Abstract
Peroxisomes are involved in multiple metabolic processes, including fatty acid oxidation, ether lipid synthesis and ROS metabolism. Recent studies suggest that peroxisomes are critical mediators of cellular responses to various forms of stress, including oxidative stress, hypoxia, starvation, cold exposure, and noise. As dynamic organelles, peroxisomes can modulate their proliferation, morphology, and movement within cells, and engage in crosstalk with other organelles in response to external cues. Although peroxisome-derived hydrogen peroxide plays a key role in cellular signaling related to stress, emerging studies suggest that other products of peroxisomal metabolism, such as acetyl-CoA and ether lipids, are also important for metabolic adaptation to stress. Here, we review molecular mechanisms through which peroxisomes regulate metabolic and environmental stress.
Keywords: Ether lipid, lipid metabolism, peroxisome, stress, plasmalogen, reactive oxygen species
A snapshot of the peroxisomal compartment
Peroxisomes are single membrane-enclosed organelles that exist in almost all eukaryotic cells. As a highly dynamic organelle, peroxisomes can adapt their shape, size, location and abundance in response to the nutritional or environmental cues [1]. Peroxisomal homeostasis is regulated by a delicate balance between formation of new peroxisomes and autophagic degradation of existing peroxisomes, or pexophagy (see Glossary)[2]. The removal of superfluous or dysfunctional peroxisomes is thought to help maintain proper peroxisome function and prevent accumulation of damage during cellular aging [3]. New peroxisomes can be derived from division of existing peroxisome or via de novo biogenesis (Box 1). Peroxisomes carry out various metabolic functions, including catabolism of very long chain fatty acids (VLCFA), branched chain fatty acids, D-amino acids, and polyamines, production and catabolism of reactive oxygen species (ROS), and biosynthesis of ether lipids and bile acids [1]. Some of these functions require cooperation with other organelles (Figure 1). For example, peroxisomal β-oxidation of VLCFA is thought to be a chain-shortening process, with the completion taking place in mitochondria (Box 2). Synthesis of ether lipids, including plasmalogens, initiates in peroxisomes and is completed in the ER (Box 3). Peroxisomes have also been shown to interact with lysosomes for transport of free cholesterol [4].
Box 1. Peroxisomal biogenesis.
Peroxisomal biogenesis, the de novo formation of peroxisomes, requires multiple proteins called peroxins or Pex proteins. Approximately 30 such proteins have been identified and many of which are conserved from yeast to mammals and control different aspects of peroxisomal biogenesis [2]. Peroxisomal matrix proteins are translated on free ribosomes in the cytoplasm and have to be imported into peroxisomes. These proteins possess either a carboxyl terminal peroxisomal targeting sequence (PTS1) consisting of the SKL tripeptide (or a conserved variant) or an internal targeting sequence (PTS2) with the consensus sequence of (R/K)(L/V/I)X5(Q/H)(L/A). Pex5 is the import receptor for PTS1-containing peroxisomal matrix proteins [14], while Pex7 is required for transport of PTS2-containing matrix proteins [68]. A longer isoform of Pex5 (Pex5L) also serves as a co-receptor for Pex7 and targets the PTS2—Pex7 complex to peroxisomes [69]. Docking of the cytosolic PTS receptor/cargo complex at the peroxisomal membrane and release of the cargo into the peroxisomal lumen requires Pex13 and Pex14 [70]. Following the import of matrix proteins, Pex5 and Pex7 are recycled back to the cytosol in a process dependent on an exporter complex consisting of Pex1 and Pex6, which are recruited to the peroxisomal membrane by Pex26 [71, 72]. Cysteine mono-ubiquitination in Pex5 mediated by the RING-type ubiquitin ligases Pex2, Pex10 and Pex12 is required for recycling of the PTS1 receptor [73, 74]. The assembly of peroxisomal membrane and import of peroxisomal membrane proteins (PMPs) necessary for the formation of functional peroxisomes capable of importing matrix proteins involves fusion of a pre-peroxisomal vesicle derived from the ER with a vesicle derived from mitochondria. In mammalian cells, Pex16 buds from the ER in a pre-peroxisomal vesicle and fuses with a Pex3-containing precursor vesicle derived from mitochondria [75, 76]. Import of newly synthesized PMPs requires Pex19, a cytosolic chaperone and import receptor [77]. Loss of functions mutations in Pex3, Pex16 or Pex19 result in a complete absence of peroxisomes and cause peroxisomal biogenesis disorders in the Zellweger spectrum, which are devastating diseases characterized by severe liver dysfunction, developmental delay, neurological abnormalities and early death (generally before 2 years of age) [78, 79].
Box 1, Figure I. Peroxisomal biogenesis pathway.
De novo formation of peroxisomes requires fusion of a pre-peroxisomal vesicle derived from the ER with a vesicle derived from the mitochondria, followed by import of peroxisomal matrix and membrane proteins. Abbreviations: PMP, peroxisomal membrane protein; PTS1, peroxisomal targeting sequence 1-containing matrix protein; PTS2, peroxisomal targeting sequence 2-containing matrix protein; Ub, ubiquitination.
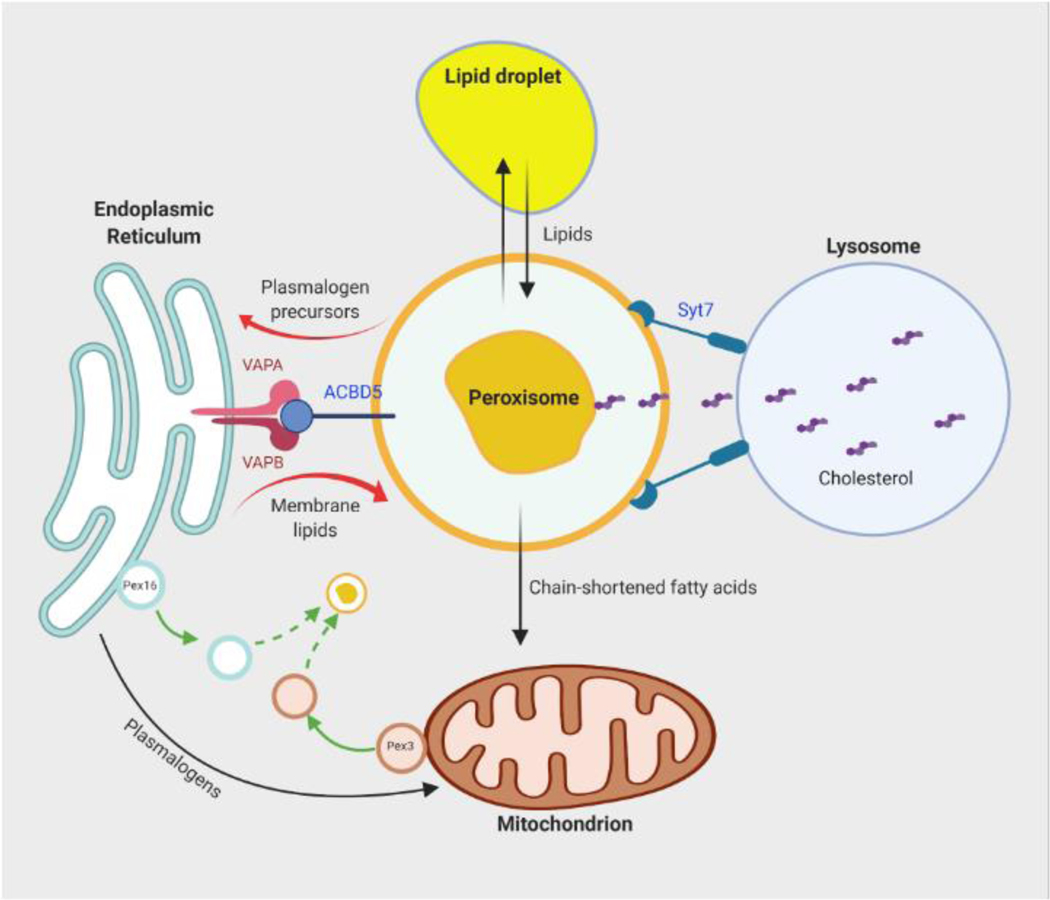
Figure 1. Crosstalk between peroxisomes and other organelles.
Peroxisomal biogenesis and functions involves inter-organelle communication. De novo formation of peroxisomes involves fusion of precursor vesicles derived from the ER and mitochondria. Peroxisomes closely interact and exchange lipids with lipid droplets. Synthesis of ether lipids, such as plasmalogens, initiates in peroxisomes but is completed in the ER. The ER-peroxisome metabolite exchange may be assisted by the bridge formed by the membrane proteins VAPA, VAPB and ACBD5. Peroxisomes are also closely associated with mitochondria. Peroxisome-derived plasmalogens are found in mitochondrial membranes and may be involved in mitochondrial dynamics. Mitochondria help to further oxidize chain-shortened fatty acids produced by peroxisomal fatty acid oxidation. Peroxisomes also interact with lysosomes through the membrane protein Syt7, which promotes transfer of free cholesterol from lysosomes to peroxisomes. Abbreviations: ER, endoplasmic reticulum; VAPA and VAPB, VAMP-associated proteins A and B; ACBD5, acyl-CoA binding domain containing protein 5; Syt7, synaptotagmin 7.
Box 2. Peroxisomal β-oxidation pathway.
In mammalian cells, fatty acids can be catabolized via β-oxidation in mitochondria and peroxisomes. Very long chain fatty acids (C≥22) are exclusively oxidized in peroxisomes [80]. This process requires the fatty acid to be first activated to an acyl-CoA by acyl-CoA synthetase (ACSL) in the presence of ATP. Acyl-CoA, transported into the peroxisomal matrix via ATP binding cassette transporter D subfamily of proteins (ABCD1–3), is oxidized to trans-2-enoyl-CoA by Acox1, an FAD-dependent oxidase that generates H2O2 as a byproduct. Related enzymes Acox2 and Acox3 are involved in oxidation of branched chain fatty acids and intermediates involved in bile acid synthesis. Trans-2-enoyl-CoA is further reacted to chiral 3-hydroxyacyl-CoA and 3-ketoacyl-CoA sequentially by the peroxisomal multifunctional enzyme 1 or 2 (MFE1/2), which possesses enoyl-CoA hydratase and 3-hydroxyacyl-CoA dehydrogenase activities. In the final step, 3-ketoacyl-CoA thiolase promotes cleavage of the terminal acetyl-CoA group, forming a new acyl-CoA molecule that has been shortened by two carbon atoms. After several rounds of peroxisomal β-oxidation, the chain-shortened fatty acid is shuttled into mitochondria by either carnitine-dependent or -independent routes to undergo further rounds of β-oxidation [81].
Box 2, Figure I. Peroxisomal β-oxidation pathway.
Very long chain fatty acids (VLCFA) are activated to acyl-CoA and imported by peroxisomes for β-oxidation. Each cycle of fatty acid oxidation shortens the acyl chain by 2 carbons with the thiolytic cleavage of the terminal acetyl-CoA.
Box 3. Plasmalogen synthetic pathway.
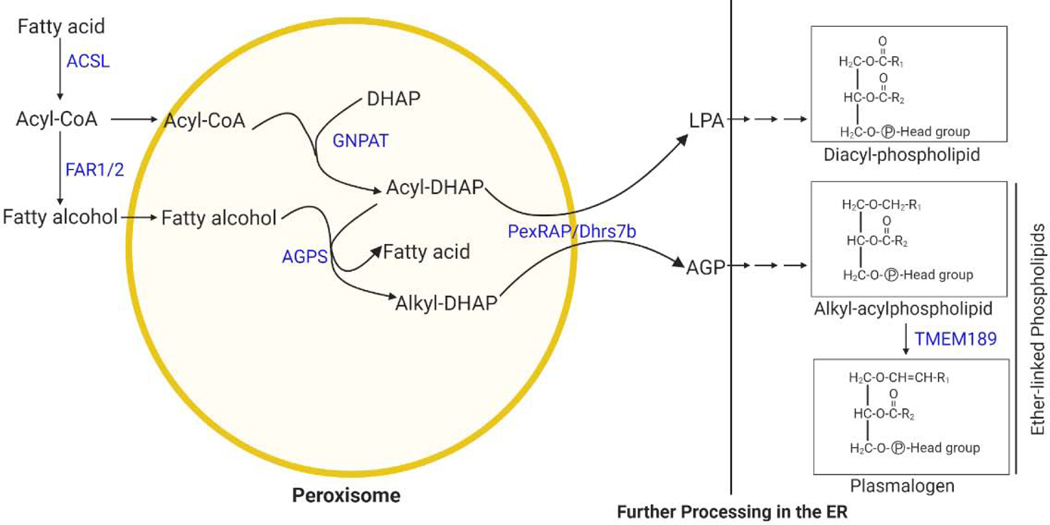
Plasmalogens are the most common form of a subclass of glycerophospholipids called ether lipids that have an alkyl chain attached to the sn-1 position of the glycerol backbone by an ether bond as opposed to an acyl chain attached by an ester bond in conventional glycerophospholipids, such as phosphatidylcholine and phosphatidylethanolamine [82]. Plasmalogens are characterized by a cis double bond adjacent to the ether linkage. Plasmalogen synthesis begins in peroxisomes, involving the acyl-dihydroxyacetone (DHAP) pathway, since the glycolysis intermediate DHAP is used as a precursor for ether lipid synthesis [83]. The process begins when glyceronephosphate O-acyltransferase (GNPAT), a peroxisomal matrix protein, esterifies DHAP with a long chain acyl-CoA at the sn-1 position. The next step is catalyzed by alkylglycerone phosphate synthase (AGPS), another peroxisomal protein, and results in the formation of the hallmark ether bond at the sn-1 position by exchanging an acyl chain for an alkyl group. The alkyl component used by AGPS is generated by a peroxisomal membrane-associated fatty acyl-CoA reductase (FAR1/FAR2), which reduces an acyl-CoA to a fatty alcohol. The final peroxisomal step is carried out by the acyl/alkyl-DHAP reductase PexRAP/DHRS7b, which reduces alkyl- or acyl-DHAP into the ether lipid precursor 1-O-alkyl glycerol-3-phosphate (AGP) or lysophophatidic acid (LPA), a precursor of conventional diacylphospholipids [84, 85]. Further steps to process AGP or LPA into their corresponding ether-linked or diacyl glycerolipids take place in the ER [83, 86]. Several potential modes have been proposed for how AGP is transported to ER for completion of plasmalogen synthesis, including involving use of a lipid-binding protein, specific shuttle vesicle, or direct membrane contact [87, 88]. In support the last mode, disruption of the VAP-ACBD5 tether between peroxisome and ER leads to decreased plasmalogen synthesis [89]. The ER steps involved in plasmalogen synthesis are less understood, but enzymes which modify the sn2 and sn3 positions during plasmalogen biosynthesis may be shared between ether- and ester-linked glycerolipids. Recently, TMEM189 was identified as the gene encoding a plasmanylethanolamine desaturase (PEDS), which is required for ethanolamine plasmalogen biosynthesis through insertion of the alk-1′-enyl ether or “vinyl-ether” double bond that is characteristic of plasmalogens [90, 91].
Box 3, Figure I. Ether lipid synthetic pathway.
The initial steps of ether lipid synthesis take place in peroxisomes, generating 1-O-alkyl glycerol-3-phosphate (AGP), a precursor of ether lipids. The subsequent steps take place in the ER. Peroxisomes can also generate lysophophatidic acid (LPA), a precursor of conventional diacylphospholipids.
In addition to these metabolic functions, emerging studies have uncovered a role for peroxisomes in metabolic adaptation to stress. In light of these recent findings, here we review molecular mechanisms through which peroxisomes regulate cellular stress response. The role of peroxisomes as sensors of stress is first briefly introduced, along with a discussion of key peroxisome-derived signaling molecules. Next, the review highlights recent important papers describing molecular mechanisms through which peroxisomes are involved in various forms of metabolic and environmental stress. The article concludes with a discussion of pertinent directions for future research and the translational potential of targeting peroxisomal regulation of stress response.
Peroxisomes as mediators of cellular stress response
All organisms have evolved a capacity to maintain homeostasis, which can be disturbed by adverse forces called stressors [5]. Stress can be caused by environmental factors or metabolic disturbances. Environmental stressors include extreme temperature, noise, toxins and pathogens. Metabolic stress is driven by metabolic disturbances and can be influenced by environmental fluctuations and include nutrient stress (excess or deficiency), oxidative stress, and hypoxia. Increasing evidence suggests that peroxisomes are critical sensors of stress. The highly plastic nature of peroxisomes, their capacity to perform a diverse variety of functions, and their ability to communicate with other organelles either via direct physical interactions or through exchange of metabolites make these organelles well-suited to serve as a signaling node that controls cellular responses to stress. Although peroxisome-derived hydrogen peroxide (H2O2) plays a key role in cellular signaling related to stress, emerging studies suggest that others products of peroxisomal metabolism, such as acetyl-CoA and ether lipids, are also important for metabolic adaptation to stress. In the next two sections, we review molecular mechanisms through which peroxisomes regulate different forms of metabolic and environmental stress.
Peroxisomal response to metabolic stress
Recent findings suggest peroxisomes are not only passive performers of various metabolic reactions, but also a signaling hub that integrates signals derived from these metabolic reactions to respond to metabolic stressors. In this section, we discuss the mechanisms through which peroxisomes act as a source or a regulator of oxidative stress, how they affect cellular fitness during hypoxic stress, and how they control lipid hydrolysis during nutrient deprivation.
Regulation of oxidative stress by peroxisomes
Peroxisomes derive their name from their role in H2O2 metabolism. Peroxisomal respiration accounts for up to 20% of total cellular oxygen consumption and produces as much as 35% of total H2O2 in certain mammalian tissues [6] through the actions of multiple FAD-dependent oxidoreductases present in these organelles. To counteract the damaging effects of ROS, peroxisomes contain several antioxidant enzymes, including catalase, which reduces H2O2 to water. Disruption of the balance between peroxisomal ROS production and removal leads to oxidative stress and is associated with a variety of age-related human diseases, including diabetes, cancer, and neurological disorders [7–9]. For example, humans with a gain of function mutation (N237S) in Acox1 (acyl CoA oxidase 1), a peroxisomal enzyme that oxidizes VLCFA and produces H2O2 as a byproduct, exhibit loss of glia and neurodegeneration. Mimicking the human disease, a Drosophila model of this mutation, which promotes dimerization of the enzyme, leads to increased ROS production in glia, resulting in glial and axonal loss and increased lethality, which could be rescued by treatment with the antioxidant N-acetyl cysteine amide [10]. Increased ROS production by the active Acox1 dimer has also been implicated in DNA damage and cancer in a process dependent on sirtuin 5 (SIRT5) [11]. SIRT5 was shown to be localized in peroxisomes, where it interacts with Acox1 and promotes its desuccinylation and inhibits its activity through suppression of dimer formation. SIRT5 protein level is downregulated in hepatocellular carcinoma tumor compared to peritumor tissues, while Acox1 activity is upregulated due to increased succinylation of the β-oxidation enzyme. Knockdown of SIRT5 in human cancer cell lines increases H2O2 production and oxidative DNA damage in an Acox1-dependent manner [11]. It remains to be determined whether the peroxisomal localization of SIRT5, a predominantly mitochondrial and cytosolic enzyme [12], is regulated by oxidative stress.
Genome-wide shRNA and CRISPR-Cas9 screens to discover regulators of oxidative stress identified known regulators of cellular redox balance, such as various antioxidant enzymes, but also led to the identification of several proteins involved in the peroxisomal import pathway, including Pex5, Pex13 and Pex14, as modifiers of cellular sensitivity to oxidative stress [13]. Of note, Pex5 is the import receptor of peroxisomal matrix proteins, including catalase, which contain a carboxyl terminal peroxisomal targeting sequence (PTS1) [14]. Paradoxically, inactivation of Pex5, which results in redistribution of catalase to the cytoplasm, increased resistance to oxidative stress caused by exogenous H2O2 treatment. Furthermore, ROS-induced phosphorylation of Pex14 at Ser232 impairs the interaction between the Pex5-Pex14 complex and catalase, confining catalase in the cytosol to counteract oxidative stress [15]. Indeed, a mutant of catalase localized to the cytoplasm was more protective against cell death caused by ROS as compared to the peroxisome-localized antioxidant enzyme [13]. This suggests that cytoplasmic catalase might be important for protection against acute oxidative stress, while peroxisomal catalase could be more involved in controlling basal oxidative stress resulting from peroxisome-generated ROS. This is consistent with previous studies suggesting that Pex5 itself is a redox-sensitive protein that exhibits reduced ability to import catalase under acute oxidative stress [16] and that the proapoptotic BCL-2 effector protein BAK promotes peroxisomal membrane permeabilization and localization of catalase in the cytosol during oxidative stress [17]. These studies support the notion that peroxisomes can function as a source or a sink of ROS, depending on physiological context [9]. The balance between ROS generation and elimination requires peroxisomal homeostasis, which in turn is maintained through a delicate balance between biogenesis and turnover of peroxisomes.
Turnover of old or dysfunctional peroxisomes has also been linked to increased peroxisomal ROS production. In this regard, ATM (Ataxia-telangiectasia mutated) kinase, a redox sensitive regulator of nuclear DNA damage response [18, 19], was recently shown to interact with Pex5 and translocate to peroxisomes to regulate pexophagy in response to ROS [20]. Activated ATM phosphorylates Pex5 at Ser 141, which in turn promotes Pex5 ubiquitination at Lys 209. Peroxisomes containing ubiquitinated Pex5 are recognized by the autophagy adaptor p62 and targeted for degradation [20]. It remains unclear whether ATM is activated by ROS endogenously produced by peroxisomes. However, supporting the possibility that endogenous ROS promotes autophagic degradation of peroxisomes, activation of pexophagy caused by knockout of the heat shock protein HSPA9 has been linked to elevated accumulation of peroxisomal ROS detected using peroxisome-localized HyPer, a redox-sensitive fluorescent protein that can detect H2O2 [21]. One potential beneficial effect of ROS-induced pexophagy mediated by ATM could be the restoration of cellular redox balance to prevent ROS-induced DNA damage. In line with this notion, knockdown of catalase leads to the impairment of peroxisomal ROS removal and causes ROS-induced pexophagy, which can be rescued by the addition of the antioxidant agent N-acetyl-L-cysteine [22].
Role of peroxisomes in metabolic adaptation to hypoxic stress
Oxygen is a critical substrate in cellular metabolism and bioenergetics. Inadequate availability of oxygen to tissues, or hypoxia, is experienced in various physiological and pathological states. For instance, tissue hypoxia is a prominent feature of most solid tumors that promotes tumor aggressiveness and metastasis [23, 24]. A genome-wide CRISPR-Cas9 growth screen by Jain et al. [25] to discover regulators of cell fitness in high and low oxygen conditions led to the identification of an essential role for peroxisomes in metabolic adaptation to hypoxic stress. Notably, the authors observed that peroxisomal biogenesis genes Pex2, Pex5, Pex7, Pex10, Pex12, and Pex13 and plasmalogen synthesis genes GNPAT, AGPS, FAR1/2 and TMEM189 were selectively essential for growth in 1% O2 versus 21% O2. Plasmalogens are peroxisome-derived phospholipids which have a vinyl ether bond at the sn-1 position as opposed to an ester bond in the conventional phospholipids (Box 3). The cell growth defect under hypoxic stress was rescued with the addition of the unsaturated fattyacid oleate, but worsened with the saturated fatty acid palmitate [25]. This is notable since previous studies suggest that stearoyl-CoA desaturase (SCD), an enzyme that introduces a single double bond in the Δ9 position of saturated fatty acids, is an oxygen-sensitive protein inhibited by hypoxia. The resulting increased ratio of saturated to unsaturated fatty acids leads to impaired cell growth, presumably due to increased membrane rigidity [26, 27]. This led Jain et al. to propose that plasmalogens, which have been implicated in membrane fluidity [28], are beneficial in hypoxia to reduce membrane rigidity. Their lipidomics analysis indicated that biosynthesis of these peroxisome-derived lipids is increased in hypoxia [25]. It remains to be determined whether plasmalogens directly influence cellular fitness in the context of hypoxic stress via control of membrane fluidity. It is possible that other mechanisms might also be at play. For example, plasmalogens are thought to function as endogenous antioxidants [29, 30], which could affect hypoxia-related lipid peroxidation [31]. In line with this possibility, a genome-wide CRISPR-Cas9 screen coupled with lipidomic profiling to discover factors that impact susceptibility to ferroptosis led to the identification of polyunsaturated ether lipids, including plasmalogens, as substrates for lipid peroxidation that promote induction of ferroptosis, which carcinoma cells can evade through downregulation of these lipids [32].
It is remarkable that while acute exposure of cells to low oxygen conditions does not decrease peroxisome abundance [25], sustained activation of hypoxia via genetic inactivation of a critical regulatory factor dramatically decreases the number of peroxisomes [33]. Hypoxia is regulated by the hypoxia inducible factor (HIF) family of transcription factors, which are active under hypoxia but targeted for degradation by the Von Hippel-Lindau (VHL) protein under oxygen replete conditions. Activated HIF transcriptionally reprogram cells to adapt to the low oxygen tension environment [34]. Interestingly, the stabilization of HIF2α resulting from VHL knockout promotes pexophagy, leading to a dramatic reduction of peroxisome abundance [33]. The precise reason for the conflicting effects on peroxisome abundance remains unclear. However, chronic hypoxia has been shown to protect against the effects of acute hypoxia [35, 36]. Thus, it is possible that induction of pexophagy as a result of sustained hypoxic stress might reflect a feedback mechanism to maintain peroxisome homeostasis.
Peroxisomal regulation of lipid homeostasis during nutrient stress
Adipose tissue stores excess energy as triglycerides within lipid droplets. This pool of intracellular lipid stores can be mobilized during nutrient deprivation through a process called lipolysis, which involves induction of β-adrenergic receptor signaling, resulting in the activation of a cAMP and protein kinase A (PKA)-dependent pathway [37]. Lipolysis is mediated by a series of lipases, including adipose triglyceride lipase (ATGL), an enzyme that catalyzes the first and rate-limiting step in the process, hydrolyzing triglycerides to diglycerides. Activities of lipolytic enzymes are regulated by nutritional status [37], but the mechanism through which these enzymes translocate onto lipid droplet and catalyze lipolysis remains ill-defined.
A recent study by Kong et al. [38] identified a role for peroxisomes in fasting-induced lipolysis in adipose tissue.Peroxisomes have long been known to associate with lipid droplets [39], but the physiological significance of this interaction has remained unclear. Kong et al. discovered that fasting stimulates physical interaction between peroxisomes and lipid droplets and induces fatty acid liberation via lipolysis in white adipose tissue (WAT) [38]. Similar results were observed in 3T3-L1 adipocytes treated with isoproterenol, a β-adrenergic receptor agonist, to mimic the fasting response. Mechanistically, the authors demonstrated that fasting promotes interaction between peroxisomes and lipid droplets by increasing kinesin-like protein KIFC3-dependent movement of peroxisomes toward lipid droplets. Moreover, they reported that the peroxisomal biogenesis factor Pex5 interacts with ATGL and recruits it to peroxisome-lipid droplet contact sites in a manner dependent on PKA activation [38]. However, it is unclear how Pex5 interacts with ATGL since the lipolytic enzyme lacks a PTS1. It is also unclear what the fate is of the fatty acids liberated by peroxisome-mediated lipolysis.
Besides lipolysis, fatty acids could be liberated from triglycerides by lipophagy, a subtype of autophagy that involves encapsulation of lipid droplets within a double-membrane autophagosome, which fuses with a lysosome, followed by hydrolysis of triglycerides by lysosomal acid lipase A (LAL) [40]. Starvation activates the autophagy pathway, but paradoxically autophagic degradation of lipid droplets is normally not sufficient to prevent hepatic steatosis that results from prolonged nutrient deprivation. Acetyl-CoA is a key metabolic regulator of autophagy whose depletion is sufficient to promote autophagy activation, albeit through a previously undefined mechanism [41].
Recent studies suggest that peroxisomal β-oxidation is a major source of cytosolic acetyl CoA that limits lipophagy activation in the liver under nutrient deprivation [42]. Hepatocyte-specific genetic inactivation of the peroxisomal β-oxidation enzyme Acox1 prevents against starvation-induced fatty liver in mice. Mechanistically, acetyl-CoA derived from Acox1-mediated fatty acid oxidation promotes acetylation of Raptor [42], a component of the mTOR (mechanistic target of rapamycin kinase) complex 1 (mTORC1) [43]. This growth and metabolism regulatory complex senses nutrients and inhibits autophagy under nutrient sufficiency by phosphorylating various autophagy-related proteins, including ULK1 (Unc-51-like autophagy-activating kinase 1) [44], which promotes autophagy initiation and autophagosome formation. Raptor regulates subcellular localization of mTOR and promotes recruitment of its substrates [45]. As a consequence of hepatic Acox1 deficiency, lysosomal localization and activation of mTOR is impaired, resulting in decreased autophagy-inhibitory Ser757 phosphorylation of ULK1 and subsequent activation of lipophagy and protection against fatty liver [42]. The activation of mTORC1 was recently shown to also be impaired in peroxisome-deficient Chinese hamster ovary cells [46]. With regard to peroxisomal β-oxidation-mediated inhibition of mTORC1, these studies suggest a peroxisome-lysosome metabolic link restricts autophagic degradation of lipid droplets in the fasted state and controls hepatic lipid homeostasis. It is noteworthy that this is in contrast to the fasting-induced peroxisome-lipid droplet interaction discussed above, which enhances fatty acid liberation via the promotion of lipolysis in adipose tissue [38] (Figure 2). These opposing effects of peroxisomes on lipid hydrolysis suggest that peroxisomal processes are calibrated in a tissue-specific manner based on nutritional status. For example, fasting increases Acox1 gene expression in the liver but not in WAT [47].
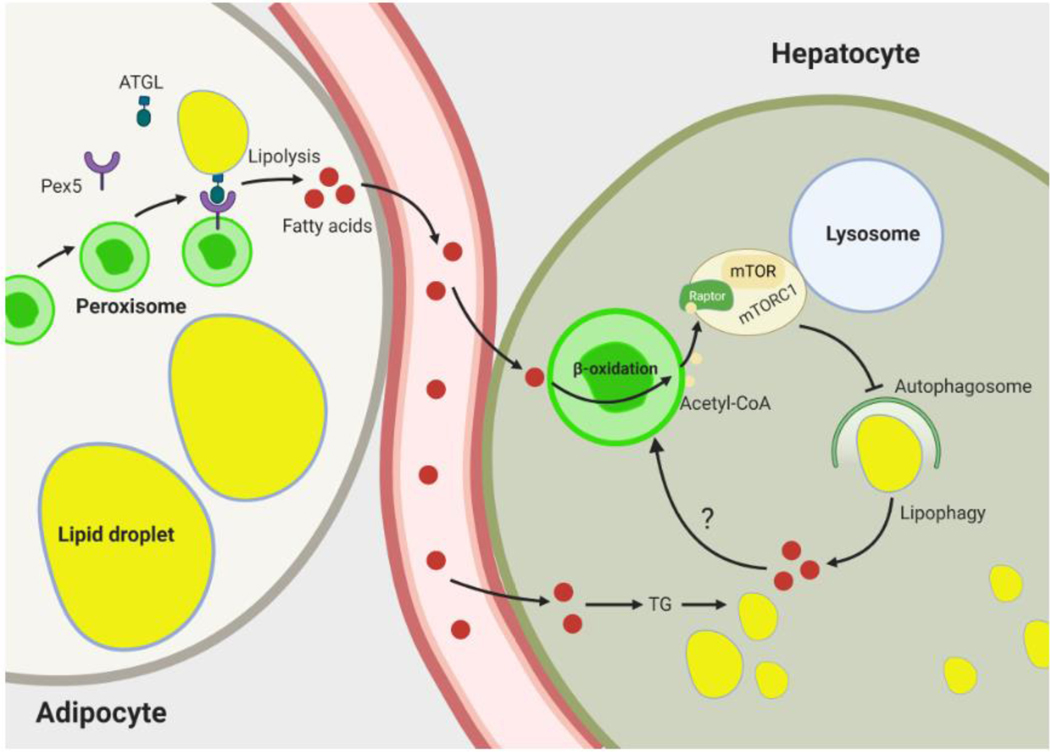
Figure 2. Role of peroxisomes in nutrient deprivation-induced lipid hydrolysis.
In adipocytes, fasting stimulates interaction between peroxisomes and lipid droplets (LD) by increasing kinesin-like protein KIFC3-dependent movement of peroxisomes toward LDs. The peroxisomal biogenesis factor Pex5 interacts with ATGL and recruits it to peroxisome-LD contact sites to induce lipolysis. The fatty acids liberated from hydrolysis of LDs are mobilized and taken up by peripheral tissues, such as the liver. In hepatocytes, these fatty acids are reesterified to triglyceride and stored in lipid droplets. Peroxisomes inhibit hydrolysis of these lipids by blocking lipophagy. This involves peroxisomal fatty acid oxidation-mediated production of acetyl-CoA, which promotes acetylation of Raptor, a component of mTORC1. Activated mTORC1 inhibits lipophagy. Fatty acids released by lipophagic hydrolysis of triglycerides may also be oxidized in peroxisomes to generate acetyl-CoA, potentially reflecting a negative feedback mechanism to limit hydrolysis of intracellular lipid stores. Abbreviations: ATGL, adipose triglyceride lipase; mTOR, mechanistic target of rapamycin; mTORC1, mechanistic target of rapamycin complex 1; TG, triglyceride.
Role of peroxisomes in environmental stress
In addition to metabolic stress, peroxisomes play a critical role in adaptation to environmental stress. In this section, we discuss the role of peroxisomes in brown fat-mediated thermogenesis in response to cold, how these organelles are involved in protection against noise-induced hearing loss caused by oxidative stress, and how they regulate innate immune response to infectious agents (Figure 3).
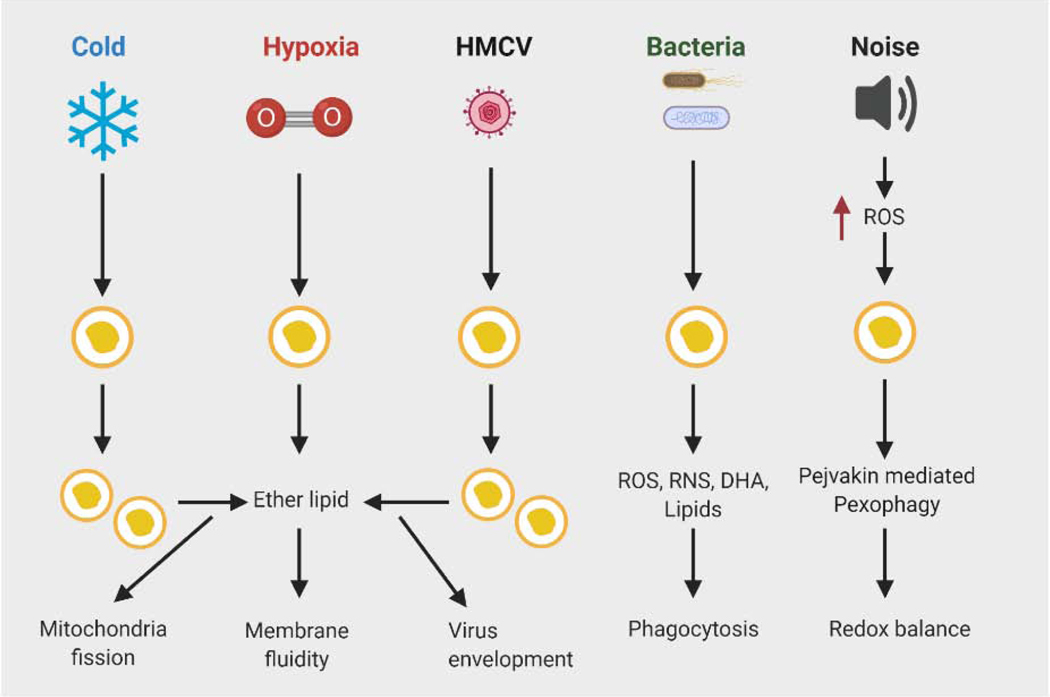
Figure 3. Role of peroxisomes in response to various stressors.
Cold induces peroxisomal biogenesis in brown fat results in increased production of plasmalogens, a form of ether lipids that are a component of mitochondrial membranes. Plasmalogens promote cold-induced mitochondrial fission to support thermogenesis. Hypoxic stress also promotes synthesis of ether lipids, which help to reduce membrane rigidity associated with low oxygen tension and improve cellular fitness. Peroxisomes are involved in innate immune response to viral infections, but some viruses have evolved a strategy to escape the antiviral effect mediated by peroxisomes. Viruses, such as HMCV promote peroxisomal production of ether lipids to support secondary envelopment of infectious particles. Peroxisomes play a protective role in bacterial infections through requirement of peroxisomal ROS and lipid metabolism in phagocytosis. Noise promotes ROS production and oxidative damage, leading to auditory hair cell damage and hearing loss. Pejvakin mediated-pexophagy is activated by noise-induced ROS in auditory hair cells to clear damaged peroxisomes, paving the way for peroxisome biogenesis. Pejvakin deficiency leads to noise-induced hearing loss. Abbreviations: DHA, docosahexaenoic acid; HMCV, human cytomegalovirus; RNS, reactive nitrogen species; ROS, reactive oxygen species.
Peroxisome-derived lipids and the regulation of thermogenesis in response to cold exposure
One of the challenges for euthermic animals is to maintain their body temperature in a cold environment. Cold-induced heat production in mammals occurs through skeletal muscle shivering [48] or by non-shivering thermogenesis, which is mediated primarily by brown adipose tissue (BAT) and the related beige adipocytes that appear within subcutaneous WAT in response to prolonged cold exposure or β-adrenergic stimulation [49]. Brown and beige fat cells are enriched in mitochondria and specialize in oxidizing fatty acids and glucose to generate heat via mitochondrial uncoupling. In addition to promoting thermogenesis through uncoupled respiration, mitochondria are highly dynamic organelles that regulate the thermogenic capacity of adipocytes through their ability to undergo fission in response to β-adrenergic stimulation. Fragmented and circular mitochondria are thought to exhibit increased uncoupling of oxidative phosphorylation by directing nutrient oxidation toward heat production instead of ATP synthesis [50].
Peroxisomes are also a dynamic organelle whose abundance in brown adipocytes increases with cold exposure [51, 52]. Recent studies indicate that cold-induced peroxisomal biogenesis is mediated by the thermogenic transcriptional co-regulator PRDM16 [53], suggesting that the transcriptional regulation of thermogenesis and de novo peroxisome formation is linked. Consistent with a role for peroxisomes in thermogenesis, disruption of peroxisomal biogenesis through adipose-specific knockout of the critical peroxisomal biogenesis factor Pex16 results in cold intolerance in mice due to inhibition of cold-induced mitochondrial fission. Mechanistically, the loss of peroxisomes decreases the mitochondrial membrane content of plasmalogens. Treatment of mice with alkyl glycerol, a plasmalogen precursor, restores cold-induced mitochondrial fragmentation and thermogenesis in Pex16 knockout mice, suggesting that peroxisomes channel lipids to mitochondria in brown and beige adipocytes to regulate mitochondrial dynamics and thermogenesis [53]. Future research will be required to understand the precise mechanism through which plasmalogens affect mitochondrial fission. One potential explanation is that these peroxisome-derived lipids are required to maintain mitochondrial membrane fluidity necessary for cold-induced mitochondrial fission. It is also possible that plasmalogens assist in the recruitment of factors involved in mitochondrial dynamics.
Peroxisomal regulation of noise-induced hearing loss
In the modern industrial society, noise is another major environmental stressor. Overexposure to noise promotes ROS production and oxidative damage, leading to auditory hair cell damage and hearing loss [54, 55], while antioxidant therapy has been shown to prevent hearing loss in mice [56]. Consistent with the important role of peroxisomes in ROS metabolism, polymorphisms in the peroxisomal antioxidant enzyme catalase are associated with noise-induced hearing loss (NIHL) in humans [57]. The initial evidence for a direct role of peroxisomes in NIHL was provided by the discovery that Pejvakin (also called DFNB59), a poorly characterized protein genetically linked to sensorineural hearing loss, is associated with peroxisomes, particularly with peroxisomal protrusions and “string-of-beads” structures indicative of peroxisome growth and fission [55]. Like humans with mutations in the gene encoding Pejvakin (PVJK), Pejvakin-deficient mice are hypervulnerable to noise. Embryonic fibroblasts from Pjvk+/+ mice stressed with H2O2 show a significant increase in peroxisome number, but those from Pjvk−/− mice remain unchanged. Furthermore, Pjvk−/− mice only display structural peroxisome abnormalities in hair cells after hearing loss onset due to the oxidative stress caused by noise exposure [55].
Additional mechanistic studies suggest that following sound-induced oxidative stress, auditory cells undergo autophagic degradation of damaged peroxisomes through pexophagy prior to peroxisome proliferation. Pejvakin contains a functional LC3-interacting region (LIR) and directly recruits the autophagosomal marker LC3B to peroxisomes in response to H2O2 treatment in HepG2 cells to facilitate pexophagy and pave the way for peroxisome proliferation [58]. Taken together, these studies establish a key pathway in which pejvakin-mediated peroxisome degradation and proliferation protect auditory hair cells against noise-induced oxidative stress and reveal that the DFNB59 form of deafness is a pexophagy disorder. As discussed above, activation of pexophagy involves Pex5-dependent peroxisomal recruitment of the ATM kinase [20]. It is unclear whether inactivation of PVJK disrupts the Pex5/ATM axis. Nevertheless, this work opens up new therapeutic avenues as antioxidant supplementation and AAV-mediated gene therapy appear to be effective treatments for the disease [55].
Role of peroxisomes in innate immune response to viral and bacterial infections
Besides extreme physical environment, pathogens, such as viruses and bacteria also contribute to environmental stress in animals. Innate immune system is the first line of defense against infections. For viral infections, the innate immune response involves recognition of viral nucleic acids by pathogen recognition receptors, including cytosolic receptors such as RIG-I-like receptors (RLRs) that activate mitochondrial antiviral signaling protein (MAVS) to destroy viruses [59]. Although originally identified as a mitochondrial membrane protein that activates NF-kB and interferon regulatory factors, resulting in the production of pro-inflammatory cytokines and type I interferons (IFN), MAVS is also located at the peroxisomal membrane and triggers expression of type III IFN and IFN-stimulated genes upon viral infections [60]. The products of IFN-stimulated genes have antiviral effects, which destroy the virus or inhibit viral replication [61]. However, some viruses have evolved a strategy to escape the antiviral effect mediated by peroxisomes. For instance, the flavivirus capsid protein promotes sequestration and degradation of Pex19, a cytoplasmic chaperone of peroxisomal membrane proteins, leading to reduction of peroxisome number and impairment of early antiviral signaling [62]. Recent studies suggest that enveloped viruses, such as human cytomegalovirus (HCMV) and herpes simplex virus type 1 (HSV-1) do not inhibit peroxisomal biogenesis, but rather increase it to support their replication [63]. The authors suggest that this leads to increased production of plasmalogens necessary for secondary envelopment and the assembly of infectious particles. However, since peroxisomal lipid synthesis also contributes to the production of conventional diacyl phospholipids [64], additional work is required to determine if plasmalogens are directly necessary for envelopment.
The peroxisomal role in innate immune response is not limited to only viral infections, but extends to bacterial infections as well. Microbial invaders are destroyed through phagocytosis, in which specialized cells use plasma membrane to engulf pathogens, giving rise to an internal compartment called the phagosome. The phagosome fuses with lysosome, forming a phagolysosome, which degrades pathogens [65]. A study by Di Cara et al. has identified a role for peroxisomes in phagocytosis [66]. Peroxisomes are localized to the sites of phagosome formation in Drosophila S2 cells and knockdown of Pex5 or Pex7 results in reduced bacterial uptake via phagocytosis. Peroxisome deficiency leads to disorganization of the actin network and a defect in phagocytic cup formation, coupled with impaired ROS metabolism. Overexpression of catalase in peroxisome deficient cells or treatment with phosphatidylinositols and DHA, lipids thought to be produced in peroxisomes, rescues phagocytosis [66]. These studies suggest that peroxisomal function in ROS and lipid metabolism is important for phagocytosis, but the detailed molecular mechanism remains to be discovered. Peroxisomal lipid metabolism has also been implicated in bactericidal activity through a different mechanism involving FAMIN, a laccase domain-containing protein that partially localizes to the cytosolic surface of peroxisomes through its interaction with fatty acid synthase. FAMIN regulates β-oxidation of de novo synthesized fatty acids. FAMIN-deficient macrophages have impaired mitochondrial ROS production, a defect in inflammasome activation and reduced bacterial clearance [67].
Concluding Remarks
Peroxisomes participate in a variety of metabolic functions. The physiological significance of these processes is only now beginning to be understood. It is becoming increasingly clear that peroxisomes are a critical signaling node that communicates with other organelles and responds to various types of cellular stress. While the role of peroxisomes in mediating and regulating redox-driven signaling events is well-appreciated, emerging evidence suggests that other products of peroxisomal metabolism, including acetyl-CoA and ether lipids, also affect cellular signaling related to stress. In the context of nutrient stress, peroxisomal β-oxidation is a major source of cytosolic acetyl CoA that controls hepatic lipid hydrolysis. Although peroxisomal β-oxidation is required for catabolism of VLCFAs, the dramatic decrease in the liver acetyl-CoA content due to hepatic Acox1 deficiency [42] suggests that the peroxisomal β-oxidation pathway might possess a broader fatty acid substrate specificity. It is also possible that fatty acids released by hepatic lipophagy are oxidized in peroxisomes to generate acetyl-CoA, potentially reflecting a negative feedback mechanism. Additional work is required to explore these possibilities (see Outstanding Questions). Future research will also be necessary to understand the molecular mechanisms through which peroxisomal lipids promote cellular fitness during hypoxic stress, control mitochondrial dynamics during cold to promote thermogenesis, and regulate innate immune responses to infectious agents, and to determine whether these peroxisomal functions could be targeted to treat human diseases. For example, it would be of great interest to determine if the immunometabolism-regulatory function of peroxisomes could be leveraged to treat obesity-associated inflammation. Regulation of cellular stress by peroxisomes is a fertile area of research. A better understanding of how these versatile organelles control stress responses could reveal new strategies to treat disease.
Outstanding Questions.
Could the role of peroxisomes in stress response be leveraged for treatment of human diseases, such as obesity, diabetes and cancer?
How is peroxisome-derived acetyl-CoA channeled toward Raptor acetylation? Are peroxisome-lysosome membrane contacts involved in this process? What other proteins are acetylated by this pool of acetyl-CoA?
As a redox-sensitive protein, does Pex5 directly regulate ROS-activated pexophagy independently of ATM?
What is the molecular basis of peroxisome-mediated regulation of mitochondrial fission?
What is the molecular mechanism of Pejvakin mediated pexophagy? Is Pejvakin an adaptor protein selectively involved in pexophagy? Is the role of Pejvakin in pexophagy specific to auditory hair cells?
Highlights.
Peroxisomes are not only passive performers of various metabolic reactions, but also a critical signaling hub that integrates signals originating from these reactions and communicates with other organelles to respond to various stressors, including oxidative stress, hypoxia, starvation, cold, noise and pathogens.
Although peroxisome-derived ROS play a key role in cellular signaling related to stress, recent studies suggest that other products of peroxisomal metabolism, such as acetyl-CoA and ether lipids, are also important for metabolic adaptation to stress.
Peroxisomes control lipid hydrolysis and mobilization during nutrient deprivation through their ability to engage in crosstalk with other organelles and generate signaling metabolites, such as acetyl-CoA.
Peroxisome-derived lipids promote cellular fitness during hypoxic stress, regulate mitochondrial dynamics to support cold-induced thermogenesis, and control innate immune responses to infectious pathogens.
Acknowledgements
This work was supported by NIH grants DK115867, DK118333, and DK020579.
Glossary
- β-oxidation
process of fatty acid catabolism, in which two carbons are sequentially removed from the carboxyl terminus of a fatty acyl CoA molecule. The process is named as such because the beta carbon of the fatty acid undergoes oxidation
- Ether lipid
peroxisome-derived glycerophospholipids in which the hydrocarbon chain at the sn-1 position of the glycerol backbone is attached by an ether bond, as opposed to an ester bond in the more common diacyl phospholipids
- Ferroptosis
an iron-dependent cell death process that is distinct from apoptosis
- HepG2 cells
a cell line derived from a patient with hepatocellular carcinoma
- Lipolysis
a cytosolic process through which triglycerides stored in lipid droplets are hydrolyzed, generating free fatty acids and glycerol
- Pexophagy
selective autophagy of peroxisomes, which involves degradation of peroxisomes through formation of a double membrane structure called autophagosome around the peroxisomal cargo, which then fuses with lysosomes
- Plasmalogen
ether lipid characterized by a vinyl ether linkage at the sn-1 position of the glycerol backbone
- Reactive Oxygen Species (ROS)
molecules containing a partially reduced form of oxygen that are unstable and chemically reactive
- Saturated fatty acid
lipid molecule consisting of a long chain of hydrocarbons to which a terminal carboxyl group is attached. They are named as such because they are saturated with hydrogens since neighboring carbons in the hydrocarbon chain are only linked by single bonds, which increases the number of hydrogens on each carbon
- Unsaturated fatty acid
fatty acid that has one or more carbon-carbon double bonds
Footnotes
Declaration of Interests
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lodhi IJ and Semenkovich CF (2014) Peroxisomes: a nexus for lipid metabolism and cellular signaling. Cell Metab 19 (3), 380–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahalingam SS et al. (2020) Balancing the Opposing Principles That Govern Peroxisome Homeostasis. Trends Biochem Sci. doi: 10.1016/j.tibs.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huybrechts SJ et al. (2009) Peroxisome dynamics in cultured mammalian cells. Traffic 10 (11), 1722–33. [DOI] [PubMed] [Google Scholar]
- 4.Chu BB et al. (2015) Cholesterol transport through lysosome-peroxisome membrane contacts. Cell 161 (2), 291–306. [DOI] [PubMed] [Google Scholar]
- 5.Chrousos GP (2009) Stress and disorders of the stress system. Nat Rev Endocrinol 5 (7), 374–81. [DOI] [PubMed] [Google Scholar]
- 6.Boveris A. et al. (1972) The cellular production of hydrogen peroxide. Biochem J 128 (3), 617–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cipolla CM and Lodhi IJ (2017) Peroxisomal Dysfunction in Age-Related Diseases. Trends Endocrinol Metab 28 (4), 297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finkel T. (2011) Signal transduction by reactive oxygen species. J Cell Biol 194 (1), 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fransen M. and Lismont C. (2019) Redox Signaling from and to Peroxisomes: Progress, Challenges, and Prospects. Antioxid Redox Signal 30 (1), 95–112. [DOI] [PubMed] [Google Scholar]
- 10.Chung HL et al. (2020) Loss- or Gain-of-Function Mutations in ACOX1 Cause Axonal Loss via Different Mechanisms. Neuron 106 (4), 589–606 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen XF et al. (2018) SIRT5 inhibits peroxisomal ACOX1 to prevent oxidative damage and is downregulated in liver cancer. EMBO Rep 19 (5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsushita N. et al. (2011) Distinct regulation of mitochondrial localization and stability of two human Sirt5 isoforms. Genes Cells 16 (2), 190–202. [DOI] [PubMed] [Google Scholar]
- 13.Dubreuil MM et al. (2020) Systematic Identification of Regulators of Oxidative Stress Reveals Non-canonical Roles for Peroxisomal Import and the Pentose Phosphate Pathway. Cell Rep 30 (5), 1417–1433 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gould SG et al. (1987) Identification of a peroxisomal targeting signal at the carboxy terminus of firefly luciferase. J Cell Biol 105 (6 Pt 2), 2923–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okumoto K. et al. (2020) The peroxisome counteracts oxidative stresses by suppressing catalase import via Pex14 phosphorylation. Elife 9. doi: 10.7554/eLife.55896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Apanasets O. et al. (2014) PEX5, the shuttling import receptor for peroxisomal matrix proteins, is a redox-sensitive protein. Traffic 15 (1), 94–103. [DOI] [PubMed] [Google Scholar]
- 17.Hosoi KI et al. (2017) The VDAC2-BAK axis regulates peroxisomal membrane permeability. J Cell Biol 216 (3), 709–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bakkenist CJ and Kastan MB (2003) DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 421 (6922), 499–506. [DOI] [PubMed] [Google Scholar]
- 19.Paull TT (2015) Mechanisms of ATM Activation. Annu Rev Biochem 84, 711–38. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J. et al. (2015) ATM functions at the peroxisome to induce pexophagy in response to ROS. Nat Cell Biol 17 (10), 1259–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jo DS et al. (2020) Loss of HSPA9 induces peroxisomal degradation by increasing pexophagy. Autophagy 16 (11), 1989–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee JN et al. (2018) Catalase inhibition induces pexophagy through ROS accumulation. Biochem Biophys Res Commun 501 (3), 696–702. [DOI] [PubMed] [Google Scholar]
- 23.Chouaib S. et al. (2017) Hypoxic stress: obstacles and opportunities for innovative immunotherapy of cancer. Oncogene 36 (4), 439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Majmundar AJ et al. (2010) Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell 40 (2), 294–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jain IH et al. (2020) Genetic Screen for Cell Fitness in High or Low Oxygen Highlights Mitochondrial and Lipid Metabolism. Cell 181 (3), 716–727 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ackerman D. et al. (2018) Triglycerides Promote Lipid Homeostasis during Hypoxic Stress by Balancing Fatty Acid Saturation. Cell Rep 24 (10), 2596–2605 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamphorst JJ et al. (2013) Hypoxic and Ras-transformed cells support growth by scavenging unsaturated fatty acids from lysophospholipids. Proc Natl Acad Sci U S A 110 (22), 8882–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagura M. et al. (2004) Alterations of fatty acid metabolism and membrane fluidity in peroxisome-defective mutant ZP102 cells. Lipids 39 (1), 43–50. [DOI] [PubMed] [Google Scholar]
- 29.Zoeller RA et al. (2002) Increasing plasmalogen levels protects human endothelial cells during hypoxia. Am J Physiol Heart Circ Physiol 283 (2), H671–9. [DOI] [PubMed] [Google Scholar]
- 30.Zoeller RA et al. (1999) Plasmalogens as endogenous antioxidants: somatic cell mutants reveal the importance of the vinyl ether. Biochem J 338 ( Pt 3), 769–76. [PMC free article] [PubMed] [Google Scholar]
- 31.Li J. et al. (2007) Hyperlipidemia and lipid peroxidation are dependent on the severity of chronic intermittent hypoxia. J Appl Physiol (1985) 102 (2), 557–63. [DOI] [PubMed] [Google Scholar]
- 32.Zou Y. et al. (2020) Plasticity of ether lipids promotes ferroptosis susceptibility and evasion. Nature 585 (7826), 603–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walter KM et al. (2014) Hif-2alpha promotes degradation of mammalian peroxisomes by selective autophagy. Cell Metab 20 (5), 882–897. [DOI] [PubMed] [Google Scholar]
- 34.Pugh CW and Ratcliffe PJ (2003) Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med 9 (6), 677–84. [DOI] [PubMed] [Google Scholar]
- 35.Kapitsinou PP et al. (2014) Endothelial HIF-2 mediates protection and recovery from ischemic kidney injury. J Clin Invest 124 (6), 2396–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reiterer M. et al. (2019) Acute and chronic hypoxia differentially predispose lungs for metastases. Sci Rep 9 (1), 10246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zechner R. (2015) FAT FLUX: enzymes, regulators, and pathophysiology of intracellular lipolysis. EMBO Mol Med 7 (4), 359–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kong J. et al. (2020) Spatiotemporal contact between peroxisomes and lipid droplets regulates fasting-induced lipolysis via PEX5. Nat Commun 11 (1), 578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Binns D. et al. (2006) An intimate collaboration between peroxisomes and lipid bodies. J Cell Biol 173 (5), 719–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zechner R. et al. (2017) Cytosolic lipolysis and lipophagy: two sides of the same coin. Nat Rev Mol Cell Biol 18 (11), 671–684. [DOI] [PubMed] [Google Scholar]
- 41.Marino G. et al. (2014) Regulation of autophagy by cytosolic acetyl-coenzyme A. Mol Cell 53 (5), 710–25. [DOI] [PubMed] [Google Scholar]
- 42.He A. et al. (2020) Acetyl-CoA Derived from Hepatic Peroxisomal beta-Oxidation Inhibits Autophagy and Promotes Steatosis via mTORC1 Activation. Mol Cell 79 (1), 30–42 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saxton RA and Sabatini DM (2017) mTOR Signaling in Growth, Metabolism, and Disease. Cell 168 (6), 960–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim J. et al. (2011) AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 13 (2), 132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Son SM et al. (2019) Leucine Signals to mTORC1 via Its Metabolite Acetyl-Coenzyme A. Cell Metab 29 (1), 192–201 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Charles KN et al. (2020) Functional Peroxisomes Are Essential for Efficient Cholesterol Sensing and Synthesis. Front Cell Dev Biol 8, 560266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Palou M. et al. (2008) Sequential changes in the expression of genes involved in lipid metabolism in adipose tissue and liver in response to fasting. Pflugers Arch 456 (5), 825–36. [DOI] [PubMed] [Google Scholar]
- 48.Haman F. and Blondin DP (2017) Shivering thermogenesis in humans: Origin, contribution and metabolic requirement. Temperature (Austin) 4 (3), 217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ikeda K. et al. (2018) The Common and Distinct Features of Brown and Beige Adipocytes. Trends Endocrinol Metab 29 (3), 191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wikstrom JD et al. (2014) Hormone-induced mitochondrial fission is utilized by brown adipocytes as an amplification pathway for energy expenditure. EMBO J 33 (5), 418–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ahlabo I. and Barnard T. (1971) Observations on peroxisomes in brown adipose tissue of the rat. J Histochem Cytochem 19 (11), 670–5. [DOI] [PubMed] [Google Scholar]
- 52.Bagattin A. et al. (2010) Transcriptional coactivator PGC-1alpha promotes peroxisomal remodeling and biogenesis. Proc Natl Acad Sci U S A 107 (47), 20376–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park H. et al. (2019) Peroxisome-derived lipids regulate adipose thermogenesis by mediating cold-induced mitochondrial fission. J Clin Invest 129 (2), 694–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ohlemiller KK et al. (1999) Early elevation of cochlear reactive oxygen species following noise exposure. Audiol Neurootol 4 (5), 229–36. [DOI] [PubMed] [Google Scholar]
- 55.Delmaghani S. et al. (2015) Hypervulnerability to Sound Exposure through Impaired Adaptive Proliferation of Peroxisomes. Cell 163 (4), 894–906. [DOI] [PubMed] [Google Scholar]
- 56.Heman-Ackah SE et al. (2010) A combination antioxidant therapy prevents age-related hearing loss in C57BL/6 mice. Otolaryngol Head Neck Surg 143 (3), 429–34. [DOI] [PubMed] [Google Scholar]
- 57.Konings A. et al. (2007) Association between variations in CAT and noise-induced hearing loss in two independent noise-exposed populations. Hum Mol Genet 16 (15), 1872–83. [DOI] [PubMed] [Google Scholar]
- 58.Defourny J. et al. (2019) Pejvakin-mediated pexophagy protects auditory hair cells against noise-induced damage. Proc Natl Acad Sci U S A 116 (16), 8010–8017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Onomoto K. et al. (2014) Antiviral innate immunity and stress granule responses. Trends Immunol 35 (9), 420–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dixit E. et al. (2010) Peroxisomes are signaling platforms for antiviral innate immunity. Cell 141 (4), 668–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schoggins JW and Rice CM (2011) Interferon-stimulated genes and their antiviral effector functions. Curr Opin Virol 1 (6), 519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.You J. et al. (2015) Flavivirus Infection Impairs Peroxisome Biogenesis and Early Antiviral Signaling. J Virol 89 (24), 12349–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jean Beltran PM et al. (2018) Infection-Induced Peroxisome Biogenesis Is a Metabolic Strategy for Herpesvirus Replication. Cell Host Microbe 24 (4), 526–541 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu XG et al. (2019) CHP1 Regulates Compartmentalized Glycerolipid Synthesis by Activating GPAT4. Mol Cell 74 (1), 45–58 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gordon S. (2016) Phagocytosis: An Immunobiologic Process. Immunity 44 (3), 463–475. [DOI] [PubMed] [Google Scholar]
- 66.Di Cara F. et al. (2017) Peroxisome-Mediated Metabolism Is Required for Immune Response to Microbial Infection. Immunity 47 (1), 93–106 e7. [DOI] [PubMed] [Google Scholar]
- 67.Cader MZ et al. (2016) C13orf31 (FAMIN) is a central regulator of immunometabolic function. Nat Immunol 17 (9), 1046–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Braverman N. et al. (1997) Human PEX7 encodes the peroxisomal PTS2 receptor and is responsible for rhizomelic chondrodysplasia punctata. Nat Genet 15 (4), 369–76. [DOI] [PubMed] [Google Scholar]
- 69.Otera H. et al. (2000) The mammalian peroxin Pex5pL, the longer isoform of the mobile peroxisome targeting signal (PTS) type 1 transporter, translocates the Pex7p.PTS2 protein complex into peroxisomes via its initial docking site, Pex14p. J Biol Chem 275 (28), 21703–14. [DOI] [PubMed] [Google Scholar]
- 70.Meinecke M. et al. (2010) The peroxisomal importomer constitutes a large and highly dynamic pore. Nat Cell Biol 12 (3), 273–7. [DOI] [PubMed] [Google Scholar]
- 71.Matsumoto N. et al. (2003) The pathogenic peroxin Pex26p recruits the Pex1p-Pex6p AAA ATPase complexes to peroxisomes. Nat Cell Biol 5 (5), 454–60. [DOI] [PubMed] [Google Scholar]
- 72.Pedrosa AG et al. (2018) Peroxisomal monoubiquitinated PEX5 interacts with the AAA ATPases PEX1 and PEX6 and is unfolded during its dislocation into the cytosol. J Biol Chem 293 (29), 11553–11563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.El Magraoui F. et al. (2012) The RING-type ubiquitin ligases Pex2p, Pex10p and Pex12p form a heteromeric complex that displays enhanced activity in an ubiquitin conjugating enzyme-selective manner. FEBS J 279 (11), 2060–70. [DOI] [PubMed] [Google Scholar]
- 74.Platta HW et al. (2009) Pex2 and pex12 function as protein-ubiquitin ligases in peroxisomal protein import. Mol Cell Biol 29 (20), 5505–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sugiura A. et al. (2017) Newly born peroxisomes are a hybrid of mitochondrial and ER-derived pre-peroxisomes. Nature 542 (7640), 251–254. [DOI] [PubMed] [Google Scholar]
- 76.van der Zand A. et al. (2012) Biochemically distinct vesicles from the endoplasmic reticulum fuse to form peroxisomes. Cell 149 (2), 397–409. [DOI] [PubMed] [Google Scholar]
- 77.Jones JM et al. (2004) PEX19 is a predominantly cytosolic chaperone and import receptor for class 1 peroxisomal membrane proteins. J Cell Biol 164 (1), 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Klouwer FC et al. (2015) Zellweger spectrum disorders: clinical overview and management approach. Orphanet J Rare Dis 10, 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Waterham HR and Ebberink MS (2012) Genetics and molecular basis of human peroxisome biogenesis disorders. Biochim Biophys Acta 1822 (9), 1430–41. [DOI] [PubMed] [Google Scholar]
- 80.Van Veldhoven PP (2010) Biochemistry and genetics of inherited disorders of peroxisomal fatty acid metabolism. J Lipid Res 51 (10), 2863–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wanders RJ (2013) Peroxisomes in human health and disease: metabolic pathways, metabolite transport, interplay with other organelles and signal transduction. Subcell Biochem 69, 23–44. [DOI] [PubMed] [Google Scholar]
- 82.Dean JM and Lodhi IJ (2018) Structural and functional roles of ether lipids. Protein Cell 9 (2), 196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hajra AK and Das AK (1996) Lipid biosynthesis in peroxisomes. Ann N Y Acad Sci 804, 129–41. [DOI] [PubMed] [Google Scholar]
- 84.Honsho M. et al. (2020) Distinct Functions of Acyl/Alkyl Dihydroxyacetonephosphate Reductase in Peroxisomes and Endoplasmic Reticulum. Front Cell Dev Biol 8, 855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lodhi IJ et al. (2012) Inhibiting adipose tissue lipogenesis reprograms thermogenesis and PPARgamma activation to decrease diet-induced obesity. Cell Metab 16 (2), 189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gibellini F. and Smith TK (2010) The Kennedy pathway--De novo synthesis of phosphatidylethanolamine and phosphatidylcholine. IUBMB Life 62 (6), 414–28. [DOI] [PubMed] [Google Scholar]
- 87.Antonenkov VD and Hiltunen JK (2012) Transfer of metabolites across the peroxisomal membrane. Biochim Biophys Acta 1822 (9), 1374–86. [DOI] [PubMed] [Google Scholar]
- 88.Lam SK et al. (2010) A vesicle carrier that mediates peroxisome protein traffic from the endoplasmic reticulum. Proc Natl Acad Sci U S A 107 (50), 21523–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hua R. et al. (2017) VAPs and ACBD5 tether peroxisomes to the ER for peroxisome maintenance and lipid homeostasis. J Cell Biol 216 (2), 367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gallego-Garcia A. et al. (2019) A bacterial light response reveals an orphan desaturase for human plasmalogen synthesis. Science 366 (6461), 128–132. [DOI] [PubMed] [Google Scholar]
- 91.Werner ER et al. (2020) The TMEM189 gene encodes plasmanylethanolamine desaturase which introduces the characteristic vinyl ether double bond into plasmalogens. Proc Natl Acad Sci U S A 117 (14), 7792–7798. [DOI] [PMC free article] [PubMed] [Google Scholar]