Abstract
Zeolites, as efficient and stable catalysts, are widely used in the environmental catalysis field. Typically, Cu-SSZ-13 with small-pore structure shows excellent catalytic activity for selective catalytic reduction of NOx with ammonia (NH3-SCR) as well as high hydrothermal stability. This review summarizes major advances in Cu-SSZ-13 applied to the NH3-SCR reaction, including the state of copper species, standard and fast SCR reaction mechanism, hydrothermal deactivation mechanism, poisoning resistance and synthetic methodology. The review gives a valuable summary of new insights into the matching between SCR catalyst design principles and the characteristics of Cu2+-exchanged zeolitic catalysts, highlighting the significant opportunity presented by zeolite-based catalysts. Principles for designing zeolites with excellent NH3-SCR performance and hydrothermal stability are proposed. On the basis of these principles, more hydrothermally stable Cu-AEI and Cu-LTA zeolites are elaborated as well as other alternative zeolites applied to NH3-SCR. Finally, we call attention to the challenges facing Cu-based small-pore zeolites that still need to be addressed.
Keywords: environmental catalysis, Cu-SSZ-13, NH3-SCR, reaction mechanism, small-pore zeolites
This review article summarizes the major advances, opportunities and challenges of Cu-based small-pore zeolites applied to environmental catalysis, which provides guidance for the design of highly efficient and stable zeolite-based catalysts.
INTRODUCTION
General introduction to zeolite catalysts, NOx pollution and SCR technology
Zeolites are a group of crystalline inorganic materials with regular pore structures that consist of connected TO4 tetrahedra (T represents the framework atom) sharing oxygen atoms. Generally, the zeolites can be divided into small-pore zeolites with 8-membered rings (pore size of ∼4.0 Å), medium-pore zeolites with 10-membered rings (pore size of ∼5.5 Å), large-pore zeolites with 12-membered rings (pore size of ∼7.5 Å) and ultra-large-pore zeolites with >12-membered rings. The pore size of zeolites is similar to molecular sizes, which endows the zeolites with a molecular sieving effect, resulting in excellent shape selectivity. Their inner channel and cavity space give zeolites huge specific surface areas. Zeolite materials also have high hydrothermal stability due to their highly crystalline framework structure. Moreover, the abundant acid sites of zeolites can be easily adjusted by ion-exchange methods to satisfy the conditions of various chemical reactions. Based on these advantages, therefore, zeolites are widely used in the petrochemical, energy and environmental fields as efficient and stable catalysts [1–3]. An atomic-scale understanding of zeolite applications in different fields is of guiding significance to the design and synthesis of zeolite catalysts. The present work focuses on the field of environmental catalysis, in which zeolite catalysts play an indispensable role.
In the environmental field, nitrogen oxides (NOx, including NO and NO2) are important precursor pollutants for haze, photochemical smog and acid rain. In China, transportation contributes ∼35% of NOx emissions, second only to industry (∼40%) as an emissions source [4]. Diesel vehicles, which account for about 10% of automobiles, produce nearly 90% of NOx (total ∼6.4 million tonnes) emitted from automobiles [5]. In addition, non-road mobile sources, which primarily use diesel engines as a power source and include engineering machinery, farming machinery and marine engines, contributed comparable NOx emissions to those of road automobiles in China. Therefore, controlling the NOx pollutants emitted from diesel engines is urgent and imperative. The SuperTruck Program and Horizon 2020 were launched by the United States and the European Union to increase engine efficiency and reduce pollutants. China will also implement the China VI Standards for emissions from diesel-fuelled heavy-duty vehicles. In the aftertreatment system, the three-way catalyst (TWC) has difficulty controlling NOx emissions due to the lean combustion conditions in diesel engines. For controlling diesel vehicle exhaust, the selective catalytic reduction of NOx with ammonia (NH3-SCR) has been successfully and commercially applied because of its high NOx efficiency in the presence of excess oxygen.
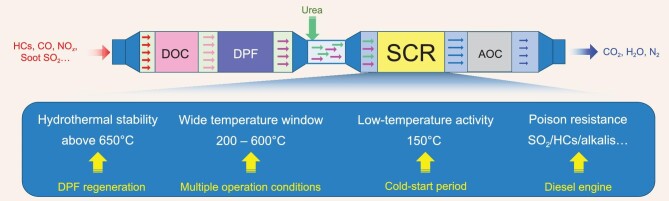
To meet progressively more rigorous legislations and policies, a complicated aftertreatment system was proposed and employed comprising multiple processing units (Fig. 1), in which the SCR unit is located in a downstream position. The DOC (diesel oxidation catalyst) is used to oxidize the hydrocarbons (HCs) and CO and partially oxidize NO to NO2 with excess oxygen. The partial oxidation of NO benefits the fast SCR reaction, which requires an equimolar mixture of NO and NO2. PMs (particulate matters) are captured and filtered by the DPF (diesel particulate filter). The reason for the placement of the AOC (ammonia oxidation catalyst) is to eliminate NH3 slip from the SCR unit. As can be seen, the SCR catalyst must have excellent hydrothermal stability due to the high-temperature environment (above 650°C) resulting from regeneration of the upstream DPF. Additionally, the complicated and changeable operation conditions of diesel vehicles expose the SCR catalyst to a wide variety of temperatures (200–600°C), including low temperatures below 200°C under cold-start and low-load conditions. Catalyst poisoning is also inevitable due to the incomplete oxidation of HCs and CO as well as the presence of S- and alkali-metal-containing diesel fuels. Therefore, SCR catalysts should be evaluated comprehensively and satisfy various operating modes in actual application. Fortunately, the zeolite catalysts are well-qualified as SCR catalysts due to their outstanding deNOx efficiency, high hydrothermal stability and optimal poison resistance.
Figure 1.
DOC + DPF + SCR + AOC aftertreatment system of diesel vehicles.
NH3-SCR reaction features and NH3-SCR catalysts
The NOx emitted from diesel engines consists of 90% NO and 10% NO2. Therefore, the primary reaction of NH3-SCR is the standard SCR reaction (SSCR, reaction (1)). In this redox reaction, 12 electrons are transferred. The N (−3 valence) in NH3 transfers 8e− and 4e− to NO and O2, respectively. Oxygen participates in the oxidation of active sites and combines with hydrogen to form H2O. Fast SCR (FSCR, reaction (2)), involving equal amounts of NO and NO2, also occurs when NO is partially oxidized in the DOC unit. Through this reaction, higher NOx conversion can usually be achieved, in which two and four electrons are transferred from NH3 to NO and NO2, respectively. Besides the standard and fast SCR reactions, there are also several side reactions (reactions (3–6)):
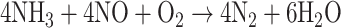 |
(1) |
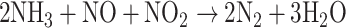 |
(2) |
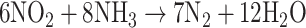 |
(3) |
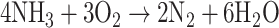 |
(4) |
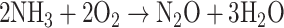 |
(5) |
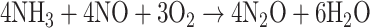 |
(6) |
The NH3-SCR reaction needs the participation of both NOx (acid oxide) and NH3 (base). Therefore, dual-functional sites are of significant importance in the SCR reaction due to the double-cycle mechanism, including the redox cycle (NOx) and acid cycle (NH3). In addition, the tight coupling of redox and acid sites (redox-acid sites) is beneficial in taking advantage of the synergistic effects of dual-sites [6,7]. For example, Fe-Ti and Ce-W oxide catalysts were developed and showed excellent NH3-SCR performance. The tight coupling of Fe (Ce) and Ti (W) is achieved by the co-precipitation method [7]. From another aspect, high dispersion and adequate exposure of functional sites, which increase the effective collision probabilities between active centres and reactants, are indispensable in every catalytic reaction.
Based on the above design principles, a series of redox-acid oxides with highly dispersed active sites were developed as novel and highly efficient NH3-SCR catalysts [7,8]. However, the thermal stability of oxide catalysts still needs to be improved due to the phase separation of the tight coupling structure of redox and acid sites at high temperatures. Since the Cu-SSZ-13 small-pore zeolite was reported to have superior activity and hydrothermal stability in the NH3-SCR reaction compared with medium- and large-pore zeolites (patent in 2009) [9–11], studies on Cu-SSZ-13 showed a dramatic increase in the past decade. The small-pore structure is primarily responsible for the high stability of the catalyst framework and active sites in Cu-SSZ-13 [11]. Concomitantly, other zeolites with similar small-pore structures, such as LTA, AEI, KFI, and RTH, also received much attention due to their comparable NH3-SCR performance and/or hydrothermal stability to Cu-SSZ-13. In this work, the selective catalytic reduction of NOx using the Cu-SSZ-13 zeolite catalyst is comprehensively reviewed with critical emphasis on the state of active sites, SCR reaction mechanism and synthetic methods, as well as poisoning resistance mechanisms (SO2, PO43−, HCs, alkali and alkaline earth metals). Based on the research methodology employed for Cu-SSZ-13, other Cu-exchanged small-pore zeolites are described in detail. Great efforts have been made to achieve matching between the characteristics of Cu2+-exchanged small-pore zeolite and highly efficient NH3-SCR catalysts, which is of great importance for the design of high-efficiency zeolitic catalysts. Furthermore, we propose basic rules for designing a zeolite catalyst for the NH3-SCR reaction as well as future efforts in this research field. We are devoted to providing an easy-to-read and systematic review of SCR of NOx using Cu2+-exchanged small-pore zeolites as catalysts and to highlighting the opportunities and challenges of Cu-based small-pore zeolites.
Cu-SSZ-13: A MODEL CASE FOR Cu2+-EXCHANGED ZEOLITE CATALYSTS FOR NH3-SCR
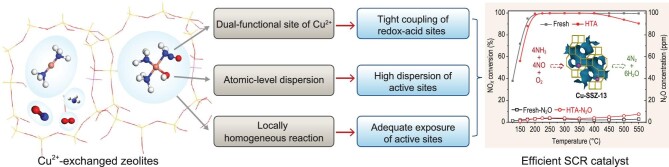
As we discussed above, the highly efficient NH3-SCR catalysts contain tightly coupled redox-acid sites as well as high dispersion and adequate exposure of these functional sites. Remarkably, the characteristics of copper ion-exchanged zeolites match perfectly with NH3-SCR catalyst requirements, as shown in Fig. 2. The Cu2+/Cu+ couple can act as both redox and acid site in the NH3-SCR reaction due to its multi-valence state and Lewis acid characteristics, where the redox cycle (NOx) and acid cycle can both be completed; moreover, the Cu2+ ions in ion-exchanged zeolites are atomically dispersed, since the metal ions compensate the positive charge of the zeolite framework by anchoring on the ion-exchange sites. More importantly, the locally homogeneous reactions in the bulk phase, resulting from the dynamic NH3-solvated Cu ions and electronic effects of the zeolite, significantly increase the effective collision probability between reactants and active species [12]. Therefore, Cu2+-exchanged zeolites achieve the required attributes of tight coupling of redox-acid sites, high dispersion and adequate exposure of functional sites. In particular, small-pore zeolites such as Cu-SSZ-13 possess better NH3-SCR performance and higher hydrothermal stability, N2 selectivity and poisoning tolerance than other zeolites due to the peculiar small-pore structure. For example, the small pores (∼4.0 Å) of Cu-based small-pore zeolites inhibit dealumination of the framework and accumulation of copper species during hydrothermal aging, which is highly beneficial for hydrothermal stability [11]. Also, some poisons, such as long-chain hydrocarbons, are prevented from entering the small pores due to their shape selectivity. The cavities of small-pore zeolites offer abundant active sites and large reaction zones, thus facilitating the chemical reactions. Therefore, the NH3-SCR reaction requirements match perfectly with the physicochemical properties of Cu2+-exchanged zeolites (especially small-pore zeolites), providing a solid foundation for highly efficient and stable NH3-SCR zeolite catalysts.
Figure 2.
Matching between the characteristics of Cu2+-exchanged zeolitic catalyst and SCR catalyst requirements. The monolithic catalyst (∼2.36 cm3) was estimated to have a total gas flow of 1.67 L/min, resulting in GHSV of ∼42 000 h−1. HTA represents that the sample was hydrothermally aged at 750°C for 16 h.
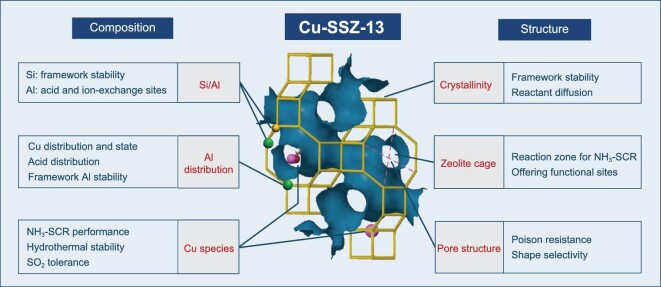
Due to the excellent deNOx efficiency and hydrothermal stability as well as the simplicity of the framework structure (only one T site), Cu-SSZ-13 zeolite, with chabazite (CHA) topology, is studied as a model case for understanding the composition-structure-activity (and stability) relationship of Cu2+-exchanged zeolite catalysts applied for NH3-SCR. As shown in Fig. 3, the CHA structure is constructed by stacking double six-membered rings (D6R) in an ABC sequence, resulting in a large CHA cavity and eight-membered ring (8MR) pore structure. The Si/Al ratio is of vital importance for the performance of Cu-SSZ-13 zeolite as an SCR catalyst. The large amount of Si in the substrate assures the stability of the zeolite framework, and the substitution of Si by Al provides acid sites due to charge compensation, which further benefits the location of active metal ions during the ion-exchange process and NH3 adsorption during the NH3-SCR reaction. Consequently, the Cu and acid site distribution as well as its stability are determined by the Al distribution in Cu-SSZ-13 zeolites. The state and location of Cu species directly affect the NH3-SCR performance, hydrothermal stability and SO2 tolerance of Cu-exchanged zeolites. As for the zeolite structure, the high crystallinity of zeolite favours framework stability and reactant diffusion. Reaction zones for NH3-SCR and adequate functional sites can be offered by the large zeolite cage (such as the CHA cavity). Importantly, the pore structure endows Cu-exchanged zeolites with shape selectivity catalysis, leading to high poison resistance, especially regarding some large molecules. After describing the general characteristics of Cu-SSZ-13, the application of Cu-SSZ-13 in the NH3-SCR reaction is elaborated in detail below.
Figure 3.
The composition-structure-activity (stability) relationship of Cu-zeolites for NH3-SCR reaction.
Copper species
The state and location of copper species are closely related to the elemental composition of the zeolite and the atmospheric environment. The substitution of Si atoms by Al atoms creates charge defects, which provide Brønsted acid sites and exchange sites for Cu ions. The isolated Cu2+ ions were initially thought to be the active centres and to be coordinated to three oxygen atoms of the six-membered rings (6MRs) [13,14]. However, Kwak et al. identified two types of Cu ions at distinct cationic positions in Cu-SSZ-13 zeolite when Cu/Al is higher than 0.2 via H2-TPR and fourier transform infrared (FTIR) measurements [15]. Afterwards, researchers depicted the location and coordination of the two types of Cu ions (Fig. 4a) [16,17]. The relatively stable Cu2+ ions, which compensate paired negative charge (labelled as Cu2+-2Al), are located in the windows of 6MRs. After saturation of paired Al, [CuOH]+ ions appear subsequently and populate single framework Al sites next to 8MRs (labelled as [Cu(OH)]+-Al). Consequently, the amount of active copper species can be adjusted by tuning the Al contents and distribution. For example, with the increase of Al content (from Si-rich zeolites to Al-rich zeolites), the capacity for Cu2+-2Al species increases due to the presence of large amounts of paired Al, and the [Cu(OH)]+-Al species appear only at high Cu/Al [17]. The Al distribution at a fixed Si/Al ratio can be adjusted by using different structure-directing agents (SDAs) to synthesize the SSZ-13 substrate. Iorio et al. found that the density of paired Al increased linearly with the Na+ content incorporated into the crystallized solid when using TMAda+ and Na+ as organic and inorganic SDA [18,19]. Zhang et al. also increased the amounts of close Al sites in SSZ-13 by adjusting the crystallization pathways using DMCHA+ as the organic template, generating more Cu2+-2Al species than found in Cu-SSZ-13 synthesized using TMAda+ as the organic template [20].
Figure 4.
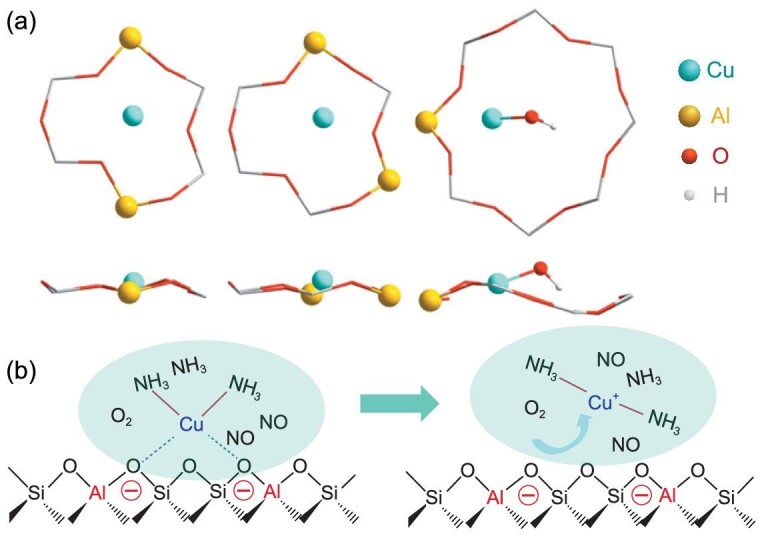
(a) Density functional theory (DFT) calculations of Cu2+ location and coordination. Adapted with permission from ref. [16]. (b) The NH3 solvation effect of Cu2+ and dynamic Cu-(NH3)n complex during SCR reaction.
The atmospheric environment can also influence the Cu state and coordination, and is closely related to the low-temperature catalytic activity of active copper species in Cu-SSZ-13 zeolite. Generally, the copper species are in the hydrated state in the ambient air, represented as [Cu(OH)(H2O)5]+ and [Cu(H2O)6]2+ [17,21]. The coordination between Cu ions and the zeolite structure can be weakened by the solvation effect of H2O. When increasing the temperature to ∼250°C, the hydrated copper species will release bound water and become bound at the ion-exchange sites in the form of [Cu(OH)]+-Al and Cu2+-2Al [21]. Similarly, the copper species are solvated by ammonia much more strongly than by H2O in the presence of NH3. The formed Cu-NH3 complex is thought to be the active site in the low-temperature NH3-SCR reaction [12,22–24]. More importantly, the active sites are movable within a limited region (∼9 Å) due to the weak bonding between Cu ions and the zeolite structure due to NH3 solvation (Fig. 4b), which further increases the effective collision possibility between active sites and reactants in a local area. Meanwhile, concomitant with the solvation of copper species by NH3, the reduction of Cu2+ ions takes place, resulting in NH3-solvated Cu+ ions [17,23]. Compared to Cu2+-2Al, [Cu(OH)]+-Al is more readily reducible by NH3 to form Cu(NH3)n+ and H2O [17]. The mobile NH3-solvated Cu+ species, which play a pivotal role in the NH3-SCR reaction, can also be generated in the actual NH3-SCR atmosphere. Researchers have also found the ‘auto-reduction’ of Cu2+ ions in a vacuum, inert atmosphere and even O2 atmosphere at high temperatures [21,25].
Standard SCR reaction mechanism
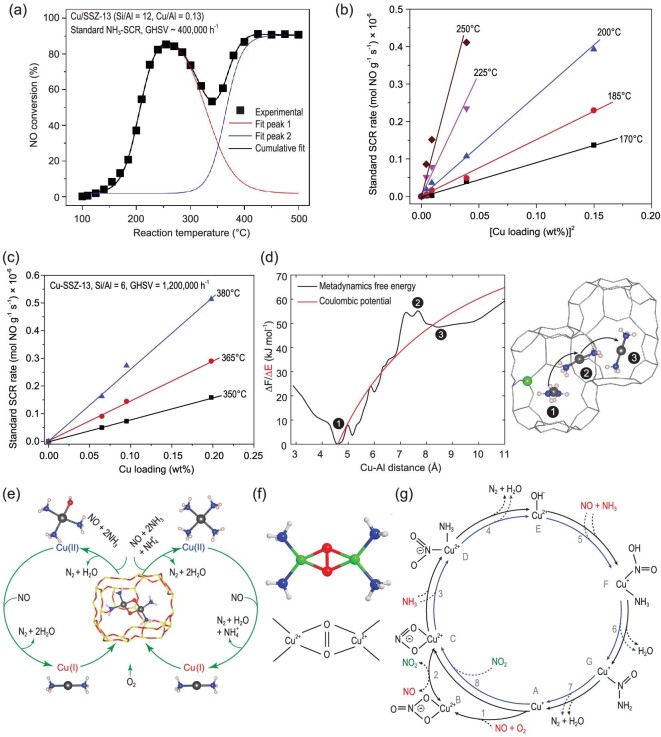
Great efforts have been devoted to uncovering the standard SCR (SSCR) reaction cycle of Cu-SSZ-13, including the reduction (Cu2+→Cu+) and oxidation (Cu+→Cu2+) half-cycles. A seagull NOx profile (Fig. 5a) with dual-maxima NOx conversion was observed during the SSCR reaction over Cu-SSZ-13 catalysts with low Cu loading [22,26]. Figure 5b and c depict the linear relationship of the SSCR rate as a function of (Cu loading)2 and (Cu loading) at low and high temperatures, respectively [27]. Both of these indicate two distinct reaction regimes that require participation of paired Cu ions and isolated Cu ions at low and high temperatures, respectively.
Figure 5.
(a) NO conversion as a function of temperature over Cu-SSZ-13. Adapted with permission from ref. [22]. SCR rate over Cu-SSZ-13 as a function of (b) (Cu loading)2 at low temperatures and (c) (Cu loading) at high temperatures. Adapted with permission from ref. [27]. (d) Free energy and diffusion of CuI(NH3)2 at different sites. Adapted with permission from ref. [12]. (e) Proposed low-temperature standard SCR catalytic cycle. Adapted with permission from ref. [12]. (f) Side-on μ-η2, η2-peroxo diamino dicopper (II) complex [Cu2(NH3)4O2]2+ species. Adapted with permission from ref. [31]. (g) Possible high-temperature SCR catalytic cycle. Adapted with permission from ref. [34].
A representative SSCR reaction cycle over Cu-SSZ-13 at low temperatures is shown in Fig. 5e. In the reduction half-cycle of SCR, Cu species which act as both redox and Lewis acid sites are readily reduced under the coexistence of NH3 and NO at low temperatures (below 250°C), leading to the formation of NH3-solvated Cu+ species, as discussed above [23,28,29]. In the oxidation half-cycle of SCR, binuclear Cu ions, which are achieved via the migration of Cu(NH3)2+, are essential for O2 activation based on the linear relationship between the standard SCR rate and (Cu loading)2 [22]. The electrostatic tethering between Cu(NH3)2+ and the zeolite structure becomes weak after NH3 coordination, resulting in dynamic multinuclear active sites in a limited volume (Fig. 5d). Paolucci et al. calculated that the diffusion radius of Cu(NH3)2+ is ∼9 Å, meaning that Cu(NH3)2+ can travel through 8MR CHA windows to form binuclear Cu ions [12]. This mechanism is a breakthrough in catalytic chemistry because it is outside the scope of traditional heterogeneous and homogeneous reactions, behaving more like a locally homogeneous reaction (Fig. 5e). The density functional theory (DFT) calculation result reveals that O2 activation (that is, formation of Cu(NH3)2-O2-Cu(NH3)2) is the rate-determining step for the NH3-SCR reaction over Cu-SSZ-13 [12,22,30]. Recently, Negri et al. identified the formation of a side-on μ-η2, η2-peroxo diamino dicopper (II) complex [Cu2(NH3)4O2]2+ (Fig. 5f) during the reaction of linear Cu(NH3)2+ with O2 via wavelet transform analysis of the extended X-ray absorption fine structure (EXAFS) data with other auxiliary measurements [31]. Four electrons are required to complete the O2 activation and dissociation. Therefore, NO was proposed to act as another electron provider in addition to two [Cu(NH3)]+, which can only deliver two electrons [22]. Using DFT calculations, Chen et al. investigated the reactivity of [Cu2(NH3)4O2]2+ with NO by proposing two cycles, in which NO is adsorbed on Cu(II) sites to form NO+ or oxygen to form NO2−. In their calculations, Brønsted acid sites play a pivotal role in the decomposition of HONO and H2NNO intermediates formed on Cu sites, and the catalytic cycle is probably controlled by the orientation of HONO and H2NNO from Cu sites to Brønsted acid sites [32]. By combining X-ray absorption spectroscopy (XAS) and ultraviolet-visible-near-infrared (UV-Vis-NIR) spectroscopies, Negri et al. found that NO + NH3 and NO can react with the [Cu2(NH3)4O2]2+ complexes, and an excess of the reactants leads to complete reduction of [Cu2(NH3)4O2]2+ to linear [Cu(NH3)]+ accompanied by the formation of N2, indicating the reaction of [Cu2(NH3)4O2]2+ with NO [31].
Turning to the reaction mechanism at elevated temperatures (>300°C), isolated Cu ions anchor on the ion-exchange sites and become fixed in the zeolite framework due to the decomposition of Cu(NH3)n species, according to NH3-TPD results [33]. The SSCR reaction activation energy at high temperatures (∼140 kJ/mol) is significantly higher than that at low temperatures (∼70 kJ/mol), indicating the presence of a different reaction pathway [27]. Janssens et al. proposed a reaction mechanism for the SSCR reaction based on isolated Cu2+/Cu+ ions [34], which is more like the reaction cycle at high temperatures (Fig. 5g). The Cu2+ ions are reduced by NO + NH3 and re-oxidized by NO + O2 or NO2. They indicated that the oxidation of an NO molecule by O2 to a form bidentate nitrate ligand is rate-determining for the SSCR.
Generally, most studies discuss the SSCR reaction cycle over the fresh Cu-SSZ-13 with low Cu loading, which simplifies the catalytic system with only a single Cu site. However, in actual application, the Cu loading of Cu-SSZ-13 is usually higher than the theoretical one. The NO reduction at high temperatures would decrease with the increase of Cu loading due to the occurrence of non-selective NH3 oxidation by oxygen at high temperatures, which leads to a lack of reductant. Moreover, some Cu species in the deep pores cannot gain access to the reactants. Therefore, controlling the amount of active Cu sites is important for actual application of Cu-SSZ-13. In another aspect, when Cu-SSZ-13 is aged, the behaviour is much more complicated due to the presence of various Cu species (such as CuOx). As a result, there have been few studies focusing on aged or high Cu-loaded Cu-SSZ-13. However, more studies should be conducted to understand the reaction process of aged or high Cu-loaded Cu-SSZ-13 zeolites, since these catalysts are closer to the actual situation in practice.
FSCR reaction and NH4NO3 formation
The FSCR reaction (reaction (2)) also plays an important role in NOx reduction due to the presence of ∼10% NO2 in actual diesel exhaust, as well as the partial oxidation of NO to NO2 in the DOC section. Normally, NO2 addition to the feed is an effective way of achieving high deNOx efficiency for NH3-SCR catalysts. Differently to most NH3-SCR catalysts, however, Cu-SSZ-13 catalysts only showed a slight improvement in NOx reduction under FSCR conditions, as reported by Kwak et al. [35]. Our group even found an inhibition effect on NOx conversion over our in situ synthesized Al-rich Cu-SSZ-13 catalyst due to the formation of ammonium nitrate (Fig. 6a) [36,37]. Therefore, the small-pore zeolites scarcely rely on NO oxidation to achieve the FSCR reaction, unlike other SCR catalysts. The formation of surface NH4NO3 from the adsorbed NO2 and NH3 is a common reaction in the FSCR according to reaction (7), and NH4NO3 decomposition primarily takes place in two ways as shown in reaction (8) and reaction (9):
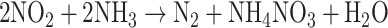 |
(7) |
Figure 6.
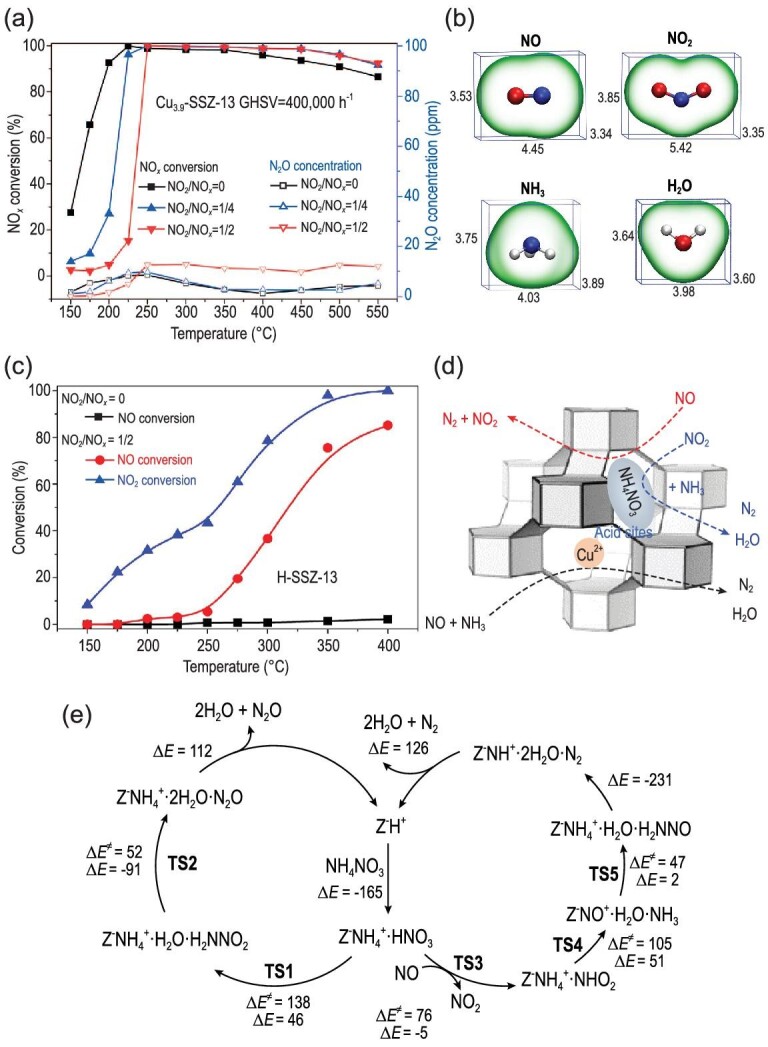
(a) NH3-SCR performance of Cu-SSZ-13 under different reaction conditions. Adapted with permission from ref. [36]. (b) Different reaction pathways of NO and NO2 over Cu-SSZ-13. Adapted with permission from ref. [37]. (c) NO, NO2 and NOx conversion on H-SSZ-13 under fast SCR conditions. Adapted with permission from ref. [37]. (d) Molecular size of NH3, NO and NO2. Adapted with permission from ref. [37]. (e) DFT-calculated reaction pathways for direct and NO-assisted NH4NO3 decomposition. Adapted with permission from ref. [39].
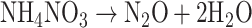 |
(8) |
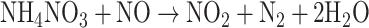 |
(9) |
The comparable geometric dimensions of the small pores (∼3.8 Å) and reactant molecules (Fig. 6b) are mainly responsible for the strong inhibition of NO conversion activity, since a slight amount of NH4NO3 accumulation can limit the diffusion of reactants as well as [Cu(NH3)2]+ species. Moreover, the NH4NO3 formed in small-pore zeolites is more stable than that in large-pore zeolites [38]. Our group investigated the effect of NO2 on the NH3-SCR reaction over Cu-SSZ-13 with varying Cu loadings [37]. We found that increased Cu loadings contributed to the SSCR reaction, while inhibiting the FSCR reaction. At low temperatures, the NO conversion activity was totally suppressed because of the formation of NH4NO3, while NO2 could react with NH3 at acid sites to contribute to NOx conversion (Fig. 6d). Further, it was observed that the deposited NH4NO3 reacted with NO over H-SSZ-13 at high temperatures (Fig. 6c), accompanied by the direct decomposition of NH4NO3 to N2O (reaction (8)). However, the N2O production is less than 25 ppm. As shown in Fig. 6e, the DFT-calculated reaction pathways demonstrated that the energy barrier (105 kJ/mol) for NO-assisted NH4NO3 decomposition (reaction (9)) is much lower compared to that (138 kJ/mol) of direct NH4NO3 decomposition (reaction (8)) [39]. The combination of reaction (9) and reaction (7) yields the FSCR reaction (2), indicating that the FSCR reaction occurs only on Brønsted acid sites, which is also testified to by computational methods [39,40]. To avoid this inhibition phenomenon, we conducted hydrothermal aging to decrease the density of Brønsted acid sites to alleviate NH4NO3 accumulation and cause mesopore formation to favour gas diffusion [41]. As expected, the inhibiting effect of NO2 on NOx conversion was weakened for the hydrothermally aged Cu-SSZ-13 catalysts. In another aspect, what should be noted is that NH4NO3 accumulated only under steady-state conditions. Bendrich et al. found that almost no NH4NO3 is predicted to form during temperature oscillation and NO/NO2 ratio oscillation [42,43]. The decomposition temperature of NH4NO3 formed on Cu-SSZ-13 is about ∼225°C, which is easily exceeded during the driving cycle [37]. As for oscillating NO/NO2 ratios, NH4NO3 can act as an NO2 buffer that stores NO2 under NO2-excess conditions and decomposes under NO2-deficient conditions.
There is a common consensus that NH4NO3 forms on Cu-SSZ-13 and inhibits NOx conversion under FSCR conditions at steady-state and low temperatures. An atomic-level picture of the FSCR reaction cycle is still lacking, especially regarding the Cu sites. Research studies have only found Cu(II) species, due to the strong oxidizing ability of NO2, and Cu(NH3)n complexes have scarcely been identified under FSCR conditions, making it difficult to investigate the reduction and oxidation half-cycle of the SCR reaction [12,44]. Moreover, the NH4NO3 formation and high reactivity of the SSZ-13 substrate limit the investigation of Cu active sites. The reaction cycle containing NO2 shown in Fig. 5g is a rational candidate due to the presence of Cu(II) species coordinated strongly to zeolite and the absence of the Cu(NH3)n complex. However, the evidence is not conclusive, and more studies are required in future.
Hydrothermal stability
For the aftertreatment system of diesel engines, excellent hydrothermal stability (HTS) is an indispensable property due to the high-temperature environment (above 650°C) resulting from regeneration of the upstream DPF. Cu-SSZ-13 small-pore zeolites were reported to have superior HTS to that of medium- and large-pore zeolites as well as most of the oxide catalysts [11]. Researchers have devoted much effort to unravelling the deactivation mechanism of hydrothermal aging (HTA).
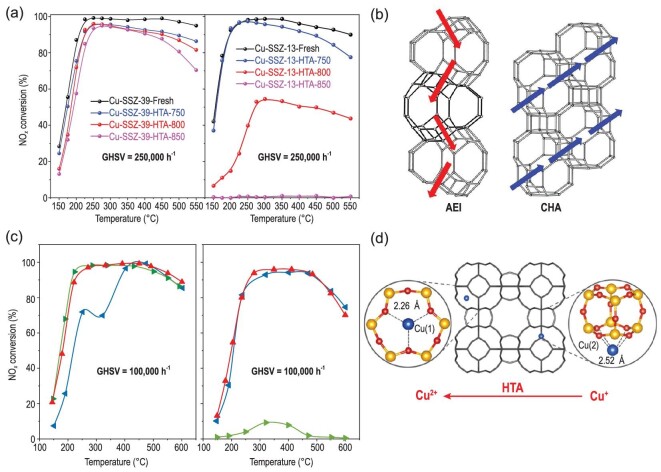
Hydrolysis of framework Al and accumulation of CuOx from Cu2+ active sites are the two key reasons for HTA deactivation. The narrow small pores (∼3.8 Å) block the diffusion of hydrolysed Al(OH)3, with large kinetic diameter of ∼5.03 Å, leading to its reattachment back into the structural defects caused by dealumination when the zeolite cools down [45]. This is the principal reason that small-pore zeolites have higher skeleton stability than medium- and large-pore zeolites during HTA. Regarding the effect of copper species, researchers found that HTS decreased with increasing Cu/Al ratio (Fig. 7a) [45–47]. As mentioned in ‘Copper species’ section, Cu2+-2Al species with higher stability primarily exist in Cu-SSZ-13 catalysts with low Cu loading. Increasing Cu loading leads to formation of [Cu(OH)]+-Al species, which easily accumulate to form CuOx. This was also proved by Gao and co-workers using electron paramagnetic resonance (EPR) as the primary measurement [48,49]. They found that mild HTA first induced some conversion of [Cu(OH)]+-Al to Cu2+-2Al species, resulting in paired Cu2+ located in a D6R prism with a distance of ∼3.90 Å [49]. Further increasing the HTA temperature, [Cu(OH)]+-Al species gradually accumulated to form CuOx clusters (Fig. 7b) [48].
Figure 7.
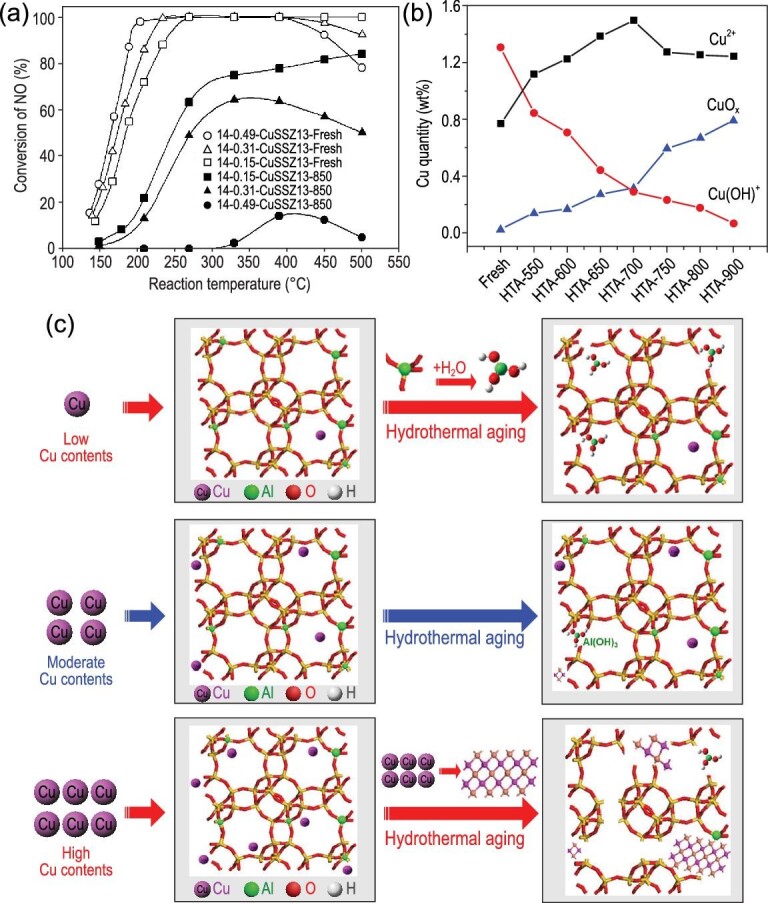
(a) Standard NH3-SCR performance of fresh and HTA Cu-SSZ-13 with different Cu/Al ratios. Adapted with permission from ref. [46]. (b) Estimation of Cu2+-2Al, [Cu(OH)]+-Al and CuOx in fresh and HTA samples. Adapted with permission from ref. [48]. (c) The deactivation mechanism of HTA of Cu-SSZ-13 with different Cu contents. Adapted with permission from ref. [51].
The Si/Al ratio can also influence the HTS of Cu-SSZ-13 by affecting the copper distribution. Al-rich zeolites favour the presence of Cu2+-2Al species, thus leading to higher HTS compared to Si-rich zeolites [46,50]. Nevertheless, the Al-rich zeolites still face the problem of dealumination, which will cause the loss of Brønsted acid sites during HTA, due to the large amount of vulnerable framework Al. We investigated the HTS of Al-rich Cu-SSZ-13 zeolite and found that Cu2+ ions inhibit dealumination of the SSZ-13 zeolite structure, while excessive quantities of them easily accumulate to form CuOx clusters, leading to collapse of long-range order (Fig. 7c) [51]. Ye et al. also reported that preservation of active Cu2+ sites is more important than preserving Brønsted acid sites [52]. This is understandable, because Brønsted acid sites only serve as an NH3 reservoir or probably as active sites for the decomposition of HONO and H2NNO intermediates, as discussed in ‘Standard SCR reaction mechanism’ section, while the Cu2+ species can act as both redox and acid sites and can complete the NH3-SCR reaction cycle on their own. Besides CuOx, CuAlOx species were also detected by researchers [53–55]. Ma et al. found that CuAlOx originating from the combination of Cu with extra-framework Al is inert, while CuOx species still have catalytic oxidation ability [55]. Schmidt et al. found evidence that Cu-SSZ-13 only shows Cu and Al clustering separately, while Cu-ZSM-5 contains large amounts of copper aluminate species after HTA, by using atom probe tomography technology, which can show the 3D distributions of component elements [54].
In summary, CuOx formation and dealumination are two reasons for HTA deactivation, and interact during HTA. CuOx accumulation can induce zeolite structure collapse, and extra-framework Al can coordinate with Cu to form CuAlOx species that are inert. As both redox and acid sites, Cu species are of vital importance and should be carefully tuned and protected. As an alternative, utilizing an Al-rich zeolite support is a simple and effective way to increase the HTS of Cu-SSZ-13 since the capacity for stable Cu2+-2Al species is large due to the presence of many paired Al atoms in Al-rich zeolites.
Sulphur poisoning and desulphurization
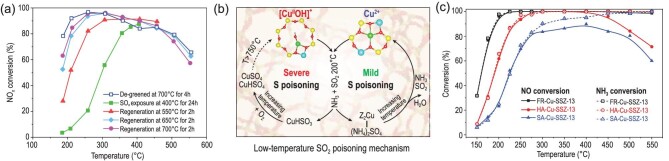
Sulphur poisoning is an inevitable challenge due to the residual sulphur in diesel fuel. Figure 8a shows the SSCR performance of fresh, sulphated and subsequently desulphated Cu-SSZ-13 catalysts [56]. The low-temperature performance was significantly inhibited on the sulphated samples compared to fresh ones, while the high-temperature performance was scarcely influenced. Thermal treatment can partially recover the deNOx efficiency of Cu-SSZ-13 and the low-temperature activity increased with progressively higher regeneration temperatures, indicating that various S-containing species, including reversible and irreversible ones, existed on the sulphated Cu-SSZ-13 catalysts.
Figure 8.
(a) Effect of SOx exposure and subsequent thermal deSOx. Adapted with permission from ref. [56]. (b) Proposed formation of sulphates during adsorption of SO2 on [Cu(OH)]+-Al and Cu2+-2Al sites. Adapted with permission from ref. [61]. (c) NH3-SCR performance of fresh Cu-SSZ-13 (FR-Cu-SSZ-13), hydrothermally aged Cu-SSZ-13 (HA-Cu-SSZ-13) and sulphur-aged Cu-SSZ-13 (SA-Cu-SSZ-13) at high temperatures (750°C). Adapted with permission from ref. [64].
Low-temperature (200°C) SO2 poisoning of Cu-SSZ-13 generally leads to the formation of H2SO4, CuSO4 and Al2(SO4)3 species, with different thermal stabilities [57]. The decomposition temperatures are ∼500°C, ∼630°C and ∼800°C for H2SO4, CuSO4 and Al2(SO4)3, respectively [57]. H2SO4 easily combines with NH3 to promote the accumulation of ammonium-sulphur species under SCR conditions [57–60]. Therefore, SO2 treatment under SCR conditions (NH3 + NO + O2 + H2O) induced more severe deactivation compared to that in the presence of O2 + H2O only [58]. Jangjou et al. found that ammonium sulphate formed on Cu2+-2Al sites and could be generally regarded as a reversible deactivation due to its facile regeneration by heat treatment [61]. However, SO2 adsorbed on [Cu(OH)]+-Al sites leads to copper bisulphite species formation, which are further oxidized to form copper bisulphate with increasing temperature (Fig. 8b) [61]. The accumulation of ammonium sulphate and copper bisulphate limits the formation and mobility of [Cu(NH3)2]+ ions that serve as dynamic active sites, thus resulting in a marked decrease in deNOx efficiency [62,63]. The copper-sulphur species need a higher temperature (>550°C) to decompose and are thus thought to result in irreversible deactivation. Al2(SO4)3 is difficult to decompose and regarded as an irreversible deactivation species due to the destruction of framework Al. When the SO2-poisoning temperature increased to 400°C, more copper-sulphur species formed due to the instability of ammonium sulphate. However, 750°C sulphur aging with H2O resulted in permanent deactivation of Cu-SSZ-13 without accumulation of reversible species, since SO2 increased the acidity of the hydrothermal atmosphere and accelerated the destruction of the zeolite structure as well as the loss of acid sites and active Cu2+ species (Fig. 7c) [64]. Compared to SO2, SO3 poisoning is more severe due to the primary formation of copper bisulphate species, which only decompose at elevated temperatures (>550°C) [59].
Turning to the solution to SO2 poisoning, heat treatment at elevated temperature (∼500°C), an easily accessible temperature in actual diesel exhaust, is generally an effective way to regenerate the deNOx efficiency of Cu-SSZ-13, since most of the total deactivation is reversible. Moreover, it should be noted that some reductants, such as NH3 and C3H6, can achieve the removal of sulphur species by chemical reaction without heat treatment at elevated temperatures [56,65]. Besides, Wei et al. found that mild HTA increased the SO2 tolerance of Cu-SSZ-13 since some sulphur-sensitive [Cu(OH)]+-Al species transformed to stable Cu2+-2Al species during mild HTA [66]. Yu et al. found that some metal oxides, which can be mixed with Cu-SSZ-13 zeolite to form hybrid catalysts, can serve as a sacrificial component to prevent Cu2+ site poisoning. However, the improvement in SO2 tolerance is still limited [67].
Effects of PO43−, HCs and metal co-cations
The exhaust from diesel engines includes various contaminants derived from the engine, urea (NH3 source), volatile engine oils, volatile precious metals and fuel additives; therefore, besides hydrothermal aging and sulphur poisoning there are also other toxic species that need to be considered in diesel exhaust, such as PO43−, HCs and alkali metals, although their quantities are small. Phosphorus mainly comes from fuels (biofuels) and some lubricating oils as well as some fuel additives. There have been several studies mainly focused on phosphorus poisoning of Cu-SSZ-13. Phosphorus poisoning is generally simulated by incipient wetness impregnation using (NH4)2HPO4 solutions as precursors. After PO43− loading, Cu-P species and Al-P species are formed and have been identified by many researchers [68–71]. The formation of Cu-P species induces a decrease in the NO and NH3 oxidation of Cu-SSZ-13, which further results in a reduction in low-temperature SCR activity due to loss of active Cu2+ sites, but improvement in high-temperature SCR activity due to the suppression of NH3 non-selective oxidation [68,71,72]. In detail, Wang et al. found that [Cu(OH)]+-Al interacts more easily with phosphorus and coordinates with only one P atom (PO3− or PO43−), while the poisoning of Cu2+-2Al involves two P atoms (i.e. P2O5). The formation of Al-P species primarily influences the HTS of Cu-SSZ-13. P loading can lead to part of the phosphorus inserting into the zeolite framework to form a localized AlPO4 phase, which is severe after HTA [72,73]. This indicates that P poisoning accelerates the collapse of the structure of Cu-SSZ-13 zeolite during HTA. However, Zhao et al. found, importantly, that an appropriate content of phosphate ions can prevent the structure collapse due to the formation of a silicoaluminophosphate interface [71]. To create conditions approaching the practical situation, Xie et al. simulated the vapour-phase phosphorus poisoning (using diluted H3PO4 solution) of Cu-SSZ-13 during NH3-SCR operating conditions [70]. Ammonium phosphate is easily formed at low temperatures but decomposes at elevated temperatures. Unfortunately, the decomposition of ammonium phosphate still deposits P in the catalysts. At high temperatures, metaphosphate (PO3−) was the dominant deposited compound compared to phosphorus oxide (P2O5) and phosphate (PO43−) during PO43− poisoning under SCR conditions. In summary, PO43− poisoning results in accumulative and permanent deactivation, although a small amount of phosphorus probably benefits the hydrothermal stability.
The effect of co-cations should also be considered since the exhaust contains some metal impurities derived from various additives, and some metals are residual species or are purposefully added in the synthetic process to improve the catalytic activity of Cu-SSZ-13. The influence of Na on Cu-SSZ-13 has been extensively studied by researchers, since Na is not only a poison but also a residual species in most synthetic methods. We first investigated the effect of Na+ on one-pot synthesized Cu-SSZ-13 and basically found that the presence of Na+ decreased the catalytic activity and hydrothermal stability [74]. However, Gao et al. found that Na+, as well as Li+ and K+, promoted the low-temperature SCR rate and HTS of Cu-SSZ-13 [75,76]. Zhao also found that an appropriate Na+ content could increase both the low-temperature activity and hydrothermal stability of their organotemplate-free synthesized Cu-SSZ-13 [77]. The primary reason for the discrepancy is varying Cu contents in the Cu-SSZ-13 catalysts. Na+, located at ion-exchange sites, weakens interactions between Cu2+ and the zeolite framework and promotes some Cu2+ accumulation during HTA, which is more pronounced in the one-pot synthesized Cu-SSZ-13 with high Cu content due to the low Si/Al ratio (∼4.2). However, if the Cu content is suitable, the weakened interaction with the zeolite framework makes Cu2+ more reducible, and a moderate amount of Na+ can prevent zeolite dealumination and stabilize the framework during HTA. Analogously, among rare earth co-cations, moderate amounts of Ce and Y ions, which locate at ion-exchange sites, were found to improve the HTS of Al-rich Cu-SSZ-13 [78–81]. Other metal co-cations (such as Cs+, Ca2+ Mg2+ etc.) generally showed a poisoning effect on Cu-SSZ-13 since they destabilized isolated Cu ions via site competition [76,82]. In summary, some co-cations have a positive effect on Cu-SSZ-13, but controlling the content of Cu ions and co-cations is the key factor.
Hydrocarbon species are primarily formed due to insufficient combustion of fuel during cold-start oxidation or when the upstream DOC is aged. Basically, heavy HCs such as C10H22 and C12H26 have no effect on the deNOx efficiency of Cu-SSZ-13, since the small channel (∼3.8 Å) prevents the long-chain HC molecules from entering into the zeolite [83,84]. As for light HCs, Cu-SSZ-13 shows a slight decrease in NO conversion in the presence of C3H6 in the medium temperature range (200–400°C). In this temperature range, C3H6 is partially oxidized and forms some carbonaceous deposits, resulting in a decrease in NO conversion [83,85]. When the temperature is above 400°C, the deposited carbon burns off and C3H6-SCR can also occur, so that the NOx conversion is only slightly changed [85–87]. When the temperature is below 200°C, there is also no adverse impact of C3H6 since the energy is insufficient to propel the C3H6 molecule (with a kinetic diameter of ∼4.5 Å) into the pores [85].
Synthetic methodology
The availability of an efficient, economical and environmentally friendly synthetic method is vitally important for the practical application of Cu-SSZ-13. In the synthesis process, the temperature, seeds and template all influence the space-time yields (STY) of CHA zeolite. Generally, Cu-SSZ-13 catalysts are prepared by an ion-exchange method, with the SSZ-13 substrate synthesized using N,N,N-trimethyl-1-adamantammonium hydroxide (TMAdaOH) as a template [88]. In consideration of the low STY (160°C for 90–120 h), high price of TMAdaOH, and complex ion-exchange process, many new synthetic methods were developed by researchers. Han et al. used coal gangue as the Al source and shortened the nucleation time of SSZ-13 to 7 h in the presence of TMAdaOH, but 36 h was still needed to prepare SSZ-13 with high crystallinity [89]. Wang et al. used a solvent-free method to successfully synthesize SSZ-13 substrate by using economical N,N,N-dimethylethylcyclohexylammonium bromide (DMCHABr) as an organic template to achieve 95.7% of efficiency of silica source usage. Interestingly, the Cu2+ ion-exchanged SSZ-13 synthesized using DMCHABr as a template showed higher HTS than Cu-SSZ-13 synthesized by the traditional method using TMAdaOH as a template, since more spatially close Al sites (favouring Cu2+-2Z as elaborated in ‘Hydrothermal stability’ section) exist in the former Cu-SSZ-13 [20,90]. Furthermore, seed-assisted rapid synthesis of SSZ-13 through interzeolite transformation from FAU zeolite was achieved in the absence of solvent, which shortened the crystallization time to 1 day [91]. Simultaneously, the solvent-free conditions benefit the stability of the TMAdaOH template at high temperatures (>200°C), so that Bian et al. shortened the synthetic time of SSZ-13 to only 1.5 h (extremely high STY) at 240°C. Besides DMCHABr, Chen et al. used economical choline chloride (CC) as a template to synthesize the SSZ-13 substrate [92]. Furthermore, they optimized this method with the assistance of fluoride and seeds, resulting in crystallinity similar to that of a sample synthesized using TMAdaOH [93]. Moreover, through the utilization of CHA seeds, an organotemplate-free synthesis of SSZ-13 was developed by Zhao et al. [77]. After Cu2+ ion-exchange, this Cu-SSZ-13 sample showed excellent catalytic activity, with NOx conversion higher than 90% in the temperature range 200–500°C, but at relatively low GHSV (80 000 h−1).
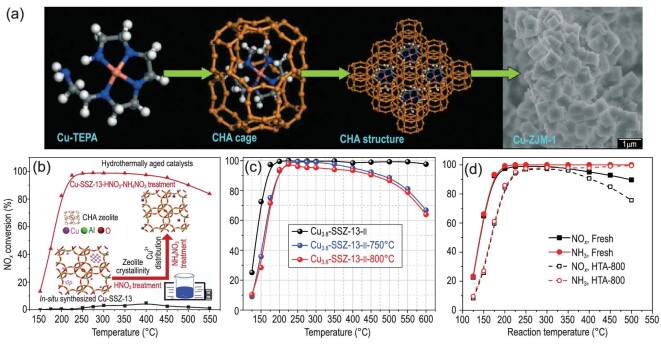
The product synthesized by all the above methods is the SSZ-13 substrate, which subsequently needs multiple steps to obtain Cu-SSZ-13 catalysts, at least including calcination, NH4+ ion-exchange, Cu2+ ion-exchange and calcination again. Therefore, a direct synthesis method for Cu-SSZ-13 catalyst is desired. Xiao and co-workers first directly synthesized Cu-SSZ-13 by using Cu-tetraethylenepentamine (Cu-TEPA) as a template to introduce the copper species in situ (Fig. 9a) [94]. Nevertheless, using Cu-TEPA as the sole template easily led to the formation of accumulation of CuOx clusters, which is detrimental to HTS and high-temperature activity (Fig. 9b, black line). Therefore, a series of aftertreatments were developed by our group to optimize the structure and copper species of in situ synthesized Cu-SSZ-13 [51,74,86]. We found that synergetic treatment by HNO3 and NH4NO3, which can accurately tune the framework crystallinity and copper state and content, markedly increased the SCR activity and hydrothermal stability (Fig. 9b, red line). Since Cu-SSZ-13 was first reported to have excellent NH3-SCR activity and hydrothermal stability in 2010, researchers have studied the physiochemical properties of Cu-SSZ-13 in detail and optimized the Si/Al ratio to ∼12 from 17.5 [48,95], and we believe that the Si/Al of Cu-SSZ-13 can be lowered further. It should be noted that the in situ synthesized Cu-SSZ-13 (Fig. 9c) showed activity superior to commercial Cu-SSZ-13 (Fig. 9d, Si/Al∼12), especially at high temperatures. This is because the in situ synthesized Cu-SSZ-13 is an Al-rich zeolite, which accommodates large amounts of paired framework Al for stable coordination of Cu2+-2Al (see details in ‘Copper species’ section), thus leading to high deNOx efficiency and HTS.
Figure 9.
(a) Mechanism of preparation of Cu-TEPA templated Cu-SSZ-13 zeolites. Adapted with permission from ref. [94]. (b) HNO3-NH4NO3 aftertreatment of in situ synthesized Cu-SSZ-13. (c) NH3-SCR performance of aftertreated Cu-SSZ-13 before and after hydrothermal aging at 750 and 800°C, GHSV = 200 000 h−1. Adapted with permission from ref. [51]. (d) NH3-SCR performance of commercial fresh and HTA-800 Cu/SSZ-13 samples (Si/Al = 12, Cu loading = 2.1%), GHSV = 200 000 h−1. Adapted with permission from ref. [48].
OTHER SMALL-PORE ZEOLITES APPLIED TO NH3-SCR
Shape selectivity of small-pore structure in NH3-SCR reaction
Cu-SSZ-13 is a Cu-exchanged small-pore zeolite with the CHA crystal structure, which contains a channel opening of about 3.8 × 3.8 Å (8MR) and a large CHA cage. As stated above, this specific structure is closely related to the properties of Cu-SSZ-13 catalysts in the NH3-SCR reaction. For example, the dealumination process of small-pore zeolites during HTA is inhibited because the constricting dimensions of the small pores limits the detachment of aluminium hydroxide [45,96]. The small-pore structure restricts large hydrocarbon species from entering the pores of the catalysts during the SCR reactions, so that Cu-SSZ-13 possesses good hydrocarbon tolerance, especially towards long-chain HCs [45]. The small-pore Cu-CHA zeolite showed very slow N2O formation in the NH3-SCR reaction because the small-pore structure can stabilize NH4NO3 [38]. Moreover, the formation of dynamic binuclear Cu ions can be achieved in the large CHA cage. Therefore, the HTS, poisoning resistance, catalytic selectivity and activity, and reaction mechanism are closely related to the shape selectivity of the SSZ-13 structure.
Based on the shape selectivity of the Cu-SSZ-13 catalyst, here, we summarize the essential characteristics of Cu-based small-pore zeolites with excellent activity and hydrothermal stability: (i) The 8MR pore structure is extremely important to its HTS due to its inhibiting effect on dealumination. Moreover, NH3-SCR reactants with small volume go through the 8MR easily while some large poisons (such as long-chain HCs) are prevented from entering the zeolite. (ii) A suitable elemental composition offers plenty of ion-exchangeable sites and Brønsted sites as well as a stable skeleton. (iii) The large cage-type structure (CHA cage of SSZ-13) provides a reaction zone where a large molecule such as dynamic Cu(NH3)2+ can favourably combine to form di-nuclear active sites to complete the O2 activation. Based on the above principles, researchers have surveyed the zeolite family, and some typical small-pore zeolites with comparable deNOx efficiency or/and HTS to Cu-SSZ-13 were developed.
Hydrothermally stable small-pore zeolites
Utilization of the Cu-SSZ-39 zeolite catalyst with AEI structure for the SCR of NOx was first reported by Moliner et al. [97]. The difference between the AEI and CHA structures is the connection mode of the D6Rs. The neighbouring D6Rs have mirror symmetry in AEI, while they are arranged in parallel in CHA, resulting in different channels and cavities. Recently, we compared the NH3-SCR activity and HTS of Al-rich Cu-SSZ-39 and Cu-SSZ-13 and found that Cu-SSZ-39 showed higher hydrothermal stability but lower deNOx efficiency (Fig. 10a), and both of them showed excellent N2 selectivity, with N2O production less than 10 ppm [98]. It was found that SSZ-39 contained more paired Al, which favoured the formation of stable Cu2+-2Al species, resulting in higher stability for active sites but lower deNOx efficiency compared to [Cu(OH)]+-Al. In another aspect, SSZ-39 has a more tortuous channel structure than SSZ-13 does (Fig. 10b). This structure can inhibit the detached Al(OH)3 from exiting the pores of the AEI zeolite framework, which would result in reincorporation of Al(OH)3 into the framework when the catalyst cools down. However, the tortuous channel is adverse to the mobility of active Cu(NH3)+ species, which further reduces the deNOx efficiency [98]. Furthermore, we investigated the SO2, alkali and alkaline earth metal resistance of Cu-SSZ-39 for NH3-SCR. Similar to Cu-SSZ-13, Cu-SSZ-39 also showed reversible (H2SO4) and irreversible (CuSO4) deactivation after SO2 poisoning. Regeneration at 600°C can recover most of the NH3-SCR activity, but decomposition of CuSO4 needs a higher temperature [99]. However, the catalytic activity and HTS were significantly decreased after alkali/alkaline earth poisoning due to the deterioration of the zeolite structure and CuOx formation from isolated Cu2+, which still should be optimized and improved [100]. To further increase the hydrothermal stability of Cu-SSZ-39, Sano and co-workers used tetraethylphosphonium (TEP) cations as a structure-directing agent to obtain P-modified Cu-SSZ-39 with excellent hydrothermal stability (hydrothermal treatment at 900°C for 4 h). However, fluoride, which is an environmental pollutant and harmful to human health, was required to accelerate mineralization, and NO conversion decreased with increasing P/Al ratio [101]. Martin et al. used N,N-dimethyl-3,5-dimethylpiperidinium and Cu-TEPA as dual OSDAs to directly synthesize Cu-SSZ-39, which showed good NH3-SCR performance, but HTS was not tested [102]. Moreover, given that SSZ-39 has traditionally been synthesized through interzeolite transformation from high-silica Y, of which the preparation is expensive and requires complex post-treatments, Xu et al. successfully synthesized SSZ-39 through interzeolite transformation from low-cost and widely used ZSM-5 and beta zeolites [103]. In summary, Cu-SSZ-39 zeolite exhibits strong potential as an NH3-SCR catalyst for actual application due to its optimal deNOx efficiency and outstanding hydrothermal stability.
Figure 10.
(a) Comparative NH3-SCR performance of Cu-SSZ-39 and Cu-SSZ-13 before and after hydrothermal aging. (b) Channel structures of AEI and CHA zeolites. Adapted with permission from ref. [98]. (c) NH3-SCR performance of Cu-LTA-16–0.48 (red), Cu-LTA-11–0.48 (green) and Cu-LTA-23–0.5 (blue) before (left) and after (right) hydrothermal aging at 900°C. Adapted with permission from ref. [104]. (d) Copper species at different locations and transformation between the two copper species during HTA. Adapted with permission from ref. [105].
Besides Cu-SSZ-39, high silica Cu-LTA is another highly stable zeolite for the NH3-SCR reaction. It was first reported by Hong et al. that fully copper-exchanged high-silica LTA zeolite possessed excellent hydrothermal stability during the NH3-SCR reaction. Even after hydrothermal aging at 900°C, the samples still showed good NOx conversion (Fig. 10c), under which conditions the Cu-SSZ-13 structure collapsed [104]. Furthermore, an increase in the low-temperature standard NH3-SCR activity was found on Cu-LTA with Si/Al of 23 and Cu/Al of 0.5 after hydrothermal aging. They ascribed this to the transformation of inactive Cu+ ions in the sod cage coordinated to four-rings to active Cu2+ ions in the single 6-rings (s6r) centre (Fig. 10d) [105]. In addition, Wang et al. found that HTA prompted the CuOx and Cu2+ to transform to Cu(OH)+ species on a Cu/LTA catalyst prepared by the incipient wetness impregnation (IWI) method, which is opposite to the behaviour of Cu-SSZ-13 [106]. Another aspect that is different from Cu-SSZ-13 is the promotion effect on NOx conversion of Cu-LTA under FSCR conditions [106,107]. Ryu et al. found that both Cu2+ and Cu(OH)+ in Cu-LTA are substantially centred on the single six-rings, where the reactant molecules should be less accessible than the eight-ring window sites, resulting in lower amounts of ammonia nitrate compared to that produced in Cu-SSZ-13 [107]. Regarding the effect of SO2, the Cu(OH)+ species in this catalyst were found to be more vulnerable to SO2 poisoning due to their weak interaction with the zeolite framework structure compared to those in Cu-SSZ-13. Nevertheless, the fresh deNOx efficiency of Cu-LTA can be totally recovered by regeneration at the elevated temperature of 750°C [108]. Although Cu-LTA zeolite showed extraordinary HTS, relatively optimized FSCR performance as well as good SO2 tolerance, the synthesis of Cu-LTA should continue to be explored in future due to the requirement of F− addition in the synthetic process [109,110].
Other alternative small-pore zeolites
Besides AEI and LTA zeolites, there are also some other small-pore zeolites that should be given attention, as shown in Table S1. The highly crystalline KFI-type zeolite was successfully synthesized via transformation of FAU-type zeolite with Na+ and K+ ions in the absence of an organic SDA (OSDA) [111]. The Cu ion-exchanged KFI catalyst showed good NH3-SCR activity and HTS, which, however, was still inferior to that of Cu-SSZ-13 [111]. Nevertheless, Han et al. synthesized high-silica Cu-KFI with Si/Al > 5 by only using K+ as a directing agent, which is more sustainable for zeolite synthesis [112]. Importantly, the high-silica Cu-KFI showed excellent HTS and maintained NOx conversion of ∼57% at 200°C even after HTA at 800°C [112]. Cu-SSZ-50 with the RTH structure, which has 2D channels with aperture sizes of 0.41 nm × 0.38 nm (8MRs) and 0.56 nm × 0.25 nm (distorted 8MRs), also showed comparable NH3-SCR activity but relatively low hydrothermal stability [113,114]. Highly active α species and inert β species both existed in Cu-SSZ-50 with high Cu loading, but the α species were easily movable and transferred to more stable sites during HTA [114]. Cu-SSZ-50 can be synthesized at high temperatures in less than 1 hour [115], which gives it significant potential for application in NH3-SCR as long as the hydrothermal stability can be improved in future. Martin et al. have investigated various cage-based small-pore catalysts, among which Cu-AFX and Cu-ERI were the first to be applied in the NH3-SCR reaction. However, although they have similar small-pore structures to Cu-SSZ-13, the SCR activity and HTS still need improvement [116]. Through transformation of FAU zeolite in the presence of an alkali metal-crown ether (AMCE) complex and RHO seeds, Ke et al. prepared high-silica RHO zeolite with Si/Al of 7.6. The copper ion-exchanged RHO catalyst showed good NH3-SCR performance with relatively high HTS at 800°C [117].
In fact, zeolites with small-pore structures and adequate ion-exchange sites have great potential for utilization as NH3-SCR catalysts with high deNOx efficiency and hydrothermal stability. Researchers have developed many small-pore zeolites in the past several years as discussed above. These zeolites, however, still require comprehensive and systematic in-depth study as well as optimization of physicochemical properties before their practical implementation. Also, it is important that the synthetic method for these developed zeolites is efficient, economical and environmentally friendly. In another aspect, moreover, development of the new type small-pore zeolites with high SCR activity and HTS is still worthwhile based on the design principles proposed above, since there is still considerable room in the small-pore zeolite family for researchers to investigate [118]. Therefore, research should still be devoted to developing and improving small-pore zeolites in the future.
SUMMARY AND PERSPECTIVE
General conclusion
This survey provides an easy-to-read and systematic overview of the reported Cu-based small-pore zeolites applied to the NH3-SCR reaction in the past decade. Using Cu-SSZ-13 as the main example, we presented an overview of the standard and fast SCR mechanisms, hydrothermal stability, chemical poisoning mechanism (SO2, PO43−, HCs, alkali and alkaline earth metals), co-cation effects and synthetic methodology. The discovery of the locally homogenous reaction mechanism (SSCR) is a big step forward in the field of catalytic chemistry. This catalytic reaction mechanism will help researchers rationally design catalysts with dynamic active sites in order to achieve high catalytic activity. For actual application of Cu-SSZ-13, the hydrothermal stability and chemical poisoning tolerance should be improved by carefully tuning the properties in the initial synthetic process and/or post-treatment. By precisely controlling the type and amount of co-cation metals, the SCR activity and hydrothermal stability can be improved. Economical and environmentally friendly synthesis routes for the zeolites mainly include the in situ method, solvent-free method and template-free method, as well as combinations of these. Each method can achieve sustainable development by using rational and economical raw materials. In addition, the unique properties of other small-pore zeolites, especially AEI- and LTA-type zeolites, were also summarized. Cu-AEI and Cu-LTA zeolites showed hydrothermal stability superior to that of Cu-SSZ-13 under certain conditions, while further breakthroughs still need to be made, such as green and sustainable synthetic methods for these other small-pore zeolites.
Opportunities
As we pointed out in the introduction section, the typical properties of Cu-exchanged small-pore zeolites match perfectly with the required characteristics of NH3-SCR catalysts, resulting in excellent deNOx efficiency in the NH3-SCR reaction. First, the dual-functional Cu2+ ion, as both redox and acid site, achieves the limiting case of tight coupling for redox-acid sites in NH3-SCR catalysts. Second, the atomic-level dispersal of Cu2+ also reaches the limits of high dispersion of active sites in NH3-SCR catalysts. Last but not least, the dynamic active sites for the copper-ammonia complex, which is mobile in the zeolite cage, adequately expose them to the reactants. The above three points determine the excellent NH3-SCR activity of Cu2+-exchanged zeolites. Besides, the shape selectivity of the small-pore structure guarantees its high hydrothermal stability and poisoning resistance. In one aspect, the narrow small pores limit the diffusion of hydrolysed Al(OH)3 and accumulation of Cu2+, resulting in outstanding hydrothermal stability of framework and active Cu2+ sites. In another aspect, some long-chain HCs and large poisoning molecules are prevented from access to the active sites. Therefore, Cu-based small-pore zeolites represent an immense opportunity as efficient and stable NH3-SCR catalysts.
In actual application, the consumption of diesel and jet fuel in 2040 will increase 75% compared with that in 2010. Therefore, diesel engines will be irreplaceable as the primary power source for the freight, navigation and marine engine industries and non-road engineering machinery for the foreseeable future, for which saving energy and restraining emissions will be the greatest challenges. Increasingly stringent emission standards are being developed across the world to reduce polluting emissions from diesel engines. In the US, the technological feasibility of achieving an emission limit of 0.02 g/bhp-hr and Low Load Cycle (LLC) limit below 0.075 g/bhp-hr has been demonstrated. In China, highway freight reached ∼40 billion tonnes, accounting for 76.9% of the total freight [5]. The China VI Standards for emissions from diesel-fuelled heavy-duty vehicles will be fully implemented on 1 July 2021. The standard explicitly forbids leakage of V-containing complexes into the atmosphere during the lifetime of vehicles that utilize the V-based SCR catalyst, which indicates that the V-based catalyst will not be applicable for exhaust purification in the future. As an alternative, the development of Cu-based small-pore zeolites with high activity and stability is of vital importance.
Challenges
Despite the great opportunities offered by Cu-based small-pore zeolites, there are still some challenges to be addressed.
The chemical poisoning tolerance (SO2, P and alkali metals etc.) should be improved. For example, although thermal treatment can recover most of the deNOx efficiency of S-poisoned zeolite-based catalysts, steady-state SO2 poisoning results in a significant decrease due to the formation of ammonium sulphate and copper bisulphite. The addition of other metals as sacrificial components is a possible way to increase the SO2 resistance. The small HCs poisoning in the medium temperature range should also be improved in future.
Although the abnormal FSCR reaction has scarcely any positive effect on the deNOx efficiency over Cu-based small-pore zeolites, NO2 is always present due to the partial oxidation of NO in the DOC part. Therefore, the NOx reduction pathway in the presence of NO2 is still worth investigating. The atomic-level understanding of the abnormal FSCR reaction process is still worthy of study, by suitable experiments accompanied by auxiliary calculations.
Besides CHA-, LTA- and AEI-type zeolites, development of other small-pore zeolites with suitable elemental composition and pore structure for the NH3-SCR reaction, which can be attractive alternative candidates for future NH3-SCR catalysts, should be pursued.
The green and sustainable synthesis of small-pore zeolites is important for actual application. Although some economical and environmentally friendly methods to synthesize Cu-SSZ-13 have been reported, they each have advantages and disadvantages. It is urgently necessary to combine the advantages of these methods, such as by combination of in situ, solvent-free and template-free methods. Green and sustainable routes for synthesizing LTA and AEI also need to be addressed in the future. Moreover, the development of ultrafast synthesis methods with various assistive technologies (such as microwave heating or seeded growth) to increase efficiency and reduce the cost is of great importance for industrial application.
Supplementary Material
Contributor Information
Yulong Shan, State Key Joint Laboratory of Environment Simulation and Pollution Control, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, Beijing 100085, China.
Jinpeng Du, State Key Joint Laboratory of Environment Simulation and Pollution Control, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, Beijing 100085, China; College of Resources and Environment, University of Chinese Academy of Sciences, Beijing 100049, China.
Yan Zhang, Center for Excellence in Regional Atmospheric Environment, Institute of Urban Environment, Chinese Academy of Sciences, Xiamen 361021, China.
Wenpo Shan, Center for Excellence in Regional Atmospheric Environment, Institute of Urban Environment, Chinese Academy of Sciences, Xiamen 361021, China.
Xiaoyan Shi, State Key Joint Laboratory of Environment Simulation and Pollution Control, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, Beijing 100085, China; College of Resources and Environment, University of Chinese Academy of Sciences, Beijing 100049, China.
Yunbo Yu, State Key Joint Laboratory of Environment Simulation and Pollution Control, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, Beijing 100085, China; Center for Excellence in Regional Atmospheric Environment, Institute of Urban Environment, Chinese Academy of Sciences, Xiamen 361021, China; College of Resources and Environment, University of Chinese Academy of Sciences, Beijing 100049, China.
Runduo Zhang, State Key Laboratory of Chemical Resource Engineering, Beijing Key Laboratory of Energy Environmental Catalysis, Beijing University of Chemical Technology, Beijing 100029, China.
Xiangju Meng, Key Laboratory of Applied Chemistry of Zhejiang Province, Department of Chemistry, Zhejiang University, Hangzhou 310007, China.
Feng-Shou Xiao, Key Laboratory of Applied Chemistry of Zhejiang Province, Department of Chemistry, Zhejiang University, Hangzhou 310007, China.
Hong He, State Key Joint Laboratory of Environment Simulation and Pollution Control, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, Beijing 100085, China; Center for Excellence in Regional Atmospheric Environment, Institute of Urban Environment, Chinese Academy of Sciences, Xiamen 361021, China; College of Resources and Environment, University of Chinese Academy of Sciences, Beijing 100049, China.
FUNDING
This work was supported by the National Natural Science Foundation of China (21637005, 21906172, 51822811 and 51978640).
Conflict of interest statement. None declared.
REFERENCES
- 1. Sun Q, Xie Z, Yu Jet al. . The state-of-the-art synthetic strategies for SAPO-34 zeolite catalysts in methanol-to-olefin conversion. Natl Sci Rev 2018; 5: 542–58. 10.1093/nsr/nwx103 [DOI] [Google Scholar]
- 2. Xie Z, Liu Z, Wang Yet al. . Applied catalysis for sustainable development of chemical industry in China. Natl Sci Rev 2015; 2: 167–82. 10.1093/nsr/nwv019 [DOI] [Google Scholar]
- 3. Zhang R, Liu N, Lei Zet al. . Selective transformation of various nitrogen-containing exhaust gases toward N2 over zeolite catalysts. Chem Rev 2016; 116: 3658–721. 10.1021/acs.chemrev.5b00474 [DOI] [PubMed] [Google Scholar]
- 4. Zheng B, Tong D, Li Met al. . Trends in China's anthropogenic emissions since 2010 as the consequence of clean air actions. Atmos Chem Phys 2018; 18: 14095–111. 10.5194/acp-18-14095-2018 [DOI] [Google Scholar]
- 5. Ministry of Ecology and Environment of the People's Republic of China . China Mobile Source Environmental Management Annual Report 2020. http://www.mee.gov.cn/xxgk2018/xxgk/xxgk15/202008/t20200810_793252.html (10 February 2021, date last accessed). [Google Scholar]
- 6. Qu W, Liu X, Chen Jet al. . Single-atom catalysts reveal the dinuclear characteristic of active sites in NO selective reduction with NH3. Nat Commun 2020; 11: 1532–8. 10.1038/s41467-020-15261-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shan W, Yu Y, Zhang Yet al. . Theory and practice of metal oxide catalyst design for the selective catalytic reduction of NO with NH3. Catal Today 2020; doi:10.1016/j.cattod.2020.05.015. doi:10.1016/j.cattod.2020.05.015 [Google Scholar]
- 8. Liu F, Yu Y, He H. Environmentally-benign catalysts for the selective catalytic reduction of NOx from diesel engines: structure-activity relationship and reaction mechanism aspects. Chem Commun 2014; 50: 8445–63. 10.1039/C4CC01098A [DOI] [PubMed] [Google Scholar]
- 9. Bull I, Xue WM, Burk Pet al. . Copper CHA zeolite catalysts. US Patent 7,610,662, 2009. [Google Scholar]
- 10. Kwak JH, Tonkyn RG, Kim DHet al. . Excellent activity and selectivity of Cu-SSZ-13 in the selective catalytic reduction of NOx with NH3. J Catal 2010; 275: 187–90. 10.1016/j.jcat.2010.07.031 [DOI] [Google Scholar]
- 11. Kwak JH, Tran D, Burton SDet al. . Effects of hydrothermal aging on NH3-SCR reaction over Cu/zeolites. J Catal 2012; 287: 203–9. 10.1016/j.jcat.2011.12.025 [DOI] [Google Scholar]
- 12. Paolucci C, Khurana I, Parekh AAet al. . Dynamic multinuclear sites formed by mobilized copper ions in NOx selective catalytic reduction. Science 2017; 357: 898–903. 10.1126/science.aan5630 [DOI] [PubMed] [Google Scholar]
- 13. Fickel DW, Lobo RF.. Copper coordination in Cu-SSZ-13 and Cu-SSZ-16 investigated by variable-temperature XRD. J Phys Chem C 2010; 114: 1633–40. 10.1021/jp9105025 [DOI] [Google Scholar]
- 14. Korhonen ST, Fickel DW, Lobo RFet al. . Isolated Cu2+ ions: active sites for selective catalytic reduction of NO. Chem Commun 2011; 47: 800–2. 10.1039/C0CC04218H [DOI] [PubMed] [Google Scholar]
- 15. Kwak JH, Zhu H, Lee JHet al. . Two different cationic positions in Cu-SSZ-13? Chem Commun 2012; 48: 4758–60. 10.1039/c2cc31184d [DOI] [PubMed] [Google Scholar]
- 16. Andersen CW, Bremholm M, Vennestrom PNet al. . Location of Cu2+ in CHA zeolite investigated by X-ray diffraction using the Rietveld/maximum entropy method. IUCrJ 2014; 1: 382–6. 10.1107/S2052252514020181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Paolucci C, Parekh AA, Khurana Iet al. . Catalysis in a cage: condition-dependent speciation and dynamics of exchanged Cu cations in SSZ-13 zeolites. J Am Chem Soc 2016; 138: 6028–48. 10.1021/jacs.6b02651 [DOI] [PubMed] [Google Scholar]
- 18. Di Iorio JR, Gounder R.. Controlling the isolation and pairing of aluminium in chabazite zeolites using mixtures of organic and inorganic structure-directing agents. Chem Mater 2016; 28: 2236–47. 10.1021/acs.chemmater.6b00181 [DOI] [Google Scholar]
- 19. Di Iorio JR, Nimlos CT, Gounder R.. Introducing catalytic diversity into single-site chabazite zeolites of fixed composition via synthetic control of active site proximity. ACS Catal 2017; 7: 6663–74. 10.1021/acscatal.7b01273 [DOI] [Google Scholar]
- 20. Zhang J, Shan Y, Zhang Let al. . Importance of controllable Al sites in CHA framework by crystallization pathways for NH3-SCR reaction. Appl Catal B 2020; 277: 119193–200. 10.1016/j.apcatb.2020.119193 [DOI] [Google Scholar]
- 21. Borfecchia E, Lomachenko KA, Giordanino Fet al. . Revisiting the nature of Cu sites in the activated Cu-SSZ-13 catalyst for SCR reaction. Chem Sci 2015; 6: 548–63. 10.1039/C4SC02907K [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gao F, Mei D, Wang Yet al. . Selective catalytic reduction over Cu/SSZ-13: linking homo- and heterogeneous catalysis. J Am Chem Soc 2017; 139: 4935–42. 10.1021/jacs.7b01128 [DOI] [PubMed] [Google Scholar]
- 23. Lomachenko KA, Borfecchia E, Negri Cet al. . The Cu-CHA deNOx catalyst in action: temperature-dependent NH3-assisted selective catalytic reduction monitored by operando XAS and XES. J Am Chem Soc 2016; 138; 12025–8. 10.1021/jacs.6b06809 [DOI] [PubMed] [Google Scholar]
- 24. Moreno-González M, Hueso B, Boronat Met al. . Ammonia-containing species formed in Cu-chabazite as per in situ EPR, solid-state NMR, and DFT calculations. J Phys Chem Lett 2015; 6: 1011–7. 10.1021/acs.jpclett.5b00069 [DOI] [PubMed] [Google Scholar]
- 25. Martini A, Borfecchia E, Lomachenko KAet al. . Composition-driven Cu-speciation and reducibility in Cu-CHA zeolite catalysts: a multivariate XAS/FTIR approach to complexity. Chem Sci 2017; 8: 6836–51. 10.1039/C7SC02266B [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fahami AR, Günter T, Doronkin DEet al. . The dynamic nature of Cu sites in Cu-SSZ-13 and the origin of the seagull NOx conversion profile during NH3-SCR. React Chem Eng 2019; 4: 1000–18. 10.1039/C8RE00290H [DOI] [Google Scholar]
- 27. Gao F, Walter ED, Kollar Met al. . Understanding ammonia selective catalytic reduction kinetics over Cu/SSZ-13 from motion of the Cu ions. J Catal 2014; 319: 1–14. 10.1016/j.jcat.2014.08.010 [DOI] [Google Scholar]
- 28. Paolucci C, Verma AA, Bates SAet al. . Isolation of the copper redox steps in the standard selective catalytic reduction on Cu-SSZ-13. Angew Chem Int Ed 2014; 126: 12022–7. 10.1002/ange.201407030 [DOI] [PubMed] [Google Scholar]
- 29. Hammershøi PS, Negri C, Berlier Get al. . Temperature-programmed reduction with NO as a characterization of active Cu in Cu-CHA catalysts for NH3-SCR. Catal Sci Technol 2019; 9: 2608–19. 10.1039/C9CY00358D [DOI] [Google Scholar]
- 30. Chen L, Falsig H, Janssens TVWet al. . Effect of Al-distribution on oxygen activation over Cu–CHA. Catal Sci Technol 2018; 8: 2131–6. 10.1039/C8CY00083B [DOI] [Google Scholar]
- 31. Negri C, Selleri T, Borfecchia Eet al. . Structure and reactivity of oxygen-bridged diamino dicopper (II) complexes in Cu-CHA catalyst for NH3-SCR. J Am Chem Soc 2020; 142: 15884–96. 10.1021/jacs.0c06270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen L, Janssens TVW, Vennestrøm PNRet al. . A complete multisite reaction mechanism for low-temperature NH3-SCR over Cu-CHA. ACS Catal 2020; 10: 5646–56. 10.1021/acscatal.0c00440 [DOI] [Google Scholar]
- 33. Luo J, Gao F, Kamasamudram Ket al. . New insights into Cu/SSZ-13 SCR catalyst acidity. Part I: nature of acidic sites probed by NH3 titration. J Catal 2017; 348: 291–9. 10.1016/j.jcat.2017.02.025 [DOI] [Google Scholar]
- 34. Janssens TVW, Falsig H, Lundegaard LFet al. . A consistent reaction scheme for the selective catalytic reduction of nitrogen oxides with ammonia. ACS Catal 2015; 5: 2832–45. 10.1021/cs501673g [DOI] [Google Scholar]
- 35. Kwak JH, Tran D, Szanyi Jet al. . The effect of copper loading on the selective catalytic reduction of nitric oxide by ammonia over Cu-SSZ-13. Catal Lett 2012; 142: 295–301. 10.1007/s10562-012-0771-y [DOI] [Google Scholar]
- 36. Xie L, Liu F, Liu Ket al. . Inhibitory effect of NO2 on the selective catalytic reduction of NOx with NH3 over one-pot-synthesized Cu–SSZ-13 catalyst. Catal Sci Technol 2014; 4: 1104–10. 10.1039/c3cy00924f [DOI] [PubMed] [Google Scholar]
- 37. Shan Y, Shi X, He Get al. . Effects of NO2 addition on the NH3-SCR over small-pore Cu–SSZ-13 zeolites with varying Cu loadings. J Phys Chem C 2018; 122: 25948–53. 10.1021/acs.jpcc.8b05930 [DOI] [Google Scholar]
- 38. Chen HY, Wei Z, Kollar Met al. . A comparative study of N2O formation during the selective catalytic reduction of NOx with NH3 on zeolite supported Cu catalysts. J Catal 2015; 329: 490–8. 10.1016/j.jcat.2015.06.016 [DOI] [Google Scholar]
- 39. Kubota H, Liu C, Toyao Tet al. . Formation and reactions of NH4NO3 during transient and steady-state NH3-SCR of NOx over H-AFX zeolites: spectroscopic and theoretical studies. ACS Catal 2020; 10: 2334–44. 10.1021/acscatal.9b05151 [DOI] [Google Scholar]
- 40. Li S, Zheng Y, Gao Fet al. . Experimental and computational interrogation of fast SCR mechanism and active sites on H-Form SSZ-13. ACS Catal 2017; 7: 5087–96. 10.1021/acscatal.7b01319 [DOI] [Google Scholar]
- 41. Shan Y, Sun Y, Du Jet al. . Hydrothermal aging alleviates the inhibition effects of NO2 on Cu-SSZ-13 for NH3-SCR. Appl Catal B 2020; 275: 119105. 10.1016/j.apcatb.2020.119105 [DOI] [Google Scholar]
- 42. Bendrich M, Scheuer A, Hayes REet al. . Unified mechanistic model for Standard SCR, Fast SCR, and NO2 SCR over a copper chabazite catalyst. Appl Catal B 2018; 222: 76–87. 10.1016/j.apcatb.2017.09.069 [DOI] [Google Scholar]
- 43. Bendrich M, Scheuer A, Hayes REet al. . Increased SCR performance of Cu-CHA due to ammonium nitrate buffer: experiments with oscillating NO/NO2 ratios and application to real driving cycles. Appl Catal B 2020; 270: 118763. 10.1016/j.apcatb.2020.118763 [DOI] [Google Scholar]
- 44. McEwen JS, Anggara T, Schneider WFet al. . Integrated operando X-ray absorption and DFT characterization of Cu–SSZ-13 exchange sites during the selective catalytic reduction of NOx with NH3. Catal Today 2012; 184: 129–44. 10.1016/j.cattod.2011.11.037 [DOI] [Google Scholar]
- 45. Fickel DW, D’Addio E, Lauterbach JAet al. . The ammonia selective catalytic reduction activity of copper-exchanged small-pore zeolites. Appl Catal B 2011; 102: 441–8. 10.1016/j.apcatb.2010.12.022 [DOI] [Google Scholar]
- 46. Kim YJ, Lee JK, Min KMet al. . Hydrothermal stability of CuSSZ13 for reducing NOx by NH3. J Catal 2014; 311: 447–57. 10.1016/j.jcat.2013.12.012 [DOI] [Google Scholar]
- 47. Han S, Ye Q, Cheng Set al. . Effect of the hydrothermal aging temperature and Cu/Al ratio on the hydrothermal stability of CuSSZ-13 catalysts for NH3-SCR. Catal Sci Technol 2017; 7: 703–17. 10.1039/C6CY02555B [DOI] [Google Scholar]
- 48. Song J, Wang Y, Walter EDet al. . Toward rational design of Cu/SSZ-13 selective catalytic reduction catalysts: implications from atomic-level understanding of hydrothermal stability. ACS Catal 2017; 7: 8214–27. 10.1021/acscatal.7b03020 [DOI] [Google Scholar]
- 49. Zhang Y, Peng Y, Li Jet al. . Probing active-site relocation in Cu/SSZ-13 SCR catalysts during hydrothermal aging by in situ EPR spectroscopy, kinetics studies, and DFT calculations. ACS Catal 2020; 10: 9410–9. 10.1021/acscatal.0c01590 [DOI] [Google Scholar]
- 50. Han S, Cheng J, Zheng Cet al. . Effect of Si/Al ratio on catalytic performance of hydrothermally aged Cu-SSZ-13 for the NH3-SCR of NO in simulated diesel exhaust. Appl Surf Sci 2017; 419: 382–92. 10.1016/j.apsusc.2017.04.198 [DOI] [Google Scholar]
- 51. Shan Y, Du J, Yu Yet al. . Precise control of post-treatment significantly increases hydrothermal stability of in-situ synthesized cu-zeolites for NH3-SCR reaction. Appl Catal B 2020; 266: 118655. 10.1016/j.apcatb.2020.118655 [DOI] [Google Scholar]
- 52. Ye X, Schmidt J, Wang RPet al. . Deactivation of Cu-exchanged automotive emissions NH3-SCR catalysts elucidated with nanoscale resolution using scanning transmission X-ray microscopy. Angew Chem Int Ed 2020; 132: 15740–7. 10.1002/ange.201916554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Albarracin-Caballero JD, Khurana I, Di Iorio JRet al. . Structural and kinetic changes to small-pore Cu-zeolites after hydrothermal aging treatments and selective catalytic reduction of NOx with ammonia. React Chem Eng 2017; 2: 168–79. 10.1039/C6RE00198J [DOI] [Google Scholar]
- 54. Schmidt JE, Oord R, Guo Wet al. . Nanoscale tomography reveals the deactivation of automotive copper-exchanged zeolite catalysts. Nat Commun 2017; 8: 1666. 10.1038/s41467-017-01765-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ma Y, Wu X, Cheng Set al. . Relationships between copper speciation and Brønsted acidity evolution over Cu-SSZ-13 during hydrothermal aging. Appl Catal A 2020; 602: 117650. 10.1016/j.apcata.2020.117650 [DOI] [Google Scholar]
- 56. Kumar A, Smith MA, Kamasamudram Ket al. . Chemical deSOx: an effective way to recover Cu-zeolite SCR catalysts from sulfur poisoning. Catal Today 2016; 267: 10–6. 10.1016/j.cattod.2016.01.033 [DOI] [Google Scholar]
- 57. Su W, Li Z, Zhang Yet al. . Identification of sulfate species and their influence on SCR performance of Cu/CHA catalyst. Catal Sci Technol 2017; 7: 1523–8. 10.1039/C7CY00302A [DOI] [Google Scholar]
- 58. Wijayanti K, Leistner K, Chand Set al. . Deactivation of Cu-SSZ-13 by SO2 exposure under SCR conditions. Catal Sci Technol 2016; 6: 2565–79. 10.1039/C5CY01288K [DOI] [Google Scholar]
- 59. Hammershøi PS, Jangjou Y, Epling WSet al. . Reversible and irreversible deactivation of Cu-CHA NH3-SCR catalysts by SO2 and SO3. Appl Catal B 2018; 226: 38–45. 10.1016/j.apcatb.2017.12.018 [DOI] [Google Scholar]
- 60. Brookshear DW, Nam JG, Nguyen Ket al. . Impact of sulfation and desulfation on NOx reduction using Cu-chabazite SCR catalysts. Catal Today 2015; 258: 359–66. 10.1016/j.cattod.2015.04.029 [DOI] [Google Scholar]
- 61. Jangjou Y, Do Q, Gu Yet al. . Nature of Cu active centers in Cu-SSZ-13 and their responses to SO2. ACS Catal 2018; 8: 1325–37. 10.1021/acscatal.7b03095 [DOI] [Google Scholar]
- 62. Hammershøi PS, Jensen AD, Janssens TVW.. Impact of SO2-poisoning over the lifetime of a Cu-CHA catalyst for NH3-SCR. Appl Catal B 2018; 238: 104–10. 10.1016/j.apcatb.2018.06.039 [DOI] [Google Scholar]
- 63. Bergman SL, Dahlin SD, Mesilov VVet al. . In-situ studies of oxidation/reduction of copper in Cu-CHA SCR catalysts: comparison of fresh and SO2-poisoned catalysts. Appl Catal B 2020; 269: 118722–32. 10.1016/j.apcatb.2020.118722 [DOI] [Google Scholar]
- 64. Shan Y, Shi X, Yan Zet al. . Deactivation of Cu-SSZ-13 in the presence of SO2 during hydrothermal aging. Catal Today 2019; 320: 84–90. 10.1016/j.cattod.2017.11.006 [DOI] [Google Scholar]
- 65. Ando R, Hihara T, Banno Yet al. . Detailed mechanism of S poisoning and de-sulfation treatment of Cu-SCR catalyst. SAE Tech Pap 2017; 2017-01-0944. [Google Scholar]
- 66. Wei L, Yao D, Wu Fet al. . Impact of hydrothermal aging on SO2 poisoning over Cu-SSZ-13 diesel exhaust SCR catalysts. Ind Eng Chem Res 2019; 58: 3949–58. 10.1021/acs.iecr.8b04543 [DOI] [Google Scholar]
- 67. Yu R, Zhao Z, Huang Set al. . Cu-SSZ-13 zeolite–metal oxide hybrid catalysts with enhanced SO2-tolerance in the NH3-SCR of NOx. Appl Catal B 2020; 269: 118825. 10.1016/j.apcatb.2020.118825 [DOI] [Google Scholar]
- 68. Xie K, Leistner K, Wijayanti Ket al. . Influence of phosphorus on Cu-SSZ-13 for selective catalytic reduction of NOx by ammonia. Catal Today 2017; 297: 46–52. 10.1016/j.cattod.2017.07.016 [DOI] [Google Scholar]
- 69. Chen Z, Fan C, Pang Let al. . The influence of phosphorus on the catalytic properties, durability, sulfur resistance and kinetics of Cu-SSZ-13 for NOx reduction by NH3-SCR. Appl Catal B 2018; 237: 116–27. 10.1016/j.apcatb.2018.05.075 [DOI] [Google Scholar]
- 70. Xie K, Wang A, Woo Jet al. . Deactivation of Cu-SSZ-13 SCR catalysts by vapor-phase phosphorus exposure. Appl Catal B 2019; 256: 117815–31. 10.1016/j.apcatb.2019.117815 [DOI] [Google Scholar]
- 71. Zhao H, Zhao Y, Liu Met al. . Phosphorus modification to improve the hydrothermal stability of a Cu-SSZ-13 catalyst for selective reduction of NOx with NH3. Appl Catal B 2019; 252: 230–9. 10.1016/j.apcatb.2019.04.037 [DOI] [Google Scholar]
- 72. Wang A, Xie K, Bernin Det al. . Deactivation mechanism of Cu active sites in Cu/SSZ-13: phosphorus poisoning and the effect of hydrothermal aging. Appl Catal B 2020; 269: 118781–92. 10.1016/j.apcatb.2020.118781 [DOI] [Google Scholar]
- 73. Xie K, Woo J, Bernin Det al. . Insights into hydrothermal aging of phosphorus-poisoned Cu-SSZ-13 for NH3-SCR. Appl Catal B 2019; 241: 205–16. 10.1016/j.apcatb.2018.08.082 [DOI] [Google Scholar]
- 74. Xie L, Liu F, Shi Xet al. . Effects of post-treatment method and Na co-cation on the hydrothermal stability of Cu–SSZ-13 catalyst for the selective catalytic reduction of NOx with NH3. Appl Catal B 2015; 179: 206–12. 10.1016/j.apcatb.2015.05.032 [DOI] [Google Scholar]
- 75. Gao F, Wang Y, Washton NMet al. . Effects of alkali and alkaline earth cocations on the activity and hydrothermal stability of Cu/SSZ-13 NH3-SCR catalysts. ACS Catal 2015; 5: 6780–91. 10.1021/acscatal.5b01621 [DOI] [Google Scholar]
- 76. Cui Y, Wang Y, Mei Det al. . Revisiting effects of alkali metal and alkaline earth co-cation additives to Cu/SSZ-13 selective catalytic reduction catalysts. J Catal 2019; 378: 363–75. 10.1016/j.jcat.2019.08.028 [DOI] [Google Scholar]
- 77. Zhao Z, Yu R, Zhao Ret al. . Cu-exchanged Al-rich SSZ-13 zeolite from organotemplate-free synthesis as NH3 -SCR catalyst: effects of Na+ ions on the activity and hydrothermal stability. Appl Catal B 2017; 217: 421–8. 10.1016/j.apcatb.2017.06.013 [DOI] [Google Scholar]
- 78. Usui T, Liu Z, Ibe Set al. . Improve the hydrothermal stability of Cu-SSZ-13 zeolite catalyst by loading a small amount of Ce. ACS Catal 2018; 8: 9165–73. 10.1021/acscatal.8b01949 [DOI] [Google Scholar]
- 79. Wang J, Peng Z, Qiao Het al. . Cerium-stabilized Cu-SSZ-13 catalyst for the catalytic removal of NOx by NH3. Ind Eng Chem Res 2016; 55: 1174–82. 10.1021/acs.iecr.5b03221 [DOI] [Google Scholar]
- 80. Wang Y, Shi X, Shan Yet al. . Hydrothermal stability enhancement of Al-Rich Cu-SSZ-13 for NH3 selective catalytic reduction reaction by ion exchange with cerium and samarium. Ind Eng Chem Res 2020; 59: 6416–23. 10.1021/acs.iecr.0c00285 [DOI] [Google Scholar]
- 81. Zhao Z, Yu R, Shi Cet al. . Rare-earth ion exchanged Cu-SSZ-13 zeolite from organotemplate-free synthesis with enhanced hydrothermal stability in NH3-SCR of NOx. Catal Sci Technol 2019; 9: 241–51. 10.1039/C8CY02033G [DOI] [Google Scholar]
- 82. Fan C, Chen Z, Pang Let al. . Steam and alkali resistant Cu-SSZ-13 catalyst for the selective catalytic reduction of NOx in diesel exhaust. Chem Eng J 2018; 334: 344–54. 10.1016/j.cej.2017.09.181 [DOI] [Google Scholar]
- 83. Luo JY, Yezerets A, Henry Cet al. . Hydrocarbon poisoning of Cu-Zeolite SCR catalysts. SAE Tech Pap 2021; 2012-01-1096. [Google Scholar]
- 84. Selleri T, Nova I, Tronconi Eet al. . The impact of light and heavy hydrocarbons on the NH3-SCR activity of commercial Cu- and Fe-zeolite catalysts. Catal Today 2019; 320: 100–11. 10.1016/j.cattod.2017.09.028 [DOI] [Google Scholar]
- 85. Ye Q, Wang L, Yang RT. Activity, propene poisoning resistance and hydrothermal stability of copper exchanged chabazite-like zeolite catalysts for SCR of NO with ammonia in comparison to Cu/ZSM-5. Appl Catal A 2012; 427–8: 24–34. 10.1016/j.apcata.2012.03.026 [DOI] [Google Scholar]
- 86. Xie L, Liu F, Ren Let al. . Excellent performance of one-pot synthesized Cu-SSZ-13 catalyst for the selective catalytic reduction of NOx with NH3. Environ Sci Technol 2014; 48: 566–72. 10.1021/es4032002 [DOI] [PubMed] [Google Scholar]
- 87. Raj R, Harold MP, Balakotaiah V.. Kinetic modeling of NO selective reduction with C3H6 over Cu-SSZ13 monolithic catalyst. Chem Eng J 2014; 254: 452–62. 10.1016/j.cej.2014.05.105 [DOI] [Google Scholar]
- 88. Zones SI. Conversion of faujasites to high-silica chabazite SSZ-13 in the presence of N,N,N-trimethyl-1-adamantammonium iodide. J Chem Soc Faraday Trans 1991; 22: 3709–16. 10.1039/ft9918703709 [DOI] [Google Scholar]
- 89. Han J, Jin X, Song Cet al. . Rapid synthesis and NH3-SCR activity of SSZ-13 zeolite via coal gangue. Green Chem 2020; 22: 219–29. 10.1039/C9GC02963J [DOI] [Google Scholar]
- 90. Shan Y, Shi X, Du Jet al. . SSZ-13 synthesized by solvent-free method: a potential candidate for NH3-SCR catalyst with high activity and hydrothermal stability. Ind Eng Chem Res 2019; 58: 5397–403. 10.1021/acs.iecr.8b05822 [DOI] [Google Scholar]
- 91. Xiong X, Yuan D, Wu Qet al. . Efficient and rapid transformation of high silica CHA zeolite from FAU zeolite in the absence of water solvent. J Mater Chem A 2017; 5: 9076–80. 10.1039/C7TA01749A [DOI] [Google Scholar]
- 92. Chen B, Xu R, Zhang Ret al. . Economical way to synthesize SSZ-13 with abundant ion-exchanged Cu+ for an extraordinary performance in selective catalytic reduction (SCR) of NOx by ammonia. Environ Sci Technol 2014; 48: 13909–16. 10.1021/es503707c [DOI] [PubMed] [Google Scholar]
- 93. Wang X, Zhang R, Wang Het al. . Strategy on effective synthesis of SSZ-13 zeolite aiming at outstanding performances for NH3-SCR process. Catal Surv Asia 2020; 24: 143–55. 10.1007/s10563-020-09300-w [DOI] [Google Scholar]
- 94. Ren L, Zhu L, Yang Cet al. . Designed copper-amine complex as an efficient template for one-pot synthesis of Cu-SSZ-13 zeolite with excellent activity for selective catalytic reduction of NOx by NH3. Chem Commun 2011; 47: 9789–91. 10.1039/c1cc12469b [DOI] [PubMed] [Google Scholar]
- 95. Schmieg SJ, Oh SH, Kim CHet al. . Thermal durability of Cu-CHA NH3-SCR catalysts for diesel NOx reduction. Catal Today 2012; 184: 252–61. 10.1016/j.cattod.2011.10.034 [DOI] [Google Scholar]
- 96. Blakeman PG, Burkholder EM, Chen HYet al. . The role of pore size on the thermal stability of zeolite supported Cu SCR catalysts. Catal Today 2014; 231: 56–63. 10.1016/j.cattod.2013.10.047 [DOI] [Google Scholar]
- 97. Moliner M, Franch C, Palomares Eet al. . Cu-SSZ-39, an active and hydrothermally stable catalyst for the selective catalytic reduction of NOx. Chem Commun 2012; 48: 8264–6. 10.1039/c2cc33992g [DOI] [PubMed] [Google Scholar]
- 98. Shan Y, Shan W, Shi Xet al. . A comparative study of the activity and hydrothermal stability of Al-rich Cu-SSZ-39 and Cu-SSZ-13. Appl Catal B 2020; 264: 118511–20. 10.1016/j.apcatb.2019.118511 [DOI] [Google Scholar]
- 99. Du J, Shi X, Shan Yet al. . Effects of SO2 on Cu-SSZ-39 catalyst for the selective catalytic reduction of NOx with NH3. Catal Sci Technol 2020; 10: 1256–63. 10.1039/C9CY02186H [DOI] [Google Scholar]
- 100. Zhu N, Shan W, Shan Yet al. . Effects of alkali and alkaline earth metals on Cu-SSZ-39 catalyst for the selective catalytic reduction of NO with NH3. Chem Eng J 2020; 388: 124250–60. 10.1016/j.cej.2020.124250 [DOI] [Google Scholar]
- 101. Sonoda T, Maruo T, Yamasaki Yet al. . Synthesis of high-silica AEI zeolites with enhanced thermal stability by hydrothermal conversion of FAU zeolites, and their activity in the selective catalytic reduction of NOx with NH3. J Mater Chem A 2015; 3: 857–65. 10.1039/C4TA05621C [DOI] [Google Scholar]
- 102. Martin N, Boruntea CR, Moliner Met al. . Efficient synthesis of the Cu-SSZ-39 catalyst for DeNOx applications. Chem Commun 2015; 51: 11030–3. 10.1039/C5CC03200H [DOI] [PubMed] [Google Scholar]
- 103. Xu H, Chen W, Wu Qet al. . Transformation synthesis of aluminosilicate SSZ-39 zeolite from ZSM-5 and beta zeolite. J Mater Chem A 2019; 7: 4420–5. 10.1039/C9TA00174C [DOI] [Google Scholar]
- 104. Ryu T, Ahn NH, Seo Set al. . Fully copper-exchanged high-silica LTA zeolites as unrivaled hydrothermally stable NH3-SCR catalysts. Angew Chem Int Ed 2017; 56: 3256–60. 10.1002/anie.201610547 [DOI] [PubMed] [Google Scholar]
- 105. Ahn NH, Ryu T, Kang Yet al. . The origin of an unexpected increase in NH3-SCR activity of aged Cu-LTA catalysts. ACS Catal 2017; 7: 6781–5. 10.1021/acscatal.7b02852 [DOI] [Google Scholar]
- 106. Wang A, Arora P, Bernin Det al. . Investigation of the robust hydrothermal stability of Cu/LTA for NH3-SCR reaction. Appl Catal B 2019; 246: 242–53. 10.1016/j.apcatb.2019.01.039 [DOI] [Google Scholar]
- 107. Ryu T, Kim H, Hong SB. Nature of active sites in Cu-LTA NH3-SCR catalysts: a comparative study with Cu-SSZ-13. Appl Catal B 2019; 245: 513–21. 10.1016/j.apcatb.2019.01.006 [DOI] [Google Scholar]
- 108. Wang A, Olsson L.. Insight into the SO2 poisoning mechanism for NOx removal by NH3-SCR over Cu/LTA and Cu/SSZ-13. Chem Eng J 2020; 395: 125048–59. 10.1016/j.cej.2020.125048 [DOI] [Google Scholar]
- 109. Jo D, Ryu T, Park GTet al. . Synthesis of high-silica LTA and UFI zeolites and NH3-SCR performance of their copper-exchanged form. ACS Catal 2016; 6: 2443–7. 10.1021/acscatal.6b00489 [DOI] [Google Scholar]
- 110. Jo D, Park GT, Ryu Tet al. . Economical synthesis of high-silica LTA zeolites: a step forward in developing a new commercial NH3-SCR catalyst. Appl Catal B 2019; 243: 212–9. 10.1016/j.apcatb.2018.10.042 [DOI] [Google Scholar]
- 111. Kim J, Cho SJ, Kim DH.. Facile synthesis of KFI-type zeolite and its application to selective catalytic reduction of NOx with NH3. ACS Catal 2017; 7: 6070–81. 10.1021/acscatal.7b00697 [DOI] [Google Scholar]
- 112. Han S, Tang X, Wang Let al. . Potassium-directed sustainable synthesis of new high silica small-pore zeolite with KFI structure (ZJM-7) as an efficient catalyst for NH3-SCR reaction. Appl Catal B 2021; 281: 119480–7. 10.1016/j.apcatb.2020.119480 [DOI] [Google Scholar]
- 113. Jo D, Lim JB, Ryu Tet al. . Unseeded hydroxide-mediated synthesis and CO2 adsorption properties of an aluminosilicate zeolite with the RTH topology. J Mater Chem A 2015; 3: 19322–9. 10.1039/C5TA03559G [DOI] [Google Scholar]
- 114. Shan Y, Shi X, Du Jet al. . Cu-exchanged RTH-type zeolites for NH3-selective catalytic reduction of NOx: Cu distribution and hydrothermal stability. Catal Sci Technol 2019; 9: 106–15. 10.1039/C8CY01933A [DOI] [Google Scholar]
- 115. Xu H, Wu Q, Chu Yet al. . Efficient synthesis of aluminosilicate RTH zeolite with good catalytic performances in NH3-SCR and MTO reactions. J Mater Chem A 2018; 6: 8705–11. 10.1039/C8TA01734D [DOI] [Google Scholar]
- 116. Martín N, Paris C, Vennestrøm PNRet al. . Cage-based small-pore catalysts for NH3-SCR prepared by combining bulky organic structure directing agents with modified zeolites as reagents. Appl Catal B 2017; 217: 125–36. 10.1016/j.apcatb.2017.05.082 [DOI] [Google Scholar]
- 117. Ke Q, Sun T, Cheng Het al. . Accelerated construction of high-silica RHO and CHA zeolites via interzeolite transformation and their NH3-SCR performances after copper exchange. Ind Eng Chem Res 2018; 57: 16763–71. 10.1021/acs.iecr.8b03907 [DOI] [Google Scholar]
- 118. International Zeolite Association Structure Commission . Database of Zeolite Structures. https://asia.iza-structure.org/IZA-SC/ftc_table.php (7 January 2021, date last accessed). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.