Abstract
Background
During the worldwide mass vaccination campaign against SARS-CoV-2, multiple side effects have been observed. We described the case of a patient who developed pure sensitive chronic inflammatory axonal polyneuropathy (CIAP) in a close temporal relationship with the administration of the BNT162b2 (Pfizer®) vaccine.
Case report
An 82-year-old woman developed lower limb sensory loss and “pricking” associated with marked gait imbalance after she had received her second dose of Pfizer-BioNTech COVID-19 vaccine. At the electroneurographic examination, the motor nerves conduction study was normal. Median, ulnar, and sural nerves sensory compound nerve action potential (CNAP) were bilaterally absent. Somatosensory evoked potentials (SSEPs) were not recordable. Spine MRI demonstrated roots enhancement from C3 to Th2 and diffuse enhancement of cauda equina nerve roots. She was treated with IV methylprednisolone whit benefit. A follow-up visit was made 4 months after the disease onset; a diagnosis of pure sensitive CIAP has been made.
Discussion
To the best of our knowledge, this is the first description of CIAP occurring in a close temporal relationship with the administration of Pfizer-BioNTech COVID-19 vaccine.
Keywords: COVID-19, Vaccination, Polyneuropathy, CIAP
Background
The huge global impact of COVID-19 pandemic has resulted in a fast-tracked development of vaccines. During the worldwide mass vaccination campaign, multiple side effects ranging from mild (fever, fatigue, injection site pain, headache) to severe (Guillain-Barrè syndrome) have been observed following the administration of COVID-19 mRNA vaccine [1–3].
As of August 31, 2021, in Italy, around 55 million doses of BNT162b1-COVID-19 vaccine (Pfizer-BioNTech) have been administered [4]. Here, we report the case of a woman who developed pure sensitive chronic inflammatory axonal polyneuropathy (CIAP) in a close temporal relationship with the second dose of Pfizer-BioNTech vaccination.
Report of case
An 82-year-old woman without significant medical comorbidities was taken to the emergency room complaining lower limb sensory loss and “pricking” associated with marked gait imbalance. Six weeks before presentation, she had received her second dose of Pfizer-BioNTech COVID-19 vaccine. During the first 3 days after vaccination, she reported fever and body pain. From the 4th day, the fever passed but the patients begone to complain worsening symptoms with slow and progressive gait difficulties until bedridden.
She was admitted to the Neurology Unit of the University-Hospital “Policlinico-San Marco” of Catania, Italy. At the neurological examination, mental status and speech were normal. Cranial nerve examination was unremarkable. Standing position was achieved with assistance; Romberg test and gait were impossible to perform; muscle tone, mass, and strength (Medical Research Council 5/5) were normal in bilateral upper and lower extremities. Deep tendon reflexes were absent. Limbs ataxia was observed at the finger-to-nose and heel-knee-shin tests. Sensation to light touch, vibration, and proprioception were reduced. Routine labs were unremarkable. Coronavirus CoV-2 PCR was negative. CSF had albumin-cytological dissociation [proteins 89.6 mg/dL (normal values 20–45 mg/dL), cell count 1 µL (normal values 0–5 µL)]. GM3 antibodies were detected in the CSF. At the electroneurographic examination, motor nerves conduction study was normal. Median, ulnar, and sural nerves sensory compound nerve action potential (CNAP) were bilaterally absent (Table 1). Somatosensory evoked potentials (SSEPs) were not recordable. Spine MRI demonstrated roots enhancement from C3 to Th2 and diffuse enhancement of cauda equina nerve roots (Fig. 1). The patient was treated with IV methylprednisolone without complications during and after treatment. Clinical improvement was appreciated after 5 days of treatment and patients became able to walk for few meters with mono-lateral support due to gait ataxia. Romberg maneuver was positive. During the recovery, the patient received physical therapy. Once discharged, she was referred to an acute rehabilitation clinic. At a follow-up visit after 4 months from the onset, she presented a further gait improvement, still ataxic. The median and ulnar nerve velocity conduction study were bilaterally normal and CNAP presented low amplitude. Sural nerves CNAP were still absent (Table 1). A diagnosis of CIAP, pure sensory variant has been made.
Table 1.
Electroneurographic evaluation at baseline and at follow-up
| Nerve stimulated | Stimulation site | Amplitude (baseline) | Amplitude (follow-up) | Latency (ms) (baseline) | Latency (ms) (follow-up) | Conduction velocity (m/s) (baseline) | Conduction velocity (m/s) (follow-up) | F wave (ms) (baseline) | F wave (ms) (follow-up) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R | L | R | L | R | L | R | L | R | L | R | L | R | L | R | L | ||
| Median (s) | Wrist | Absent | Absent | 7.5 | 5 | 40 | 41 | ||||||||||
| Ulnar (s) | Wrist | Absent | Absent | 6.6 | 4 | 39 | 39 | ||||||||||
| Sural (s) | Calf | Absent | Absent | Absent | Absent | ||||||||||||
| Median (m) | Wrist | 9 | 5 | 8 | 6 | 2.8 | 2.8 | 2.7 | 2.9 | 23 | 25 | 24 | 24 | ||||
| Elbow | 7 | 4 | 8 | 5 | 60 | 51 | 62 | 58 | |||||||||
| Axilla | 7 | 4 | 8 | 5 | 65 | 55 | 63 | 62 | |||||||||
| Ulnar (m) | Wrist | 6 | 5 | 7 | 7 | 2.2 | 2.1 | 2.3 | 2.2 | 25 | 26 | 25 | 25 | ||||
| Below elbow | 5 | 5 | 7 | 6 | 50 | 49 | 51 | 52 | |||||||||
| Above elbow | 5 | 5 | 6 | 6 | 60 | 58 | 55 | 56 | |||||||||
| Axilla | 5 | 5 | 6 | 6 | 52 | 64 | 57 | 62 | |||||||||
| Tibial (m) | Ankle | 4 | 5 | 5 | 5 | 5.2 | 3.6 | 4.9 | 4.0 | 45 | 48 | 44 | 46 | ||||
| Popliteal fossa | 4 | 5 | 5 | 4 | 40 | 42 | 41 | 44 | |||||||||
| Peroneal (m) | Ankle | 4 | 3 | 3.9 | 4.2 | 4.1 | 4.2 | 46 | 49 | 45 | 44 | ||||||
| Below fibula | 4 | 3 | 48 | 40 | 44 | 45 | |||||||||||
| Above fibula | 4 | 3 | 47 | 42 | 47 | 48 | |||||||||||
Legend: R, right; L, left; amplitude motor = mV, sensory = µV; m, motor conduction study; s, sensory conduction study (antidromic). Pathological values are marked in bold.
Fig. 1.
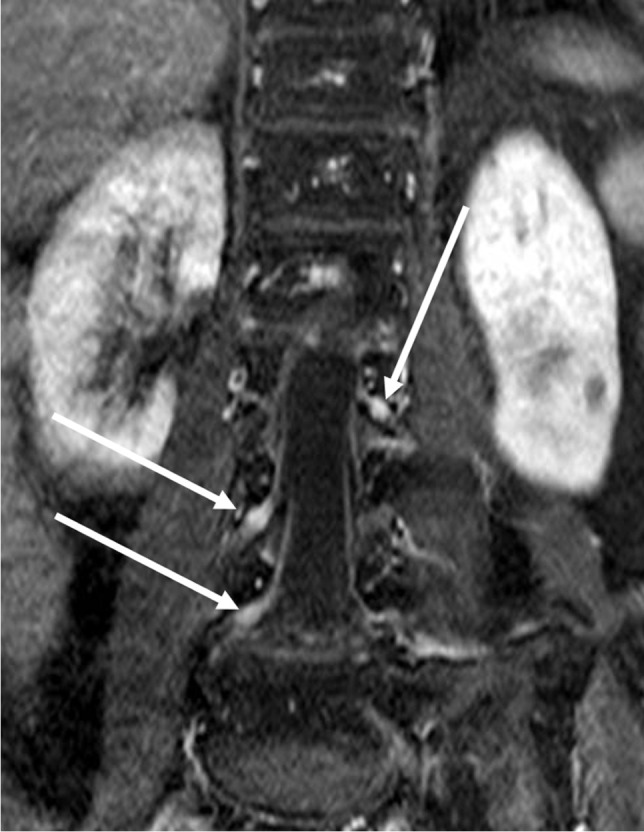
Nerve roots enhancement at spine MRI
The case was notified to the pharmacovigilance unit. Written informed consent for the publication of this case was obtained by the patient.
Comment
CIAP represents an uncommon variant of chronic inflammatory demyelinating polyneuropathy (CIDP) and is mainly characterized by progressive course (at least 2 months), electrophysiological evidence of axonal neuropathy in at least two nerves, and good response to immunotherapy. The presence of a high CSF protein concentration represents a supportive criterion for the diagnosis [5]. To the best of our knowledge, to date, no cases of chronic inflammatory polyneuropathy, neither CIAP nor CIDP, following COVID-19 vaccine have been reported. Although we are aware that the close temporal relationship between vaccination and CIAP development does not necessarily imply a causal relationship, it could be hypothesized that the immune response consequent to vaccination could trigger, in some patients, autoimmune processes leading to acute and/or chronic polyneuropathies. Nevertheless, considering the very low risk of vaccine-associated complications, we strongly encourage and support mass COVID-19 vaccination program.
Data availability
Anonymized data will be available if requested.
Code availability
Not applicable.
Declarations
Ethical approval
Not applicable for case report.
Consent to participate
Not applicable.
Consent for publication
Consent for publication was obtained from the patient.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;31:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waheed S, Bayas A, Hindi F, Rizvi Z, Espinosa PS. Neurological complications of COVID-19: Guillain-Barre syndrome following Pfizer COVID-19 vaccine. Cureus. 2021;13:e13426. doi: 10.7759/cureus.13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trimboli M, Zoleo P, Arabia G, Gambardella A. Guillain-Barré syndrome following BNT162b2 COVID-19 vaccine. Neurol Sci Aug. 2021;4:1–2. doi: 10.1007/s10072-021-05523-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.COVID-19 vaccination status. https://ourworldindata.org/covid-vaccinations. Accessed August 31, 2021.
- 5.Oh SJ, Lu L, Alsharabati M, Morgan MB, King P. Chronic inflammatory axonal polyneuropathy. J Neurol Neurosurg Psychiatry. 2020;91(11):1175–1180. doi: 10.1136/jnnp-2020-323787. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be available if requested.
Not applicable.


