Abstract
Objective
The primary objective of this study was to investigate adverse effects of ambient particulate matter of various sizes on the incidence of the prevalent autoimmune rheumatic diseases (AIRDs): RA, AS and SLE.
Methods
We investigated 230 034 participants in three metropolitan cities of South Korea from the National Health Insurance Service–National Sample Cohort (NHIS-NSC). Starting from January 2010, subjects were followed up until the first event of prevalent AIRDs, death, or December 2013. The 2008–2009 respective averages of particulate matter2.5 (<2.5 μm) and particulate mattercoarse (2.5 μm to 10 μm) were linked with participants’ administrative district codes. Adjusted hazard ratios (aHRs) and 95% CIs were estimated using Cox regression analysis in one- and two-pollutant models.
Results
Adjusted for age, sex, region, and household income, in the two-pollutant model, RA incidence was positively associated with the 10 μg/m³ increment of particulate matter2.5 (aHR = 1.74, 95% CI: 1.06, 2.86), but not with particulate mattercoarse (aHR = 1.27, 95% CI: 0.87, 1.85). In the one-pollutant model, the elevated incidence rate of RA was slightly attenuated (particulate matter2.5 aHR = 1.61, 95% CI: 0.99, 2.61; particulate mattercoarse aHR = 1.13, 95% CI: 0.80, 1.61), with marginal statistical significance for particulate matter2.5. The RA incidence was also higher in the 4th quartile group of particulate matter2.5 compared with the first quartile group (aHR = 1.83, 95% CI: 1.07, 3.11). Adverse effects from particulate matter were not found for AS or SLE in either the one- or two-pollutant models.
Conclusion
The important components of particulate matter10 associated with RA incidence were the fine fractions (particulate matter2.5); no positive association was found between particulate matter and AS or SLE.
Keywords: rheumatoid arthritis, ankylosing spondylitis, systemic lupus erythematosus, particulate matter, epidemiology
Rheumatology key messages
Particulate matter less than 2.5 μm was found to be associated with incidence of RA.
Incidences of AS and SLE were not positively associated with particulate matter.
Differential effects of particulate matter on incidence of RA were observed, based on its size.
Introduction
Particulate matter is a mixture of liquid droplets, metals, organic compounds, and ions like sulfates and nitrates from combustion and traffic-related sources [1]. Particulate matter is classified into four groups based on its size: particulate matter10 (<10 μm in aerodynamic diameter), particulate matter2.5 (<2.5 μm; fine particles), particulate mattercoarse (particulate matter ranging from 2.5 μm to 10 μm), and ultrafine particulate matter (<0.1 μm). Since particulate matter can induce systemic inflammation by depositing in airways and circulating through the bloodstream, its pernicious impacts on the circulatory, respiratory and nervous systems have been well established [2–4]. However, only a few studies have been reported on the association between particulate matter and autoimmune rheumatic diseases (AIRDs).
AIRDs are a complex set of disorders caused by the failure of immunological tolerance that suppresses lymphocytes reacting to self-antigens, leading to the impairment of musculoskeletal systems [5, 6]. RA, AS, SLE, SSc, primary SS (pSS), scleroderma, idiopathic inflammatory myositis (IIM) and systemic vasculitides (SV) are included as AIRDs. Increased prevalence of AIRDs (and medical expenses per patient) has been demonstrated in Korea, which makes AIRDs an important aspect of public health [7].
Although the pathogenic mechanisms of AIRDs are still not very clear, one possible environmental factor associated with AIRDs is particulate air pollution; therefore, a few studies have been conducted to investigate any linkage between the two [8–13]. However, these studies have reported widely varying outcomes and have mainly focused solely on RA. For instance, Bernatsky et al. and Zhao et al. reported a positive association between long-term industrial particulate matter2.5 levels and ACPAs [14, 15]. In contrast, Chang et al. suggested there was no significant increased incidence of RA in a highly particulate matter2.5–exposed group, using a retrospective cohort in Taiwan [8]. Hart et al. also found that, in a US cohort of female nurses, ambient particulate matter was not related to RA incidence in middle-aged, socio-economically advantaged women [10].
In addition, differential health impacts of particulate matter on AIRDS according to particle size are still not clear. Shin et al. mainly studied the impact of particulate matter10 on RA [12], but there is a further need to separate out the effects of fine and coarse particles in research, since they differ in their constituents, source and properties [16]. As no highly representative cohort studies of urban populations with data for both particulate matter2.5 and particulate matter10 levels have been conducted so far, we aimed to evaluate whether long-term exposure to ambient particulate matter increased the incidence rate of AIRDs using the National Health Insurance Service (NHIS) database.
Materials and methods
Study population
The National Health Insurance Service (NHIS) of South Korea is an insurance coverage service for various medical practices. NHIS covers ∼97% of citizens in South Korea, at the same time collecting their personal data and information on medical procedures [17]. In addition, the NHIS offers biannual health screening examinations for insured Koreans aged 40 years or more. For those who undergo screening examinations, health measures such as smoking, drinking habits, and physical activities are collected, via self-reported questionnaires. Fasting serum glucose and cholesterol levels are collected via lab test. The NHIS–National Sample Cohort (NHIS-NSC) is a highly representative population-based cohort that contains these NHIS-associated records. A total of 1 025 340 participants were randomly selected in 2002 using the proportional allocation method, which comprises 2.2% of the target population, and they were followed for 13 years.
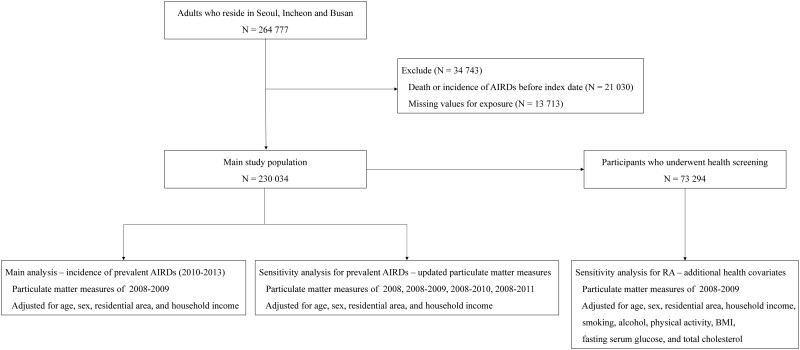
Of the 998 527 subjects from the NHIS-NSC followed for 11 years (2002–2013), 663 090 were initially excluded in this study, since these participants did not reside in metropolitan cities for which particulate matter2.5 levels were available: Seoul, Busan and Incheon. We additionally excluded 70 660 participants who were aged <20 years, 21 030 subjects diagnosed with AIRDs [RA, SLE, AS, pSS, SSc, IIM or SV; based on the International Code of Diseases (ICD)-10] or who died before the index date of 1 January 2010, and 13 713 individuals with missing exposure data. The final study population consisted of 230 034 participants. A flow chart of the study population and a simplified methodology is shown in Fig. 1.
Fig. 1.
Flow chart of study population and methodology
RA, AS and SLE are denoted as prevalent AIRDs.
NHIS-NSC: National Health Insurance Service–National Sample Cohort; N: number of participants; AIRDs: autoimmune rheumatic diseases.
The retrospective cohort used in the study were de-identified, and direct contact with separate patients was not applicable. The requirement for informed consent was waived as the NHIS database is anonymized according to strict confidentiality guidelines prior to distribution.
Exposure measurements and cohort follow-up
We obtained air pollutant levels for administrative district areas in South Korea on a daily basis from the Air Korea database. It collects particulate matter10 data from >300 local district monitoring stations across the country. Unlike particulate matter10, particulate matter2.5 levels were only available for 64 monitoring sites in three major cities—Seoul, Busan and Incheon—starting from 2008, allowing us to use the 2-year (2008–2009) respective averages of the particulate matter10, particulate mattercoarse and particulate matter2.5 levels. Particulate mattercoarse levels were calculated by subtracting the particulate matter2.5 levels from the particulate matter10 levels. These cities have total of 50 administrative districts, and over a third of the South Korean citizens reside within them. For 14 districts with two monitoring stations, we averaged the exposure values from those two monitoring stations. The mean (SD) area for 50 districts was 55.1 (79.9) km2 [18]. All participants were assigned the 2-year averages of the data from the fixed monitoring stations based on their administrative province codes in the NHIS-NSC.
Among numerous AIRDs, three of them—RA, AS and SLE—were selected to be investigated, based on their prevalence and severity in Korea [7]. From the index date of 1 January 2010 to the end point of 31 December 2013, all subjects were followed until their death or diagnosis of one of the AIRDs above. Since the NHIS-NSC data only provided the month and year of death, the first day of the month was assigned as the date of death for those who died after the index date. The mean follow-up time for the 230 034 subjects was 3.95 years. Of the 230 034 subjects, 254, 88 and 40 were diagnosed with RA, AS and SLE, respectively. The median time to diagnosis for RA, AS and SLE was 1.85, 1.82 and 2.26 years, respectively.
‘Prescription of DMARDs or biologics under diagnosis codes for RA (M05 and M06 in ICD-10)’ was used as a diagnosis criteria for RA in this study, as this has been suggested as a great screening strategy for RA in the NHIS-NSC database, with an accuracy of >90% [19]. However, there were no previous studies that suggested a thoroughly proven screening method for AS or SLE in the NHIS-NSC database. Thus, we adopted operational definitions for AS and SLE based on previous studies on the NHIS-NSC [20, 21]. Lee et al. and Choi et al. defined AS and SLE diagnosis in the NHIS database using the diagnosis code for AS and SLE (M45 and M32 in ICD-10, respectively). Lee et al. further ascertained the diagnostic criteria for AS by considering the place of diagnosis; thus, in this study we adopted the diagnostic criteria for AS as ‘patients who were diagnosed in general hospital under the diagnosis code for AS’. Choi et al. further considered the number of outpatient visits in the screening of SLE patients. In reference to this, we ascertained the SLE criteria as ‘≥1 admission or ≥3 outpatient department visits within 1 year for diagnosis codes for SLE’. The validity of our operational definitions were checked by comparing the sociodemographic characteristics of screened patients with well-recognized epidemiology of RA, AS, and SLE. The incidence date of these AIRDs was defined as the date of the first outpatient visit in the past at which the abovementioned operational definitions were satisfied.
Statistical analysis
Descriptive statistics were used to summarize particulate matter exposures and population characteristics. Differences in the mean concentrations of particulate matter according to the levels of covariates were assessed using the t test and analysis of variance. We considered the following four covariates for the main analysis: age (categorical: 20–34, 35–49, 50–64 and ≥65); sex (categorical: men and women); area of residence (categorical: Seoul, Busan and Incheon); and household income derived from the insurance premium (categorical: first, second, third and 4th quartiles), which represented the participants’ status in December 2009 and which had been adjusted in previous studies [8, 12, 13].
The one-pollutant model was the simplest model for determining the impact of an air pollutant, by including one exposure variable with confounders in the model. However, it may reflect the effect of mixtures of pollutants not included in the regression model, rather than that of the single pollutant itself. While the two-pollutant model, including two exposure variables with confounders in the model, had the limitation of possible high correlation between pollutants, it was useful for separating the independent impact of particulate matters of various sizes [3, 22, 23]. In order to assess the differential size effect of particulate matter on prevalent AIRDs, we fitted one-pollutant models for particulate matter2.5 and particulate mattercoarse and a two-pollutant model for both sizes. A multivariate Cox proportional hazard model was applied to evaluate the adjusted hazard ratios (aHRs) and 95% CIs for the 10 μg/m³ increase in particulate matter2.5 and particulate mattercoarse from the one- and two-pollutant models, with statistical significance α = 0.05. Additional analysis was also performed by dividing into quartiles the levels of particulate matter2.5 and particulate mattercoarse from the two-pollutant model.
Two methods of analysing the sensitivity of the association of particulate matter2.5 and particulate mattercoarse with RA were performed. The first method was to consider the time-varying property of the exposure; thereby, updated particulate matter2.5 and particulate mattercoarse measures for the two-pollutant model were adopted: exposure averages for 1 year (2008), 2 years (2008–2009; baseline), 3 years (2008–2010) and 4 years (2008–2011). In order to obtaining exposure averages for 3 years and 4 years, we excluded subjects in the main study population who were diagnosed with the prevalent AIRDs within 1 year (N = 95) and 2 years (N = 203) after the index date. The second method was to subset those who underwent health screening within 2 years prior to the index date (2008–2009). These participants (N = 73 294) had additional health measures from health examinations: smoking (categorical: never, former, and current smoker); alcohol intake (categorical: hardly none, 2–3 per month, 1–2 per week, 3–4 per week, and almost every day); physical activity per week (categorical: none, 1–2, 3–4, 5–6, and almost every day); BMI (continuous); fasting serum glucose (continuous); and total cholesterol (continuous), which may confound the relationship between particulate matter levels and AIRDs, according to previous studies [12, 24, 25]. In this group, several models with different confounders were subsequently adopted: Model 1: age, sex, region, and household income; Model 2: model 1 + life style behaviours (smoking, alcohol, and physical activity); Model 3: model 2 + health status (body mass index, fasting serum glucose, and total cholesterol). All analyses were performed by using R and STATA software.
The IRB number for this study is E-1905-148-1035.
Results
Descriptive characteristics of the main study population are shown in Table 1. The differences in the mean concentrations of particulate matter based on the levels of covariates other than sex were all statistically significant. Large heterogeneity was found according to region, with subjects in Seoul comprising more than half of the study population (66.4%; N = 152 804). Incheon was the city with the highest mean levels of particulate matter10 and particulate matter2.5 (58.2 μg/m³ and 31.7 μg/m³, respectively), whereas the mean level of particulate mattercoarse was highest in Seoul (28.5 μg/m³). The sociodemographic characteristics of the groups diagnosed with RA, AS and SLE are shown in Supplementary Table S1, available at Rheumatology online. The distributions of age and sex in the screened patients were consistent with well-known epidemiology for AIRDs [20, 26, 27].
Table 1.
Descriptive characteristics of the main study population
| Number of people (%) | Particulate matter (μg/m³), mean (SD) |
|||
|---|---|---|---|---|
| Particulate matter10 | Particulate matter2.5 | Particulate mattercoarse | ||
| Age | ||||
| 20–34 | 71 770 (31.2) | 54.3 (4.4) | 26.9 (3.3) | 27.4 (3.8) |
| 35–49 | 77 987 (33.9) | 54.4 (4.7) | 27.0 (3.5) | 27.3 (3.8) |
| 50–64 | 52 974 (23.0) | 54.1 (4.5) | 26.8 (3.3) | 27.3 (3.9) |
| ≥65 | 27 303 (11.9) | 54.2 (4.5) | 26.8 (3.3) | 27.4 (3.9) |
| P-value | <0.001 | <0.001 | <0.001 | |
| Sex | ||||
| Men | 116 076 (50.5) | 54.3 (4.5) | 26.9 (3.4) | 27.4 (3.8) |
| Women | 113 958 (49.5) | 54.3 (4.5) | 26.9 (3.3) | 27.4 (3.8) |
| P-value | 0.033 | 0.014 | 0.710 | |
| Region | ||||
| Seoul | 152 804 (66.4) | 54.6 (2.1) | 26.1 (2.2) | 28.5 (2.6) |
| Busan | 38 181 (16.6) | 49.1 (7.1) | 25.3 (3.8) | 23.8 (6.1) |
| Incheon | 39 049 (17.0) | 58.2 (3.6) | 31.7 (2.4) | 26.5 (2.4) |
| P-value | <0.001 | <0.001 | <0.001 | |
| Household income, quartile | ||||
| 1st (lowest) | 51 475 (22.4) | 54.3 (4.7) | 26.9 (3.4) | 27.2 (4.0) |
| 2nd | 62 019 (27.0) | 54.3 (4.5) | 27.1 (3.4) | 27.3 (3.9) |
| 3rd | 49 866 (21.7) | 54.4 (4.7) | 27.0 (3.4) | 27.3 (3.9) |
| 4th (highest) | 66 674 (29.0) | 54.3 (4.4) | 26.7 (3.2) | 27.6 (3.7) |
| P-value | <0.001 | <0.001 | <0.001 | |
Particulate matter levels determined by the 2-year average levels of 2008–2009. Particulate mattercoarse was calculated by subtracting the particulate matter2.5 levels from the particulate matter10 levels. P-values were calculated by analysis of variance for variables with more than two levels and a t test for variables with two levels.
Table 2 shows the number of events and aHRs with 95% CIs obtained from the one- and two-pollutant models. Adjusting for age, sex, region, and household income, for the two-pollutant model for particulate matter2.5 and particulate mattercoarse (correlation coefficient = −0.21), the 10 μg/m³ increment of particulate matter2.5 showed a positive association with RA (aHR = 1.74, 95% CI: 1.06, 2.86), but not with AS (aHR = 1.19, 95% CI: 0.52, 2.70) or SLE (aHR = 0.72, 95% CI: 0.21, 2.55). On the other hand, a 10 μg/m³ increase in particulate mattercoarse did not elevate the incidence of the prevalent AIRDs: RA (aHR = 1.27, 95% CI: 0.87, 1.85), AS (aHR = 0.86, 95% CI: 0.47, 1.55) and SLE (aHR = 0.53, 95% CI: 0.20, 1.41). For the one-pollutant model, the statistical significance for the effect of particulate matter2.5 on RA was slightly attenuated, but marginal (aHR = 1.61, 95% CI: 0.99, 2.61). AS (aHR = 1.25, 95% CI: 0.56, 2.76) and SLE (aHR = 0.87, 95% CI: 0.26, 2.90) were not associated with the particulate matter2.5 level. In accordance with the results for the two-pollutant model, a 10 μg/m³ increase in particulate mattercoarse showed no meaningful relationship with the prevalent AIRDs: RA (aHR = 1.13, 95% CI: 0.80, 1.61), AS (aHR = 0.83, 95% CI: 0.47, 1.48) and SLE (aHR = 0.56, 95% CI: 0.21, 1.46). The effect of the 10 μg/m³ increment of particulate matter10 from the one-pollutant model is also shown in Supplementary Table S2, available at Rheumatology online, which included an adverse effect on RA (aHR = 1.40, 95% CI: 1.00, 1.96), but not on AS (aHR = 0.95, 95% CI: 0.56, 1.60) or SLE (aHR = 0.59, 95% CI: 0.26, 1.35). Table 3 shows the results of the two-pollutant model for particulate matter2.5 and particulate mattercoarse by dividing the levels of particulate matter into quartiles. An increment in the quartile of the particulate mattercoarse levels did not elevate the incidence of the prevalent AIRDs, and RA was the only disorder that showed a positive association with particulate matter2.5 in the 4th quartile group (aHR = 1.83, 95% CI: 1.07, 3.11, compared with the reference group). An increasing trend in aHRs for RA with increasing increment in the quartile groups was also found (P < 0.05).
Table 2.
Association of particulate matter2.5 and particulate mattercoarse with prevalent AIRDs in one- and two-pollutant models
| Adjusted hazard ratio (95% CI) |
||
|---|---|---|
| Particulate matter2.5 | Particulate mattercoarse | |
| RA (Events = 254) | ||
| Two-pollutant model | 1.74 (1.06, 2.86) | 1.27 (0.87, 1.85) |
| One-pollutant model | 1.61 (0.99, 2.61) | 1.13 (0.80, 1.61) |
| AS (events = 88) | ||
| Two-pollutant model | 1.19 (0.52, 2.70) | 0.86 (0.47, 1.55) |
| One-pollutant model | 1.25 (0.56, 2.76) | 0.83 (0.47, 1.48) |
| SLE (events = 40) | ||
| Two-pollutant model | 0.72 (0.21, 2.55) | 0.53 (0.20, 1.41) |
| One-pollutant model | 0.87 (0.26, 2.90) | 0.56 (0.21, 1.46) |
A Cox proportional hazard model was applied to estimate the coefficients and 95% CIs. Adjusted hazard ratios are shown for the rate of outcome per 10 μg/m³ increase in particulate matter and were calculated after adjustments for age, sex, region, and household income. Particulate matter levels were determined from the 2-year average levels of 2008–2009. Particulate mattercoarse was calculated by subtracting the particulate matter2.5 levels from the particulate matter10 levels.
Table 3.
Association of particulate matter2.5 and particulate mattercoarse with prevalent AIRDs according to particulate matter quartiles in the two-pollutant model
| Particulate matter2.5 |
Particulate mattercoarse |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1st group | 2nd group | 3rd group | 4th group | P for trend | 1st group | 2nd group | 3rd group | 4th group | P for trend | |
| Range (μg/m³) | (20.8, 24.6) | (24.6, 26.8) | (26.8, 29.2) | (29.2, 34.6) | (16.6, 25.8) | (25.8, 27.7) | (27.7, 29.1) | (29.1, 35.4) | ||
| RA | ||||||||||
| Events (%) | 63 (24.8) | 61 (24.0) | 63 (24.8) | 67 (26.4) | 58 (22.8) | 65 (25.6) | 66 (26.0) | 65 (25.6) | ||
| Person-years | 243 956 | 214 175 | 230 270 | 221 138 | 231 512 | 247 593 | 209 685 | 220 749 | ||
| aHR (95% CI) | 1.00 | 1.31 | 1.32 | 1.83 | 0.035 | 1.00 | 1.04 | 1.22 | 1.47 | 0.053 |
| (reference) | (0.85, 2.03) | (0.81, 2.15) | (1.07, 3.11) | (reference) | (0.70, 1.54) | (0.83, 1.78) | (0.94, 2.28) | |||
| AS | ||||||||||
| Events (%) | 20 (22.7) | 23 (26.1) | 31 (35.2) | 14 (15.9) | 23 (26.1) | 21 (23.9) | 28 (31.8) | 16 (18.2) | ||
| Person-years | 243 956 | 214 175 | 230 270 | 221 138 | 231 512 | 247 593 | 209 685 | 220 749 | ||
| aHR (95% CI) | 1.00 | 1.26 | 1.49 | 0.95 | 0.327 | 1.00 | 1.07 | 1.09 | 0.90 | 0.818 |
| (reference) | (0.60, 2.65) | (0.69, 3.23) | (0.35, 2.56) | (reference) | (0.60, 1.90) | (0.64, 1.88) | (0.46, 1.74) | |||
| SLE | ||||||||||
| Events (%) | 8 (20.0) | 11 (27.5) | 11 (27.5) | 10 (25.0) | 16 (40.0) | 10 (25.0) | 7 (17.5) | 7 (17.5) | ||
| Person-years | 243 956 | 214 175 | 230 270 | 221 138 | 231 512 | 247 593 | 209 685 | 220 749 | ||
| aHR (95% CI) | 1.00 | 1.55 | 1.49 | 0.94 | 0.767 | 1.00 | 0.53 | 0.42 | 0.52 | 0.075 |
| (reference) | (0.52, 4.61) | (0.46, 4.77) | (0.21, 4.21) | (reference) | (0.22, 1.30) | (0.16, 1.11) | (0.17, 1.60) | |||
A Cox proportional hazard model was applied to estimate the coefficients and 95% CIs. Adjusted hazard ratios were calculated after adjustments for age, sex, region, and household income. Particulate matter levels were determined from the 2-year average levels of 2008–2009. Particulate mattercoarse was calculated by subtracting the particulate matter2.5 levels from the particulate matter10 levels. aHR: adjusted hazard ratio.
Table 4 shows the results of sensitivity analysis on the association of particulate matter2.5 and particulate mattercoarse with RA according to particulate matter exposure period and among those who underwent health screening with additional adjustments for lifestyle behaviour and health status. One year exposure to particulate matter2.5 was positively associated with RA (aHR = 1.68, 95% CI: 1.07, 2.64), but exposure to particulate mattercoarse was not (aHR = 1.17, 95% CI: 0.83, 1.66). Though statistical significance was attenuated, the trend of association between pollutants (particulate matter2.5 and particulate mattercoarse) and RA in the baseline model (2 years exposure) was still conserved in the results for 3 years exposure (particulate matter2.5 aHR = 1.59, 95% CI: 0.85, 2.99; particulate mattercoarse aHR = 1.10, 95% CI: 0.70, 1.73) and 4 years exposure (particulate matter2.5 aHR = 1.58, 95% CI: 0.77, 3.24; particulate mattercoarse aHR = 1.03, 95% CI: 0.60, 1.77). For those who underwent health examinations within 2 years prior to the index date, adjustment for age, sex, region, and household income yielded a similar trend to that of these results, though statistical significance was decreased (particulate matter2.5 aHR = 1.82, 95% CI: 0.82, 4.00; particulate mattercoarse aHR = 1.17, 95% CI: 0.66, 2.09). The results of additional adjustment for life style behaviours (particulate matter2.5 aHR = 1.79, 95% CI: 0.81, 3.95; particulate mattercoarse aHR = 1.17, 95% CI: 0.66, 2.09) and health status with life style behaviours (particulate matter2.5 aHR = 1.81, 95% CI: 0.82, 3.98; particulate mattercoarse aHR = 1.18, 95% CI: 0.66, 2.10) showed stable results. Since smoking is a relatively well-established risk factor for RA, we additionally investigated the influence of the smoking variable on the association of particulate matter with RA (see Supplementary Table S3, available at Rheumatology online) [28]. The sensitivity analysis for AS and SLE according to particulate matter exposure period is reported in Supplementary Table S4, available at Rheumatology online, showing similar trends to those in Tables 2 and 3.
Table 4.
Sensitivity analysis of the association of particulate matter2.5 and particulate mattercoarse with RA in the two-pollutant model
| aHR (95% CI) |
||||
|---|---|---|---|---|
| N | Events | Particulate matter2.5 | Particulate mattercoarse | |
| Particulate matter exposure period | ||||
| 1 year (2008) | 230 034 | 254 | 1.68 (1.07, 2.64) | 1.17 (0.83, 1.66) |
| 2 years (2008–2009; baseline) | 230 034 | 254 | 1.74 (1.06, 2.86) | 1.27 (0.87, 1.85) |
| 3 years (2008–2010) | 228 793 | 190 | 1.59 (0.85, 2.99) | 1.10 (0.70, 1.73) |
| 4 years (2008–2011) | 227 474 | 116 | 1.58 (0.77, 3.24) | 1.03 (0.60, 1.77) |
| Participants who underwent health screening | ||||
| Model 1 | 73 294 | 93 | 1.82 (0.82, 4.00) | 1.17 (0.66, 2.09) |
| Model 2 | 73 294 | 93 | 1.79 (0.81, 3.95) | 1.17 (0.66, 2.09) |
| Model 3 | 73 294 | 93 | 1.81 (0.82, 3.98) | 1.18 (0.66, 2.10) |
Sensitivity analysis according to the particulate matter exposure period or among those who underwent health screening with additional adjustments for lifestyle, behavior, and health status. Adjusted hazard ratios are shown for the rate of outcome per 10 μg/m³ increase in particulate matter. The 3- and 4-year averages for particulate matter were adopted by excluding participants diagnosed with prevalent AIRDs within 1 year (N = 95) and 2 years (N = 203). Model 1: age, sex, region, and household income. Model 2: model 1 + life style behaviours (smoking, alcohol, and physical activity). Model 3: model 2 + health status (BMI, fasting serum glucose, and total cholesterol). N: number of participants; aHR: adjusted hazard ratio.
Discussion
To the best of the authors’ knowledge, this is the first cohort study in an Asian population that has assessed the association between AIRDs and particulate matter, including both particulate matter2.5 and particulate mattercoarse. We discovered long-term exposure to particulate matter2.5 was associated with the incidence of RA, but not with that of AS or SLE. In the one-pollutant model, an elevated incidence of RA by particulate matter10 was also observed. Since particulate mattercoarse was not positively associated with RA, unlike particulate matter2.5, adverse effects of particulate matter10 might be derived from those due to particulate matter2.5. Due to possible differential effects of particulate matter by its size, a two-pollutant model for particulate matter2.5 and particulate mattercoarse was subsequently applied to investigate the independent effects of the particulate air pollutants, and estimates from this model are likely to be more valid than from the one-pollutant model [29]. The positive association between particulate matter2.5 and RA from the two-pollutant model shown in Tables 2 and 3 suggests that the important component of particulate matter10 for RA resides in the fine fraction (particulate matter2.5), and that the size of the particulate matter does matter in determining whether there is an abnormal autoimmune response. Although the positive association was attenuated in the sensitivity analysis due to low number of events, the trend remained consistent in Table 4. Furthermore, smoking, a well-known risk factor for RA, seemed not to influence the association of particulate matter with RA, based on the stable results obtained when adjusting for all covariates, with or without the smoking variable, and there was no statistical significance for an effect on the interaction, as shown in Supplementary Table S3, available at Rheumatology online.
Previous findings have indicated that there is no significant evidence that particulate matter10 is a risk factor for RA [10–12, 30]. Considering particulate mattercoarse was not positively associated with the prevalent AIRDs, the effect of particulate matter10 on RA might be masked if the particulate matter2.5:particulate matter10 ratio was relatively low. Some previous studies have also reported inconsistent results for particulate matter2.5 [8, 10, 14, 15]. Difference in exposure measurement, constituents, and operational definition might lead to this heterogeneity. Hart et al. for example, adopted a land use regression model, estimating concentration of air pollutants in a particular area using local emission sources and various environmental variables [10]. Chang et al. used an operational definition for RA based on the ICD-9 code from the Longitudinal Health Insurance Database (LHID) [8]. While an association between particulate matter2.5 and RA is still controversial, the present study supports the potential for particulate matter2.5 as being a risk factor for RA, using a population-based cohort with adequate representativeness.
Some possible mechanisms have been suggested for particulate matter2.5 inducing an abnormal autoimmune response. Pulmonary inflammation after inhaling particulate matter may be the first step in triggering the cascade. It can induce bronchus-associated lymphoid tissue, a tertiary lymphoid structure that is associated with producing autoantibodies and proinflammatory cytokines such as TNF-α, IL-1, IL-6 and IL-8 [31, 32]. The balance of helper T cells can also be modified by air pollutants, activating NF-κB to regulate Th1 and binding to the aryl hydrocarbon receptor, which regulates Th17 and Treg cells [33, 34]. Decreased methylation at CpG loci of inflammation-related genes is another suggested cause of an abnormal autoimmune response to particulate matter2.5 [35].
Compared with particulate matter10, fine particles have a large total surface area, include many toxic materials, and display greater airway deposition, inducing pulmonary inflammation [36, 37]. For example, particulate matter2.5 penetrates our body more deeply, reaching small airways and the alveoli, from which the particles are eliminated more slowly than they are from the upper airways, where the coarse fraction deposits [38–40]. One study also showed that particulate matter2.5 displayed a 10 000 times greater particle number dose per macrophage than particulate mattercoarse does [41]. Furthermore, a genomic study found that the coarse and fine particles induced dissimilar gene expression patterns, indicating their different roles in various types of pathogenesis [42].
The result that particulate matter2.5 levels were associated with RA but not with other prevalent AIRDs suggests that the fine fraction of particulate matter might have a specific role in the pathogenesis of RA. ACPAs, highly specific in RA [43], may be the related factor. Experimental studies showed that nanomaterials of air pollution can induce protein citrullination [44]. Observational studies also corroborate the hypothesis of air pollution–induced ACPAs by reporting a positive association between ACPAs and industrial particulate matter2.5 exposures [14, 15]. Thus, a mixed effect of induced helper T cell imbalance and increased ACPAs due to particulate matter2.5 may contribute to the increased incidence of RA in the highly exposed group. Meanwhile, a marginal protective effect of particulate mattercoarse on SLE can be seen in Table 3. However, a low number of cases (N = 7) in some exposure groups may cause a bias. Another explanation is that particulate matter can have a shielding effect against ultraviolet light [45, 46], a well-known risk factor for SLE, which could lead to a marginal protective effect. Future studies adjusting for meteorological variables, including ultraviolet radiation, are needed.
In this study, the stability and robustness of the dataset were assured, since the NHIS-NSC applied a proportional allocation method with an adequate sample size. This highly representative population-based cohort has an advantage over other occupation-based cohorts due to reduced sampling bias. In addition, we applied the suggested screening method for RA based on a previous study, thereby increasing accuracy compared with just using the ICD-10 code. While the lack of proven diagnostic criteria for AS and SLE is one limitation, we adopted strict operational definitions for AS and SLE based on the previous literature and checked whether the sociodemographic characteristics of the screened patients were consistent with well-known epidemiology.
A limitation of this study is the potential for bias when dropping 34 743 subjects from the study population. Since missings in the exposure data stem from unavailable data for specific locations, heterogeneity in the distribution of the covariates between the main study population and the dropped population was found (see Supplementary Table S5, available at Rheumatology online). Furthermore, smoking variable, a well-recognized environmental factor affecting the incidence of RA, is only available in subgroup for sensitivity analysis (N = 73 294). Thus, a selection bias may have arisen to adjust health measures, including smoking, since the subgroup who underwent health examinations would likely tend to pay more attention to health. These intrinsic limitations of the NHIS database should be considered when interpreting and generalizing the results. Another limitation was the unavailability of particulate matter2.5 levels before 2008; even the data for 2008 onwards only included that for three large cities, so generalization to rural areas may not be possible. Gaps in the exposure data might have led to decreased statistical significance in the sensitivity analysis. In addition, the incidence of the prevalent AIRDs was mutually exclusive in the study, as subjects were followed up until the first event of RA, AS or SLE. Finally, the particulate matter levels used in the study may not fully represent the real exposure level of the subjects, since indoor air quality was not considered.
The present paper demonstrated that ambient exposure of particulate matter was associated with an increased incidence of RA, but not of AS or SLE, and that the size of the particulate matter was significant in the exposure. The level of the particulate matter <2.5 μm in aerodynamic diameter was potentially linked to the incidence of RA, while particulate matter larger than that showed no association. The impact of air pollution on AIRDs is still controversial. Additional studies would be necessary to reduce the public health burden of AIRDs and further elucidate the biological effect of particulate matter on the pathogenesis of RA.
Funding: No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
Disclosure statement: The authors have declared no conflicts of interest.
Data availability statement
The datasets were derived from sources in the public domain: National Health Insurance Service (NHIS) of South Korea, http://nhis.or.kr/nhis/index.do.
Supplementary data
Supplementary data are available at Rheumatology online
Supplementary Material
References
- 1. Adams K, Greenbaum DS, Shaikh R, van Erp AM, Russell AG.. Particulate matter components, sources, and health: systematic approaches to testing effects. J Air Waste Manag Assoc 2015;65:544–58. [DOI] [PubMed] [Google Scholar]
- 2. Chen C-H, Wu C-D, Chiang H-C. et al. The effects of fine and coarse particulate matter on lung function among the elderly. Sci Rep 2019;9:14790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Genc S, Zadeoglulari Z, Fuss SH, Genc K.. The adverse effects of air pollution on the nervous system. J Toxicol 2012;2012:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Puett RC, Hart JE, Suh H, Mittleman M, Laden F.. Particulate matter exposures, mortality, and cardiovascular disease in the health professionals follow-up study. Environ Health Perspect 2011;119:1130–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goldblatt F, O’Neill SG.. Clinical aspects of autoimmune rheumatic diseases. Lancet 2013;382:797–808. [DOI] [PubMed] [Google Scholar]
- 6. Romagnani S. Immunological tolerance and autoimmunity. Intern Emerg Med 2006;1:187–96. [DOI] [PubMed] [Google Scholar]
- 7. Kim H, Cho SK, Kim JW. et al. An increased disease burden of autoimmune inflammatory rheumatic diseases in Korea. Semin Arthritis Rheum 2020;50:526–33. [DOI] [PubMed] [Google Scholar]
- 8. Chang KH, Hsu CC, Muo CH. et al. Air pollution exposure increases the risk of rheumatoid arthritis: a longitudinal and nationwide study. Environ Int 2016;94:495–9. [DOI] [PubMed] [Google Scholar]
- 9. Bernatsky S, Fournier M, Pineau CA. et al. Associations between ambient fine particulate levels and disease activity in patients with systemic lupus erythematosus (SLE). Environ Health Perspect 2011;119:45–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hart JE, Kallberg H, Laden F. et al. Ambient air pollution exposures and risk of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2013;65:1190–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hart JE, Kallberg H, Laden F. et al. Ambient air pollution exposures and risk of rheumatoid arthritis: results from the Swedish EIRA case–control study. Ann Rheum Dis 2013;72:888–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shin J, Lee J, Lee J, Ha EH.. Association between exposure to ambient air pollution and rheumatoid arthritis in adults. Int J Environ Res Public Health 2019;16:1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bernatsky S, Smargiassi A, Barnabe C. et al. Fine particulate air pollution and systemic autoimmune rheumatic disease in two Canadian provinces. Environ Res 2016;146:85–91. [DOI] [PubMed] [Google Scholar]
- 14. Bernatsky S, Smargiassi A, Joseph L. et al. Industrial air emissions, and proximity to major industrial emitters, are associated with anti-citrullinated protein antibodies. Environ Res 2017;157:60–3. [DOI] [PubMed] [Google Scholar]
- 15. Zhao N, Smargiassi A, Hatzopoulou M. et al. Long-term exposure to a mixture of industrial SO2, NO2, and particulate matter2.5 and anti-citrullinated protein antibody positivity. Environ Health 2020;19:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wilson WE, Suh HH.. Fine particles and coarse particles: concentration relationships relevant to epidemiologic studies. J Air Waste Manag Assoc 1997;47:1238–49. [DOI] [PubMed] [Google Scholar]
- 17. Lee YH, Han K, Ko SH, Ko KS, Lee KU, Taskforce Team of Diabetes Fact Sheet of the Korean Diabetes Association. Data analytic process of a nationwide population-based study using national health information database established by National Health Insurance Service. Diabetes Metab J 2016;40:79–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Choi S, Kim KH, Kim K. et al. Association between post-diagnosis particulate matter exposure among 5-year cancer survivors and cardiovascular disease risk in three metropolitan areas from South Korea. Int J Environ Res Public Health 2020;17:2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cho SK, Sung YK, Choi CB. et al. Development of an algorithm for identifying rheumatoid arthritis in the Korean National Health Insurance claims database. Rheumatol Int 2013;33:2985–92. [DOI] [PubMed] [Google Scholar]
- 20. Lee JS, Oh BL, Lee HY, Song YW, Lee EY.. Comorbidity, disability, and healthcare expenditure of ankylosing spondylitis in Korea: a population-based study. PLoS One 2018;13:e0192524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Choi CW, Eun SH, Choi KH, Bae JM.. Increased risk of comorbid rheumatic disorders in vitiligo patients: a nationwide population-based study. J Dermatol 2017;44:909–13. [DOI] [PubMed] [Google Scholar]
- 22. Hoek G, Brunekreef B, Verhoeff A, van Wijnen J, Fischer P.. Daily mortality and air pollution in The Netherlands. J Air Waste Manag Assoc 2000;50:1380–9. [DOI] [PubMed] [Google Scholar]
- 23. Vedal S, Kaufman JD.. What does multi-pollutant air pollution research mean? Am J Respir Crit Care Med 2011;183:4–6. [DOI] [PubMed] [Google Scholar]
- 24. Lu MC, Yan ST, Yin WY, Koo M, Lai NS.. Risk of rheumatoid arthritis in patients with type 2 diabetes: a nationwide population-based case–control study. PLoS One 2014;9:e101528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Andersen CJ. Impact of dietary cholesterol on the pathophysiology of infectious and autoimmune disease. Nutrients 2018;10:764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Choi IA, Lee JS, Song YW, Lee EY.. Mortality, disability, and healthcare expenditure of patients with seropositive rheumatoid arthritis in Korea: a nationwide population-based study. PLoS One 2019;14:e0210471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ju JH, Yoon SH, Kang KY. et al. Prevalence of systemic lupus erythematosus in South Korea: an administrative database study. J Epidemiol 2014;24:295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Baka Z, Buzas E, Nagy G.. Rheumatoid arthritis and smoking: putting the pieces together. Arthritis Res Ther 2009;11:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tolbert PE, Klein M, Peel JL, Sarnat SE, Sarnat JA.. Multipollutant modeling issues in a study of ambient air quality and emergency department visits in Atlanta. J Expo Sci Environ Epidemiol 2007;17:S29–35. [DOI] [PubMed] [Google Scholar]
- 30. Jung CR, Hsieh HY, Hwang BF.. Air pollution as a potential determinant of rheumatoid arthritis: a population-based cohort study in Taiwan. Epidemiology 2017;28: S54–9. [DOI] [PubMed] [Google Scholar]
- 31. Sigaux J, Biton J, Andre E, Semerano L, Boissier MC.. Air pollution as a determinant of rheumatoid arthritis. Joint Bone Spine 2019;86:37–42. [DOI] [PubMed] [Google Scholar]
- 32. Steenhof M, Gosens I, Strak M. et al. In vitro toxicity of particulate matter collected at different sites in the Netherlands is associated with particulate matter composition, size fraction and oxidative potential–the RAPTES project. Part Fibre Toxicol 2011;8:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Veldhoen M, Hirota K, Westendorf AM. et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 2008;453:106–9. [DOI] [PubMed] [Google Scholar]
- 34. Quintana FJ, Basso AS, Iglesias AH. et al. Control of Treg and TH17 cell differentiation by the aryl hydrocarbon receptor. Nature 2008;453:65–71. [DOI] [PubMed] [Google Scholar]
- 35. Wang C, Chen R, Shi M. et al. Possible mediation by methylation in acute inflammation following personal exposure to fine particulate air pollution. Am J Epidemiol 2018;187:484–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dockery DW. Health effects of particulate air pollution. Ann Epidemiol 2009;19:257–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Valavanidis A, Fiotakis K, Vlachogianni T.. Airborne particulate matter and human health: toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 2008;26:339–62. [DOI] [PubMed] [Google Scholar]
- 38. Host S, Larrieu S, Pascal L. et al. Short-term associations between fine and coarse particles and hospital admissions for cardiorespiratory diseases in six French cities. Occup Environ Med 2007;65:544–51. [DOI] [PubMed] [Google Scholar]
- 39. Dominici F, Peng RD, Bell ML. et al. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA 2006;295:1127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Englert N. Fine particles and human health—a review of epidemiological studies. Toxicol Lett 2004;149:235–42. [DOI] [PubMed] [Google Scholar]
- 41. Venkataraman C, Kao AS.. Comparison of particle lung doses from the fine and coarse fractions of urban particulate matter-10 aerosols. Inhal Toxicol 1999;11:151–69. [DOI] [PubMed] [Google Scholar]
- 42. Huang YC, Karoly ED, Dailey LA. et al. Comparison of gene expression profiles induced by coarse, fine, and ultrafine particulate matter. J Toxicol Environ Health A 2011;74:296–312. [DOI] [PubMed] [Google Scholar]
- 43. Vander Cruyssen B, Peene I, Cantaert T. et al. Anti-citrullinated protein/peptide antibodies (ACPA) in rheumatoid arthritis: specificity and relation with rheumatoid factor. Autoimmun Rev 2005;4:468–74. [DOI] [PubMed] [Google Scholar]
- 44. Valesini G, Gerardi MC, Iannuccelli C. et al. Citrullination and autoimmunity. Autoimmun Rev 2015;14:490–7. [DOI] [PubMed] [Google Scholar]
- 45. Deng X, Zhou X, Tie X. et al. Attenuation of ultraviolet radiation reaching the surface due to atmospheric aerosols in Guangzhou. 2012;57:2759–66. [Google Scholar]
- 46. Gilcrease GW, Padovan D, Heffler E, UNESCO Chair, Department of Culture, Politics and Society, University of Turin, Italy et al. Is air pollution affecting the disease activity in patients with systemic lupus erythematosus? State of the art and a systematic literature review. Eur J Rheumatol 2020;7:31–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets were derived from sources in the public domain: National Health Insurance Service (NHIS) of South Korea, http://nhis.or.kr/nhis/index.do.