Abstract
Background
An excess risk of Bell's palsy has been suggested after mRNA vaccines. We examined the association between the BNT162b2 mRNA COVID-19 vaccine and Bell's palsy.
Methods
Using the database of the largest healthcare provider in Israel, we retrieved data from different periods in 2018-2021. Observed cases of Bell's palsy occurring within 21-days after the first vaccine dose and within 30-days after the second vaccine dose were compared to the expected cases, based on the experience of the population in 2019. Standardized incidence ratios (SIRs) and attributable risks (ARs) were computed.
Findings
Overall, 132 cases of Bell's palsy were reported in 2,594,990 vaccinees with the first dose, and 152 cases in 2,434,674 vaccinees after the second dose. The age and sex weighted SIRs were 1.36(95% CI, 1.14-1.61) and 1.16(0.99-1.36) after the first and second vaccine dose, respectively. SIRs tended to be higher in older age groups after the first and second vaccine doses. The estimates were more pronounced in older females after the first vaccine dose; SIR=1.71(1.10-2.54) at age 45-64, and 2.51(1.65-3.68) at age ≥65 years. The highest AR was 4.46 per 100,000 vaccinees detected in females aged ≥65 years. In patients with previous history of Bell's palsy, only 4 cases of Bell's palsy were reported in 7,567 vaccinees and 10 cases in 7,045 vaccinees after the first and the second dose, respectively. The age and sex weighted SIRs were 1.15(0.36-2.76) and 2.15(1.09-3.83) after the first and second vaccine dose, respectively.
Interpretation
This study suggests that the BNT162b2 mRNA COVID-19 vaccine might be associated with increased risk of Bell's palsy. The small estimated attributable risks suggest that the impact on public health is relatively minor. The benefits of vaccinations explicitly outweigh the possible link to Bell's palsy that has high recovery rate if timely treated with corticosteroids.
Funding
No external funding was available for this study.
Keywords: adverse events, Bell's palsy, facial paralysis, BNT162b2 mRNA COVID-19 vaccine, COVID-19, mRNA vaccines
Research in context.
Evidence before this study
Safety data from Pfizer-BioNTech and Moderna mRNA COVID-19 vaccine pre-licensure trials revealed a higher number of incident cases of Bell's palsy in the vaccine groups compared to the placebo groups. Yet, the numbers were small and insufficient to conclude about a causal relationship between vaccination and Bell's palsy. After the emergency authorization of these vaccines, there were some case reports and case series describing Bell's palsy onset following vaccine receipt. Current studies which investigate this association are limited. A small single hospital-based case control study from Israel found no association between BNT162b2 vaccine and Bell's palsy. A recently published population-based case control study from Hong Kong found an increased risk of Bell's palsy after CoronaVac vaccine but not after BNT162b2 vaccine.
The literature search was conducted at PubMed. We used the search terms “COVID-19 or COVID or Coronavirus or SARS-CoV-2” AND “vaccine” or “Vaccination” AND “Bell's palsy” or “facial paralysis” or “facial paresis” or “facial palsy”. The search was updated till July 25, 2021. No limitation applied to the language of publications.
Added value of this study
To our knowledge, this is the first large population-based study examining the association between BNT162b2 mRNA COVID-19 vaccine and Bell's palsy and providing estimates by age, sex and history of Bell's palsy. Our findings suggest that BNT162b2 mRNA COVID-19 vaccine is associated with increased risk of Bell's palsy. The association appears to be more pronounced in older females after the first vaccine dose. Nonetheless, the magnitude of the estimated risk that might be attributed to vaccine receipt appear to be small.
Implication of all the available evidence
Although the current study reveals an association between BNT162b2 mRNA COVID-19 vaccine and Bell's palsy, from a public health perspective and assuming a causal relationship, our findings are reassuring and show that the number of Bell's palsy cases which can be attributed to the BNT162b2 mRNA COVID-19 vaccine among vaccinees is relatively small.
Alt-text: Unlabelled box
Introduction
On March 11, 2020, the World Health Organization (WHO) has declared novel Coronavirus disease 2019 (COVID-19) outbreak a global pandemic [1]. To defuse the pandemic, extraordinary efforts were made to develop vaccines against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), the causative agent of COVID-19. The Pfizer-BioNTech BNT162b2 mRNA vaccine was the first COVID-19 vaccine to receive emergency use authorization by the US Food and Drug Administration (FDA) and the European Commission on December, 2020. Shortly thereafter, the mRNA-1273 vaccine by Moderna was also authorized [2], [3], [4].
The safety databases from both mRNA vaccine trials revealed an imbalance between the vaccine and placebo arms of incident cases of Bell's palsy which is defined as spontaneous idiopathic facial paralysis. Seven cases of Bell's palsy were reported among participants in the vaccine groups (four from Pfizer-BioNTech trial and three from Moderna trial) compared with one case among participants in the placebo groups (none from Pfizer-BioNTech trial and one from Moderna trial) [5,6]. The resulting rate ratio of 7.0 (P = 0.07) may indicate that vaccination is associated with Bell's palsy. Furthermore, the observed incidence rate of Bell's palsy following vaccination in these trials was 3.5- 7 times higher than the reported background incidence rate of Bell's palsy in the general population which is estimated at 15 to 30 cases per 100 000 person-years [7]. Yet, these data were in fact re-elaborated, and estimates cut down by a subsequent letter [8]. Due to the small numbers of cases derived from the safety data of phase 3 trials it was insufficient to conclude that the Bell's palsy cases were causally associated to the vaccine receipt. A post authorization safety monitoring for Bell's palsy following vaccination was recommended in order to evaluate any possible causal relationship [5,6].
As yet, published studies which investigate the association between Bell's palsy and mRNA COVID 19 vaccines are limited. A recent safety analysis using the WHO pharmacovigilance database indicates that the reporting rate of facial paralysis after mRNA COVID-19 vaccination is not higher than the observed rate with influenza vaccines and other viral vaccines [9]. However, this does not mean that there is no association, and the analysis does not provide a risk estimate because the population exposed to the vaccine is unknown. A hospital-based case control study from Israel found no association between BNT162b2 vaccine and Bell's palsy [10]. A recently published population-based case control study from Hong Kong found an increased risk of Bell's palsy after CoronaVac vaccine but not after BNT162b2 vaccine [11].
In view of the given data, it is crucial to evaluate the safety of mRNA COVID-19 vaccines in the context of Bell's palsy by further studies including population based analyses.
Israel set to be the first country to initiate a nationwide mass vaccination program against COVID-19 using the BNT162b2 mRNA vaccine. As of 30 April, 2021, a total of 5,405,152 had received at least one dose of the vaccine [12]. In the current study we take advantage of the electronic data bases of the largest health organization in Israel to assess the association between vaccination with the BNT162b2 mRNA COVID-19 vaccine and Bell's palsy.
Methods
Source of data
This study is based on data from the computerized database of Clalit Health Services (CHS) which provides inclusive health care for more than half of the Israeli population (∼4.7 million). Health care coverage in Israel is mandatory according to the National Health Insurance Law (1995) and is provided by four groups akin to not-for-profit health maintenance organizations (HMOs), which are charged with providing a broad package of benefits stipulated by the government. The four HMOs are both a health-care insurers and a providers, thus financing and supplying health services. Membership in a specific HMO is voluntary and members can freely switch to another HMO. All members of the different HMOs have a similar health insurance plan and similar access to health services, including low medications copayment. CHS maintains a database that receives data from multiple sources including records of primary care physicians, community specialty clinics, hospitalizations, laboratories, and pharmacies. Designed for purposes of administrative and clinical management, the database is available for clinical studies. Several high quality population-based studies have been conducted based on data retrieved from this database [13,14].
Study design
We performed a retrospective cohort study with nonconcurrent historic comparative group. In this approach the observed cases of Bell's palsy appearing after vaccination were compared to the expected cases of Bell's palsy as estimated based on the experience of the CHS population in 2019 prior to COVID-19 epidemic and vaccine introduction.
Study population
To estimate the observed cases of Bell's palsy after the first vaccine dose, we identified all CHS members aged ≥16 years who received the first dose of the vaccine starting from 20 December 2020, the start date of the mass COVID-19 vaccination campaign in Israel, till 30 April 2021. Identified subjects constituted the population for the estimation of the standardized incidence ratio (SIR) of Bell's palsy after the first vaccine dose. Among them, those who received the second vaccine dose by 30 April 2021 constituted the population for the estimation of SIR after the second vaccine dose.
Study variables
Identification of patients with Bell's palsy was based on the search of ICD-9 code (351.0) from multiple sources, including discharge diagnosis, emergency department, community specialty clinics, and primary physician visits. Identified cases from these sources were cross-linked with the medication prescription computerized database, and only patients who filled a prescription of prednisone within two weeks after diagnosis were included. To evaluate the validity of this algorithm the first author reviewed the medical files of 165 patients, selected at random (using SPSS) from a list of Bell's palsy patients identified with this algorithm from the database. The diagnosis of Bell's palsy relied on the consistency of clinical notes with Bell's palsy as judged by the attending physician and the absence of alternative diagnosis. Of the 165 reviewed medical files, eight patients had facial palsy from other reasons and 157 had documentation consistent with Bell's palsy, yielding a positive predictive value (PPV) of 95.1%. A patient was considered to have previous history of Bell's palsy if we found a documentation of Bell's palsy diagnosis in the previous five years in conjunction with purchasing prednisone, like the algorithm we used for incident cases. For vaccinated subjects the five years refers to the period before vaccination date, and for the reference population in 2019 the five years refers to 2014-2018.
Data on the receipt of BNT162b2 mRNA COVID-19 vaccine were retrieved from CHS database. This data are considered complete because since the start of the COVID-19 pandemic, the Israeli Ministry of Health (MOH) has been collecting all COVID-19 related data and activities including vaccination status to a national database. The collected data are transferred daily to the healthcare providers.
Statistical methods
The observed number of cases of Bell's palsy occurring within 21 days after the first vaccine dose and within 30 days after the second vaccine dose were each compared to the expected cases, based on estimation from historic data. Observed cases after the first vaccine dose were assessed in those who received the first dose between 20 December 2020 and 30 April 2021, and the observed cases after the second vaccine dose were assessed in those who received the second dose between 10 January 2021 and 30 April 2021. Both cohorts were retrospectively followed through 31 May 2021 for Bell's palsy ascertainment. The expected incidence rate of Bell's palsy was estimated based on the experience of the CHS population in 2019 during the same period (January-May). These rates were applied to estimate the number of Bell's palsy cases that were expected to occur within 21 days and within 30 days after the first and second vaccine dose, respectively. Standardized incidence ratios (SIRs) were computed by dividing the observed by the expected number of Bell's palsy cases for each vaccine dose, for each sex, for age groups 16-44, 45-64, and ≥65 years as well as for the total population (weighted for sex and age). The 95% confidence intervals for the standardized incidence ratios (SIRs) were computed using the Mid-P exact test (OpenEpi, Version 3). We calculated the attributable risk fraction (ARF) among vaccinated as (SIR-1)/SIR and computed attributable risks (ARs) for 100,000 vaccine doses by multiplying the risk after each vaccine dose by ARF.
We performed several sensitivity analyses; firstly, we computed SIR for follow-up of over 21 days up to 60 days after the first vaccine dose. This analysis relied on data from 132,014 who have not developed Bell's palsy within 21 days after the first vaccine dose and have not received the second vaccine dose during follow-up. Secondly, we computed SIR for follow-up of 60 days after the second vaccine dose instead of the 30 days used in the main analysis. This analysis relied on data from 2,310,675 subjects who received the second vaccine dose by 31 March 2021. We performed another sensitivity analysis in which the expected rates were estimated based on the population experience in 2018.
All analysis were performed and presented separately for subjects without a previous history of Bell's palsy (main analysis) and for subjects with a previous history of Bell's palsy (secondary analysis). However, because of the small number of observed cases of Bell's palsy in vaccinated subjects with previous history of Bell's palsy, only age and sex weighted SIRs are presented. To calculate the SIRs for the first and second vaccine dose among subjects with previous history of Bell's palsy we used as reference the 2019 population with previous history of Bell's palsy.
Ethics
The study was approved by the Review Board of the Lady Davis Medical Centre and conducted in accordance with the Declaration of Helsinki.
Role of the funding source
There was no external funding for this study.
Results
Subjects without previous history of Bell's palsy
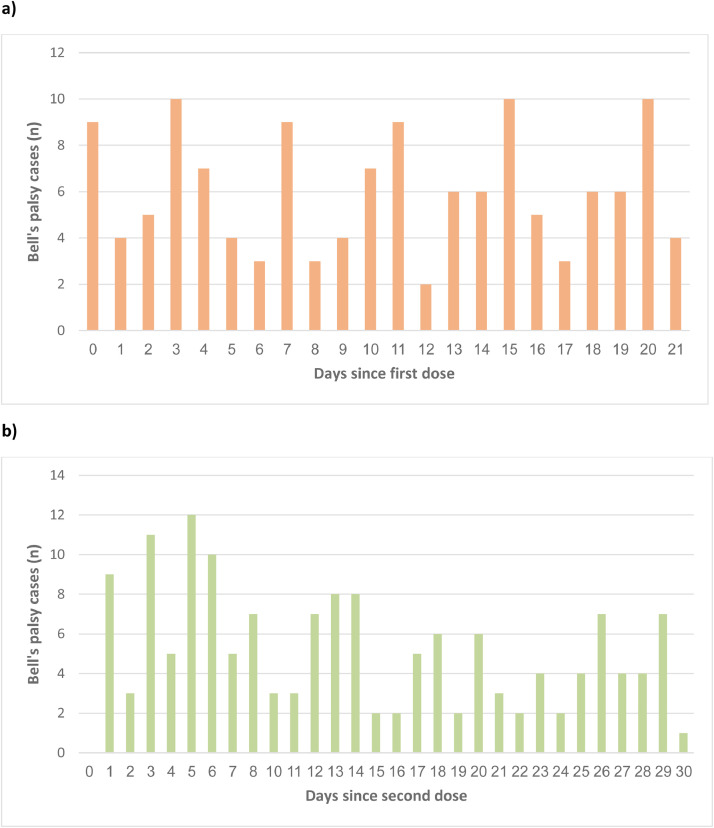
Overall, 2,594,990 CHS members with mean age 46.8 ± 19.6 years (51.6% females), without prior history of Bell's palsy, received the first dose of BNT162b2 mRNA COVID-19 vaccine between 20 December 2020 and 30 April 2021. Of them 2,434,674 received the second vaccine dose between 10 January 2021 and 30 April 2021. Bell's palsy was reported in 132 patients within 21 days after the first vaccine dose and in 152 patients within 30 days after the second vaccine dose, reflecting a 21-day risk of 5.09 per 100,000 and 30-day risk of 6.24 per 100,000, respectively (Tables 1 and 2). Of the 132 patients with Bell's palsy within 21 days after receiving the first dose 37 (28.0%) have not received the second dose during follow-up, compared to 5.1% who have not received the second dose in those without Bell's palsy. The distribution of observed Bell's palsy cases by time (days) since the receipt of each vaccine dose is depicted in Figure 1. Person times at risk and incidence rates, for the vaccinees and for the reference population in 2019 (without previous Bell's palsy) are shown in Table 3.
Table 1.
Standardized incidence ratio (SIR), attributable risk fraction (ARF), and attributable risk (AR) for the first vaccine dose, stratified by sex and age groups.
| Sex and age group (years) | Vaccinated with the first dose1(n) | Observed cases2(n) | Risk per 100,000 vaccinees3 | Expected cases (2019 reference)4(n) | SIR5 (95% CI) | ARF6 | AR per 100,000 vaccinees7 |
|---|---|---|---|---|---|---|---|
| All8 | 2594990 | 132 | 5.09 | 97.10 | 1.36 (1.14-1.61) | 0.26 | 1.35 |
| Males(n=1,256,958) | |||||||
| 16-44 | 654040 | 22 | 3.36 | 20.81 | 1.06 (0.68-1.57) | 0.06 | 0.19 |
| 45-64 | 339849 | 23 | 6.77 | 21.44 | 1.07 (0.70-1.58) | 0.07 | 0.44 |
| 65+ | 263069 | 17 | 6.46 | 12.72 | 1.34 (0.80-2.10) | 0.25 | 1.64 |
| Females(n=1,338,032) | |||||||
| 16-44 | 659597 | 24 | 3.64 | 19.67 | 1.22 (0.80-1.79) | 0.18 | 0.66 |
| 45-64 | 354617 | 22 | 6.20 | 12.89 | 1.71 (1.10-2.54) | 0.42 | 2.58 |
| 65+ | 323818 | 24 | 7.41 | 9.56 | 2.51 (1.65-3.68) | 0.60 | 4.46 |
Number of those who received the first vaccine dose between 20 December 2020 and 30 April 2021
Number of observed cases of Bell's palsy that occurred within 21 days after the receipt of the first dose
The risk of Bell's palsy within 21 days after the first vaccine dose, estimated by dividing the number of observed cases by the number of vaccinees and reported per 100,000 vaccinees
Expected number of cases of Bell's palsy as estimated based on the experience of CHS population between January-May 2019
Standardized incidence ratio (SIR), computed by dividing the observed cases by the expected cases of Bell's palsy.
Attributable risk fraction (ARF) among exposed (vaccinated), calculated as (SIR-1)/SIR
Attributable risk (AR) per 100,000 vaccinees, estimated by multiplying ARF by the risk of Bell's palsy among vaccinated.
Age and sex weighted
Table 2.
Standardized incidence ratio (SIR), attributable risk fraction (ARF), and attributable risk (AR) for the second vaccine dose, stratified by sex and age groups.
| Sex and age group (years) | Vaccinated with the second dose1(n) | Observed cases2(n) | Risk per 100,000 vaccinees3 | Expected cases (2019 reference)4(n) | SIR5 (95% CI) | ARF6 | AR per 100,000 vaccinees7 |
|---|---|---|---|---|---|---|---|
| All8 | 2434674 | 152 | 6.24 | 130.49 | 1.16 (0.99-1.36) | 0.14 | 0.86 |
| Males(n=1,178,077) | |||||||
| 16-44 | 602455 | 36 | 5.98 | 27.39 | 1.13 (0.93-1.80) | 0.12 | 0.69 |
| 45-64 | 321982 | 29 | 9.01 | 29.02 | 1.00 (0.68-1.42) | 0.00 | 0.00 |
| 65+ | 253640 | 26 | 10.25 | 17.53 | 1.48 (0.99-2.14) | 0.32 | 3.32 |
| Females(n=1,256,597) | |||||||
| 16-44 | 609135 | 21 | 3.45 | 25.96 | 0.81 (0.51-1.21) | -0.23 | -0.81 |
| 45-64 | 335129 | 22 | 6.56 | 17.40 | 1.26 (0.81-1.88) | 0.21 | 1.35 |
| 65+ | 312333 | 18 | 5.76 | 13.18 | 1.37 (0.83-2.12) | 0.27 | 1.56 |
Number of those who received the second vaccine dose between 10 January 2021 and 30 April 2021.
Number of observed cases of Bell's palsy that occurred within 30 days after the receipt of the second dose.
The risk of Bell's palsy within 30 days after the second vaccine dose, estimated by dividing the number of observed cases by the number of vaccinees and reported per 100,000 vaccinees.
Expected number of cases of Bell's palsy as estimated based on the experience of CHS population between January-May 2019.
Standardized incidence ratio (SIR), computed by dividing the observed cases by the expected cases of Bell's palsy.
Attributable risk fraction (ARF) among exposed (vaccinated), calculated as (SIR-1)/SIR.
Attributable risk (AR) per 100,000 vaccinees, estimated by multiplying ARF by the risk of Bell's palsy among vaccinated.
Age and sex weighted.
Figure 1.
Epidemic curves depicting the distribution of Bell's palsy cases by time (days) since each vaccine dose receipt, a) within 21 days after the first vaccine dose, and b) within 30 days after the second vaccine dose.
Table 3.
Incidence rates of Bell's palsy after the first and second vaccine doses and in 2019 (the reference population)
| Sex and age group (years) | 2019 reference data (January 1 to May 31) |
First vaccine dose data1 (within 21 days after) |
Second vaccine dose data2 (within 30 days after) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Person years | Bell's palsy number | Incidence Rate (per 100,000 P-Y) | Person years | Bell's palsy number | Incidence Rate (per 100,000 P-Y) | Person years | Bell's palsy number | Incidence Rate (per 100,000 P-Y) | |
| All | |||||||||
| 16-44 | 720499 | 386 | 53.57 | 75561 | 46 | 60.88 | 99580 | 57 | 57.24 |
| 45-64 | 330499 | 283 | 85.63 | 39944 | 45 | 112.66 | 54007 | 51 | 94.43 |
| 65+ | 260218 | 171 | 65.71 | 33757 | 41 | 121.46 | 46516 | 44 | 94.59 |
| Total | 1311217 | 840 | 64.06 | 149262 | 132 | 88.43 | 200103 | 152 | 75.96 |
| Males | |||||||||
| 16-44 | 357923 | 198 | 55.32 | 37620 | 22 | 58.48 | 49515 | 36 | 72.70 |
| 45-64 | 159559 | 175 | 109.68 | 19547 | 23 | 117.67 | 26463 | 29 | 109.59 |
| 65+ | 114163 | 96 | 84.09 | 15131 | 17 | 112.35 | 20846 | 26 | 124.72 |
| Total | 631646 | 469 | 74.25 | 72297 | 62 | 85.76 | 96824 | 91 | 93.98 |
| Females | |||||||||
| 16-44 | 362576 | 188 | 51.85 | 37941 | 24 | 63.26 | 50065 | 21 | 41.95 |
| 45-64 | 170940 | 108 | 63.18 | 20398 | 22 | 107.85 | 27544 | 22 | 79.87 |
| 65+ | 146055 | 75 | 51.35 | 18626 | 24 | 128.85 | 25670 | 18 | 70.12 |
| Total | 679570 | 371 | 54.59 | 76965 | 70 | 90.95 | 103279 | 61 | 59.06 |
includes subjects who received the first vaccine dose between 20 December 2020 and 30 April 2021.
includes subjects who received the second vaccine dose between 10 January 2021 and 30 April 2021.
Using the experience of the population in 2019 as reference, the age and sex weighted SIRs were 1.36 (95% CI, 1.14-1.61) for the first vaccine dose and 1.16 (95% CI, 0.99-1.36) for the second dose (Tables 1 and 2). Stratified analysis by sex and age groups showed that the magnitude of the SIRs tends to be higher in older age groups both in females and males after the first and second vaccine doses. In females the most pronounced estimates were detected after the first vaccine dose; SIR 1.71 (95% CI, 1.10-2.54) at age 45-64 years, and 2.51 (95% CI, 1.65-3.68) at age ≥65 years (Table 1). In males the most pronounced result was detected after the second dose in subjects aged ≥65 years; SIR 1.48 (95% CI, 0.99-2.14) (Table 2). The attributable risks (ARs) were generally small with the highest AR of 4.46 per 100,000 vaccinees detected in females aged ≥65 years (Table 1). The pattern of the association was very similar especially after the first vaccine dose when we repeated the analysis by using the experience of the population in 2018 as reference (Table 1S and 2S).
A total of 132,014 subjects who have not received the second vaccine dose were included in the assessment of the association with follow-up of over 21 days up to 60 days after the first vaccine dose. Only 15 of them developed Bell's palsy during follow-up, yielding an age and sex weighted SIR of 1.78 (95% CI, 1.03-2.87) which is in line with the results for 21 days follow-up. A total of 273 cases of Bell's palsy were detected in 2,310,675 subjects included in a sensitivity analysis in which we extended the follow-up for 60 days after the second vaccine dose. The results were very similar to the results for 30 days follow-up with age and sex weighted SIR of 1.10 (95% CI, 0.97-1.23).
Subjects with previous history of Bell's palsy
Overall, 7,567 adults with mean age 50.2 ± 18.7 years (44.8% females), with a prior history of Bell's palsy, received the first dose of BNT162b2 mRNA COVID-19 vaccine. Of them 7,045 received the second vaccine dose. Bell's palsy was reported in 4 patients within 21 days after the first vaccine dose and in 10 patients within 30 days after the second vaccine dose, reflecting a 21-day risk of 52.86 per 100,000 and 30-day risk of 141.94 per 100,000, respectively (Table 4). The 2019 reference population with previous history of Bell's palsy included 8,784 patients. Of them 29 patients had recurrent Bell's palsy during follow-up reflecting an incidence rate of 803 per 100,000 person-years as compared to incidence rate of 919 per 100,0000 person-years after the first vaccine dose, and 1,727 per 100,000 person years after the second vaccine dose. Using the experience of the population (with previous Bell's palsy) in 2019 as reference, the age and sex weighted SIRs were 1.15 (95% CI, 0.36-2.76) for the first vaccine dose and 2.15 (95% CI, 1.09-3.83) for the second dose (Tables 4). In these patients the attributable risk (ARs) after the second dose was 75.95 per 100,000 vaccinees (Table 4). The pattern of the association after the second vaccine dose was similar but less marked when we repeated the analysis by using the experience of the population in 2018 as reference (Table 3S).
Table 4.
Age and sex weighted standardized incidence ratio (SIR), attributable risk fraction (ARF), and attributable risk (AR) for the first and second vaccine dose among subjects with a previous history of Bell's palsy (2019 reference)
| Number of vaccinated 1(n) | Observed cases2(n) | Risk per 100,000 vaccinees3 | Expected cases (2019 reference)4(n) | SIR5 (95% CI) | ARF6 | AR per 100,000 vaccinees7 | |
|---|---|---|---|---|---|---|---|
| First vaccine dose | 7567 | 4 | 52.86 | 3.51 | 1.15 (0.36-2.76) | 0.13 | 6.70 |
| Second vaccine dose | 7045 | 10 | 141.94 | 4.67 | 2.15 (1.09-3.83) | 0.54 | 75.95 |
Number of those who received the first vaccine dose, between 20 December 2020 and 30 April 2021, and the second vaccine dose between 10 January 2021 and 30 April 2021, and had a previous history of Bell's palsy
Number of observed cases of Bell's palsy that occurred within 21 days and 30 days after the receipt of the first and second vaccine dose, respectively.
The risk of Bell's palsy within 21 days after the first vaccine dose and 30 days after the second vaccine dose, estimated by dividing the number of observed cases by the number of vaccinees and reported per 100,000 vaccinees
Expected number of cases of Bell's palsy as estimated based on the experience of CHS population between January-May 2019. For this purpose, we used as reference the 2019 population with a previous history of Bell's palsy.
Standardized incidence ratio (SIR), computed by dividing the observed cases by the expected cases of Bell's palsy.
Attributable risk fraction (ARF) among exposed (vaccinated), calculated as (SIR-1)/SIR
Attributable risk (AR) per 100,000 vaccinees, estimated by multiplying ARF by the risk of Bell's palsy among vaccinated.
Discussion
A safety concern linking between mRNA COVID-19 vaccines and the onset of Bell's palsy has been raised when data from mRNA COVID-19 pre licensure trials signaled an imbalance in Bell's palsy occurrence between the vaccine and the placebo arms [5,6]. The association of Bell's palsy is not specific for COVID-19 vaccines and has been reported with other vaccines such as the intranasal seasonal influenza vaccine used in Switzerland during 2000-2001 [15].
Our observed-to-expected analysis refines the reported imbalance in mRNA COVID-19 pre licensure trials and suggests an association between the BNT162b2 mRNA COVID-19 vaccine and Bell's palsy. According to our findings, the overall observed rate of Bell's palsy after vaccination was higher than the expected rates. However, when looking at different strata of age groups, gender and vaccine dose it is notable that the existence and the strength of the association vary. The most marked association was observed among women ≥ 65 years within a time interval of 21 days after receipt of the first vaccine dose, however these numbers were small. In patients with a previous history of Bell's palsy, the association appears to be more pronounced after the second vaccine dose. We have no explanation for why the risk varies between people with and without a previous history of Bell's palsy in relation to the order of the vaccine dose. We are aware that the number of patients with recurrent Bell's palsy is small and therefore our estimates may be unstable. Yet, to the best of our knowledge, this study is the first to evaluate this association in patients with history of Bell's palsy. Further studies are needed to clarify this association.
Bell's palsy reported in the clinical trial were presumably more often reported after the second dose (2 of the 4 cases in Pfizer trial and all 3 cases in Moderna trials) [5,6]. Whereas in a case series from Israel 6 of the 9 described cases of Bell's palsy were reported after the first vaccine dose [16], a clue that might suggests a higher risk of Bell's palsy after the first vaccine dose consistent with our finding.
Sato and colleagues investigated the association between COVID-19 mRNA vaccines (BNT162b2 and mRNA-1273) and Bell's palsy, using large self-reporting data from the United States (Vaccine Adverse Event Reporting System [VAERS]). They observed significantly high reporting of Bell's palsy with reporting OR of 1.84 (95% CI, 1.65-2.06) and 1.54 (95% CI, 1.39-1.70) following the administration of BNT162b2 and mRNA-1273 vaccines, respectively [17]. These findings are in line with our results. However, due to the nature of self-reporting, this approach has several limitation. A recently published case control study examined the association between Bell's palsy and BNT162b2 mRNA COVID-19 vaccination based on a comparison between new Bell's palsy cases admitted to an emergency department at a single hospital in Israel, and controls admitted for other reasons during January and February, 2021. BNT162b2 mRNA COVID-19 vaccination was defined as receipt of either the first or the second vaccine dose 30 days prior to Bell's palsy onset. Unlike our findings, no significant association was observed between Bell's palsy and recent receipt of BNT162b2 mRNA COVID-19 vaccine [10]. A population-based nested case control study that included 298 cases of Bell's palsy and 1,181 control, showed an overall increased risk of Bell's palsy after CoronaVac vaccine but not after BNT162b2. However, in a post-hoc analysis, subjects who completed the first and second BNT162b2 vaccine dose were significantly at higher risk for Bell's palsy, compared to not vaccinated (OR 3.16, 95% CI 1.24-8.04) [11]. It should be noted, however, that our study is based on large-scale set of population data and therefore has more power to identify smaller differences between subgroups and after each single vaccine dose.
Although our results reveal a significant association between BNT162b2 mRNA COVID-19 vaccine and Bell's palsy as reflected by the relative differences between the observed and the expected rates, a reference to this result alone may be misleading. From a public health perspective and assuming a causal relationship, our findings show that the number of Bell's palsy cases which can be attributed to the vaccine among vaccine recipients without prior history of Bell's palsy is small. Even in elderly females where the strongest association was observed within 21 days after the first vaccine dose, the excess risk of Bell's palsy was estimated as ∼ 4.5 cases per 100 000 vaccinees. From our point of view, the benefits of vaccinations against COVID-19 explicitly outweigh the possible link to Bell's palsy which is in any case uncommon and with good prognosis with a recovery rate reaching ∼90% within 9 months, if timely treated with corticosteroids [18]. It should be emphasized that the prognosis of COVID-19 vaccine related Bell's palsy remains unknown and needs continued monitoring. Our speculations are further supported by a preliminary analysis from the TriNetX, a global federated research network, which suggests a higher risk pf Bell's palsy in patients with COVID-19 compared to those who were vaccinated [19]. A special attention may be given to people with a previous history of Bell's palsy. In this group, our estimated excess risk of Bell's palsy was ∼ 76 per 100 000 vaccinees.
Our study has some limitations. As with any retrospective cohort study that is based on data from administrative databases, a possible limitation may be related to the quality of the data. Yet, information about the receipt of BNT162b2 mRNA COVID-19 vaccine is considered to be complete because these data are prospectively collected by the Israeli Ministry of Health. Moreover, in order to increase the specificity of acute Bell's palsy case identification, our algorithm relied on ICD-9 codes for Bell's palsy combined with prescription of steroids yielding a high PPV of ∼95%. Although this algorithm might reduce sensitivity of detecting Bell's palsy any misclassification related to this algorithm is likely nondifferential. Another potential limitation is the possibility of detection bias due to differences between vaccinated and unvaccinated patients in terms of seeking medical care after Bell's palsy. However, the likelihood that a patient with an episode of facial paralysis is not seen by a physician, regardless of the vaccination status, is small and thus we assume that the influence of this bias is minimal. Although our large sample size has allowed us to conduct stratified analysis by age and sex and compute weighted SIRs, residual confounding remains a major concern of the current study as we have not controlled for other potential confounders such obesity, hypertension and diabetes mellitus which are known risk factors for Bell's palsy and might differ between subjects voluntary vaccinated and the general population. In addition, by limiting the search of previous Bell's palsy to the last five years we might have missed a large number of patients with prior history of Bell's palsy. Another limitation of our study is that SIRs might be unstable estimates, particularly when the observed case and/or the denominator (expected case) are small. Our study was conducted only in Israel, which may impair the generalizability of its results to other populations. Finally, this was a retrospective cohort study with non-concurrent historic comparative group, the lack of a concurrent control group might introduce bias due to secular trends and changes of treatment and in diagnostic criteria over time. However, we relied on the most recent years immediately before COVID- 19 pandemic, and therefore no major temporal differences are expected.
In summary, our findings suggest that the mRNA COVID-19 vaccine might be associated with increased risk of Bell's palsy, and that this association appears to be more pronounced in older females after the first vaccine dose. The small estimated attributable risks suggest that the impact on public health is minor. More studies are needed to examine this association especially in patients with previous history of Bell's palsy.
Contributors
RS, OB and WS conceived the study, conducted the analysis, and edited the final manuscript. All authors contributed to study design, revising the manuscript for important intellectual content, were responsible for the decision to submit for publication, and approved the final submitted version of the manuscript. All authors had full access to the deidentified and aggregated data in the study. RS, OB, and WS accessed and verified the data underlying the study and take responsibility for the data.
Data availability statement
Individual level data used in this study are sensitive and cannot be shared. Aggregated data will be provided upon request through contacting the corresponding author.
Declaration of Interests
All authors declare that there is no conflict of interest
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lanepe.2021.100236.
Appendix. Supplementary materials
References
- 1.https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—11-march-2020 (accessed July, 1 2021).
- 2.https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine (accessed July, 1 2021).
- 3.https://ec.europa.eu/info/live-work-travel-eu/coronavirus-response/public-health/eu-vaccines-strategy_en#authorised-vaccines (accessed July, 1 2021).
- 4.https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/moderna-covid-19-vaccine (accessed July, 1 2021).
- 5.Vaccines and Related Biological Products Advisory Committee meeting. December 10, 2020. https://www.fda.gov/media/144245/download FDA briefing document. Pfizer BioNTech COVID-19 vaccine. (accessed July, 1 2021) [DOI] [PubMed] [Google Scholar]
- 6.Vaccines and Related Biological Products Advisory Committee meeting. December 17, 2020. https://www.fda.gov/media/144434/download FDA briefing document. Moderna COVID-19 vaccine. (accessed July, 1 2021) [DOI] [PubMed] [Google Scholar]
- 7.Ozonoff A, Nanishi E, Levy O. Bell's palsy and SARS-CoV-2 vaccines. Lancet Infect Dis. 2021;21:450–452. doi: 10.1016/S1473-3099(21)00076-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cirillo N, Doan R. Bell's palsy and SARS-CoV-2 vaccines-an unfolding story. Lancet Infect Dis. 2021;21:1210–1211. doi: 10.1016/S1473-3099(21)00273-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Renoud L, Khouri C, Revol B. Association of facial paralysis with mRNA COVID-19 Vaccines: a disproportionality analysis using the World Health Organization pharmacovigilance database. JAMA Intern Med. 2021;27 doi: 10.1001/jamainternmed.2021.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shemer A, Pras E, Einan-Lifshitz A, Dubinsky-Pertzov B, Hecht I. Association of COVID-19 vaccination and facial nerve palsy: a case-control Study. JAMA Otolaryngol Head Neck Surg. 2021 doi: 10.1001/jamaoto.2021.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wan EYF, Chui CSL, Lai FTT. Bell's palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and nested case-control study. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00451−5. S1473−3099(21)00451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Public Health Services, Israel Ministry of Health. A report about the side effects following COVID 19 vaccine. April 30, 2021. https://www.gov.il/BlobFolder/reports/vaccine-efficacy-safety-follow-up-committee/he/files_publications_corona_side-effects-after-vaccination-30042021.pdf (accessed July, 1 2021).
- 13.Saliba W, Barnett-Griness O, Gronich N. Association of diabetes and glycated hemoglobin with the risk of intracerebral hemorrhage: a population-based cohort study. Diabetes care. 2019;42:682–688. doi: 10.2337/dc18-2472. [DOI] [PubMed] [Google Scholar]
- 14.Dagan N, Barda N, Kepten E. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mutsch M, Zhou W, Rhodes P. Use of the inactivated intranasal influenza vaccine and the risk of Bell's palsy in Switzerland. N Engl J Med. 2004;350:896–903. doi: 10.1056/NEJMoa030595. [DOI] [PubMed] [Google Scholar]
- 16.Shemer A, Pras E, Hecht I. Peripheral facial nerve palsy following BNT162b2 (COVID-19) vaccination. Isr Med Assoc J. 2021;23:143–144. [PubMed] [Google Scholar]
- 17.Sato K, Mano T, Niimi Y, Toda T, Iwata A, Iwatsubo T. Facial nerve palsy following the administration of COVID-19 mRNA vaccines: analysis of self-reporting database. Int J Infect Dis. 2021;4 doi: 10.1016/j.ijid.2021.08.071. S1201-9712(21)00704-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sullivan FM, Swan IR, Donnan PT. Early treatment with prednisolone or acyclovir in Bell's palsy. N Engl J Med. 2007;357:1598–1607. doi: 10.1056/NEJMoa072006. [DOI] [PubMed] [Google Scholar]
- 19.Tamaki A, Cabrera CI, Li S. Incidence of Bell's palsy in patients with COVID-19. JAMA Otolaryngol Head Neck Surg. 2021 doi: 10.1001/jamaoto.2021.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Individual level data used in this study are sensitive and cannot be shared. Aggregated data will be provided upon request through contacting the corresponding author.