Summary
Granulosa cells (GCs) are somatic cells essential for establishing and maintaining bi-directional communication with the oocytes. This connection has a profound importance for the delivery of energy substrates, structural components and ions to the maturing oocyte through gap junctions. Cumulus cells, group of closely associated GCs, surround the oocyte and can diminished the effect of harmful environmental insults. Both GCs and oocytes prefer different energy substrates in their cellular metabolism: GCs are more glycolytic, whereas oocytes rely more on oxidative phosphorylation pathway. The interconnection of these cells is emphasized by the fact that GCs supply oocytes with intermediates produced in glycolysis. The number of GCs surrounding the oocyte and their age affect the energy status of oocytes. This review summarises available studies collaboration of cellular types in the ovarian follicle from the point of view of energy metabolism, signaling and protection of toxic insults. A deeper knowledge of the underlying mechanisms is crucial for better methods to prevent and treat infertility and to improve the technology of in vitro fertilization.
Keywords: Cumulus cells, Granulosa cells, Oocytes, Energy metabolism, Mitochondria
Introduction
Human oocytes develop in ovarian follicles surrounded by granulosa cells (GC). The coordinated development of all cell types in a follicle is crucial for the possibility of ovulating a fertile egg and therefore for normal or assisted reproduction. The details of the metabolic and signaling interplay within the follicle are therefore useful for the prevention and treatment of infertility and for the improvement of methods of assisted reproduction.
Granulosa cells create a kind of protective barrier and communication platform between the oocyte and extraovarian microenvironment. They deliver nutrients and signal molecules to the oocyte and they play an essential role during sperm penetration (Tanghe et al. 2002). These facts make granulosa cells attractive for research because the knowledge of their essential function in reproduction and of their metabolism can give us better view how GCs protect oocyte during maturation and how they contribute to the normal development of a human embryo. In this review we focus on the metabolic role of GCs and their participation in the oocyte developmental competence and protection.
Follicle growth and development
Ovarian primordial follicles are present at birth as a reserve of primary oocytes for the female reproductive life cycle. Oocytes in the primordial follicular stage are arrested in the prophase of the first meiotic division and they are surrounded by a single layer of squamous granulosa cells (De La Fuente and Eppig 2001). The maturation of ovarian follicles (folliculogenesis) continues by the development of primary primordial follicles into large antral secondary follicles. The follicles start as spheroidal sacs filled with the follicular fluid and containing oocytes. Oocytes in the preantral primary follicles are surrounded by a single layer of cuboidal cells called as preantral granulosa cells (Diaz et al. 2006). The primary follicles gradually develop into secondary follicles by forming the antrum, while at the same time the oocytes grow in size. During this process granulosa cells increase their mitotic activity. They divide into two subpopulations: (1) mural granulosa cells (MGCs) lining the wall of antrum and (2) cumulus granulosa cells (CCs) population surrounding the oocyte (Diaz et al. 2007).
In humans when a follicle reaches the size of a Graafian follicle and the oocyte grows to about 100 μm in diameter (Cavilla et al. 2008), ovulation is initiated after a preovulatory surge of luteinizing hormone (LH). Oocytes are arrested in the second meiotic metaphase (Madgwick and Jones 2007). The ovulation is coordinated by physiological and biochemical processes with an active participation of the cumulus granulosa cells. A few hours before ovulation the cumulus-oocyte complex initiates the synthesis and assembly of a highly viscoelastic extracellular matrix rich in proteins and proteoglycans such as hyaluronan, a non-sulfated glycosaminoglycan important for sperm penetration (Ikawa et al. 2010). Its main component, hyaluronic acid (HA), a large polyanionic glycosaminoglycan, is synthesized by both MGCs and CCs and is responsible for the expansion of the cumulus oophorus (Eppig 1979, Salustri et al. 1992, Salustri et al. 2004). The expanded oocyte complex is ovulated from the Graafian follicle and then fertilized by a sperm in the oviduct. Not only the mature oocyte but also the surrounding cumulus cells secrete sperm chemoattractants, to which human sperm cells are sensitive (Sun et al. 2005). One example is progesterone, which acts as a chemoattractant for mammalian sperma-tozoa at picomolar concentrations (Teves et al. 2006).
Oocyte maturation – role of the granulosa cells
Oocyte maturation is a short and dynamic process, which occurs with a synchronous nuclear and cytoplasmic maturation and many biochemical changes (Eppig 1996). During hormone-regulated transition from a primary into a mature secondary follicle, the oocyte has to increase its RNA synthesis and accumulation of RNAs and proteins (Picton et al. 1998). The first meiotic division includes the formation of the metaphase I spindle and its migration to the cortex in an actin-dependent manner with subsequent polar body extrusion (FitzHarris et al. 2007, Maro et al. 1984, Maro and Verlhac 2002). A dramatic reorganization of the endomembrane system occurs; similarly the Golgi apparatus undergoes a substantial re-organization between the germinal vesicle (GV) stage and the metaphase II (MII) stage (Moreno et al. 2002, Racedo et al. 2012). During the oocyte maturation a reorganization of the endoplasmic reticulum (ER) occurs including a remodelling of the ER into the characteristic cortical clusters. This process is among the essential modifications in the ooplasm prior to fertilization (FitzHarris et al. 2007).
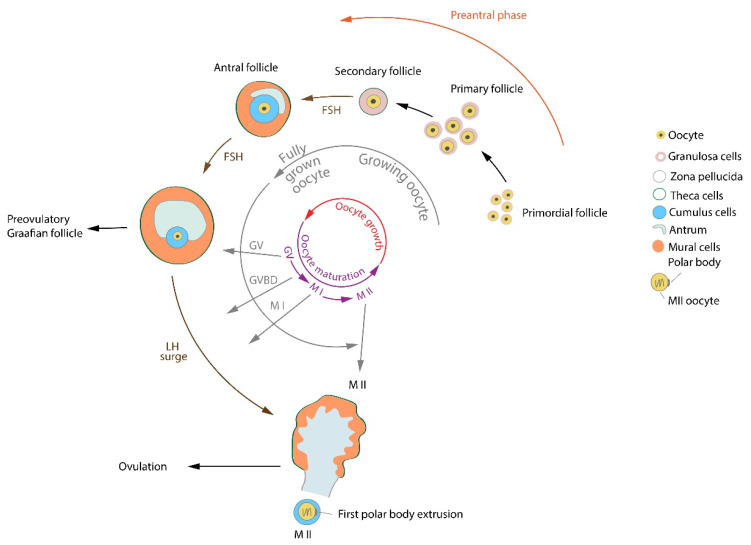
Cumulus granulosa cells play a crucial role in environmental support of the maturing oocytes. Virant-Klun et al. (2018) showed an improved maturation of human oocytes in vitro when co-cultured with cumulus cells isolated from mature oocytes. In growing oocytes there is a decondensed chromatin configuration (non-surrounded nucleolus, NSN), while in a fully grown oocyte before oocyte maturation the transcriptional activity winds down, the chromatin in the germinal vesicle (GV) surrounds the nucleolus (surrounded nucleolus – SN) and becomes progressively condensed (Debey et al. 1993, Inoue et al. 2008, De La Fuente et al. 2004). During this transcriptionally quiescent period CCs provide the oocyte with nutrients and regulatory signals molecules, CCs contribute to the promotion of nuclear and cytoplasmic oocyte maturation (Kolesarova et al. 2015, Sanchez and Smitz 2012). Metabolites of glucose (e.g. pyruvate), lipids, nucleotides and cholesterol provided by granulosa cells are important for the successful transition from prophase-I to germinal vesicle breakdown (GVBD), to undergo metaphase-I, and to progress through anaphase-I/telophase-I to metaphase-II (MII) (Eppig 1996, Xie et al. 2016). A scheme of the oocyte/follicle development is shown in Figure 1.
Fig. 1.
Processes and signaling molecules in oocyte/follicle development. FSH – follicle-stimulating hormone, GV – germinal vesicle, GVBD – germinal vesicle breakdown, LH – luteinizing hormone, MI – metaphase I oocyte, MII – metaphase II oocyte.
There is a strong evidence that during the oocyte maturation cumulus cells secrete factors important for oocyte development to the medium (Gilchrist et al. 2008). Uhde et al. (2018) described detailed metabolomic profiles of bovine CCs and the cumulus-oocyte complex conditioned medium during a 23-hour maturation period in vitro (IVM). Researchers have detected 369 different biochemical components in the CCs, of which 173 biochemical components were detected in the maturation medium. Significant changes were described in both the maturation medium and CCs during maturation with most of the changes related to amino acid, saccharide and lipid metabolism. In the CCs an increase of most of amino acids (esp. glutamine, leucine and isoleucine during the first 8 h), UDP-glucose and UDP-galactose (esp. during the first 8 h), lactate and pyruvate (during the final 15 h) and sphingomyelin, glycerophosphorylcholine and N-palmitoyl-sphinganine was described. On the contrary, a decrease of levels in CCs was detected for betaine, taurine and ophthalmate, for intermediates of pathways of saccharide metabolism (e.g. phosphoenolpyruvate and 3-phosphoglycerate) and for the majority of intermediates in fatty acid/phospholipid metabolic pathways (the lowest for acetylcarnitine, octanoylcarnitine and carnitine). In the maturation medium the most important increases were detected for creatinine (increased 427-fold after 8 h and 516-fold after 23 h), various amino acids (incl. hypotau-rine and ornithine) and urea (10-fold rise), for lactate (with 667-fold increase after 8 h and 1227-fold after 23 h), mannitol/sorbitol, ribitol and pyruvate and for cholinephosphate, myo-inositol, glycerol and carnitine. A decline in the medium was detected for glucose (indicating that it was consumed by the COCs) (Uhde et al. 2018). In a further investigation it was found that during oocyte in vitro maturation a supplementation of a fresh maturation medium with carnitine (concentration 2.5, 5 or 10 mmol/l), but not with creatine (concentration 5, 10 or 20 mmol/l) improves the developmental competence of denuded oocytes (Uhde et al. 2018).
The communicative interconnection and metabolic cooperation between the oocyte and cumulus cells consist of interactions mediated by paracrine factors and by gap junctions through connexin 37 (Anderson and Albertini 1976, Kidder and Mhawi 2002, Gilchrist et al. 2008). First, there are homologous gap junctions between granulosa cells themselves. The second type of connection – heterologous junctions – is between granulosa cells and the oocyte (Larsen and Wert 1988, Downs 1995). Small molecules are moving directly through gap junctions between the cells leading to the development of a metabolic coupling (Gilula et al. 1972). A whole spectrum of nutrients e.g. energy substrates, nucleotides, and amino acids are taken up into the oocyte (Buccione et al. 1990). These gap junction channels seem also to have signaling functions. They enable the exchange of low molecular weight compounds such as cAMP through the plasma membrane between the oocyte and cumulus cells, and between cumulus cells themselves (Eppig 1991). There is a clear evidence in the literature that cAMP and other purine derivatives (e.g. guanyl compounds) play a crucial role in the maintenance of oocyte arrest in meiosis I prophase (Downs et al. 1988). The disassembly of gap junctions between the mural and cumulus cells results in a reduced supply of meiosis-arresting substances to the oocyte leading to the occurrence of GVBD (Eppig 1991, Wert and Larsen 1990).
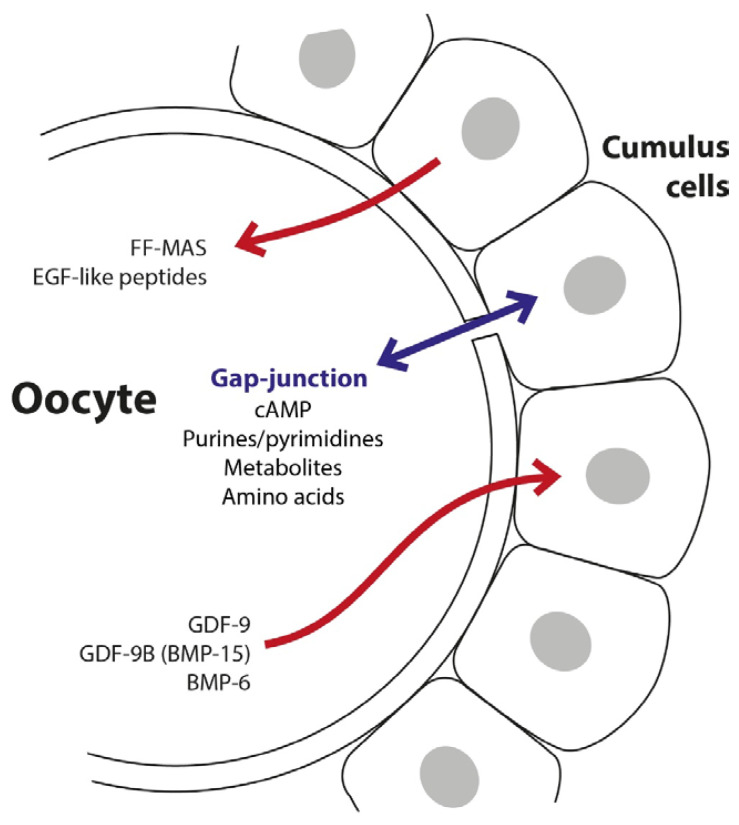
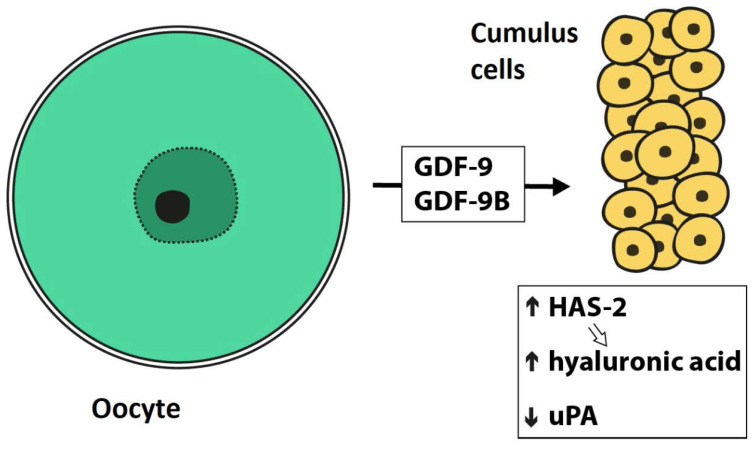
The regulatory interaction between granulosa cells and oocytes is bi-directional. Human oocytes express and secrete factors, which regulate MGCs and CCs function in vitro: namely GDF-9 (growth-differentiation factor 9) and GDF-9B (growth-differentiation factor 9B=BMP-15 – bone morphogenetic protein 15) (Dube et al. 1998, Aaltonen et al. 1999, Teixeira et al. 2002). These molecules activate signaling pathways important for CC differentiation (Gilchrist et al. 2008) and the presence of exogenous GDF-9 in media during in vitro maturation enhances embryo development (Yeo et al. 2008). Cumulus cells denuded from oocytes becomes unable to synthesize hyaluronic acid and hence fail to undergo expansion. The synthesis of hyaluronic acid by cumulus cells in response to the presence of follicle-stimulating hormone (FSH) is restored if they are co-cultured with isolated oocytes (Salustri et al. 1990, Buccione et al. 1990). A scheme of types of mutual interconnections and communication between oocytes and cumulus cells is shown in Figure 2. Figure 3 summarizes the processes related to the production of hyaluronic acid in cumulus cells.
Fig. 2.
Transfer of molecules between the oocyte and cumulus cells (modified from Gilchrist et al. 2004). BMP-6 – bone morphogenetic protein 6, cAMP – cyclic adenosine monophosphate, FF-MAS – follicular fluid meiosis-activating sterol, GDF-9 – growth-differentiation factor 9, GDF-9B – growth-differentiation factor 9B, BMP-15 – bone morphogenetic protein 15.
Fig. 3.
Signaling processes related to the production of hyaluronic acid in cumulus cells (modified from Gilchrist et al. 2004). GDF-9 – growth-differentiation factor 9, GDF-9B – growth-differentiation factor 9B, HAS-2 – hyaluronan synthase 2, uPA – urokinase-type plasminogen activator.
Energy metabolism of granulosa cells and oocytes
The oocyte growth and development depend significantly on interconnections with GCs, which supply them with energy substrates via gap junctional communi-cation (Orisaka et al. 2009). The two cells types show different preferences for energy substrates in order to produce ATP. GCs have been described to be more dependent on glycolysis, whereas oocytes rely more on mitochondrial oxidative phosphorylation (Kansaku et al. 2017a, Xie et al. 2016, Sutton-McDowall et al. 2010). It was shown that GC proliferation is dependent on an up-regulation of HIF1α-VEGF-Akt pathway and the activation of glycolysis (Shiratsuki et al. 2016). The importance of the mutual interconnection of these cells is emphasized by the fact that GCs supply oocytes with intermediates produced in the glycolytic pathway, e.g. pyruvate and lactate. Several studies have shown that the number of GCs surrounding the oocyte plays an important (and positive) role in its energy status (ATP levels) (Munakata et al. 2016a, Sugiyama et al. 2016). Mitochondria are essential for the normal development and viability of oocytes. Their low number is associated with a significant negative impact (Wai et al. 2010, Stojkovic et al. 2001).
Ageing has a well described effect on mitochondrial quantity and quality (Pantos et al. 1999, Iwata et al. 2011, Bartmann et al. 2004). The oocyte quality decreases with maternal age and the age-associated mitochondrial deterioration is closely related to this decline in oocyte quality (Pantos et al. 1999, Tilly and Sinclair 2013, Kansaku et al. 2017b). Several studies described a decrease in the mitochondrial DNA copy number, decline in the ATP content and increased levels of reactive oxygen species (ROS) in oocytes in different animal species (Simsek-Duran et al. 2013, Rambags et al. 2014, Kansaku et al. 2017b). Kansaku et al. (2017b) described a lower level of mitochondrial biogenesis and an inadequate activation of mitochondrial biogenesis in response to uncoupler-induced mitochondrial dysfunction in oocytes of aged cows. They hypothesized that high ROS and SIRT1 (silent mating type information regulation 2 homolog 1) levels in aged bovine oocytes restrain the proper activation of the mitochondrial quality control system and might cause aged-associated decline in oocyte quality.
A proper coordination of metabolic processes is crucial for the correct pace of maturation of oocytes (Funahashi et al. 2008). Levels of ATP and the gene expression of key proteins of the electron transport chain (ETC) fluctuate during the maturation process in oocytes, with high levels of ATP at the GVBD stage and early MII stage (Yu et al. 2010, Yamamoto et al. 2010). Gap junctions between CCs and oocytes are open at the beginning of oocyte maturation, whereas in its later stages they close thus reducing the importance of the mutual interconnections and the delivery of substrates to oocytes (Appeltant et al. 2015, Ozawa et al. 2008). An RNA-sequencing analysis of oocytes and GCs revealed an increased expression of genes associated with glycolysis in GCs and genes associated with ETC in oocytes during the final stages of maturation (Munakata et al. 2016b, Kansaku et al. 2017a). These findings explain, why mitochondrial dysfunction affects differently the ATP content in oocytes depending on the stage of their maturation (Kansaku et al. 2017a).
Oxygen is the most important and essential nutrient for the cellular growth and energy metabolism and the number of granulosa cells and their metabolism determine the follicular oxygen concentration (Li et al. 2013, Clark et al. 2006). Oxygen levels in human follicular fluid increase rapidly during the early antral stages of follicle growth, then decline to low levels in the late antral and pre-ovulatory phases (Redding et al. 2008). Hypoxia activates glycolysis and the gene analysis revealed that HIF1-associated genes are upregulated in the granulosa cells during the normal follicle development. HIF1 is the key regulator of glycolysis and oxidative phosphorylation in granulosa cells and oocytes (Takahashi et al. 2016, Shiratsuki et al. 2016).
Metabolism of saccharides and lipids
Mitochondria of female mammalian germ cells undergo important changes during the oogenesis, maturation and fertilization. They show dynamic morphological changes as they increase in number and populate different cell domains within the oocyte. At the same time mitochondria form complex relationships with other cellular organelles (e.g. smooth endoplasmic reticulum and vesicles), according to different energetic/metabolic needs of the cell. At the end they are inherited by the developing embryo, where they assume a more typical somatic cell form. (Motta et al. 2000). Their main role is in providing ATP molecules through the metabolism of saccharides and lipids contained within the cell cytoplasm and in the medium (Wilding et al. 2001). Embryos generate a large amount of lactate during in vitro culture with a very low intensity of glucose oxidation, perhaps only 10 % of total glucose utilization (Wales et al. 1987, Thompson et al. 1991, Bavister and Squirrell 2000). These data might indicate that the mitochondrial metabolic activity is low during early embryo development, or may reflect a restricted metabolism of glucose via glycolysis (Wales et al. 1987) or a competition for mitochondrial metabolism by other oxidizable substrates (glutamine, pyruvate) provided in the culture medium (Thompson et al. 1991, Bavister and Squirrell 2000).
Glucose is taken up by cumulus cells via the GLUT transporters and converted to pyruvate. It can be then directly transferred into the oocyte through gap junctions, or it can also be secreted by the cumulus cells and taken up from the surrounding medium by the oocyte (Wang et al. 2012, Su et al. 2009). Observations showed that the maturation of oocytes is possible in a glucose-containing medium but only in the presence of follicle granulosa cells producing pyruvate and/or oxaloacetate (Donahue and Stern 1968, Leese and Barton 1985, Downs and Hudson 2000). On the other hand, there are studies describing the presence of hexokinase, glucose-6-phosphate dehydrogenase, enzymes of the pentose phosphate pathway, as well as GLUTs, in oocytes suggests a possible involvement of glucose metabolism in some oocyte functions (Wang et al. 2012). Pyruvate transferred from CCs together with fatty acids are the most important sources of substrates for ATP production in the mitochondria of maturing oocytes (Simerman et al. 2015). Uhde et al. (2018) describe that during the maturation of cumulus-oocyte complexes (COCs) for 23 h there was a significant increase of concentrations of both pyruvate and lactate in the medium, while glucose concentrations decreased. In particular the lactate concentration in the maturation medium increased 667-fold after an 8-hour maturation in vitro (Uhde et al. 2018). Oocytes are rich in members of the monocarboxylic acid transporters (MCT) family that enable the transport of lactate (Uhde et al. 2018, Herubel et al. 2002).
Lipids are important in terms of energy production during the oocyte maturation and the establishment of a developmentally competent oocyte (Uhde et al. 2018). The molar yield of ATP is much higher for β-oxidation than for glycolysis. Lipid droplets have been found in CCs and β-oxidation takes place predominantly in them (Dunning et al. 2014b, Aardema et al. 2013). Fatty acid oxidation (assessed by the production 3H2O formed via oxidation of 9,10-[3H]palmitate) in CCs is increased in cumulus-oocyte complexes matured in vivo and conversely deficient during in vitro maturation (Dunning et al. 2014a). In vitro, the supplementation of maturation medium with palmitic and stearic acids resulted in a detrimental effect on oocyte maturation, whereas linolenic acid was described to have a positive effect on oocyte maturation and embryo development (Marei et al. 2009, Aardema et al. 2011, Uhde et al. 2018). An optimization of oocyte maturation in vitro still has a way to go in order to improve reproductive technology.
Effect of inhibitors of energy metabolism
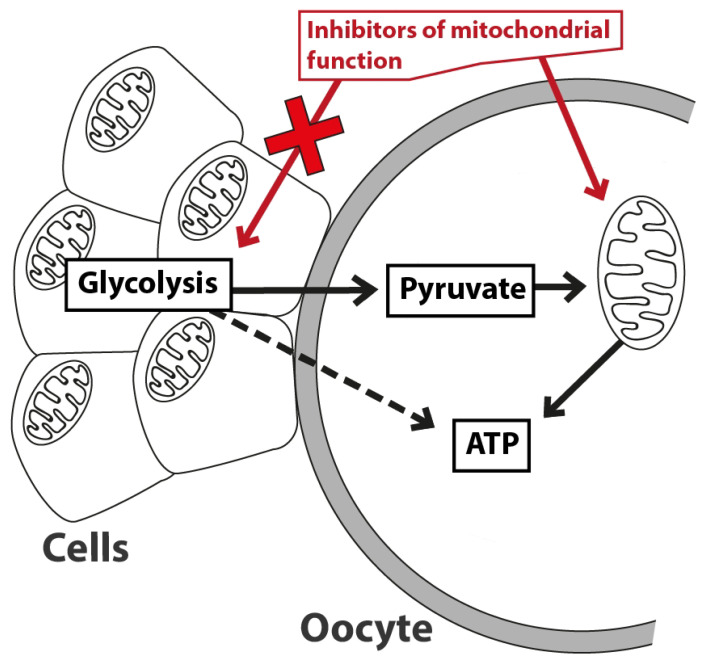
Kansaku et al. (2017a) studied in detail the effects of different mitochondrial inhibitors, an inhibitor of glycolysis (bromopyruvic acid – BA) and a gap-junction inhibitor (18α-glycyrrhetinic acid – 18-GA) on the ATP content in GCs and oocytes (Kansaku et al. 2017a). Basic types of mitochondrial inhibitors/modulators (uncoupler CCCP, complex III inhibitor antimycin A and ATP-synthase inhibitor oligomycin) have profound negative effects on the ATP production of denuded oocytes, whereas BA caused no negative effect. GCs showed an opposite result: BA reduced the ATP content, whereas mitochondrial inhibitors did not change it. COCs treated with CCCP or 18-GA showed a reduced ATP content in oocytes, where 18-GA caused only slight decrease. A higher number of GCs was able partially to mitigate the negative effect of CCCP on the ATP production in oocytes (rich vs. poor COCs), a co-treatment with 18-GA abolished the effect. These results suggest that oocytes primarily depend on ATP production via the electron transport chain, whereas GCs depend mainly on glycolysis. A mitochondrial dysfunction therefore only affects the energy status of oocytes, allowing GCs to act as an ATP provider for oocytes (via working gap junctions), even following treatment with the uncoupler CCCP. Oocytes depend on both: self-production of ATP through mitochondria and ATP provided by GCs (Kansaku et al. 2017a). A mutual exchange of metabolic substrates is shown in Figure 4.
Fig. 4.
Exchange of energy substrates between the oocyte and cumulus cells as described by Kansaku et al. (2017a).
Similar results were described by Dalton et al. (2014), who studied the effect of the ATP synthase inhibitor oligomycin and the inhibitor of glycolysis iodoacetic acid (inhibits glyceraldehyde 3-phosphate dehydrogenase) on ATP levels (Dalton et al. 2014) using a recombinant FRET-based probe. Oligomycin caused a significant decline of ATP content in oocytes, whereas iodoacetic acid caused no decrease. An inhibition of gap junction transport abolished the higher level of ATP in cumulus enclosed oocytes (Dalton et al. 2014). The uncoupler CCCP was shown to enhance mitochondrial biogenesis and degradation in oocytes, marked by an increased mitochondrial DNA copy number and an increased expression of mitochondrial transcription factor A (TFAM), a key regulator of mitochondrial biogenesis (Itami et al. 2015).
There are, however, some methodological limitations to these studies: (1) measurement of ATP content instead of ATP/ADP ratio is not an accurate parameter for the evaluation of the cellular energy state and (2) some inhibitors used in these studies are not specific enough for the processes that were assessed. Therefore, more detailed studies are necessary for a deeper understanding of the effects of the inhibition of the mitochondrial ETC and other metabolic pathways in COCs.
Reactive oxygen species and antioxidant mechanisms
The role of reactive oxygen species in the physiological and pathological processes in the human body has been well described (Dröge 2002, Valko et al. 2007). Together with antioxidants they play an important part in the regulation of reproductive processes and fertility in humans. The oxidant/antioxidant imbalance has been shown to be involved in the development of a number of female reproductive diseases, including the polycystic ovary syndrome, endometriosis and preeclampsia (Lu et al. 2018). Defects of the mitochondrial respiratory chain cause severe ATP deficiencies and increased generation of ROS (Schatten et al. 2014). The overproduction of ROS leads among other things to a damage of mtDNA with severe consequences on mitochondrial functions (Chappel 2013). Endometriosis is an illustrative example of a disease with a known pathogenesis involving a ROS overproduction. The follicular fluid from patients with endometriosis contains ROS and damages nuclear DNA in the oocyte (Hamdan et al. 2016). The disease can result in cumulus cell mitochondrial dysfunction and cause defective apoptosis and increased oxidative stress (Zhang et al. 2017). As a consequence, cumulus cells of women with endometriosis have a lower ATP production and a reduced ability to protect the developing oocyte with a negative impact on the fertility of the patients (Hsu et al. 2015). However, cumulus cells have a significant protective role against the oxidative damage caused by ROS. They show an ability to protect the oocyte against lower levels of H2O2 and •OH (Shaeib et al. 2016). When the concentration of H2O2 increases, it dramatically affects the cumulus cell number and this reduction in the antioxidant capacity makes the oocyte more susceptible to oxidative damage (Shaeib et al. 2016).
Cumulus cells synthesize glutathione (GSH), a key antioxidant molecule of cells (Luberda 2005). It protects cells from oxidative damage and supports the maintenance of redox homeostasis (Meister 1983, Forman et al. 2009). Glutathione is a tripeptide thiol found in mammalian cells, where it plays major roles in the redox metabolism, detoxification of xenobiotics and amino acid transport. It has been shown that glutathione is an important cytoplasmic factor of the oocyte. A modulation of GSH levels during the oocyte maturation and fertilization is crucial for sperm chromatin decondensation and male pronucleus formation after sperm penetration in some animal species (Calvin et al. 1986, Perreault et al. 1988, Yoshida 1993, Sutovsky and Schatten 1997). Human spermatozoa decondense also in vitro in the presence of physiological concentrations of heparin and GSH (Reyes et al. 1989, Caglar et al. 2005). When the maturation of the oocyte begins there is an evidence of an increasing content of GSH in cumulus cells (Zuelke et al. 2003). In an in vitro maturation medium supplemented with a higher concentration of cysteamine as a precursor for the GSH synthesis cumulus cells have higher levels of GSH (de Matos et al. 1997).
However, the protection of oocytes against ROS harmful effect provided by cumulus cells has some limitations. Lower concentrations of H2O2 and •OH can be effectively detoxified, whereas the effects of higher concentrations of these or other ROS such as HClO could not be prevented (Shaeib et al. 2016).
Granular cells as targets of environmental toxins
The human body is exposed to a variety of environmental insults e.g. air pollution, food additives, toxins and radiation leading to genotoxicity. These factors induce a damage at different levels: damage to oocyte DNA or to surrounding granulosa cells (Winship et al. 2018). The follicular fluid, which is the most important environment for a proper oocyte development, is also negatively affected by these factors (Younglai et al. 2002). With the increasing incidence of cancer during recent years and the corresponding increase in the use of effective non-surgical cancer therapy (chemotherapy and radiotherapy) the risk ovarian failure due to these treatments becomes a highly relevant problem (Molina et al. 2005, Roness et al. 2014, Warne et al. 1973). Growing cells in both mature and immature follicles are the initial targets of a long-term exposure to chemotherapeutic agents (Jeruss and Wooddruff 2009). Pyknosis indicating granulosa cells death is detectable within a few hours after irradiation (Stroud et al. 2009).
Environmental toxins known as endocrine-disrupting compounds such as bisphenol A (BPA), a component of plastics widely found in water, air and food, decrease the viability of GCs by inducing their apoptosis and a G2-to-M arrest (Xu et al. 2002). Similarly, bisphenol S (BPS) causes a failure of microtubule formation and disrupts cumulus cells expansion (Zalmanova et al. 2017). The impact of endocrine disruptors on newborn steroidogenesis was shown in a study of Kolatorova et al. (2018), which confirmed accumulation of phthalate in the fetal area via measurements in maternal plasma and in the cord blood of their newborns. These facts taken together have a significant impact on quality of oocytes and success of human reproduction. Further studies should be made to elucidate the mechanism of action of these compounds and their effect on the collaborative follicular niche.
Conclusions
Follicle growth and the development and maturation of oocytes are highly dependent on and regulated by the metabolic and signaling coupling between oocytes and granulosa cells. The bi-directional communication is mediated by a wide spectrum of signaling molecules regulating the processes in the partner cells. This coordinated interconnection is crucial for the delivery of energy substrates and other important components. Both GCs and oocytes prefer different energy substrates in covering their needs: GCs are more glycolytic, whereas oocytes depend more on the oxidative phosphorylation pathway. At the same time, oocytes are supplied with intermediates produced in glycolysis by GCs. Environmental insults, genetic predisposition and some therapeutic methods can interrupt these processes leading to changes of the cellular metabolic and signaling profiles leading to a decreased quality of oocytes, development of diseases of the female reproductive tract and to lower fertility. Further investigations are necessary for a deeper understanding of these harmful mechanism in order to develop treatments to limit their effects and improve methods of in vitro fertilization.
Acknowledgements
The authors would like to thank Martin Pavelka and Daniel Nezbeda for help in preparing the schemes and figures in this paper. This study was supported by the Charles University funding PROGRES Q36, Czech Health Research Council (NV18-01-00544), Ministry of Agriculture of the Czech Republic – institutional support MZE-RO0718 and European Human Biomonitoring Initiative HBM4EU provided by H2020.
Abbreviations
- 18-GA
18α-glycyrrhetinic acid
- Akt
protein kinase B
- ATP
adenosine triphosphate
- BA
bromopyruvic acid
- BMP-6
bone morphogenetic protein 6
- BMP-15
bone morphogenetic protein 15
- BPA
bisphenol A
- BPS
bisphenol S
- cAMP
cyclic adenosine monophosphate
- CCCP
carbonyl cyanide m-chlorophenyl hydrazone
- CCs
cumulus granulosa cell
- COCs
cumulus-oocyte complexes
- ER
endoplasmic reticulum
- ETC
electron transport chain
- FF-MAS
follicular fluid meiosis-activating sterol
- FSH
follicle stimulating hormone
- GCs
granulosa cells
- GDF-9
growth-differentiation factor 9
- GDF-9B
growth-differentiation factor 9B
- GLUT
glucose transporter
- GSH
glutathione
- GV
germinal vesicle
- GVBD
germinal vesicle breakdown
- HA
hyaluronic acid
- HAS-2
hyaluronan synthase 2
- HIF-1α
hypoxia-inducible factor 1-alpha
- HClO
hypochlorous acid
- IVM
in vitro maturation
- LH
luteinizing hormone
- MI
metaphase I stage oocyte
- MII
metaphase II stage oocyte
- MCT
monocarboxylic acid transporters
- MGCs
mural granulosa cells
- ROS
reactive oxygen species
- SER
smooth endoplasmic reticulum
- SIRT-1
sirtuin (silent mating type information regulation 2 homolog) 1
- TFAM
transcription factor A
- uPA
urokinase-type plasminogen activator
- VEGF
vascular endothelial growth factor.
Footnotes
Conflict of interest
There is no conflict of interest.
References
- AALTONEN J, LAITINEN MP, VUOJOLAINEN K, JAATINEN R, HORELLI-KUITUNEN N, SEPPÄ L, LOUHIO H, TUURI T, SJÖBERG J, BÜTZOW R, HOVATA O, DALE L, RITVOS O. Human growth differentiation factor 9 (GDF-9) and its novel homolog GDF-9B are expressed in oocytes during early folliculogenesis. J Clin Endocrinol Metab. 1999;84:2744–2750. doi: 10.1210/jc.84.8.2744. [DOI] [PubMed] [Google Scholar]
- AARDEMA H, LOLICATO F, Van de LEST CHA, BROUWERS JF, VAANDRAGER AB, Van TOL HTA, ROELEN BAJ, VOS PLAM, HELMS JB, GADELLA BM. Bovine cumulus cells protect maturing oocytes from increased fatty acid levels by massive intracellular lipid storage. Biol Reprod. 2013;88:164–164. doi: 10.1095/biolreprod.112.106062. [DOI] [PubMed] [Google Scholar]
- AARDEMA H, VOS PLAM, LOLICATO F, ROELEN BAJ, KNIJN HM, VAANDRAGER AB, HELMS JB, GADELLA BM. Oleic acid prevents detrimental effects of saturated fatty acids on bovine oocyte developmental competence. Biol Reprod. 2011;85:62–69. doi: 10.1095/biolreprod.110.088815. [DOI] [PubMed] [Google Scholar]
- ANDERSON E, ALBERTINI DF. Gap junctions between the oocyte and companion follicle cells in the mammalian ovary. J Cell Biol. 1976;71:680–686. doi: 10.1083/jcb.71.2.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APPELTANT R, SOMFAI T, NAKAI M, BODÓ S, MAES D, KIKUCHI K, Van SOOM A. Interactions between oocytes and cumulus cells during in vitro maturation of porcine cumulus-oocyte complexes in a chemically defined medium: effect of denuded oocytes on cumulus expansion and oocyte maturation. Theriogenology. 2015;83:567–576. doi: 10.1016/j.theriogenology.2014.10.026. [DOI] [PubMed] [Google Scholar]
- BARTMANN AK, ROMAO GS, RAMOS EDA S, FERRIANI RA. Why do older women have poor implantation rates? A possible role of the mitochondria. J Assist Reprod Genet. 2004;21:79–83. doi: 10.1023/B:JARG.0000027018.02425.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAVISTER BD, SQUIRRELL JM. Mitochondrial distribution and function in oocytes and early embryos. Hum Reprod. 2000;15:189–198. doi: 10.1093/humrep/15.suppl_2.189. [DOI] [PubMed] [Google Scholar]
- BUCCIONE R, SCHROEDER AC, EPPIG JJ. Interactions between somatic cells and germ cells throughout mammalian oogenesis. Biol Reprod. 1990;43:543–547. doi: 10.1095/biolreprod43.4.543. [DOI] [PubMed] [Google Scholar]
- CAGLAR GS, HAMMADEH M, ASIMAKOPOULOS B, NIKOLETTOS N, DIEDRICH K, AL-HASSANI S. In vivo and In vitro decondensation of human sperm and assisted reproduction technologies. In Vivo. 2005;19:623–630. [PubMed] [Google Scholar]
- CALVIN HI, GROSSHANS K, BLAKE EJ. Estimation and manipulation of glutathione levels in prepuberal mouse ovaries and ova: Relevance to sperm nucleus transformation in the fertilized egg. Gamete Res. 1986;14:265–275. doi: 10.1002/mrd.1120140310. [DOI] [Google Scholar]
- CAVILLA JL, KENNEDY CR, BYSKOV AG, HARTSHORNE GM. Human immature oocytes grow during culture for IVM. Hum Reprod. 2008;23:37–45. doi: 10.1093/humrep/dem178. [DOI] [PubMed] [Google Scholar]
- CHAPPEL S. The role of mitochondria from mature oocyte to viable blastocyst. Obstet Gynecol Int. 2013;2013:1–10. doi: 10.1155/2013/183024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARK AR, STOKES YM, LANE M, THOMPSON JG. Mathematical modelling of oxygen concentration in bovine and murine cumulus-oocyte complexes. Reproduction. 2006;131:999–1006. doi: 10.1530/rep.1.00974. [DOI] [PubMed] [Google Scholar]
- DALTON CM, SZABADKAI G, CARROLL J. Measurement of ATP in single oocytes: impact of maturation and cumulus cells on levels and consumption. J Cell Physiol. 2014;229:353–361. doi: 10.1002/jcp.24457. [DOI] [PubMed] [Google Scholar]
- DEBEY P, SZÖLLÖSI MS, SZÖLLÖSI D, VAUTIER D, GIROUSSE A, BESOMBES D. Competent mouse oocytes isolated from antral follicles exhibit different chromatin organization and follow different maturation dynamics. Mol Reprod Dev. 1993;36:59–74. doi: 10.1002/mrd.1080360110. [DOI] [PubMed] [Google Scholar]
- De la FUENTE R, EPPIG JJ. Transcriptional activity of the mouse oocyte genome: companion granulosa cells modulate transcription and chromatin remodeling. Dev Biol. 2001;229:224–236. doi: 10.1006/dbio.2000.9947. [DOI] [PubMed] [Google Scholar]
- De la FUENTE R, VIVEIROS MM, BURNS KH, ADASHI EY, MATZUK MM, EPPIG JJ. Major chromatin remodeling in the germinal vesicle (GV) of mammalian oocytes is dispensable for global transcriptional silencing but required for centromeric heterochromatin function. Dev Biol. 2004;75:447–458. doi: 10.1016/j.ydbio.2004.08.028. [DOI] [PubMed] [Google Scholar]
- De MATOS DG, FURNUS CC, MOSES DF. Glutathione synthesis during in vitro maturation of bovine oocytes: role of cumulus cells. Biol Reprod. 1997;57:1420–1425. doi: 10.1095/biolreprod57.6.1420. [DOI] [PubMed] [Google Scholar]
- DIAZ FJ, O’BRIEN MJ, WIGGLESWORTH K, EPPIG JJ. The preantral granulosa cell to cumulus cell transition in the mouse ovary: development of competence to undergo expansion. Dev Biol. 2006;299:91–104. doi: 10.1016/j.ydbio.2006.07.012. [DOI] [PubMed] [Google Scholar]
- DIAZ FJ, WIGGLESWORTH K, EPPIG JJ. Oocytes are required for the preantral granulosa cell to cumulus cell transition in mice. Dev Biol. 2007;305:300–311. doi: 10.1016/j.ydbio.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DONAHUE RP, STERN S. Follicular cell support of oocyte maturation: production of pyruvate in vitro. J Reprod Fertil. 1968;17:395–398. doi: 10.1530/jrf.0.0170395. [DOI] [PubMed] [Google Scholar]
- DOWNS SM, DANIEL SAJ, EPPIG JJ. Induction of maturation in cumulus cell-enclosed mouse oocytes by follicle-stimulating hormone and epidermal growth factor: evidence for a positive stimulus of somatic cell origin. J Exp Zool. 1988;245:86–96. doi: 10.1002/jez.1402450113. [DOI] [PubMed] [Google Scholar]
- DOWNS SM, HUDSON ED. Energy substrates and the completion of spontaneous meiotic maturation. Zygote. 2000;8:339–351. doi: 10.1017/S0967199400001131. [DOI] [PubMed] [Google Scholar]
- DOWNS SM. The influence of glucose, cumulus cells, and metabolic coupling on the ATP levels and meiotic control in the isolated mouse oocyte. Dev Biol. 1995;167:502–512. doi: 10.1006/dbio.1995.1044. [DOI] [PubMed] [Google Scholar]
- DRÖGE W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- DUBE JL, WANG P, ELVIN J, LYONS KM, CELESTE AJ, MATZUK MM. The bone morphogenetic protein 15 gene is X-linked and expressed in oocytes. Mol Endocrinol. 1998;12:1809–1817. doi: 10.1210/mend.12.12.0206. [DOI] [PubMed] [Google Scholar]
- DUNNING KR, ANASTASI MR, ZHANG VJ, RUSSELL DL, ROBKER RL. Regulation of fatty acid oxidation in mouse cumulus-oocyte complexes during maturation and modulation by PPAR agonists. PLoS One. 2014a;9:e87327. doi: 10.1371/journal.pone.0087327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNNING KR, RUSSELL DL, ROBKER RL. Lipids and oocyte developmental competence: the role of fatty acids and β-oxidation. Reproduction. 2014b;148:R15–R27. doi: 10.1530/REP-13-0251. [DOI] [PubMed] [Google Scholar]
- EPPIG JJ. FSH stimulates hyaluronic acid synthesis by oocyte-cumulus cell complexes from mouse preovulatory follicles. Nature. 1979;281:483–484. doi: 10.1038/281483a0. [DOI] [PubMed] [Google Scholar]
- EPPIG JJ. Intercommunication between mammalian oocytes and companion somatic cells. Bioessays. 1991;13:569–574. doi: 10.1002/bies.950131105. [DOI] [PubMed] [Google Scholar]
- EPPIG JJ. Coordination of nuclear and cytoplasmic oocyte maturation in eutherian mammals. Reprod Fertil Dev. 1996;8:485–489. doi: 10.1071/RD9960485. [DOI] [PubMed] [Google Scholar]
- FITZHARRIS G, MARANGOS P, CARROLL J. Changes in endoplasmic reticulum structure during mouse oocyte maturation are controlled by the cytoskeleton and cytoplasmic dynein. Dev Biol. 2007;305:133–144. doi: 10.1016/j.ydbio.2007.02.006. [DOI] [PubMed] [Google Scholar]
- FORMAN HJ, ZHANG H, RINNA A. Glutathione: overview of its protective roles, measurement, and biosynthesis. Mol Aspects Med. 2009;30:1–12. doi: 10.1016/j.mam.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUNAHASHI H, KOIKE T, SAKAI R. Effect of glucose and pyruvate on nuclear and cytoplasmic maturation of porcine oocytes in a chemically defined medium. Theriogenology. 2008;70:1041–1047. doi: 10.1016/j.theriogenology.2008.06.025. [DOI] [PubMed] [Google Scholar]
- GILCHRIST RB, LANE M, THOMPSON JG. Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality. Hum Reprod Update. 2008;14:159–177. doi: 10.1093/humupd/dmm040. [DOI] [PubMed] [Google Scholar]
- GILCHRIST RB, RITTER LJ, ARMSTRONG DT. Oocyte-somatic cell interactions during follicle development in mammals. Anim Reprod Sci. 2004;82–83:431–446. doi: 10.1016/j.anireprosci.2004.05.017. [DOI] [PubMed] [Google Scholar]
- GILULA NB, REEVES OR, STEINBACH A. Metabolic coupling, ionic coupling and cell contacts. Nature. 1972;235:262–265. doi: 10.1038/235262a0. [DOI] [PubMed] [Google Scholar]
- HAMDAN M, JONES KT, CHEONG Y, LANE SIR. The sensitivity of the DNA damage checkpoint prevents oocyte maturation in endometriosis. Sci Rep. 2016;6:36994. doi: 10.1038/srep36994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HÉRUBEL F, El MOUATASSIM S, GUÉRIN P, FRYDMAN R, MÉNÉZO Y. Genetic expression of monocarboxylate transporters during human and murine oocyte maturation and early embryonic development. Zygote. 2002;10:175–181. doi: 10.1017/S096719940200223X. [DOI] [PubMed] [Google Scholar]
- HSU AL, TOWNSEND PM, OEHNINGER S, CASTORA FJ. Endometriosis may be associated with mitochondrial dysfunction in cumulus cells from subjects undergoing in vitro fertilization-intracytoplasmic sperm injection, as reflected by decreased adenosine triphosphate production. Fertil Steril. 2015;103:347–352. doi: 10.1016/j.fertnstert.2014.11.002. [DOI] [PubMed] [Google Scholar]
- INOUE A, NAKAJIMA R, NAGATA M, AOKI F. Contribution of the oocyte nucleus and cytoplasm to the determination of meiotic and developmental competence in mice. Hum Reprod. 2008;23:1377–1384. doi: 10.1093/humrep/den096. [DOI] [PubMed] [Google Scholar]
- IKAWA M, INOUE N, BENHAM AM, OKABE M. Fertilization: a sperm’s journey to and interaction with the oocyte. J Clin Invest. 2010;120:984–994. doi: 10.1172/JCI41585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ITAMI N, SHIRATSUKI S, SHIRASUNA K, KUWAYAMA T, IWATA H. Mitochondrial biogenesis and degradation are induced by CCCP treatment of porcine oocytes. Reproduction. 2015;150:97–104. doi: 10.1530/REP-15-0037. [DOI] [PubMed] [Google Scholar]
- IWATA H, GOTO H, TANAKA H, SAKAGUCHI Y, KIMURA K, KUWAYAMA T, MONJI Y. Effect of maternal age on mitochondrial DNA copy number, ATP content and IVF outcome of bovine oocytes. Reprod Fertil Dev. 2011;23:424–432. doi: 10.1071/RD10133. [DOI] [PubMed] [Google Scholar]
- JERUSS JS, WOODRUFF TK. Preservation of fertility in patients with cancer. N Engl J Med. 2009;360:902–911. doi: 10.1056/NEJMra0801454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANSAKU K, ITAMI N, KAWAHARA-MIKI R, SHIRASUNA K, KUWAYAMA T, IWATA H. Differential effects of mitochondrial inhibitors on porcine granulosa cells and oocytes. Theriogenology. 2017a;103:98–103. doi: 10.1016/j.theriogenology.2017.07.049. [DOI] [PubMed] [Google Scholar]
- KANSAKU K, TAKEO S, ITAMI N, KIN A, SHIRASUNA K, KUWAYAMA T, IWATA H. Maternal aging affects oocyte resilience to carbonyl cyanide-m-chlorophenylhydrazone-induced mitochondrial dysfunction in cows. PLoS One. 2017b;12:e0188099. doi: 10.1371/journal.pone.0188099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIDDER GM, MHAWI AA. Gap junctions and ovarian folliculogenesis. Reproduction. 2002;123:613–620. doi: 10.1530/reprod/123.5.613. [DOI] [PubMed] [Google Scholar]
- KOLATOROVA L, VITKU J, VAVROUS R, HAMPL R, ADAMCOVA K, SIMKOVA M, PARIZEK A, STARKA L, DUSKOVA M. Phthalate metabolites in maternal and cord plasma and their relations to other selected endocrine disruptors and steroids. Physiol Res. 2018;67(Suppl 3):S473–S487. doi: 10.33549/physiolres.933962. [DOI] [PubMed] [Google Scholar]
- KOLESAROVA A, SIROTKIN AV, MELLEN M, ROYCHOUDHURY S. Possible intracellular regulators of female sexual maturation. Physiol Res. 2015;64:379–386. doi: 10.33549/physiolres.932838. [DOI] [PubMed] [Google Scholar]
- LARSEN WJ, WERT SE. Roles of cell junctions in gametogenesis and in early embryonic development. Tissue Cell. 1988;20:809–848. doi: 10.1016/0040-8166(88)90025-0. [DOI] [PubMed] [Google Scholar]
- LEESE HJ, BARTON AM. Production of pyruvate by isolated mouse cumulus cells. J Exp Zool. 1985;234:231–236. doi: 10.1002/jez.1402340208. [DOI] [PubMed] [Google Scholar]
- LI D, REDDING GP, BRONLUND JE. Oxygen consumption by bovine granulosa cells with prediction of oxygen transport in preantral follicles. Reprod Fertil Dev. 2013;25:1158–1164. doi: 10.1071/RD12283. [DOI] [PubMed] [Google Scholar]
- LUBERDA Z. The role of glutathione in mammalian gametes. Reprod Biol. 2005;5:5–17. [PubMed] [Google Scholar]
- LU J, WANG Z, CAO J, CHEN Y, DONG Y. A novel and compact review on the role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 2018;16:80. doi: 10.1186/s12958-018-0391-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MADGWICK S, JONES KT. How eggs arrest at metaphase II: MPF stabilisation plus APC/C inhibition equals cytostatic factor. Cell Div. 2007;2:4. doi: 10.1186/1747-1028-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAREI WF, WATHES DC, FOULADI-NASHTA AA. The effect of linolenic Acid on bovine oocyte maturation and development. Biol Reprod. 2009;81:1064–1072. doi: 10.1095/biolreprod.109.076851. [DOI] [PubMed] [Google Scholar]
- MARO B, JOHNSON MH, PICKERING SJ, FLACH G. Changes in actin distribution during fertilization of the mouse egg. J Embryol Exp Morphol. 1984;81:211–237. [PubMed] [Google Scholar]
- MARO B, VERLHAC M-H. Polar body formation: new rules for asymmetric divisions. Nat Cell Biol. 2002;4:E281–E283. doi: 10.1038/ncb1202-e281. [DOI] [PubMed] [Google Scholar]
- MEISTER A. Selective modification of glutathione metabolism. Science. 1983;220:472–477. doi: 10.1126/science.6836290. [DOI] [PubMed] [Google Scholar]
- MOLINA JR, BARTON DL, LOPRINZI CL. Chemotherapy-induced ovarian failure. Drug Saf. 2005;28:401–416. doi: 10.2165/00002018-200528050-00004. [DOI] [PubMed] [Google Scholar]
- MORENO RD, SCHATTEN G, RAMALHO-SANTOS J. Golgi apparatus dynamics during mouse oocyte in vitro maturation: effect of the membrane trafficking inhibitor brefeldin A. Biol Reprod. 2002;66:1259–1266. doi: 10.1095/biolreprod66.5.1259. [DOI] [PubMed] [Google Scholar]
- MOTTA PM, NOTTOLA SA, MAKABE S, HEYN R. Mitochondrial morphology in human fetal and adult female germ cells. Hum Reprod. 2000;15:129–147. doi: 10.1093/humrep/15.suppl_2.129. [DOI] [PubMed] [Google Scholar]
- MUNAKATA Y, ICHINOSE T, OGAWA K, ITAMI N, TASAKI H, SHIRASUNA K, KUWAYAMA T, IWATA H. Relationship between the number of cells surrounding oocytes and energy states of oocytes. Theriogenology. 2016a;86:1789–1798. doi: 10.1016/j.theriogenology.2016.05.036. [DOI] [PubMed] [Google Scholar]
- MUNAKATA Y, KAWAHARA-MIKI R, SHIRATSUKI S, TASAKI H, ITAMI N, SHIRASUNA K, KUWAYAMA T, IWATA H. Gene expression patterns in granulosa cells and oocytes at various stages of follicle development as well as in in vitro grown oocyte-and-granulosa cell complexes. J Reprod Dev. 62:359–366. doi: 10.1262/jrd.2016-022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORISAKA M, TAJIMA K, TSANG BK, KOTSUJI F. Oocyte-granulosa-theca cell interactions during preantral follicular development. J Ovarian Res. 2009;2:9. doi: 10.1186/1757-2215-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OZAWA M, NAGAI T, SOMFAI T, NAKAI M, MAEDOMARI N, FAHRUDIN M, KARJA NWK, KANEKO H, NOGUCHI J, OHNUMA K, YOSHIMI N, MIYAZAKI H, KIKUCHI K. Comparison between effects of 3-isobutyl-1-methylxanthine and FSH on gap junctional communication, LH-receptor expression, and meiotic maturation of cumulus-oocyte complexes in pigs. Mol Reprod Dev. 2008;75:857–866. doi: 10.1002/mrd.20820. [DOI] [PubMed] [Google Scholar]
- PANTOS K, ATHANASIOU V, STEFANIDIS K, STAVROU D, VAXEVANOGLOU T, CHRONOPOULOU M. Influence of advanced age on the blastocyst development rate and pregnancy rate in assisted reproductive technology. Fertil Steril. 1999;71:1144–1146. doi: 10.1016/S0015-0282(99)00121-1. [DOI] [PubMed] [Google Scholar]
- PERREAULT SD, BARBEE RR, SLOTT VL. Importance of glutathione in the acquisition and maintenance of sperm nuclear decondensing activity in maturing hamster oocytes. Dev Biol. 1988;125:181–186. doi: 10.1016/0012-1606(88)90070-X. [DOI] [PubMed] [Google Scholar]
- PICTON H, BRIGGS D, GOSDEN R. The molecular basis of oocyte growth and development. Mol Cell Endocrinol. 1998;145:27–37. doi: 10.1016/S0303-7207(98)00166-X. [DOI] [PubMed] [Google Scholar]
- RACEDO SE, RAWE VY, NIEMANN H. Dynamic changes of the Golgi apparatus during bovine in vitro oocyte maturation. Reproduction. 2012;143:439–447. doi: 10.1530/REP-11-0492. [DOI] [PubMed] [Google Scholar]
- RAMBAGS BP, Van BOXTEL DC, THARASANIT T, LENSTRA JA, COLENBRANDER B, STOUT TA. Advancing maternal age predisposes to mitochondrial damage and loss during maturation of equine oocytes in vitro. Theriogenology. 2014;81:959–965. doi: 10.1016/j.theriogenology.2014.01.020. [DOI] [PubMed] [Google Scholar]
- REDDING GP, BRONLUND JE, HART AL. Theoretical investigation into the dissolved oxygen levels in follicular fluid of the developing human follicle using mathematical modelling. Reprod Fertil Dev. 2008;20:408–417. doi: 10.1071/RD07190. [DOI] [PubMed] [Google Scholar]
- REYES R, ROSADO A, HERNÁNDEZ O, DELGADO NM. Heparin and glutathione: physiological decondensing agents of human sperm nuclei. Gamete Res. 1989;23:39–47. doi: 10.1002/mrd.1120230105. [DOI] [PubMed] [Google Scholar]
- RONESS H, KALICH-PHILOSOPH L, MEIROW D. Prevention of chemotherapy-induced ovarian damage: possible roles for hormonal and non-hormonal attenuating agents. Hum Reprod Update. 2014;20:759–774. doi: 10.1093/humupd/dmu019. [DOI] [PubMed] [Google Scholar]
- SALUSTRI A, GARLANDA C, HIRSCH E, De ACETIS M, MACCAGNO A, BOTTAZZI B, DONI A, BASTONE A, MANTOVANI G, BECK PECCOZ P, SALVATORI G, MAHONEY DJ, DAY AJ, SIRACUSA G, ROMANI L, MANTOVANI A. PTX3 plays a key role in the organization of the cumulus oophorus extracellular matrix and in in vivo fertilization. Development. 2004;131:1577–1586. doi: 10.1242/dev.01056. [DOI] [PubMed] [Google Scholar]
- SALUSTRI A, YANAGISHITA M, HASCALL VC. Mouse oocytes regulate hyaluronic acid synthesis and mucification by FSH-stimulated cumulus cells. Dev Biol. 1990;138:26–32. doi: 10.1016/0012-1606(90)90173-G. [DOI] [PubMed] [Google Scholar]
- SALUSTRI A, YANAGISHITA M, UNDERHILL CB, LAURENT TC, HASCALL VC. Localization and synthesis of hyaluronic acid in the cumulus cells and mural granulosa cells of the preovulatory follicle. Dev Biol. 1992;151:541–551. doi: 10.1016/0012-1606(92)90192-J. [DOI] [PubMed] [Google Scholar]
- SÁNCHEZ F, SMITZ J. Molecular control of oogenesis. Biochim Biophys Acta. 2012;1822:1896–1912. doi: 10.1016/j.bbadis.2012.05.013. [DOI] [PubMed] [Google Scholar]
- SCHATTEN H, SUN QY, PRATHER R. The impact of mitochondrial function/dysfunction on IVF and new treatment possibilities for infertility. Reprod Biol Endocrinol. 2014;12:111. doi: 10.1186/1477-7827-12-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAEIB F, KHAN SN, ALI I, THAKUR M, SAED MG, DAI J, AWONUGA AO, BANERJEE J, ABU-SOUD HM. The defensive role of cumulus cells against reactive oxygen species insult in metaphase II mouse oocytes. Reprod Sci. 2016;23:498–507. doi: 10.1177/1933719115607993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIRATSUKI S, HARA T, MUNAKATA Y, SHIRASUNA K, KUWAYAMA T, IWATA H. Low oxygen level increases proliferation and metabolic changes in bovine granulosa cells. Mol Cell Endocrinol. 2016;437:75–85. doi: 10.1016/j.mce.2016.08.010. [DOI] [PubMed] [Google Scholar]
- SIMERMAN AA, HILL DL, GROGAN TR, ELASHOFF D, CLARKE NJ, GOLDSTEIN EH, MANRRIQUEZ AN, CHAZENBALK GD, DUMESIC DA. Intrafollicular cortisol levels inversely correlate with cumulus cell lipid content as a possible energy source during oocyte meiotic resumption in women undergoing ovarian stimulation for in vitro fertilization. Fertil Steril. 2015;103:249–257. doi: 10.1016/j.fertnstert.2014.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMSEK DURAN F, LI F, FORD W, SWANSON RJ, JONES HW, JR, CASTORA FJ. Age-associated metabolic and morphologic changes in mitochondria of individual mouse and hamster oocytes. PLoS One. 2013;8:e64955. doi: 10.1371/journal.pone.0064955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOJKOVIC M, MACHADO SA, STOJKOVIC P, ZAKHARTCHENKO V, HUTZLER P, GONÇALVES PB, WOLF E. Mitochondrial distribution and adenosine triphosphate content of bovine oocytes before and after in vitro maturation: correlation with morphological criteria and developmental capacity after in vitro fertilization and culture. Biol Reprod. 2001;64:904–909. doi: 10.1095/biolreprod64.3.904. [DOI] [PubMed] [Google Scholar]
- STROUD JS, MUTCH D, RADER J, POWELL M, THAKER PH, GRIGSBY PW. Effects of cancer treatment on ovarian function. Fertil Steril. 2009;92:417–427. doi: 10.1016/j.fertnstert.2008.07.1714. [DOI] [PubMed] [Google Scholar]
- SUGIYAMA M, SUMIYA M, SHIRASUNA K, KUWAYAMA T, IWATA H. Addition of granulosa cell mass to the culture medium of oocytes derived from early antral follicles increases oocyte growth, ATP content, and acetylation of H4K12. Zygote. 2016;24:848–856. doi: 10.1017/S0967199416000198. [DOI] [PubMed] [Google Scholar]
- SUN F, BAHAT A, GAKAMSKY A, GIRSH E, KATZ N, GIOJALAS LC, TUR-KASPA I, EISENBACH M. Human sperm chemotaxis: both the oocyte and its surrounding cumulus cells secrete sperm chemoattractants. Hum Reprod. 2005;20:761–767. doi: 10.1093/humrep/deh657. [DOI] [PubMed] [Google Scholar]
- SUTOVSKY P, SCHATTEN G. Depletion of glutathione during bovine oocyte maturation reversibly blocks the decondensation of the male pronucleus and pronuclear apposition during fertilization. Biol Reprod. 1997;56:1503–1512. doi: 10.1095/biolreprod56.6.1503. [DOI] [PubMed] [Google Scholar]
- SUTTON-McDOWALL ML, GILCHRIST RB, THOMPSON JG. The pivotal role of glucose metabolism in determining oocyte developmental competence. Reproduction. 2010;139:685–695. doi: 10.1530/REP-09-0345. [DOI] [PubMed] [Google Scholar]
- SU Y-Q, SUGIURA K, EPPIG J. Mouse oocyte control of granulosa cell development and function: paracrine regulation of cumulus cell metabolism. Semin Reprod Med. 2009;27:32–42. doi: 10.1055/s-0028-1108008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKAHASHI N, DAVY PM, GARDNER LH, MATHEWS J, YAMAZAKI Y, ALLSOPP RC. Hypoxia inducible factor 1 alpha is expressed in germ cells throughout the murine life cycle. PLoS One. 2016;11:e0154309. doi: 10.1371/journal.pone.0154309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TANGHE S, Van SOOM A, NAUWYNCK H, CORYN M, De KRUIF A. Minireview: Functions of the cumulus oophorus during oocyte maturation, ovulation, and fertilization. Mol Reprod Dev. 2002;61:414–424. doi: 10.1002/mrd.10102. [DOI] [PubMed] [Google Scholar]
- TEIXEIRA FILHO FL, BARACAT EC, LEE TH, SUH CS, MATSUI M, CHANG RJ, SHIMASAKI S, ERICKSON GF. Aberrant expression of growth differentiation factor-9 in oocytes of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87:1337–1344. doi: 10.1210/jcem.87.3.8316. [DOI] [PubMed] [Google Scholar]
- TEVES ME, BARBANO F, GUIDOBALDI HA, SANCHEZ R, MISKA W, GIOJALAS LC. Progesterone at the picomolar range is a chemoattractant for mammalian spermatozoa. Fertil Steril. 2006;86:745–749. doi: 10.1016/j.fertnstert.2006.02.080. [DOI] [PubMed] [Google Scholar]
- THOMPSON JG, SIMPSON AC, PUGH PA, WRIGHT RW, JR, TERVIT HR. Glucose utilization by sheep embryos derived in vivo and in vitro. Reprod Fertil Dev. 1991;3:571–576. doi: 10.1071/RD9910571. [DOI] [PubMed] [Google Scholar]
- TILLY JL, SINCLAIR DA. Germline energetics, aging, and female infertility. Cell Metab. 2013;17:838–850. doi: 10.1016/j.cmet.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UHDE K, Van TOL HTA, STOUT TAE, ROELEN BAJ. Metabolomic profiles of bovine cumulus cells and cumulus-oocyte-complex-conditioned medium during maturation in vitro. Sci Rep. 2018;8:9477. doi: 10.1038/s41598-018-27829-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VALKO M, LEIBFRITZ D, MONCOL J, CRONIN MT, MAZUR M, TELSER J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- VIRANT-KLUN I, BAUER C, STÅHLBERG A, KUBISTA M, SKUTELLA T. Human oocyte maturation in vitro is improved by co-culture with cumulus cells from mature oocytes. Reprod Biomed Online. 2018;36:508–523. doi: 10.1016/j.rbmo.2018.01.011. [DOI] [PubMed] [Google Scholar]
- WAI T, AO A, ZHANG X, CYR D, DUFORT D, SHOUBRIDGE EA. The role of mitochondrial DNA copy number in mammalian fertility. Biol Reprod. 2010;83:52–62. doi: 10.1095/biolreprod.109.080887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALES RG, WHITTINGHAM DG, HARDY K, CRAFT IL. Metabolism of glucose by human embryos. J Reprod Fertil. 1987;79:289–297. doi: 10.1530/jrf.0.0790289. [DOI] [PubMed] [Google Scholar]
- WANG Q, CHI MM, SCHEDL T, MOLEY KH. An intercellular pathway for glucose transport into mouse oocytes. Am J Physiol Endocrinol Metab. 2012;302:E1511–E1518. doi: 10.1152/ajpendo.00016.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARNE GL, FAIRLEY KF, HOBBS JB, MARTIN FIR. Cyclophosphamide-induced ovarian failure. N Engl J Med. 1973;289:1159–1162. doi: 10.1056/NEJM197311292892202. [DOI] [PubMed] [Google Scholar]
- WERT SE, LARSEN WJ. Preendocytotic alterations in cumulus cell gap junctions precede meiotic resumption in the rat cumulus-oocyte complex. Tissue Cell. 1990;22:827–851. doi: 10.1016/0040-8166(90)90047-D. [DOI] [PubMed] [Google Scholar]
- WILDING M, DALE B, MARINO M, Di MATTEO L, ALVIGGI C, PISATURO ML, LOMBARDI L, De PLACIDO G. Mitochondrial aggregation patterns and activity in human oocytes and preimplantation embryos. Hum Reprod. 2001;16:909–917. doi: 10.1093/humrep/16.5.909. [DOI] [PubMed] [Google Scholar]
- WINSHIP AL, STRINGER JM, LIEW SH, HUTT KJ. The importance of DNA repair for maintaining oocyte quality in response to anti-cancer treatments, environmental toxins and maternal ageing. Hum Reprod Update. 2018;24:119–134. doi: 10.1093/humupd/dmy002. [DOI] [PubMed] [Google Scholar]
- XIE H-L, WANG Y-B, JIAO G-Z, KONG D-L, LI Q, LI H, ZHENG L-L, TAN J-H. Effects of glucose metabolism during in vitro maturation on cytoplasmic maturation of mouse oocytes. Sci Rep. 2016;6:20764. doi: 10.1038/srep20764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XU J, OSUGA Y, YANO T, MORITA Y, TANG X, FUJIWARA T, TAKAI Y, MATSUMI H, KOGA K, TAKETANI Y, TSUTSUMI O. Bisphenol A induces apoptosis and G2-to-M arrest of ovarian granulosa cells. Biochem Biophys Res Commun. 2002;292:456–462. doi: 10.1006/bbrc.2002.6644. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO T, IWATA H, GOTO H, SHIRATUKI S, TANAKA H, MONJI Y, KUWAYAMA T. Effect of maternal age on the developmental competence and progression of nuclear maturation in bovine oocytes. Mol Reprod Dev. 2010;77:595–604. doi: 10.1002/mrd.21188. [DOI] [PubMed] [Google Scholar]
- YEO CX, GILCHRIST RB, THOMPSON JG, LANE M. Exogenous growth differentiation factor 9 in oocyte maturation media enhances subsequent embryo development and fetal viability in mice. Hum Reprod. 2008;23:67–73. doi: 10.1093/humrep/dem140. [DOI] [PubMed] [Google Scholar]
- YOSHIDA M. Role of glutathione in the maturation and fertilization of pig oocytes in vitro. Mol Reprod Dev. 1993;35:76–81. doi: 10.1002/mrd.1080350113. [DOI] [PubMed] [Google Scholar]
- YOUNGLAI EV, FOSTER WG, HUGHES EG, TRIM K, JARRELL JF. Levels of environmental contaminants in human follicular fluid, serum, and seminal plasma of couples undergoing in vitro fertilization. Arch Environ Contam Toxicol. 2002;43:121–126. doi: 10.1007/s00244-001-0048-8. [DOI] [PubMed] [Google Scholar]
- YU Y, DUMOLLARD R, ROSSBACH A, LAI FA, SWANN K. Redistribution of mitochondria leads to bursts of ATP production during spontaneous mouse oocyte maturation. J Cell Physiol. 2010;224:672–680. doi: 10.1002/jcp.22171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ŽALMANOVÁ T, HOŠKOVÁ K, NEVORAL J, ADÁMKOVÁ K, KOTT T, ŠULC M, KOTÍKOVÁ Z, PROKEŠOVÁ Š, JÍLEK F, KRÁLÍČKOVÁ M, PETR J. Bisphenol S negatively affects the meotic maturation of pig oocytes. Sci Rep. 2017;7:485. doi: 10.1038/s41598-017-00570-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG D, KEILTY D, ZHANG ZF, CHIAN RC. Mitochondria in oocyte aging: current understanding. Facts Views Vis Obgyn. 2017;9:29–38. [PMC free article] [PubMed] [Google Scholar]
- ZUELKE KA, JEFFAY SC, ZUCKER RM, PERREAULT SD. Glutathione (GSH) concentrations vary with the cell cycle in maturing hamster oocytes, zygotes, and pre-implantation stage embryos. Mol Reprod Dev. 2003;64:106–112. doi: 10.1002/mrd.10214. [DOI] [PubMed] [Google Scholar]