Abstract
Objective
To further characterize the effect of guselkumab, a selective IL-23p19-subunit inhibitor approved for PsA, on enthesitis and assess relationships between enthesitis resolution and patient status/outcomes.
Methods
Adults with active PsA despite standard therapies in the phase 3 DISCOVER-1 and DISCOVER-2 studies were randomized 1:1:1 to guselkumab 100 mg every 4 weeks (Q4W); guselkumab 100 mg at week 0, week 4, Q8W; or placebo through week 20 followed by guselkumab 100 mg Q4W. Independent assessors evaluated enthesitis using the Leeds Enthesitis Index (LEI; total score 0–6). Enthesitis findings through week 24 were pre-specified to be pooled across studies; post hoc and week 52 analyses also employed pooled data.
Results
Among 1118 randomized, treated patients in DISCOVER-1 and 2 who had ≥1 LEI site evaluated, 65% had enthesitis at baseline. These patients exhibited numerically more swollen and tender joints, systemic inflammation and impaired physical function than patients without enthesitis. Guselkumab Q4W and Q8W were superior to placebo in resolving pre-existing enthesitis at week 24 (45 and 50% vs 29%; both adjusted P = 0.0301). Enthesitis resolution rates continued to rise; 58% of guselkumab-randomized patients achieved resolution at week 52, including patients with mild (LEI = 1; 70–75%), moderate (LEI = 2; 69–73%) or severe (LEI = 3–6; 42–44%) enthesitis at baseline. Among guselkumab-randomized patients with resolved enthesitis at week 24, 42% achieved minimal disease activity at week 52, vs 17% of patients with unresolved enthesitis.
Conclusion
Guselkumab resulted in higher proportions of PsA patients with resolved enthesitis by week 24, with maintenance of resolution rates through 1 year. As enthesitis confers greater disease burden, sustained resolution could portend better patient outcomes.
Clinical trial registration
DISCOVER 1 (NCT03162796) and DISCOVER 2 (NCT03158285)
Keywords: psoriatic arthritis, biologic, entheses, interleukin-23, p19 protein, spondyloarthritis
Rheumatology key messages
Patients with enthesitis exhibited more active psoriatic arthritis and impaired physical function vs those without enthesitis.
Guselkumab provided higher enthesitis resolution rates at week 24, with maintenance of rates through 1 year.
Patients with resolved enthesitis were more likely to achieve minimal disease activity state.
Introduction
PsA is associated with inflammation within the joints (synovitis), the entheses (enthesitis) and the spine (spondylitis) [1, 2]. Enthesitis, defined as inflammation of tendon, ligament or joint capsule insertion sites to bone [3], is an important clinical finding in PsA and is part of the inflammatory articular disease stem in the Classification for Psoriatic Arthritis criteria [4]. Its prevalence varies, with enthesitis reported in up to 54% of PsA patients [5]. As a potential antecedent to inflammatory and structural changes in the joint and a predominant source of pain, enthesitis confers greater disease burden in patients with PsA [5–7]. Resolution of enthesitis is associated with improvements in function, health-related quality-of-life (HRQoL) and pain [8]. A recent meta-analysis reported that TNF inhibitors (TNFi) and antibodies to IL-17 or IL-12/23 are effective in treating enthesitis [2]. As such, the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis and EULAR now recommend biologic therapy for patients with active enthesitis despite receiving NSAIDs or local steroid injections [9, 10]. Accordingly, evaluation of enthesitis as an end point in PsA clinical trials has become increasingly important.
Basic science and research data implicate the IL‐23–IL‐17 axis in the pathogenesis of psoriasis and PsA. Genetic polymorphisms in the IL-23 pathway are associated with both disorders. In murine enthesitis, IL-23 receptor-expressing innate cells show downstream IL-17 and IL-22 expression and a primary enthesitis-dependent inflammatory arthritis [11, 12]. The normal human enthesis harbours myeloid cells that are capable of producing IL-23 protein [13]. Type-3 innate lymphoid cells and some γδ T cell populations, important to barrier tissue homeostasis, repair and inflammation, are present at human entheseal sites and respond to IL‐1β/IL‐23 signalling with IL‐17 or IL‐22 production [14]. The normal human enthesis also contains conventional Th17 CD4+ T cells, an important target cell population of IL-23 stimulation [15], but the role of such resident IL-23–IL-17-axis cell populations in disease pathogenesis awaits further investigation.
Guselkumab is a high-affinity, human, anti-IL-23p19-subunit monoclonal antibody that is approved to treat moderate-to-severe psoriasis and PsA [16]. The pivotal, placebo-controlled, DISCOVER-1 [17] and DISCOVER-2 trials [18] demonstrated favourable benefit–risk profiles through week 24. Of note, significantly higher rates of enthesitis resolution were observed among guselkumab- than placebo-treated patients at week 24 when, as pre-specified, data were pooled across the trials [18]. We further analysed the pooled DISCOVER-1 and DISCOVER-2 data, to comprehensively investigate the efficacy of guselkumab in both resolving and preventing enthesitis. We also assessed the relationship of both enthesitis status at baseline and enthesitis resolution to the achievement of important patient outcomes through 1 year.
Methods
Patients and study designs
The DISCOVER-1 (NCT03162796) and DISCOVER-2 (NCT03158285) studies, both multicentre, randomized, double-blind, placebo-controlled, phase 3 studies of guselkumab, enrolled adults with active PsA despite standard therapies [conventional synthetic DMARDs (csDMARDs), apremilast, NSAIDs]. In DISCOVER-1, the 381 participants had ≥3 tender and ≥3 swollen joints and CRP ≥0.3 mg/dl. The study allowed ∼30% of enrolled patients to have previously taken one or two TNFi [17]. In DISCOVER-2, 739 biologic-naïve patients with ≥5 tender and ≥5 swollen joints and CRP ≥0.6 mg/dl were enrolled [18].
In both studies, patients were permitted to continue stable use of selected standard treatments, including NSAIDs or other analgesics up to the regional marketed dose approved; oral corticosteroids (≤10 mg/day of prednisone or equivalent dose); or one csDMARD, limited to MTX ≤25 mg/week, SSZ ≤3 g/day, HCQ ≤400 mg/day or LEF ≤20 mg/day. Patients also had to meet screening criteria for laboratory assessments and tuberculosis history, testing and treatment (for latent tuberculosis).
In both studies, patients were randomized to subcutaneous guselkumab 100 mg every 4 weeks (Q4W), guselkumab 100 mg at weeks 0 and 4, then every 8 weeks (Q8W), or placebo followed by crossover to guselkumab 100 mg Q4W at week 24 (Placebo→Q4W). Treatment continued through week 48 in DISCOVER-1, and through week 100 in DISCOVER-2. Efficacy data collected through week 52 are included in the current analyses.
The studies were conducted in compliance with the Declaration of Helsinki and International Council for Harmonization Guidelines for Good Clinical Practice. The protocols were approved by each site’s governing ethical body. Additional study details have previously been reported [17–20].
Assessments
Independent assessors determined tender joint counts (TJCs, 0–68), swollen joint counts (SJCs, 0–66, excluding hips) and evaluated patients for the presence of enthesitis and dactylitis. Enthesitis was assessed in both studies using the Leeds Enthesitis Index (LEI), a tool specifically validated for PsA patients to document the absence (0) or presence (1) of painful entheses among the left and right lateral epicondyle humeri, left and right medial femoral condyle and left and right Achilles tendon insertions (LEI score range: 0–6) [21].
Additionally, patients reported their pain level [0–10 cm visual analogue scale (VAS)], global impression of disease activity (0–10 cm VAS), and physical function (HAQ-Disability Index; 0–3). Investigators completed the global assessment of disease activity (0–10 cm VAS) and assessed the severity of skin disease using the Investigator’s Global Assessment (IGA) of psoriasis [total score 0–4 averaged across induration, erythema and scaling considered to be cleared (0), minimal (1), mild (2), moderate (3) or severe (4)]. The Psoriasis Area and Severity Index (PASI, total score 0–72) assessed the extent (percentage of body surface area affected) and degree of associated redness, thickness and scaling [each graded from none (0) to maximum (4)]. Serum CRP (mg/dl) was determined. The 36-item Short-Form Health Survey (SF-36) physical and mental component summary (PCS and MCS) scores were used to assess HRQoL.
Data analyses
As pre-specified, pooled data from DISCOVER-1 and 2 were employed to assess changes from baseline in the number of tender LEI sites [i.e. LEI entheseal count (EC)] and proportions of patients achieving enthesitis resolution (LEI EC = 0) at week 24 among patients with enthesitis (LEI EC ≥1) at baseline [18]. For consistency, these same data at week 52 were also pooled across DISCOVER-1 and 2. Of note, 10 patients in DISCOVER-2 had only 4 of 6 LEI sites evaluated at baseline, including eight with enthesitis (considered to have enthesitis based on LEI EC ≥1 and included in analyses, but with an incomplete and thus missing actual baseline LEI score) and two with no enthesitis identified (excluded from analyses). Treatment failure (TF) rules were applied through week 24 as pre-specified [18] and after week 24 were applied post hoc [19]. Patients meeting TF criteria were considered non-responders. Missing binary data were imputed as non-response; continuous data were imputed as no change from baseline if missing following early discontinuation or using multiple imputation (assumed to be missing-at-random) if missing for other reasons. Also, as pre-specified, the observed number of LEI sites with newly developed enthesitis was determined through week 52 among patients with no enthesitis at baseline.
We undertook several post hoc analyses of pooled data from patients with enthesitis at baseline. Key baseline characteristics were summarized for patients with enthesitis (LEI EC ≥1), including those with mild (LEI score = 1), moderate (LEI score = 2) or severe (LEI score = 3–6) enthesitis and patients without enthesitis at baseline (LEI EC = 0). The least squares (LS) mean change in LEI score from baseline to week 24 was determined for patients with mild, moderate or severe enthesitis at baseline. Enthesitis resolution rates at week 24, with application of TF rules and imputation of missing data as described above, were evaluated in subgroups of patients defined by baseline demographic and clinical disease characteristics, including sex (male, female), age (<45, 45 to <65, ≥65 years), body weight (≤90, >90 kg), BMI (<25, 25 to <30, ≥30 kg/m2), PsA duration (<1, 1 to <3, ≥3 years), swollen and tender joints (<10, 10–15, >15), CRP level (<1, 1 to ≤2, >2 mg/dl), IGA score (<2, ≥2), PASI score (<12, 12 to 20, ≥20) and baseline use of csDMARDs including MTX (yes/no). Utilizing observed data, the median time to first resolution of enthesitis for each treatment group was determined by Kaplan–Meier analysis.
In shift analyses of pooled patients with mild, moderate or severe enthesitis at baseline (categories assigned post hoc), observed post-baseline status was categorized as resolved (post-baseline LEI score = 0), stable or partially improved (0 < post-baseline LEI score ≤ baseline LEI score) or worse (post-baseline LEI score > baseline LEI score) at weeks 4, 8, 16, 24 and 52.
The observed patient-level enthesitis resolution status over time through week 52 was categorized as full (post-baseline LEI EC = 0), partial (post-baseline LEI EC < baseline LEI EC) or stable/worse (post-baseline LEI EC ≥ baseline LEI EC) and displayed by a heatmap.
Additional post hoc analyses of other selected clinical outcomes at weeks 24 and 52 were conducted in the pooled subgroups of patients with (LEI EC ≥1) and without (LEI EC = 0) enthesitis at baseline. Clinical outcomes assessed included ≥20%/50%/70% improvement in the ACR response criteria (ACR20/ACR50/ACR70); mean change from baseline in the 28-joint DAS using CRP (DAS28-CRP); IGA response (IGA = 0/1 and ≥2-point reduction from baseline) in patients with ≥3% of body surface area affected with psoriasis and baseline IGA score ≥2; ≥75%/90%/100% improvement in PASI (PASI75/PASI90/PASI100) in patients with ≥3% of body surface area psoriasis involvement and baseline IGA ≥2; mean changes from baseline in HAQ-DI, SF-36 PCS and SF-36 MCS scores; and achievement of minimal disease activity (MDA) [22].
The relationships between enthesitis resolution and the following outcomes, at both weeks 24 and 52, were assessed using χ2 analysis: MDA; normalized HAQ-DI (score <0.5; in patients with baseline HAQ-DI ≥0.5); ACR50 response; and SJC and TJC ≤1.
The guselkumab effect size for the mean change from baseline in LEI score within each treatment group was determined at week 24 using Cohen’s D, defined as the difference between the mean LEI scores at baseline and week 24 divided by the pooled standard deviation of the scores at baseline and week 24. Effect size was calculated for all patients with enthesitis at baseline and also by baseline concomitant MTX use (yes/no).
Results
Baseline demographic characteristics
Among pooled DISCOVER-1 and DISCOVER-2 patients, 728 (65%) had enthesitis based on evaluation of ≥1/6 LEI sites at baseline (i.e. LEI EC ≥1), including 243 patients in the Q4W, 230 in the Q8W and 255 in the Placebo→Q4W groups. As previously reported, baseline demographic and disease characteristics were generally consistent across randomized treatment groups in both studies [17, 18]. Relative to pooled patients without enthesitis (LEI EC = 0) at baseline, the subgroup of patients with enthesitis at baseline (mean LEI score = 2.8) was characterized by somewhat higher proportions of female patients and patients with BMI ≥30, dactylitis, IGA score = 4 and PASI score ≥20; numerically higher mean values for indicators of more active disease (e.g. swollen and tender joint counts, DAS28-CRP score, serum CRP levels, PASI score); and impaired physical function (Table 1).
Table 1.
Baseline characteristics in PsA patients with or without enthesitis at baseline
| Characteristic | All patients | Patients with enthesitis (LEI EC ≥1) | Patients without enthesitis (LEI EC = 0) |
|---|---|---|---|
| Pooled randomized, treated patients, n | 1120a | 728b | 390c |
| Age, mean (s.d.), years | 46.6 (11.7) | 46.4 (11.4) | 47.0 (12.1) |
| Sex, % | |||
| Male | 52.1 | 49.9 | 56.4 |
| Female | 47.9 | 50.1 | 43.6 |
| Weight, mean (s.d.), kg | 84.9 (19.3) | 85.1 (20.1) | 84.5 (17.7) |
| BMI, mean (s.d.), kg/m2 | 29.2 (6.1) | 29.4 (6.4) | 28.8 (5.5) |
| Normal (<25), % | 25.5 | 25.8 | 25.1 |
| Overweight (≥25 and <30), % | 34.5 | 32.4 | 38.2 |
| Obese (≥30), % | 40.0 | 41.8 | 36.7 |
| PsA disease duration, mean (s.d.), years | 5.9 (6.1) | 6.1 (6.4) | 5.6 (5.4) |
| Joint counts, mean (s.d.) | |||
| Swollen (0–66) | 11.4 (7.4) | 12.3 (7.9) | 9.8 (6.0) |
| Tender (0–68) | 20.6 (13.3) | 23.8 (14.2) | 14.6 (8.6) |
| DAS28-CRP, mean (s.d.) | 5.1 (1.0) | 5.3 (1.0) | 4.7 (1.0) |
| Dactylitis at baselinec, % | 42.3 | 48.5 | 30.8 |
| Dactylitis severity score (1–60)c, mean (s.d.) | 8.2 (9.6) | 9.1 (10.3) | 5.6 (6.4) |
| Enthesitis (LEI) score (1–6), mean (s.d.) | 2.8 (1.6) d | 2.8 (1.6)e | — |
| HAQ-DI (0–3), mean (s.d.) | 1.2 (0.6)f | 1.3 (0.6) | 1.1 (0.6) |
| CRP, mean (s.d.), mg/dl | 1.8 (2.3) | 2.0 (2.5) | 1.5 (1.8) |
| IGA score (0–4), % | |||
| Cleared (0) | 2.4 | 2.9 | 1.5 |
| Minimal (1) | 16.5 | 15.2 | 19.0 |
| Mild (2) | 36.2 | 35.0 | 38.5 |
| Moderate (3) | 36.5 | 37.0 | 35.4 |
| Severe (4) | 8.4 | 9.9 | 5.6 |
| PASI score (0–72), mean (s.d.) | 9.5 (10.6) | 10.2 (11.3) | 8.1 (9.1) |
| <12, % | 74.4 | 72.4 | 78.2 |
| ≥12 and <20, % | 12.9 | 13.6 | 11.5 |
| ≥20, % | 12.7 | 14.0 | 10.3 |
| csDMARD use at baseline, % | 67.8 | 68.7 | 66.2 |
| MTX | 58.4 | 59.1 | 57.2 |
| Other | 9.4 | 9.6 | 9.0 |
Results are pooled across DISCOVER-1 and DISCOVER-2.
Among 1120 patients, 1118 were included in enthesitis analyses, eight of whom had enthesitis noted among the 4/6 LEI sites assessed at baseline (LEI EC ≥1, but actual LEI score incomplete and considered missing), and two with no enthesitis noted among the 4/6 LEI sites assessed at baseline were excluded from enthesitis analyses.
Includes eight patients with LEI EC ≥1 at baseline based on only 4/6 LEI sites evaluated.
Excludes two patients with no enthesitis noted at 4/6 LEI sites or no dactylitis noted at DSS sites assessed at baseline.
Among 1110 patients with non-missing/complete LEI scores at baseline.
Among 720 patients with non-missing/complete LEI scores at baseline.
n = 1119.
csDMARD: conventional synthetic DMARD; DSS: Dactylitis Severity Score; EC: enthesitis count; IGA: Investigator’s Global Assessment of psoriasis; LEI: Leeds Enthesitis Index; PASI: Psoriasis Area and Severity Index.
Baseline characteristics in patients with mild, moderate or severe enthesitis indicated that patients with more severe enthesitis comprised a larger proportion of females and obese patients, and had longer duration of PsA, a lower CRP level, higher joint counts and more extensive psoriasis skin involvement (Supplementary Table S1, available at Rheumatology online).
Enthesitis resolution/improvement
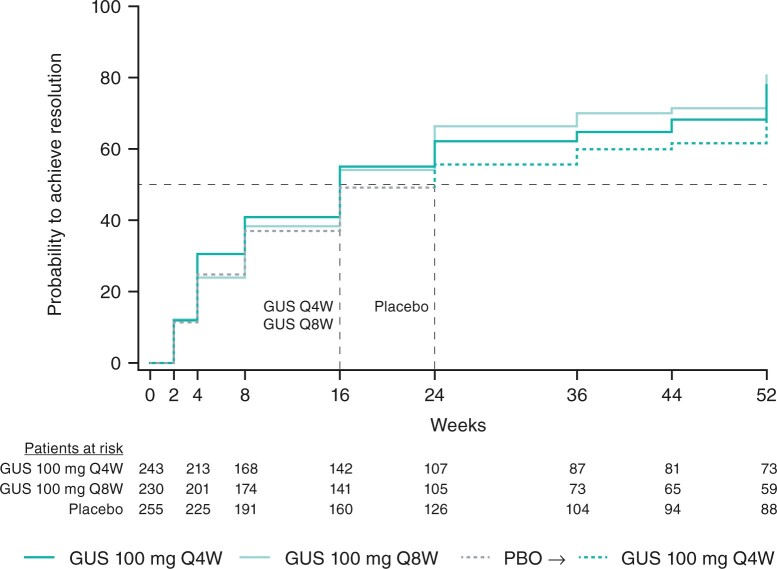
As previously reported [18], significantly higher proportions of pooled patients with enthesitis at baseline (LEI EC ≥1) achieved resolution (LEI EC = 0) at week 24 in the guselkumab Q4W (45%, 109/243; multiplicity-adjusted P = 0.0301 vs placebo) and Q8W (50%, 114/230; P = 0.0301 vs placebo) than placebo (29%, 75/255) groups (Table 2). The time to the first resolution of enthesitis in pooled patients with enthesitis at baseline is shown in Fig. 1. The median time to enthesitis resolution was shorter in patients receiving guselkumab Q4W or Q8W (both 16 weeks) than placebo (24 weeks).
Table 2.
Enthesitis resolution/improvementa through week 52 in patients with enthesitis at baseline and clinical responsea through week 52 in PsA patients with or without enthesitis at baseline
| Outcome | Patients with enthesitis (LEI EC ≥1) |
Patients without enthesitis (LEI EC = 0) |
|||||
|---|---|---|---|---|---|---|---|
| Guselkumab 100 mg |
Placebo (wks 0–24)→Q4W (wks 24–52) | Guselkumab 100 mg |
Placebo (wks 0–24)→Q4W (wks 24–52) | ||||
| Week | Q4W | Q8W | Q4W | Q8W | |||
| Randomized, treated pts, n | 243 | 230 | 255 | 130 | 144 | 116 | |
| Enthesitis resolution (LEI = 0), % (95% CI)b; adjusted P-value vs placeboc | 24 | 44.9 (38.4, 51.3); 0.0301 | 49.6 (42.9, 56.2); 0.0301 | 29.4 (23.6, 35.2) | — | — | — |
| 52 | 57.6 (51.2, 64.4) | 57.8 (51.2, 64.4) | 61.6 (55.4, 67.7) | — | — | — | |
| Randomized, treated pts with evaluable data at week 52, n | 237 | 225 | 251 | — | — | — | |
| 24 | −1.6 (−1.8, −1.4) | −1.5 (−1.7, −1.3) | −1.0 (−1.2, −0.8) | — | — | — | |
| 52 | −1.8 (−2.0, −1.6) | −1.8 (−2.0, −1.6) | −1.8 (−2.0, −1.7) | — | — | — | |
| Clinical outcomes in randomized, treated pts, n | 243 | 230 | 255 | 130 | 144 | 116 | |
| ACR20, % (95% CI)b | 24 | 61.3 (55.0, 67.6) | 59.6 (53.0, 66.1) | 29.8 (24.0, 35.6) | 63.8 (55.2, 72.5) | 60.4 (52.1, 68.8) | 27.6 (19.0, 36.2) |
| 52 | 72.4 (66.6, 78.3) | 68.3 (62.0, 74.5) | 58.0 (51.8, 64.3) | 70.0 (61.7, 78.3) | 71.5 (63.8, 79.2) | 69.0 (60.1, 77.8) | |
| ACR50, % (95% CI)b | 24 | 31.3 (25.2, 37.3) | 27.4 (21.4, 33.4) | 13.3 (9.0, 17.7) | 39.2 (30.5, 48.0) | 36.8 (28.6, 45.0) | 10.3 (4.4, 16.3) |
| 52 | 46.9 (40.4, 53.4) | 42.6 (36.0, 49.2) | 33.3 (27.4, 39.3) | 51.5 (42.6, 60.5) | 49.3 (40.8, 57.8) | 45.7 (36.2, 55.2) | |
| ACR70, % (95% CI)b | 24 | 11.1 (7.0, 15.3) | 11.7 (7.4, 16.1) | 5.1 (2.2, 8.0) | 23.8 (16.1, 31.6) | 23.6 (16.3, 30.9) | 3.4 (0.0, 7.2) |
| 52 | 24.7 (19.1, 30.3) | 25.7 (19.8, 31.5) | 15.7 (11.0, 20.3) | 31.5 (23.2, 39.9) | 29.9 (22.0, 37.7) | 20.7 (12.9, 28.5) | |
| DAS28-CRP, LS mean (95% CI)b change from baselined | 24 | −1.6 (−1.8, −1.5) | −1.5 (−1.7, −1.4) | −0.9 (−1.1, −0.8) | −1.5 (−1.7, −1.3) | −1.5 (−1.7, −1.3) | −0.8 (−1.0, −0.6) |
| 52 | −2.0 (−2.2, −1.9) | −1.9 (−2.1, −1.8) | −1.8 (−2.0, −1.7) | −1.9 (−2.1, −1.7) | −1.9 (−2.1, −1.6) | −1.8 (−2.0, −1.6) | |
| 24 | −0.4 (−0.4, −0.3) | −0.4 (−0.4, −0.3) | −0.1 (−0.2, 0.0) | −0.4 (−0.5, −0.3) | −0.3 (−0.4, −0.2) | −0.1 (−0.2, −0.0) | |
| 52 | −0.5 (−0 6, −0.4) | −0.4 (−0.5, −0.4) | −0.3 (−0.4, −0.2) | −0.5 (−0.6, −0.4) | −0.4 (−0.5, −0.3) | −0.3 (−0.4, −0.3) | |
| 24 | 6.7 (5.8, 7.7) | 7.0 (6.0, 7.9) | 3.3 (2.4, 4.2) | 6.9 (5.6, 8.2) | 6.4 (5.1, 7.7) | 1.9 (0.5, 3.4) | |
| 52 | 8.5 (7.4, 9.6) | 8.1 (7.0, 9.2) | 6.9 (5.8, 7.9) | 8.1 (6.6, 9.5) | 7.4 (5.9, 8.8) | 6.2 (4.6, 7.8) | |
| SF-36 MCS, LS mean (95% CI)b change from baselined | 24 | 4.1 (3.0, 5.2) | 4.1 (2.9, 5.2) | 1.8 (0.7, 2.9) | 3.5 (2.0, 4.9) | 3.3 (1.9, 4.7) | 2.6 (1.1, 4.2) |
| 52 | 4.7 (3.6, 5.8) | 5.0 (3.9, 6.1) | 4.0 (2.9, 5.0) | 3.8 (2.3, 5.2) | 3.4 (2.0, 4.8) | 4.3 (2.7, 5.8) | |
| MDA, % (95% CI)b | 24 | 16.9 (12.0, 21.8) | 19.1 (13.8, 24.4) | 7.1 (3.7, 10.4) | 33.8 (25.3, 42.4) | 32.6 (24.6, 40.6) | 9.5 (3.7, 15.2) |
| 52 | 30.9 (24.9, 36.9) | 26.5 (20.6, 32.4) | 23.5 (18.1, 28.9) | 45.4 (36.4, 54.3) | 37.5 (29.2, 45.8) | 37.9 (28.7, 47.2) | |
| Skin responses in randomized, treated pts with ≥3% BSA psoriasis and IGA ≥2 at baseline, n | 187 | 162 | 182 | 86 | 96 | 79 | |
| IGA 0/1, % (95% CI)b | 24 | 71.1 (64.4, 77.9) | 64.8 (57.2, 72.5) | 17.6 (11.8, 23.4) | 69.8 (59.5, 80.1) | 68.8 (59.0, 78.5) | 19.0 (9.7, 28.3) |
| 52 | 80.2 (74.2, 86.2) | 71.6 (64.4, 78.9) | 76.4 (69.9, 82.8) | 80.2 (71.2, 89.2) | 69.8 (60.1, 79.5) | 74.7 (64.5, 84.9) | |
| PASI75, % (95% CI)b | 24 | 81.8 (76.0, 87.6) | 77.2 (70.4, 83.9) | 18.7 (12.7, 24.6) | 79.1 (69.9, 88.2) | 79.2 (70.5, 87.8) | 24.1 (14.0, 34.1) |
| 52 | 89.3 (84.6, 94.0) | 81.5 (75.2, 87.8) | 81.9 (76.0, 87.7) | 87.2 (79.6, 94.8) | 82.3 (74.1, 90.4) | 74.7 (64.5, 84.9) | |
| PASI90, % (95% CI)b | 24 | 62.6 (55.4, 69.8) | 61.7 (53.9, 69.5) | 8.8 (4.4, 13.2) | 59.3 (48.3, 70.3) | 64.6 (54.5, 74.7) | 13.9 (5.7, 22.2) |
| 52 | 77.0 (70.7, 83.3) | 71.0 (63.7, 78.3) | 69.2 (62.3, 76.2) | 74.4 (64.6, 84.2) | 68.8 (59.0, 78.5) | 68.4 (57.5, 79.2) | |
| PASI100, % (95% CI)b | 24 | 44.4 (37.0, 51.8) | 35.8 (28.1, 43.5) | 2.2 (0.0, 4.6) | 45.3 (34.2, 56.5) | 44.8 (34.3, 55.3) | 7.6 (1.1, 14.1) |
| 52 | 57.8 (50.4, 65.1) | 48.1 (40.1, 56.2) | 49.5 (41.9, 57.0) | 64.0 (53.2, 74.7) | 53.1 (42.6, 63.6) | 58.2 (46.7, 69.7) | |
Results are pooled across DISCOVER-1 and DISCOVER-2.
Patients meeting treatment failure criteria and with missing dichotomous end point data were considered non-responders. Continuous end point data missing following discontinuation or for other reasons were imputed as no change from baseline or using MI (assumed to be missing-at-random), respectively. Patients who received placebo only before discontinuing treatment had missing data imputed as non-response or no improvement through week 52.
95% confidence intervals based on the Wald statistic.
P-value adjusted for multiplicity [18]. Treatment difference assessed via a Cochran–Mantel–Haenszel test.
LS mean determined using an analysis of covariance model.
ACR20/50/70: ≥20/50/70% improvement per ACR response criteria; BSA: body surface area; EC: enthesitis count; IGA 0/1: Investigator’s Global Assessment of psoriasis score of 0 or 1 and ≥ 2-point reduction from baseline; LEI: Leeds Enthesitis Index; LS: least squares; MDA: Minimal Disease Activity; MI: multiple imputation; PASI75/90/100: ≥75/90/100% improvement in the Psoriasis Area and Severity Index; pts: patients; Q4/8W: every 4/8 weeks; SF-36 PCS/MCS: 36-item short form health survey physical/mental component summary; wk: week.
Fig. 1.
Time to first enthesitis resolution through week 52 in PsA patients with enthesitis at baseline
Observed resolution status pooled across DISCOVER-1 and DISCOVER-2. The intersections of the dashed lines represent the time points at which 50% of patients achieved resolution of enthesitis in the Q4W and Q8W groups (week 16) and in the placebo-crossover group (week 24). Enthesitis defined as LEI EC ≥ 1; enthesitis resolution defined as LEI EC = 0. Patients ‘at risk’ at each visit are those still with enthesitis. GUS: guselkumab; EC: enthesitis count; LEI: Leeds Enthesitis Index; PBO, placebo; Q4W/Q8W: every 4/8 weeks.
Among these same patients (and with patients who discontinued study agent considered non-responders thereafter), rates of resolution and improvements in LEI ECs seen at week 24 in the guselkumab Q4W and Q8W groups were maintained at week 52, when 58% had enthesitis resolution and, on average, LEI ECs improved by 64% from baseline. Consistent results were seen in patients who crossed over from Placebo→Q4W (Table 2).
Enthesitis resolution/improvement at week 24 by baseline characteristics
Higher rates of enthesitis resolution with guselkumab Q4W and Q8W than placebo at week 24 were consistently seen across most demographic and clinical disease characteristics subgroups evaluated, including in patients with longer duration of PsA and more extensive joint and skin involvement. Exceptions included the relatively small subgroups of patients receiving guselkumab Q4W with PsA duration <1 year and no csDMARD use at baseline and patients receiving guselkumab Q8W with <10 tender joints at baseline (Fig. 2).
Fig. 2.
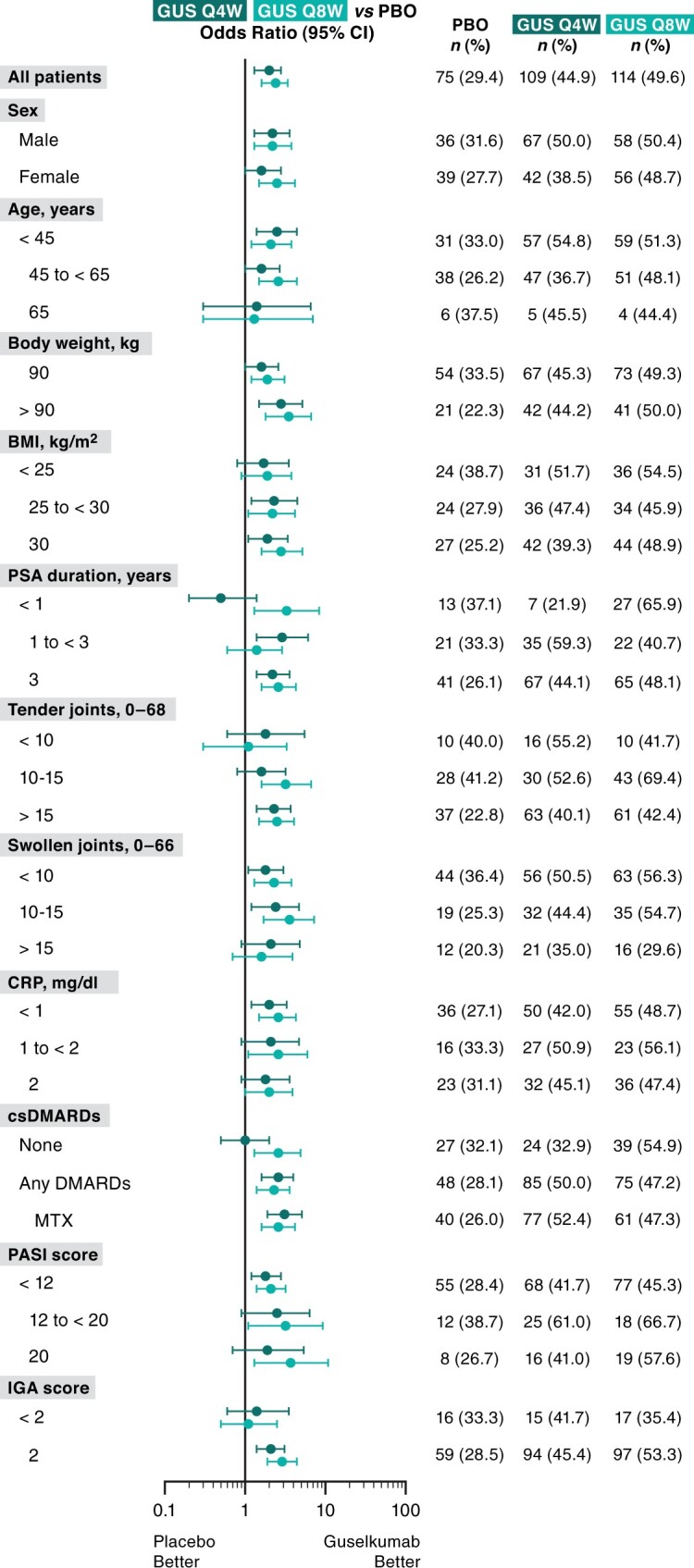
Enthesitis resolution at week 24 by baseline characteristics in PsA patients with enthesitis at baseline
Data pooled across DISCOVER-1 and DISCOVER-2. Enthesitis defined as LEI EC ≥1; enthesitis resolution defined as LEI EC = 0 (patients meeting TF criteria were considered non-responders). Q4W, n = 243; Q8W, n = 230; PBO, n = 255. csDMARD: conventional synthetic DMARD; EC: enthesitis count; GUS: guselkumab; IGA: Investigator’s Global Assessment of psoriasis; LEI: Leeds Enthesitis Index; PASI: Psoriasis Area and Severity Index; PBO: placebo; Q4W/Q8W: every 4/8 weeks.
LS mean changes in LEI score at week 24 for patients with mild, moderate or severe enthesitis at baseline are shown in Supplementary Table S2, available at Rheumatology online. Improvements in LS mean LEI score were greater in both guselkumab groups compared with placebo, with the greatest improvements seen in patients with severe enthesitis.
Effect size
Cohen’s D-values for mean change from baseline in LEI score at week 24 were 1.07 in the Q4W group and 0.91 in the Q8W group, indicating a large effect size (Supplementary Table S3, available at Rheumatology online). Cohen’s D-value for the placebo group was 0.67 (medium effect size). Similar effect sizes were observed for patients who were and were not receiving concomitant MTX at baseline.
Shift in enthesitis severity from baseline through week 52
Among pooled patients with mild enthesitis (LEI score = 1) at baseline, numerical treatment differences in the proportions of guselkumab Q4W and Q8W-treated patients achieving resolution were observed beginning at week 8. By week 24, 68 and 66%, respectively, of these patients, compared with 43% of placebo-treated patients, had resolved enthesitis. By week 52, 70–75% of guselkumab-randomized patients with mild enthesitis at baseline, and 80% of such patients who crossed over to guselkumab Q4W at week 24, achieved enthesitis resolution (Supplementary Fig. S1, available at Rheumatology online).
Similar response patterns were observed in pooled patients with moderate (LEI score = 2) enthesitis at baseline. In such patients, treatment effects were also evident by week 8, and more than two-thirds had achieved enthesitis resolution by week 52. Patients starting the study with severe enthesitis (LEI score = 3–6), who as noted previously comprised larger proportions of females and obese patients, with lower CRP levels, longer PsA duration, higher joint counts, more severe dactylitis and more extensive psoriasis skin involvement (Supplementary Table S1, available at Rheumatology online), demonstrated substantially lower rates of enthesitis resolution throughout the study. Numerical differences from placebo were not evident until week 16 and fewer than half of these patients achieved enthesitis resolution by week 52 (Supplementary Fig. S1, available at Rheumatology online).
Enthesitis changes in individual patients
Employing a heat map analysis to evaluate patient-level response in DISCOVER-1 and DISCOVER-2 patients with enthesitis at baseline, it can be seen that more placebo- than guselkumab-treated patients had stable or worse enthesitis through week 24. At week 52, most guselkumab-treated patients, including those in the Placebo→Q4W group, had improved or resolved enthesitis (Fig. 3).
Fig. 3.
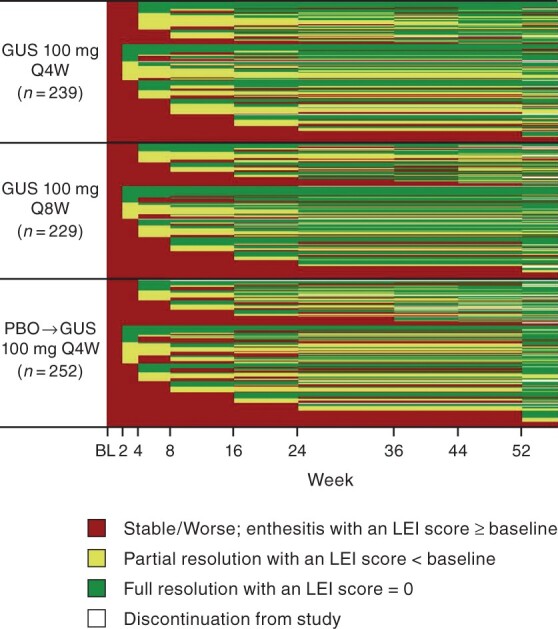
Heat-map of patient-level changes in enthesitis through week 52 in PsA patients with enthesitis at baseline
Observed scores pooled across DISCOVER-1 and DISCOVER-2. Enthesitis defined as LEI score ≥1 (n = 720, excludes eight patients with assessment of only 4/6 LEI entheseal sites). Patients with missing data following discontinuation are shown as white. BL: baseline; GUS: guselkumab; LEI: Leeds Enthesitis Index; Q4W/Q8W: every 4/8 weeks.
New-onset enthesitis
Through week 52, the vast majority of pooled patients without enthesitis at baseline did not develop the condition. Numerical differences in the proportions of patients with no new-onset enthesitis were evident at week 24 between patients receiving guselkumab Q4W (89%) and Q8W (92%) and placebo-treated patients (81%). At week 52, 83–87% of all guselkumab-treated patients, including those crossing over from placebo, without enthesitis at baseline remained so (Supplementary Fig. S2, available at Rheumatology online).
Relationships between enthesitis status and extra-entheseal clinical response
Extra-entheseal clinical response by baseline enthesitis status
An ACR20 response at week 24, the primary end point of DISCOVER-1 and DISCOVER-2, was achieved by similar proportions of guselkumab-randomized patients with (60–61%) and without (60–64%) enthesitis at baseline. Efficacy was sustained through 52 weeks in both enthesitis cohorts, at which time 68–72% of guselkumab-randomized patients with enthesitis and 70–72% of those without enthesitis at baseline achieved ACR20 response. Findings were similar for mean changes in DAS28-CRP scores. The proportion of patients achieving the more stringent ACR50 and ACR70 response criteria appeared to be somewhat lower in the subgroup of patients with enthesitis at baseline, possibly owing to the more established and potentially refractory disease seen in such patients at study outset. Robust and sustained improvements in skin psoriasis were also observed across baseline enthesitis cohorts. Of note, 38–45% of guselkumab-randomized patients without enthesitis, and 27–31% of those with enthesitis, at baseline achieved MDA at week 52 (Table 2).
Guselkumab treatment effects were also consistent for patients with and without enthesitis at baseline when assessing improvements in physical function (HAQ-DI score) and HRQoL (SF-36 PCS and MCS scores) at week 24, although guselkumab-randomized patients with enthesitis at baseline appeared to have more improvement through week 52 than patients without enthesitis at baseline in mental aspects of HRQoL (SF-36 MCS LS mean changes: 4.7–5.0 vs 3.4–3.8) (Table 2).
Relationships between enthesitis resolution and clinical response
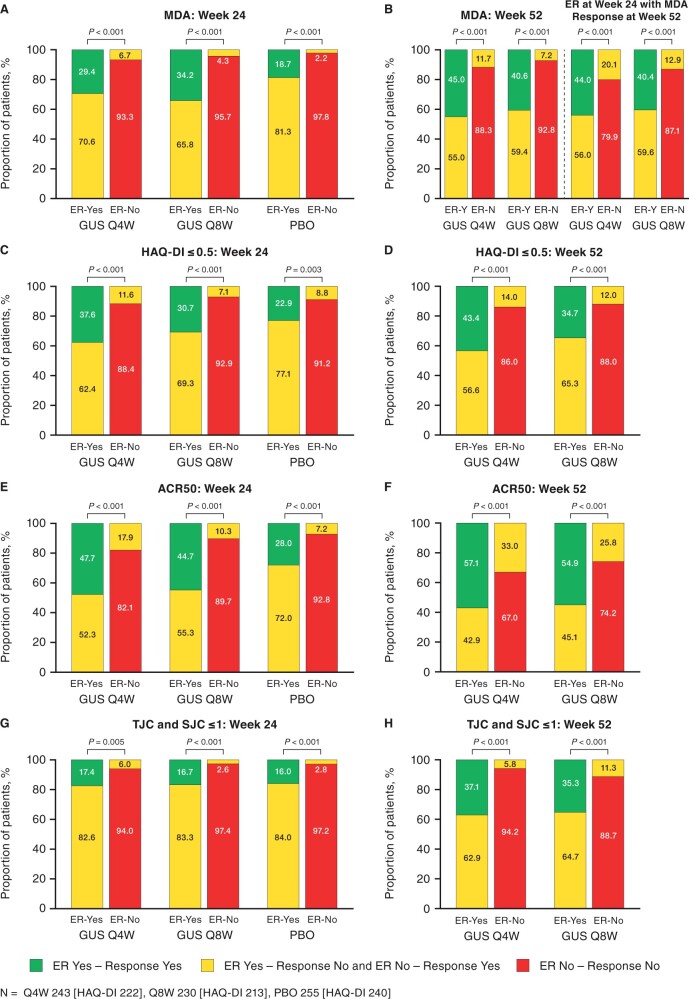
At week 24, patients who achieved enthesitis resolution were more likely to also achieve clinical response as measured by MDA, normalized HAQ-DI, ACR50, and both SJC and TJC ≤1 than patients who did not have resolution of enthesitis (Fig. 4). Similar findings were observed for enthesitis resolution and clinical responses at week 52. Additionally, patients achieving enthesitis resolution at week 24 were also more likely to achieve MDA at week 52 compared with patients who did not have enthesitis resolution.
Fig. 4.
Clinical response at weeks 24 and 52 by enthesitis resolution status
Data pooled across DISCOVER-1 and DISCOVER-2. Enthesitis defined as LEI EC ≥1; enthesitis resolution defined as LEI EC = 0 (patients meeting treatment failure criteria were considered non-responders). ACR50: ≥50% improvement in ACR response criteria; EC: enthesitis count; ER: enthesitis resolution; GUS: guselkumab; LEI: Leeds Enthesitis Index; MDA: minimal disease activity; PBO: placebo; Q4W/Q8W: every 4/8 weeks; SJC: swollen joint count; TJC: tender joint count; Y/N: yes/no.
Discussion
Enthesitis is not only a hallmark of PsA, but may also be a progenitor of the structural joint damage seen in patients with PsA [3]. Specifically, biomechanical stress is proposed to trigger the release of cytokines into the synovio-entheseal complex based on a popular model, which then induce an articular inflammatory response [23, 24]. Indeed, the presence and extent of enthesitis have demonstrated positive associations with greater peripheral and axial joint damage, impaired quality of life and function, sleep disturbance and patient-reported pain [5, 25, 26]. Underscoring the importance of effectively treating this key PsA manifestation, which is a component of inflammatory articular disease as described in the stem of the Classification for Psoriatic Arthritis criteria [4], biologic agents are recommended to treat enthesitis [9, 10]. As such, enthesitis has come under increasingly intense investigation in phase 3 clinical trial programmes [8, 27, 28].
Both animal model and human data support IL-23 as a central cytokine associated with enthesitis pathogenesis [29, 30]. We comprehensively evaluated the effects of guselkumab, the first selective anti-IL-23p19 mAb approved to treat PsA, on this important manifestation of PsA, by pooling data across two phase 3 studies. Despite not being an entry requirement, enthesitis was a common finding, affecting 65% of participants. This prevalence is generally comparable to other studies evaluating biologic agents in patients with PsA [8, 28, 31–35]. Consistent with previous observations that PsA patients with enthesitis manifest more severe disease [5, 26], patients from the pooled DISCOVER-1 and DISCOVER-2 trials with enthesitis at baseline were characterized by more active disease and functional impairment, as well as a higher prevalence of factors that can signal more aggressive disease and portend poorer outcome, e.g. female, higher BMI, more severe dactylitis [36, 37], relative to patients without enthesitis at baseline. Of note, obesity has been previously associated with sonographically determined entheseal changes in otherwise healthy subjects, suggesting that damage due to mechanical or degenerative stress may occur in addition to inflammatory changes in these patients [38].
Among patients with enthesitis at baseline, significantly higher proportions of guselkumab Q4W- and Q8W- than placebo-treated patients experienced enthesitis resolution by week 24. This treatment effect was largely consistent across numerous baseline demographic and clinical disease characteristics. Resolution response rates among patients who continued guselkumab through 1 year reached 58%. The median time to resolution was shorter with both guselkumab dosing regimens (16 weeks) than placebo (24 weeks). Guselkumab Q4W and Q8W both resolved enthesitis in substantial proportions of patients regardless of its baseline severity, although resolution rates were higher in patients who presented with mild or moderate than severe enthesitis. In addition, enthesitis resolution was correlated with achieving other measures of clinical response at weeks 24 and 52. Guselkumab treatment effects at week 24 were largely consistent across the enthesitis/no enthesitis cohorts for a range of clinical outcomes. Guselkumab-randomized patients with enthesitis at baseline, however, appeared to have more improvement through 1 year than patients without enthesitis in mental aspects of HRQoL, potentially highlighting the impact of enthesitis on mental health, the duration of treatment needed to effect such change, and the broader implications of resolving this particular manifestation of PsA.
In patients without enthesitis at baseline, most (>80%) did not develop enthesitis through week 52. While this suggests that guselkumab may inhibit the development of enthesitis, it may also be due to inherent patient and disease differences between those with and without enthesitis. However, as noted, guselkumab was similarly effective in improving joint signs and symptoms, psoriasis, physical function and physical aspect of HRQoL in patients with or without enthesitis.
While some data on the efficacy of biologics approved for PsA in treating enthesitis have been published, it is difficult to compare across studies due to differences in study designs, measurement tools and data analyses. Earlier studies were often limited in the numbers of patients with enthesitis and the extent of enthesitis data collected and analysed [31–35, 39, 40]. More recently, results of a meta-analysis indicated that inhibitors of TNF, IL-17 and IL-12/23 were effective in treating enthesitis [2]. Based on the central role of IL-23 in enthesitis, it is not surprising that results of comparative studies of PsA patients with enthesitis showed both ustekinumab (anti-IL-12/23 agent) and ixekizumab (anti-IL-17 agent acting downstream of IL-23) to be more effective than a TNFi in treating enthesitis [6, 27]. These limited active comparator data, together with the findings reported here and those recently reported for anti-IL-17 agents [8, 28], support the central role of the IL-23 and IL-17 pathways in PsA enthesitis pathogenesis and may inform treatment choices for PsA patients with enthesitis.
An important limitation of the study is the lack of an active comparator. It will be important to further investigate whether targeting the IL-23p19 subunit is superior to inhibition of TNF and IL-17 in treating PsA patients with enthesitis. The results presented here, which largely derive from post hoc analyses of pooled data from two phase 3 studies, would be strengthened by those from a prospective randomized trial of enthesitis patients. Results reported herein are based on clinical evaluations aimed at detecting entheseal tenderness. To identify subclinical enthesitis and more specific characteristics of the disorder, e.g. tendon thickening, bursitis, bone erosions, enthesophytes and calcifications, imaging studies are needed [3]. While the placebo-controlled period extended only to week 24, this is widely accepted as the longest ethically feasible period to delay treatment.
Thus, by targeting the IL-23p19 subunit, guselkumab 100 mg given Q4W or Q8W offers a new mechanism of action by which to effect sustained resolution of enthesitis in patients with active PsA. Enthesitis resolution was associated with achieving important patient outcomes. While patients with more severe enthesitis had lower rates of resolution, the efficacy of guselkumab in improving other disease domains was consistent across patients with or without enthesitis at baseline. The patients with the most severe disease as determined by higher overall LEI scores tended to be female and have a higher BMI, longer disease duration and normal CRP, potentially pointing towards mechanical factors and chronic pain contributing to entheseal area tenderness that may not be modifiable with biological therapy. This is an important area for further research for appropriate therapy initiation and understanding underlying mechanisms associated with treatment response.
Supplementary Material
Acknowledgements
The authors thank Cynthia Guzzo MD, a paid consultant of Janssen, and Michelle Perate MS and Rebecca Clemente PhD of Janssen Scientific Affairs for assistance in developing and submitting this manuscript. All authors, including Janssen employees (M.S., S.K., C.S.K., A.P.K., E.C.H., X.L.X., S.S., P.A., B.Z.), were involved in data collection, analysis and/or interpretation; trial design; manuscript preparation and deciding to submit it for publication. The corresponding author (D.M.) had full access to all study data and had final responsibility to submit for publication.
Funding: This work was supported by Janssen Research & Development, LLC. Janssen funded professional medical writers to help prepare and submit the manuscript.
Disclosure statement: D.M. has received research grants and/or honoraria from AbbVie, Amgen, Bristol Myers Squibb, Celgene, Eli Lilly, Gilead, Janssen, Novartis, Pfizer and UCB.
I.B.M. has received research grants and/or honoraria from AbbVie, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly, Janssen, Novartis, Pfizer and UCB.
A.D. has received grants and research support paid to University from Bristol Myers Squibb, Eli Lilly, GlaxoSmithKline, Novartis, Pfizer and UCB, as well as honoraria or consultation fees paid to self from AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, GlaxoSmithKline, Janssen, Novartis, Pfizer and UCB.
G.S. has received grants and research support paid to University from Bristol Myers Squibb, Eli Lilly, GlaxoSmithKline, Novartis and UCB, as well as honoraria or consultation fees paid to self from AbbVie, Amgen, Bristol Myers Squibb, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Novartis, Pfizer and UCB.
M.S., S.K., C.S.K., A.P.K., E.C.H., X.L.X., S.S., P.A. and B.Z. are or were employees of Janssen (a subsidiary of Johnson & Johnson) at the time the work was performed and own Johnson & Johnson stock or stock options.
C.T.R. has received research funding from AbbVie, Amgen, and UCB and consulting fees from AbbVie, Amgen, Eli Lilly, Janssen, Novartis, Pfizer, and UCB.
P.R. has received consulting fees from Abbott, AbbVie, Amgen, Bristol Myers Squibb, Celgene, Eli Lilly, Janssen, Novartis and Pfizer, and has also received a research grant from Janssen.
P.J.M. has received research grants and/or consultation/speaker honoraria from AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly, Galapagos, Genentech, Gilead, GlaxoSmithKline, Janssen, Novartis, Pfizer, Sun Pharmaceuticals and UCB.
Data availability statement
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Dougados M, Baeten D.. Spondyloarthritis. Lancet 2011;377:2127–37. [DOI] [PubMed] [Google Scholar]
- 2. Mourad A, Gniadecki R.. Treatment of dactylitis and enthesitis in psoriatic arthritis with biologic agents: a systematic review and metaanalysis. J Rheumatol 2020;47:59–65. [DOI] [PubMed] [Google Scholar]
- 3. Kaeley GS, Eder L, Aydin SZ, Gutierrez M, Bakewell C.. Enthesitis: a hallmark of psoriatic arthritis. Semin Arthritis Rheum 2018;48:35–43. [DOI] [PubMed] [Google Scholar]
- 4. Taylor W, Gladman D, Helliwell P. et al. ; CASPAR Study Group. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 2006;54:2665–73. [DOI] [PubMed] [Google Scholar]
- 5. Polachek A, Li S, Chandran V, Gladman DD.. Clinical enthesitis in a prospective longitudinal psoriatic arthritis cohort: incidence, prevalence, characteristics, and outcome. Arthritis Care Res (Hoboken) 2017;69:1685–91. [DOI] [PubMed] [Google Scholar]
- 6. Mease PJ, van der Heijde D, Ritchlin CT. et al. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann Rheum Dis 2017;76:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tan AL, McGonagle D.. Psoriatic arthritis: correlation between imaging and pathology. Joint Bone Spine 2010;77:206–11. [DOI] [PubMed] [Google Scholar]
- 8. Gladman DD, Orbai AM, Klitz U. et al. Ixekizumab and complete resolution of enthesitis and dactylitis: integrated analysis of two phase 3 randomized trials in psoriatic arthritis. Arthritis Res Ther 2019;21:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coates LC, Kavanaugh A, Mease PJ. et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol 2016;68:1060–71. [DOI] [PubMed] [Google Scholar]
- 10. Gossec L, Baraliakos X, Kerschbaumer A. et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis 2020;79:700–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reinhardt A, Yevsa T, Worbs T. et al. Interleukin-23-dependent γ/δ T cells produce interleukin-17 and accumulate in the enthesis, aortic valve, and ciliary body in mice. Arthritis Rheumatol 2016;68:2476–86. [DOI] [PubMed] [Google Scholar]
- 12. Sherlock JP, Joyce-Shaikh B, Turner SP. et al. IL-23 induces spondyloarthropathy by acting on ROR-γt+ CD3+CD4−CD8− entheseal resident T cells. Nat Med 2012;18:1069–76. [DOI] [PubMed] [Google Scholar]
- 13. Bridgewood C, Watad A, Russell T. et al. Identification of myeloid cells in the human enthesis as the main source of local IL-23 production. Ann Rheum Dis 2019;78:929–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cuthbert RJ, Watad A, Fragkakis EM. et al. Evidence that tissue resident human enthesis γδT-cells can produce IL-17A independently of IL-23R transcript expression. Ann Rheum Dis 2019;78:1559–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Watad A, Rowe H, Newton D, Bridgewood C, McGonagle DG.. Is a human in vitro enthesitis model relevant to SpA-associated enthesitis? Response to: ‘Beware of wolves in sheep’s clothing: immune cell plasticity and instability in health and disease' by Alunno et al. Ann Rheum Dis 2020, doi: 10.1136/annrheumdis-2020-218151. [DOI] [PubMed] [Google Scholar]
- 16.Janssen Biotech, Inc. Tremfya: package insert. Horsham, PA: Janssen Biotech, Inc., 2020; https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/TREMFYA-pi.pdf (April 2021, date last accessed). [Google Scholar]
- 17. Deodhar A, Helliwell PS, Boehncke WH. et al. Guselkumab in patients with active psoriatic arthritis who were biologic-naive or had previously received TNFa inhibitor treatment (DISCOVER-1): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet 2020;395:1115–25. [DOI] [PubMed] [Google Scholar]
- 18. Mease PJ, Rahman P, Gottlieb AB. et al. Guselkumab in biologic-naive patients with active psoriatic arthritis (DISCOVER-2): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet 2020;395:1126–36. [DOI] [PubMed] [Google Scholar]
- 19. McInnes IB, Rahman P, Gottlieb AB. et al. Efficacy and safety of guselkumab, an interleukin-23p19-specific monoclonal antibody, through 1 year in biologic-naïve psoriatic arthritis patients. Arthritis Rheumatol 2021;73:604–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ritchlin CR, Helliwell PS, Boehncke WH. et al. Guselkumab, an inhibitor of the IL-23p19-subunit, provided sustained improvement in signs and symptoms of active psoriatic arthritis: one year results of a phase 3 randomized study of patients who were biologic-naïve or TNFα inhibitor-experienced. Ann Rheum Dis 2020;79:1148–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Healy PJ, Helliwell PS.. Measuring clinical enthesitis in psoriatic arthritis: assessment of existing measures and development of an instrument specific to psoriatic arthritis. Arthritis Rheum 2008;59:686–91. [DOI] [PubMed] [Google Scholar]
- 22. Coates LC, Fransen J, Helliwell PS.. Defining minimal disease activity in psoriatic arthritis: a proposed objective target for treatment. Ann Rheum Dis 2010;69:48–53. [DOI] [PubMed] [Google Scholar]
- 23. McGonagle D. Enthesitis: an autoinflammatory lesion linking nail and joint involvement in psoriatic disease. J Eur Acad Dermatol Venereol 2009;23 (Suppl 1): 9–13. [DOI] [PubMed] [Google Scholar]
- 24. Kehl AS, Corr M, Weisman MH.. Review: Enthesitis: new insights into pathogenesis, diagnostic modalities, and treatment. Arthritis Rheumatol 2016;68:312–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gezer O, Batmaz I, Sariyildiz MA. et al. Sleep quality in patients with psoriatic arthritis. Int J Rheum Dis 2017;20:1212–8. [DOI] [PubMed] [Google Scholar]
- 26. Mease PJ, Karki C, Palmer JB. et al. Clinical characteristics, disease activity, and patient-reported outcomes in psoriatic arthritis patients with dactylitis or enthesitis: results from the Corrona Psoriatic Arthritis/Spondyloarthritis Registry. Arthritis Care Res (Hoboken) 2017;69:1692–9. [DOI] [PubMed] [Google Scholar]
- 27. Araujo EG, Englbrecht M, Hoepken S. et al. Effects of ustekinumab versus tumor necrosis factor inhibition on enthesitis: results from the enthesial clearance in psoriatic arthritis (ECLIPSA) study. Semin Arthritis Rheum 2019;48:632–7. [DOI] [PubMed] [Google Scholar]
- 28. Coates LC, Wallman JK, McGonagle D. et al. Secukinumab efficacy on resolution of enthesitis in psoriatic arthritis: pooled analysis of two phase 3 studies. Arthritis Res Ther 2019;21:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bridgewood C, Sharif K, Sherlock J, Watad A, McGonagle D.. Interleukin-23 pathway at the enthesis: the emerging story of enthesitis in spondyloarthropathy. Immunol Rev 2020;294:27–47. [DOI] [PubMed] [Google Scholar]
- 30. Gravallese EM, Schett G.. Effects of the IL-23-IL-17 pathway on bone in spondyloarthritis. Nat Rev Rheumatol 2018;14:631–40. [DOI] [PubMed] [Google Scholar]
- 31. Mease PJ, Gladman DD, Ritchlin CT. et al. ; Adalimumab Effectiveness in Psoriatic Arthritis Trial Study Group. Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: results of a double-blind, randomized, placebo-controlled trial. Arthritis Rheum 2005;52:3279–89. [DOI] [PubMed] [Google Scholar]
- 32. Gottlieb A, Menter A, Mendelsohn A. et al. Ustekinumab, a human interleukin 12/23 monoclonal antibody, for psoriatic arthritis: randomised, double-blind, placebo-controlled, crossover trial. Lancet 2009;373:633–40. [DOI] [PubMed] [Google Scholar]
- 33. Kavanaugh A, van der Heijde D, McInnes IB. et al. Golimumab in psoriatic arthritis: one-year clinical efficacy, radiographic, and safety results from a phase III, randomized, placebo-controlled trial. Arthritis Rheum 2012;64:2504–17. [DOI] [PubMed] [Google Scholar]
- 34. McInnes IB, Kavanaugh A, Gottlieb AB. et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet 2013;382:780–9. [DOI] [PubMed] [Google Scholar]
- 35. Mease PJ, Fleischmann R, Deodhar AA. et al. Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24-week results of a Phase 3 double-blind randomised placebo-controlled study (RAPID-PsA). Ann Rheum Dis 2014;73:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gladman DD, Antoni C, Mease P, Clegg DO, Nash P.. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis 2005;64: ii14–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bergman M, Lundholm A.. Mitigation of disease- and treatment-related risks in patients with psoriatic arthritis. Arthritis Res Ther 2017;19:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Eder L, Jayakar J, Thavaneswaran A. et al. Is the MAdrid Sonographic Enthesitis Index useful for differentiating psoriatic arthritis from psoriasis alone and healthy controls? J Rheumatol 2014;41:466–72. [DOI] [PubMed] [Google Scholar]
- 39. Mease PJ, Kivitz AJ, Burch FX. et al. Etanercept treatment of psoriatic arthritis: safety, efficacy, and effect on disease progression. Arthritis Rheum 2004;50:2264–72. [DOI] [PubMed] [Google Scholar]
- 40. Antoni CE, Kavanaugh A, Kirkham B. et al. Sustained benefits of infliximab therapy for dermatologic and articular manifestations of psoriatic arthritis: results from the infliximab multinational psoriatic arthritis controlled trial (IMPACT). Arthritis Rheum 2005;52:1227–36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.