Abstract
Even though no definitive link has been established, Bell's palsy has been described as a potential side effect of SARS-CoV-2 mRNA vaccines in a few reports, and the US Food and Drug Administration has recommended strict surveillance of its occurrence in the vaccinated general population.
We present the case of a previously healthy 35-year-old female patient who developed Bell's palsy 12 h after receiving the first dose of the mRNA-1273 vaccine. Her general practitioner performed the diagnosis, and corticosteroid treatment was initiated, with slow symptoms improvement. The neurologist's evaluation and a contrast-enhanced brain Magnetic Resonance Imaging revealed a subtle enhancement of the left facial nerve, confirming the diagnosis of Bell's palsy.
Keywords: Bell's palsy, SARS-CoV-2 vaccine, Moderna vaccine, Facial nerve paralysis, COVID-19 vaccine side effects
1. Introduction
Two vaccines using mRNA technology are currently available against SARS-CoV-2 infection: BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna). Bell's palsy has been observed as a potential side effect of both but without any defined causal relationship.1 This disorder, also known as idiopathic peripheral facial paralysis, consists of rapid onset facial nerve paralysis. The peak age of presentation is between 15 and 50 years, with about 25 cases per 100,000 per year, without gender predominance.2 It is currently a diagnosis of exclusion, supported by a typical presentation and usually encounters spontaneous resolution within 6–8 weeks in 70% of the cases. Timely treatment with corticosteroids raises the chance of full recovery to more than 90%.2
Two large phase 3 vaccine trials, based on data from 73,799 volunteers, 36,901 of whom got at least one vaccine dose, identified eight occurrences of probable Bell's palsy: seven from vaccinated participants and one from placebo recipients3: four incidences of Bell's palsy were recorded in the mRNA-1273 vaccine group, three in vaccine recipients and one in the placebo group4; whereas, in the case of the BNT162b2 vaccine, four vaccinated participants developed Bell's palsy.1., 3.
Even if a clear causal relationship between the administration of mRNA SARS-CoV-2 have not been identified, the US Food and Drug Administration recommended monitoring in the general population should be performed to determine the side effects profile of each vaccine.
We report the case of a 35-year-old previously healthy female who developed symptoms of left Bell's palsy after receiving the first dose of the mRNA-1273 vaccine. Brain Magnetic Resonance Imaging (MRI) demonstrated a subtle contrast enhancement of the left facial nerve, typical for this disorder. She received an oral steroid treatment with progressive clinical resolution.
2. Case presentation
A previously healthy 35-year-old Caucasian female, with no previous history of Bell's palsy or Herpes Simplex Virus type 1 infection, received the first dose of mRNA-1273 vaccination on August 6, at 9 a.m. On the same day, about 12 h later, she developed a deep left laterocervical pain and stiffness, followed by the sensation of not holding liquids properly in her mouth. On the next morning, after about 26 h from the injection, she noticed a left facial droop with a slight difficulty in speaking and eating, and started experiencing during the day inability to close the ipsilateral eye completely. All her symptoms developed without occurrence of pain, general malaise or fever.
A diagnosis of Bell's palsy was made by the general practitioner, and treatment with 50 mg of daily prednisone for two weeks was prescribed. The patient came to our attention 10 days after the onset of the symptoms for a neurological evaluation. At the clinical examination, the patient was alert and oriented. Vital parameters were within the normal range. Cranial nerve examination showed isolated left VII nerve palsy of lower motor neuron type; a slight asymmetry of the left corner of the mouth was detectable when smiling, and the patient was able to completely close the left eye only with maximum effort (House-Brackmann grade III). No objective weakness in all four limbs was observed, and the rest of her physical examination was unremarkable. Even though the symptoms were still present, they were described as improved since their onset. After 12 days from the vaccine injection, the patient underwent a contrast-enhanced brain MRI confirming the diagnosis of Bell's palsy and demonstrating only a subtle enhancement of the left facial nerve (Fig. 1, Fig. 2 ). On a second neurological visit, performed seven days after the MRI, the patient showed complete resolution of the symptomatology.
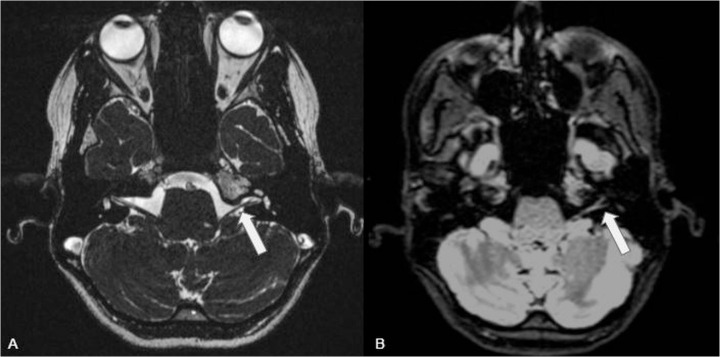
Fig. 1.
CISS (A) (field of view 180 × 180 mm, matrix 256 × 256, voxel size 0.6 × 0.6 × 0.6 mm, TR 5.63 ms, TE 2.48 ms, flip angle 70°) and dark fluid para-axial reconstruction (B) (Field of view 280 × 245 mm, matrix 218 × 256, voxel size 1.1 × 1.1 × 1.0, TR 6000 ms, TE 359 ms) shows the facial and vestibulocochlear nerves entering the internal auditory meatus with regular morphology and thickness (arrows).
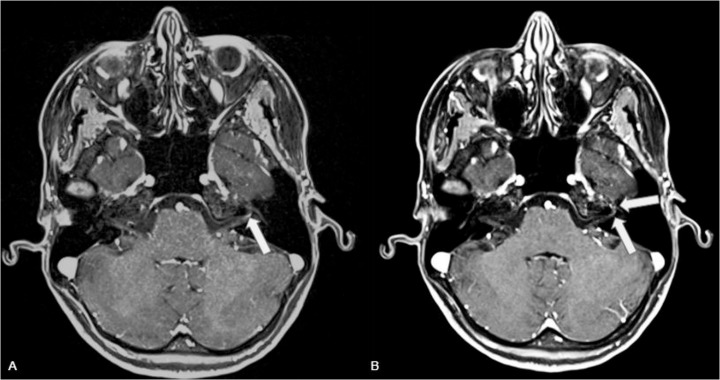
Fig. 2.
(A) post contrast axial T1-weighted sequence (A) (field of view 250 × 180 mm), matrix 290 × 448, voxel size 0.6 × 0.6 × 0.6 mm, TR 8.16 ms, TE 3.15 ms, flip angle (15°) and reconstruction at 3 mm thickness (B) showing contrast enhancement of the distal intracanalicular and labyrinthine segments of the left VII nerve (arrows).
3. Discussion
Bell's palsy is defined as an acute unilateral facial paralysis; the etiology is still unclear, but the potential mechanisms of development include immune, infective, and ischemic mechanisms. The most accredited hypotheses are the reactivation of latent Herpes Simplex Virus type 1 in the geniculate ganglia of the facial nerves, an autoimmune mechanism through the mimicry of host molecules by the antigens of the vaccines, or an immune-mediated demyelination analogous to Guillain-Barrè syndrome.5
Until recently, the data available in the literature about the development of Bell's palsy after administration of post-mRNA vaccines in the general population were limited, consisting mainly of case reports5., 6., 7., 8.; a recent study analyzed the reported frequency of neuralgic amyotrophy, Bell's palsy, and Guillain-Barrè syndrome in VigiBase, the World Health Organization's (WHO) global database of suspected adverse drug reactions, in patients who received COVID-vaccine.9
Regarding Bell'palsy, the authors stated that this disorder was disproportionately more frequently reported with COVID-19 vaccines than with other viral vaccines over the full database, with a higher frequency in males and in the age subgroup 65–74 years and ≥75 years. The characteristics of the affected patients, as well as the incidence for the type of vaccine are listed in Table 1 . According to this study, male patients aged ≥65 years has higher risk of post vaccine Bell's palsy.
Table 1.
Patients' demographics, time of occurrence, and types of COVID-19 vaccines, in patients who developed a Bell's palsy after COVID- 19 vaccine, according to data collected in a recent study from the VigiBase, the WHO global database of suspected adverse drug reactions.9 The total number of considered reported Bell's palsy was 33209.
| Number of patients (%) | |
|---|---|
| Sex | |
| Male | 1994 (60.1%) |
| Female | 1280 (38.6%) |
| NR | 46 (1.4%) |
| Age range | |
| <18 years | 8 (0.2%) |
| 18–44 years | 891 (26.8%) |
| 45–64 years | 1197 (36.1%) |
| 65–74 years | 479 (14.4%) |
| ≥75 years | 446 (13.4%) |
| NR | 51 (8.1%) |
| COVID-19 vaccine | |
| Pfizer BioNTech | 1822 (54.9%) |
| Moderna | 623 (18.8%) |
| Vaxzevria (ex COVID-19 AstraZeneca) | 671 (20.2%) |
| Janssen | 162 (4.9%) |
| Coronavac | 13 (0.4%) |
| Convidecia | 1 (0%) |
| Sputnik V | 1 (0%) |
| NR | 27 (0.8%) |
| Outcome | |
| Recovery | 544 (16.4%) |
| Recovery with sequeale | 20 (0.6%) |
| Healing (recovering at the report time) | 423 (12.7%) |
| Absent recovery | 647 (19.5%) |
| Death | 2 (0%) |
| NR | 1684 (50.7%) |
| Days (IQR) | |
|---|---|
| Median time to occurrencea | |
| After the 2nd dose | 4 (1−12) |
| After the 1st/2nd dose (not specified in the report) | 10 (1−22) |
NR = not reported.
IQR = interquartile range.
For the time to onset, the reported cases after the second dose were 49; cases reported after the 1st or 2nd dose, without the specification of which dose, were 2767. In 504 cases, the time of the onset of the symptoms was not reported.
The authors instead observed an incidence of neuralgic amyotrophy and Guillian Barrè post-CVOID-19 vaccine similar to that previously observed for other viral vaccines, without any sex or age predisposition.9 Most of the neurological adverse effects were related with the BNT162b2 (Pfizer-BioNTech)9., 10. and mRNA-1273 (Moderna),9., 11. and the COVID-19 Vaccine Vaxzevria (ex-COVID-19 AstraZeneca), whilst less frequently were associated with the Ad26.COV2.S (Janssen) vaccination,9., 12. A case report described Bell's palsy after mRNA-1273 vaccine administration, reporting the case of a 36-year-old male who developed facial palsy after one day from the second vaccine dose; in this case, brain MRI was unremarkable.7
In our case, the patient complained of symptoms at 12 h from the injection. We can assume that the timing of Bell's palsy onset after mRNA vaccine administration varies, with early manifestation occurring as early as 5 h after vaccination, as reported in a male patient who received BNT162b2 and developed recurrent Bell's palsy after the second dose of vaccine.8 In this case, the first episode occurred 5 h after the first dose was administered, and the second occurred 2 days later.
The reported mean time-to-onset was 4 days (range: 1–12 days).9
The mechanism of Bell's palsy in patients post-vaccination still remains unclear. The first hypothesis is an autoimmune reaction occurring via either mimicry of host molecules by the vaccine antigen or bystander activation of dormant auto-reactive T-cells.13 A second theory is that the vaccine causes innate immune activation by a combination of mRNA and lipids, which could include interferon synthesis, exceeding the peripheral tolerance.14., 15. Another possible etiopathogenesis is the reactivation of latent Herpes Simplex Type 1 infections of the geniculate ganglia of facial nerves: a previous study by Murakami et al. on 14 patients with Bell's palsy, 9 patients with the Ramsay-Hunt syndrome, and 12 controls, demonstrated the presence of Herpes simplex virus type 1 genomes in the 79% of Bell's palsy patients, but not in patients with the Ramsay-Hunt syndrome or in other controls.16
If this hypothesis is confirmed by future studies, previous Herpes Simplex Virus 1 infection should be considered as a risk factor for the development of post-vaccine Bell's palsy.
Moreover, in a study comparing Bell's palsy patients treated with acyclovir-prednisone versus patients treated with placebo-prednisone, the first treatment was significantly more effective in returning volitional muscle motion and in preventing partial nerve degeneration than placebo-prednisone treatment.17
Contrast-enhanced brain MRI can help confirm the diagnosis or exclude other anomalies, through the assessment of the morphology and normal enhancement pattern of facial nerve along its course, including the geniculate ganglion and mastoid segments. The normal facial nerve faintly enhances in the geniculate ganglion, tympanic, and mastoid segments, but not in the cisternal, intracanalicular, labyrinthine, or parotid portions.
In Bell's palsy, increased nerve enhancement has been reported in a variable rate, ranging from 57 to 100%18; the affected segments of the facial nerve enhance in a linear fashion, more intensely than the contralateral non-affected side. The mastoid and extratemporal segments are less frequently involved.19 The enlargement or thickening of the facial nerve should be instead considered suspicious for a neoplastic process.
The treatment is based on steroids administration. Patients presented to the Emergency Departments are usually treated with intravenous methylprednisolone (500 mg) twice daily for three days plus 400 mg of acyclovir four times daily, followed by prednisone per os associated with 400 mg of acyclovir three times daily, as for idiopathic Bell's Palsy.20 Patients who directly refer the general practitioner are usually treated with 50–60 mg of daily oral prednisolone for one week associated or not with acyclovir with the above reported dosage.7
For what concerning the prognosis of these patients, Noseda et al.9 reported a recovery in 16.4% of patients, a recovery with sequelae in the 0.6% of patients, and an absent recovery in 19.5% of patients. Renoud et al.21 in an analysis of the data collected in the VigiBase on March 9, 2021, described a recovery in the 19.8% of patients, a recovery with sequelae in the 0.3% and an absent recovery in the 23.9% of patients.
4. Conclusion
Reports of Bell's palsy following mRNA SARS-CoV-2 vaccines in the general population are beginning to emerge in the literature. The exact causal relationship between the mRNA vaccines and the onset of Bell's palsy needs to be investigated further. Our case emphasizes the importance of collecting vaccine history in patients with Bell's palsy. Brain MRI can be used to confirm a diagnosis and rule out other disorders.
Declaration of competing interest
The authors declare that they have no conflict of interest.
References
- 1.Baden L.R., El Sahly H.M., Essink B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zandian A., Osiro S., Hudson R., et al. The neurologist's dilemma: a comprehensive clinical review of Bell's palsy, with emphasis on current management trends. Med Sci Monit. 2014;20:83–90. doi: 10.12659/MSM.889876. Published 2014 Jan 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cirillo N. Reported orofacial adverse effects of COVID-19 vaccines: the knowns and the unknowns. J Oral Pathol Med. 2021;50(4):424–427. doi: 10.1111/jop.13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.FDA Briefing document Pfizer-BioNTech Covid-19 vaccine. 2020. https://www.fda.gov/media/144245/download Available:
- 5.Wan E.Y.F., Chui C.S.L., Lai F.T.T., et al. Bell's palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and nested case-control study. Lancet Infect Dis. 2021;S1473–3099(21):00451–00455. doi: 10.1016/S1473-3099(21)00451-5.5. Aug 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin-Villares C., Vazquez-Feito A., Gonzalez-Gimeno M.J., de la Nogal-Fernandez B. Bell's palsy following a single dose of mRNA SARS-CoV-2 vaccine: a case report. J Neurol. 2021:1–2. doi: 10.1007/s00415-021-10617-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iftikhar H., Noor S.M.U., Masood M., Bashir K. Bell's palsy after 24 hours of mRNA-1273 SARS-CoV-2 vaccine. Cureus. 2021;13(6) doi: 10.7759/cureus.15935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burrows A., Bartholomew T., Rudd J., Walker D. Sequential contralateral facial nerve palsies following COVID-19 vaccination first and second doses. BMJ Case Rep. 2021;14(7) doi: 10.1136/bcr-2021-243829. Jul 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noseda R., Ripellino P., Ghidossi S., Bertoli R., Ceschi A. Reporting of acute inflammatory neuropathies with COVID-19 vaccines: subgroup disproportionality analyses in VigiBase. Vaccines (Basel). 2021 Sep 14;9(9):1022. doi: 10.3390/vaccines9091022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colella G., Orlandi M., Cirillo N. Bell's palsy following COVID-19 vaccination. J Neurol. 2021 Oct;268(10):3589–3591. doi: 10.1007/s00415-021-10462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin-Villares C., Vazquez-Feito A., Gonzalez-Gimeno M.J., de la Nogal-Fernandez B. Bell's palsy following a single dose of mRNA SARS-CoV-2 vaccine: a case report. J Neurol. 2021;25:1–2. doi: 10.1007/s00415-021-10617-3. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishizawa Y., Hoshina Y., Baker V. Bell's palsy following the Ad26.COV2.S COVID-19 vaccination. QJM. 2021 doi: 10.1093/qjmed/hcab143. hcab143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Principi N., Esposito S. Do vaccines have a role as a cause of autoimmune neurological syndromes? Front Public Health. 2020;28(8):361. doi: 10.3389/fpubh.2020.00361. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hwang I., Calvit T.B., Cash B.D., Holtzmuller K.C. Bell's palsy: a rare complication of interferon therapy for hepatitis C. Dig Dis Sci. 2004;49:619–620. doi: 10.1023/b:ddas.0000026389.56819.0c. [DOI] [PubMed] [Google Scholar]
- 15.Yalçindağ F.N., Alay C. Bell's palsy during interferon alpha 2a treatment in a case with Behçet uveitis. F1000 Res. 2013;2:245. doi: 10.12688/f1000research.2-245.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murakami T.S., Mizobuchi M., Nakashiro Y., Doi T., Hato N., Yanagihara N. Bell palsy and herpes simplex virus: identification of viral DNA in endoneurial fluid and muscle. Ann Intern Med. 1996;124:27–30. doi: 10.7326/0003-4819-124-1_part_1-199601010-00005. [DOI] [PubMed] [Google Scholar]
- 17.Adour K.K., Ruboyianes J.M., Von Doersten P.G. Bell's palsy treatment with acyclovir and prednisone compared with prednisone alone: a double-blind, randomized, controlled trial. Ann Otol Rhinol Laryngol. 1996;105:371–378. doi: 10.1177/000348949610500508. [DOI] [PubMed] [Google Scholar]
- 18.Karaca H., Soydan L., Yildiz S., Toros S.Z. Measurement of the depth of facial nerve at the level of stylomastoid foramen using MR imaging in Bell's palsy. Clin Imaging. 2019;58:34–38. doi: 10.1016/j.clinimag.2019.06.008. Nov-Dec. [DOI] [PubMed] [Google Scholar]
- 19.Al-Noury K., Lotfy A. Normal and pathological findings for the facial nerve on magnetic resonance imaging. Clin Radiol. 2011;66(8):701–707. doi: 10.1016/j.crad.2011.02.012. Aug. [DOI] [PubMed] [Google Scholar]
- 20.Mason M.C., Liaqat A., Morrow J., Basso R., Gujrati Y. Bilateral facial nerve palsy and COVID-19 vaccination: causation or coincidence? Cureus. 2021;13(8) doi: 10.7759/cureus.17602. Aug 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Renoud L., Khouri C., Revol B., Lepelley M., Perez J., Roustit M., Cracowski J.L. Association of facial paralysiswith mRNA COVID-19 vaccines: a disproportionality analysis using the World Health Organization pharmacovigilance database. JAMA Intern Med. 2021 Sep 1;181(9):1243–1245. doi: 10.1001/jamainternmed.2021.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]