Abstract
We compared chronic graft-versus-host disease (cGvHD) following umbilical cord blood (UCBT) and matched sibling donor peripheral blood transplant (MSD). 145 patients (2010–2017) with cGvHD after MSD (n = 104) and UCBT (n = 41) were included. Prior acute GvHD was less frequent in MSD (55% vs. 85%; p = 0.01). Severe cGvHD (32% vs. 15%, p = 0.01) and de-novo onset (45% vs. 15%, p < 0.01) were more frequent following MSD. Liver was more frequently involved in MSD recipients (38% vs. 6%); and GI in UCBT (33% vs. 63%), both p < 0.01. Overall response (CR + PR) was similar between both cohorts. 2-year CR was higher in UCBT (14% vs 33%, p = 0.02). Karnofsky score (KPS) ≥ 90 at cGvHD diagnosis was associated with higher odds of response (95%CI: 1.42–10, p < 0.01). The cumulative incidence of durable discontinuation of immune-suppressive therapy, failure-free survival (FFS) and NRM at 2-years were similar between cohorts. KPS < 90 (95%CI: 3.1–24.9, p < 0.01) and platelets <100 × 10e9/L (95%CI: 1.25–10, p = 0.01) were associated with higher risk of NRM. UCBT patients were more likely to have a prior acute GvHD, less severe cGvHD and more likely to attain CR. Despite differences, both cohorts had similar NRM and FFS. High-risk groups, including those with platelets <100 × 10e9/L and KPS < 90, need careful monitoring and intensified therapy.
Introduction
Chronic Graft-versus-Host Disease (cGvHD) remains a major cause of morbidity and mortality following allogeneic hematopoietic stem cell transplant (allo-HCT). cGvHD occurs in ~30–50% of patients after allo-HCT and is a leading cause of late nonrelapse mortality (NRM) [1, 2]. It is also associated with an inferior quality of life [3]. The commonly identified risk factors for cGvHD include transplant from an human leukocyte antigen (HLA) mismatched or unrelated donor, older age at HCT, HCT from an alloimmunized female donor to male recipient, peripheral blood stem cell (PBSC) graft compared to bone marrow or umbilical cord blood, and history of prior acute GvHD (aGvHD) [1, 4-8]. Recently, GvHD prophylaxis strategies including anti-thymocyte globulin (ATG) and post-HCT cyclophosphamide have been shown to be associated with a lower incidence of cGvHD [9, 10].
Although the incidence of cGvHD is reportedly lower after umbilical cord blood transplantation (UCBT) [11-13], studies evaluating comparative outcomes after diagnosis of cGvHD are limited. We hence performed a retrospective cohort study of patients, who developed cGvHD following a UCBT or MSD PBSCT, treated uniformly in a similar manner at a single center (University of Minnesota) and present results of a comparative analysis of clinical presentation, response to treatment, duration of immune-suppresive therapy (IST), NRM and failure free survival (FFS).
Methods and definitions
This retrospective cohort study included all patients ≥18 years of age, who developed cGvHD after MSD PBSCT or double/single UCBT at University of Minnesota between 2010 and 2017. The cohort included 145 patients (104 patients following MSD PBSCT and 41 after UCBT). Patients received an unrelated donor single or double UCBT based on cell dose and HLA matching criteria as previously published [14]. The University of Minnesota Institutional Review Board (IRB) reviewed and approved this study. Patient demographic and transplant characteristics, diagnosis and date of cGvHD and survival information were retrieved from the University of Minnesota BMT database. This was supplemented with chart reviews for cGvHD characteristics, response, immune-suppression, complications, survival and underlying malignancy relapse.
Definitions: The 2014 NIH Consensus Criteria [15] was used to retrospectively reclassify cGvHD cases, organ involvement, and overall cGvHD severity at diagnosis. Onset of cGvHD was defined as progressive if cGvHD developed without resolution of prior acute GvHD, de-novo if cGvHD developed without prior acute GvHD and quiescent if cGvHD developed after complete resolution of prior acute GvHD [16]. Patients, who had a relapse of underlying malignancy or donor lymphocyte infusion (DLI) prior to the development of cGvHD, were excluded from this study.
Evaluation of response
Response was assessed at 6-months, 1-year and 2-years after cGvHD diagnosis retrospectively [17] as defined below. Wide window periods of ±4 weeks were used to maximize response capture. (a) Complete response (CR) was defined as resolution of all reversible signs and symptoms of cGvHD, (b) Partial response (PR) included those with improvement in one or more organs of involvement and no evidence of worsening in any other organ, (c) Flare was defined as worsening of cGvHD that was less severe than at the baseline evaluation in patients with previous CR or PR, (d) Progression was defined as manifestations that were worse than at baseline evaluation, including new organ involvement, and (e) Mixed response (MR) included patients with CR or PR in 1 or more organs and worsening in one or more organs compared to baseline.
Systemic IST was considered as any form of systemic treatment, including extracorporeal photopheresis (ECP) to treat cGvHD. Topical immune-suppressive therapy for skin, oral, ophthalmic involvement and isolated topical gastrointestinal treatment with budesonide was not considered as systemic immune-suppression. Treatment type, duration and time to discontinuation of IST were captured. Durable discontinuation of steroids was defined as no treatment with steroids for at least 6 consecutive months after discontinuation. Durable discontinuation of all IST was defined as no exposure to any systemic IST for at least 6 months after stopping all IST.
Treatment: most patients initiated treatment with (i) either weekly pulsed methylprednisolone (15 mg/kg/week) × 8 weeks (n = 71, 50 MSD, 21 UCBT), along with prednisone (0.5 mg/kg every other day) and calcineurin inhibitor or sirolimus or (ii) prednisone 1 mg/kg/day (n = 74, 54 MSD, 20 UCBT) and calcineurin inhibitor or sirolimus. Taper was initiated over 4–8 weeks to reach a dose of 0.5 mg/kg every other day, with the tapering starting within 2 weeks after the first evidence of cGvHD improvement. Once an every-other-day prednisone (or equivalent) regimen was achieved, this dose remained constant for 10–12 weeks until all reversible cGvHD manifestations resolved. A second taper was then initiated to taper and stop over the next 12 weeks. Once prednisone was discontinued, taper of calcineurin inhibitor or sirolimus was initiated and tapered off over the next 3 months. Additional lines of systemic immune-suppression were defined as a change/ or addition of a new systemic treatment to treat an inadequate response or progression of cGvHD. Increase in prednisone dose by ≥25% was considered as an additional line of treatment.
Failure free survival (FFS) was defined as a composite end point including the absence of second-line treatment, NRM, and recurrent malignancy during initial systemic treatment of cGvHD [2]. FFS was calculated at 2 years after initiation of cGvHD therapy.
Statistical analysis
The study is a retrospective cohort study. The primary endpoints were response to IST at 1 year and NRM at 2 years in UCBT vs. MSD PBSCT. Secondary endpoints included clinical presentation (organ involvement, NIH global severity) and duration of IST. All endpoints were assessed from diagnosis of cGvHD. Variables related to patient, disease, and transplantation characteristics were described using descriptive statistics. Proportion of patients within each category of response were compared using chi-square test. Cumulative incidence for NRM was calculated treating disease progression/relapse as the competing risk. Cumulative incidence was also used to determine discontinuation of steroids and discontinuation of all immune-suppression treating relapse/disease progression and death as competing risks.
Logistic regression was employed to evaluate the independent effect of variables on treatment response. Fine and Gray regression was used for analysis of nonrelapse mortality and time to discontinuation of immunosuppressive therapy. SAS 9.4 (SAS Institute, Inc., Cary, NC) was used for all statistical analyses.
The following predictors were evaluated in multivariate analysis of response and NRM (assessed from diagnosis of cGvHD): gender, age at transplant (continuous variable), donor type (matched sibling vs. single/double UCBT), conditioning regimen (myeloablative vs. reduced intensity conditioning), diagnosis (acute leukemia vs. chronic leukemia vs. lymphoma vs. other), lymphocytes at cGvHD diagnosis (<0.8 × 10e9/L vs. ≥0.8 × 10e9/L), eosinophils at cGvHD diagnosis (<0.7 × 10e9/L vs. ≥0.7 × 10e9/L), platelets at cGvHD diagnosis (<100 × 10e9/L vs. ≥100 × 10e9/L), cGvHD onset (de-novo vs. quiescent vs. progressive), type of cGvHD (classic vs. overlap), cGvHD global severity at diagnosis (mild vs. moderate vs. severe), number of organs involved at diagnosis (1-2 vs. 3+), days from transplant to diagnosis of cGvHD, Karnofsky score (KPS) at cGvHD diagnosis (<90 vs. ≥90), recipient (R)/donor (D) CMV serostatus (R+/D+ vs. R+/D− vs. R−/D+ vs. R−/D−), and HCT-comorbidity index (low vs. intermediate vs. high risk).
Results
The 2-year cumulative incidence of cGvHD following MSD PBSCT was 104/340 (31%; 95% CI: 26–36%) vs 41/391 (10%; 95% CI: 7–14%) in UCBT.
Table 1 shows the characteristics of patients with cGvHD. Of 145 patients, 104 (72%) received MSD PBSCT and 41 (28%) UCBT. Pre-HCT conditioning was myeloablative in 34% vs. 59% of MSD PBSCT and UCBT groups respectively, p = 0.02. The median time from HCT to cGvHD was similar, at 218 days (range, 88–1111) versus 224 days (range, 110–903) in the MSD PBSCT and UCBT cohorts, respectively. Prior acute GvHD was seen in 55% after MSD PBSCT vs. 85% after UCBT, p < 0.01. Classic cGvHD was seen in 68% vs. 63% and overlap cGvHD developed in 32 and 37% of patients in MSD and UCBT cohorts, respectively (p = 0.58). Diagnosis of cGvHD was confirmed with biopsy in 102 cases (70%), including liver biopsy in 17 cases (17%), GI biopsy in 15 cases (15%), minor salivary gland biopsy in 16 cases (16%), lower lip biopsy in 28 (27%) and skin biopsy in 26 (25%) cases. Thrombocytopenia at diagnosis of cGvHD (platelet count <100 × 10e9/L) was similar in MSD PBSCT (24%) and UCBT (32%) (p = 0.34). Onset of cGvHD (Fig. 1A) and organ involvement are shown in Fig. 1B, C. De-novo onset of cGvHD was more frequent following MSD PBSCT (45%), compared to UCBT (15%), p < 0.01. As shown in Fig. 1B, liver involvement (38% vs. 6%, p < 0.01) was more frequent after MSD PBSCT, whereas gastrointestinal (GI) involvement was significantly more frequent after UCBT (33% in MSD vs. 63% in UCBT, p < 0.01). Involvement of ≥3 organs with cGvHD was similar between MSD PBSCT vs UCBT (53% vs. 37%), p = 0.08. Characteristics of organ involvement at the onset of cGvHD are shown in Fig. 1C. cGvHD was more severe after MSD PBSCT compared to UCBT (32% vs 15%, p = 0.01) (Table 1).
Table 1.
Demographic and clinical characteristics.
| MSD PBSCT, n (%) |
UCBT, n (%) | p value | |
|---|---|---|---|
| N | 104 | 41 | |
| Age | 0.06 | ||
| Age, median (range) | 54 (23–74) | 50 (19–69) | |
| Gender | 0.74 | ||
| Male | 69 (66%) | 26 (63%) | |
| Diagnosis | |||
| Acute leukemia | 57 (55%) | 29 (71%) | |
| Chronic leukemia (CML/CLL) | 4 (4%) | 2 (5%) | |
| Lymphoma | 21 (20%) | 4 (10%) | |
| Other | 22 (21%) | 6 (15%) | |
| Donor type | |||
| Matched sibling | 104 (100%) | 0 (0%) | |
| sUCBT | 0 (0%) | 9 (22%) | |
| dUCBT | 0 (0%) | 32 (78%) | |
| Conditioning regimen | 0.02 | ||
| Myeloablative with total body irradiation | 35 (34%) | 24 (59%) | |
| Myeloablative without total body irradiation | 3 (3%) | 1 (2%) | |
| Reduced intensity conditioning | 66 (63%) | 16 (39%) | |
| HCT- Comorbidity Index | 0.75 | ||
| Low risk: 0 | 41 (39%) | 19 (46%) | |
| Intermediate risk: 1–2 | 31 (30%) | 11 (27%) | |
| High risk: 3+ | 32 (31%) | 11 (27%) | |
| Days from HCT to cGvHD | |||
| Days (median range) | 218 (88–1111) | 224 (110–903) | |
| GvHD prophylaxis | <0.01 | ||
| CNI + MTX | 50 (48%) | 0 (0%) | |
| CNI + MMF | 54 (52%) | 33 (80%) | |
| Sirolimus+MMF | 0 (0%) | 8 (20%) | |
| CMV, Recepient/Donor (R/D) | |||
| R+/D+ | 29 (28%) | 0 (0%) | |
| R+/D− | 26 (25%) | 26 (63%) | |
| R−/D− | 34 (33%) | 15 (37%) | |
| R−/D+ | 15 (14%) | 0 (0%) | |
| HLA match | |||
| 8/8 HLA matched | 104 (100%) | 0 (0%) | |
| sUCBT | 0 (0%) | 9 (22%) | |
| 4/6 HLA matched | 2 (22%) | ||
| 5/6 HLA matched | 5 (56%) | ||
| 6/6 HLA matched | 2 (22%) | ||
| dUCBT | 0 (0%) | 32 (78%) | |
| 4/6 + 4/6 HLA matched | 9 (28%) | ||
| 4/6 + 5/6 HLA matched | 5 (16%) | ||
| 4/6 + 6/6 HLA matched | 0 (0%) | ||
| 5/6 + 5/6 HLA matched | 10 (31%) | ||
| 5/6 + 6/6 HLA matched | 6 (19%) | ||
| 6/6 + 6/6 HLA matched | 2 (6%) | ||
| Prior aGvHD by grade | <0.01 | ||
| No aGvHD | 47 (45%) | 6 (15%) | |
| Grade 1 | 6 (6%) | 2 (5%) | |
| Grade 2 | 30 (29%) | 12 (29%) | |
| Grade 3 | 15 (14%) | 17 (41%) | |
| Grade 4 | 6 (6%) | 4 (10%) | |
| Karnofsky score at cGvHD diagnosis | 0.75 | ||
| <90 at cGvHD diagnosis | 28 (27%) | 10 (24%) | |
| Cumulative incidence of cGvHD | 104/340 (31%) | 41/391(10%) | |
| cGvHD onset | <0.01 | ||
| De-Novo | 47 (45%) | 6 (15%) | |
| Quiescent | 34 (33%) | 23 (56%) | |
| Progressive | 23 (22%) | 12 (29%) | |
| cGvHD type | 0.58 | ||
| Classic | 71 (68%) | 26 (63%) | |
| Overlap | 33 (32%) | 15 (37%) | |
| cGvHD global severity at diagnosis | 0.01 | ||
| Mild | 12 (12%) | 12 (29%) | |
| Moderate | 59 (57%) | 23 (56%) | |
| Severe | 33 (32%) | 6 (15%) | |
| Numbers of organs at cGvHD diagnosis | 0.08 | ||
| 1 or 2 | 49 (47%) | 26 (63%) | |
| 3+ | 55 (53%) | 15 (37%) | |
| Platelets count at cGvHD diagnosis | 0.34 | ||
| <100 × 10e9/L | 25 (24%) | 13 (32%) | |
| ≥100 × 10e9/L | 79 (76%) | 28 (68%) | |
| Eosinophil count at cGvHD diagnosis | 0.05 | ||
| <0.7 × 10e9/L | 79 (76%) | 37 (90%) | |
| ≥0.7 × 10e9/L | 25 (24%) | 4 (10%) | |
| Lymphocyte count at cGvHD diagnosis | 0.63 | ||
| <0.8 × 10e9/L | 27 (26%) | 12 (29%) | |
| ≥0.8–5.3 × 10e9/L | 77(74%) | 29 (71%) | |
| cGvHD treatment | 0.33 | ||
| Systemic | 26 (25%) | 13 (32%) | |
| Topical | 7 (7%) | 5 (12%) | |
| Both (topical & systemic) | 71 (68%) | 23 (56%) |
CML chronic myelogenous leukemia, CLL chronic lymphocytic leukemia, UCBT umbilical cord blood transplant, sUCBT single UCBT, dUCBT double UCBT, HCT-CI Hematopoietic Cell Transplantation - comorbidity index, CNI calcineurin inhibitor, MTX Methotrexate, MMF mycophenolate mofetil, CMV cytomegalovirus, HLA human leukocyte antigen, MSD PBSCT matched sibling donor peripheral blood stem cell transplant, cGvHD chronic graft-versus-host disease, aGvHD acute graft-versus-host disease.
Fig. 1. Chronic GvHD characterisitcs.
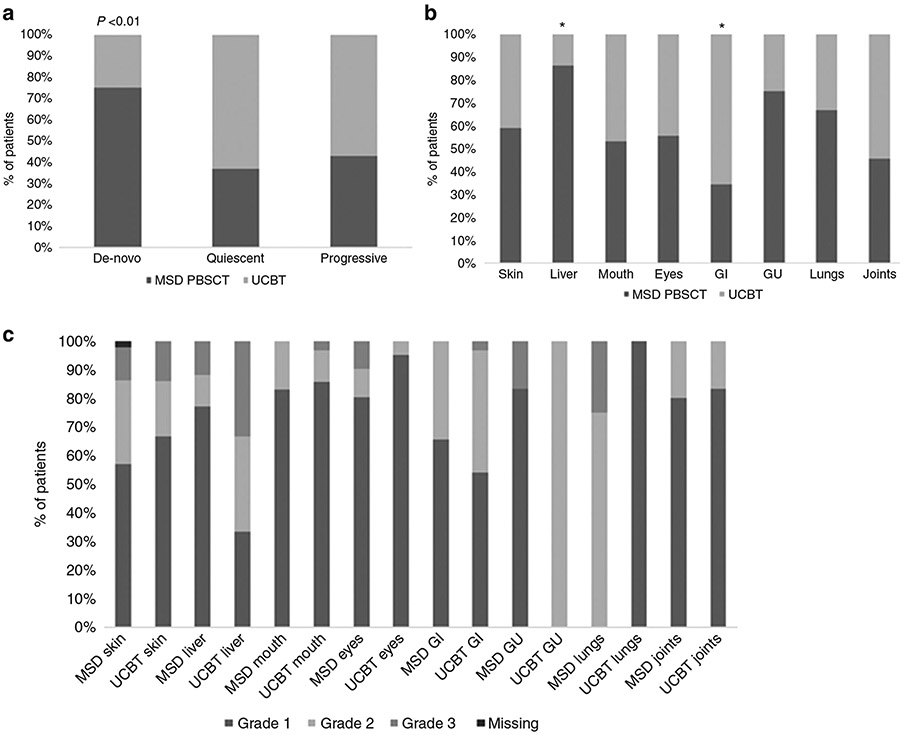
A Type of onset of chronic GvHD; B Clinical presentation of chronic GvHD at diagnosis; C Grade of organ involvement at the onset of chronic GvHD. *Statistically significant (p < 0.01). cGvHD chronic graft-versus-host disease, MSD PBSCT matched sibling donor peripheral blood stem cell transplant, UCBT umbilical cord blood transplant, GI gastrointestinal, GU genito-urinary.
Response to therapy
Response was assessed at 6-months, 1-year and 2-years from diagnosis of cGvHD. No difference was seen in overall response rates (CR + PR) at 6-months (78% vs. 78%; p = 0.98), 1-year (69% vs. 73%; p = 0.72) and 2-years (67% vs. 67%; p = 0.99) in MSD vs. UCBT respectively. Attainment of CR was significantly higher in UCBT group compared to MSD PBSCT by 2-years, (33% vs 14%, p = 0.02) (Table 2).
Table 2.
Response at 6 months, 1 year and 2 years.
| 6 months |
1 year |
2 years |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| MSD (n = 104) |
UCBT (n = 41) |
p | MSD (n = 98) |
UCBT (n = 40) |
p | MSD (n = 90) |
UCBT (n = 36) |
p | |
| CR + PR | 81 (78%) | 32 (78%) | 0.98 | 68 (69%) | 29 (73%) | 0.72 | 60 (67%) | 24 (67%) | 0.99 |
| CR | 1 (1%) | 2 (5%) | 0.19 | 5 (5%) | 4 (10%) | 0.28 | 11 (14%) | 11 (33%) | 0.02 |
| Other | 23 (22%) | 9 (22%) | 30 (31%) | 11 (28%) | 30 (33%) | 12 (33%) | |||
Other includes following: mixed response, flare, progression, death or relapse of underlying malignancy.
MSD matched sibling donor, UCBT umbilical cord blood transplant, CR complete response, PR partial response
Multivariate logistic regression at 1-year (Table 3), showed that UCBT recipients had similar odds of CR + PR (OR: 0.9 (95% CI: 0.4–2.1, p = 0.9) compared to MSD PBSCT. KPS ≥ 90 at the time of diagnosis of cGvHD was associated with 3-fold higher odds of response to therapy (95% CI: 1.42–10.0, p < 0.01).
Table 3.
Multivariate analysis of response to therapy at 1 year.
| Factor | Odds Ratio of CR + PR (95% CI) |
P value |
|---|---|---|
| Donor type | 0.09 | |
| MSD PBSCT | 1.0 (reference) | |
| UCBT | 0.9 (0.4–2.1) | |
| Karnofsky performance score | <0.01 | |
| 90–100 | 1.0 (reference) | |
| <90 | 0.3 (01–0.7) |
MSD PBSCT matched sibling donor peripheral blood stem cell transplant, UCBT umbilical cord blood transplant, CR complete response, PR partial response.
Overall survival from diagnosis of cGvHD was 79% (95% CI: 70–86%) vs 71% (95% CI: 54–83%), p = 0.29, in MSD PBSCT vs UCBT cohorts, respectively. Causes of death are described in Table 4.
Table 4.
Causes of death (CoD).
| MSD, n (%) | UCBT, n (%) | |
|---|---|---|
| Graft failure | 1 (5%) | 0 |
| Bacterial infection | 1 (5%) | 0 |
| Fungal infection | 1 (5%) | 0 |
| Other infection | 3 (15%) | 0 |
| GvHD | 3 (15%) | 5 (45%) |
| Relapse of hematologic malignancy | 9 (45%) | 3 (27%) |
| Multiorgan failure | 0 | 1 (9%) |
| Hemorrhage | 0 | 1 (9%) |
| Unknown causes | 2 (10%) | 0 |
MSD matched sibling donor, UCBT umbilical cord blood transplant.
Overall infections were seen in 91/104 MSD PBSCT vs 36/41 UCBT patients in the 2 groups. The incidence density of bacterial infections per 1000 patient-days within 2 years in MSD PBSCT vs UCBT cohorts was 2.83 vs 3.18, viral infections 2.09 vs 2.16, fungal infections 0.44 vs 0.33 in MSD PBSCT vs UCBT respectively. The overall incidence density of infections within 2 years was 5.37 vs 5.67 in MSD PBSCT vs UCBT groups (all p > 0.4).
Nonrelapse mortality
The overall incidence of NRM at 2 years following cGvHD was 15% (95% CI: 9–21%). The 2-year cumulative incidence of NRM was similar in the 2 cohorts: MSD PBSCT [13% (95% CI: 7–20%)] vs. UCBT [20% (95% CI: 8–33%), p = 0.29)], as shown in Fig. 2. In multivariate analysis, UCBT recipients had a similar risk of NRM at 2 years as compared to MSD PBSCT recipients (RR: 1.9, 95% CI: 0.8–4.3, p = 0.15). Across both cohorts KPS < 90 was associated with an 8.8-fold higher risk of NRM (95% CI: 3.1–24.9, p < 0.01) and platelet count of <100 × 10e9/L was associated with 3-fold higher risk of NRM (95% CI: 1.25–10, p = 0.01) (Table 5).
Fig. 2. Nonrelapse mortality by donor type.
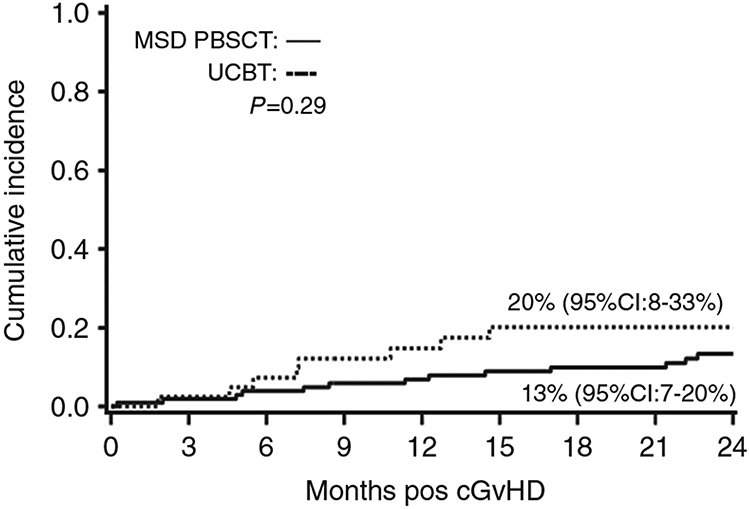
2-year cumulative incidence of NRM in MSD PBSCT and UCBT cohorts. cGvHD chronic graft-versus-host disease, MSD PBSCT matched sibling donor peripheral blood stem cell transplant, UCBT umbilical cord blood transplant.
Table 5.
Multivariate analysis of 2-year nonrelapse mortality.
| Factors | Hazard ratio (95 CI%) | p value |
|---|---|---|
| Donor type | 0.15 | |
| MSD PBSCT | 1.0 (reference) | |
| UCBT | 1.9 (0.8–4.3) | |
| Karnofsky performance score | <0.01 | |
| 90–100 | 1.0 (reference) | |
| <90 | 8.8 (3.1–24.9) | |
| Platelets | 0.01 | |
| <100 × 10e9/L | 1.0 (reference) | |
| ≥100 × 10e9/L | 0.3 (0.1–0.8) |
MSD PBSCT matched sibling donor peripheral donor stem cell transplant, UCBT umbilical cord blood transplant.
66 (46%) patients required additional lines of immune-suppressive therapy within 2 years from diagnosis of cGvHD. The treatments were initiated as per physician preference except in 7 patients (10%) (4 patients were enrolled in a trial testing ROCK2 inhibitor, and 3 patients were enrolled in a trial testing ibrutinib). Of the remaining 59 patients – 24 (36%) had ≥25% increase in prednisone dose, 12 (18%) were started on sirolimus, 7 (11%) were started on tacrolimus, 4 (6%) received mycophenolate, 4 (6%) received extracorporeal photopheresis, 3 (4%) were started on cyclosporine, 2 (3%) were given rituximab, 1 (2%) was started on ofatumumab, 1 (2%) received ruxolitinib, and 1 (2%) patient received imatinib.
Discontinuation of IST
The cumulative incidence of durable discontinuation of steroids at 2 years was 53% (95% CI: 43–63%) and was similar after MSD PBSCT [53% (95% CI: 41–64%)] and UCBT [55% (95% CI: 35–74%)], p = 0.63. Median time to discontinuation of steroids was 213 days (range, 49–1581 days) in MSD PBSCT group vs. 280 days (range, 30–2063 days) in UCBT (p = 0.76).
The cumulative incidence of durable discontinuation of all IST at 2 years was 42% (95% CI: 32–51%) and was similar after MSD PBSCT [39% (95% CI: 28–49%)] and UCBT [50% (95% CI: 32–69%)], p = 0.63 (Fig. 3). Median time to discontinuation of IST was 347 days (range, 59–686 days) in MSD PBSCT group vs 387 days (range, 30–676 days) in UCBT (p = 0.93).
Fig. 3. Discontinuation of Immune-suppression by donor type.
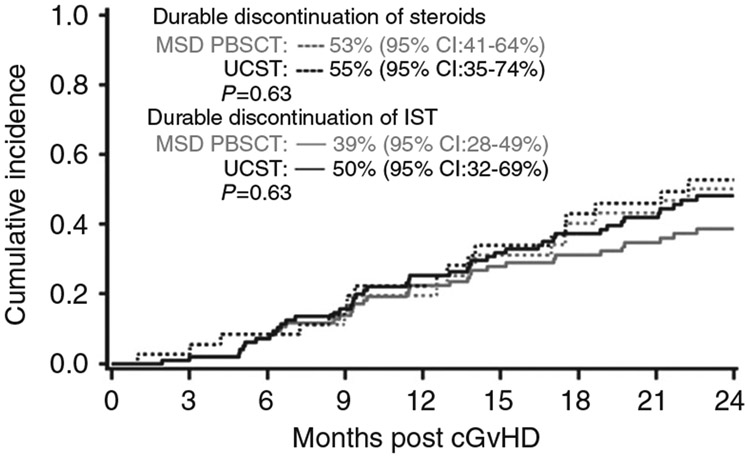
The cumulative incidence of durable discontinuation of all IST at 2 years. cGvHD chronic graft-versus-host disease, MSD PBSCT matched sibling donor peripheral blood stem cell transplant, UCBT umbilical cord blood transplant, IST immune-suppression therapy.
Failure free survival
The FFS at 2 years after initial treatment was 36% (95% CI: 26–45%) in MSD PBSCT group vs. 32% (95% CI: 18–47%) in UCBT group (p = 0.66). In MSD cohort (n = 104), 46 patients (44%, 95% CI: 34–54%) received additional line(s) of therapy; 3 patients (3%, 95% CI: 1–6%) experienced NRM and 16 patients (15%, 95% CI: 8–22%) developed recurrent malignancy. In UCBT cohort (n = 41), 20 patients (49%, 95% CI: 33–64%) had addition of a second line of the therapy; 3 patients (7%, 95% CI: 1–15%) experienced NRM and 4 patients (10%, 95% CI: 1–19%) developed recurrent malignancy (Fig. 4).
Fig. 4. Failure free survival by donor type.
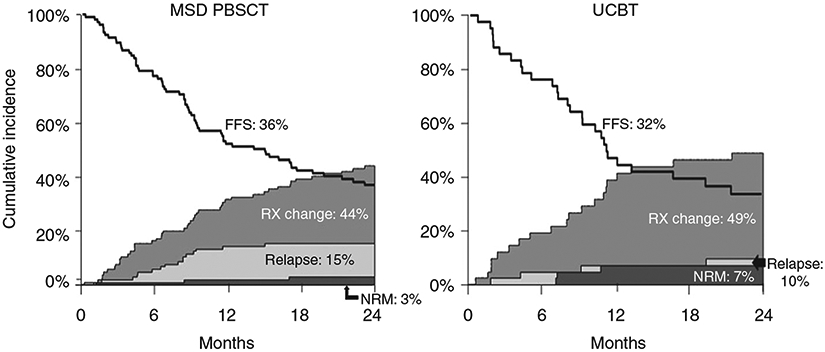
The FFS at 2 years after initial treatment in MSD PBSCT and UCBT cohorts. FFS Failure free survival, Rx change addition of second line of treatment, NRM nonrelapse mortality, MSD PBSCT matched sibling donor peripheral blood stem cell transplant, UCBT umbilical cord blood transplant.
Discussion
We report a comparative retrospective analysis of presentation and outcomes in patients with cGvHD following UCBT and MSD PBSCT. We observed significant differences in presentation and severity of cGvHD between the 2 groups. In the UCBT cohort, patients diagnosed with cGvHD mostly had prior acute GvHD (85%). This is similar to a prior report where ~60% of the patients had prior acute GvHD [18]. Also similar to prior reports, we noted higher GI involvement in UCBT recipients [7, 18]. This may partly be due to continuation of GI GvHD from prior acute GvHD [18], as indicated by larger numbers of progressive or quiescent onset, and less de-novo chronic GvHD. Also similar to the prior study, cGvHD was more frequently mild or moderate (vs. severe) in the UCBT cohort [18].
Some prior studies have identified myeloablative conditioning as a risk factor for both acute and chronic GvHD [19]. Yet, in our study, despite more patient in the UCBT cohort receiving myeloablative conditioning compared to MSD PBSCT group, the frequency and severity of cGvHD in the UCBT cohort was lower.
Overall, in both MSD PBSCT and UCBT cohorts we report a lower frequency of skin and liver involvement. In prior reports from CIBMTR [1] and the Consortium [20] a higher frequency of skin and liver involvement was seen. Also, there were more patients with overlap cGvHD (86%) in the study from cGvHD consortium as compared to our study (32 and 37%). This is likely because of the lower incidence of liver cGvHD in our cohort. We reported liver cGvHD based on physician’s assessment of whether GvHD contributed to liver involvement. If the assessment clearly indicated a different etiology (medications/infections) of liver disease, we excluded these incidents.
Despite the difference in HLA match and clinical presentation, cGvHD following UCBT had similar NRM and FFS to that following MSD PBSCT. Although we observed a statistically higher CR rate in UCBT group compared to MSD PBSCT, the number of patients attaining this was small, and this did not translate to better outcomes.
We observed a higher response in both MSD PBSCT and UCBT recipients than reported previously [21]. Differences in patient populations and the retrospective nature of our study without prospectively recorded response assessments could account for these differences. Similar to recent studies we observed a lower NRM of 13 and 20% at 2 years in MSD PBSCT and UCBT, respectively, likely reflecting improvements in supportive care in the modern era [1, 20].
Similar to prior reports, we noted no difference in the time to discontinuation of IST between the two groups [7]. At the 2-year landmark more than half of patients (58%) were still on IST. This is similar to a recent report, where 30% were still on immune-suppression at 5.6 years [22]. Similar to prior reports, high risk groups identified included those with thrombocytopenia and a KPS < 90 [20, 23].
FFS rate at 24 months was similar between 2 groups. Overall FFS at 2 years in both groups was 35% (95% CI: 27–43%). The study by Inamoto et al. reported FFS rate of 43% at 24 months [2] with further treatment changes accounting substantially to the FFS (37%). The contributions of NRM (10%) and recurrent malignancy (10%), to FFS in that study, were lower compared to the treatment change and need for the second line of therapy. Similarly, treatment change (44% in MSD PBSCT and 49% in UCBT) was the major contributor of FFS in our study compared to NRM and recurrent malignancy - both in MSD PBSCT and UCBT.
Overall, this study highlights several unique features of the UCBT cohort. They are less likely to have de-novo onset of cGvHD, and more frequently have gut involvement suggesting a distinct pathophysiology and need for unique preventive and treatment strategies. Though the clinical presentation of cGvHD was less severe and more likely to attain CR, this did not translate to improved NRM, or FFS. High risk groups, including platelets count of <100 × 10e9/L and KPS < 90 points, need careful monitoring and possible intensified therapy. Prolonged immune-suppression is needed by a significant fraction of the patients signifying need for more effective treatment strategies and ongoing monitoring.
Highlights.
cGvHD after UCBT was less severe, and had more CR to therapy than MSD transplant.
NRM was similar in patients with cGvHD after UCBT and MSD transplant.
FFS was similar in patients with cGvHD after UCBT and MSD transplant.
Footnotes
Conflict of interest DJW - research support from Incyte. JEW - advisor at Magenta Therapeutics. BRB - founder of Tmunity Therapeutics, advisory board member for Kadmon Pharmaceuticals, Magenta Therapeutics, and BlueRock Therapeutics and receives research funding from BlueRock Therapeutics. SGH – consultant for Incyte, Bristol Meyers Squibb, and Generon.
References
- 1.Arai S, Arora M, Wang T, Spellman SR, He W, Couriel DR, et al. Increasing incidence of chronic graft-versus-host disease in allogeneic transplantation: a report from the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transpl. 2015;21:266–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inamoto Y, Flowers ME, Sandmaier BM, Aki SZ, Carpenter PA, Lee SJ, et al. Failure-free survival after initial systemic treatment of chronic graft-versus-host disease. Blood. 2014;124:1363–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pidala J, Kurland B, Chai X, Majhail N, Weisdorf DJ, Pavletic S, et al. Patient-reported quality of life is associated with severity of chronic graft-versus-host disease as measured by NIH criteria: report on baseline data from the Chronic GVHD Consortium. Blood. 2011;117:4651–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flowers ME, Inamoto Y, Carpenter PA, Lee SJ, Kiem HP, Petersdorf EW, et al. Comparative analysis of risk factors for acute graft-versus-host disease and for chronic graft-versus-host disease according to National Institutes of Health consensus criteria. Blood. 2011;117:3214–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frey NV, Porter DL. Graft-versus-host disease after donor leukocyte infusions: presentation and management. Best Pr Res Clin Haematol. 2008;21:205–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Higman MA, Vogelsang GB. Chronic graft versus host disease. Br J Haematol. 2004;125:435–54. [DOI] [PubMed] [Google Scholar]
- 7.Newell LF, Flowers ME, Gooley TA, Milano F, Carpenter PA, Martin PJ, et al. Characteristics of chronic GVHD after cord blood transplantation. Bone Marrow Transplant. 2013;48:1285–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Styczynski J, Tridello G, Gil L, Ljungman P, Hoek J, Iacobelli S, et al. Impact of donor Epstein-Barr virus serostatus on the incidence of graft-versus-host disease in patients with acute leukemia after hematopoietic stem-cell transplantation: a study from the acute leukemia and infectious diseases working parties of the European Society for Blood and Marrow Transplantation. J Clin Oncol. 2016;34:2212–20. [DOI] [PubMed] [Google Scholar]
- 9.Kanakry CG, O’Donnell PV, Furlong T, Lima MJD, Wei W, Medeot M, et al. Multi-Institutional study of post-transplantation cyclophosphamide as single-agent graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation using myeloablative busulfan and fludarabine conditioning. J Clin Oncol. 2014;32:3497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohty M, Malard F. Antithymocyte globulin for graft-versus-host disease prophylaxis after allogeneic hematopoietic stem-cell transplantation. J Clin Oncol. 2017;35:3993–5. [DOI] [PubMed] [Google Scholar]
- 11.Gutman JA, Ross K, Smith C, Myint H, Lee CK, Salit R, et al. Chronic graft versus host disease burden and late transplant complications are lower following adult double cord blood versus matched unrelated donor peripheral blood transplantation. Bone Marrow Transpl. 2016;51:1588–93. [DOI] [PubMed] [Google Scholar]
- 12.Kanda J, Nakasone H, Atsuta Y, Toubai T, Yokoyama H, Fukuda T, et al. Risk factors and organ involvement of chronic GVHD in Japan. Bone Marrow Transplant. 2014;49:228–35. [DOI] [PubMed] [Google Scholar]
- 13.Konuma T, Tsukada N, Kanda J, Uchida N, Ohno Y, Miyakoshi S, et al. Comparison of transplant outcomes from matched sibling bone marrow or peripheral blood stem cell and unrelated cord blood in patients 50 years or older. Am J Hematol. 2016;91: E284–E92. [DOI] [PubMed] [Google Scholar]
- 14.Barker JN, Weisdorf DJ, DeFor TE, Blazar BR, McGlave PB, Miller JS, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105:1343–7. [DOI] [PubMed] [Google Scholar]
- 15.Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transpl. 2015;21:389–401.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lazaryan A, Arora M. Evolving concepts in prognostic scoring of chronic GvHD. Bone Marrow Transpl. 2017;52:1361–6. [DOI] [PubMed] [Google Scholar]
- 17.Lee SJ, Wolff D, Kitko C, Koreth J, Inamoto Y, Jagasia M, et al. Measuring therapeutic response in chronic graft-versus-host disease. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: IV. The 2014 Response Criteria Working Group report. Biol Blood Marrow Transpl. 2015;21:984–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ponce DM, Gonzales A, Lubin M, Castro-Malaspina H, Giralt S, Goldberg JD, et al. Graft-versus-host disease after double-unit cord blood transplantation has unique features and an association with engrafting unit-to-recipient HLA match. Biol Blood Marrow Transpl. 2013;19:904–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scott BL, Pasquini MC, Logan BR, et al. Myeloablative versus reduced-intensity hematopoietic cell transplantation for acute myeloid leukemia and myelodysplastic syndromes. J Clin Oncol. 2017;35:1154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pidala J, Vogelsang G, Martin P, Chai X, Storer B, Pavletic S, et al. Overlap subtype of chronic graft-versus-host disease is associated with an adverse prognosis, functional impairment, and inferior patient-reported outcomes: a Chronic Graft-versus-Host Disease Consortium study. Haematologica. 2012;97:451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carpenter PA, Logan BR, Lee SJ, Weisdorf DJ, Johnston L, Costa LJ, et al. A phase II/III randomized, multicenter trial of prednisone/sirolimus versus prednisone/ sirolimus/calcineurin inhibitor for the treatment of chronic graft-versus-host disease: BMT CTN 0801. Haematologica. 2018;103:1915–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee SJ, Nguyen TD, Onstad L, Bar M, Krakow EF, Salit RB, et al. Success of immunosuppressive treatments in patients with chronic graft-versus-host disease. Biol Blood Marrow Transpl. 2018;24:555–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arora M, Klein JP, Weisdorf DJ, Hassebroek A, Flowers ME, Cutler CS, et al. Chronic GVHD risk score: a center for international blood and marrow transplant research analysis. Blood 2011;117:6714–20. [DOI] [PMC free article] [PubMed] [Google Scholar]


