Summary
Sex-related differences were observed not only in human but also in experimental hypertension. The aim of our study was to compare blood pressure (BP) of aged male and female heterozygous transgenic rats (TGR) harboring Ren-2 mouse gene, with their normotensive Hannover Sprague-Dawley (HanSD) controls. At the age of 9 months, systolic (SBP) and diastolic blood pressure (DBP) were measured by a direct puncture of carotid artery in rats awaking from isoflurane anesthesia. Thiobarbituric acid-reactive species (TBARS) formation was monitored as indicator of lipid peroxidation damage in heart, kidney and liver, whereas intracellular content of reduced glutathione was determined in the same organs as the main intracellular antioxidant. Furthermore, plasma triglycerides and total cholesterol as well as high-density lipoprotein (HDL) and low-density lipoprotein (LDL) fractions of cholesterol were measured. As compared to HanSD rats, we found significantly elevated BP only in male TGR (MAP: 123±1 vs. 171±5, SBP: 150±2 vs. 208±7, and DBP: 99±3 vs. 140±4 mm Hg), but not between TGR and HanSD females, which were both normotensive. We also did not find any significant differences in TBARS and reduced glutathione in the three above mentioned organs as well as in plasma cholesterol or its HDL and LDL fractions between transgene-negative HanSD and TGR animals of either sex. However, we found significant sex differences in TBARS, glutathione and plasma lipids in both rat strains. Our results confirmed that aged TGR exhibit a marked sexual BP dimorphism, which does not seem to be dependent on oxidative stress or abnormal cholesterol metabolism.
Keywords: Hypertension, Thiobarbituric acid-reactive species (TBARS), Reduced glutathione, Total plasma cholesterol, HDL and LDL cholesterol fractions
Introduction
Over the years plenty of data on blood pressure (BP) has been gathered in men and women. Generally, aging is usually associated with an increase in BP and hypertension becomes the most common chronic disease in the world. Men have higher incidence of hypertension compared with age-matched women until the onset of menopause. Prominent sex-related differences in hypertension were observed repeatedly not only in humans but also in experimental animals such as dog, rabbit, mouse or rat and even in birds (Blank et al. 2016, Lerman et al. 2019, Sandberg and Ji 2012). Animal models are inevitable for both the understanding hypertension pathogenesis and possible novel treatment options. Rats are often used as hypertension animal model. There are several rat models in which sex differences in BP were described – genetic hypertension in spontaneously hypertensive rats (Maris et al. 2005), NO-deficient hypertension induced by chronic L-NAME administration (Sainz et al. 2004) or salt hypertension in salt-sensitive Dahl rats (Zicha et al. 2012). There is an interesting model of hypertension in transgenic Ren-2 rats (TGR), in which mouse Ren-2 renin gene was inserted into the genome of normotensive Hannover Sprague-Dawley rats (HanSD). The overexpression of mouse renin gene induces the development of angiotensin II-dependent hypertension with endogenous activation of renin-angiotensin system (RAS), which plays a central role in blood pressure control and its long-term dysregulation causes sustained BP elevation. Therefore, the pharmacological RAS targeting is widely used in the clinical hypertension treatment and permanently tested in different experimental trials (Laurent 2017).
For a better understanding on the role of RAS in the pathogenesis of hypertension we can use TGR as a well-defined monogenetic model of hypertension (Mullins et al. 1990). Homozygous TGR display severe malignant hypertension accompanied by organ damage and they die at 7–13 weeks of age (Vernerová et al. 2009). On the other hand, heterozygous TGR develop a less severe hypertension, which allows long-term studies (Vaněčková et al. 2011). It was described that the established hypertension in male TGR is maintained or only a slight BP decrease occurs in the late adulthood (Lee et al. 1998, Springate et al. 1992, Vaněčková et al. 2011). In contrast, BP decline in female TGR can even yield normotensive values (Cargnelli et al. 1998, Lee et al. 1998, Vaněčková et al. 2011).
The aim of our study was to search for sex differences a) in blood pressure of 9-month-old male and female TGR, b) in the content of TBARS (oxidative stress marker) or reduced glutathione (intracellular antioxidant) in heart, kidney and liver, and c) in plasma lipids (triglycerides and cholesterol).
Material and Methods
Animals
Adult male and female heterozygous (mRen-2) 27 transgenic rats (TGR) aged 9 months were housed at 23 °C under a 12 h light/dark cycle periods, fed a standard rat chow Altromin and given to tap water ad libitum. Transgene-negative Hannover Sprague-Dawley rats (HanDS) served as controls. At the end of the experiment blood pressure was measured. After blood collection, kidney, heart and liver were excised and used for tissue analysis. Plasma samples (in the EDTA presence) were prepared and stored at −80 °C until further analysis.
All the procedures and experimental protocols were performed in accordance with guidelines and practice established by the Ethical Committee of the Institute of Physiology CAS, conformed to the European Convention on Animal Protection and Guidelines on Research Animal Use.
Measurement of blood pressure
Mean arterial (MAP), systolic (SBP) and diastolic (DBP) blood pressure and heart rate were measured by a direct puncture of carotid artery under isoflurane anesthesia (2.5 % isoflurane) and in awaking animals (0.5 % isoflurane). To eliminate the influence of circadian blood pressure (BP) variation, the measurements were always done approximately at the same time of day (between 8:00 and 10:00 a.m.).
Determination of thiol concentration
The intracellular content of reduced glutathione (GSH) in heart, kidney and liver was determined immediately in fresh tissues according to the methods described earlier (Ellman 1959). Briefly, the tissue samples were homogenized in 3 % sulfosalicylic acid and 10 % homogenates were centrifuged for 10 min at 3000 g. A portion of the supernatant was mixed with 0.02 M 5, 5′-dithiobis-(2-nitrobenzoic acid) in 0.1 M phosphate buffer (pH 8). The absorbance of a colored product was read at 412 nm, the concentration of GSH was calculated from calibration curve prepared by serial dilution of 1 mM stock solution. The results were expressed as μmol GSH/g tissue.
Measurement of lipid peroxidation
Lipid peroxidation in the samples was monitored by measuring thiobarbituric acid-reactive substances (TBARS) formation (Ohkawa et al. 1979). The frozen-thawed 10 % homogenates were incubated with thiobarbituric and acetic acid at 95 °C for 45 min. Absorbance was measured at 535 nm using Tecan Infinite M200 multimode microplate spectrofluorometer. The results were expressed as nmol of TBARS/mg of protein.
Biochemical parameters
Folin method was used for the determination of protein concentration using bovine serum albumin as standard (Lowry et al. 1951).
The concentrations of plasma triglycerides (TG), total cholesterol (TC), and high-density lipoprotein-cholesterol (HDL) were estimated using commercial kits (Pliva-Lachema Diagnostika, Brno, Czech Republic). Low-density lipoprotein-cholesterol (LDL) was estimated indirectly using the formula: LDL = TC - (TG/5) - HDL.
Results
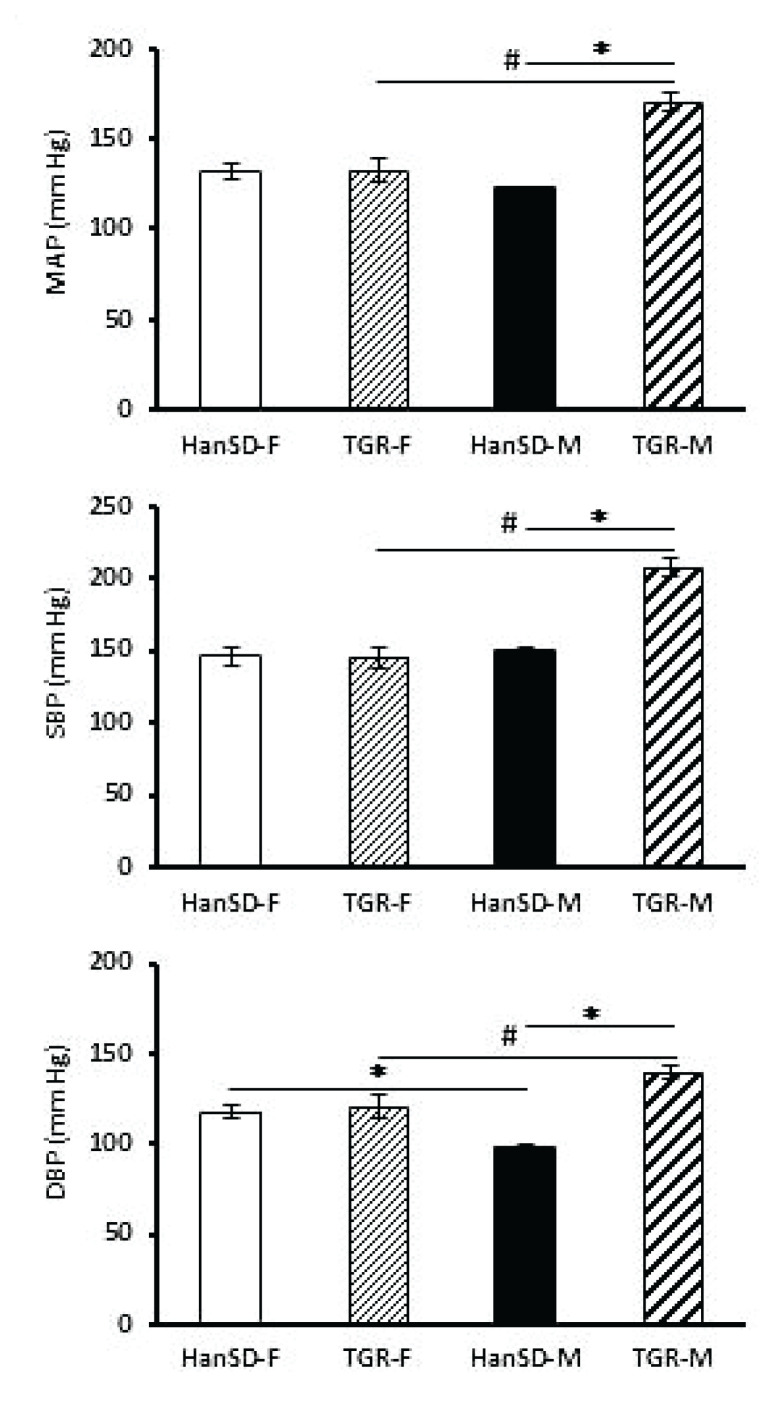
Figure 1 shows systolic (SBP), mean arterial (MAP) and diastolic (DBP) blood pressures in 9-month-old male and female TGR and their HanSD controls which were recorded in awaking animals. In TGR females, the blood pressures were decreased to normotensive values seen in HanSD females. On the other hand, older TGR males had still a considerable hypertension, their SBP and DBP being substantially elevated compared to their HanSD controls.
Fig. 1.
Mean (MAP), systolic (SBP) and diastolic (DBP) blood pressure in awaking female (F) and male (M) Hannover Sprague-Dawley (HanSD) and transgenic rats (TGR) aged 9 months. All values are mean ± SEM. Significantly different: *P≤0.05 vs. HanSD, #P≤0.05 vs. females.
Body weights were higher in male but not in female TGR as compared to sex-matched HanSD animals (Table 1). The absolute weights of hearts and kidneys were always greater in males than in females of both genotypes, but there were no significant differences in the relative weights of hearts and kidneys between TGR and their sex- and age-matched HanSD controls. Similarly, 9-month-old male TGR had greater absolute liver weight than HanSD rats, but their relative liver weight was not increased.
Table 1.
General characteristics of female and male Hannover Sprague-Dawley (HanSD) and transgenic rats (TGR) aged 9 months.
| Females | HanSD (n=8) | TGR (n=8) |
|---|---|---|
| Body weight (BW; g) | 353 ± 12 | 382 ± 8 |
| Heart weight (HW; mg) | 1165 ± 24 | 1253 ± 24 |
| Relative heart weight (HW/BW; mg/g | 3.33 ± 0.12 | 3.31 ± 0.13 |
| Kidney weight (KW; mg) | 2071 ± 46 | 2272 ± 46* |
| Relative kidney weight (KW/BW; mg/g) | 5.93 ± 0.29 | 5.97 ± 0.31 |
|
| ||
| Males | HanSD (n=6) | TGR (n=5) |
|
| ||
| Body weight (BW; g) | 690 ± 1# | 818 ± 24*# |
| Heart weight (HW; mg) | 1756 ± 21# | 2130 ± 55*# |
| Relative heart weight (HW/BW; mg/g | 2.55 ± 0.03# | 2.61 ± 0.09# |
| Left ventricular weight (LVW; mg) | 1376 ± 34 | 1720 ± 89* |
| Relative left ventricular weight (LVW/BW; mg/g) | 2.00 ± 0.02 | 2.12 ± 0.06 |
| Kidney weight (KW; mg) | 4289 ± 227# | 4762 ± 212# |
| Relative kidney weight (KW/BW; mg/g) | 6.22 ± 0.34 | 5.83 ± 0.26 |
| Liver weight (LW; mg) | 23757 ± 1921 | 28376 ± 2163* |
| Relative liver weight (LW/BW; mg/g) | 34.5 ± 2.9 | 34.7 ± 2.3 |
All values are mean ± SEM, n, number of animals is given in parentheses. Significantly different:
P ≤ 0.05 vs. HanSD,
P ≤ 0.05 vs. females.
To evaluate the degree of organ damage by lipid peroxidation we measured TBARS concentrations as an indirect marker of lipid peroxidation in the heart, kidney and liver (Table 2). We did not find any significant difference in TBARS concentrations in the particular tissues between HanSD and TGR rats, but we found significantly lower TBARS concentrations in all examined organs of male rats, irrespective of the genotype.
Table 2.
Thiobarbituric acid-reactive substances in heart, kidney and liver of female and male Hannover Sprague-Dawley (HanSD) and transgenic rats (TGR) aged 9 months.
| Females | HanSD (n=8) | TGR (n=8) |
|---|---|---|
| Heart (μmol/mg protein) | 15.93 ± 0.90 | 15.98 ± 1.01 |
| Kidney (μmol/mg protein) | 28.79 ± 2.20 | 28.96 ± 1.34 |
| Liver (μmol/mg protein) | 13.46 ± 0.98 | 15.13 ± 1.77 |
|
| ||
| Males | HanSD (n=6) | TGR (n=5) |
|
| ||
| Heart (μmol/mg protein) | 11.70 ± 1.52# | 11.74 ± 1.23# |
| Kidney (μmol/mg protein) | 18.55 ± 0.11# | 20.36 ± 1.21# |
| Liver (μmol/mg protein) | 8.80 ± 0.45# | 8.52 ± 2.19# |
All values are mean ± SEM, n, number of animals is given in parentheses Significantly different:
P ≤ 0.05 vs. females.
We observed the lowest concentration of thiol groups in the heart and the highest one in the liver (Table 3). Concentrations of thiol groups were not significantly different between TGR and HanSD animals. However, male rats of both genotypes had significantly higher heart and liver SH concentration but a significantly lower kidney SH concentration than corresponding females.
Table 3.
Concentration of thiol groups in heart, kidney and liver of female and male Hannover Sprague-Dawley (HanSD) and transgenic rats (TGR) aged 9 months.
| Females | HanSD (n=8) | TGR (n=8) |
|---|---|---|
| Heart (μmol/g) | 1.73 ± 0.08 | 1.54 ± 0.05 |
| Kidney (μmol/g) | 4.10 ± 0.17 | 4.24 ± 0.17 |
| Liver (μmol/g) | 4.89 ± 0.39 | 4.82 ± 0.28 |
|
| ||
| Males | HanSD (n=6) | TGR (n=5) |
|
| ||
| Heart (μmol/g) | 2.13 ± 0.08# | 2.35 ± 0.05# |
| Kidney (μmol/g) | 3.19 ± 0.18# | 3.36 ± 0.15# |
| Liver (μmol/g) | 8.58 ± 0.29# | 8.52 ± 2.19# |
All values are mean ± SEM, n, number of animals is given in parentheses. Significantly different:
P ≤ 0.05 vs. females.
Male rats of both genotypes had significantly higher values of serum triglycerides (TG), total cholesterol (TC) and low-density lipoprotein-cholesterol (LDL) as well as the ratio between total cholesterol and its HDL fraction (TC/HDL, atherogenic index) than corresponding females (Table 4). However, there were no significant differences in the above parameters between TGR and HanSD controls.
Table 4.
Plasma lipids in female and male Hannover Sprague-Dawley (HanSD) and transgenic rats (TGR) aged 9 months.
| Females | HanSD (n=8) | TGR (n=8) |
|---|---|---|
| Triglycerides (mmol/l) | 0.26 ± 0.01 | 0.26 ± 0.03 |
| Total cholesterol (mmol/l) | 1.96 ± 0.14 | 1.84 ± 0.10 |
| HDL (mmol/l) | 1.49 ± 0.15 | 1.44 ± 0.08 |
| LDL (mmol/l) | 0.42 ± 0.09 | 0.35 ± 0.04 |
| TC/HDL ratio | 1.36 ± 0.11 | 1.28 ± 0.03 |
|
| ||
| Males | HanSD (n=6) | TGR (n=5) |
|
| ||
| Triglycerides (mmol/l) | 0.85 ± 0.06# | 0.79 ± 0.15# |
| Total cholesterol (mmol/l) | 2.37 ± 0.08# | 2.59 ± 0.18# |
| HDL (mmol/l) | 1.28 ± 0.03 | 1.37 ± 0.12 |
| LDL (mmol/l) | 0.91 ± 0.04# | 1.06 ± 0.13# |
| TC/HDL ratio | 1.84 ± 0.02# | 1.91 ± 0.12# |
All values are mean ± SEM, n, number of animals is given in parentheses. Significantly different:
P ≤ 0.05 vs. females.
HDL, high-density lipoprotein-cholesterol; LDL, low-density lipoprotein-cholesterol; TC, total cholesterol
Discussion
TGR represents a model of hypertension with a well-defined genetic background. We confirmed a marked sexual dimorphism in this model because our data obtained in awaking rats indicated BP elevation only in TGR males, whereas BP of TGR females was close to that of HanSD females. Sex-related differences in BP values were reported earlier in this strain. Our SBP values correspond well with previous two long-term studies (lasting 10–12 months) that followed both male and female TGR in comparison with their sex- and age-matched HanSD controls (Lee et al. 1996, Vaněčková et al. 2011). In both mentioned studies maximal SBP values were higher and the phase of established hypertension was longer in males than females. Cargnelli et al. (1998) showed that the established period of hypertension was followed by a progressive decrease of SBP towards the normal values in 35-week-old heterozygous females TGR, while SBP values of HanSD females were unaffected by ageing. Springate et al. (1994) described diminished SBP in 8-month-old male TGR compared to 2- and 4-month-old rats of this strain. We extended our study by measuring of MAP and DBP in two different conditions, i.e. under deep isoflurane anesthesia (data not shown) and in animals awaking from this anesthesia. The differences in BP were more pronounced in awaking animals.
TGR with elevated endogenous angiotensin II were sporadically described as a suitable model of spontaneous liver fibrosis and portal hypertension (non-alcoholic fatty liver disease), which is associated with hepatic mitochondrial oxidative damage and impaired mitochondrial fatty acid oxidation (Klein et al. 2019, Wei et al. 2008, 2009). However, our group of aged TGR males showed neither the higher relative liver weight nor the increased level of TBARS in liver.
Oxidative or nitrosative stress, which represents the imbalance between production and elimination of reactive oxygen or nitrogen species, is often considered as an important factor contributing to the pathogenesis of different forms of hypertension (Guzik and Touyz 2017, Reckelhoff et al. 2019). To examine the role of oxidative damage, we measured TBARS levels in three different organs (liver, heart and kidney). Vokurková et al. (2015) disclosed higher concentration of TBARS in renal cortex in 3-month-old TGR males but this difference was not confirmed by the parallel measurements of conjugated dienes or oxidative index. However, we did not find significant differences in renal, cardiac or liver levels of TBARS between TGR and HanSD rats of either sex. Kopkan et al. (2009) reported increased levels of malondialdehyde in the whole kidney and left heart ventricle of 3-month-old male TGR and significantly reduced levels of malondialdehyde after the chronic administration of O2− scavenger tempol or NADPH oxidase inhibitor apocynin but this malondialdehyde decrease was not associated with any SBP change. Interestingly, in spite of higher blood pressure in our TGR males we found significantly lower concentrations of TBARS than in females for which we cannot offer a plausible explanation.
The main intracellular antioxidant reduced glutathione (GSH) plays important roles in peroxide detoxification, recycling of vitamins C and E, cysteine storage and other biochemical reactions (Aquilano et al. 2014, Gould and Pazdro 2019). We found sex-different GSH concentrations in all examined tissues (heart, kidney, liver). Males had always higher GHS concentrations in heart and liver but lower values in the kidney. On the other hand, GSH concentrations were not influenced by rat genotype. Similar GSH concentrations were also obtained in 3-month-old TGR males (Vokurková et al. 2015b) and 5-month-old Dahl males (Vokurková et al. 2015a).
All parameters of lipid metabolism (with the exception of HDL cholesterol) were significantly higher in older males than females. This is a rather surprising finding because there are several reports of higher plasma cholesterol levels in female than male Sprague Dawley or Lewis rats of various age (Lee et al. 2008, Borbélyová et al. 2017). On the other hand, there were no differences between HanSD and TGR animals of either sex. To our knowledge, there are no data on alterations of lipid metabolism in TGR. The only relevant study (Vettor et al. 1994) reported similar fasting levels of plasma triglycerides in TGR and HanSD rats aged 5 months.
In summary, the present study shows a sexual dimorphism in blood pressure of heterozygous TGR. Additionally, we did not find any corresponding difference in TBARS concentrations and reduced glutathione levels between transgene-negative normotensive (HanSD) and hypertensive Ren-2 transgenic rats (TGR).
Acknowledgements
The study was supported by an international research grant GACR 19-08260J and by an institutional support (RV0:67985823). A part of research results was presented on FEPS meeting in Bologna, Italy (Rauchová et al. 2019).
Footnotes
Conflict of interest
There is no conflict of interest.
References
- AQUILANO K, BALDELLI S, CIRIOLO MR. Glutathione: new roles in redox signaling for an old antioxidant. Front Pharmacol. 2014;5:196. doi: 10.3389/fphar.2014.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLENCK CL, HARVEY PA, RECKELHOFF JF, LEINWAND LA. The importance of biological sex and estrogen in rodent models of cardiovascular health and disease. Circ Res. 2016;118:1294–1312. doi: 10.1161/CIRCRESAHA.116.307509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORBÉLYOVÁ V, DOMONKOS E, BÁBÍČKOVÁ J, TÓTHOVÁ Ľ, KAČMÁROVÁ M, ULIČNÁ O, OSTATNÍKOVÁ D, HODOSY J, CELEC P. Does long-term androgen deficiency lead to metabolic syndrome in middle-aged rats? Exp Gerontol. 2017;98:38–46. doi: 10.1016/j.exger.2017.08.016. [DOI] [PubMed] [Google Scholar]
- CARGNELLI G, ROSSI GP, PESSINA AC, LUCIANI S, DEBETTO P, GANTEN D, PETERS J, BOVA S. Changes of blood pressure and aortic strip contractile responses to ET-1 of heterozygous female transgenic rats, TGR(mRen2)27. Pharmacol Res. 1998;37:207–211. doi: 10.1006/phrs.1998.0287. [DOI] [PubMed] [Google Scholar]
- ELLMAN GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- FORTEPIANI LA, RECKELHOFF JF. Role of oxidative stress in the sex differences in blood pressure in spontaneously hypertensive rats. J Hypertens. 2005;23:801–805. doi: 10.1097/01.hjh.0000163149.05083.13. [DOI] [PubMed] [Google Scholar]
- GOULD RL, PAZDRO R. Impact of supplementary amino acids, micronutrients, and overall diet on glutathione homeostasis. Nutrients. 2019;11:1056. doi: 10.3390/nu11051056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUZIK TJ, TOUYZ RM. Oxidative stress, inflammation, and vascular aging in hypertension. Hypertension. 2017;70:660–667. doi: 10.1161/HYPERTENSIONAHA.117.07802. [DOI] [PubMed] [Google Scholar]
- KASPER SO, CARTER CS, FERRARIO CM, GANTEN D, FERDER LF, SONNTAG WE, GALLAGHER PE, DIZ DI. Growth, metabolism, and blood pressure disturbances during aging in transgenic rats with altered brain renin-angiotensin systems. Physiol Genomics. 2005;23:311–317. doi: 10.1152/physiolgenomics.00163.2005. [DOI] [PubMed] [Google Scholar]
- KLEIN S, KLEINE CE, PIEPER A, GRANZOW M, GAUTSCH S, HIMMIT M, KAHRMANN K, SCHIERWAGEN R, USCHNER FE, MAGDALENO F, NAOUM ME, KRISTIANSEN G, WALTHER T, BADER M, SAUERBRUCH T, TREBICKA J. TGR(mREN2)27 rats develop non-alcoholic fatty liver disease-associated portal hypertension responsive to modulations of Janus-kinase 2 and Mas receptor. Sci Rep. 2019;9:11598. doi: 10.1038/s41598-019-48024-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOPKAN L, HUSKOVÁ Z, VAŇOURKOVÁ Z, THŮMOVÁ M, ŠKAROUPKOVÁ P, MALÝ J, KRAMER HJ, DVOŘÁK P, ČERVENKA L. Reduction of oxidative stress does not attenuate the development of angiotensin II-dependent hypertension in Ren-2 transgenic rats. Vascul Pharmacol. 2009;51:175–181. doi: 10.1016/j.vph.2009.06.001. [DOI] [PubMed] [Google Scholar]
- LAURENT S. Antihypertensive drugs. Pharmacol Res. 2017;124:116–125. doi: 10.1016/j.phrs.2017.07.026. [DOI] [PubMed] [Google Scholar]
- LEE CE, KANG JS, KIM KI. Effects of gender, gonadectomy and sex hormones on growth and plasma cholesterol level in rats. Ann Nutr Metab. 2008;53:1–5. doi: 10.1159/000152867. [DOI] [PubMed] [Google Scholar]
- LEE MA, BÖHM M, PAUL M, BADER M, GANTEN U, GANTEN D. Physiological characterization of the hypertensive transgenic rat TGR(mREN2)27. Am J Physiol. 1996;270:E919–E929. doi: 10.1152/ajpendo.1996.270.6.E919. [DOI] [PubMed] [Google Scholar]
- LERMAN LO, KURTZ TW, TOUYZ RM, ELLISON DH, CHADE AR, CROWLEY SD, MATTSON DL, MULLINS JJ, OSBORN J, EIRIN A, RECKELHOFF JF, IADECOLA C, COFFMAN TM. Animal models of hypertension: A scientific statement from the American Heart Association. Hypertension. 2019;73:e87–e120. doi: 10.1161/HYP.0000000000000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY OH, ROSEBROUGH NJ, FARR AL, RANDALL RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- MARIS ME, MELCHERT RB, JOSEPH J, KENNEDY RH. Gender differences in blood pressure and heart rate in spontaneously hypertensive and Wistar-Kyoto rats. Clin Exp Pharmacol Physiol. 2005;32:35–39. doi: 10.1111/j.1440-1681.2005.04156.x. [DOI] [PubMed] [Google Scholar]
- MULLINS JJ, PETERS J, GANTEN D. Fulminant hypertension in transgenic rats harbouring the mouse Ren-2 gene. Nature. 1990;344:541–544. doi: 10.1038/344541a0. [DOI] [PubMed] [Google Scholar]
- NAUTIYAL M, KATAKAM PV, BUSIJA DW, GALLAGHER PE, TALLANT EA, CHAPPELL MC, DIZ DI. Differences in oxidative stress status and expression of MKP-1 in dorsal medulla of transgenic rats with altered brain renin-angiotensin system. Am J Physiol Regul Integr Comp Physiol. 2012;303:R799–R806. doi: 10.1152/ajpregu.00566.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OHKAWA H, OHISHI N, YAGI K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- RAUCHOVÁ H, HOJNÁ S, KADLECOVÁ M, VANĚČKOVÁ I, ZICHA J. Sex-related differences of blood pressure in older Ren-2 transgenic rats. Acta Physiol. 2019;227(Suppl 718):173. [Google Scholar]
- RECKELHOFF JF, ROMERO DG, YANES CARDOZO LL. Sex, oxidative stress, and hypertension: insights from animal models. Physiology (Bethesda) 2019;34:178–188. doi: 10.1152/physiol.00035.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SÁINZ J, OSUNA A, WANGENSTEEN R, DE DIOS LUNA J, RODRÍGUEZ-GÓMEZ I, DUARTE J, MORENO JM, VARGAS F. Role of sex, gonadectomy and sex hormones in the development of nitric oxide inhibition-induced hypertension. Exp Physiol. 2004;89:155–162. doi: 10.1113/expphysiol.2003.002652. [DOI] [PubMed] [Google Scholar]
- SANDBERG K, JI H. Sex differences in primary hypertension. Biol Sex Differ. 2012;3:7. doi: 10.1186/2042-6410-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPRINGATE JE, FELD LG, GANTEN D. Renal function in hypertensive rats transgenic for mouse renin gene. Am J Physiol. 1994;266:F731–F737. doi: 10.1152/ajprenal.1994.266.5.F731. [DOI] [PubMed] [Google Scholar]
- VANĚČKOVÁ I, HUSKOVÁ Z, VAŇOURKOVÁ Z, ČERVENKA L. Castration has antihypertensive and organoprotective effects in male but not in female heterozygous Ren-2 rats. Kidney Blood Press Res. 2011;34:46–52. doi: 10.1159/000322618. [DOI] [PubMed] [Google Scholar]
- VERNEROVÁ Z, KUJAL P, KRAMER HJ, BÄCKER A, ČERVENKA L, VANĚČKOVÁ I. End-organ damage in hypertensive transgenic Ren-2 rats: influence of early and late endothelin receptor blockade. Physiol Res. 2009;58(Suppl 2):S69–S78. doi: 10.33549/physiolres.931640. [DOI] [PubMed] [Google Scholar]
- VETTOR R, CUSIN I, GANTEN D, ROHNER-JEANRENAUD F, FERRANNINI E, JEANRENAUD B. Insulin resistance and hypertension: studies in transgenic hypertensive TGR(mREN-2)27 rats. Am J Physiol. 1994;267:R1503–R1509. doi: 10.1152/ajpregu.1994.267.6.R1503. [DOI] [PubMed] [Google Scholar]
- VOKURKOVÁ M, RAUCHOVÁ H, ŘEZÁČOVÁ L, VANĚČKOVÁ I, ZICHA J. ROS production is increased in the kidney but not in the brain of Dahl rats with salt hypertension elicited in adulthood. Physiol Res. 2015a;64:303–312. doi: 10.33549/physiolres.933054. [DOI] [PubMed] [Google Scholar]
- VOKURKOVÁ M, RAUCHOVÁ H, ŘEZÁČOVÁ L, VANĚČKOVÁ I, ZICHA J. NADPH oxidase activity and reactive oxygen species production in brain and kidney of adult male hypertensive Ren-2 transgenic rats. Physiol Res. 2015b;64:849–856. doi: 10.33549/physiolres.933254. [DOI] [PubMed] [Google Scholar]
- WEI Y, CLARK SE, MORRIS EM, THYFAULT JP, UPTERGROVE GM, WHALEY-CONNELL AT, FERRARIO CM, SOWERS JR, IBDAH JA. Angiotensin II-induced non-alcoholic fatty liver disease is mediated by oxidative stress in transgenic TG(mRen2)27(Ren2) rats. J Hepatol. 2008;49:417–428. doi: 10.1016/j.jhep.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEI Y, CLARK SE, THYFAULT JP, UPTERGROVE GM, LI W, WHALEY-CONNELL AT, FERRARIO CM, SOWERS JR, IBDAH JA. Oxidative stress-mediated mitochondrial dysfunction contributes to angiotensin II-induced nonalcoholic fatty liver disease in transgenic Ren2 rats. Am J Pathol. 2009;174:1329–1337. doi: 10.2353/ajpath.2009.080697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZICHA J, DOBEŠOVÁ Z, VOKURKOVÁ M, RAUCHOVÁ H, HOJNÁ S, KADLECOVÁ M, BEHULIAK M, VANĚČKOVÁ I, KUNEŠ J. Age-dependent salt hypertension in Dahl rats: fifty years of research. Physiol Res. 2012;61(Suppl 1):S35–S87. doi: 10.33549/physiolres.932363. [DOI] [PubMed] [Google Scholar]