Abstract
Mechanoperception, the ability to perceive and respond to mechanical stimuli, is a common and fundamental property of all forms of life. Vascular plants such as Mimosa pudica use this function to protect themselves against herbivory. The mechanical stimulus caused by a landing insect triggers a rapid closing of the leaflets that drives the potential pest away. While this thigmonastic movement is caused by ion fluxes accompanied by a rapid change of volume in the pulvini, the mechanism responsible for the detection of the mechanical stimulus remains poorly understood. Here, we examined the role of mechanosensitive ion channels in the first step of this evolutionarily conserved defense mechanism: the mechanically evoked closing of the leaflet. Our results demonstrate that the key site of mechanosensation in the Mimosa leaflets is the pulvinule, which expresses a stretch-activated chloride-permeable mechanosensitive ion channel. Blocking these channels partially prevents the closure of the leaflets following mechanical stimulation. These results demonstrate a direct relation between the activity of mechanosensitive ion channels and a central defense mechanism of M. pudica.
One-sentence summary: Vascular plants use mechanosensitive ion channels to move their leaflets in response to external mechanical stimuli.
Introduction
All plants respond to mechanical stimuli. Slow mechanically evoked responses (thigmotropism) are known to occur in the below- and above-ground parts of plants such as vines or coiling tendrils (Reinhold, 1967; Riehl and Jaffe, 1984; Monshausen and Gilroy, 2009; Monshausen and Haswell, 2013). When an elongating root comes in contact with an object, e.g. a rock, it grows away from the object. However, some species such as the carnivorous plants venus fly trap (Dionaea muscipula; Darwin, 1875; Scherzer et al., 2019), Utricularia (Plachno et al., 2006), and Drosera (Poppinga et al., 2013) have developed rapid mechanisms that allow an immediate, mechanically evoked response (thigmonasty or seismonasty), which is employed to capture and eventually digest prey. Others, such as Mimosa pudica, have used these seismonastic behaviors to close their leaflets and drop their petiole to fend off insects that would otherwise feed on the leaves (Fondeville et al., 1966; De Luccia and Friedman, 2011; Abramson and Chicas-Mosier, 2016).
First described by Robert Hooke in the 17th century (Hooke, 1665), the mechanisms underlying the folding of the Mimosa leaflets have since been studied focusing mostly on the bending movements. These involve a rapid, typically <1 s, hydraulic motion within the pulvinus, or hinge, to cause leaflet bending and the dramatic change of the relative angle between petiole and stem (Kumon and Tsurumi, 1984; Tamiya et al., 1988; Visnovitz et al., 2007; Volkov et al., 2013; Geitmann 2016; Hagihara and Toyota, 2020). In response to mechanical stimuli, sudden changes of turgor pressure occur in the motor cells at the base of each leaflet. These turgor variations are mainly due to an increase of the permeability of the cell membranes involving the activity of aquaporin water channels (Moran, 2007). Such increased permeability causes water and ion redistribution in the pulvinus (Abe, 1981; Samejima and Sibaoka, 1982; Kumon and Tsurumi, 1984), resulting in the shrinking of the motor cells and the movement of the leaflet (Kumon and Tsurumi, 1984; Tamiya et al., 1988). Despite these advances in our knowledge of the seismonastic behavior of the Mimosa, our understanding of the initial trigger for these responses, i.e. the sensation of mechanical stimuli, has remained limited. The conduction of the signals involves the generation of action potentials (Scherzer et al., 2019) and recently, magnetic signals have been detected during the rapid leaf folding response of the venus fly trap (Fabricant et al., 2021). The possible contribution of ion channels capable of converting mechanical stimuli into electrical signals was proposed almost 30 years ago (Cosgrove and Hedrich, 1991), yet definite proof of their involvement in the mechanosensing properties of the Mimosa leaflets has been elusive.
In the present study, we used a combination of behavioral, electrophysiological, and pharmacological approaches to characterize the mechanosensitivity of the Mimosa leaflets, demonstrate the presence of mechanosensitive ion channels (MSCs) in the leaflets, and provide direct evidence for the role of these channels in the seismonastic behavior of the plant.
Results
The pulvinule is the primary site of mechanosensation
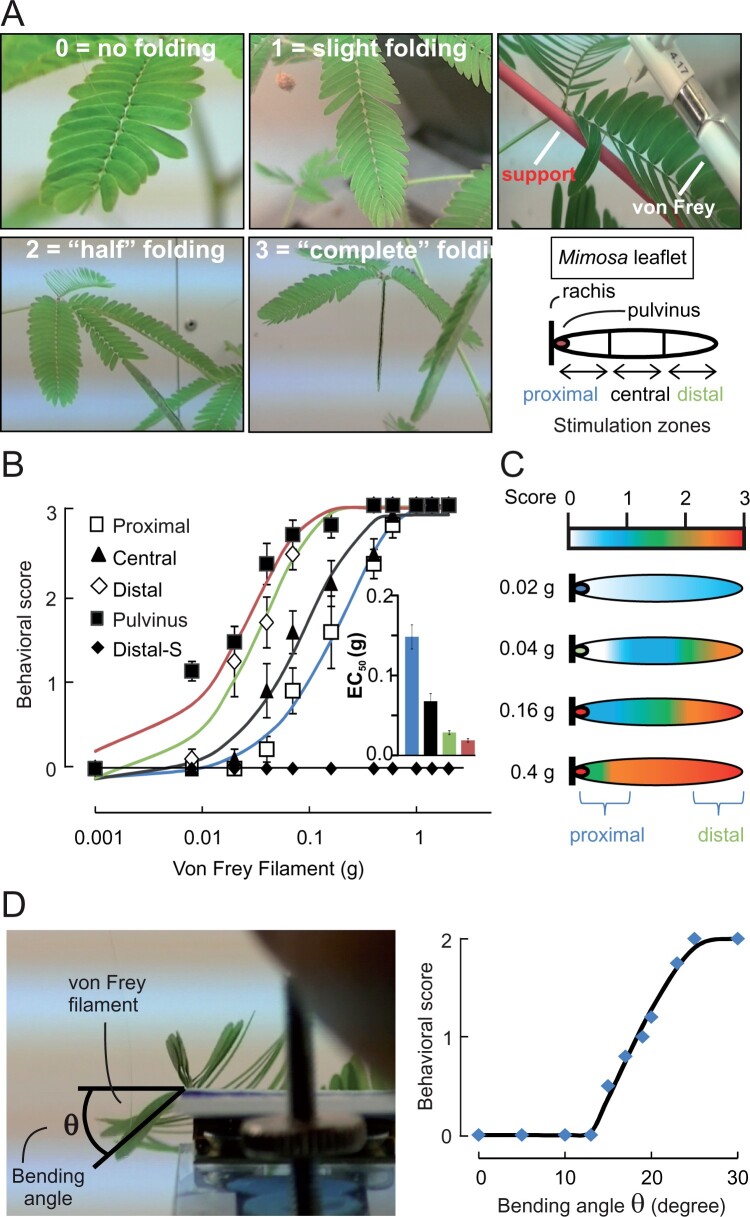
The rapid leaflet closure of Mimosa in response to touch has been demonstrated to be dependent on ion fluxes leading to water movement (Samejima and Sibaoka, 1982; Tamiya et al., 1988). To thoroughly characterize the responses of the Mimosa leaflets to mechanical stimuli, we recorded the movement of the leaflets in response to graded mechanical stimulation or leaflet bending angle. Mechanical stimuli of increasing intensities were delivered with von Frey filaments, which are routinely used for determining mechanical thresholds in human patients and rodents (Armstrong et al., 1998; Le Bars et al., 2001; Bradman et al., 2015). Mechanically evoked responses were scored based on their amplitude, ranging from no folding to complete folding of the leaf (Figure 1A; see Supplemental Videos S1–S5). Furthermore, to examine whether leaflets possess zones of high mechanosensitivity on their adaxial surfaces, we applied the stimuli on the tertiary pulvinus (called pulvinule), or on the proximal, central, or distal regions of the secondary leaflet (Figure 1A). Our data demonstrate that the application of mechanical stimuli could elicit leaflet folding when applied on all four regions of the secondary leaflet, with the pulvinule being the zone of highest mechanical sensitivity, followed by the distal to proximal zones (Figure 1B). This is further demonstrated by extracting the stimulus intensity that produced half of the maximal response (see inset of Figure 1B). The compartmentalized mechanical sensitivity is also demonstrated by heat maps of the zones producing the strongest behavioral responses, highlighting the distal end of the secondary leaflet and the pulvinule as primary sites at which a mechanical trigger leads to mechanosensation (Figure 1C).
Figure 1.
Leaflet folding is induced after perception of the mechanical stress at the pulvinule (tertiary pulvinus). A, Degrees of leaflet folding after mechanical stimulation with von Frey filament expressed as a score between 0 and 3 (left) and locations of applied stimulation on the leaflet (right). B, Leaflet folding in response to touching with calibrated von Frey filament. Results are mean ± standard error of the mean (sem), n = 9. C, Heat map of the folding response after mechanical stimulation. D, Picture showing the angle measurement applied with the 1-g von Frey filament (left) and the corresponding folding response (right).
We speculated that this spatial pattern of mechanosensitivity is typical of a hinge effect, in which the primary site of mechanosensation is the pulvinule and the sensitivity to a trigger applied to the distal zone of the secondary leaflet is not perceived at that zone directly but results from a lever effect on the hinge, which would be the pulvinule. Therefore, the determining factor for eliciting leaflet bending would not be the intensity of the mechanical stimulus at the touched leaflet surface but rather the degree of bending it produces at the pulvinule. We examined the relation between bending angle and leaflet closure and demonstrated that the minimum bending angle necessary to produce a behavioral response (score of 1) ranges between 15 ± 1° < θ < 23 ± 2° and a score of 2 is reached with an angle θ > 23 ± 2° (Figure 1D). To corroborate that the bending angle is the critical parameter, we speculated that preventing the mechanical stimulus applied to the distal zone of a secondary leaflet from being transmitted to the pulvinule through a lever effect would abolish the leaflet closure response. Indeed, when a support rod was placed under the proximal to central zone of the leaflet (see top right panel of Figure 1A), even the strongest mechanical stimuli applied to the distal zone could not provoke leaflet closure (Figure 1B). These data indicate that the pulvinule is the primary site of mechanosensation on the leaflet.
Stretch-activated MSCs are present in the leaflet
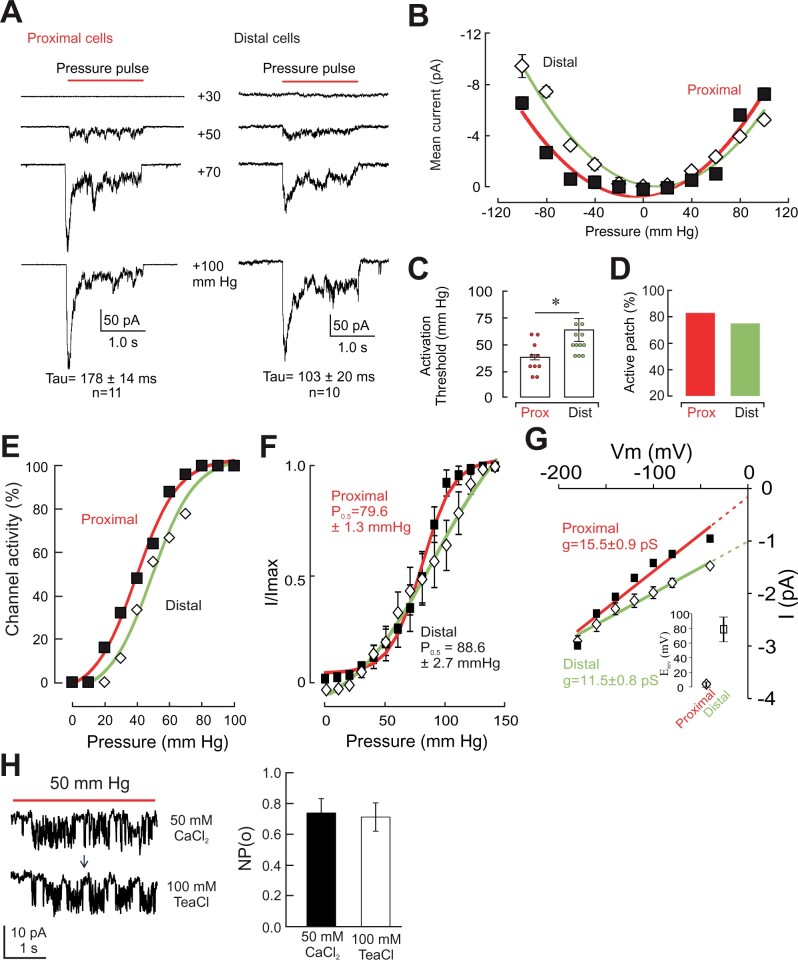
Mechanically evoked behaviors are typically initiated by the activation of MSCs, which activate a signaling cascade that leads to the generation of the appropriate behaviors. This is exemplified in several mammalian physiological systems such as the myogenic tone of resistance arteries (Sharif-Naeini et al., 2009), and the pain (He et al., 2017) and touch (Ranade et al., 2014) response to high- and low-intensity stimuli, respectively. We examined whether proximal adaxial cells stimulated by von Frey filaments express MSCs. We focused in particular on the tannin-containing red-colored cells located in the epidermis of the pulvinule, as these had previously been suggested to be the potential mechanoreceptor cells (Visnovitz et al., 2007). These cells seem to be derived from stomatal subsidiary cells and are absent from other regions of the leaflet epidermis. Using a previously validated approach (Tran et al., 2017), we recorded in the excised outside-out patch clamp configuration the activity of MSCs in protoplasts isolated from these cells harvested from the proximal (pulvinule) region of the leaflet. Mesophyll cells from the distal regions of the secondary leaflet served as controls. Our results demonstrate that cells from both cell types express mechanically activated ion currents (Figure 2A). Both currents displayed rapid adaptation kinetics with slightly slower time constants in proximal cells (103 ± 20 ms, n = 10; versus 178 ± 14 ms, n = 11 in proximal cells). Mechanically activated ion currents can be either activated or inactivated by stretch (Bourque et al., 2002; Sachs, 2010). To further characterize the MSCs observed in proximal and distal cells of the Mimosa leaflet, we recorded mechanically evoked currents at both negative and positive pipette pressures, aimed at producing concave or convex deformation of the membrane, respectively. Our results indicate the MSCs found in both proximal and distal protoplasts behave as stretch-activated ion currents, with the minimal channel activity near 0 mmHg (Figure 2B).
Figure 2.
Mechanosensitive channels are present in cells of the Mimosa leaflet. A, Representative patch-clamp recording of proximal (left) and distal (right) cells in outside-out configuration at a pressure pulse of 30, 50, 70, and 100 mmHg. Time constants of inactivation (Tau) are indicated in insets (mean ± sem). B, Mechanosensitive ion channels found in both proximal (red) and distal (green) cells display a stretch-activated mode of activation. Mean (± sem) mechanically evoked current elicited at different pressures. C, Proximal cells have a lower threshold than distal cells in response to pressure pulse stimulation. Bars represent mean ± sem, n ≥ 12. A paired t test was used to compare means (*P < 0.05). D, Proportion of patches with at least one active channel in proximal and distal cells. E, Maximum current–pressure relationships recorded in different patches and fitted with a Boltzmann function. Data represent mean ± sem (n = 10). F, Imax normalized current–pressure relationship of stretch-activated currents and fitted with a Boltzmann equation. P0.5 of 79.6 ± 1.3 mmHg and 88.6.3 ± 5.6 mmHg for proximal and distal cells, respectively, are the average values determined for individual cells. Data represent mean ± sem (n = 10). G, Single channel I/V curves from MSCs of proximal and distal cells show higher conductance in proximal cells (squares) than in distal cells (diamonds). Values are means ± sem (n = 6); Extrapolations of currents were used to obtain single channel conductance and reversal potential values (inset). H, Representative single channel recording (left) in response to positive pressure in proximal cells of Mimosa, in excised outside-out configuration, in bath solution containing 50-mM CaCl2 (top) or 100-mM TeaCl (bottom). Mean (± sem) MSC activities (right) are detectable upon membrane stretching in both conditions and show similar open probabilities (NPo). The arrow indicates the transition from one bath solution to another. The membrane potential was held at −186 mV, except for (G). Ionic conditions are described in the “Materials and methods”.
An important characteristic of MSCs is their activation threshold. Channels with low activation threshold can be readily activated and may mediate different types of stimuli, such as touch-sensing in mammals (Ranade et al., 2014). High-threshold MSCs, on the other hand, require mechanical stimuli of greater intensities to open, and can be found in mammalian pain-transmitting neurons (Sharif-Naeini, 2015). Our data indicate that MSCs found in proximal cells have a relatively low activation threshold (37.8 ± 4.9 mmHg, n = 10) whereas those found in distal cells have a significantly higher activation threshold (53.0 ± 3.5 mmHg, n = 11, P < 0.05, Student’s t test; Figure 2C). Although the difference in activation threshold can suggest that a different MSC is expressed in distal or proximal cells, this property can also be modulated by accessory proteins such as the cytoskeleton (Zhang et al., 2007). Another feature of the MSCs that can be used to distinguish two different channels is their respective single-channel conductance (Cho et al., 2002). We recorded single channel currents in proximal and distal cells while holding the membrane at different voltages. The slope generated by the I–V relations, and their extrapolation, provides us with the single channel conductance, as well as an approximation of the reversal potential of the current permeating through them (Figure 2G). Our data indicate the MSCs found in proximal cells have a single channel conductance of 15.5 ± 0.9 pS and a reversal potential of approximately ∼3.4 ± 3.1 mV (n = 22; ECl = 9.8 mV) in near-symmetrical (110 versus 162.4 mM) chloride concentrations, indicating that mechanically evoked inward currents result from the efflux of chloride ions. Consistent with this hypothesis, we found that the single channel amplitude and open probability are not modified when Ca2+ ions are replaced by TEA+ in the bath solution (Figure 2H). In distal cells, however, MSCs have a single channel conductance of 11.5 ± 0.8 pS and a reversal potential extrapolated to be approximately ∼77.5 ± 14.7 mV. Given that ECl− is at +9.8 mV and ECa2+ is at +136 mV (based on internal concentration of free Ca2+ of 1.6 µM), our results suggest that the channel present in distal cells is likely Ca2+-permeable. Ion substitution experiments with varying concentrations of calcium will be required for further validation. These observations indicate that proximal and distal cells likely respond to mechanical stimuli through different sets of MSCs.
Furthermore, the cellular response to a mechanical stimulus will depend on the expression level of MSCs. Cells with low density of membrane MSCs will show a smaller mechanically evoked response compared to those with a higher number of membrane MSCs. An indirect method of assessing the plasma membrane expression of MSCs is to examine the percentage of patches in which at least one MSC is activated by the mechanical stimulation protocol. Our results indicate that proximal and distal cells express comparable densities of MSCs on their membranes (83% versus 75% in proximal and distal cells, respectively; Figure 2D). Despite the similarity in active patches, our analysis of the relation between percent active patches and stimulus intensity (Figure 2E) indicates that at low mechanical stimuli (i.e. 20 mmHg), MSCs of distal cells remain closed, whereas nearly 20% of MSCs in proximal cells have already opened and could potentially contribute to the mechanically evoked behavior. This observation is also illustrated in the relative current (Figure 2F), where despite having similar P0.5 values, channels of proximal cells display a steeper activation range, reaching maximal activation at ∼100 mmHg.
Stretch-activated channels are essential to trigger the mechanically evoked leaflet closure
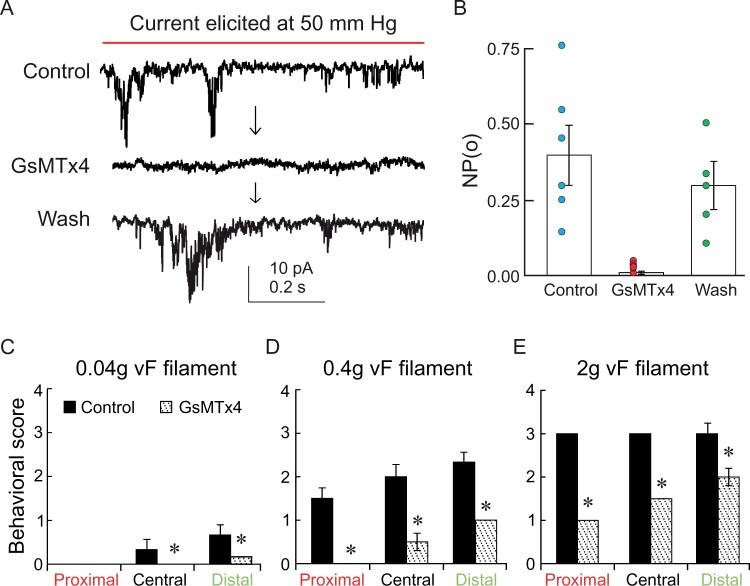
Mechanosensitive channels are known to be involved in the mechanotransduction processes in living organisms (Hamill and Martinac, 2001; Ranade et al., 2015) and could be implicated in the leaflet folding of M. pudica. Definite proof that these channels are an integral part of the mechanically evoked leaflet movement can be obtained through pharmacological treatment with known inhibitors of MSCs. To test this hypothesis, we investigated the effect of GsMTx4, which is known to specifically affect mechanically activated channel activity in animal cells (Gottlieb et al., 2007). In proximal cells, stretch-activated currents were abolished by the presence of GsMTx4 (5 µM), and could subsequently be restored by washing out the toxin (5 min, Figure 3A). This was also demonstrated by examining the mean open probability (NPo) of the channels (Figure 3B). To determine the contribution of these MSCs to the mechanically evoked whole-plant behavior, we then tested the effect of GsMTx4 on the whole plant. The peptide GsMTx4 was directly applied on the pulvinule and the leaflet tested with von Frey filaments 30 min later. This delay was necessary to allow the leaflet to re-open following its closure triggered by the application of the GsMTx4 on the epidermis. Our results indicate that a mechanical stimulation of 0.04 g, administered in the middle and distal zones of the secondary leaflet, induced a slight folding of the leaflet (score <1) with a control physiological solution supplemented only with the detergent (0.01% Triton). This response was strongly inhibited in the presence of GsMTx4 (10 µM; Figure 3C). The inhibitory effect of GsMTx4 on leaflet mechanosensing was more pronounced with higher mechanical stimuli. For instance, a stimulation administered by touching the distal zone with a 0.4 g filament induced a score above 2 in control conditions, whereas in the presence of GsMTx4, the folding response had a score below 1 (Figure 3D). Stimulation with a 2 g filament induced a complete folding of the leaflet (score = 3) regardless of the zone stimulated in control conditions (Figure 3E). Those responses were significantly reduced in leaflets pretreated with GsMTx4. Altogether, our results support the idea that the mechanical stress is perceived at the proximal zone in the pulvinule. Furthermore, our data suggest that MSCs in the proximal cells are involved in the mechanotransduction process leading to folding of the leaflet.
Figure 3.
GsMTx4 inhibits the stretch-activated channel activity and the folding response of leaflets. A, Typical patch-clamp recording of proximal cells in outside-out configuration in control condition, in the presence of GsMTx4 (10 µM), then after wash-out and B, their corresponding mean (± sem) open probability (NPo). C–E, Application of GsMTx4 to the pulvinule inhibits the folding of the leaflet in response to mechanical stimulation in the different secondary leaflet zones with (C) 0.04-g, (D) 0.4-g, and (E) 2-g von Frey filament. Bars represent mean ± sem, n = 8. A paired t test was used to compare means (*P < 0.05).
Discussion
We provide compelling evidence to support the hypothesis that mechanical strain in M. pudica is perceived in the pulvinule through the action of MS channels. In this study, we have shown that stretch-activated channels are present in the plasma membrane of leaflet cells, with cells at the proximal locations of the secondary leaflet showing a more sensitive behavioral profile. These channels appear to be permeant to anions. Indeed, the observation that single channel amplitudes did not vary when the external cation was changed to the quaternary ammonium compound TEA-Cl (Tetraethylammonium chloride) suggests that the mechanically evoked currents are elicited by a chloride-permeable channel. It remains possible that the mechanically evoked current could be due to the influx of TEA. A similar observation was made with the larger conductance channel Piezo1 (Gnanasambandam et al., 2015), where channel conductance was reduced by half upon switching from CaCl2 to TEA-Cl. In our recordings, where the channel conductance is ∼15 pS, mechanically evoked TEA permeation through the channel would at least result in a noticeable decrease in conductance and therefore a visible decrease in single channel amplitude. The absence of such decrease further supports the possibility of a mechanically activated chloride channel. Nonetheless, additional ion substitution experiments with varying concentrations of chloride will be required to further validate this.
Furthermore, these channels are blocked by GsMTx4, an inhibitor known to be specific to MS channel in animal cells (Gottlieb et al., 2007). In addition, at the whole plant level, we used von Frey filaments to deliver calibrated and reproducible mechanical stimuli. We showed that stimulation at the pulvinule of the leaflet was the most efficient site to induce the folding response, and that a touch at a distal location was able to trigger a response as well, albeit at higher force levels. Importantly, this response to a distally applied trigger was eliminated when the bending at the pulvinule was prevented mechanically. Considering the lever principle, this behavior suggests that mechanical strain is perceived and translated into an electrochemical signal at the proximal zone of the secondary leaflet and more specifically at the pulvinule. In the primary pulvini, ultrastructural studies with X-ray imaging and tomography revealed a bendable xylem, shrinkable/expendable epidermis, small wrinkles for surface modification, and a xylem vessel network for efficient water transport during the motile mechanism (Song et al., 2014). The mechanosensitivity of M. pudica leaflets could therefore be generated by a combination of leaflet anatomy and biophysical properties of the cells in the proximal region. This is reminiscent of the mechanosensitivity of the venus fly trap plant, in which a single-layered ring of as few as 50 mechanosensitive cells near the base of the sensory hair is responsible for the mechanotransduction process (Buchen et al., 1983; Iosip et al., 2020). The critical role of this zone for the transduction process in Dionaea was identified based on the juxtaposition of its anatomically strategic location with the expression of MS channels (Iosip et al., 2020). Specifically, the conversion of a bending trigger into shear stress was found to be optimal at this constriction zone. The parallels between Mimosa and Dionaea point to a potential convergent evolution of the structural apparatus necessary for mechanotransduction in vascular plants in which the mechanosensitive cells are placed at an optimal anatomical site where minute displacement of a physical structure such as a leaflet or hair is translated into large shear stress through a lever effect to initiate the translation of the mechanical trigger into an electrophysiological signal. In M. pudica, further biophysical studies and modeling of the strain/stress distribution on the pulvinule will provide a better understanding of this process and the potential structural features involved.
At the plasma membrane level, we provide evidence that MS channels are present in M. pudica and are crucial in the mechanotransduction process since the folding of the leaflet in response to mechanical stimulation is strongly inhibited by pulvinule application of GsMTx4. Due to their position in the plasma membrane, MS channels are among the early actors in the mechanotransduction. It is known that MS channels are activated either by a tension in the membrane plane or by local bending of the membrane. We also know that a mechanical stimulation, in order to be efficient (in terms of physiological response), should produce a tissue/cell deformation (Coutand et al., 2000; Moulia et al., 2015). This deformation can be relayed by anchor proteins such as wall-associated kinase or cell wall sensing receptor like kinase (RLK). For example, the RLK protein FERONIA has been shown to be important in mechanoperception; the mutant shows altered Ca2+ signaling (Shih et al., 2014). The MS channels in the proximal cells mediate anionic current which is in accordance with the one recorded in Arabidopsis thaliana i.e. MSL channel family (Haswell et al., 2008; Maksaev and Haswell, 2012; Hamilton et al., 2015). The anionic nature of the current is favorable to generate a rapid membrane depolarization leading to a cascade of signalization such as reactive oxygen species with activation of NADPH oxidase, activation of voltage-gated channels (Ca2+ and/or K+), or activation of protein kinase (Sierla et al., 2016). However, the coupling of an initial depolarization with second messengers triggering raises fundamental questions that cannot be answered yet in the frame of plant MS channel study, which is still in its infancy. Our work strongly suggests a key role of MS channels in mechanotransduction signaling and paves the way to the identification of other actors in physical perception in plants.
Materials and Methods
Plants
The seeds of M. pudica were soaked in lukewarm water (27°C) for 48 h. They were grown in growth chambers in well-drained peat moss at 27°C with a 16-h light/8-h dark cycle. During the 16-h light period, they were irradiated with light of 800–1,500 µmol photons m−2 s−1. The humidity averaged 60%–65%. The plants were watered every 2 d. Two- to three-month-old plants were used for the experiments. All experiments were performed on healthy adult specimens.
Behavior experiment
von Frey filaments (Aesthesio von Frey filaments, Bioseb, France) were used to apply force on the leaflet in a calibrated manner as shown in Figure 1. Starting with a low-weight filament able to exert a force corresponding to 8 mg of weight, each filament was applied perpendicularly against the respective zone of the secondary leaflet. The response was scored as listed in Table 1; sample videos of the mechanically evoked behaviors are provided in supplementary materials. Although a score of 4 could be seen upon strong mechanical stimulation, the fine sensitivity of the leaflets could be observed with filament intensities below 1 g of weight, which plateaued at a score of 3. We therefore we never observed scores of 4 in our experiments.
Table 1.
Scoring categories for leaf response to mechanical trigger.
| Score | Response |
|---|---|
| 0 | No perceptible folding |
| 1 | Slight folding of one or few leaflets on one pinna |
| 2 | Complete folding of approximately half of the leaflets on one pinna |
| 3 | Complete folding of all the leaflets on one pinna |
| 4 | Complete folding of all leaflets of all pinnae of a leaf |
For inhibition experiments, bath solution containing (mM): 50 CaCl2, 5 MgCl2, and 10 MES–Tris (pH 5.6) supplemented with 0.05% v/v of Triton X-100 was applied directly on the pulvinule with or without 0.01 mM of GsMTx4.
Protoplast preparation
To prepare motor cell protoplasts, pulvinules were excised from the leaflet of primary leaves using a razor blade. The pulvinal slices were placed in incubation buffer (2-mM CaCl2, 2-mM MgCl2, 1-mM KCl, 1% w/v bovine serum albumin, 25-mM MES (pH 5.6) that contained (in w/v): 2% Cellulase Onozuka R-10, 2% Cellulase Onozuka RS, 1% w/v Macerozyme Onozuka R-10 (Yakult), and 0.1% Cellulysin at 21°C in the dark for 15 h and mannitol to 720 mOsmol). For enzyme removal, the preparation was washed twice with 2-mM CaCl2, 2-mM MgCl2, 1-mM KCl, and 25-mM MES (pH 5.6) with mannitol added to adjust the osmolarity at 720 mOsmol. For protoplast release, the preparation was incubated with 2-mM CaCl2, 2-mM MgCl2, 1-mM KCl, and 25-mM MES (pH 5.6), and mannitol (450 mOsmol). The suspension was filtered through a 50-µm nylon mesh. For proximal cells, a paradermal shaving was made from the surface of the tertiary pulvini. For distal cells, small pieces of tissue at the distal tip of the secondary leaflet were excised. Protoplast preparations were done separately for each location. For patch clamp recording, cells were selected as follows: for proximal cells, nonchlorophyllous cells were selected, i.e. either red or colorless cells. For distal cell samples, only green cells were patched. Eighty-six percent (43 out of 50) of the recordings from proximal cells showed MSC activity, whereas for distal cells this percentage was only 63% (21 out of 33 patches).
Electrophysiology
Patch-clamp experiments were performed at room temperature with a patch-clamp amplifier (Axopatch 2B, Axon Instruments, Foster City, CA, USA) and a Digidata 1440A interface (Axon Instruments). Currents were filtered at 2 kHz, digitized at 10 kHz, and analyzed with pCLAMP8.1 and Clampfit10 software. During patch-clamp recordings, cells were held at a holding potential (corrected from liquid junction potential) of −180 mV depending on the composition of the pipette solution and pressure stimulus was applied with a High-Speed Pressure-Clamp system (ALA Scientific Instrument, NY, USA), allowing the application of precise and controlled pulses in the pipette.
For Cl− current recordings, bath solutions contained (mM): 50 CaCl2, 5 MgCl2, 10 MES–Tris (pH 5.6); while pipettes were filled with (mM): 150 CsCl, 2 MgCl2, 5 EGTA, 4.2 CaCl2, and 10 Tris–HEPES (pH 7.2), supplemented with 5-mM MgATP. To remove Ca2+, a solution containing (mM): 100 TeaCl, 5 MgCl2, and 10 MES–Tris (pH 5.6) was used. The mean current elicited by the mechanical stimulus was calculated by averaging the current value during the entire stimulation period. Furthermore, recordings in which a basal current was present prior to the application of a mechanical stimulus were not taken into consideration.
For inhibitor treatments, 0.25-mM GdCl3 or 0.01-mM of GsMTx4 were added to the bath solution, osmolarity was adjusted with mannitol to 720 mOsmol for the bath solution and to 750 mOsmol for the pipette solution using a vapor pressure osmometer (VAPRO 5520). GigaOhm resistance seals between pipettes (pipette resistance, 0.8–1.5 MΩ) pulled from capillaries (Borosilicate capillaries, A-M Systems #8520) and protoplast membranes were obtained with gentle suction leading to the whole-cell configuration, and then excised to an outside-out configuration. The current inactivation kinetics were fitted with a mono exponential function: F(t)= A * e(−t/tau)+ C, where A is the coefficient, t is the time constant, tau is the decay constant, and C represents the maximum current intensity.
Statistical analyses
Datasets were analyzed using Student’s t test or analysis of variance using Prism Software.
Accession numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers NC_003074.8 (FERONIA Arabidopsis thaliana), NC_003075.7 (Wall-Associated Kinase, Arabidopsis thaliana), and NM_001142864.4 (Piezo1 Homo sapiens).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Videos S1–S5. Mechanically evoked folding of the Mimosa leaflets. Presented are sample videos of the types of leaflet folding elicited by the application of a mechanical stimulus with von Frey filaments. Videos 1–5 describe behaviors that are scored from 0 to 4, respectively.
Supplementary Material
Funding
This work was funded by a team grant from the Fonds de Recherche du Québec-Nature et Technologies (# 245089) to A.G and R.S.N.
Conflict of interest statement. The authors declare no competing financial interest.
D.T. and H.P. performed the experiments; D.T., H.P., Y.C., A.G., and R.S.N. were involved in the study design; D.T., H.P., and R.S.N. analyzed the data; D.T., R.S.N., and A.G. wrote the paper. All authors discussed the results and commented on the manuscript.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys/pages/general-instructions) is: Reza Sharif-Naeini (reza.sharif@mcgill.ca).
References
- Abe T (1981) Chloride ion efflux during an action potential in the main pulvinus of Mimosa pudica. Bot Mag 94: 379–383 [Google Scholar]
- Abramson CI, Chicas-Mosier AM (2016) Learning in plants: lessons from Mimosa pudica. Front Psychol 7: 417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong DG, Lavery LA, Vela SA, Quebedeaux TL, Fleischli JG (1998) Choosing a practical screening instrument to identify patients at risk for diabetic foot ulceration. Arch Intern Med 158: 289–292 [DOI] [PubMed] [Google Scholar]
- Bourque CW, Voisin DL, Chakfe Y (2002) Stretch-inactivated cation channels: cellular targets for modulation of osmosensitivity in supraoptic neurons. Prog Brain Res 139: 85–94 [DOI] [PubMed] [Google Scholar]
- Bradman MJ, Ferrini F, Salio C, Merighi A (2015) Practical mechanical threshold estimation in rodents using von Frey hairs/Semmes-Weinstein monofilaments: towards a rational method. J Neurosci Methods 255: 92–103 [DOI] [PubMed] [Google Scholar]
- Buchen B, Hensel D, Sievers A (1983) Polarity in mechanoreceptor cells of trigger hairs of Dionaea muscipula Ellis. Planta 158: 458–468 [DOI] [PubMed] [Google Scholar]
- Cho H, Shin J, Shin CY, Lee SY, Oh U (2002) Mechanosensitive ion channels in cultured sensory neurons of neonatal rats. J Neurosci 22: 1238–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove DJ, Hedrich R (1991) Stretch-activated chloride, potassium, and calcium channels coexisting in plasma membranes of guard cells of Vicia faba L. Planta 186: 143–153 [DOI] [PubMed] [Google Scholar]
- Coutand C, Julien JL, Moulia B, Mauget JC, Guitard D (2000) Biomechanical study of the effect of a controlled bending on tomato stem elongation: global mechanical analysis. J Exp Bot 51: 1813–1824 [DOI] [PubMed] [Google Scholar]
- Darwin C (1875) Insectivorous Plants. John Murray, London
- De Luccia TP, Friedman P (2011) Boolean function applied to Mimosa pudica movements. Plant Signal Behav 6: 1361–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabricant A, Iwata GZ, Scherzer S, Bougas L, Rolfs K, Jodko-Wladzinska A, Voigt J, Hedrich R, Budker D (2021) Action potentials induce biomagnetic fields in carnivorous Venus flytrap plants. Sci Rep 11: 1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fondeville JC, Borthwick HA, Hendricks SB (1966) Leaflet movement of Mimosa pudica L. indicative of phytochrome action. Planta 69: 357–364 [DOI] [PubMed] [Google Scholar]
- Geitmann A (2016) Actuators acting without actin. Cell 166: 15–17 [DOI] [PubMed] [Google Scholar]
- Gnanasambandam R, Bae C, Gottlieb PA, Sachs F (2015) Ionic selectivity and permeation properties of human PIEZO1 channels. PLoS One 10: e0125503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb PA, Suchyna TM, Sachs F (2007) Properties and mechanism of the mechanosensitive ion channel inhibitor GsMTx4, a therapeutic peptide derived from Tarantula venom. Curr Top Membr 59: 81–109 [DOI] [PubMed] [Google Scholar]
- Hagihara T, Toyota M (2020) Mechanical Signaling in the Sensitive Plant Mimosa pudica L. Plants 9: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Martinac B (2001) Molecular basis of mechanotransduction in living cells. Physiol Rev 81: 685–740 [DOI] [PubMed] [Google Scholar]
- Hamilton ES, Jensen GS, Maksaev G, Katims A, Sherp AM, Haswell ES (2015) Mechanosensitive channel MSL8 regulates osmotic forces during pollen hydration and germination. Science 350: 438–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haswell ES, Peyronnet R, Barbier-Brygoo H, Meyerowitz EM, Frachisse JM (2008) Two MscS homologs provide mechanosensitive channel activities in the Arabidopsis root. Curr Biol 18: 730–734 [DOI] [PubMed] [Google Scholar]
- He BH, Christin M, Mouchbahani-Constance S, Davidova A, Sharif-Naeini R (2017) Mechanosensitive ion channels in articular nociceptors drive mechanical allodynia in osteoarthritis. Osteoarthritis Cartilage 25: 2091–2099 [DOI] [PubMed] [Google Scholar]
- Hooke R (1665) Micrographia, or, Some Physiological Descriptions of Minute Bodies Made by Magnifying Glasses: With Observations and Inquiries Thereupon. Jo. Martyn and Ja. Allestry, London
- Iosip AL, Bohm J, Scherzer S, Al-Rasheid KAS, Dreyer I, Schultz J, Becker D, Kreuzer I, Hedrich R (2020) The Venus flytrap trigger hair-specific potassium channel KDM1 can reestablish the K+ gradient required for hapto-electric signaling. PLoS Biol 18: e3000964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumon K, Tsurumi S (1984) Ion efflux from pulvinar cells during slow downward movement of the petiole of Mimosa pudica L. induced by photostimulation. J Plant Physiol 115: 439–443 [DOI] [PubMed] [Google Scholar]
- Le Bars D, Gozariu M, Cadden SW (2001) Animal models of nociception. Pharmacol Rev 53: 597–652 [PubMed] [Google Scholar]
- Maksaev G, Haswell ES (2012) MscS-Like10 is a stretch-activated ion channel from Arabidopsis thaliana with a preference for anions. Proc Natl Acad Sci USA 109: 19015–19020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monshausen GB, Gilroy S (2009) Feeling green: mechanosensing in plants. Trends Cell Biol 19: 228–235 [DOI] [PubMed] [Google Scholar]
- Monshausen GB, Haswell ES (2013) A force of nature: molecular mechanisms of mechanoperception in plants. J Exp Bot 64: 4663–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran N (2007) Osmoregulation of leaf motor cells. FEBS Lett 581: 2337–2347 [DOI] [PubMed] [Google Scholar]
- Moulia B, Coutand C, Julien JL (2015) Mechanosensitive control of plant growth: bearing the load, sensing, transducing, and responding. Front Plant Sci 6: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plachno BJ, Adamec L, Lichtscheidl IK, Peroutka M, Adlassnig W, Vrba J (2006) Fluorescence labelling of phosphatase activity in digestive glands of carnivorous plants. Plant Biol (Stuttg) 8: 813–820 [DOI] [PubMed] [Google Scholar]
- Poppinga S, Hartmeyer SR, Masselter T, Hartmeyer I, Speck T (2013) Trap diversity and evolution in the family Droseraceae. Plant Signal Behav 8: e24685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranade SS, Syeda R, Patapoutian A (2015) Mechanically activated ion channels. Neuron 87: 1162–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranade SS, Woo SH, Dubin AE, Moshourab RA, Wetzel C, Petrus M, Mathur J, Begay V, Coste B, Mainquist J, et al. (2014) Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature 516: 121–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhold L (1967) Induction of coiling in tendrils by auxin and carbon dioxide. Science 158: 791–793 [DOI] [PubMed] [Google Scholar]
- Riehl TE, Jaffe MJ (1984) Physiological studies on pea tendrils: XIV. Effects of mechanical perturbation, light, and 2-deoxy-d-glucose on callose deposition and tendril coiling. Plant Physiol 75: 679–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs F (2010) Stretch-activated ion channels: what are they? Physiology (Bethesda) 25: 50–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samejima M, Sibaoka T (1982) Membrane potentials and resistances of excitable cells in the petiole and main pulvinus of Mimosa pudica. Plant Cell Physiol 23: 459–465 [Google Scholar]
- Scherzer S, Federle W, Al-Rasheid KAS, Hedrich R (2019) Venus flytrap trigger hairs are micronewton mechano-sensors that can detect small insect prey. Nat Plants 5: 670–675 [DOI] [PubMed] [Google Scholar]
- Sharif-Naeini R (2015) Contribution of mechanosensitive ion channels to somatosensation. Prog Mol Biol Transl Sci 131: 53–71 [DOI] [PubMed] [Google Scholar]
- Sharif-Naeini R, Folgering JH, Bichet D, Duprat F, Lauritzen I, Arhatte M, Jodar M, Dedman A, Chatelain FC, Schulte U, et al. (2009) Polycystin-1 and -2 dosage regulates pressure sensing. Cell 139: 587–596 [DOI] [PubMed] [Google Scholar]
- Shih HW, Miller ND, Dai C, Spalding EP, Monshausen GB (2014) The receptor-like kinase FERONIA is required for mechanical signal transduction in Arabidopsis seedlings. Curr Biol 24: 1887–1892 [DOI] [PubMed] [Google Scholar]
- Sierla M, Waszczak C, Vahisalu T, Kangasjarvi J (2016) Reactive oxygen species in the regulation of stomatal movements. Plant Physiol 171: 1569–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song K, Yeom E, Lee SJ (2014) Real-time imaging of pulvinus bending in Mimosa pudica. Sci Rep 4: 6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamiya T, Miyazaki T, Ishikawa H, Iriguchi N, Maki T, Matsumoto JJ, Tsuchiya T (1988) Movement of water in conjunction with plant movement visualized by NMR imaging. J Biochem 104: 5–8 [DOI] [PubMed] [Google Scholar]
- Tran D, Galletti R, Neumann ED, Dubois A, Sharif-Naeini R, Geitmann A, Frachisse JM, Hamant O, Ingram GC (2017) A mechanosensitive Ca(2+) channel activity is dependent on the developmental regulator DEK1. Nat Commun 8: 1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visnovitz T, Vilagi I, Varro P, Kristof Z (2007) Mechanoreceptor cells on the tertiary pulvini of Mimosa pudica L. Plant Signal Behav 2: 462–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkov AG, O'Neal L, Volkova MI, Markin VS (2013) Morphing structures and signal transduction in Mimosa pudica L. induced by localized thermal stress. J Plant Physiol 170: 1317–1327 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Kindrat AN, Sharif-Naeini R, Bourque CW (2007) Actin filaments mediate mechanical gating during osmosensory transduction in rat supraoptic nucleus neurons. J Neurosci 27: 4008–4013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.