Abstract
The enzymatic hydrolysis of cellulose into glucose, referred to as saccharification, is severely hampered by lignins. Here, we analyzed transgenic poplars (Populus tremula × Populus alba) expressing the Brachypodium (Brachypodium distachyon) p-coumaroyl-Coenzyme A monolignol transferase 1 (BdPMT1) gene driven by the Arabidopsis (Arabidopsis thaliana) Cinnamate 4-Hydroxylase (AtC4H) promoter in the wild-type (WT) line and in a line overexpressing the Arabidopsis Ferulate 5-Hydroxylase (AtF5H). BdPMT1 encodes a transferase which catalyzes the acylation of monolignols by p-coumaric acid (pCA). Several BdPMT1-OE/WT and BdPMT1-OE/AtF5H-OE lines were grown in the greenhouse, and BdPMT1 expression in xylem was confirmed by RT-PCR. Analyses of poplar stem cell walls (CWs) and of the corresponding purified dioxan lignins (DLs) revealed that BdPMT1-OE lignins were as p-coumaroylated as lignins from C3 grass straws. For some transformants, pCA levels reached 11 mg·g−1 CW and 66 mg·g−1 DL, exceeding levels in Brachypodium or wheat (Triticum aestivum) samples. This unprecedentedly high lignin p-coumaroylation affected neither poplar growth nor stem lignin content. Interestingly, p-coumaroylation of poplar lignins was not favored in BdPMT1-OE/AtF5H-OE transgenic lines despite their high frequency of syringyl units. However, lignins of all BdPMT1-OE lines were structurally modified, with an increase of terminal unit with free phenolic groups. Relative to controls, this increase argues for a reduced polymerization degree of BdPMT1-OE lignins and makes them more soluble in cold NaOH solution. The p-coumaroylation of poplar samples improved the saccharification yield of alkali-pretreated CW, demonstrating that the genetically driven p-coumaroylation of lignins is a promising strategy to make wood lignins more susceptible to alkaline treatments used during the industrial processing of lignocellulosics.
The expression of a grass p-coumaroyl-CoA:monolignol transferase induces high p-coumaroylation of poplar lignins and better saccharification of alkali-pretreated poplar wood without growth penalty.
Introduction
Wood appears as a major feedstock for traditional or innovative biorefineries producing pulp, chemicals, or fermentable sugars. However, most industrial fractionations of lignocellulosics are detrimentally affected by lignins. For instance, the enzymatic hydrolysis of cellulose into glucose, referred to as saccharification, is severely hampered by lignins that hinder the accessibility of enzymes to cell wall (CW) polysaccharides. Indeed, the economically effective production of cellulosic ethanol necessitates costly, polluting and energy-intensive pretreatments that most often aim at reducing the lignin shield effect (Yang and Wyman, 2008; Sun et al., 2016). Since the last decades, lignin engineering in trees has been the subject of intensive studies to produce tailor-made wood more amenable to efficient deconstruction by milder processes (Pilate et al., 2012; Chanoca et al., 2019; Mahon and Mansfield, 2019). However, lignins play key roles in wood and sufficient lignin amounts are required to warrant tree growth, development and defense. On this basis, reducing lignin content may result in impaired tree growth and redesigning lignin structure appears as a better strategy to obtain wood biomass more adapted to industrial deconstruction without yield penalty.
Lignins primarily result from the enzymatically driven oxidation of monolignols, mainly coniferyl alcohol and sinapyl alcohol that give rise to guaiacyl (G) and syringyl (S) units, respectively. It is now well established that lignin biosynthesis is very plastic and that, besides the main monolignols, a number of other molecules may participate in the formation of lignin polymers (Mottiar et al., 2016; del Río et al., 2020). For instance, p-coumaroylated sinapyl alcohol and, to a lower extent, p-coumaroylated coniferyl alcohol, are naturally incorporated into grass lignins (Grabber et al., 1996; Lu and Ralph, 1999; Hatfield et al., 2009; Ralph, 2010). This p-coumaroylation of grass monolignols is specifically catalyzed by a p-coumaroyl-coenzyme A monolignol transferase (PMT) studied in various grass species (Hatfield et al., 2009; Withers et al., 2012; Marita et al., 2014; Petrik et al., 2014). The p-coumaroylation of dicot lignins was recently achieved by introducing the rice (Oryza sativa) PMT gene into poplar and Arabidopsis plants (Smith et al., 2015), but the p-coumaroylation level of transgenic dicot CW reported in this study was modest (varying from 1 to 3.5 mg·g−1 CW) and much lower than that of lignified grass stems (p-coumaric acid [pCA] ranging from 6 to 39 mg·g−1 CW; Hatfield et al., 2009). In contrast, the introduction of two different Brachypodium PMT genes (BdPMT1 or BdPMT2) under the control of the AtC4H promoter into various Arabidopsis lines boosted the p-coumaroylation of mature stem lignins up to the grass lignin level (Sibout et al., 2016). In addition to a high pCA content, the Arabidopsis BdPMT1-OE lignins displayed other traits specific to grass lignins, i.e. a high frequency of free phenolic units in lignins and an increased solubility in cold alkali.
In this work, we explored the potential of introducing the proAtC4H::BdPMT1 construct into poplar in order to beneficially tailor lignin structure without biomass penalty. To this end, BdPMT1 was expressed not only in the poplar wild-type (WT) background, but also in a transgenic poplar line overexpressing the AtF5H gene (AtF5H-OE). By so doing, we obtained several independent transformants that were grown in the greenhouse together with the corresponding controls during 3 months. In this study, we first evaluated the growth of the BdPMT1-OE lines and the p-coumaroylation of their stem lignins, as compared to control trees. We then investigated the effect of the BdPMT1 expression on lignin content and structure before subjecting the transgenic and control poplar stems to alkali-solubilization assays and saccharification tests.
Results and discussion
The expression of heterologous BdPMT1 gene under the control of the AtC4H promoter does not alter poplar growth
The BdPMT1 acyltransferase (referred to as Bradi2g36910) has been shown to be specific to monolignol p-coumaroylation (Petrik et al., 2014). BdPMT1 is a close homolog of the rice OsPMT that was introduced by Smith et al. (2015) into poplar and Arabidopsis plants. We used the AtC4H promoter to drive the expression of BdPMT1 as it is highly expressed in Arabidopsis xylem tissues during lignification (Bell-Lelong et al., 1997) and is also efficient to drive transgene expression in poplar wood (Franke et al., 2000). The transformation was performed in two poplar genetic backgrounds, the WT line and a transgenic line overexpressing the AtF5H gene. The AtF5H expression was driven by a poplar cellulose synthase A4 promoter, known to be highly active in the fibers and vessels of poplar developing xylem (Hai et al., 2016). The AtF5H-OE poplar line was chosen to test the hypothesis that the p-coumaroylation of poplar lignins may be favored by a high frequency of S units based on the two following published data: (1) the p-coumaroylation of grass lignins mostly occurs on S units (reviewed in Ralph, 2010; Karlen et al., 2018) and (2) overexpressing the AtF5H gene in poplar substantially increases the frequency of S lignin units (Franke et al., 2000).
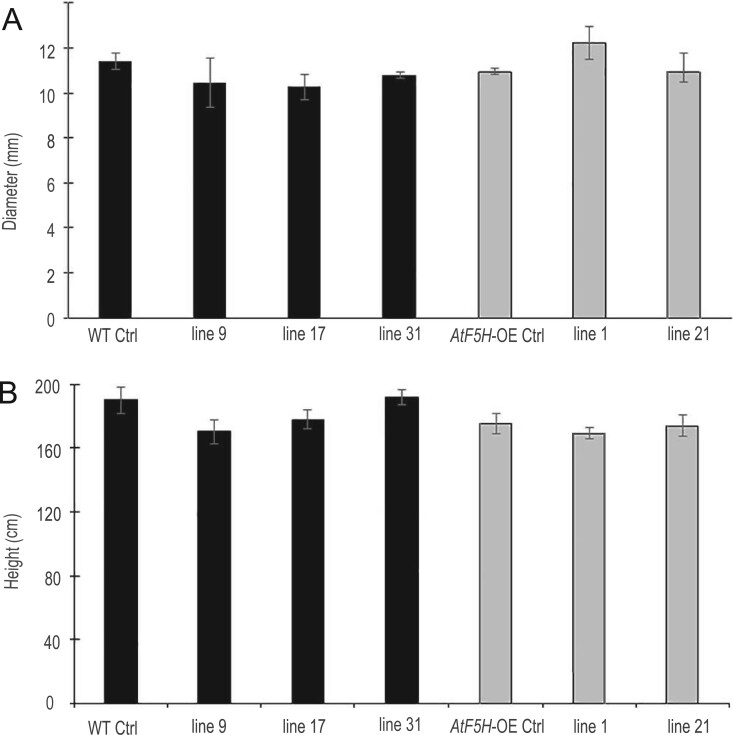
The Agrobacterium tumefaciens-mediated transformation yielded several independent transformants in the WT background (referred to as BdPMT1-OE/WT lines) and in the AtF5H-overexpressing background (referred to as BdPMT1-OE/AtF5H-OE lines). Three BdPMT1-OE/WT lines and two BdPMT1-OE/AtF5H-OE lines were randomly chosen for further analyses: They were acclimatized and grown for 3 months in the greenhouse together with corresponding control plants (Supplemental Figure S1A). Semi-quantitative RT-PCR with BdPMT1 specific primers revealed a substantial BdPMT1 transcript abundance in developing xylem of BdPMT1-OE lines, with some variations between lines, whereas no BdPMT1 expression could be detected in the WT or AtF5H-OE control trees (Supplemental Figure S1B). Likewise, when using primers directed to AtF5H, a strong RT-PCR signal was observed in the AtF5H-OE transgenic lines (Supplemental Figure S1C). Relative to the control trees, the BdPMT1-OE did not induce any significant difference in height and diameter (Figure 1) and the transgenic poplar plants did not show any obvious phenotype difference when compared to WT trees.
Figure 1.
Growth response to the introduction of the proAtC4H::BdPMT1 construct into the poplar WT background (black bars) and into the AtF5H-OE background (gray bars), as compared to control (Ctrl) trees. The basal diameter (A) and the tree height (B) were measured on 3-month-old greenhouse-grown trees. Data are means (sd) values of three or four biological replicates. Duncan tests (at P < 0.05) did not reveal any significant differences between poplar lines.
The BdPMT1-OE poplar stems and their corresponding purified dioxane lignin fractions are p-coumaroylated to the levels of C3 grass samples
CW samples from the stems of 3-month-old greenhouse-grown poplar trees were subjected to mild alkaline hydrolysis to quantify p-hydroxybenzoic acid (Bz), pCA, and ferulic acid (FA) ester-linked to CW polymers. Poplar wood is typified by the occurrence of Bz ester-linked to lignins (Smith, 1955; Venverloo, 1969) and preferentially to the γ position of S lignin units (Lu et al., 2004; Morreel et al., 2004). Most BdPMT1-OE poplar samples displayed similar p-hydroxybenzoylation levels as their corresponding controls (Table 1). In addition to Bz, mild alkaline hydrolysis of poplar samples released small amounts of FA consistently and significantly in slightly smaller quantities in all BdPMT1-OE/WT lines compared to the WT (Table 1). In plant CW, FA preferentially acylates noncellulosic polysaccharides (Ishii, 1997). Some of these FA esters can be oxidatively coupled to monolignols and act as lignin nucleation sites (Ralph, 2010). The small differences of FA esters between poplar lines might reflect some variations in the feruloylation degree of CWs polymers and/or in their ferulate mediated cross-linking.
Table 1.
Amount of Bz, pCA, and FA released by mild alkaline hydrolysis of extract-free poplar stems (referred to as CWs) from BdPMT1-OE lines obtained in the WT and AtF5H-OE backgrounds, as compared to their respective controls
| Line (n Replicates) | Bz | pCA | FA |
|---|---|---|---|
| mg·g−1 CW | mg·g−1 CW | mg·g−1 CW | |
| WT control (3) | 3.86 (0.10)a,b | 0.01 (0.00)f | 0.22 (0.00)a |
| BdPMT1-OE/WT line 9 (3) | 3.21 (0.51)b | 7.12 (0.49)b | 0.15 (0.02)c |
| BdPMT1-OE/WT line 17 (3) | 3.66 (0.28)a,b | 10.69 (0.49)a | 0.18 (0.01)b |
| BdPMT1-OE/WT line 31 (3) | 3.57 (0.11)ab | 3.63 (0.42)d | 0.10 (0.00)d |
| AtF5H-OE control (4) | 3.29 (0.14)b | 0.01 (0.00)f | 0.06 (0.00)e |
| BdPMT1-OE/AtF5H-OE line 1 (4) | 3.06 (0.56)b | 0.76 (0.04)e | 0.07 (0.01)de |
| BdPMT1-OE/AtF5H-OE line 21 (3) | 4.35 (0.08)a | 4.92 (0.19)c | 0.13 (0.02)c |
The data represent mean (sd) values from n biological replicates. Different letters in columns indicate significant differences (Duncan test, P < 0.01).
While expressing the BdPMT1 gene both in the WT and the AtF5H-OE backgrounds had no effect on Bz units ester-linked to poplar CW, this transformation dramatically increased CW p-coumaroylation with up to 1,000-fold higher levels compared to the trace amounts of the controls (Table 1). Remarkably enough, this quantity was boosted up to about 11 mg·g−1 CW in BdPMT1-OE/WT line 17. As compared to grass mature stems, the pCA levels of this poplar line exceeded those of most C3 grass CW, but remained lower than those of C4 grass CW (Supplemental Table S1). With the exception of line 1, the obtained BdPMT1-OE poplar lines were as p-coumaroylated as extract-free proAtC4H::BdPMT1 Arabidopsis mature stems (pCA amounts ranging between 3.5 and 12.6 mg·g−1 CW; Sibout et al., 2016). In contrast, these levels were much higher than the values reported for OsPMT-OE poplar lines (pCA range: 1.2–3.5 mg·g−1 CW) or for OsPMT-OE Arabidopsis lines (pCA range: 1.0–2.0 mg·g−1 CW) when OsPMT expression was driven by the 35S CAMV promoter or by the CELLULOSE SYNTHASE7 promoter (Smith et al., 2015). In agreement with Smith et al. (2015), the p-coumaroylation of poplar CW did not affect their p-hydroxybenzoylation (Table 1). The high p-coumaroylation of poplar CWs obtained in the present work is very likely related to the efficiency of the AtC4H promoter, in agreement with recent data obtained with BdPMT1-OE Arabidopsis lines (Sibout et al., 2016).
Isolation of dioxan lignin (DL) fractions followed by their mild alkaline hydrolysis recently proved to be an efficient strategy to demonstrate that pCA units introduced in BdPMT1-transformed Arabidopsis plants are ester-linked to lignins (Sibout et al., 2016). The isolation method consists in mild acidolysis (refluxing CW samples in dioxane/0.2 M aq. HCl for 30 min under N2), which provides a rough lignin extract then purified to recover DL fractions. This isolation method relies on the hydrolysis of some ether bonds in lignins to make the insoluble native lignin polymers partially soluble into the reaction medium. The purified DL fractions contain a low amount of sugar contaminants (<10% by weight) and the mild isolation procedure mostly preserves lignin-linked pCA esters, if present (Chazal et al., 2014). Purified poplar DL fractions were isolated from a few control and BdPMT1-OE poplar lines and then subjected to mid-infrared (IR) spectroscopy. Their mid-IR spectra not only confirmed their low contamination by sugar components, but also suggested that the lignin fractions isolated from BdPMT1-OE/WT and BdPMT1-OE/AtF5H-OE lines were enriched in pCA esters (Supplemental Figure S2). Relative to their respective controls, the IR spectra from BdPMT1-OE lines displayed increased signals at 1,604, 1,164, and 833 cm−1, which can be assigned to the occurrence of pCA units (Chazal et al., 2014). More importantly, high pCA amounts (from 31 to 66 mg·g−1 DL, Table 2) were released by mild alkaline hydrolysis of the purified DL fractions isolated from BdPMT1-OE poplar lines, as confirmed by both high performance liquid chromatography (HPLC) and gas chromatography/mass spectrometry (GC/MS) analyses (Supplemental Figure S3). The upper values were similar to the pCA levels of DL fractions isolated from C3 grass CW, but remained lower than those of DL fractions isolated from C4 grass species (Supplemental Table S1). Alkaline hydrolysis of the DL fractions isolated from control samples released very low amounts of pCA units (Table 2), which reveals that pCA acylates poplar lignins to a weak extent and is in agreement with results obtained for Arabidopsis lignins (Sibout et al., 2016). The pCA contents of DL fractions from BdPMT1-OE poplar line were found to be 6- to 10-fold higher than those from the corresponding CW (Table 1). Such an outstanding enrichment further establishes that most pCA units introduced in the transgenic poplars are ester-linked to lignins.
Table 2.
Amount of pCA released by mild alkaline hydrolysis of DL fractions isolated from control and BdPMT1-OE lines obtained in the WT and AtF5H-OE backgrounds
| Line | pCA mg·g−1 DL |
|---|---|
| WT control | 3.21 (0.17) |
| BdPMT1-OE/WT line 9 | 50.00 (0.77) |
| BdPMT1-OE/WT line 17 | 66.52 (0.47) |
| BdPMT1-OE/WT line 31 | 31.36 (0.43) |
| AtF5H-OE control | 0.87 (0.04) |
| BdPMT1-OE/AtF5H-OE line 21 | 33.98 (0.53) |
The data represent mean (sd) values from technical duplicates.
Analytical pyrolysis further confirms the high p-coumaroylation of BdPMT1-OE poplar lines
The main advantages of the pyrolysis-GC/MS (Py-GC/MS) method is its high-throughput screening capabilities together with its low sample demand (Ralph and Hatfield, 1991; Lapierre, 1993). When subjected to this method, lignified CW samples provide lignin-derived phenolics originating from G and S lignin units. In addition, during pyrolysis, ester-linked Bz and pCA units (if present) are decarboxylated to produce phenol and 4-vinylphenol, respectively. The relative abundances (area %) of the main G and S pyrolysis products and of phenol and 4-vinylphenol generated from the poplar CW samples are listed in Table 3.
Table 3.
Relative percentage values of the peaks assigned to the main phenolics released by Py-GC/MS of poplar CWs from BdPMT1-OE lines obtained in the WT and AtF5H-OE backgrounds, as compared to their respective controls
| Line (n Replicates) | Phenol | 4-Vinylphenol | G Compoundsa | S Compoundsb | S/G Ratio |
|---|---|---|---|---|---|
| WT control (4) | 5.33 (0.46)a,b | 0.21 (0.07)e | 24.76 (1.31)a | 69.71 (1.57)b | 2.82 (0.21)b |
| BdPMT1-OE/WT line 9 (4) | 4.60 (0.94)a,b | 10.73 (0.35)b | 21.84 (0.80)b | 62.83 (1.11)c | 2.88 (0.13)b |
| BdPMT1-OE/WT line 17 (4) | 5.43 (0.43)a,b | 15.44 (0.71)a | 20.69 (0.78)bc | 58.44 (0.43)d | 2.83 (0.12)b |
| BdPMT1-OE/WT line 31 (4) | 5.06 (0.56)a,b | 5.40 (1.46)d | 24.10 (0.89)a | 65.44 (2.40)bc | 2.72 (0.19)b |
| AtF5H-OE control (4) | 4.99 (0.70)a,b | 0.10 (0.04)e | 18.64 (0.94)cd | 76.38 (1.53)a | 4.11 (0.29)a |
| BdPMT1-OE/AtF5H-OE line 1 (4) | 4.40 (1.17)b | 1.15 (0.010)e | 17.86 (1.69)d | 76.59 (2.74)a | 4.32 (0.52)a |
| BdPMT1-OE/AtF5H-OE line 21 (3) | 6.21 (0.42)a | 7.43 (0.25)c | 16.40 (0.85)d | 69.95 (0.56)b | 4.27 (0.24)a |
These area values are expressed as percentage of the total area per sample (set to 100). The data represent mean (SD) values from n biological replicates. Different letters in columns indicate significant differences (Duncan test, P < 0.01)
G compounds include: guaiacol, 4-methylguaiacol, 4-ethylguaiacol, 4-vinylguaiacol, 4-allylguaiacol (two isomers), vanillin, acetoguaiacone, guaiacylacetone.
S compounds include: syringol, 4-methylsyringol, 4-ethylsyringol, 4-vinylsyringol, 4-allylsyringol (two isomers), syringaldehyde, acetosyringone, syringylacetone.
The pyrolysis S/G ratio calculated from the relative amount of lignin-derived S and G pyrolysis compounds was not significantly affected in the BdPMT1-OE/WT lines (Table 3). This result suggests that the proportion of G and S lignin unit is not affected by the introduction of BdPMT1 in poplar. In agreement with literature data (Franke et al., 2000; Stewart et al., 2009), this ratio was substantially increased in the AtF5H-OE control line as well as in the BdPMT1-OE/AtF5H-OE lines 1 and 21.
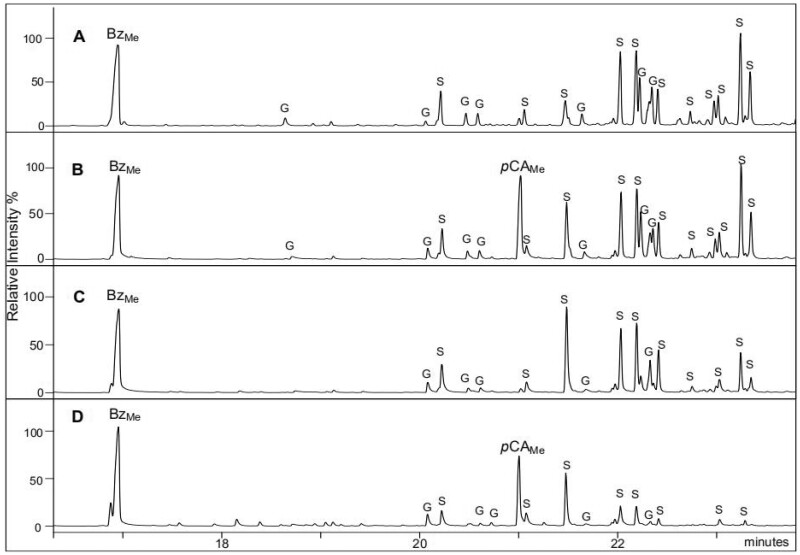
The relative percentage of pyrolysis-derived phenol did not discriminate the various transgenic samples from their control. This result is quite consistent with mild alkaline hydrolysis which provided similar Bz amounts from most transgenic lines and their respective controls. In contrast, the relative amount of 4-vinylphenol was dramatically increased in the BdPMT1-OE lines as compared to their controls. Such a relative increase concomitantly decreased the relative percentage of the lignin-derived pyrolysis G and/or S compounds (Table 3). Even though the pyrolysis-derived 4-vinylphenol might originate from tyrosine residues of putatively present protein contaminants, it is essentially produced from the decarboxylation of CW-linked pCA units (Ralph and Hatfield, 1991). The relative abundance of 4-vinylphenol was found to nicely echo the level of alkali-releasable pCA, as revealed by the positive correlation between pCA amount and the 4-vinylphenol % (R2 = 0.982; Supplemental Figure S4). In other words, the relative amount of pyrolysis-derived 4-vinylphenol may be viewed as a good signature of the CW p-coumaroylation level. To further confirm that 4-vinylphenol prominently originates from pCA decarboxylation, a few pyrolysis assays were carried out in the presence of tetramethylammonium hydroxyde (TMAH). The TMAH-Py-GC/MS method yields methyl 4-methoxybenzoate (BzMe) and methyl 4-methoxy-p-coumarate (pCAMe) from Bz and pCA units, respectively (Kuroda et al., 2001, 2002). As shown in the pyrograms outlined in Figure 2, the relative intensity of the BzMe peak was similar in the BdPMT1-OE and in their corresponding controls whereas the pCAMe peak was prominent in the BdPMT-OE poplar lines.
Figure 2.
Traces of poplar CWs after Py-GC/MS in the presence of TMAH. (A) WT control, (B) BdPMT1-OE/WT line 9, (C) AtF5H-OE control, and (D) BdPMT1-OE/AtF5H-OE line 21. BzMe, 4-methoxybenzoate; pCAMe, methyl 4-methoxy-p-coumarate; peaks quoted G and S correspond to methylated G and S compounds, respectively.
The expression of BdPMT1 transformation has no or little effect on the lignin content of poplar stems, but a strong impact on lignin structure
The most p-coumaroylated transgenic poplar lines were analyzed for their lignin content, using both the Klason lignin (KL) and the acetyl bromide lignin (ABL) methods. As shown in Table 4, the BdPMT1 transformation had no impact on the lignin content of the poplar stem CW. This result contrasts with those obtained for proAtC4H::BdPMT1 Arabidopsis transformants provided with similar p-coumaroylation levels as these poplar transgenics, but with 10%–30% lower lignin contents than their controls (Sibout et al., 2016). Introducing the proAtC4H::BdPMT1 into Arabidopsis plants seemed to affect the metabolic flux to lignins and thereby the stem lignin content whereas such an effect was not observed in the BdPMT1-OE poplar lines.
Table 4.
Lignin content of extract-free poplar stems from BdPMT1-OE lines obtained in the WT and AtF5H-OE backgrounds, as compared to their respective controls
| Line | KL (%) | ABL (%) |
|---|---|---|
| WT control | 21.82 (0.21)a | 19.18 (0.33)ab |
| BdPMT1-OE/WT line 9 | 21.22 (0.09)a | 18.77 (0.50)b |
| BdPMT1-OE/WT line 17 | 21.60 (0.21)a | 19.47 (0.44)ab |
| BdPMT1-OE/WT line 31 | 21.09 (0.43)a | 19.27 (0.28)ab |
| AtF5H-OE control | 20.86 (0.23)a | 19.95 (0.23)a |
| BdPMT1-OE/AtF5H-OE line 21 | 20.87 (0.69)a | 19.86 (0.13)a |
The data represent mean (sd) values from biological triplicates. Different letters in columns indicate significant differences (Duncan test, P < 0.01). The lignin content is expressed as weight percentage of the sample and was determined using the KL and the ABL methods
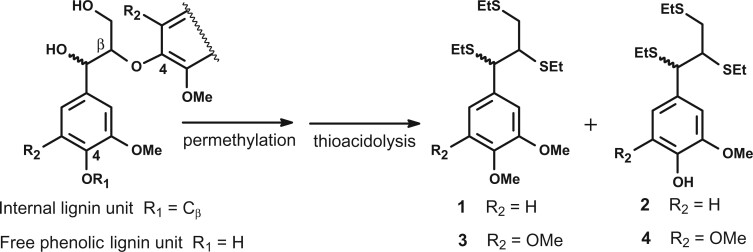
A major structural trait of native lignins is their percentage of free phenolic groups, which has a strong impact on lignin susceptibility towards industrial alkaline or oxidative treatments. When thioacidolysis is performed on CW exhaustively permethylated with diazomethane or trimethylsilyldiazomethane, the percentages of free phenolic groups in β-O-4 linked G or S lignin units, referred to as %GOH or %SOH, can be evaluated. These percentages have been shown to nicely parallel that of the whole polymer (Lapierre, 2010). With the objective to evaluate the impact of the BdPMT1 transformation on the structure of poplar native lignins, we employed this analytical approach, the principle of which is outlined in Figure 3. Past studies have shown that the thioacidolysis yield is not affected by the mild permethylation procedure (Lapierre et al., 1988; Lapierre, 2010). Whatever the sample, the p-hydroxyphenyl (H) thioacidolysis monomers were found to be obtained as trace components (<1% of the monomer yield) and, in consequence, these minor H units were not considered in the following. In agreement with the Py-GC/MS data, the thioacidolysis S/G ratio was not affected by the BdPMT1 transformation in the WT background (Table 5). Consistently with the pyrolysis data (Table 3) and as compared to the WT, the thioacidolysis S/G ratio was found to be drastically increased in the AtF5H-OE samples (Table 5).
Figure 3.
Principle of the evaluation of free phenolic units in lignin by thioacidolysis of permethylated samples. Lignin units only involved in β-O-4 bonds give rise to thioacidolysis guaiacyl (R2 = H) and syringyl (R2 = OMe) monomers. Terminal G and S units with free phenolic group (R1 = H) are first methylated at C4, then degraded to monomers 1 and 3 (erythro/threo mixture), respectively. Internal G and S units (R1 = Cβ of another lignin sidechain) are degraded to monomers 2 and 4, respectively (erythro/threo mixture), EtS = SEt = thio-ethyl.
Table 5.
Thioacidolysis of TMSD-methylated poplar CWs from BdPMT1-OE lines obtained in the WT and AtF5H-OE backgrounds, as compared to their respective controls
| Line | S/G Molar Ratio | % Free Phenolic Units in β-O-4 Linked G or S Units |
|
|---|---|---|---|
| (3 + 4)/(1 + 2) | %GOH | %SOH | |
| 100 × 1/(1 + 2) | 100 × 3/(3 + 4) | ||
| WT control | 2.05 (0.03)b | 19.45 (0.22)d | 2.81 (0.07)e |
| BdPMT1-OE/WT line 9 | 2.09 (0.18)b | 22.85 (0.12)b | 3.65 (0.19)bc |
| BdPMT1-OE/WT line 17 | 2.12 (0.19)b | 23.65 (0.48)a | 4.44 (0.25)a |
| BdPMT1-OE/WT line 31 | 2.08 (0.06)b | 21.09 (0.26)c | 3.44 (0.10)cd |
| AtF5H-OE control | 3.12 (0.13)a | 20.85 (0.44)c | 3.26 (0.03)d |
| BdPMT1-OE/AtF5H-OE line 21 | 2.96 (0.15)a | 23.10 (0.49)b | 4.02 (0.21)bc |
The S/G molar ratio corresponds to the ratio of the S monomers (3 + 4) to the G monomers (1 + 2; monomers shown in Figure 3). The molar % of free phenolic groups in β-O-4 linked G or S units, referred to as %GOH or %SOH, is calculated according to the outlined formula. The data represent mean (sd) values from biological triplicates. Different letters in columns indicate significant differences (Duncan test, P < 0.01).
At this stage of the study and from the simultaneous examination of both thioacidolysis S/G ratio (Table 5) and pCA level (Table 1), our anticipated hypothesis that a high frequency of sinapyl alcohol in AtF5H-OE poplar lines might increase the BdPMT1-induced acylation of poplar lignins is most likely to be ruled out. This conclusion is consistent with literature data reporting on the impact of F5H overexpression in plant species provided with acylated S lignin units. For instance, upregulating F5H in poplar increased the S frequency up to 97.5%, whereas the incorporation of p-hydroxybenzoic acid in lignins was two-fold lower than the control level (Stewart et al., 2009). Upregulating F5H in rice also increased the frequency of S units up to 89%, whereas pCA levels were similar in the transgenic and control plants (Takeda et al., 2017).
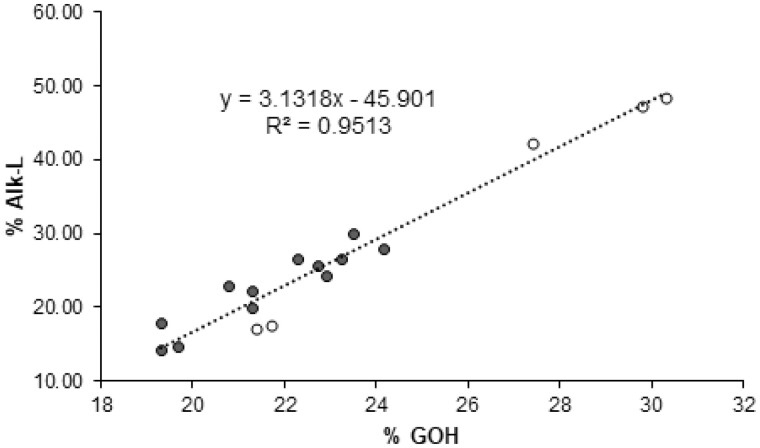
In agreement with literature data (Lapierre, 1993, 2010), the control poplar samples displayed a %GOH and a %SOH close to 20% and 3%, respectively, which confirms that S units essentially are internal units. Even though the impact of F5H upregulation on poplar lignins is out of the main scope of this study, the data of Table 5 revealed that, in addition to the expected higher S/G ratio, lignins from AtF5H-OE control plants have more terminal units with free phenolic groups than WT lignins (Table 5). This result is consistent with a published paper about lignin structure in F5H-upregulated poplars (Stewart et al., 2009). In this study and relative to the WT samples, lignin fractions isolated from F5H-upregulated poplars were shown to concomitantly have a twice higher frequency of phenolic OH and a lower degree of polymerization (Stewart et al., 2009). More strikingly and whatever the genetic background, both %GOH and %SOH were significantly increased in the p-coumaroylated lignins of the BdPMT1-OE poplar lines (Table 5). The increase in %GOH or in %SOH was found to be nicely correlated to the pCA level of the BdPMT1-OE/WT lines (R2 = 0.95 for %GOH and 0.93 for %SOH; Figure 4). This result means that the incorporation of p-coumaroylated monolignols in poplar lignins increases the frequency of free phenolic terminal units relative to internal units. Such a structural change may be accounted for by the occurrence of lignin polymers with lower polymerization degree and/or with a higher content of biphenyl or biphenyl ether branching structures.
Figure 4.
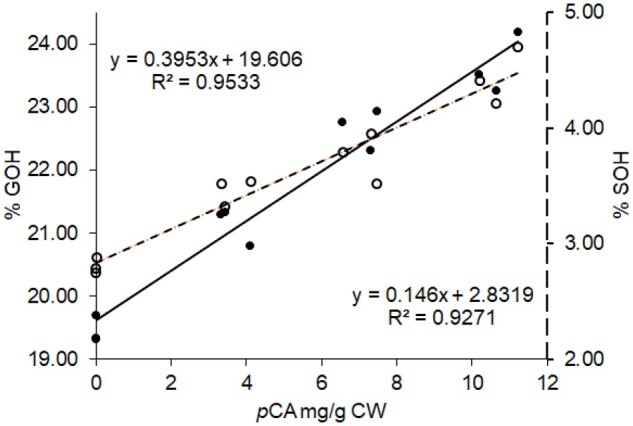
Relationships between the pCA amounts in poplar CWs and the percentage of G lignin units with free phenolic groups (%GOH, black circles, full line) or the percentage of S lignin units with free phenolic groups (%SOH, white circles, dotted lines). The lignin structural traits %GOH and %SOH are evaluated by thioacidolysis of permethylated samples for BdPMT1-OE poplars and their WT controls
The alkaline hydrolysis of the DL fractions isolated from the BdPMT1-OE poplar lines revealed that their pCA units were primarily ester-linked to lignins. With the objective to more precisely localize these pCA esters on lignin units, we subjected the BdPMT1-OE/WT line 17 and the WT samples to 1-h long thioacidolysis experiments, followed by Raney nickel desulfuration in order to identify the syringylpropanol and/or guaiacylpropanol units acylated by p-dihydrocoumaric acid (diHCA). This short thioacidolysis time is necessary as pCA esters do not survive the standard 4-h long thioacidolysis method (Lapierre, 1993; Sibout et al., 2016). When applied to the BdPMT1-OE/WT line 17, the method provided substantial amount of syringylpropanol acylated by diHCA while this dimer could not be observed with a longer thioacidolysis duration (Supplemental Figure S5 and Supplemental Table S2). Interestingly enough and in contrast to the results reported by Smith et al. (2015), its G analogue could not be detected. Taken together and similarly to grass lignins, these results support the hypothesis that the p-coumaroylation of lignins in the BdPMT1-OE/WT line 17 primarily involves S lignin units.
The analysis of the lignin-derived dimers obtained with the standard thioacidolysis method followed by Raney nickel desulfuration was comparatively performed for the WT line and for the BdPMT1-OE/WT line 17. With the caveat that the results were obtained from four technical replicates of WT and line 17 samples, this analysis supported the following conclusions. When expressed as relative percentage of the total area of the main dimers (set to 100; Supplemental Table S2), the relative amount of dimers with biphenyl or biphenyl ether bonds was not increased by the presence of BdPMT1 and suggests that BdPMT1-OE/WT line 17 does not contain lignins more branched than the WT ones. In contrast, the relative percentage of the syringaresinol-derived dimers displayed a 1.4-fold increase in the case of the BdPMT1-OE/WT line 17 sample relative to the WT level (Supplemental Table S2). The syringaresinol structures exclusively originate from the dimerization of sinapyl alcohol and are thus starting points for lignin growth (Ralph et al., 2004). Their higher relative recovery from the BdPMT1-OE/WT line 17 further argues for the occurrence of lignin polymers with lower polymerization degrees than in the WT sample.
The BdPMT1-driven substantial p-coumaroylation of poplar samples makes their lignins more easily solubilized in cold alkali
The enrichment in free phenolic G and S units is very likely to improve the lignin susceptibility to alkaline treatments that are employed in chemical pulping or in the cellulose-to-ethanol conversion process. The beneficial impact of lignin terminal units with free phenolic groups on the CW delignification induced by alkaline treatment has been established for a long time for grass samples (Lapierre et al., 1989; Lapierre, 2010) and confirmed for poplar trees deficient in cinnamyl alcohol dehydrogenase (CAD) activity (Lapierre et al., 1999, 2004; Van Acker et al., 2017), for tobacco (Nicotiana tabacum) plants deficient in cinnamoyl-coenzyme A reductase (CCR) activity (O'Connell et al., 2002) and for BdPMT1-transformed Arabidopsis lines (Sibout et al., 2016). The results of a mild alkaline treatment applied to the poplar samples are shown in Table 6. The residue recovered after this treatment, referred to as the saponified residue (SR), was obtained with similar yields whatever the line. The percentage of the alkali-soluble lignin (%Alk-L) was calculated from the SR recovery yield and the lignin amount of the CW and SR samples. From the data of Table 6, we can see that the lignins from the AtF5H-OE control samples are more alkali-soluble than the lignins from the WT samples. This result is consistent with the higher frequency of terminal units with free phenolic groups in the AtF5H-OE control samples as compared to the WT samples (Table 5). Whatever the genetic background, the percentage of the alkali-soluble lignin (%Alk-L) revealed that the BdPMT1-OE lines are more easily delignified by the employed mild alkaline treatment compared with their control lines. Whereas 15%–20% of the lignin polymers were solubilized by cold alkali for the controls, the %Alk-L was substantially increased in the BdPMT1-OE lines (up to 26%–28% in lines 9, 17, and 21, Table 6). As reported for transgenic CAD- or CCR-deficient plants (Lapierre et al., 1999; O'Connell et al., 2002), increasing the percentage of lignin units with free phenolic groups has beneficial effects on the kraft pulping properties of the lignocellulosic biomass, thereby decreasing the energy and environmental costs of this industrial process. The introduction of BdPMT1 in trees would likely improve the pulping properties of poplar wood.
Table 6.
Impact of a mild alkaline treatment (aq. NaOH 1 M, overnight, room temperature) on extract-free poplar stems from control and BdPMT1-OE lines obtained in the WT and AtF5H-OE backgrounds
| Line | %SR | %ABL in SR | %Alk-L |
|---|---|---|---|
| WT control | 68.03 (0.88)a | 23.69 (0.04)a | 15.5 (2.1)d |
| BdPMT1-OE/WT line 9 | 67.17 (0.46)a | 20.85 (0.47)b | 25.5 (1.1)a,b |
| BdPMT1-OE/WT line 17 | 65.25 (0.73)b | 21.52 (0.24)a,b | 28.1 (1.7)a |
| BdPMT1-OE/WT line 31 | 68.17 (0.22)a | 22.11 (0.75)a,b | 21.7 (1.6)b,c |
| AtF5H-OE control | 69.02 (0.65)a | 22.96 (0.28)a,b | 20.5 (1.3)c |
| BdPMT1-OE/AtF5H-OE line 21 | 67.65 (1.28)a | 21.52 (0.13)a,b | 26.4 (1.7)a |
The percentage of the recovered saponified residue (%SR) is expressed relative to the initial sample. The lignin content of the SR sample is measured as acetyl bromide lignin (%ABL). The percentage of alkali-soluble lignins (%Alk-L) is calculated from the ABL content of the CW and from the %SR recovery yield. The data represent mean (sd) values from biological triplicates. Different letters in columns indicate significant differences (Duncan test, P < 0.01).
The relationship of the free phenolic groups in poplar lignins to their susceptibility towards cold alkaline treatment is further illustrated in Figure 5. On this scheme, we have gathered the data from 17 different poplar lines, comprising the current BdPMT1-OE/WT lines and CAD-deficient ones (Lapierre et al., 2004), together with their respective controls. The effect of the %GOH structural property onto the solubility of poplar lignins in cold alkali is supported by the positive correlation between %GOH and %Alk-L (R2 = 0.9513; Figure 5).
Figure 5.
Relationship between the percentage of G lignin units with free phenolic groups (%GOH) and the solubility of poplar lignins in cold alkali (%Alk-L). The data correspond to BdPMT1-OE trees and their WT controls (black circles) as well as to CAD-deficient trees and their corresponding controls (white circles).
The BdPMT1-driven p-coumaroylation of poplar samples results in improved saccharification after cold alkaline pretreatment
It is well established that the detrimental role of lignins on the cost-effective enzymatic conversion of lignocellulosic polysaccharides into fermentable sugars makes necessary the use of pretreatments (Yang and Wyman, 2008; Wang et al., 2015; Sun et al., 2016). Among these pretreatments, alkaline technologies with sodium hydroxide or with lime have emerged as major procedures for the conversion of lignocellulosic biomass (Kim et al., 2016). Most alkaline pretreatments are carried out under mild conditions (temperature below 60°C) and with moderate alkaline charge (0.5%–10% NaOH w/v) and long reaction time (several hours to several days; Carvalho et al., 2016; Kim et al., 2016; Moreno and Olsson, 2017; Rezania et al., 2020). These simple processes improve saccharification by partially removing lignins and hemicelluloses and by cellulose swelling (Carvalho et al., 2016). Sodium hydroxide pretreatments are more effective with grass feedstocks than on woody ones, which is related to the higher solubility of grass lignins in alkali (Beckmann et al., 1923; Scalbert et al., 1986; Lapierre et al., 1989). From these various literature data about NaOH pretreatments and from the analytical results that we obtained so far on the BdPMT1-OE poplar lines, we could anticipate that an alkaline pretreatment would be well suited to reduce the lignin-related recalcitrance of poplar wood to saccharification. Accordingly, the saccharification experiments run on the poplar samples were preceded by a cold alkaline pretreatment (aq. NaOH 1M, overnight, room temperature). Even though the optimization of this pretreatment was out of the scope of this study, we selected reaction duration and temperature as well as NaOH charge that were similar to literature data about alkaline pretreatment technologies (Carvalho et al., 2016; Kim et al., 2016). The saccharification efficiency was evaluated both by the weight loss (%WL) and by the amount of released glucose (Glc; Table 7). In agreement with their higher level of alkali-soluble lignins, the alkali-pretreated AtF5H-OE control samples displayed a higher saccharification efficiency than the alkali-pretreated WT samples (Table 7). More importantly, the saccharification efficiency was higher in the alkali-pretreated BdPMT1-OE lines, compared with their corresponding control (Table 7). In contrast, when the assays were carried out without any alkali pretreatment, low saccharification yields were observed and the transgenic samples were not significantly different from their controls (Supplemental Table S3). Not unexpectedly, in the WT background, the best saccharification results from alkali-pretreated samples were obtained for the lines provided with the concomitant highest pCA level, %GOH and %Alk-L. The enrichment of poplar lignins in free and readily ionizable phenolic groups favored lignin solubilization in alkali, which consequently improved the saccharification of alkali-pretreated samples. Taken together, these results reveal that the lignins from the current BdPMT1-OE poplar plants share common features with grass lignins. As compared to nongrass lignins from WT plants, these common features are (1) a substantial p-coumaroylation of S lignin units, (2) a higher level of free phenolic units, and (3) a higher solubility in cold alkali. At this point, we may hypothesize that, similar to grass lignins, lignins from the BdPMT1-OE poplar lines obtained herein are distributed in the CWs as small lignin domains which are both rich in free phenolic groups and more easily extracted by cold alkali treatment (Lapierre, 2010).
Table 7.
Saccharification of the poplar SR obtained after a mild alkaline treatment (aq. NaOH 1 M, overnight, room temperature) and corresponding to BdPMT1-OE lines obtained in the WT and AtF5H-OE backgrounds, as compared to their respective controls
| SR from Line | %WL | Glc | Glc |
|---|---|---|---|
| mg·g−-1 SR | mg·g−-1 CW | ||
| WT control | 39.8 (1.3)d | 307.7 (16.0)e | 210.1 (11.3)e |
| BdPMT1-OE/WT line 9 | 52.1 (1.6)a,b | 417.5 (23.2)b,c | 280.1 (17.4)b,c |
| BdPMT1-OE/WT line 17 | 55.1 (2.0)a | 452.4 (17.7)a,b | 294.2 (8.3)a,b |
| BdPMT1-OE/WT line 31 | 45.8 (0.3)c,d | 369.3 (17.9)d | 251.8 (11.9)d |
| AtF5H-OE control | 44.2 (2.2)c,d | 401.4 (6.7)c,d | 277.1 (6.6)c,d |
| BdPMT1-OE/AtF5H-OE line 21 | 49.4 (1.5)b,c | 461.7 (22.4)a | 312.4 (16.5)a |
The saccharification efficiency is evaluated both by the weight loss (%WL) and by the released glucose (Glc). Glc yields are expressed either relative to the SR samples or to the initial CW samples. The data represent mean (sd) values from biological triplicates. Different letters in columns indicate significant differences (Duncan test, P < 0.01).
Conclusion
In this study, we have shown that p-coumaroylating poplar lignins up to the level of grass lignins has consequences that go far beyond a simple lignin decoration and that deeply change not only lignin structural traits, but also important industrial potentialities of lignified CW. Remarkably enough the expression of BdPMT1 under the control of the AtC4H promoter introduced neither any growth penalty, nor reduced lignin content in the various transgenic greenhouse-grown poplar lines that were obtained in two genetic backgrounds. In agreement with a recent study (Sibout et al., 2016), choosing the lignin-specific AtC4H promoter to drive the heterologous expression of BdPMT1 in dicot CW had very likely a key role in changing wood properties.
Since the last decades and with the objective to facilitate the industrial conversion of lignocellulosics into pulp or into bioethanol, many approaches have been used to genetically modify lignin content and/or structure (reviewed in Boerjan and Ralph, 2019; Halpin, 2019; Mahon and Mansfield, 2019; Ralph et al., 2019). Among the lignin structural traits that can be affected by the genetic transformation of angiosperm species, the S/G ratio is probably the most systematically scrutinized one (Chanoca et al., 2019). In contrast, the relative frequency of free phenolic units in native lignins is a key structural trait, which is surprisingly overlooked despite its biological significance and its major effect on the susceptibility of lignins to alkaline or oxidative treatments. In past studies, redesigning native lignins with more free phenolic groups (and therefore with increased alkali-solubility) could be obtained with other genetic transformations, such as CCR or CAD downregulation (O'Connell et al., 2002; Lapierre et al., 2004). In this work, we provide another compelling evidence that the genetically driven increase of free phenolic units in lignins is an efficient strategy for the rational design of lignocellulosics more adapted to industrial biorefineries.
Materials and methods
Production of plant materials
The BdPMT1 expression in poplar was conducted using the same molecular construct as described in Sibout et al. (2016), with the BdPMT1 sequence inserted into the pCC0996 vector under the control of the AtC4H promoter (Weng et al., 2008). This construct was introduced using A. tumefaciens cocultivation into the hybrid poplar clone INRA 717-1B4 as well as in a 717-1B4 transgenic line named AtF5H-OE, according to the method described in Leplé et al. (1992). The AtF5H-OE line was previously transformed with an AtF5H gene inserted into the pH7m24GW vector (Karimi et al., 2007) under the control of the promoter 1.3-kb upstream of the hybrid poplar CesA4 gene (Potri.002G257900). Several transgenic lines from both genetic backgrounds were selected for further analyses. Three to five ramets of each line were acclimatized and grown in a S2 greenhouse for 3 months, from April until July following a random design plantation. Height and stem diameter were measured before plant sampling for molecular and biochemical analyses.
Differentiating xylem samples were collected by a light scraping at the surface of the debarked stem. Samples were immediately frozen in liquid nitrogen and stored at −80°C until use. DNA was prepared using Nucleospin DNA Plant II kit (Macherey-Nagel, Hoerdt, France) and the integration of BdPMT1 and F5H genes was verified by PCR using the following primers pairs: PMT 5′-CCTCATCATGCAGGTGACAG-3′ and 5′-GAAGCAGTTGCCGTAGAACC-3′; F5H 5′-ACGGCTCTTGTCATCGTTGT-3′; and 5′-GTTATGTTGCGGGTCAGTGC-3′. Likewise, RNA was extracted from differentiating xylem using a Nucleospin RNA Plant kit (Macherey-Nagel, Hoerdt, France). The expression level of the BdPMT1 and AtF5H gene in each tree was evaluated by semi-quantitative RT-PCR performed in standard conditions on 1-µg total RNA using the same primers as above.
Analyses of CW phenolics
Preparation of CW samples and dioxane lignins
Most analyses of CW phenolics were carried out from biological replicates (three or four per line) harvested from 3-month-old poplar trees. For each tree, the 20-cm-long basal part of the stem was collected, manually debarked, air--dried and ground to 0.5 mm. Extract-free samples were prepared by exhaustive water and ethanol extraction in an accelerated solvent extractor (ASE350, Dionex). The dried and extract-free samples are referred to as CW samples.
The isolation of DL fractions was performed from 1 to 2 g of CW as previously described (Sibout et al., 2016). Fourier transform infrared (FTIR) spectra of DL fractions were run on a Thermo Scientific Nicolet IS5 spectrophotometer and in KBr pellets.
Analytical pyrolysis
Py-GC/MS was done using a CDS model 5250 pyroprobe autosampler interfaced to an Agilent 6890/5973 GC/MS. The CW samples (about 300 µg) were pyrolyzed in a quartz tube at 500°C for 15 s. The pyrolysis products were separated on a capillary column (5% phenyl methyl siloxane, 30 m, 250 μm i.d., and 0.25-μm film thickness) using helium as the carrier gas with a flow rate of 1 mL·min−1. The pyrolysis and GC/MS interfaces were kept at 290°C and the GC was programmed from 40°C (1 min) to 130°C at +6°C min−1, then from 130 to 250°C at +12°C min−1 and finally from 250°C to 300°C at +30°C min−1 (3 min at 300°C). The various phenolic pyrolysis compounds were identified by comparison to published spectra (Ralph and Hatfield, 1991). Py-GC/MS in the presence of TMAH was similarly performed but with addition of 3 µL of a 25% (w/v) TMAH methanolic solution (Aldrich) onto the CW sample. The methylated pyrolysis products were identified by comparison of their mass spectra with those of the NIST MS library or with published TMAH-pyrograms (Kuroda et al., 2001, 2002).
Determination of lignin content
The determination of KL content was perfomed from about 300 mg of CW (weighted to the nearest 0.1 mg) and as previously described (Méchin et al., 2014). The quantitation of ABL was done from about 5 mg of CW (weighted to the nearest 0.01 mg) according to a recently published procedure (Sibout et al., 2016).
Determination of ester-linked p-hydroxybenzoic and p-hydroxycinnamic acids by mild alkaline hydrolysis
About 5–10 mg of poplar CW or DL samples were put into 2-mL Eppendorf tube together with 1 mL of 1 M NaOH and 0.1 mL of o-coumaric internal standard (IS) methanolic solution. The IS amount was 0.05 mg for CW samples and 0.25 mg for DL ones. Mild alkaline hydrolysis was proceeded on a carousel overnight and at room temperature. After acidification (0.2 mL of 6 M HCl) and centrifugation (1,500g, 10 min), the supernatant was subjected to solid phase extraction as previously described (Ho-Yue-Kuang et al., 2016). The recovered methanolic samples were analyzed by HPLC combined with diode array detection (HPLC–DAD). For HPLC separation, 1 μL of sample was injected onto an RP18 column (4 × 50 mm, 2.7-μm particle size, Nucleoshell, Macherey-Nagel) with a flow rate of 0.25 mL min−1. The eluents were 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B), and the gradient was as follows: 0 min 5% B; 12 min, 20% B; 14 min, 80% B; 16 min, 5% B. The quantitative determination of alkali-released Bz, pCA, and FA was performed from the 250–400 nm DAD chromatograms and after calibration with authentic compounds.
Analysis of lignin structure by thioacidolysis
Thioacidolysis (4-h long) followed by GC/MS of the trimethylsilylated lignin-derived compounds was carried out from about 10 mg of CW samples using the simplified procedure previously published (Méchin et al., 2014), with some adaptations to the CW type concerning the IS amount and the reagent to sample ratio. In brief, 5–10 mg (weighed to the nearest 0.1 mg) were put together with 2 mL of freshly prepared thioacidolysis reagent and 0.1 mL of IS solution (heinecosane C21, 5 mg·mL−1 in CH2Cl2) in a glass tube (Teflon-lined screwcap). The closed tubes were then heated at 100°C (oil bath) and for 4 h with occasional gentle shaking. After tube cooling, 2 mL of 0.2 M NaHCO3 were added to destroy the excess of BF3 etherate. Then, 0.025 mL of 6 M HCl was added to ensure that the pH was less than 3, before the addition of 2-mL CH2Cl2 and tube mixing. A small amount (about 0.5 mL) of the lower organic phase was withdrawn with a glass Pasteur pipette, dried over anhydrous Na2SO4 and then directly subjected to trimethylsilylation. This silylation was performed with 10 µL of the solution together with 100-µL BSTFA (Sigma-Aldrich) and 10 µL of GC-grade pyridine (1 h at room temperature). The GC/MS analyses were carried out as previously described (Méchin et al., 2014). Some short thioacidolysis assays (1-h long) were also carried out and were followed by desulfuration experiments according to a published method (Lapierre et al., 1995). In addition, thioacidolysis from exhaustively permethylated CW samples was run according to Sibout et al. (2016) and using the same thioacidolysis and GC/MS conditions.
Investigation of some CW properties
Alkali solubilization assays
About 300 mg of poplar CW were subjected to mild alkaline hydrolysis in 10 mL of 1 M NaOH, into a 25-mL plastic tube agitated overnight on a carousel and at room temperature. The alkali-treated residue, referred to as the SR, was recovered by centrifugation (2,000g, 20 min), washed with 1 M HCl before centrifugation and then with water (three times with centrifugation following each washing step). The final residue was freeze-dried, weighted to calculate its recovery yield and subjected to KL or ABL determination. The weight percentage of alkali-soluble lignin (%Alk-L) was calculated from the weight percentages of ABL in CW (%ABLCW) and in SR (%ABLSR) samples and from the SR recovery yield (%SR), as follows:
Saccharification assays
Saccharification experiments were performed from about 30 mg of samples (weighed to the nearest 0.1 mg) under the conditions previously described (Sibout et al., 2016). Saccharification efficency was calculated both from the weight loss and from the glucose yield.
Statistical analyses
Statistical analyses were performed with R software (version 3.2.3). Duncan’s multiple range tests were performed with the package agricolae (https://cran.r-project.org/web/packages/agricolae/).
Accession numbers
BdPMT1 = Bradi2g36910, accession number NM_00128785 in the Genbank database.
AtF5H = accession number U38416 in the GenBank database.pCesA4 = Potri.002G257900, accession number AC21305 in the GenBank database.
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1. Molecular analyses on greenhouse-grown plant material.
Supplemental Figure S2. IR spectra (KBr pellet) of DL fractions isolated from BdPMT1-OE/WT and BdPMT1-OE/AtF5H-OE lines as compared to their controls.
Supplemental Figure S3 . HPLC and GC/MS analyses of low-molecular weight phenolics released by alkaline hydrolysis of DL fractions isolated from WT and BdPMT1-OE/WT lines.
Supplemental Figure S4 . Correlation between the amount of ester-linked pCA and the relative % of 4-vinylphenol released by analytical pyrolysis of BdPMT1-OE poplar trees.
Supplemental Figure S5 . Partial GC/MS chromatograms of the main dimers obtained after 1- or 4-h-long thioacidolysis followed by Raney nickel desulfuration from WT or BdPMT1-OE/WT lines.
Supplemental Table S1. Amount of pCA ester-linked to grass CWs and to the corresponding purified DL fractions.
Supplemental Table S2. Relative amount (% area) of the main dimers obtained after thioacidolysis and Raney nickel desulfuration of extract-free poplar stems.
Supplemental Table S3. Saccharification of extract-free poplar stems with no pretreatment.
Supplementary Material
Acknowledgments
We thank Armelle Delile, Orlane Touzet (INRAE Biofora) and Anita Rinfray (GBFOR, INRAE, Forest Genetics and Biomass Facility, https://doi.org/10.15454/1.5572308287502317E12) for their technical contribution to produce and characterize the plant material. Likewise, the technical assistance of Frédéric Legée for the determination of KL is gratefully acknowledged. The production of transgenic material has benefited from the LICA (Laboratoire d'Ingéniérie Cellulaire de l'Arbre) equipments.
Funding
The AtF5H-OE line was generated in the frame of the European ENERGYPOPLAR project (FP7-211917). The acquisition of the Py-GC/MS equipment was supported by the 3BCAR Carnot Institute.
Conflict of interest statement. The authors declare they have no conflict of interest.
C.L., R.S., and G.P. conceived the original research plans; G.P. and M.C.L. performed the plant transformation and production; C.L. performed the analyses of CW phenolics; C.L. and G.P. analyzed the data, and wrote the article with contributions of all the authors; R.S. and A.D. contributed to the research and complemented the writing; F.L. performed the image analyzes; G.P. agrees to serve as the author responsible for contact and ensures communication.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys/pages/General-Instructions) is: G. Pilate (gilles.pilate@inrae.fr).
References
- Beckmann E, Liesche O, Lehman F (1923) Qualitative und quantitative Unterschiede der Lignine einiger Holz- und Stroharten. Biochem Z 739:491–508 [Google Scholar]
- Bell-Lelong DA, Cusumano JC, Meyer K, Chapple C (1997) Cinnamate-4-hydroxylase expression in Arabidopsis (regulation in response to development and the environment). Plant Physiol 113:729–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerjan W, Ralph J (2019) Editorial overview: plant biotechnology - lignin engineering. Curr Opin Biotechnol 56:iii–v [DOI] [PubMed] [Google Scholar]
- Carvalho DMD, Queiroz JHd, Colodette JL (2016) Assessment of alkaline pretreatment for the production of bioethanol from eucalyptus, sugarcane bagasse and sugarcane straw. Ind Crops Prod 94:932–941 [Google Scholar]
- Chanoca A, de Vries L, Boerjan W (2019) Lignin engineering in forest trees. Front Plant Sci 10 [PMC][10.3389/fpls.2019.00912] [31404271] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazal R, Robert P, Durand S, Devaux MF, Saulnier L, Lapierre C, Guillon F (2014) Investigating lignin key features in maize lignocelluloses using infrared spectroscopy. Appl Spectrosc 68:1342–1347 [DOI] [PubMed] [Google Scholar]
- del Río J, Rencoret J, Gutiérrez A, Elder T, Kim H, Ralph J (2020) Lignin monomers from beyond the canonical monolignol biosynthetic pathway: another brick in the wall. ACS Sustain Chem Eng 8:4997–5012 [Google Scholar]
- Franke R, McMichael CM, Meyer K, Shirley AM, Cusumano JC, Chapple C (2000) Modified lignin in tobacco and poplar plants over-expressing the Arabidopsis gene encoding ferulate 5-hydroxylase. Plant J 22:223–234 [DOI] [PubMed] [Google Scholar]
- Grabber JH, Quideau S, Ralph J (1996) p-coumaroylated syringyl units in maize lignin: implications for beta-ether cleavage by thioacidolysis. Phytochemistry 43:1189–1194 [Google Scholar]
- Hai G, Jia Z, Xu W, Wang C, Cao S, Liu J, Cheng Y (2016) Characterization of the Populus PtrCesA4 promoter in transgenic Populus alba × P. glandulosa. Plant Cell Tissue Organ Cult 124:495–505 [Google Scholar]
- Halpin C (2019) Lignin engineering to improve saccharification and digestibility in grasses. Curr Opin Biotechnol 56:223–229 [DOI] [PubMed] [Google Scholar]
- Hatfield RD, Marita JM, Frost K, Grabber J, Ralph J, Lu FC, Kim H (2009) Grass lignin acylation: p-coumaroyl transferase activity and cell wall characteristics of C3 and C4 grasses. Planta 229:1253–1267 [DOI] [PubMed] [Google Scholar]
- Ho-Yue-Kuang S, Alvarado C, Antelme S, Bouchet B, Cézard L, Le Bris P, Legée F, Maia-Grondard A, Yoshinaga A, Saulnier L, et al. (2016) Mutation in Brachypodium caffeic acid O-methyltransferase 6 alters stem and grain lignins and improves straw saccharification without deteriorating grain quality. J Exp Bot 67:227–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T (1997) Structure and functions of feruloylated polysaccharides. Plant Sci 127:111–127 [Google Scholar]
- Karimi M, Bleys A, Vanderhaeghen R, Hilson P (2007) Building blocks for plant gene assembly. Plant Physiol 145:1183–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlen SD, Free HCA, Padmakshan D, Smith BG, Ralph J, Harris PJ (2018) Commelinid monocotyledon lignins are acylated by p-Coumarate. Plant Physiol 177:513–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Lee YY, Kim TH (2016) A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour Technol 199:42–48 [DOI] [PubMed] [Google Scholar]
- Kuroda K, Ozawa T, Ueno T (2001) Characterization of sago palm (Metroxylon sagu) lignin by analytical pyrolysis. J Agric Food Chem 49:1840–1847 [DOI] [PubMed] [Google Scholar]
- Kuroda Ki, Nishimura N, Izumi A, Dimmel DR (2002) Pyrolysis of lignin in the presence of tetramethylammonium hydroxide: a convenient method for S/G ratio determination. J Agric Food Chem 50:1022–1027 [DOI] [PubMed] [Google Scholar]
- Lapierre C (1993) Applications of new methods for the investigation of lignin structure. InJung HG, Buxton DR, Hatfield RD, Ralph J, eds, Forage Cell Wall Structure and Digestibility. American Society of Agronomy Inc., Madison, pp 133–163 [Google Scholar]
- Lapierre C (2010) Determining lignin structure by chemical degradations. InHeitner C, Dimmel D, Schmidt JA, eds, Lignin and Lignans – Advances in Chemistry. CRC Press – Taylor and Francis Group, Boca Raton, London, New-York, pp 11–48 [Google Scholar]
- Lapierre C, Jouin D, Monties B (1989) On the molecular origin of the alkali solubility of gramineae lignins. Phytochemistry 28:1401–1403 [Google Scholar]
- Lapierre C, Monties B, Rolando C (1988) Thioacidolyses of diazomethane-methylated pine compression wood and wheat straw in situ lignins. Holzforschung 42:409–411 [Google Scholar]
- Lapierre C, Pilate G, Pollet B, Mila I, Leple JC, Jouanin L, Kim H, Ralph J (2004) Signatures of cinnamyl alcohol dehydrogenase deficiency in poplar lignins. Phytochemistry 65:313–321 [DOI] [PubMed] [Google Scholar]
- Lapierre C, Pollet B, Petit-Conil M, Toval G, Romero J, Pilate G, Leple J-C, Boerjan W, Ferret V, De Nadai V, et al. (1999) Structural alterations of lignins in transgenic poplars with depressed cinnamyl alcohol dehydrogenase or caffeic acid O-methyltransferase activity have an opposite impact on the efficiency of industrial kraft pulping. Plant Physiol 119:153–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapierre C, Pollet B, Rolando C (1995) New insights into the molecular architecture of hardwood lignins by chemical degradative methods. Res Chem Intermed 21:397–412 [Google Scholar]
- Leplé JC, Brasileiro ACM, Michel MF, Delmotte F, Jouanin L (1992) Transgenic poplars: expression of chimeric genes using four different constructs. Plant Cell Rep 11:137–141 [DOI] [PubMed] [Google Scholar]
- Lu F, Ralph J (1999) Detection and determination of p-coumaroylated units in lignins. J Agric Food Chem 47:1988–1992 [DOI] [PubMed] [Google Scholar]
- Lu FC, Ralph J, Morreel K, Messens E, Boerjan W (2004) Preparation and relevance of a cross-coupling product between sinapyl alcohol and sinapyl p-hydroxybenzoate. Organic Biomol Chem 2:2888–2890 [DOI] [PubMed] [Google Scholar]
- Mahon EL, Mansfield SD (2019) Tailor-made trees: engineering lignin for ease of processing and tomorrow’s bioeconomy. Curr Opin Biotechnol 56:147–155 [DOI] [PubMed] [Google Scholar]
- Marita JM, Hatfield RD, Rancour DM, Frost KE (2014) Identification and suppression of the p-coumaroyl CoA:hydroxycinnamyl alcohol transferase in Zea mays L. Plant J 78:850–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méchin V, Laluc A, Legée F, Cézard L, Denoue D, Barrière Y, Lapierre C (2014) Impact of the brown-midrib bm5 mutation on maize lignins. J Agric Food Chem 62:5102–5107 [DOI] [PubMed] [Google Scholar]
- Moreno AD, Olsson L (2017) Pretreatment of lignocellulosic feedstocks. InSani RK, Krishnaraj RN, eds, Extremophilic Enzymatic Processing of Lignocellulosic Feedstocks to Bioenergy. Springer International Publishing, Cham, pp 31–52 [Google Scholar]
- Morreel K, Ralph J, Kim H, Lu FC, Goeminne G, Ralph S, Messens E, Boerjan W (2004) Profiling of oligolignols reveals monolignol coupling conditions in lignifying poplar xylem. Plant Physiol 136:3537–3549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottiar Y, Vanholme Ruben, Boerjan W, Ralph J, Mansfield SD (2016) Designer lignins: harnessing the plasticity of lignification. Curr Opin Biotechnol 37:190–200 [DOI] [PubMed] [Google Scholar]
- O'Connell A, Holt K, Piquemal J, Grima-Pettenati J, Boudet A, Pollet B, Lapierre C, Petit-Conil M, Schuch W, Halpin C (2002) Improved paper pulp from plants with suppressed cinnamoyl-CoA reductase or cinnamyl alcohol dehydrogenase. Transgenic Res 11:495–503 [DOI] [PubMed] [Google Scholar]
- Petrik DL, Karlen SD, Cass CL, Padmakshan D, Lu F, Liu S, Le Bris P, Antelme S, Santoro N, Wilkerson CG, et al. (2014) p-Coumaroyl-CoA:monolignol transferase (PMT) acts specifically in the lignin biosynthetic pathway in Brachypodium distachyon. Plant J 77:713–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilate G, Déjardin A, Leplé J-C (2012) Field trials with lignin modified transgenic trees. InJouanin L, Lapierre C, eds, Lignins: Biosynthesis, Biodegradation and Bioengineering, Vol 61. Academic Press/Elsevier, London, pp 1–36 [Google Scholar]
- Ralph J (2010) Hydroxycinnamates in lignification. Phytochem Rev 9:65–83 [Google Scholar]
- Ralph J, Hatfield RD (1991) Pyrolysis-GC-MS characterization of forage materials. J Agric Food Chem 39:1426–1437 [Google Scholar]
- Ralph J, Lapierre C, Boerjan W (2019) Lignin structure and its engineering. Curr Opin Biotechnol 56:240–249 [DOI] [PubMed] [Google Scholar]
- Ralph J, Lundquist K, Brunow G, Lu F, Kim H, Schatz PF, Marita JM, Hatfield RD, Ralph SA, Christensen JH, et al. (2004) Lignins: natural polymers from oxidative coupling of 4-hydroxyphenyl-propanoids. Phytochem Rev 3:29–60 [Google Scholar]
- Rezania S, Oryani B, Cho J, Talaiekhozani A, Sabbagh F, Hashemi B, Rupani PF, Mohammadi AA (2020) Different pretreatment technologies of lignocellulosic biomass for bioethanol production: an overview. Energy 199:117457 [Google Scholar]
- Scalbert A, Monties B, Guittet E, Lallemand JY (1986) Comparison of wheat straw lignin preparations. 1. CHEMICAL AND SPECTROSCOPIC CHARACTERIZATIONS. Holzforschung 40:119–127 [Google Scholar]
- Sibout R, Le Bris P, Legée F, Cézard L, Renault H, Lapierre C (2016) Structural redesigning Arabidopsis lignins into alkali-soluble lignins through the expression of p-coumaroyl-CoA: monolignol transferase PMT. Plant Physiol 170:1358–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DCC (1955) Ester groups in lignin. Nature 176:267–268 [Google Scholar]
- Smith RA, Gonzales-Vigil E, Karlen SD, Park J-Y, Lu F, Wilkerson CG, Samuels L, Ralph J, Mansfield SR (2015) Engineering monolignol p-coumarate conjugates into Poplar and Arabidopsis lignins. Plant Physiol 169:2992–3001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JJ, Akiyama T, Chapple C, Ralph J, Mansfield SD (2009) The effects on lignin structure of overexpression of ferulate 5-hydroxylase in hybrid Poplar1. Plant Physiol 150:621–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Sun S, Cao X, Sun R (2016) The role of pretreatment in improving the enzymatic hydrolysis of lignocellulosic materials. Bioresour Technol 199:49–58 [DOI] [PubMed] [Google Scholar]
- Takeda Y, Koshiba T, Tobimatsu Y, Suzuki S, Murakami S, Yamamura M, Rahman MM, Takano T, Hattori T, Sakamoto M, et al. (2017) Regulation of coniferaldehyde 5-hydroxylase expression to modulate cell wall lignin structure in rice. Planta 246:337–349 [DOI] [PubMed] [Google Scholar]
- Van Acker R, Dejardin A, Desmet S, Hoengenaert L, Vanholme R, Morreel K, Laurans F, Kim H, Santoro N, Foster C, et al. (2017) Different routes for conifer- and sinapaldehyde and higher saccharification upon deficiency in the dehydrogenase CAD1. Plant Physiol 175:1018–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venverloo CJ (1969) The lignin of Populus nigra L C.v. "Italica". A comparative study of the lignified structures in tissues cultures and the tissues of the tree. Acta Bot Neerlandica 18:241–314 [Google Scholar]
- Wang P, Dudareva N, Morgan JA, Chapple C (2015) Genetic manipulation of lignocellulosic biomass for bioenergy. Curr Opin Chem Biol 29:32–39 [DOI] [PubMed] [Google Scholar]
- Weng J-K, Li X, Stout J, Chapple C (2008) Independent origins of syringyl lignin in vascular plants. Proc Natl Acad Sci USA 105:7887–7892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withers S, Lu F, Kim H, Zhu Y, Ralph J, Wilkerson CG (2012) Identification of grass-specific enzyme that acylates monolignols with p-coumarate. J Biol Chem 287:8347–8355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Wyman CE (2008) Pretreatment: the key to unlocking low-cost cellulosic ethanol. Biofuels Bioprod Bioref 2:26–40 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.