Abstract
Grasslands dominate the terrestrial landscape, and grasses have evolved complex and elegant strategies to overcome abiotic stresses. The C4 grasses are particularly stress tolerant and thrive in tropical and dry temperate ecosystems. Growing evidence suggests that the presence of C4 photosynthesis alone is insufficient to account for drought resilience in grasses, pointing to other adaptations as contributing to tolerance traits. The majority of grasses from the Chloridoideae subfamily are tolerant to drought, salt, and desiccation, making this subfamily a hub of resilience. Here, we discuss the evolutionary innovations that make C4 grasses so resilient, with a particular emphasis on grasses from the Chloridoideae (chloridoid) and Panicoideae (panicoid) subfamilies. We propose that a baseline level of resilience in chloridoid ancestors allowed them to colonize harsh habitats, and these environments drove selective pressure that enabled the repeated evolution of abiotic stress tolerance traits. Furthermore, we suggest that a lack of evolutionary access to stressful environments is partially responsible for the relatively poor stress resilience of major C4 crops compared to their wild relatives. We propose that chloridoid crops and the subfamily more broadly represent an untapped reservoir for improving resilience to drought and other abiotic stresses in cereals.
Chloridoid grasses have evolved unique adaptations to adverse environments and represent an untapped reservoir for improving resilience to drought and other abiotic stresses in cereals.
Introduction
The earliest grasses emerged between 55 and 70 million years ago, and now dominate ecosystems covering 30%–40% of ice-free land (Linder and Ferguson, 1985; Jacobs et al., 1999; Kellogg, 2001; Blair et al., 2014). Grasslands typify environments that are too stressful to support trees. In the Arctic, grasses prevail north of the boundary where low temperatures and permafrost prevent tree growth (Scheffer et al., 2012), while in warmer regions, lower mean annual precipitation and/or frequent disturbances such as wildfires, favor open savannas over wooded ecosystems (Higgins et al., 2000; Sankaran et al., 2005). The ability of grasses to colonize these relatively harsh environments is enabled by a network of unique anatomical, physiological, and molecular adaptations that combat issues related to water, temperature, salinity, and excess light stresses (Linder et al., 2018). Much of the resilience in grasses has been attributed to the evolution of C4 photosynthesis (Osborne and Freckleton, 2009; Christin et al., 2013), an optimized carbon concentration mechanism that reduces photorespiration and improves water-use efficiency (WUE). Other adaptations such as low critical leaf water potential and a modified leaf anatomy also contribute to drought tolerance in grasses (Balsamo et al., 2006; Nunes et al., 2020). Most resilience traits are either conserved or widespread in the grass family. For instance, grasses share a unique stomatal structure, which is thought to be more efficient than the stomata of other plants (Stebbins and Shah, 1960; Franks and Farquhar, 2007; Chen et al., 2017; Nunes et al., 2020). Similarly, C4 photosynthesis is widespread in the grass family, representing ∼42% of all grass species (Osborne et al., 2014). Tolerance to abiotic stressors is evolutionarily labile in grasses, despite the prevalence of underlying traits that enable stress tolerance. Cold, salt, and desiccation tolerance are all thought to have evolved independently multiple times within grasses (Bennett et al., 2013; Gaff and Oliver, 2013; Schubert et al., 2019).
The majority of species in the grass family falls into two evolutionarily and phenotypically distinct clades, BOP (Bambusoideae, Oryzoideae, and Pooideae subfamilies) and PACMAD (Panicoideae, Arundinoideae, Chloridoideae, Micrairoideae, Aristidoideae, and Danthonioideae subfamilies), named for the subfamilies they contain (Figure 1; Grass Phylogeny Working Group II, 2012). Most species in the BOP clade are classified as cool-season grasses with distributions in temperate climates, where C3 outperforms C4 photosynthesis. Within BOP, the Bambusoideae and Oryzoideae subfamilies are generally native to warmer climates and include the agronomically important species bamboos (Bambusa sp.) and rice (Oryza sativa), respectively. Pooideae is the largest subfamily of grasses and includes the temperate cereals wheat (Triticum aestivum), barley (Hordeum vulgare), oat (Avena sativa), and rye (Secale cereale), as well as most pasture grasses. All BOP clade grasses utilize the C3 pathway of photosynthesis, and most independent origins of frost tolerance in grasses are found within the Pooideae (Schubert et al., 2019). Conversely, grasses in the PACMAD clade are mostly distributed in warm temperate and tropical regions. PACMAD contains all known origins of C4 photosynthesis in grasses, the majority of salt tolerance origins, and all but one origin of desiccation tolerance (Sage, 2004; Bennett et al., 2013; Marks et al., 2021). The agriculturally important PACMAD grasses belong to two subfamilies: sugarcane (Saccharum officinarum), maize (Zea mays), sorghum (Sorghum bicolor), and various millets are in Panicoideae and the under-resourced grain crops finger millet (Eleusine coracana) and teff (Eragrostis tef) are in the Chloridoideae. In this review, we focus on the PACMAD grasses and the evolution of the abiotic stress tolerance that empowered their dominance and diversification. We ask which factors fostered the evolution of stress tolerance in these grasses, and why all C4 PACMAD grasses are not drought-tolerant.
Figure 1.
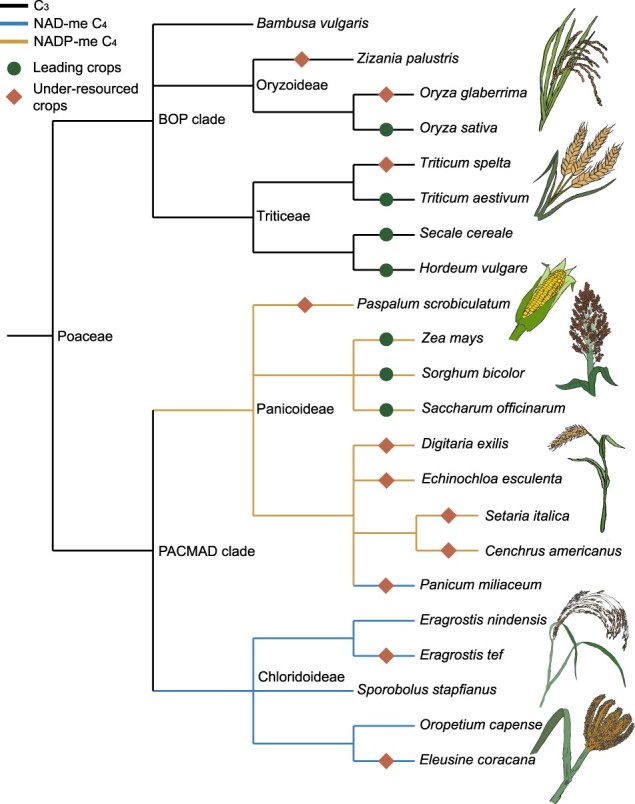
Phylogeny of agronomically important C3 and C4 grasses. The two major clades of grasses, the BOP and PACMAD are shown. Leading or underresourced crop species are highlighted by green circles and orange diamonds, respectively.
The Panicoideae and Chloridoideae have very different evolutionary histories that have shaped their respective aridity tolerance. In general, panicoid grasses are taller, more ecologically dominant, and less stress-tolerant than the shorter, more stress-resilient chloridoid grasses (Liu et al., 2012; Liu and Osborne, 2015). Panicoid grasses are also better represented among major crops, and of nine C4 grasses in the United Nations Food and Agriculture Organization global crop production database, only two, finger millet and teff, are chloridoid grasses (Sage and Zhu, 2011). The underrepresentation of chloridoid grasses among worldwide crops may be explained by the hypothesis of a tradeoff between yield and stress tolerance (Silva et al., 2019). If true, perhaps chloridoid grasses have underlying traits that simultaneously confer drought tolerance but also limit production, making them less suitable crop plants. Alternatively, the low number of domesticated chloridoid grasses may stem from their ecological underrepresentation in the main centers of origin for crop plants. Panicoid species are dominant in mesic environments that were most conducive to the development of agriculture, compared to the more arid regions where chloridoid grasses dominate (Liu and Osborne, 2015). If this is the case, chloridoid species may represent an untapped resource for developing the next generation of climate-resilient crops. Here, we highlight the resilience traits distinguishing panicoid and chloridoid grasses and discuss the potential of using chloridoid species to improve the climate resilience of agriculture.
What makes PACMAD grasses so resilient?
Grasses have evolved unique anatomical, physiological, molecular, and life history traits to thrive in poor or dynamic environments. Evolutionary innovations underlying resilience, such as modified stomata, C4 photosynthesis with Kranz anatomy, salt glands, and desiccation tolerance, arose independently in grasses (Sage, 2004; Grass Phylogeny Working Group II, 2012; Bennett et al., 2013; Gaff and Oliver, 2013). Other adaptations such as high WUE, improved leaf water potential under drought conditions, and deep fibrous root systems in grasses represent stepwise improvements on conserved mechanisms found in all plants. Some of these traits are conserved widely across grasses, but many are uniquely or more frequently found in the PACMAD clade. The C4 members of the PACMAD clade are especially drought-resilient compared to C3 members of the clade (Pau et al., 2013; Taylor et al., 2014).
Grasses with C4 photosynthesis cover ∼18% of vegetated land area, especially in tropical, arid, and semi-arid regions (Still et al., 2003). C4 grasses are also crucial for agriculture, with two C4 species (maize and sugarcane) leading all other plants in terms of global production. Water availability is thought to have been a major driving force of C4 grass evolution and diversification (Osborne and Sack, 2012). However, not all C4 grasses are from arid environments, and tolerance to drought varies widely across C4 grasses. For example, the so-called resurrection grasses, such as lovegrass (Eragrostis nindensis) and Oropetium capense, are able to equilibrate to low atmospheric moisture for months without dying, while other C4 species such as switchgrass (Panicum hemitomon) are semi-aquatic, requiring regular flooding to survive (Gaff, 1971; Kirkman and Sharitz, 1993). This raises the question: what factors enable drought tolerance in the C4 PACMAD grasses, if C4 photosynthesis per se is not the sole driver of stress tolerance?
The 19th century architect Louis Sullivan famously stated that “form ever follows function” (Tubbs, 2015). This saying has long been applied to biology to describe how structure and function are related. This principle applies particularly well to abiotic stress adaptation among PACMAD grasses, where their anatomy is intimately linked to their resilience. Stomatal anatomy is one example of an anatomical trait conferring resilience across all grasses. Grasses have a unique stomatal structure with elongated dumbbell-shaped guard cells and two subsidiary cells (Stebbins and Shah, 1960; Figure 2). This morphology allows faster stomatal responses than those of the kidney-shaped guard cells of eudicots and most nongrass monocots, resulting in higher WUE (McAusland et al., 2016; Lawson and Vialet-Chabrand, 2019). In addition to the structure of guard cells, the arrangement and density of stomatal pores is another important factor in determining drought tolerance. The majority of grasses have either hypostomatic leaves, where the stomatal pores are primarily on the abaxial leaf surface, or amphistomatic leaves, where the pores are roughly equally distributed between the adaxial and abaxial surfaces. Amphistomatic leaves allow for more efficient CO2 diffusion into the leaf and therefore greater maximum photosynthetic rates (Hardy et al., 1995). In eudicots with dorsoventral leaf anatomy, leaves are often held perpendicular to the axis of irradiance and amphistomaty comes at the cost of greater evapotranspiration. However, grasses have isobilateral leaves that are often held parallel to the axis of irradiance. The deeper placement of veins in isobilateral leaves and the more vertical leaf angle of these grasses overcome the WUE cost of amphistomatic leaves (Drake et al., 2019). Among grasses, amphistomatic leaves are more prevalent among C4 species, particularly among those adapted to arid environments with high irradiance (Mott et al., 1982; Drake et al., 2019).
Figure 2.
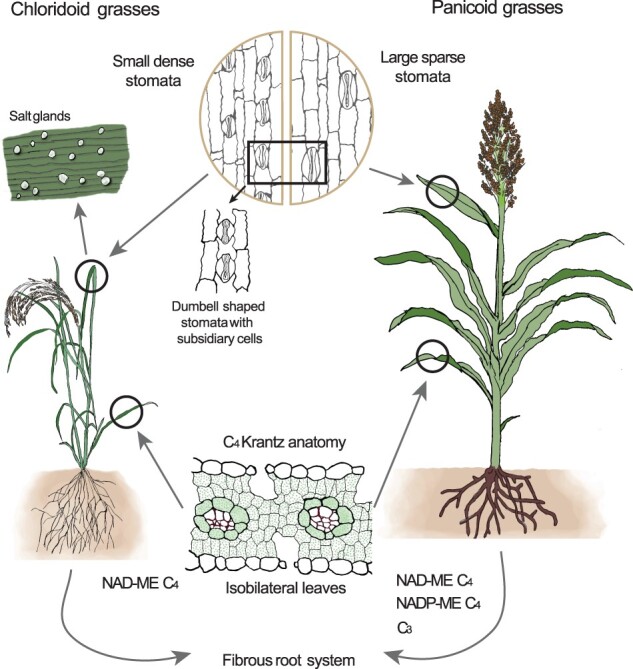
Evolutionary innovations contributing to stress tolerance in C4 grasses. Several shared and unique adaptations in chloridoid grasses (left) and panicoid grasses (right) are shown.
Contribution of the “C4 syndrome” to water deficit stress tolerance
C4 photosynthesis is a central trait that has enabled PACMAD grasses to survive arid environments. At its heart, C4 photosynthesis is a carbon concentrating mechanism; however, it is not exclusively a biochemical trait, as a modified leaf anatomy is needed for C4 to operate efficiently. Thus, C4 photosynthesis has been labeled as a “syndrome” of both anatomical and biochemical traits (Laetsch, 1974). In 1884, the botanist Gotleib Haberlandt described the “Kranz” anatomy of certain plants, whereby a ring of large bundle sheath cells around the vascular bundles containing many chloroplasts is surrounded by a second, sparser, ring of smaller mesophyll cells (Figure 2) (Haberlandt, 1884; Lundgren et al., 2014). This anatomy was later associated with C4 photosynthesis, and the majority of C4 species, including grasses, has the Kranz-type leaf anatomy (El‐Sharkawy and Hesketh, 1965; Edwards et al., 2004; Lundgren et al., 2014). While primarily thought of as supporting C4 biochemistry, Kranz anatomy also influences WUE and drought tolerance. Indeed, the large bundle sheath cells increase the hydraulic capacitance of the leaf, which may help buffer against the sudden increases in evapotranspiration that are common in open environments (Sage, 2001). C4 species with Kranz type anatomy have shorter interveinal distances than in C3 species (Sage, 2001; Griffiths et al., 2013), and an increased vein density facilitates C4 biochemistry by minimizing the diffusion distance between mesophyll and bundle sheath cells. Shorter interveinal distances also result in higher leaf hydraulic conductance. In C3 species, leaf hydraulic conductance is positively correlated with maximum CO2 assimilation rates (Brodribb et al., 2007), which is thought to result from higher stomatal and mesophyll conductance of CO2 in plants with higher leaf hydraulic conductance. However, under dry conditions (high vapor pressure deficit), increased leaf hydraulic conductance results in lower WUE (Sinclair et al., 2008; Rzigui et al., 2018). Thus, C3 species experience a tradeoff between carbon gain and WUE. However, in C4 species, net assimilation is decoupled from hydraulic conductance (Ocheltree et al., 2016). C4 biochemistry can tolerate reduced stomatal conductance, which conserves water. Furthermore, C4 species adapted to dryer environments have greater mesophyll conductance and lower hydraulic conductance as compared to C4 species from wet environments (Pathare et al., 2020). Therefore, these species can increase hydraulic safety while maintaining high CO2 assimilation rates.
The anatomical traits that enable C4 biochemistry are thought to predate the evolution of the biochemical carbon concentrating mechanism, which has evolved at least 22 times independently within the grass family (Grass Phylogeny Working Group II, 2012). In the C3 ancestors of these modern C4 lineages, higher hydraulic conductance likely came at the cost of WUE. However, after the carbon concentrating mechanism arose, there has consistently been selection for lower leaf hydraulic conductance while maintaining maximum CO2 assimilation rate within C4 grasses (Zhou et al., 2020). Thus, lineages where C4 arose earlier, and those with faster evolutionary rates, tend to have lower leaf hydraulic conductance and higher WUE (Zhou et al., 2020). The Chloridoideae subfamily likely contains the oldest origin of C4 photosynthesis and, unlike the Panicoideae subfamily, the ancestor of core chloridoid grasses was likely C4 (Christin et al., 2008). Thus, chloridoid lineages have had the greatest amount of time since the introduction of C4 biochemistry, and thus the most time to respond to the strong selective pressure favoring reduced hydraulic conductance. Consistent with this phylogenetic history, modern chloridoid grasses generally have lower leaf hydraulic conductance than panicoid grasses (Liu and Osborne, 2015).
Relation between the C4 biochemical pathway and WUE
The C4 pathway optimizes WUE, and C4 grasses tend to occupy drier and more exposed habitats than their C3 relatives (Edwards and Still, 2008). In C4 photosynthesis, phosphoenolpyruvate carboxylase (PEPcase) catalyzes the reaction fixing inorganic bicarbonate (HCO3), which is in equilibrium with CO2, into organic acids. These organic acids are then transported to bundle sheath cells where they are decarboxylated, raising the bundle sheath CO2 concentration and allowing Rubisco to operate more efficiently (Kellogg, 2013). The higher affinity of PEPcase for its substrate HCO3 compared to that of Rubsico for CO2, along with the C4 carbon concentrating mechanism more generally, allows C4 plants to operate at lower mesophyll CO2 concentrations than C3 plants. Consequently, they are able to maintain lower stomatal conductance, resulting in higher instantaneous WUE (Ghannoum et al., 2011). Under drought stress, leaf-level WUE often increases, as water savings from stomatal closure are greater than the reduction in CO2 assimilation due to inhibition of photosynthesis.
C4 grasses are classified into three distinct subtypes based on their biochemistry: NADP-dependent malic enzyme (NADP-me), NAD-dependent malic enzyme (NAD-me), and phosphoenolpyruvate carboxykinase (PCK). In NADP-me plants, malate is the primary C4 acid being transported between mesophyll and bundle sheath cells, while aspartate is the primary transported acid in NAD-me and PCK C4 grasses. WUE is correlated with the C4 subtype, with NAD-me grasses having higher WUE under drought stress than NADP-me grasses (Ghannoum et al., 2002). NAD-me C4 grasses are more abundant in arid regions, while NADP-me species tend to inhabit more mesic environments (Taub, 2000; Liu and Osborne, 2015). There is only a weak correlation between the distribution of PCK-based C4 grasses and precipitation gradients (Ghannoum, 2009). The PCK pathway is thought to be an addition to both the NAD-me and NADP-me pathways and is found at relatively equal frequency across both panicoid and chloridoid grasses. In contrast, the distribution of NADP-me and NAD-me C4 pathways mostly follows phylogenetic lineages. All species within the Chloridoideae subfamily are either of the NAD-me or PCK subtypes. The NAD-me subtype is thought to be the ancestral state of chloridoid grasses, from which PCK grasses arose. Panicoid grasses are mostly NADP-me with a minority of NAD-me and PCK species. These factors make it difficult to separate the influence of phylogeny and selective pressures on their biochemistry.
Distinguishing features underlying stress tolerance in chloridoid grasses
Many of the most stress-tolerant grasses belong to the PACMAD clade, but this resilience is not uniform, and substantial variation exists between and within these clades. The Chloridoideae subfamily is arguably the most stress tolerant subfamily of PACMADs, and dominates in arid and resource-poor subtropical and tropical deserts that are inhospitable to most grasses (Clayton and Renvoize, 1986). The degree of tolerance in chloridoid grasses is linked to their C4 subtype, as taxa with the NAD-me subtype thrive in hot and dry climates whereas PCK taxa are more common in mesic habitats. NAD-me chloridoids have an additional column of cells between the vascular bundles that is missing from PCK species and promotes leaf rolling, thus limiting transpirational water loss (Peterson et al., 2007). This anatomical adaptation facilitates tighter leaf rolling in NAD-me chloridoid grasses compared to other C4 grasses (Liu and Osborne, 2015). The majority of chloridoid species are classified as the NAD-me photosynthetic subtype as are some of the most resilient panicoid grasses, raising the question as to whether biochemical subtype or phylogeny is a more important predictor of resilience. Habitat aridity is correlated with subfamily in C4 grasses, with chloridoid species occupying drier niches. However, other factors such as a preference for open habitats and shorter stature in chloridoid compared to panicoid species are also correlated with phylogeny and contribute to the overall habitat preference of chloridoid grasses for dry open environments (Liu et al., 2012). At the leaf level, anatomical traits are also strongly correlated with phylogeny; chloridoid species have higher specific leaf area, higher stomatal density, and smaller stomata than panicoid species. However, physiological traits such as leaf water potential under ambient and saturating conditions are more strongly influenced by photosynthetic subtype than phylogeny (Liu and Osborne, 2015).
It is difficult to separate the effects of phylogeny and photosynthetic subtype on habitat preference, as the two are intertwined. For example, there is a significant interaction between phylogeny and the photosynthetic subtype for certain leaf hydraulic traits (Liu and Osborne, 2015). Turgor loss point is the leaf water potential at which wilting occurs and is a function of both leaf osmotic potential and tissue flexibility (Cosgrove, 1988; Bartlett et al., 2012). Surprisingly, NAD-me species in both the chloridoid and panicoid lineages have less negative turgor loss points than either PCK or NADP-me species. However, PCK chloridoid species have more negative turgor loss points than panicoid PCK species (Liu and Osborne, 2015). A more negative osmotic potential under saturating conditions is correlated with greater osmotic adjustment under drought stress, allowing plants to maintain turgor at lower leaf water potentials. Leaf flexibility, as measured by bulk modulus of elasticity (ε), is the ratio of change in cell turgor divided by the change in relative cell volume (Steudle and Zimmermann, 1977; Touchette et al., 2014). A higher ε value indicates more rigid cells and theoretically would result in a more negative turgor loss point. However, in a meta-analysis of 372 species, osmotic potential at saturation was shown to be the primary driver of turgor loss point, not bulk modulus of elasticity (Bartlett et al., 2012). Plants with more flexible cells (lower ε) are able to maintain lower relative water content at the turgor loss point and contribute to a greater capacity to maintain leaf integrity under adverse osmotic conditions (Bartlett et al., 2012; Liu and Osborne, 2015). Likewise, chloridoid PCK species were shown to have more negative saturated osmotic potential and higher ε, while chloridoid NAD-me species had less negative osmotic potential and lower ε (Liu and Osborne, 2015), possibly a result of different drought response strategies, with PCK chloridoid species exhibiting tolerance through osmotic adjustment while NAD-me species employ an avoidance strategy through a higher capacity to buffer against adverse osmotic conditions (Liu and Osborne, 2015). Given that NAD-me chloridoid species tend to occur in drier habitats than PCK species, it is unexpected that they would also be less able to tolerate drought stress at a physiologically relevant level and instead employ strategies to avoid water stress. One explanation is that the prevalence of NAD-me chloridoids in dry habitats is driven not by their inherent stress tolerance, but by another feature that afforded chloridoids the ecological opportunity to radiate into dry environments. Ancestral state reconstruction indicated that the C3 ancestor of the Chloridoideae subfamily likely lived in dry areas (Osborne and Freckleton, 2009). The paleontologist Gaylord Simpson originally proposed the idea that “evolutionary access to ecological opportunity” may drive adaptive radiation (Simpson, 1953; Edwards and Donoghue, 2013; Stroud and Losos, 2016). In the case of the NAD-me chloridioids, perhaps features such as their preference for high-irradiance, open environments, gave these early chloridoid species evolutionary access to dry environments. Subsequent adaptations to their primarily arid environment then led to the resilience observed in this group today.
The resilience of the chloridoid subfamily is not limited to ordinary drought tolerance. Chloridoid grasses are also well represented among halophytes and desiccation-tolerant species. Drought and high salinity often co-occur, and both can cause osmotic stress in plants. Thus, cross-tolerance is common. Salinity tolerance is widely distributed across the grass phylogeny and is thought to have arisen independently over 70 times (Bennett et al., 2013). Most origins of salinity tolerance in the grass family are relatively recent, resulting in numerous small clades of halophyte grasses. However, the Chloridoideae subfamily is the exception, and likely contains ancient origins of salt tolerance (Bennett et al., 2013). Is it possible that drought tolerance in the Chloridoideae evolved through a common mechanism with salt tolerance or that one trait enabled the evolution of the other? Salinity tolerance is more prevalent among C4 lineages as compared to C3 lineages within the PACMAD clade (Bromham and Bennett, 2014). The correlation between C4 and salinity tolerance within PACMAD grasses has both physiological and evolutionary explanations. Salt tolerance is conferred through both ion exclusion and osmotic adjustment. Grasses with the C4 pathway are in general more efficient in their water use than their C3 counterparts, translating into the uptake of fewer ions per fixed carbon. However, many chloridoid grasses adapted to saline environments take up sodium ions but then excrete them through specialized salt glands. Salt glands are seemingly unrelated to water-deficit stress caused by drought, while osmotic adjustment is an important response to water-deficit. While all chloridoid species accumulate compatible solutes when grown in saline conditions, the primary salt tolerance mechanism is thought to be excretion through bicellular salt glands (Marcum and Murdoch, 1994; Marcum, 1999). Thus, cross tolerance alone is likely insufficient to explain the prevalence of both drought and salt tolerance within the chloridoid subfamily. Alternatively, salt tolerance, the C4 pathway, and drought tolerance may be correlated traits because dry environments and saline environments often co-occur. Therefore, species living in these environments face selective pressures that make all three traits adaptive (Bennett et al., 2013). Consequently, chloridoid grasses may have evolved these traits because they had the evolutionary access to overcome a selective pressure.
The idea that evolutionary access drives the prevalence of stress tolerance traits in Chloridoideae may explain the likely multiple independent origins of desiccation tolerance in this subfamily (Gaff and Oliver, 2013; Pardo et al., 2020). Desiccation tolerance is the ability of vegetative tissue to survive drying, often defined as equilibration with 50% relative humidity air or drying to 10% absolute water content, without dying (Bewley, 1979; Alpert, 2005). Vegetative desiccation tolerance relies on a combination of anatomical, biochemical, and molecular adaptations (Vander Willigen et al., 2001; Costa et al., 2017; VanBuren et al., 2017). Studies examining gene expression of vegetative tissues in desiccation-tolerant species repeatedly find high expression of genes normally expressed during seed maturation and dehydration (Mitra et al., 2013; Costa et al., 2017; VanBuren et al., 2017). It is often hypothesized that the repurposing of seed desiccation pathways for vegetative tissues drove the evolution of desiccation tolerance (Oliver et al., 2000; VanBuren, 2017). However, the transcriptional network responsible for coordinating the seed dehydration response is not activated in the leaves of the desiccation-tolerant monocot Xerophyta humilis (Lyall et al., 2019). Furthermore, we previously found that across five grass species, more components of the seed dehydration pathway are expressed in leaves of all species under severe drought stress, irrespective of their desiccation tolerance or susceptibility (Pardo et al., 2020). The overlap between desiccation-sensitive and -tolerant species suggests that underlying conserved drought responses allowed the subsequent evolution of desiccation tolerance. Vegetative desiccation tolerance is an uncommon trait among grasses, with only nine genera within Poaceae containing desiccation-tolerant species (Gaff and Oliver, 2013). However, most of these desiccation-tolerant genera (seven) are found within the Chloridoideae subfamily (Marks et al., 2021). This clumped distribution of desiccation tolerance across the grass family may indicate the predisposition of chloridoid grasses to evolve this trait. However, the superior drought tolerance of chloridoid grasses may have also enabled the evolution of desiccation tolerance in this group by allowing chloridoid ancestors to grow in environments where desiccation tolerance is adaptive. The ancestors of desiccation-tolerant chloridoid grasses had the evolutionary access to the selective pressure that made vegetative desiccation tolerance an adaptive trait. Desiccation-tolerant species from only distantly related lineages often co-occur in rocky, dry areas and are even the dominant flora in these specialized habitats (Conceição et al., 2007; Alcantara et al., 2015). In addition to a lack of moisture, these rocky dry areas are also open, exposing plants to high irradiance. This is perhaps also key to the evolution of desiccation tolerance, as photoprotective mechanisms are thought to play a major role in desiccation tolerance (Huang et al., 2012; Verhoeven et al., 2018; VanBuren et al., 2019). Given that desiccation-tolerant grasses are rare outside these conducive environments, it is likely that the trait is only adaptive under a particular set of environmental conditions. Thus, at a minimum, access to those environments is likely necessary, if not sufficient, to afford the opportunity to evolve desiccation tolerance. Chloridoid grasses radiated in open, high-light, dry environments, and high light is an important component of their ecological niche (Osborne and Freckleton, 2009; Liu et al., 2012). Other lineages of desiccation-sensitive PACMAD grasses cohabitate regions with tolerant Chloridoid species, but they may lack the prerequisite traits to evolve desiccation tolerance. Adaptation to high light and arid environments possibly drove the evolution of these enabling traits, which then allowed for the subsequent repeated evolution of desiccation tolerance in Chloridoideae. More broadly, once a species is established in a particular environment, it is subjected to selective pressures, which then drive adaptations to the conditions prevalent in that environment. C4 grasses, and particularly the Chloridoideae subfamily, diversified in dry, open, and sometimes salty environments. They therefore evolved traits to cope with these pressures, resulting in a reservoir of resilience within this group of grasses.
Is resilience a roadblock for domestication in grasses, or a source of untapped genetic potential?
Water deficit is the greatest abiotic threat to global food production. A single drought event reduces the gross agricultural production of a nation by an average of 0.8%, according to global data collected between 1983 and 2009 (Kim et al., 2019). The prevalence and severity of drought events are forecasted to increase in many agricultural areas over the next century, and drought-associated losses will be amplified under the changing climate (Dai, 2011). The evolution and diversification of C4 lineages was driven largely by exposure to arid environments, and C4 cereals can thrive in hot, dry conditions that are too extreme for other cereals and staple crops (Osborne and Sack, 2012). Thus, C4 cereals should be a more central component of a stable and resilient food system under a changing climate.
The yield per hectare of C4 cereals and biomass grasses far exceeds that of most other crops, yet despite their relative efficiency and resiliency, this level of productivity still requires a substantial amount of water. C4 staples of the global food system such as maize and sugarcane are among the most water-intensive crops. High-yielding commercial maize hybrids require ∼500–750 mm of precipitation over the course of the growing season, with a peak water use of ∼7.5 mm per day (Kranz et al., 2008). To meet their water requirements, dryland maize requires a minimum of ∼600 mm of precipitation over the growing season. Sugarcane requires 1,200–2,700 mm of water over its 11- to 18-month growing season, with a peak daily water use of ∼6 mm per day. The extensive water requirements of sugarcane limit its production to areas with greater than 1,000–1,200 mm of annual precipitation (Yates and Taylor, 1986). WUE for staple C4 crops such as maize is high despite their high absolute water requirements. However, the maximum WUE requires substantial water input and WUE drops substantially in environments with less water (Fang et al., 2017). High precipitation or irrigation requirements are not universal across C4 grasses, and other less widely grown C4 cereals such as the chloridoids teff and finger millet and the panicoids proso millet (Panicum miliaceum) and fonio millet (Digitaria exilis) use far less water (Table 1). Teff is grown primarily in the arid highlands and lowlands of Ethiopia and Eritrea and requires only ∼300 mm of water during the growing season. Similarly, proso millet is regarded as having the lowest water requirement of any grain crop, using just 200–300 mm. Collectively, multiple grain species categorized as millets constitute an important global crop; however, the total production of all millet species is still far short of that from maize (Habiyaremye et al., 2016). Given the limitation that drought imposes on agricultural yields, it is surprising that less water stress-tolerant crop species dominate in terms of acreage planted. One possible explanation is a tradeoff between stress tolerance and growth. Such a tradeoff has been hypothesized to account for the generally slow growth of desiccation-tolerant species (Alpert, 2005, 2006). If a tradeoff between yield and stress tolerance exists, perhaps the most productive C4 cereal crops are inevitably less stress-tolerant than lower yielding but more resilient C4 species.
Table 1.
Comparison of C4 crop water use and yield. Global average and Least Developed Countries yield data (Tonnes per Hectare) are adapted from the FAOstat database for 2019 crop yields (Food and Agriculture Organization of the United Nations, 2019). The minimum and maximum yield range (Tonnes per Hectare), growing season water requirements (mm), as well as the growing season length for each crop are adapted from the Useful Tropical Plants Database (Fern and Fern, 2014)
| Crop | Scientific Name | Water Requirement (mm) | Growing Season Length (months) | Global Average Yield (T·H−1) | Least Developed Countries Yield (T·H−1) | Minimum Yield Range (T·H−1) | Maximum Yield Range (T·H−1) |
|---|---|---|---|---|---|---|---|
| Maize | Zea mays | 500–750 | 4–5 | 5.8 | 1.95 | 1 | 20 |
| Sugarcane | Saccharum officinarum | 1,200–2,700 | 11–18 | 72.8 | 57.74 | 50 | 150 |
| Sorghum | Sorghum bicolor | 450–650 | 3–4 | 1.45 | 0.89 | 2 | 6 |
| Teff | Eragrostis tef | 300 | 2–5 | 0.89a | 0.67a | 0.2 | 4.5 |
| Finger millet | Eleusine coracana | 350 | 3–6 | 0.89a | 0.67a | 0.25 | 5 |
| Proso millet | Panicum miliaceum | 200–300 | 2–3 | 0.89a | 0.67a | 0.45 | 2 |
| Pearl millet | Cenchrus americanus | 350 | 2–3 | 0.89a | 0.67a | 0.25 | 8 |
| Fonio | Digitaria exilis | 250–350 | 2–3 | 0.76 | 0.81 | 0.6 | 1 |
All millets including pearl millet, proso millet, finger millet, and teff are grouped together in the FAOSTAT Database.
Drought resilience does not always negatively correlate with yield. For example, yield comparisons of the C4 panicoid crops maize, sorghum, and pearl millet (Cenchrus americanus) in a semi-arid environment revealed that maize is the highest yielding crop, followed by sorghum, with pearl millet having the lowest yield (Muchow, 1989), despite the fact that sorghum and pearl millet are more drought-resistant than maize. However, in drier environments where maize yields dropped below 6.4 metric tons per hectare, sorghum was more productive (Staggenborg et al., 2008). Cross-species comparisons are suggestive of a tradeoff between yield and stress tolerance, whereby the higher yielding species outperform the more stress-tolerant cereals in all but the most stressful environments. However, within-species analysis suggests otherwise. An examination of the commercial “drought-tolerant” maize hybrids from three major seed companies found that the drought-tolerant lines outperformed drought-susceptible cultivars in dry environments with no yield penalty under adequate moisture (Adee et al., 2016). Similarly, adoption of drought-tolerant maize lines developed by the International Maize and Wheat Improvement Center (CIMMYT) increased maize yield in Uganda by 15% (Simtowe et al., 2019). In sorghum, the stay-green phenotype, which confers resistance to senescence under terminal drought, is associated with increased yield under dry conditions but has a minimal to no yield penalty under adequate moisture (Sabadin et al., 2012).
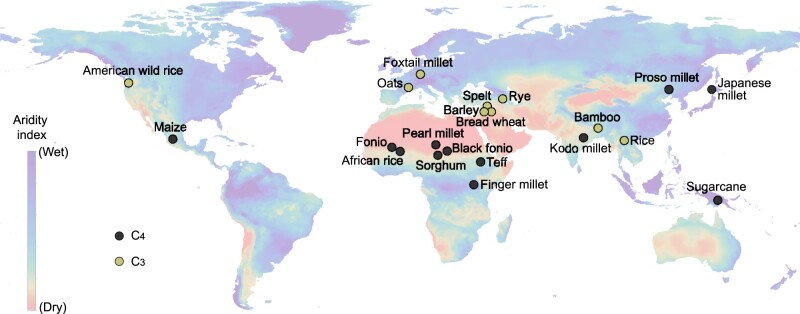
If there is not necessarily a tradeoff between yield and drought stress resilience, what other factors might explain the relative lack of water-stress tolerance among C4 global staples? In the case of maize and sorghum, their respective domestication histories may explain their differences in drought resilience. Maize was domesticated in the central Balsas valley in what is now southwest Mexico (Figure 3). Today, this region receives ∼1,200 mm of rainfall annually, 80% of which falls during the wet season from June to October (Piperno et al., 2007). By contrast, sorghum was domesticated in the Kassala region of Sudan (Fuller and Stevens, 2018). This region receives only 100–400 mm of precipitation annually. Thus, the wild progenitor of sorghum was selected in a much drier environment than the maize progenitor. While the origin of divergent drought tolerance levels in maize and sorghum may be ancient, the differences in yield between the two species are actually rather recent developments. In the USA, for example, maize and sorghum yields were very similar until 1960 (Staggenborg et al., 2008). Since that time, maize yields have increased rapidly relative to those of sorghum. This yield increase might be attributed to greater funding and efforts focused on maize improvement rather than a tradeoff resulting from their diverging stress tolerance. However, even among the generally more drought-tolerant species such as sorghum and the C4 millet species, water availability often limits production. For example, despite its low water requirement, proso millet frequently experiences yield losses from drought due to its shallow root system (Habiyaremye et al., 2016). Alternatively, perhaps major agricultural crops are less stress-tolerant because early farmers lived in more mesic environments, and thus domesticated less stress-tolerant plants from the local flora. The emergence of agricultural societies is linked to domestication centers rich in species with abundant resources such as the Fertile Crescent in the Middle East (Figure 3; Harlan and Others, 1992; Lev-Yadun et al., 2000). In Western Africa, yam (Dioscorea sp.), African rice (Oryza glaberrima), pearl millet, and cowpea (Vigna unguiculata) were domesticated around the Niger River, likely in the early Holocene when the “green Sahara” slowly desertified (Hély et al., 2009; Scarcelli et al., 2019). Proso millet was domesticated in Neolithic China ∼10,000 years ago and is the earliest dry farming crop in East Asia. Proso millet was historically grown in the dryer interior regions of China, which receive 350–450 mm of water annually compared to the later domesticated Foxtail millet that dominated the wetter eastern areas of China with an average of 450–550 mm water per year (Lu et al., 2009). It is possible that C4 crops domesticated in drier areas experienced stronger selection for drought tolerance over yield. Consequently, the more resilient C4 crops were possibly not selected as intensely for yield.
Figure 3.
Domestication and origin of major C3 and C4 crops and cereals. The putative centers of origin for major domesticated grasses are shown with C4 species highlighted in black and C3 species highlighted in yellow. The aridity index is overlaid; blue regions are the least arid and orange regions the most arid. Data for the crop origins were adapted from (Milla, 2020).
The traits that shaped the domestication of millets are different from the key innovations that characterize C3 and C4 cereals. Most millets were domesticated in semi-arid regions of Africa and India, where selection favored stable and reliable yields in drought-plagued and low rainfall areas (Figure 3; Doggett, 1989). Finger millet was domesticated in the dry highlands of Ethiopia and Uganda, with a second center of diversity in the Himalayas of Nepal and India (Hilu and De Wet, 1976; Hilu et al., 1979). Teff was domesticated in similarly arid regions of the Ethiopian highlands (Costanza et al., 1979; D’Andrea, 2008). Fonio (D. exilis) and iburu (D. iburua) were domesticated in the central delta and Jos plateau of Nigeria, respectively, and drought-tolerant iburu is intercropped with fonio as a fail-safe under low rainfall (De Wet, 1986). Millets are often referred to as ‘orphan crops’ because their yield is considerably lower than leading cereals, and they have undergone less intensive breeding and selection than other cereal crops. This term is somewhat of a misnomer; however, as most millets underwent intensive selection during early domestication, but the target traits are not normally associated with high-yielding cereals. Millets like teff and fonio have small grains, are susceptible to shattering and lodging, and are generally low-yielding, but they produce dependable yields under arid and poor conditions that are unsuitable for other cereals (Jideani and Akingbala, 1993; Tadesse, 1993). Teff and fonio are fast-maturing, and teff is often used as a “rescue crop” for a late season harvest after another crop fails due to drought (Tefera et al., 2001). The fast maturation of millets may come as a tradeoff, as a shorter vegetative stage means less net assimilation across the growing season, and ultimately lower crop yields. Teff is still morphologically similar to its wild progenitor Eragrostis pilosa, with overlapping ranges in plant architecture and seed size, but larger and more numerous panicles (Ingram and Doyle, 2003). The natural stress resilience observed in E. pilosa has been maintained throughout domestication and selection in teff, presumably in parallel with modest gains in yield. This suggests that resilience is not a roadblock in grasses and that cereals can be selected for higher yields while maintaining stress tolerance.
Researchers and businesses have expended considerable effort to improve resilience of major crops such as maize. For example, 20% of US corn belt acres are now planted with drought-tolerant maize hybrids (McFadden et al., 2019; Messina et al., 2020). The focus on improving drought tolerance in maize has come at the expense of the production of more drought-tolerant cereals such as sorghum (Bhagavatula et al., 2013; Mundia et al., 2019). Despite the improvements in resilience of major crops, naturally resilient cereals maintain a greater degree of stress tolerance than drought-tolerant maize. These cereals can be used to reclaim semi-arid or resource-poor land that is typically not suitable for agriculture. They also represent an opportunity to improve the resilience of agriculture more generally. Chloridoid grasses dominate in stressful environments, providing them evolutionary access to the ecological conditions necessary to evolve stress adaptations. The crop plants derived from this group share many of those adaptations, providing a strong base of resilience within these species. Conventional breeding is constrained by the available pool of genetic variation for drought tolerance traits within a given species. Efforts to improve the resilience of major crops such as maize are consequently also constrained by genetic variation, and biotech-based approaches are needed to exceed the natural tolerance found within existing germplasm. Conversely, a renewed focus on improvement of agronomic traits in naturally stress-tolerant cereals may lead to the development of crops that are simultaneously productive and resilient. Thus, we propose that more research focus is warranted on stress-tolerant cereals generally and the chloridoid subfamily in particular.
Funding
This work was supported by NSF Grant MCB‐1817347 (to R.V.) and by predoctoral training award T32-GM110523 from the National Institute of General Medical Sciences of the NIH (to J.P.).
Conflict of interest statement. None declared.
Senior author.
J.P. and R.V. designed and conceived the review topics and wrote the paper.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plcell) is: Robert VanBuren (bobvanburen@gmail.com).
References
- Adee E, Roozeboom K, Balboa GR, Schlegel A, Ciampitti IA (2016) Drought-tolerant corn hybrids yield more in drought-stressed environments with no penalty in non-stressed environments. Front Plant Sci 7: 1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcantara S, Mello‐Silva R, Teodoro GS, Drequeceler K, Ackerly DD, Oliveira RS (2015) Carbon assimilation and habitat segregation in resurrection plants: a comparison between desiccation‐ and non‐desiccation‐tolerant species of Neotropical Velloziaceae (Pandanales). Funct Ecol 29: 1499–1512 [Google Scholar]
- Alpert P (2006) Constraints of tolerance: why are desiccation-tolerant organisms so small or rare? J Exp Biol 209: 1575–1584 [DOI] [PubMed] [Google Scholar]
- Alpert P (2005) The limits and frontiers of desiccation-tolerant life. Integr Comp Biol 45: 685–695 [DOI] [PubMed] [Google Scholar]
- Balsamo RA, Willigen CV, Bauer AM, Farrant J (2006) Drought tolerance of selected Eragrostis species correlates with leaf tensile properties. Ann Bot 97: 985–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett MK, Scoffoni C, Sack L (2012) The determinants of leaf turgor loss point and prediction of drought tolerance of species and biomes: a global meta-analysis. Ecol Lett 15: 393–405 [DOI] [PubMed] [Google Scholar]
- Bennett TH, Flowers TJ, Bromham L (2013) Repeated evolution of salt-tolerance in grasses. Biol Lett 9: 20130029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley JD (1979) Physiological aspects of desiccation tolerance. Annu Rev Plant Physiol 30: 195–238 [Google Scholar]
- Bhagavatula S, Rao PP, Basavaraj G, Nagaraj N (2013) Sorghum and Millet Economies in Asia – Facts, Trends and Outlook. International Crops Research Institute for the Semi-Arid Tropics, Patancheru, India
- Blair J, Nippert J, Briggs J (2014) Grassland ecology. InMonson R, ed, Ecology and the Environment. Springer, New York, 2014, pp 389–423 [Google Scholar]
- Brodribb TJ, Feild TS, Jordan GJ (2007) Leaf maximum photosynthetic rate and venation are linked by hydraulics. Plant Physiol 144: 1890–1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromham L, Bennett TH (2014) Salt tolerance evolves more frequently in C4 grass lineages. J Evol Biol 27: 653–659 [DOI] [PubMed] [Google Scholar]
- Chen Z-H, Chen G, Dai F, Wang Y, Hills A, Ruan Y-L, Zhang G, Franks PJ, Nevo E, Blatt MR (2017) Molecular evolution of grass stomata. Trends Plant Sci 22: 124–139 [DOI] [PubMed] [Google Scholar]
- Christin P-A, Besnard G, Samaritani E, Duvall MR, Hodkinson TR, Savolainen V, Salamin N (2008) Oligocene CO2 decline promoted C4 photosynthesis in grasses. Curr Biol 18: 37–43 [DOI] [PubMed] [Google Scholar]
- Christin P-A, Osborne CP, Chatelet DS, Columbus JT, Besnard G, Hodkinson TR, Garrison LM, Vorontsova MS, Edwards EJ (2013) Anatomical enablers and the evolution of C4 photosynthesis in grasses. Proc Natl Acad Sci U S A 110: 1381–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton WD, Renvoize SA (1986) Genera Graminum. Grasses of the World, Vol 13. The University of the Chicago Press, Chicago, IL [Google Scholar]
- Conceição AA, Pirani JR, Meirelles ST (2007) Floristics, structure and soil of insular vegetation in four quartzite-sandstone outcrops of “Chapada Diamantina”, Northeast Brazil. Braz J Bot 30: 641–656 [Google Scholar]
- Cosgrove DJ (1988) In defence of the cell volumetric elastic modulus. Plant Cell Environ 11: 67–69 [PubMed] [Google Scholar]
- Costa M-CD, Artur MAS, Maia J, Jonkheer E, Derks MFL, Nijveen H, Williams B, Mundree SG, Jiménez-Gómez JM, Hesselink T, et al. (2017). A footprint of desiccation tolerance in the genome of Xerophyta viscosa. Nat Plants 3: 17038. [DOI] [PubMed] [Google Scholar]
- Costanza SH, Dewet JMJ, Harlan J (1979) Literature review and numerical taxonomy of Eragrostis tef (T’ef). Econ Bot 33: 413–424 [Google Scholar]
- Dai A (2011) Drought under global warming: a review: drought under global warming. Wiley Interdiscip Rev Clim Change 2: 45–65 [Google Scholar]
- D’Andrea AC (2008) T’ef (Eragrostis tef) in ancient agricultural systems of highland Ethiopia. Econ Bot 62: 547–566 [Google Scholar]
- da Silva AC, de Freitas Lima M, Eloy NB, Thiebaut F, Montessoro P, Hemerly AS, Ferreira PCG (2019) The Yin and Yang in plant breeding: the trade-off between plant growth yield and tolerance to stresses. Biotechnol Res Innov 3: 73–79 [Google Scholar]
- De Wet JMJ (1986) Origin, evolution and systematics of minor cereals. InSeetharam A, Riley KW, Harinarayana G, eds, Proceedings of the 1st International Small Millets Workshop, Bangalore, India [Google Scholar]
- Doggett H (1989) Small millets—a selective overview. InSeetharam A, Riley KW, Harinarayana G, eds, Small Millets in Global Agriculture. Oxford & IBH, New Delhi, pp 59–70 [Google Scholar]
- Drake PL, de Boer HJ, Schymanski SJ, Veneklaas EJ (2019) Two sides to every leaf: water and CO2 transport in hypostomatous and amphistomatous leaves. New Phytol 222: 1179–1187 [DOI] [PubMed] [Google Scholar]
- Edwards EJ, Donoghue MJ (2013) Is it easy to move and easy to evolve? Evolutionary accessibility and adaptation. J Exp Bot 64: 4047–4052 [DOI] [PubMed] [Google Scholar]
- Edwards EJ, Still CJ (2008) Climate, phylogeny and the ecological distribution of C4 grasses. Ecol Lett 11: 266–276 [DOI] [PubMed] [Google Scholar]
- Edwards GE, Franceschi VR, Voznesenskaya EV (2004) Single-cell C4 photosynthesis versus the dual-cell (Kranz) paradigm. Annu Rev Plant Biol 55: 173–196 [DOI] [PubMed] [Google Scholar]
- El‐Sharkawy M, Hesketh J (1965) Photosynthesis among species in relation to characteristics of leaf anatomy and CO2 diffusion resistance 1. Crop Sci 5: 517–521 [Google Scholar]
- Fang Q, Ma L, Ahuja LR, Trout TJ, Malone RW, Zhang H, Gui D, Yu Q (2017) Long-term simulation of growth stage-based irrigation scheduling in maize under various water constraints in Colorado, USA. Front Agric Sci Eng 4: 172 [Google Scholar]
- Fern K, Fern A (2014) Useful Tropical Plants Database. http://tropical.theferns.info/
- Food and Agriculture Organization of the United Nations (2019) FAOSTAT http://www.fao.org/faostat/en/#data/QC (July 12, 2021)
- Franks PJ, Farquhar GD (2007) The mechanical diversity of stomata and its significance in gas-exchange control. Plant Physiol 143: 78–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DQ, Stevens CJ (2018) Sorghum domestication and diversification: a current archaeobotanical perspective. InMercuri AM, D’Andrea AC, Fornaciari R, Höhn A, eds, Plants and People in the African Past: Progress in African Archaeobotany. Springer International Publishing, Cham, pp 427–452 [Google Scholar]
- Gaff DF (1971) Desiccation-tolerant flowering plants in southern Africa. Science 174: 1033–1034 [DOI] [PubMed] [Google Scholar]
- Gaff DF, Oliver M (2013) The evolution of desiccation tolerance in angiosperm plants: a rare yet common phenomenon. Funct Plant Biol 40: 315. [DOI] [PubMed] [Google Scholar]
- Ghannoum O (2009) C4 photosynthesis and water stress. Ann Bot 103: 635–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghannoum O, von Caemmerer S, Conroy JP (2002) The effect of drought on plant water use efficiency of nine NAD-ME and nine NADP-ME Australian C4 grasses. Funct Plant Biol 29: 1337. [DOI] [PubMed] [Google Scholar]
- Ghannoum O, Evans JR, von Caemmerer S (2011) Nitrogen and water use efficiency of C4 plants. InRaghavendra AS, Sage RF, eds, C4 Photosynthesis and Related CO2 Concentrating Mechanisms. Springer Netherlands, Dordrecht, pp 129–146 [Google Scholar]
- Grass Phylogeny Working Group II (2012) New grass phylogeny resolves deep evolutionary relationships and discovers C4 origins. New Phytol 193: 304–312 [DOI] [PubMed] [Google Scholar]
- Griffiths H, Weller G, Toy LFM, Dennis RJ (2013) You’re so vein: bundle sheath physiology, phylogeny and evolution in C3 and C4 plants. Plant Cell Environ 36: 249–261 [DOI] [PubMed] [Google Scholar]
- Haberlandt G (1884) Physiologische Pflanzenanatomie. Wilhelm Engelmann, Leipzig
- Habiyaremye C, Matanguihan JB, D’Alpoim Guedes J, Ganjyal GM, Whiteman MR, Kidwell KK, Murphy KM (2016) Proso millet (Panicum miliaceum L.) and its potential for cultivation in the Pacific Northwest, U.S.: a review. Front Plant Sci 7: 1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy JP, Anderson VJ, Gardner JS (1995) Stomatal characteristics, conductance ratios, and drought-induced leaf modifications of semiarid grassland species. Am J Bot 82: 1–7 [Google Scholar]
- Harlan JR (1992) Crops and Man. American Society of Agronomy, Madison, WI
- Hély C, Braconnot P, Watrin J, Zheng W (2009) Climate and vegetation: simulating the African humid period. CR Geosci 341: 671–688 [Google Scholar]
- Higgins SI, Bond WJ, Trollope WSW (2000) Fire, resprouting and variability: a recipe for grass–tree coexistence in savanna. J Ecol 88: 213–229 [Google Scholar]
- Hilu KW, De Wet JMJ (1976) Domestication of Eleusine coracana. Econ Bot 30: 199–208 [Google Scholar]
- Hilu KW, de Wet JMJ, Harlan JR (1979) Archaeobotanical studies of eleusine Coracana ssp. Coracana (finger millet). Am J Bot 66: 330–333 [Google Scholar]
- Huang W, Yang S-J, Zhang S-B, Zhang J-L, Cao K-F (2012) Cyclic electron flow plays an important role in photoprotection for the resurrection plant Paraboea rufescens under drought stress. Planta 235: 819–828 [DOI] [PubMed] [Google Scholar]
- Ingram AL, Doyle JJ (2003) The origin and evolution of Eragrostis tef (Poaceae) and related polyploids: evidence from nuclear waxy and plastid rps16. Am J Bot 90: 116–122 [DOI] [PubMed] [Google Scholar]
- Jacobs BF, Kingston JD, Jacobs LL (1999) The origin of grass-dominated ecosystems. Ann Mo Bot Gard 86: 590–643 [Google Scholar]
- Jideani AI, Akingbala JO (1993) Some physicochemical properties of acha (digitaria exilis stapf) and iburu (Digitaria iburua stapf) grains. J Sci Food Agric 63: 369–374 [Google Scholar]
- McFadden J, Smith D, Wechsler S, Wallander S (2019) Development, Adoption, and Management of Drought-Tolerant Corn in the United States. U.S. Department of Agriculture Economic Research Service, Washington, DC
- Kellogg EA (2013) C4 photosynthesis. Curr Biol 23: R594–R599 [DOI] [PubMed] [Google Scholar]
- Kellogg EA (2001) Evolutionary history of the grasses. Plant Physiol 125: 1198–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W, Iizumi T, Nishimori M (2019) Global patterns of crop production losses associated with droughts from 1983 to 2009. J Appl Meteorol Climatol 58: 1233–1244 [Google Scholar]
- Kirkman LK, Sharitz RR (1993) Growth in controlled water regimes of three grasses common in freshwater wetlands of the southeastern USA. Aquat Bot 44: 345–359 [Google Scholar]
- Kranz WL, Specialist I, Engineer I (2008) Irrigation Management for Corn. University of Nebraska Extension Publications, Lincoln, NE
- Laetsch WM (1974) The C4 syndrome: a structural analysis. Annu Rev Plant Physiol 25: 27–52 [Google Scholar]
- Lawson T, Vialet-Chabrand S (2019) Speedy stomata, photosynthesis and plant water use efficiency. New Phytol 221: 93–98 [DOI] [PubMed] [Google Scholar]
- Lev-Yadun S, Gopher A, Abbo S (2000) The cradle of agriculture. Science 288: 1602–1603 [DOI] [PubMed] [Google Scholar]
- Linder HP, Ferguson IK (1985) Notes on the pollen morphology and phylogeny restionales and poales. Grana 24: 65–76 [Google Scholar]
- Linder HP, Lehmann CER, Archibald S, Osborne CP, Richardson DM (2018) Global grass (Poaceae) success underpinned by traits facilitating colonization, persistence and habitat transformation. Biol Rev 93: 1125–1144 [DOI] [PubMed] [Google Scholar]
- Liu H, Edwards EJ, Freckleton RP, Osborne CP (2012) Phylogenetic niche conservatism in C4 grasses. Oecologia 170: 835–845 [DOI] [PubMed] [Google Scholar]
- Liu H, Osborne CP (2015) Water relations traits of C4 grasses depend on phylogenetic lineage, photosynthetic pathway, and habitat water availability. J Exp Bot 66: 761–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Messager E, Bedianashvili G, Rusishvili N, Lebedeva E, Longford C, Hovsepyan R, Bitadze L, Chkadua M, Vanishvili N, et al. (2009) Earliest domestication of common millet (Panicum miliaceum) in East Asia extended to 10,000 years ago. Proc Natl Acad Sci U S A 106: 7367–7372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren MR, Osborne CP, Christin P-A (2014) Deconstructing Kranz anatomy to understand C4 evolution. J Exp Bot 65: 3357–3369 [DOI] [PubMed] [Google Scholar]
- Lyall R, Schlebusch SA, Proctor J, Prag M, Hussey SG, Ingle RA, Illing N (2019) Vegetative desiccation tolerance in the resurrection plant Xerophyta humilis has not evolved through reactivation of the seed canonical LAFL regulatory network. Plant J 101: 1349–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcum KB (1999) Salinity tolerance mechanisms of grasses in the subfamily Chloridoideae. Crop Sci 39: 1153–1160 [Google Scholar]
- Marcum KB, Murdoch CL (1994) Salinity tolerance mechanisms of six C4 turfgrasses. J Am Soc Hortic Sci 119: 779–784 [Google Scholar]
- Marks RA, Farrant JM, Nicholas McLetchie D, VanBuren R (2021) Unexplored dimensions of variability in vegetative desiccation tolerance. Am J Bot 108: 346–358 [DOI] [PubMed] [Google Scholar]
- McAusland L, Vialet‐Chabrand S, Davey P, Baker NR, Brendel O, Lawson T (2016) Effects of kinetics of light‐induced stomatal responses on photosynthesis and water‐use efficiency. New Phytol 211: 1209–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina CD, Cooper M, Hammer GL, Berning D, Ciampitti I, Clark R, Diepenbrock C, Gho C, Jines M, Lee T, et al. (2020) Two decades of creating drought tolerant maize and underpinning prediction technologies in the US corn-belt: review and perspectives on the future of crop design. Cold Spring Harbor Laboratory: 2020.10.29.361337 [Advance access publication date: October 30, 2020].
- Milla R (2020) Crop Origins and Phylo Food: a database and a phylogenetic tree to stimulate comparative analyses on the origins of food crops. Glob Ecol Biogeogr 29: 606–614 [Google Scholar]
- Mitra J, Xu G, Wang B, Li M, Deng X (2013) Understanding desiccation tolerance using the resurrection plant Boea hygrometrica as a model system. Front Plant Sci 4: 446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott KA, Gibson AC, O’leary JW (1982) The adaptive significance of amphistomatic leaves. Plant Cell Environ 5: 455–460 [Google Scholar]
- Muchow RC (1989) Comparative productivity of maize, sorghum and pearl millet in a semi-arid tropical environment I. Yield potential. Field Crops Res 20: 191–205 [Google Scholar]
- Mundia CW, Secchi S, Akamani K, Wang G (2019) A regional comparison of factors affecting global sorghum production: the case of North America, Asia and Africa’s Sahel. Sustain Sci Pract Policy 11: 2135 [Google Scholar]
- Nunes TDG, Zhang D, Raissig MT (2020) Form, development and function of grass stomata. Plant J 101: 780–799 [DOI] [PubMed] [Google Scholar]
- Ocheltree TW, Nippert JB, Prasad PVV (2016) A safety vs efficiency trade-off identified in the hydraulic pathway of grass leaves is decoupled from photosynthesis, stomatal conductance and precipitation. New Phytol 210: 97–107 [DOI] [PubMed] [Google Scholar]
- Oliver MJ, Tuba Z, Mishler BD (2000) The evolution of vegetative desiccation tolerance in land plants. Plant Ecol 151: 85–100 [Google Scholar]
- Osborne CP, Freckleton RP (2009) Ecological selection pressures for C4 photosynthesis in the grasses. Proc Biol Sci 276: 1753–1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne CP, Sack L (2012) Evolution of C4 plants: a new hypothesis for an interaction of CO2 and water relations mediated by plant hydraulics. Philos Trans R Soc Lond B Biol Sci 367: 583–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne CP, Salomaa A, Kluyver TA, Visser V, Kellogg EA, Morrone O, Vorontsova MS, Clayton WD, Simpson DA (2014) A global database of C4 photosynthesis in grasses. New Phytol 204: 441–446 [DOI] [PubMed] [Google Scholar]
- Pardo J, Man Wai C, Chay H, Madden CF, Hilhorst HWM, Farrant JM, VanBuren R (2020) Intertwined signatures of desiccation and drought tolerance in grasses. Proc Natl Acad Sci U S A 117: 10079–10088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathare VS, Sonawane BV, Koteyeva N, Cousins AB (2020) C4 grasses adapted to low precipitation habitats show traits related to greater mesophyll conductance and lower leaf hydraulic conductance. Plant Cell Environ 43: 1897–1910 [DOI] [PubMed] [Google Scholar]
- Pau S, Edwards EJ, Still CJ (2013) Improving our understanding of environmental controls on the distribution of C3 and C4 grasses. Glob Chang Biol 19: 184–196 [DOI] [PubMed] [Google Scholar]
- Peterson PM, Columbus JT, Pennington SJ (2007) Classification and biogeography of New World grasses: Chloridoideae. Aliso 23: 580–594 [Google Scholar]
- Piperno DR, Moreno JE, Iriarte J, Holst I, Lachniet M, Jones JG, Ranere AJ, Castanzo R (2007) Late Pleistocene and Holocene environmental history of the Iguala Valley, Central Balsas Watershed of Mexico. Proc Natl Acad Sci U S A 104: 11874–11881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzigui T, Jazzar L, Baaziz Khaoula B, Fkiri S, Nasr Z (2018) Drought tolerance in cork oak is associated with low leaf stomatal and hydraulic conductances. IForest 11: 728–733 [Google Scholar]
- Sabadin PK, Malosetti M, Boer MP, Tardin FD, Santos FG, Guimarães CT, Gomide RL, Andrade CLT, Albuquerque PEP, Caniato FF, et al. (2012) Studying the genetic basis of drought tolerance in sorghum by managed stress trials and adjustments for phenological and plant height differences. Theor Appl Genet 124: 1389–1402 [DOI] [PubMed] [Google Scholar]
- Sage RF (2001) Environmental and evolutionary preconditions for the origin and diversification of the C4 photosynthetic syndrome. Plant Biol 3: 202–213 [Google Scholar]
- Sage RF (2004) The evolution of C4 photosynthesis. New Phytol 161: 341–370 [DOI] [PubMed] [Google Scholar]
- Sage RF, Zhu X-G (2011) Exploiting the engine of C4 photosynthesis. J Exp Bot 62: 2989–3000 [DOI] [PubMed] [Google Scholar]
- Sankaran M, Hanan NP, Scholes RJ, Ratnam J, Augustine DJ, Cade BS, Gignoux J, Higgins SI, Le Roux X, Ludwig F, et al. (2005) Determinants of woody cover in African savannas. Nature 438: 846–849 [DOI] [PubMed] [Google Scholar]
- Scarcelli N, Cubry P, Roland A, Thuillet A-C, Obidiegwu J, Baco MN, Otoo E, Sonké B, Dansi A, Djedatin G, et al. (2019). Yam genomics supports West Africa as a major cradle of crop domestication. Sci Adv 5: eaaw1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer M, Hirota M, Holmgren M, Van Nes EH, Chapin FS III (2012) Thresholds for boreal biome transitions. Proc Natl Acad Sci U S A 109: 21384–21389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert M, Grønvold L, Sandve SR, Hvidsten TR, Fjellheim S (2019) Evolution of cold acclimation and its role in niche transition in the temperate grass subfamily Pooideae. Plant Physiol 180: 404–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson GG (1953) The Major Features of Evolution. Columbia University Press, New York, NY [Google Scholar]
- Simtowe F, Amondo E, Marenya P, Rahut D, Sonder K, Erenstein O (2019). Impacts of drought-tolerant maize varieties on productivity, risk, and resource use: evidence from Uganda. Land Use Policy 88: 104091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair TR, Zwieniecki MA, Holbrook NM (2008) Low leaf hydraulic conductance associated with drought tolerance in soybean. Physiol Plant 132: 446–451 [DOI] [PubMed] [Google Scholar]
- Staggenborg SA, Dhuyvetter KC, Gordon WB (2008) Grain sorghum and corn comparisons: yield, economic and environmental responses. Agron J 100: 1600–1604 [Google Scholar]
- Stebbins GL, Shah SS (1960) Developmental studies of cell differentiation in the epidermis of monocotyledons: II. Cytological features of stomatal development in the Gramineae. Dev Biol 2: 477–500 [Google Scholar]
- Steudle E, Zimmermann U (1977) Effect of turgor pressure and cell size on the wall elasticity of plant cells. Plant Physiol 59: 285–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Still CJ, Berry JA, Collatz GJ, DeFries RS (2003) Global distribution of C3 and C4 vegetation: carbon cycle implications. Glob Biogeochem Cycles 17: 6–14 [Google Scholar]
- Stroud JT, Losos JB (2016) Ecological opportunity and adaptive radiation. Annu Rev Ecol Evol Syst 47: 507–532 [Google Scholar]
- Tadesse D (1993) Study on genetic variation of landraces of teff (Eragrostis tef (Zucc.) Trotter) in Ethiopia. Genet Resour Crop Evol 40: 101–104 [Google Scholar]
- Taub DR (2000) Climate and the US distribution of C4 grass subfamilies and decarboxylation variants of C4 photosynthesis. Am J Bot 87: 1211–1215 [PubMed] [Google Scholar]
- Taylor SH, Ripley BS, Martin T, De-Wet L-A, Ian Woodward F, Osborne CP (2014) Physiological advantages of C 4 grasses in the field: a comparative experiment demonstrating the importance of drought. Glob Chang Biol 20: 1992–2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tefera H, Belay G, Sorrells ME (2001) Narrowing the rift: Tef Research and Development. In Proceedings of the “International Workshop on Tef Genetics and Improvement”, Debre Zeit, Ethiopia, 16–19 October 2000, Ethiopian Agricultural Research Organization
- Touchette BW, Marcus SE, Adams EC (2014) Bulk elastic moduli and solute potentials in leaves of freshwater, coastal and marine hydrophytes. Are marine plants more rigid? AoB Plants 6: plu014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubbs SR (2015) Form follows function or does it? Clin Anat 28: 955–955 [DOI] [PubMed] [Google Scholar]
- VanBuren R (2017) Desiccation tolerance: seedy origins of resurrection. Nat Plants 3: 17046. [DOI] [PubMed] [Google Scholar]
- VanBuren R, Pardo J, Man Wai C, Evans S, Bartels D (2019) Massive tandem proliferation of ELIPs supports convergent evolution of desiccation tolerance across land plants. Plant Physiol 179: 1040–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanBuren R, Wai CM, Zhang Q, Song X, Edger PP, Bryant D, Michael TP, Mockler TC, Bartels D (2017) Seed desiccation mechanisms co-opted for vegetative desiccation in the resurrection grass Oropetium thomaeum. Plant Cell Environ 40: 2292–2306 [DOI] [PubMed] [Google Scholar]
- Vander Willigen C, Pammenter NW, Mundree S, Farrant J (2001) Some physiological comparisons between the resurrection grass, Eragrostis nindensis, and the related desiccation-sensitive species, E. curvula. Plant Growth Regul 35: 121–129 [Google Scholar]
- Verhoeven A, García-Plazaola JI, Fernández-Marín B (2018) Shared mechanisms of photoprotection in photosynthetic organisms tolerant to desiccation or to low temperature. Environ Exp Bot 154: 66–79 [Google Scholar]
- Yates RA, Taylor RD (1986) Water use efficiencies in relation to sugarcane yields. Soil Use Manag 2: 70–76 [Google Scholar]
- Zhou H, Akcay E, Edwards E, Helliker B (2020) The legacy of C4 evolution in the hydraulics of C3 and C4 grasses. BioRxiv 10.1101/2020.05.14.097030 [DOI]