG, LANDSBERGIS P, CIRILLO PM, COHN BA, LIU X, FACTOR-LITVAK P. Effects of work and life stress on semen quality. Fertil Steril. 2014;102:530–538. doi: 10.1016/j.fertnstert.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALANTARIDOU SN, ZOUMAKIS E, MAKRIGIANNAKIS A, LAVASIDIS LG, VREKOUSSIS T, CHROUSOS GP. Corticotropin-releasing hormone, stress and human reproduction: an update. J Reprod Immunol. 2010;85:33–39. doi: 10.1016/j.jri.2010.02.005. [DOI] [PubMed] [Google Scholar]
- KOPERA IA, BILINSKA B, CHENG CY, MRUK DD. Sertoli-germ cell junctions in the testis: a review of recent data. Philos Trans R Soc Lond B Biol Sci. 2010;365:1593–1605. doi: 10.1098/rstb.2009.0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU W, WANG X, MEI Z, GONG J, GAO X, ZHAO Y, MA J, XIE F, QIAN L. Chronic stress promotes the progression of pressure overload-induced cardiac dysfunction through inducing more apoptosis and fibrosis. Physiol Res. 2015;64:325–334. doi: 10.33549/physiolres.932778. [DOI] [PubMed] [Google Scholar]
- NESTLER EJ, HYMAN SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICOLAIDES NC, KYRATZI E, LAMPROKOSTOPOULOU A, CHROUSOS GP, CHARMANDARI E. Stress, the stress system and the role of glucocorticoids. Neuroimmunomodulation. 2015;22:6–19. doi: 10.1159/000362736. [DOI] [PubMed] [Google Scholar]
- PARHIZKAR S, YUSOFF MJ, DOLLAH MA. Effect of Phaleria macrocarpa on Sperm Characteristics in Adult Rats. Adv Pharm Bull. 2013;3:345–352. doi: 10.5681/apb.2013.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIBEIRO CT, de SOUZA DB, COSTA WS, SAMPAIO FJB, PEREIRA-SAMPAIO MA. Immediate and late effects of chronic stress in the testes of prepubertal and adult rats. Asian J Androl. 2018;20:385–390. doi: 10.4103/aja.aja_68_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYGULA R, ABUMARIA N, FLUGGE G, HIEMKE C, FUCHS E, RUTHER E, HAVEMANN-REINECKE U. Citalopram counteracts depressive-like symptoms evoked by chronic social stress in rats. Behav Pharmacol. 2006;17:19–29. doi: 10.1097/01.fbp.0000186631.53851.71. [DOI] [PubMed] [Google Scholar]
- SAKI G, RAHIM F, ALIZADEH K. Effect of forced swimming stress on count, motility and fertilization capacity of the sperm in adult rats. J Hum Reprod Sci. 2009;2:72–75. doi: 10.4103/0974-1208.57226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHMIDT MV, SCHARF SH, STERLEMANN V, GANEA K, LIEBL C, HOLSBOER F, MULLER MB. High susceptibility to chronic social stress is associated with a depression-like phenotype. Psychoneuroendocrinology. 2010;35:635–643. doi: 10.1016/j.psyneuen.2009.10.002. [DOI] [PubMed] [Google Scholar]
- TAO J, LIN M, SHA J, TAN G, SOONG TW, LI S. Separate locations of urocortin and its receptors in mouse testis: function in male reproduction and the relevant mechanisms. Cell Physiol Biochem. 2007;19:303–312. doi: 10.1159/000100649. [DOI] [PubMed] [Google Scholar]
- TOYAMA Y, SUZUKI-TOYOTA F, MAEKAWA M, ITO C, TOSHIMORI K. Disruption of ectoplasmic specializations between Sertoli cells and maturing spermatids by anti-nectin-2 and anti-nectin-3 antibodies. Asian J Androl. 2008;10:577–584. doi: 10.1111/j.1745-7262.2008.00357.x. [DOI] [PubMed] [Google Scholar]
- WANG B, ZHOU J, ZHUANG YY, WANG LL, PU JX, HUANG YH, XIA F, LV JX. The Non-Peptide Vasopressin V1b Receptor Antagonist, SSR149415, Ameliorates Spermatogenesis Function in a Mouse Model of Chronic Social Defeat Stress. J Cell Biochem. 2017a;118:3891–3898. doi: 10.1002/jcb.26040. [DOI] [PubMed] [Google Scholar]
- WANG FF, WANG Q, CHEN Y, LIN Q, GAO HB, ZHANG P. Chronic stress induces ageing-associated degeneration in rat Leydig cells. Asian J Androl. 2012;14:643–648. doi: 10.1038/aja.2011.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG XD, CHEN Y, WOLF M, WAGNER KV, LIEBL C, SCHARF SH, HARBICH D, MAYER B, WURST W, HOLSBOER F, DEUSSING JM, BARAM TZ, MULLER MB, SCHMIDT MV. Forebrain CRHR1 deficiency attenuates chronic stress-induced cognitive deficits and dendritic remodeling. Neurobiol Dis. 2011;42:300–310. doi: 10.1016/j.nbd.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG XD, SU YA, WAGNER KV, AVRABOS C, SCHARF SH, HARTMANN J, WOLF M, LIEBL C, KUHNE C, WURST W, HOLSBOER F, EDER M, DEUSSING JM, MULLER MB, SCHMIDT MV. Nectin-3 links CRHR1 signaling to stress-induced memory deficits and spine loss. Nat Neurosci. 2013;16:706–713. doi: 10.1038/nn.3395. [DOI] [PubMed] [Google Scholar]
- WANG XX, LI JT, XIE XM, GU Y, SI TM, SCHMIDT MV, WANG XD. Nectin-3 modulates the structural plasticity of dentate granule cells and long-term memory. Transl Psychiatry. 2017b;7:e1228. doi: 10.1038/tp.2017.196. [DOI] [PMC free article] [PubMed] [Google Scholar]