Abstract
Objective
To investigate the long-term outcomes of patients with RA after myocardial infarction (MI).
Methods
All-comer, real-life MI patients with RA (n = 1614, mean age 74 years) were retrospectively compared with propensity score (1:5) matched MI patients without RA (n = 8070) in a multicentre, nationwide, cohort register study in Finland. The impact of RA duration and the usage of corticosteroids and antirheumatic drugs on RA patients’ outcomes were also studied. The median follow-up was 7.3 years.
Results
RA was associated with an increased 14-year mortality risk after MI compared with patients without RA [80.4% vs 72.3%; hazard ratio (HR) 1.25; CI: 1.16, 1.35; P <0.0001]. Patients with RA were at higher risk of new MI (HR 1.22; CI: 1.09, 1.36; P =0.0001) and revascularization (HR 1.28; CI: 1.10, 1.49; P =0.002) after discharge from index MI. Cumulative stroke rate after MI did not differ between RA and non-RA patients (P =0.322). RA duration and corticosteroid usage before MI, but not use of methotrexate or biologic antirheumatic drugs, were independently associated with higher mortality (P <0.001) and new MI (P =0.009). A higher dosage of corticosteroids prior to MI was independently associated with higher long-term mortality (P =0.002) and methotrexate usage with lower stroke rate (P =0.034). Serological status of RA was not associated with outcomes.
Conclusion
RA is independently associated with poorer prognosis after MI. RA duration and corticosteroid usage and dosage were independent predictors of mortality after MI in RA. Special attention is needed for improvement of outcomes after MI in this vulnerable population.
Keywords: Cohort study, coronary artery disease, myocardial infarction, outcomes, rheumatoid arthritis
Rheumatology key messages
Patients with rheumatoid arthritis (RA) have impaired long-term outcomes after myocardial infarction (MI).
Glucocorticoid use before MI is associated with higher mortality and recurrent MI.
Secondary prevention of cardiovascular disease should be a treatment priority after MI in RA patients.
Introduction
Cardiovascular disease (CVD) is the leading cause of death in patients with RA [1], with approximately a 50% higher CVD mortality rate compared with the general population [2]. A multitude of studies among RA cohorts demonstrate an excess risk of most types of CVD events, with a 1.7-fold myocardial infarction (MI) risk [3]. Alongside traditional CVD risk factors, some of which are overrepresented among RA patients [4], RA disease activity and systemic inflammation are key elements underlying the increased atherosclerotic burden [5]. Randomized controlled trials are lacking, yet observational studies point to a reduction in CVD events among RA patients using effective, DMARDs such as methotrexate or TNF inhibitors, whereas glucocorticoids are associated with an increased risk of CVD events [6]. A definite unmet need exists to improve CVD outcomes in this high-risk patient population [7].
The increased prevalence of atherosclerotic CVD and MI among patients with RA is well documented, but less is known about the outcomes after MI. Several studies have focussed on short-term outcomes after MI and most [8–10], but not all [11, 12], have reported increased in-hospital or 30-day mortality among RA patients compared with controls without RA. A few small studies on the long-term outcomes of MI among RA patients have reported excess mortality and recurrent ischaemia [8, 13, 14]. To our knowledge, only a few large-scale registry studies from Sweden [15, 16] and Taiwan [17] have reported impaired long-term survival among RA patients after acute coronary syndrome, and they did not explore the effects of DMARDs or glucocorticoids on survival. Conflicting results exist on whether RA patients are undertreated in secondary prevention after MI compared with patients without RA [15, 18, 19].
In this nationwide study, we investigated long-term outcomes of MI among RA patients, including mortality, recurrent MI, stroke and revascularization. The impact of glucocorticoids and DMARDs on outcomes after MI was also explored.
Methods
Study design and population
We performed a nationwide registry study on the outcomes after MI among prevalent RA patients compared with propensity-matched, non-RA patients. Predictors of long-term outcomes in RA patients after MI were also studied. The primary outcome of interest was all-cause mortality. Secondary outcomes were new MI, stroke, revascularization and cardiovascular medication usage after MI.
Consecutive MI patients aged ≥18 years admitted between 1 January 2005 and 31 December 2014 to participating hospitals were retrospectively recognized from The Care Register for Healthcare in Finland (CRHF). MI was detected by ICD-10 code I21 as the primary discharge diagnosis. The CRHF is a nationwide registry that includes prospectively registered data on all hospital admissions and major surgical procedures in Finland [20]. All hospitals with a coronary catheterization laboratory that treats MI patients in Finland (n = 20) were included. Only patients admitted to medical, surgical or ICU wards through emergency department or paramedic services were included to capture only incident MIs. Patients lost to mortality follow-up (0.5%) and those treated with aortic or valvular surgery were excluded.
Patients with prevalent RA were recognized from the registry database using ICD-10 codes M05 and M06 for seropositive and seronegative RA, respectively. All patients in Finland with appropriately diagnosed RA requiring DMARDs are entitled to special reimbursement for medication. A rheumatologist or a doctor working at a rheumatology clinic writes a medical certificate describing the rationale for the diagnosis, and the certificate is then delivered to the Social Insurance Institution of Finland to receive this reimbursement. Only patients with the special reimbursement for antirheumatic medications were included in the RA group (Supplementary Fig. S1, available at Rheumatology online).
Outcomes, co-morbidities, baseline features and prescription drugs are defined in Supplementary Data S1 and Supplementary Tables S1–S3, available at Rheumatology online. Usage of DMARD and corticosteroid prior to MI was defined as prescription drug purchase within 180 days prior to MI. The cumulative usage of oral corticosteroids as prednisolone equivalents [21] per day was determined from drug purchases made within the 12 months prior to MI. Cardiovascular medication usage after MI was defined as prescription drug purchase within 90 days from hospital discharge. RA duration was defined as the time from DMARD reimbursement approval to MI. Hospital transfers after MI admission were combined.
Data sources and permissions
The CRHF registry data were obtained from the National Institute for Health and Welfare of Finland. Prescription drug purchase data (including ATC-code, dosage, pack size and purchase date) and medication reimbursement entitlement data were obtained from the nationwide registry held by the Social Insurance Institution of Finland. Intravenous infusions, which are only administered in hospital, were not captured in the prescription drug registry. Mortality data were obtained from the cause of death registry held by Statistics Finland. These registries are mandated by law and have a full coverage of hospital admissions, drug purchases, entitlements for reimbursements and deaths in the Finnish population. Follow-up ended on 31 December 2018. The study was approved by the National Institute for Health and Welfare of Finland (permission no: THL/2245/5.05.00/2019), Statistics Finland (TK-53–484-20) and the Social Insurance Institution of Finland (91/522/2015). This was a retrospective register study; thus, no informed consent was required, and the participants were not contacted. The legal basis for processing personal data is public interest and scientific research (EU General Data Protection Regulation 2016/679 (GDPR), Article 6(1)(e) and Article 9(2)(j); Data Protection Act, Sections 4 and 6).
Statistical analysis
Between group differences were analysed with t test and χ2 test (non-matched groups) or paired t test, Wilcoxon signed-rank test, McNemar’s test and McNemar–Bowker’s test (matched groups). Effect sizes in baseline characteristics between groups were evaluated by standardized mean difference (SMD). A propensity score based on age, sex, alcohol abuse, anaemia, atrial fibrillation, cerebrovascular disease, coagulopathy, dementia, diabetes, heart failure, hypertension, hypothyroidism, liver disease, malignancy, paralysis, peripheral vascular disease, prior coronary artery bypass, prior MI, psychotic disorder, renal disease, valvular disease, revascularization, ST-elevation MI, treating hospital and study year (Table 1) was created using logistic regression. Patients (n = 209 without RA) with non-overlapping propensity scores were excluded. The trimmed propensity score was used for local optimal 1:5 caliper matching without replacing the use of 0.05 caliper width of the logit of the s.d. [22]. Outcomes were studied with the Kaplan–Meier method and Cox regression. Mortality predictors in RA patients were studied using multivariate Cox regression. Proportional hazard assumptions were confirmed by visual examination of Schoenfeld residuals. Cause-specific hazard models for competing risk due to death were applied in analysis of other long-term outcomes. Logistic regression was used in analysing cardiovascular medication usage. Matched regression models were used in analysing propensity matched groups. Median follow-up time for survivors was 7.3 years (min 4.0, max 14.0 years). Results are given as the mean (s.d.), median ± interquartile range (IQR), percentage, hazard ratio (HR) or odds ratio (OR) with 95% CI. Statistical significance was inferred at P-value <0.05. Analyses were performed with SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Table 1.
Baseline features of myocardial infarction patients with and without RA
| All patients |
Matched patients |
|||||||
|---|---|---|---|---|---|---|---|---|
| RA | Control | RA | Control | |||||
| Variable | n = 1614 | n = 58 832 | P-value | |SMD| | n = 1614 | n = 8070 | P-value | |SMD| |
| Age, years (s.d.) | 73.6 (10.3) | 70.9 (13.0%) | <0.0001 | 0.197 | 73.6 (10.3) | 73.9 (10.8) | 0.667 | 0.007 |
| Men | 663 (41.2%) | 37083 (63.0%) | <0.0001 | 0.450 | 663 (41.2%) | 3253 (40.3%) | 0.169 | 0.016 |
| Alcohol abuse | 20 (1.2%) | 1561 (2.7%) | 0.0004 | 0.103 | 20 (1.2%) | 108 (1.3%) | 0.568 | 0.009 |
| Anaemia (history of) | 119 (7.4%) | 2102 (3.6%) | <0.0001 | 0.168 | 119 (7.4%) | 556 (6.9%) | 0.199 | 0.019 |
| Atrial fibrillation | 334 (20.7%) | 9469 (16.1%) | <0.0001 | 0.119 | 334 (20.7%) | 1666 (20.6%) | 0.937 | 0.001 |
| Cerebrovascular disease | 231 (14.3%) | 6897 (11.7%) | 0.002 | 0.077 | 231 (14.3%) | 1139 (14.1%) | 0.716 | 0.006 |
| Chronic pulmonary disease | 288 (17.8%) | 7632 (13.0%) | <0.0001 | 0.135 | 288 (17.8%) | 1435 (17.8%) | 0.918 | 0.002 |
| Coagulopathy | 12 (0.7%) | 203 (0.4%) | 0.008 | 0.054 | 12 (0.7%) | 51 (0.6%) | 0.389 | 0.013 |
| Dementia | 111 (6.9%) | 3878 (6.6%) | 0.648 | 0.011 | 111 (6.9%) | 576 (7.1%) | 0.516 | 0.010 |
| Depression | 211 (13.1%) | 5792 (9.8%) | <0.0001 | 0.102 | 211 (13.1%) | 1032 (12.8%) | 0.584 | 0.009 |
| Diabetes | 364 (22.6%) | 13 913 (23.7%) | 0.306 | 0.026 | 364 (22.6%) | 1856 (23.0%) | 0.500 | 0.011 |
| Insulin dependent | 122 (7.6%) | 4675 (8.0%) | 0.570 | 0.014 | 122 (7.6%) | 639 (7.9%) | 0.395 | 0.013 |
| Non-insulin dependent | 258 (16.0%) | 9908 (16.8%) | 0.364 | 0.023 | 258 (16.0%) | 1291 (16.0%) | 0.983 | <0.001 |
| Heart failure | 526 (32.6%) | 15 188 (25.8%) | <0.0001 | 0.149 | 526 (32.6%) | 2632 (32.6%) | 0.972 | 0.001 |
| Hypertension | 901 (55.8%) | 29839 (50.7%) | <0.0001 | 0.103 | 901 (55.8%) | 4561 (56.5%) | 0.367 | 0.014 |
| Hypothyroidism | 130 (8.1%) | 2678 (4.6%) | <0.0001 | 0.145 | 130 (8.1%) | 681 (8.4%) | 0.361 | 0.014 |
| Liver disease | 21 (1.3%) | 515 (0.9%) | 0.072 | 0.041 | 21 (1.3%) | 101 (1.3%) | 0.778 | 0.004 |
| Malignancy (history of) | 203 (12.6%) | 7052 (12.0%) | 0.471 | 0.018 | 203 (12.6%) | 1048 (13.0%) | 0.435 | 0.012 |
| Paralysis | 10 (0.6%) | 222 (0.4%) | 0.121 | 0.034 | 10 (0.6%) | 47 (0.6%) | 0.761 | 0.005 |
| Peripheral vascular disease | 193 (12.0%) | 4584 (7.8%) | <0.0001 | 0.140 | 193 (12.0%) | 978 (12.1%) | 0.747 | 0.005 |
| Prior CABG | 54 (3.4%) | 2043 (3.5%) | 0.784 | 0.007 | 54 (3.4%) | 255 (3.2%) | 0.508 | 0.011 |
| Prior myocardial infarction | 388 (24.0%) | 14004 (23.8%) | 0.826 | 0.006 | 388 (24.0%) | 1994 (24.7%) | 0.315 | 0.016 |
| Psychotic disorder | 34 (2.1%) | 1853 (3.2%) | 0.017 | 0.065 | 34 (2.1%) | 156 (1.9%) | 0.430 | 0.012 |
| Renal failure | 95 (5.9%) | 2130 (3.6%) | <0.0001 | 0.107 | 95 (5.9%) | 473 (5.9%) | 0.946 | 0.001 |
| Valvular disease | 146 (9.1%) | 3298 (5.6%) | <0.0001 | 0.132 | 146 (9.1%) | 753 (9.3%) | 0.521 | 0.010 |
| Revascularization | 694 (43.0%) | 28 873 (49.1%) | <0.0001 | 0.122 | 694 (43.0%) | 3393 (42.0%) | 0.214 | 0.019 |
| PCI | 633 (39.2%) | 25 239 (42.9%) | 0.003 | 0.075 | 633 (39.2%) | 3121 (38.7%) | 0.473 | 0.011 |
| CABG | 61 (3.8%) | 3708 (6.3%) | <0.0001 | 0.116 | 61 (3.8%) | 275 (3.4%) | 0.195 | 0.019 |
| STEMI | 592 (36.7%) | 22 238 (37.8%) | 0.360 | 0.023 | 592 (36.7%) | 2939 (36.4%) | 0.730 | 0.005 |
| Anteriora | 301 (50.8%) | 11 453 (51.5%) | 0.752 | 0.013 | 301 (50.8%) | 1483 (50.5%) | 0.656 | 0.008 |
| Inferiora | 230 (38.9%) | 8490 (38.2%) | 0.739 | 0.014 | 230 (38.9%) | 1156 (39.3%) | 0.892 | 0.010 |
| Lateral/othera | 61 (10.3%) | 2295 (10.3%) | 0.990 | 0.001 | 61 (10.3%) | 300 (10.2%) | 0.837 | 0.003 |
| Treating hospital (n = 20) | <0.0001 | 0.213 | 0.816 | 0.018 | ||||
| Study year | 0.001 | 0.108 | 0.734 | 0.007 | ||||
| Medicationb | ||||||||
| Corticosteroid | 782 (48.5%) | 3095 (5.3%) | <0.0001 | 1.116 | 782 (48.5%) | 538 (6.7%) | <0.0001 | 1.058 |
| Methotrexate | 553 (34.3%) | 57 (0.1%) | <0.0001 | 1.016 | 553 (34.3%) | 10 (0.1%) | <0.0001 | 1.015 |
| Biological drugc | 48 (3.0%) | 5 (0.01%) | <0.0001 | 0.247 | 48 (3.0%) | 0 (0.0%) | <0.0001 | 0.248 |
Cohorts of all patients and propensity score matched (1:5) patients. CABG: coronary artery bypass grafting surgery; PCI: percutaneous coronary intervention; SMD: standardized mean difference; STEMI: ST-elevation myocardial infarction. aOf STEMI patients. bPresciption drug purchase within 180 days prior to MI. cIntravenous infusions, which are only administered in hospital, were not captured in the prescription drug registry (infliximab, rituximab, and intravenous formulations of tosilizumab and abatacept).
Results
Of all the included MI patients [n = 60 446, 62.1% men, median age 73 (range 18–103) years], 2.7% (n = 1614) had RA with entitlement for drug reimbursement. Patients with RA were older, more often female and had more comorbidities than non-RA patients (Table 1). Of the studied co-morbidities, only alcohol abuse and psychotic disorders were less frequent in the RA group. MI was more frequently non-ST-segment elevation MI in the RA group. Revascularization by either percutaneous coronary intervention (PCI) or CABG was performed less frequently in RA patients with MI (Table 1). Propensity score matching (1:5) identified 8070 non-RA patients with features comparable to RA patients (Table 1). Median duration of hospital admission after MI was 7 (IQR 4–12) days in RA patients vs 6 (IQR 4–11) days in controls (P <0.0001). Based on ICD-10 codes, the majority of RA patients (80.9%) were seropositive. The median RA duration before MI was 14.4 (IQR 7.3–27.7) years.
Oral corticosteroids were used by 48.5%, methotrexate by 34.3% and biological drugs by 3.0% of RA patients within 180 days before MI. The median average corticosteroid dosage was 4.1 (IQR 2.7–5.5) mg/day among those who used corticosteroids in the year preceding MI. Table 2 presents post-discharge cardiovascular medication usage after MI. Patients with RA received statin treatment less frequently compared with matched non-RA controls after MI (Table 2). The intensity of the used statin therapy or the usage of other cardiovascular medications did not differ between groups.
Table 2.
Usage of post-discharge cardiovascular prescription medication in hospital surviving RA patients and matched controls after myocardial infarction
| RA | Matched controls | |||
|---|---|---|---|---|
| n = 1366 | n = 6995 | OR (95% CI) | P-value | |
| ADP-inhibitor | 827 (60.5%) | 4288 (61.3%) | 0.96 (0.85, 1.09) | 0.521 |
| Anticoagulant | 205 (15.0%) | 1067 (15.3%) | 1.00 (0.84, 1.18) | 0.957 |
| Antidiabetic | 231 (16.9%) | 1260 (18.0%) | 0.91 (0.78, 1.07) | 0.251 |
| Insulin | 111 (8.1%) | 587 (8.4%) | 0.98 (0.79, 1.22) | 0.832 |
| Non-insulin | 151 (11.1%) | 873 (12.5%) | 0.84 (0.70, 1.02) | 0.071 |
| ACEi or ARB | 855 (62.6%) | 4561 (65.2%) | 0.91 (0.80, 1.03) | 0.117 |
| Aldosterone antagonist | 59 (4.3%) | 277 (4.0%) | 1.10 (0.82, 1.48) | 0.509 |
| Antiarrhythmic | 11 (0.8%) | 90 (1.3%) | 0.65 (.0.34, 1.23) | 0.187 |
| Beta-blocker | 1147 (84.0%) | 5764 (82.4%) | 1.11 (0.94, 1.30) | 0.217 |
| Ca-blocker | 253 (18.5%) | 1310 (18.7%) | 0.97 (0.84, 1.14) | 0.739 |
| Digitalis | 62 (4.5%) | 307 (4.4%) | 1.04 (0.78, 1.38) | 0.788 |
| Diuretic | 634 (46.4%) | 3176 (45.4%) | 1.06 (0.94, 1.19) | 0.351 |
| Ezetimibe | 28 (2.1%) | 166 (2.4%) | 0.86 (0.57, 1.30) | 0.463 |
| Nitrate | 960 (70.3%) | 4863 (69.5%) | 1.03 (0.91, 1.17) | 0.651 |
| Statin | 998 (73.1%) | 5410 (77.3%) | 0.79 (0.69, 0.91) | 0.001 |
| Intensity of statin therapy | 0.281 | |||
| Low | 49 (4.9%) | 311 (5.8%) | ||
| Moderate | 804 (80.6%) | 4272 (79.0%) | ||
| High | 145 (14.5%) | 827 (15.3%) |
ACEi: angiotensin-converting-enzyme inhibitor; ADP: adenosine diphosphate; ARB: angiotensin receptor blocker; OR: odds ratio.
Mortality
A total of 5468 deaths (1016 in the RA group) occurred during the follow-up. Cumulative mortality in RA patients was 13.8% at 30 days, 25.8% at 1 year, 51.0% at 5 years and 68.6% at 10 years after MI (Fig. 1). Corresponding mortality in non-RA controls was 12.8% (P =0.344 vs RA) at 30 days, 22.7% at 1 year, 43.0% at 5 years and 60.8% at 10 years after MI. The cumulative all-cause mortality rate at the end of a 14.0-year follow-up after MI admission was 80.4% in RA patients and 72.3% in controls (HR 1.25; CI: 1.16, 1.35; P <0.0001). RA duration, corticosteroid usage, and average corticosteroid dose prior to MI, but not serological status of RA, were independently associated with higher long-term mortality in RA patients (Table 3).
Fig. 1.
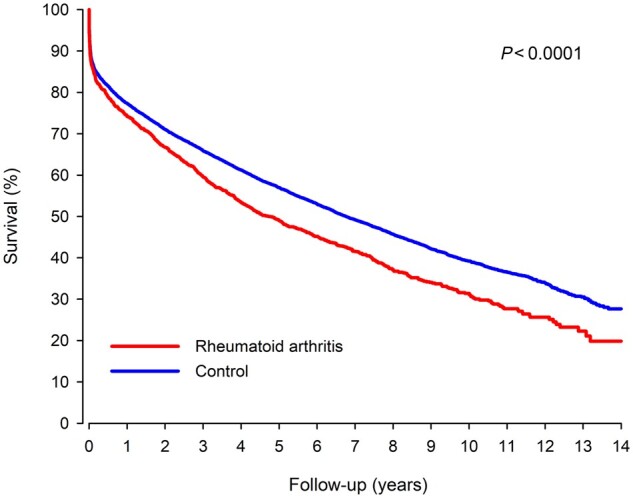
Survival in RA and matched control patients after myocardial infarction
Table 3.
Association of RA duration, drug usage and seropositivity with long-term outcomes in RA patients after myocardial infarction
| Mortality |
||||||
|---|---|---|---|---|---|---|
| Univariate |
Model 1 |
Model 2 |
||||
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Duration of RA per 5-year increment | 1.09 (1.07, 1.12) | <0.0001 | 1.07 (1.05, 1.10) | <0.0001 | 1.06 (1.04, 1.09) | <0.0001 |
| Corticosteroid usage | 1.52 (1.34, 1.72) | <0.0001 | 1.31 (1.15, 1.49) | <0.001 | 1.27 (1.11, 1.45) | 0.001 |
| Corticosteroid dosage per 1 mg/daya | 1.05 (1.03, 1.08) | <0.0001 | 1.05 (1.02, 1.08) | 0.001 | 1.05 (1.02, 1.08) | 0.002 |
| Methotrexate usage | 0.63 (0.44, 0.90) | 0.018 | 0.91 (0.79, 1.04) | 0.166 | 0.90 (0.78, 1.03) | 0.134 |
| Biological drug usageb | 0.35 (0.20, 0.60) | 0.0002 | 1.07 (0.61, 1.87) | 0.823 | 1.02 (0.58, 1.80) | 0.948 |
| Seropositivity | 0.98 (0.84, 1.14) | 0.789 | 1.17 (0.99, 1.37) | 0.062 | 1.10 (0.93, 1.30) | 0.259 |
| New myocardial infarction |
||||||
|---|---|---|---|---|---|---|
| Univariate |
Model 1 |
Model 2 |
||||
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Duration of RA per 5-year increment | 1.07 (1.03, 1.11) | 0.001 | 1.05 (1.01, 1.10) | 0.009 | 1.05 (1.01, 1.09) | 0.018 |
| Corticosteroid usage | 1.39 (1.15, 1.68) | 0.001 | 1.27 (1.04, 1.54) | 0.019 | 1.25 (1.02, 1.53) | 0.029 |
| Corticosteroid dosage per 1 mg/daya | 1.03 (0.99, 1.07) | 0.211 | 1.01 (0.97, 1.06) | 0.634 | 1.01 (0.96, 1.05) | 0.796 |
| Methotrexate usage | 0.78 (0.64, 0.95) | 0.014 | 0.94 (0.76, 1.16) | 0.573 | 0.94 (0.76, 1.17) | 0.583 |
| Biological drug usageb | 0.63 (0.34, 1.19) | 0.153 | 1.25 (0.65, 2.39) | 0.498 | 1.21 (0.63, 2.33) | 0.559 |
| Seropositivity | 0.89 (0.71, 1.12) | 0.890 | 0.99 (0.78, 1.26) | 0.915 | 0.93 (0.73, 1.19) | 0.929 |
| Stroke |
||||||
|---|---|---|---|---|---|---|
| Univariate |
Model 1 |
Model 2 |
||||
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Duration of RA per 5-year increment | 1.06 (1.00, 1.12) | 0.046 | 1.04 (0.98, 1.11) | 0.192 | 1.03 (0.97, 1.10) | 0.286 |
| Corticosteroid usage | 1.22 (0.91, 1.64) | 0.194 | 1.07 (0.78, 1.47) | 0.671 | 1.11 (0.80, 1.54) | 0.516 |
| Corticosteroid dosage per 1 mg/daya | 1.07 (1.01, 1.13) | 0.023 | 1.08 (1.01, 1.15) | 0.034 | 1.07 (1.01, 1.15) | 0.044 |
| Methotrexate usage | 0.54 (0.38, 0.75) | 0.0003 | 0.65 (0.45, 0.92) | 0.015 | 0.65 (0.45, 0.92) | 0.016 |
| Biological drug usageb | 0.43 (0.14, 1.36) | 0.152 | 0.72 (0.22, 2.33) | 0.584 | 0.73 (0.26, 2.36) | 0.596 |
| Seropositivity | 0.94 (0.65, 1.35) | 0.730 | 0.96 (0.66, 1.41) | 0.844 | 0.98 (0.66, 1.45) | 0.909 |
| Revascularization after index MI admission |
||||||
|---|---|---|---|---|---|---|
| Univariate |
Model 1 |
Model 2 |
||||
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Duration of RA per 5-year increment | 0.98 (0.93, 1.04) | 0.997 | 1.01 (0.95, 1.07) | 0.718 | 1.01 (0.95, 1.07) | 0.705 |
| Corticosteroid usage | 1.08 (0.84, 1.40) | 0.544 | 1.14 (0.87, 1.49) | 0.353 | 1.15 (0.87, 1.52) | 0.327 |
| Corticosteroid dosage per 1 mg/daya | 0.99 (0.94, 1.06) | 0.854 | 0.98 (0.92, 1.04) | 0.475 | 0.98 (0.92, 1.04) | 0.472 |
| Methotrexate usage | 0.92 (0.70, 1.20) | 0.531 | 0.94 (0.71, 1.24) | 0.635 | 0.93 (0.70, 1.24) | 0.928 |
| Biological drug usageb | 1.32 (0.72, 2.42) | 0.367 | 1.11 (0.59, 2.10) | 0.746 | 1.10 (0.58, 2.08) | 0.781 |
| Seropositivity | 0.91 (0.67, 1.26) | 0.579 | 0.88 (0.63, 1.22) | 0.877 | 0.87 (0.62, 1.22) | 0.413 |
Model 1 was adjusted for age, sex, alcohol abuse, anaemia, atrial fibrillation, cerebrovascular disease, chronic pulmonary disease, coagulopathy, dementia, depression, diabetes, heart failure, hypertension, hypothyroidism, liver disease, malignancy, paralysis, peripheral vascular disease, prior coronary artery bypass, prior MI, psychotic disorder, revascularization by percutaneous coronary intervention, revascularization by coronary bypass, type of myocardial infarction and treating hospital. Model 2 = Model 1 + duration of RA, serological status, usage of corticosteroids, usage of methotrexate and usage of biological drug. aAverage cumulative corticosteroid dosage as prednisolone equivalents mg/day during the year before myocardial infarction (MI) in patients with corticosteroid purchase. bIntravenous infusions, which are only administered in hospital, were not captured in the prescription drug registry (infliximab, rituximab, and intravenous formulations of tosilizumab and abatacept).
New myocardial infarction
New MI occurred in 2412 patients (437 in the RA group) during the follow-up after index hospital discharge. The cumulative rate of all new MIs in RA patients was 17.3% at 1-year, 29.4% at 5-year, 42.5% at 10-year and 46.6% at 14-year follow-up (Fig. 2). Occurrence of new MI in matched controls was 14.0% at 1-year, 25.9% at 5-year, 35.1% at 10-year and 40.9% at 14-year follow-up. Patients with RA had a higher hazard of new MI during the follow-up (HR 1.22; 1.09–1.36; P =0.001). Cumulative rate of new MI admission during follow-up was 42.4% in RA and 36.1% in control groups (HR 1.20; CI: 1.06, 1.35; P =0.004). The occurrence of fatal MI after index discharge was 19.5% in the RA group and 17.0% in the control group (HR 1.22; CI: 1.01, 1.47; P =0.037). RA duration and corticosteroid usage prior to MI were independently associated with occurrence of new MI in long-term follow-up (Table 3).
Fig. 2.
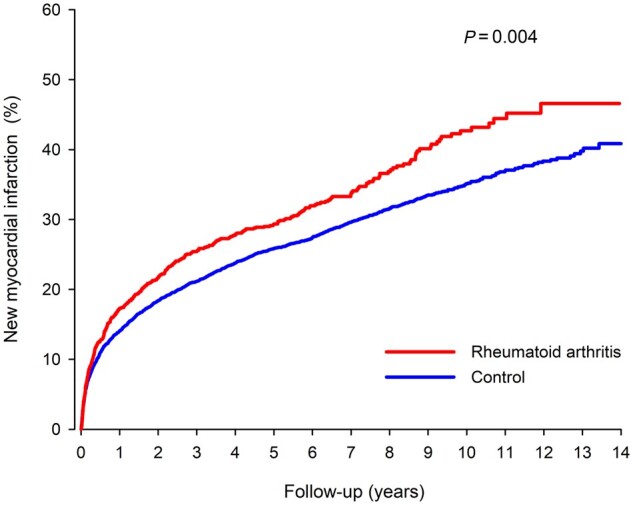
Cumulative occurrence of new myocardial infarction in RA and matched control patients after index myocardial infarction
Stroke
Of discharged MI patients, 1066 (174 in the RA group) had a stroke during follow-up. The cumulative rate of any stroke in the RA group was 4.6% at 1-year, 13.4% at 5-year and 20.3% at 10-year follow-up (Supplementary Fig. S2, available at Rheumatology online). The corresponding rates in the non-RA group were 3.8%, 12.0% and 19.6%. The cumulative incidence of any stroke was 27.0% in the RA group vs 25.9% in the non-RA group (HR 1.09; CI: 0.92, 1.31; P =0.322) at the end of follow-up. The cumulative stroke admission rate was 26.7% in the RA group vs 25.0% in non-RA group (P =0.173). The fatal stroke rate was 6.4% in the RA group vs 7.7% in the non-RA group (P =0.437). Methotrexate usage was independently associated with lower stroke occurrence after MI (Table 3). The hazard of stroke after MI increased with dosage (Table 3) if corticosteroids were used.
Revascularization
Coronary artery revascularization was performed on 1249 patients (235 in RA group) during follow-up after index MI discharge. The cumulative rate of revascularization after index MI admission was 29.7% in RA patients and 22.9% in non-RA controls (HR 1.27; CI: 1.09, 1.49; P =0.002, Fig. 3). Percutaneous coronary intervention was performed on 26.8% of RA and 19.2% of non-RA patients (P <0.0001). Coronary artery bypass surgery was performed on 4.6% of RA and 4.8% of non-RA patients (P =0.427). RA duration, antirheumatic medication, or seropositivity were not associated with revascularization after index MI (Table 3).
Fig. 3.
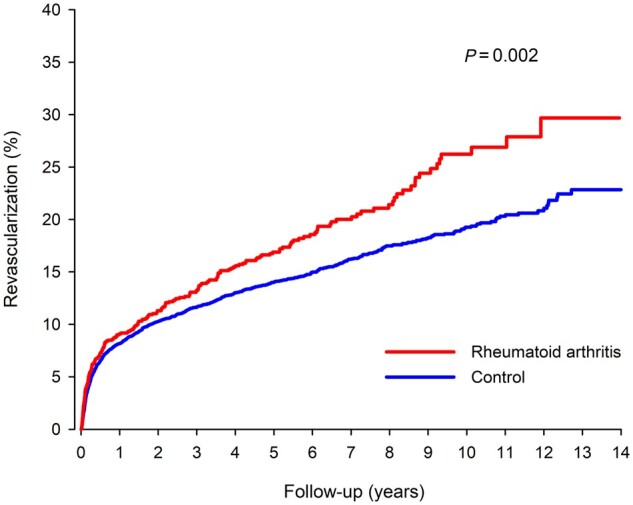
Cumulative rate of revascularization after admission for index myocardial infarction in RA and matched control patients
Discussion
Our multicentre nationwide retrospective cohort study showed that during a median follow-up of 7.3 years, patients with RA had a significantly increased long-term mortality after MI compared with baseline-matched patients without RA. Additionally, the risk for recurrent MI and revascularization was higher in patients with RA during follow-up. Longer RA disease duration and oral glucocorticoid use before MI were associated with increased long-term mortality, while methotrexate use was associated with a lower stroke risk.
Our study comprised 1614 RA patients experiencing MI, and it is one of the largest studies to date assessing long-term outcomes of MI in patients with RA. We accounted for numerous competing risk factors and characteristics of the index event with propensity score matching to estimate the independent impact of RA on outcomes. We observed significantly increased long-term mortality, risk for recurrent MI and revascularization. Mantel and colleagues [15] previously studied 1135 Swedish RA patients and 3184 matched controls with first-time acute coronary syndrome (ACS) in 2007–2010. They reported that, among RA patients compared with controls, the risk for recurrent ACS was increased by 25% and risk for all-cause mortality by 50% in a model adjusted for age, sex, pre-existing comorbidities, pharmacotherapies and ACS type [15]. Similar estimates were reported by a Taiwanese nationwide registry study exploring outcomes in 748 RA patients with first-time MI [17]. A few smaller studies have also indicated that RA patients have impaired long-term prognosis after MI [8, 13, 14]. Impaired 30-day and 1-year survival after the first MI has been reported not only in RA but across a wide range of autoimmune rheumatic diseases, including ankylosing spondylitis, psoriatic arthritis, SLE and other less common rheumatic diseases [23]. Our results, which further confirm an increased mortality and recurrent MI risk among RA patients after MI during a long-term 14-year follow-up, add to the previous literature by exploring the impact of DMARDs on long-term MI outcomes.
We did not observe a difference in 30-day mortality after MI between RA patients and matched controls with similar baseline and MI characteristics and rates of revascularization. Previous studies on short-term MI outcomes have shown conflicting results, which may at least partly be explained by differences in revascularization rates and ACS phenotype between RA cases and controls. An Australian population-based study found that CVD mortality after MI was almost doubled among patients with RA compared with controls, a finding that was accompanied with reduced PCI rates among RA patients [10]. In contrast, analyses of the National Inpatient Sample database from the US have revealed improved in-hospital mortality after ACS and higher rates of reperfusion therapies among RA patients compared with controls [12, 24, 25]. In our study, RA patients had reduced rates of PCI and CABG compared with MI patients without RA, but we accounted for this by propensity score matching. In line with our results, Isogai and coworkers reported that RA presence did not affect the likelihood of 30-day mortality among Japanese patients with MI who had good access to coronary revascularization (coronary angiography was performed in >90% during hospitalization) [11]. A Swedish registry study reported that RA patients’ 30-day mortality after ACS was increased by ∼50%, but their findings were not explained by differences in revascularization rates [9]. The authors concluded that a more severe clinical ACS phenotype among RA patients partly accounted for worse outcomes, and proposed that inflammation-induced mechanisms such as impaired coronary collateral development, more vulnerable atherosclerotic plaques and hypercoagulability may contribute to increased short-term mortality [9]. Our results indicate that while MI short-term outcomes are comparable among patients with and without RA who have similar MI characteristics and rates of revascularization, the major difference in outcomes comes in the long term, offering a window of opportunity for secondary prevention.
Glucocorticoid use and dosage were strongly associated with mortality after MI in our study. To our knowledge, only one previous study with 77 RA patients has assessed the impact of DMARDs and glucocorticoids on long-term outcomes of MI, but it was underpowered to detect associations [14]. Many previous studies have shown that glucocorticoid use in RA is associated with increased CVD [26, 27] and mortality risks [28]. Glucocorticoids can also indicate poor RA control and high disease activity, which can partially explain the observed association by confounding by indication [29]. Glucocorticoid-free remission is an important treatment target in current RA treatment guidelines [30], which is also a key part of the strategy to reduce the CVD risk in RA patients [7].
Use of methotrexate or biologic DMARDs were associated with better survival in univariate models, but the observed associations disappeared after adjustment for potential confounders, with the exception that methotrexate use was associated with a reduced stroke risk. There was also a statistically nonsignificant trend towards decreased mortality among methotrexate users. The finding that longer RA duration was a predictor of both mortality and new MI may reflect the effect of cumulative disease activity. Systemic inflammation is a key element of accelerated atherosclerosis among RA patients [5], and high disease activity is associated with increased risk of cardiovascular events [29]. Effective antirheumatic treatment seems to decrease CVD burden in RA: methotrexate and TNF-inhibitors are associated in observational studies with a decreased risk of CVD events [6]. RA disease activity should be optimally controlled among all RA patients to lower CVD risk [7]. Methotrexate is the anchor drug of RA treatment [30], and our results support the role of methotrexate also among RA patients with previous MI.
There are numerous potential explanations for impaired survival and excess ACS recurrence after MI among RA patients. RA patients in our study used less often statins than well-matched controls during 6 months after MI, which could speculatively be linked to concerns regarding musculoskeletal side effects or polypharmacy in this patient group with pre-existing musculoskeletal complaints. Statin discontinuation has been linked to increased risk of recurrent MI and mortality both in general population [31] and among RA patients [32]. Perhaps due to varying geographic regions, study settings and methodologies, some previous studies have reported reduced initiation and adherence to secondary preventive drugs among patients with RA compared with controls [18, 19], whereas others report no difference [14, 15]. RA patients with acute or stable coronary disease may have worse PCI outcomes than patients without rheumatic diseases [33]. Inflammation is linked to vulnerability of atherosclerotic plaques, and RA patients may have more vulnerable atherosclerotic plaques than controls [34]. RA patients may also experience atypical symptoms of MI [35], and this might cause delay in admission to hospital.
Of note, the study population comprised elderly, multimorbid patients with only 37.5% using methotrexate and 3% using biologic DMARDs during the year preceding index MI. Up to one-third of patients also had a heart failure diagnosis, more than half had hypertension and a quarter had suffered a previous MI. Our results may not be applicable to younger RA patients with fewer comorbidities who are, on average, treated with more effective treatment regimens.
Seropositivity has previously been associated with an increased risk of CVD and mortality [36–38], although some results also indicate that seropositivity might not be an independent predictor of CVD after adjustment for traditional risk factors, medications and diseases activity, at least in early RA [39, 40]. Seropositivity was not associated with a worse prognosis in our study. This finding warrants CVD risk management equally in seropositive and seronegative RA patients.
Our study has many important strengths. We used a combination of previously validated nationwide registries, which are mandated by law in Finland [41]. We included only patients with special reimbursement for medication to ensure reliability of the RA diagnosis, and we had detailed data on MI characteristics and treatment. Propensity score matching was used to balance differences in major risk factors between the study groups. It is possible that unrecognised residual confounders may impact the results, although propensity score matching is one of the strongest methods to control confounding factors. Smoking is a potential residual confounder in our study, as it is associated with both RA and CVD. Even so, we consider it is unlikely that smoking alone would explain our study’s main findings. Medication usage was estimated with the standard method of studying drug purchase data from databases covering all prescription drug purchases in Finland [42]. We, however, have no data about the actual usage of purchased medications. We did not have access to data on intravenously administered biologic DMARDs. However, the majority of biologic DMARDs in Finland were administered subcutaneously between 2005 and 2014. Based on data on RA patients identified from the National Register for Biologic Treatment in Finland (ROB-FIN) 2004–2014, infliximab comprised 12% of all TNF inhibitor treatment periods [43], and infliximab, rituximab, abatacept and tocilizumab (all administered mostly intravenously before 2015) comprised approximately one-third of all biologic DMARD treatment periods (Dan Nordström and Nina Trokovic, personal communication, 1 February 2021). We had no data about RA disease activity or BMI. Inaccuracies in administrative databases are a limitation that is likely to affect RA patients and controls in a similar fashion and cause bias towards the null hypothesis; thus, they are not expected to interfere with the essential findings of this study.
Conclusion
In conclusion, our study demonstrated that RA is independently associated with a worse prognosis after MI during long-term follow-up. Longer RA disease duration and glucocorticoid use were independent predictors of mortality, while methotrexate use was associated with a lower stroke risk. Secondary prevention with statins was less frequent in patients with RA, and given the survival disadvantage, secondary prevention in RA warrants particular attention. Patients with RA who suffer a myocardial infarction could benefit from a comprehensive evaluation and optimization of treatment to improve long-term outcomes.
Supplementary Material
Acknowledgements
We wish to thank Dan Nordström and Nina Trokovic for their kind help with the estimation of the intravenous biologic DMARD use within the National Register for Biologic Treatment in Finland (ROB-FIN) during our study period. A.M.K. has been supported by a research grant from FOREUM Foundation for Research in Rheumatology.
Disclosure statement: A.P. has received grants from the Finnish Medical Foundation, the Finnish Foundation for Cardiovascular Research and the Turku University Hospital Research Foundation, a consulting fee from Pfizer, a lecture fee from MSD, Pfizer and Sanofi, and travel expenses from Bristol-Myers-Squib and Novartis. A.M.K. has received speaker fees from Boehringer-Ingelheim, has attended advisory boards of Pfizer, Gilead and Boehringer-Ingelheim, and received congress sponsorship from Pfizer, Celgene, UCB Pharma, Mylan and Roche. M.M. has received travel grants and congress sponsorship (Abbott, Boston Lifesciences, Medtronic). V.K. has received scientific consultancy fees (AstraZeneca), speaker fees (Bayer, Boehringer-Ingelheim, Roche) and travel grants and congress sponsorship (AstraZeneca, Boehringer-Ingelheim, Bayer, Pfizer). The other author has declared no conflicts of interest.
Funding: This study was supported by grant funding from the Finnish Cultural Foundation, the Paulo Foundation, and the Finnish Governmental VTR-funding.
Data availability statement
The data and study materials will be made available to those who fulfil the requirements of applicable Finnish laws and regulations for purposes of reproducing the results or replicating the procedure.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Houge IS, Hoff M, Thomas R, Videm V.. Mortality is increased in patients with rheumatoid arthritis or diabetes compared to the general population – the Nord-Trøndelag Health Study. Sci Rep 2020;10:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aviña-Zubieta JA, Choi HK, Sadatsafavi M. et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Care Res 2008;59:1690–7. [DOI] [PubMed] [Google Scholar]
- 3. Avina-Zubieta JA, Thomas J, Sadatsafavi M, Lehman AJ, Lacaille D.. Risk of incident cardiovascular events in patients with rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis 2012;71:1524–9. [DOI] [PubMed] [Google Scholar]
- 4. Boyer JF, Gourraud PA, Cantagrel A, Davignon JL, Constantin A.. Traditional cardiovascular risk factors in rheumatoid arthritis: a meta-analysis. Jt Bone Spine 2011;78:179–83. [DOI] [PubMed] [Google Scholar]
- 5. Choy E, Ganeshalingam K, Semb AG, Szekanecz Z, Nurmohamed M.. Cardiovascular risk in rheumatoid arthritis: recent advances in the understanding of the pivotal role of inflammation, risk predictors and the impact of treatment. Rheumatology 2014;53:2143–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Roubille C, Richer V, Starnino T. et al. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Ann Rheum Dis 2015;74:480–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Agca R, Heslinga SC, Rollefstad S. et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis 2017;76:17–28. [DOI] [PubMed] [Google Scholar]
- 8. Sodergren A, Stegmayr B, Lundberg V, Ohman M-L, Wallberg-Jonsson S.. Increased incidence of and impaired prognosis after acute myocardial infarction among patients with seropositive rheumatoid arthritis. Ann Rheum Dis 2006;66:263–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mantel Ä, Holmqvist M, Jernberg T, Wållberg-Jonsson S, Askling J.. Rheumatoid arthritis is associated with a more severe presentation of acute coronary syndrome and worse short-term outcome. Eur Heart J 2015;36:3413–22. [DOI] [PubMed] [Google Scholar]
- 10. Van Doornum S, Brand C, King B, Sundararajan V.. Increased case fatality rates following a first acute cardiovascular event in patients with rheumatoid arthritis. Arthritis Rheum 2006;54:2061–8. [DOI] [PubMed] [Google Scholar]
- 11. Isogai T, Matsui H, Tanaka H. et al. Treatments and in-hospital mortality in acute myocardial infarction patients with rheumatoid arthritis: a nationwide retrospective cohort study in Japan. Clin Rheumatol 2017;36:995–1004. [DOI] [PubMed] [Google Scholar]
- 12. Elbadawi A, Ahmed HMA, Elgendy IY. et al. Outcomes of acute myocardial infarction in patients with rheumatoid arthritis. Am J Med 2020;133:1168–79.e4. [DOI] [PubMed] [Google Scholar]
- 13. Douglas KMJ, Pace AV, Treharne GJ. et al. Excess recurrent cardiac events in rheumatoid arthritis patients with acute coronary syndrome. Ann Rheum Dis 2006;65:348–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McCoy SS, Crowson CS, Maradit-Kremers H. et al. Longterm outcomes and treatment after myocardial infarction in patients with rheumatoid arthritis. J Rheumatol 2013;40:605–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mantel Ä, Holmqvist M, Jernberg T, Wållberg-Jonsson S, Askling J.. Long-term outcomes and secondary prevention after acute coronary events in patients with rheumatoid arthritis. Ann Rheum Dis 2017;76:2017–24. [DOI] [PubMed] [Google Scholar]
- 16. Skielta M, Söderström L, Rantapää-Dahlqvist S, Jonsson SW, Mooe T.. Trends in mortality, co-morbidity and treatment after acute myocardial infarction in patients with rheumatoid arthritis 1998–2013. Eur Hear J Acute Cardiovasc Care 2020;9:931–8. [DOI] [PubMed] [Google Scholar]
- 17. Lai CH, Lai CH, Lai CH. et al. Outcomes of acute cardiovascular events in rheumatoid arthritis and systemic lupus erythematosus: a population-based study. Rheumatology 2020;59:1355–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lindhardsen J, Ahlehoff O, Gislason GH. et al. Initiation and adherence to secondary prevention pharmacotherapy after myocardial infarction in patients with rheumatoid arthritis: a nationwide cohort study. Ann Rheum Dis 2012;71:1496–501. [DOI] [PubMed] [Google Scholar]
- 19. Van Doornum S, Brand C, Sundararajan V, Ajani AE, Wicks IP.. Rheumatoid arthritis patients receive less frequent acute reperfusion and secondary prevention therapy after myocardial infarction compared with the general population. Arthritis Res Ther 2010;12:R183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kytö V, Myllykangas ME, Sipilä J. et al. Long-term outcomes of mechanical Vs biologic aortic valve prosthesis in patients older than 70 years. Ann Thorac Surg 2019;108:1354–60. [DOI] [PubMed] [Google Scholar]
- 21. Mager DE, Lin SX, Blum RA, Lates CD, Jusko WJ.. Dose equivalency evaluation of major corticosteroids: pharmacokinetics and cell trafficking and cortisol dynamics. J Clin Pharmacol 2003;43:1216–27. [DOI] [PubMed] [Google Scholar]
- 22. Malmberg M, Gunn J, Sipilä J. et al. Comparison of long-term outcomes of patients having surgical aortic valve replacement with versus without simultaneous coronary artery bypass grafting. Am J Cardiol 2020;125:964–9. [DOI] [PubMed] [Google Scholar]
- 23. Van Doornum S, Bohensky M, Tacey MA. et al. Increased 30-day and 1-year mortality rates and lower coronary revascularisation rates following acute myocardial infarction in patients with autoimmune rheumatic disease. Arthritis Res Ther 2015;17:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Edigin E, Shaka H, Eseaton P. et al. Rheumatoid arthritis is not associated with increased inpatient mortality in patients admitted for acute coronary syndrome. Cureus 2020;12:e9799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Francis ML, Varghese JJ, Mathew JM. et al. Outcomes in patients with rheumatoid arthritis and myocardial infarction. Am J Med 2010;123:922–8. [DOI] [PubMed] [Google Scholar]
- 26. Wilson JC, Sarsour K, Gale S. et al. Incidence and risk of glucocorticoid-associated adverse effects in patients with rheumatoid arthritis. Arthritis Care Res 2019;71:498–511. [DOI] [PubMed] [Google Scholar]
- 27. Aviña-zubieta JA, Abrahamowicz M, De vera MA. et al. Immediate and past cumulative effects of oral glucocorticoids on the risk of acute myocardial infarction in rheumatoid arthritis: a population-based study. Rheumatology 2013;52:68–75. [DOI] [PubMed] [Google Scholar]
- 28. Del Rincón I, Battafarano DF, Restrepo JF, Erikson JM, Escalante A.. Glucocorticoid dose thresholds associated with all-cause and cardiovascular mortality in rheumatoid arthritis. Arthritis Rheumatol 2014;66:264–72. [DOI] [PubMed] [Google Scholar]
- 29. Solomon DH, Reed GW, Kremer JM. et al. Disease activity in rheumatoid arthritis and the risk of cardiovascular events. Arthritis Rheumatol 2015;67:1449–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Smolen JS, Landewé RBM, Bijlsma JWJ. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 2020;79:685–99. [DOI] [PubMed] [Google Scholar]
- 31. Ho PM, Spertus JA, Masoudi FA. et al. Impact of medication therapy discontinuation on mortality after myocardial infarction. Arch Intern Med 2006;166:1842. [DOI] [PubMed] [Google Scholar]
- 32. De Vera MA, Choi H, Abrahamowicz M. et al. Statin discontinuation and risk of acute myocardial infarction in patients with rheumatoid arthritis: a population-based cohort study. Ann Rheum Dis 2011;70:1020–4. [DOI] [PubMed] [Google Scholar]
- 33. Nochioka K, Biering-Sørensen T, Hansen KW. et al. Long-term outcomes in patients with rheumatologic disorders undergoing percutaneous coronary intervention: a BAsel Stent Kosten-Effektivitäts Trial-PROspective Validation Examination (BASKET-PROVE) sub-study. Eur Hear J Acute Cardiovasc Care 2017;6:778–86. [DOI] [PubMed] [Google Scholar]
- 34. Aubry MC, Maradit-Kremers H, Reinalda MS. et al. Differences in atherosclerotic coronary heart disease between subjects with and without rheumatoid arthritis. J Rheumatol 2007;34:937–42. [PubMed] [Google Scholar]
- 35. Maradit-Kremers H, Crowson CS, Nicola PJ. et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum 2005;52:402–11. [DOI] [PubMed] [Google Scholar]
- 36. Gonzalez A, Icen M, Kremers HM. et al. Mortality trends in rheumatoid arthritis: the role of rheumatoid factor. J Rheumatol 2008;35:1009–14. [PMC free article] [PubMed] [Google Scholar]
- 37. Ajeganova S, Andersson MLE, Frostegård J, Hafström I.. Disease factors in early rheumatoid arthritis are associated with differential risks for cardiovascular events and mortality depending on age at onset: a 10-year observational cohort study. J Rheumatol 2013;40:1958–66. [DOI] [PubMed] [Google Scholar]
- 38. Kerola AM, Nieminen TVM, Virta LJ. et al. No increased cardiovascular mortality among early rheumatoid arthritis patients: a nationwide register study in 2000-2008. Clin Exp Rheumatol 2015;33:391–8. [PubMed] [Google Scholar]
- 39. Innala L, Möller B, Ljung L. et al. Cardiovascular events in early RA are a result of inflammatory burden and traditional risk factors: a five year prospective study. Arthritis Res Ther 2011;13:R131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kerola AM, Kerola T, Kauppi MJ. et al. Cardiovascular comorbidities antedating the diagnosis of rheumatoid arthritis. Ann Rheum Dis 2013;72:1826–9. [DOI] [PubMed] [Google Scholar]
- 41. Sund R. Quality of the Finnish Hospital Discharge Register: a systematic review. Scand J Public Health 2012;40:505–15. [DOI] [PubMed] [Google Scholar]
- 42. Prami T, Khanfir H, Hasvold P. et al. Concomitant use of drugs known to cause interactions with oral antiplatelets—polypharmacy in acute coronary syndrome outpatients in Finland. Eur J Clin Pharmacol 2020;76:257–65. [DOI] [PubMed] [Google Scholar]
- 43. Aaltonen KJ, Joensuu JT, Pirilä L. et al. Drug survival on tumour necrosis factor inhibitors in patients with rheumatoid arthritis in Finland. Scand J Rheumatol 2017;46:359–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data and study materials will be made available to those who fulfil the requirements of applicable Finnish laws and regulations for purposes of reproducing the results or replicating the procedure.


