Abstract
Objective
To predict the target of the active ingredient of lotus leaf for lowering fat and losing weight. Explore its multicomponent, multitarget, multipath mechanism.
Methods
Screen the main active ingredients of lotus leaves through the TCMSP database, and use the TCMSP database to predict the potential targets of the active ingredients. Obtain obesity-related targets from the human genome annotation (GeneCards) database. Use Venn software to take the intersection of the two to obtain the effect target of the lotus leaf lipid-lowering and weight-reducing effects. Use Cytoscape 3.6.0 software to construct an effective ingredient-target network. Use the STRING database to construct an intersection target protein interaction (PPI) network, visualize it with Cytoscape 3.6.0 software, and perform network topology analysis to obtain the core target. Use the DAVID database to perform gene ontology (GO) and metabolic pathway (KEGG) enrichment analysis for the above targets. Use AutoDockTools software for molecular docking to verify the binding strength.
Results
A total of 15 main active ingredients such as quercetin, isorhamnetin, sitosterol, and kaempferol were obtained, which can act on 135 targets related to obesity. These targets are significantly enriched in multiple GO and KEGG entries such as hypoxia response, positive regulation of gene expression, response to toxic substances, aging, and positive regulation of RNA polymerase II promoter transcription. Molecular docking shows that flavonoids such as quercetin have better binding to the target protein Akt1.
Conclusion
The lipid-lowering and weight-reducing effects of lotus leaf embody the characteristics of multicomponent, multitarget, and multipathway of traditional Chinese medicine, which provides a certain scientific basis for the screening and in-depth study of the effective ingredients of lotus leaf.
1. Introduction
The lotus leaf belongs to the leaf of the Nymphaeaceae plant lotus, which is a medicinal and food homologous plant published by the Ministry of Health [1]. The lotus leaf mainly contains lotus leaf essential oil, lotus leaf alkaloids, lotus leaf polysaccharides, and lotus leaf flavonoids. Lotus leaf flavone is its main active substance, which has the effects of antioxidation, stabilizing blood vessels, dredging blood vessels, and lowering blood pressure. At the same time, it is also a good medicine for weight loss, has the effect of lowering blood lipids, and is often used clinically for the treatment of obesity [2–5]. The fat-lowering and weight-reducing effects of lotus leaves have long been recorded in ancient Chinese medicine books. Due to the complex composition and variety of active ingredients, lotus leaves and their crude extracts have been used for overall functional research for a long time. They all show very good fat-lowering and weight-loss effects [6, 7].
Traditional Chinese medicine has the characteristics of multicomponent, multitarget, and synergistic effects between each component. Network pharmacology is based on the theory of systems biology, and it is a new technology to explain the drug's action and its mechanism [8–10]. Integrating computational virtual computing and network database methods to construct a “medicine-component-target” network, analyze the pathway mechanism and provide guidance for the prediction of the mechanism of traditional Chinese medicine and the multidirectional treatment of diseases [11]. In this study, the network pharmacology method was used to study the characteristics of the multicomponent, multitarget, and multipathway effects of the lotus leaf and to explore the material basis and mechanism of the lotus leaf's lipid-lowering and weight-reducing effects.
2. Analysis Method
2.1. Screening of Active Ingredients and Targets
Use the Computational Systems Biology Laboratory (TCMSP) to retrieve all the active ingredient data of lotus leaves. It is an important evaluation index for drugs to participate in the process of absorption, distribution, metabolism, and excretion. Taking oral bioavailability (OB) and drug-like properties (DL) as screening conditions, set oral bioavailability (OB) ≥30%, and drug-like properties (DL) ≥0.18 to obtain active compounds that meet the conditions. Obtain the corresponding target points of each active ingredient in TCMSP [12, 13].
2.2. Screening Obesity-Related Disease Targets and Lotus Leaf Weight Loss Targets
Search for obesity-related disease targets through the GeneCards database (https://www.genecards.org). Deduplicate, set the correlation score to be greater than 1, and screen out disease targets [14]. Draw a Venn diagram to obtain the intersection of the lipid-lowering and weight-reducing effects of the lotus leaf and the obesity-related targets, which is the pharmacological target of the lotus leaf's lipid-lowering and weight-reducing effects.
2.3. Lotus Leaf-Composition-Target-Disease Network Construction
The intersection of the active ingredients of the lotus leaf, obesity, obesity, and the active ingredients obtained from the above screening is taken as the node. Cytoscape 3.6.0 software was used to visually analyze the process of reducing fat and losing weight in lotus leaves. Construct a lotus leaf-component-target-obesity network [15, 16].
2.4. Construction of the Protein Interaction (PPI) Network
Enter the lotus leaf lipid-lowering and weight-loss targets in the STRING database (https://string-db.org/). The minimum interaction threshold is set to “highest confidence” (>0.4) to obtain the PPI network and obtain protein interaction information. Use Cytoscape 3.6.0 software to draw the PPI network diagram, and screen out the core targets according to the node degree value [17].
2.5. Target Enrichment Analysis and Visualization
Enter the intersection target of obesity and lotus leaf active ingredients in the DAVID database, and perform GO enrichment analysis and KEGG pathway enrichment analysis, respectively. According to the P value, the top 10 GO items are selected for analysis. Carry out KEGG enrichment analysis on the signal pathway in which the target participates. According to the P value, list the top 10 items for analysis, and draw the core signal pathway diagram [18].
2.6. Molecular Docking
Enter the main active ingredients selected under 1.1 into the PubChem (https://pubchem.ncbi.nlm.nih.gov/) database to download the structure of small molecule ligands. Enter the core target protein screened under item 1.2 into the RCSB PDB (http://www.rcsb.org/) database, and download the 3D structure of the target protein. Prepare ligand files and receptor files, and use AutoDockTools software for molecular docking [19].
3. Results and Analysis
3.1. The Main Active Ingredients of Lotus Leaf
A total of 93 compounds in lotus leaves were retrieved through the TCMSP database, and 15 active ingredients were screened based on oral bioavailability (OB) ≥30% and drug-like activity (DL) ≥0.18. The results are given in Table 1.
Table 1.
Basic information of the main active ingredients in lotus leaves.
| Number | Id | Compound | OB | DL |
|---|---|---|---|---|
| 1 | MOL000098 | Quercetin | 46.43 | 0.28 |
| 2 | MOL000354 | Isorhamnetin | 49.6 | 0.31 |
| 3 | MOL000359 | Sitosterol | 36.91 | 0.75 |
| 4 | MOL000422 | Kaempferol | 41.88 | 0.24 |
| 5 | MOL006405 | (1S)-1-(4-Hydroxybenzyl)-2-methyl-3,4-dihydro-1H-isoquinoline-6,7-diol | 67.14 | 0.23 |
| 6 | MOL003578 | Cycloartenol | 38.69 | 0.78 |
| 7 | MOL007206 | Armepavine | 69.31 | 0.29 |
| 8 | MOL007207 | Machiline | 79.64 | 0.24 |
| 9 | MOL007210 | o-Nornuciferine | 33.52 | 0.36 |
| 10 | MOL007213 | Nuciferin | 34.43 | 0.4 |
| 11 | MOL007214 | (+)-Leucocyanidin | 37.61 | 0.27 |
| 12 | MOL007217 | Leucodelphinidin | 30.02 | 0.31 |
| 13 | MOL007218 | Remerin | 40.75 | 0.52 |
| 14 | MOL000073 | Ent-epicatechin | 48.96 | 0.24 |
| 15 | MOL000096 | (−)-Catechin | 49.68 | 0.24 |
3.2. Drug-Disease Intersection Target Acquisition
Through the TSMSP database, 189 target proteins of 15 active ingredients in the lotus leaf were retrieved, and the target proteins and gene information were corrected through the STRING database [20]. The GeneCards (https://www.genecards.org) database was searched with “Obesity” as the keyword, and 9,510 obesity-related target genes were obtained. Take 2569 genes with a correlation score greater than 1 and draw a Venn diagram of the targets of the active ingredients in the lotus leaf and the targets of obesity-related diseases. The results are shown in Figure 1. There are 135 intersection targets between component targets and disease targets. The results showed that the lipid-lowering and weight-reducing effects of lotus leaves are related to the 15 active ingredients in lotus leaves and the above 135 targets.
Figure 1.
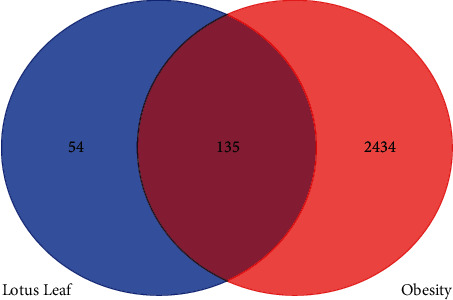
Venn diagram of the common target of lotus leaf and obesity.
3.3. Lotus Leaf-Components-Target-Disease Network Construction
The lotus leaf-components-target-obesity network was constructed by Cytoscape 3.6.0 software. The results are shown in Figure 2.
Figure 2.
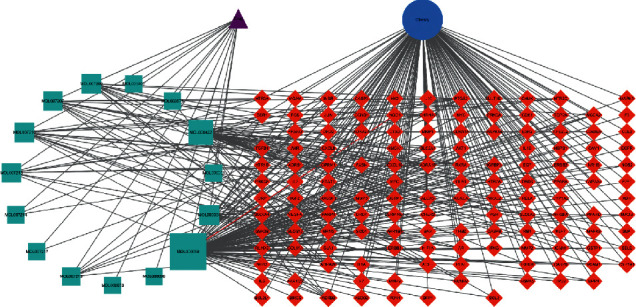
“Lotus leaf-components-target-obesity” interaction network.
It can be seen from Figure 2 that the active ingredients and most targets have more interactions. This fully shows that the lotus leaf has the effect of reducing fat and losing weight through multicomponent, multitarget, and multichannel synergistic action. Among the active ingredients, the top 5 active ingredients ranked by the degree value are quercetin, kaempferol, isorhamnetin, O-nornopine, and papaverine. It shows that these ingredients are the main active ingredients of the lotus leaf's lipid-lowering and weight-loss effect [21].
3.4. PPI Network Construction and Topology Analysis
Import the obtained 135 intersection targets into the STRING database to obtain the PPI relationship. The results are shown in Figure 3. Figure 3 shows that the network has a total of 135 nodes and 2348 edges. According to the degree value, the top 10 key target proteins are screened out. The results are given in Table 2.
Figure 3.
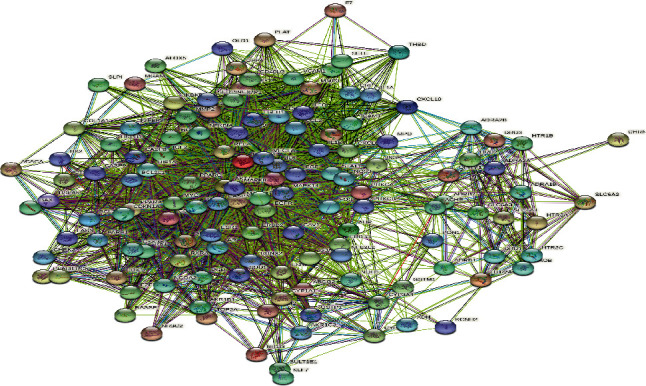
PPI network diagram of the intersection target of lotus leaf and obesity.
Table 2.
Key targets of the lotus leaf weight loss PPI network.
| Number | Target | Degree |
|---|---|---|
| 1 | Akt1 | 103 |
| 2 | IL6 | 92 |
| 3 | TP53 | 89 |
| 4 | VEGFA | 87 |
| 5 | CASP3 | 83 |
| 6 | JUN | 78 |
| 7 | MYC | 76 |
| 8 | EGFR | 74 |
| 9 | EGF | 74 |
| 10 | MAPK8 | 74 |
The top 10 targets in terms of the degree value are protein kinase B1 (Akt1), interleukin 6 (IL6), tumor necrosis protein p53 (TP53), caspase 3 (CASP3), JUN protein (JUN), myeloma virus oncogene homolog (MYC), epidermal growth factor receptor (EGFR), epidermal growth factor (EGF), and mitogen activated protein kinase 8 (MAPK8). These targets may be the key targets for the lotus leaf to lower fat and lose weight.
3.5. GO Enrichment Analysis Results
A total of 622 GO entries were screened in the DAVID database (P < 0.05). GO analysis consists of three parts: biological process (BP), cellular component (CC), and molecular function (MF). Among them, there are 479 BP entries, 44 CC entries, and 99 MF entries [22]. According to the size of the P value, the top 10 GO items are listed, respectively, as shown in Figure 4.
Figure 4.
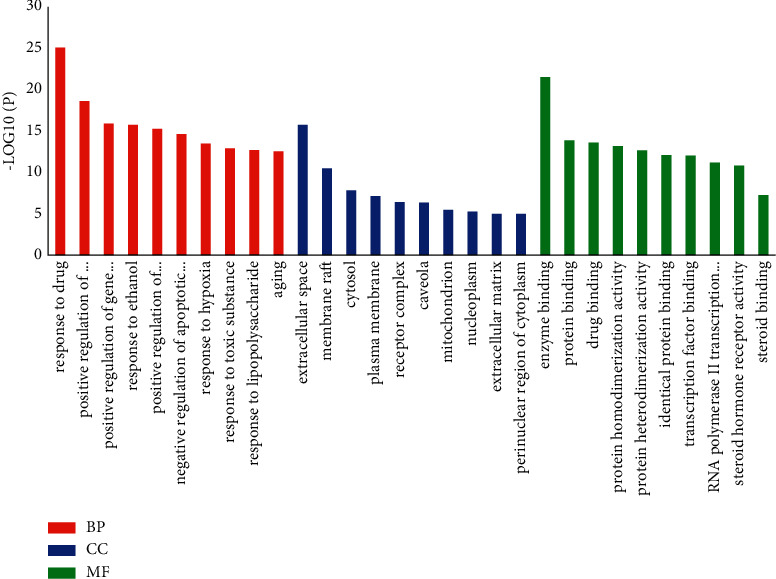
GO enrichment analysis diagram of the lotus leaf's weight-reducing effect.
It can be seen from Figure 2 that biological process notes mainly include response to hypoxia, positive regulation of gene expression, response to toxic substance, aging, and positive regulation of transcription from RNA polymerase II promoter. The annotation of cell components indicates that the relevant mechanism mainly occurs in the extracellular space, cytosol, plasma membrane, mitochondrion, and extracellular matrix. Molecular functions mainly include enzyme binding, protein binding, identical protein binding, transcription factor binding, and steroid hormone receptor activity.
3.6. KEGG Enrichment Analysis
112 KEGG-enriched signal pathways were screened by the DAVID database (P < 0.05) [23]. Select the first 10 KEGG signal pathways. Table 3 provides the details of the pathways.
Table 3.
KEGG channel details.
| Number | Pathway | P value |
|---|---|---|
| hsa05200 | Pathways in cancer | 2.20E−24 |
| hsa05161 | Hepatitis B | 1.16E−20 |
| hsa04668 | TNF signaling pathway | 1.08E−16 |
| hsa05219 | Bladder cancer | 1.21E−16 |
| hsa05212 | Pancreatic cancer | 6.35E−16 |
| hsa05142 | Chagas disease (American trypanosomiasis) | 1.05E−15 |
| hsa04066 | HIF-1 signaling pathway | 3.19E−15 |
| hsa05145 | Toxoplasmosis | 4.41E−14 |
| hsa05215 | Prostate cancer | 1.54E−13 |
| hsa05210 | Colorectal cancer | 2.55E−12 |
Analysis shows that the targets are significantly enriched in the hepatitis B signaling pathway, hypoxia-inducible factor-1 (HIF-1) signaling pathway, tumor necrosis factor (TNF) signaling pathway, Chagas disease signaling pathway, and multiple signaling pathways related to cancer. The HIF-1 signaling pathway is shown in Figure 5.
Figure 5.
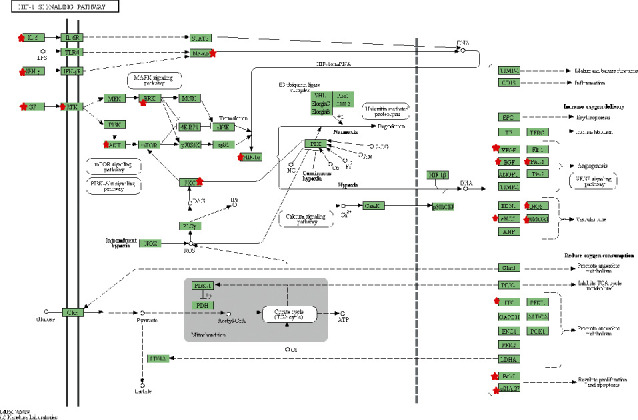
HIF-1 signal pathway diagram of the lotus leaf weight loss effect.
3.7. Molecular Docking
Search the three-dimensional structure of the interaction between quercetin (MOL000098), the active ingredient of lotus leaf, and the target Akt1 through the PDB database, download the MOL structural formula of the compound, and import them into the AutoDockTools software, respectively [24]. The binding energy is −6.2. This shows that the receptor and the ligand can bind spontaneously and the result of the binding of quercetin to the target Akt1 target protein (Figure 6).
Figure 6.
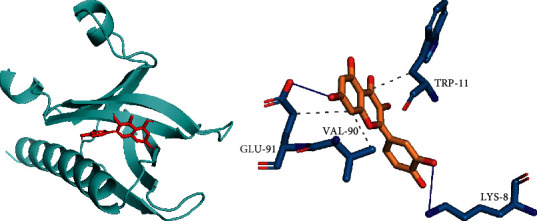
Docking analysis diagram of quercetin and Akt1.
4. Discussion
This study uses the method of network pharmacology to explore the mechanism of the effect of lotus leaf in reducing fat and reducing weight. From the “lotus leaf-components-targets-obesity” network diagram, it can be seen that compounds such as quercetin, kaempferol, isorhamnetin, O-nornopine, and papaverine are important nodes in the network. It is speculated that these compounds may be the material basis of lotus leaf's lipid-lowering and weight-reducing effects. The PPI network of the intersection target of lotus leaf and obesity shows that protein kinase B1 (Akt1), interleukin 6 (IL6), tumor necrosis protein p53 (TP 53), caspase 3 (CASP3), JUN protein (JUN), myeloma virus oncogene homolog (MYC), epidermal growth factor receptor (EGFR), epidermal growth factor (EGF), and mitogen-activated protein kinase 8 (MAPK8) interact with multiple compounds and have high degrees and play a key role in the network diagram. It is suggested that it may be the key target of lotus leaf's lipid-lowering and weight-reducing effects. The GO function is significantly enriched in biological processes such as the positive regulation of gene expression and the positive regulation of RNA polymerase II promoter transcription. KEGG pathway enrichment analysis results show that lotus leaf can play a weight loss effect through the hypoxia-inducible factor-1 (HIF-1) signaling pathway, tumor necrosis factor (TNF) signaling pathway, and multiple signaling pathways related to cancer. The results of molecular docking show that flavonoids such as quercetin have good binding to the target protein Akt1, which provides a strong basis for network pharmacology to predict the reliability of the target. On the basis of network pharmacology, this study discussed the active ingredients of lotus leaf and its multitarget and multipathway characteristics in the process of reducing fat and losing weight. Preliminary explanation of the mechanism of the lotus leaf weight loss effect is provided for the further in vivo and in vitro experimental verification of the lotus leaf weight loss activity and the screening and evaluation of traditional Chinese medicine for weight loss.
Acknowledgments
This research was funded by the Modern Chinese Medicine Technical Innovation and Social Service Team of Bozhou Vocational and Technical College (yptd001), the Natural Science Research Foundation of the Department of Education of Anhui Province (KJ2020A0789), Anhui Provincial-Level Quality Engineering Project (2020jyxm0790, 2020zyrc077, and gxbjZD2021089), and school-level grassroots teaching and research office demonstration project of Anhui Xinhua University (2019jyssfx02).
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- 1.Tan Y., Deng F. Research progress in lotus leaf components and biological functions. Food Research and Development . 2020;41(10):193–197. [Google Scholar]
- 2.Fan Y., Cao L. Study on the extraction process of lotus leaf total flavonoids and its antioxidant activity. Anhui Chemical Industry . 2021;47(2):55–58. [Google Scholar]
- 3.Chen Y., Tang L., Liang J., Zhang S., Chen B., Chen J. Nuciferine promotes the proliferation of human aortic endothelial cells by up-regulating the SDF-1/CXCR4 signaling pathway. Chinese Journal of Arteriosclerosis . 2020;28(11):981–985. [Google Scholar]
- 4.Zhou G., Li X.-W., Li J.-C., Feng X.-H. Study on preparation technology and physical fingerprint of chuilian jianpi granules based on QbD. Complexity . 2021;2021:9. doi: 10.1155/2021/9992022.9992202 [DOI] [Google Scholar]
- 5.Xuehua F., Zhou G., Ali T. Establishment of the quantitative analysis of multiindex in Euphorbia lathyris by the single marker method for Euphorbia lathyris based on the quality by design concept. Journal of Analytical Methods in Chemistry . 2021;2021:8. doi: 10.1155/2021/4311934.4311934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang C., Yang Y. Research progress on the chemical constituents and pharmacological activities of lotus leaves. Modern Chinese Medicine Research and Practice . 2020;34(4):74–81. [Google Scholar]
- 7.Li T., Xiao Y., Chen Z., et al. Research progress in lipid-lowering effects and active ingredients of Chinese medicine for food and medicine. Strait Pharmacy . 2020;32(9):42–46. [Google Scholar]
- 8.Tang J., Zheng S., Cao X. Research on the mechanism of action of “dandelion-prunella” based on network pharmacology in the treatment of breast cancer. Evaluation and Analysis of Drug Use in Chinese Hospitals . 2020;20(1):44–49. [Google Scholar]
- 9.Ji X., Hou C., Gao Y., Xue Y., Yan Y., Guo X. Metagenomic analysis of gut microbiota modulatory effects of jujube (Ziziphus jujuba Mill.) polysaccharides in a colorectal cancer mouse model. Food & Function . 2020;11(1):163–173. doi: 10.1039/c9fo02171j. [DOI] [PubMed] [Google Scholar]
- 10.Ji X., Peng B., Ding H., Cui B., Nie H., Yan Y. Purification, structure and biological activity of pumpkin (Cucurbita moschata) polysaccharides: a review. Food Reviews International . 20211904973 [Google Scholar]
- 11.Shang Y., Li C., Luqiu W. A preliminary study on the blood-enriching mechanism of jujube based on network pharmacology. Chinese Food and Nutrition . 2021;27(5):58–62. [Google Scholar]
- 12.Yuan J., Li X., Chen C., Song X., Wang S. Prediction of the effective components and potential targets of Ginkgo biloba in the treatment of cardiovascular and cerebrovascular diseases based on network pharmacology methods. Chinese Journal of Experimental Formulas . 2014;20(11):208–212. [Google Scholar]
- 13.An J., Sun W., Wang Y. Based on network pharmacology to analyze the mechanism of action of Panax notoginseng in the treatment of hepatocellular carcinoma. Journal of Chinese Medicine . 2020;35(4):848–852. [Google Scholar]
- 14.Huang X., Zou M., Chen Y. Based on network pharmacology and molecular docking analysis of the mechanism of action of Astragalus in the treatment of ulcerative colitis. Chinese Medicine New Drugs and Clinical Pharmacology . 2021;32(6):815–824. [Google Scholar]
- 15.Liao J., Qin Z., Yang Q., Li R., Xu W. Study on the mechanism of action of spatholobi in the treatment of hepatocellular carcinoma based on network pharmacology. Guangxi Medicine . 2020;42(14):1840–1845. [Google Scholar]
- 16.Wang Y., Xue W., Liu P. Discussion on the mechanism of action of astragalus on pancreatic cancer based on network pharmacology. Journal of Precision Medicine . 2019;34(6):522–527. [Google Scholar]
- 17.Xia Y., Liu T., Gong F. Based on network pharmacology to explore the mechanism of blood pressure reduction of mulberry branch. Journal of Jiangxi University of Traditional Chinese Medicine . 2021;33(3):77–84. [Google Scholar]
- 18.Xu H., Wang S., Yang H. Based on network pharmacology, research on the mechanism of compound donkey-hide gelatin in adjuvant treatment of tumors. Chinese Journal of Chinese Materia Medica . 2014;39(16):3148–3151. [PubMed] [Google Scholar]
- 19.Yao J., Yuan Y., Wang Q. Research on the mechanism of Scutellaria baicalensis-Coptis in the treatment of ulcerative colitis based on network pharmacology. Journal of Clinical Drug Therapy . 2021;19(7):48–54. [Google Scholar]
- 20.Liu Y., Liu L. Based on network pharmacology and molecular docking technology to explore the mechanism of the effect of active ingredients of Shenmai injection on cytochrome P450. China Pharmaceuticals . 2021;30(13):27–31. [Google Scholar]
- 21.Da Y. Discussion on the mechanism of agarwood in the treatment of abdominal pain based on network pharmacology. Bachu Medicine . 2021;4(2):78–84. [Google Scholar]
- 22.Yang Q., Mao Y. Discussion on the mechanism of Aconite-Asarum on hypertension based on network pharmacology. Hunan Journal of Traditional Chinese Medicine . 2021;37(6):149–155. [Google Scholar]
- 23.Jin H., Hui C., Zhang H. Research on the anti-tumor active ingredients and mechanism of Jue Chuang based on network pharmacology. Chinese Journal of Modern Medicine . 2021;23(6):1–6. [Google Scholar]
- 24.Yilimire·Wufuer K.·Y., Guo L. etc. Study on the network pharmacological mechanism of the antitumor effect of snow chrysanthemum total flavonoids on colon cancer cells. Journal of Xinjiang Medical University . 2021;44(6):670–678. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


