Abstract
Objective
The prevalence and associations of leucopenia in SLE remain incompletely understood. We evaluated associations of disease activity and medication use with leucopenia (lymphopenia and neutropenia) in a multinational, prospectively followed SLE cohort.
Methods
Data from the Asia Pacific Lupus Collaboration cohort, in which disease activity and medications were prospectively captured from 2013 to 2018, were used. Predictors of lymphopenia (lymphocyte count <0.8 × 109/l) and neutropenia (neutrophil count <1.5 × 109/l) were examined using multiple failure, time-dependent survival analyses.
Results
Data from 2330 patients and 18 287 visits were analysed. One thousand and eighteen patients (43.7%) had at least one episode of leucopenia; 867 patients (37.2%) had lymphopenia, observed in 3065 (16.8%) visits, and 292 (12.5%) patients had neutropenia, in 622 (3.4%) visits. After multivariable analyses, lymphopenia was associated with overall disease activity, ESR, serology, prednisolone, AZA, MTX, tacrolimus, CYC and rituximab use. MTX and ciclosporin were negatively associated with neutropenia. Lupus low disease activity state was negatively associated with both lymphopenia and neutropenia.
Conclusion
Both lymphopenia and neutropenia were common in SLE patients but were differentially associated with disease and treatment variables. Lymphopenia and neutropenia should be considered independently in studies in SLE.
Keywords: leucopenia, lymphopenia, neutropenia, SLE disease activity, LLDAS, medications
Rheumatology key messages
The associations with disease activity and medication use were different between lymphopenia and neutropenia.
Disease activity was consistently and significantly associated with lymphopenia.
Prednisolone, immunosuppressants and rituximab were associated with lymphopenia; methotrexate and ciclosporin were protective in neutropenia.
Introduction
Leucopenia is commonly observed in patients with SLE, with a reported prevalence of leucopenia between 22 and 42% [1]. The presence of leucopenia is incorporated in many versions of SLE classification criteria [2–5], but sub-setting of leucopenia in these is mostly in relation to lymphopenia. Less is known about neutropenia in SLE.
The pathogenesis of leucopenia in SLE is not well understood; however, different mechanisms have been proposed. Peripheral destruction from lymphotoxic or anti-neutrophil antibody effects, complement-mediated cell lysis and excessive neutrophil apoptosis, as well as marrow suppression, have been postulated [6, 7]. The attribution of leucopenia to SLE disease activity is relatively easy to recognize before treatment but can become challenging when patients have been treated with immunosuppressive therapy with potential myelosuppressive effects. There remain knowledge gaps regarding the prevalence and clinical associations of leucopenia in SLE. In this study, we sought to examine the associations of disease characteristics and medication use with lymphopenia and neutropenia in a multi-ethnic international longitudinal lupus cohort.
Methods
Patients
Data from the Asia Pacific Lupus Collaboration (APLC) patient cohort collected prospectively between 2013 and 2018 using pre-determined data collection forms were used to conduct this study [8]. Study participants were recruited from 16 sites across nine countries (Supplementary Fig. 1, available at Rheumatology online). All APLC patients were consenting adults who met either the 1997 ACR Modified Classification Criteria for SLE [3] or the SLICC 2012 Classification Criteria [4]. Each APLC site has local ethics approval for patient recruitment and to contribute to the centralized APLC dataset. Individual centres obtain valid written informed consent in accordance with the local authority regarding ethical conduct of human research. Monash University Human Research Ethics Committee has approved storage of the central dataset in Monash University's secure servers and the performance of analyses using collective data.
Variables
The APLC cohort prospectively captures patient demographics including gender, ethnicity, and years of birth, SLE onset and SLE diagnosis, and diagnostic criteria (ACR [3] and SLICC [4]) at recruitment. Data on disease activity, pathology and medications were prospectively captured at every visit using standard data collection forms, with medication documentation recording what the patients were taking at the time of the visit. Disease activity was measured using SLEDAI-2K [9], which requires assignment to lupus activity, and a physician global assessment (PGA) on a scale of 0 (no activity) to 3 (maximum activity) [10]. Disease flares were captured using the SELENA flare index (SFI) [11]. Irreversible organ damage was captured using the SLICC-ACR Damage Index (SDI) [12], measured at recruitment and at each annual visit. LLDAS attainment was determined at each visit as published by Golder et al. [13].
The following medications were recorded at each visit: prednisolone (or equivalent), HCQ, chloroquine, MTX, AZA, MMF, mycophenolic acid (MPA), LEF, ciclosporin (CyA), tacrolimus (TAC), CYC use in preceding 6 months (Y/N), rituximab (RTX) use in preceding 6 months (Y/N) and belimumab (Y/N).
Leucopenia was defined according to the National Cancer Institute's Common Terminology Criteria for Adverse Events (NCI CTCAE v5.0) into different severity categories (mild, moderate, severe) based on peripheral blood total white cell count (WCC) as well as lymphocyte and neutrophil cell counts. Moderate (grade 2) leukopenia was used as the primary outcome for this study, and was defined as WCC <3.0 × 109/l, and/or lymphocyte count <0.8 × 109/l and/or neutrophil count <1.5 × 109/l.
Statistical analysis
Statistical analysis was performed using Stata version 15.1 (StataCorp, College Station, TX, USA). Patient characteristics were described as summary statistics, stratified according to the presence of grade 2 leucopenia at least once (leucopenia ever). Continuous variables were expressed as median [inter-quartile range (IQR), (range)] and compared using the Wilcoxon rank sum test. Categorical variables were described as frequency (%) and compared using the chi-square test. Multivariate survival analysis incorporating conditional risk set modelling (Prentice, Williams and Peterson model including gap time [14]) was carried out to examine longitudinal associations of recurrent events of leucopenia. Clustering was specified in the Cox regression models to account for intragroup correlation. The results are presented as the hazard ratio (HR) with corresponding 95% CI, and P-values ≤0.05 are considered statistically significant.
Results
Patient characteristics
Data from 2330 patients and 18 287 visits from the APLC 2013–18 dataset were analysed, restricting the analysis to patients with ≥2 visits and with per-visit data on peripheral blood white blood cell counts available. Patients were followed-up for a median (IQR) (range) of 840 (378, 1281) (21, 1765) days.
Frequency of leucopenia
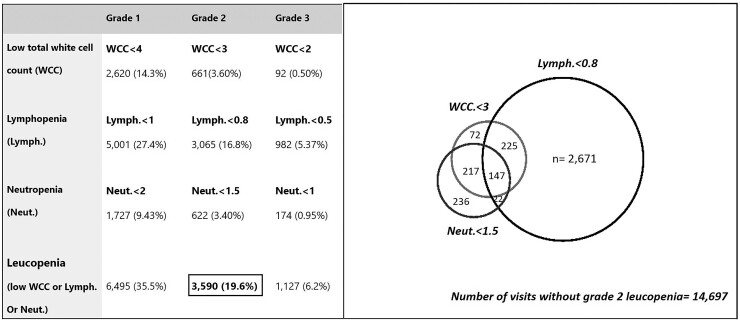
Figure 1 summarizes the frequency and proportion [n (%)] of visits with different grades of leukopenia. In total, 1018 patients (43.7%) experienced leucopenia of at least grade 2, in 3590 (19.6%) visits. Of the patients who experienced leucopenia, ∼66% (n = 676) had leucopenia in two or more visits [median (IQR) (range) number of leucopenia visits = 2 (1, 5) (1, 21)]. A total of 867 (37.2%) patients experienced lymphopenia in 3065 (16.8%) visits and 292 (12.5%) patients experienced neutropenia in 622 (3.4%) visits; ∼61% (618/1018) of leucopenic patients experienced lymphopenia only, while a further 24.5% (n = 249) experienced lymphopenia in combination with either neutropenia or low total WCC or both. In contrast, visits affected by neutropenia were less common and were frequently associated with a corresponding drop in total white cell count and/or lymphopenia (Fig. 1). Overall, 7.3% (n = 74) of leucopenic patients had neutropenia only, and another 21.4% (n = 218) had neutropenia with lymphopenia and/or low WCC.
Fig. 1.
Frequency (n) and proportion of visits (%) of leucopenic grades (cell counts ×109/l)
Patient characteristics of those who experienced leucopenia
Demographics and disease characteristics between patients who never experienced leucopenia and those who had leucopenia at least once during study observation period were compared. As shown in Table 1, leucopenic patients were younger at SLE diagnosis and recruitment and had longer study observation period. A significantly higher proportion of leucopenic patients demonstrated serological activity and medications use including prednisolone, anti-malarials (AM) and immunosuppressive agents (IS). Leucopenic patients also had significantly higher median time adjusted SLEDAI-2K and PGA scores, indicating overall higher disease activity. A higher proportion of these patients experienced flare and accrued irreversible damage. In contrast, significantly fewer leucopenic patients achieved LLDAS during the study period (Table 1).
Table 1.
Patient characteristics, stratified by presence of grade 2 leucopenia at least once (ever) during study observation period
| All patients | Leucopoenia—never | Leucopoenia—ever | P-value | |
|---|---|---|---|---|
| (n = 2330) | (n = 1312) | (n = 1018) | ||
| Demographics | ||||
| Age at enrolment, median (IQR), years | 39 (30, 50) | 41 (31, 52) | 38 (29, 48) | <0.001 |
| Age at SLE diagnosis, median (IQR), years | 29 (21, 39) | 30 (22, 41) | 28 (21, 37) | 0.002 |
| Disease duration, median (IQR), years | 8 (3, 14) | 8 (3, 14) | 7 (3, 13) | 0.07 |
| Study observation period, median (IQR), years | 2.3 (1.0, 3.5) | 2.1 (0.7, 3.4) | 2.6 (1.6, 3.7) | <0.001 |
| No. of visits, median (IQR) | 7 (4, 11) | 5 (3, 9) | 9 (5, 12) | <0.001 |
| Visits per study years, median (IQR) | 3.8 (2.6, 4.7) | 3.6 (2.4, 4.7) | 4.0 (2.9, 4.7) | <0.001 |
| Asian ethnicity, n (%) | 2055 (89) | 1173 (90) | 882 (88) | 0.09 |
| Females, n (%) | 2171 (93) | 1218 (93) | 953 (94) | 0.4 |
| Current smoker at enrolment, n (%) | 113 (5.3) | 65 (5.6) | 48 (4.9) | 0.5 |
| Family history of SLE, n (%) | 177 (8.3) | 104 (9.0) | 73 (7.5) | 0.2 |
| Tertiary education, n (%) | 1069 (48) | 609 (49) | 460 (47) | 0.3 |
| Serology, n (%) | ||||
| Low complement (C3/C4) | 1767 (76) | 889 (68) | 878 (87) | <0.001 |
| Anti-dsDNA positivity | 1406 (61) | 698 (53) | 708 (70) | <0.001 |
| ESR ≥25 | 1280 (62) | 636 (56) | 644 (69) | <0.001 |
| Medications use evera, n (%) | ||||
| Prednisolone | 1989 (85) | 1042 (79) | 947 (93) | <0.001 |
| TAM prednisolone, median (IQR), mg/d | 5.3 (2.5, 9.4) | 5.0 (1.1, 7.8) | 7.3 (4.4, 10.3) | <0.001 |
| Anti-malarial drugs | 1734 (74) | 1002 (76) | 732 (72) | 0.014 |
| HCQ | 1612 (69) | 933 (71) | 679 (68) | 0.020 |
| Chloroquine | 167 (7.1) | 92 (7.0) | 74 (7.3) | 0.8 |
| Immunosuppressants | 1661 (71) | 831 (63) | 830 (82) | <0.001 |
| Mycophenolate | 820 (35) | 403 (31) | 417 (41) | <0.001 |
| Mycophenolic acid | 132 (5.7) | 60 (4.6) | 72 (7.1) | 0.010 |
| AZA | 688 (30) | 317 (24) | 371 (36) | <0.001 |
| Ciclosporin | 170 (7.3) | 78 (5.9) | 92 (9.0) | 0.004 |
| MTX | 166 (7.1) | 84 (6.4) | 82 (8.1) | 0.12 |
| Tacrolimus | 88 (3.8) | 35 (2.7) | 53 (5.2) | 0.001 |
| LEF | 66 (2.8) | 29 (2.2) | 37 (3.6) | 0.040 |
| Mizoribine | 13 (0.5) | 3 (0.2) | 10 (1.0) | 0.015 |
| CYC | 229 (11) | 96 (8.2) | 133 (14) | <0.001 |
| Biologics (any) ever | 76 (3.3) | 20 (1.5) | 56 (5.5) | <0.001 |
| Rituximab | 50 (2.4) | 12 (1.0) | 38 (4.0) | <0.001 |
| Belimumab | 31 (1.5) | 9 (0.8) | 22 (2.3) | 0.003 |
| Clinical indicators | ||||
| TAM SLEDAI-2K, median (IQR) | 3.1 (1.5, 5.1) | 2.5 (1.0, 4.5) | 3.7 (2.0, 5.8) | <0.001 |
| SLEDAI ≥ 6 ever, n (%) | 1196 (51) | 584 (44) | 612 (60) | <0.001 |
| TAM PGA, median (IQR) | 0.4 (0.2, 0.8) | 0.4 (0.2, 0.8) | 0.5 (0.3, 0.9) | <0.001 |
| Mild/moderate/severe flare evera, n (%) | 1257 (54) | 606 (46) | 651 (64) | <0.001 |
|
Baseline organ damage present (SDI > 0 at recruitment), n (%) |
916 (39) | 517 (39) | 399 (39) | 0.9 |
| Damage accrual during study period, n (%) | 332 (14) | 154 (12) | 178 (18) | <0.001 |
| Achieved LLDAS evera, n (%) | 1697 (73) | 1004 (77) | 693 (68) | <0.001 |
| Percentage time spent in LLDAS during study period, median (IQR) |
44.6 (0, 76.9) |
50.5 (8.5, 88.3) |
28.9 (0, 58.3) |
<0.001 |
Used/achieved at least once during study observation period. P-values for comparing categorical variables were derived using Pearson’s chi-square test. P-values for comparing continuous variables were derived using the Wilcoxon rank-sum test. IQR: interquartile range; LLDAS: lupus low disease activity state; PGA: physician global assessment; TAM: time adjusted mean.
We also separately examined patient characteristics by lymphopenia (lymphopenia–never vs lymphopenia–ever) and neutropenia (neutropenia–never vs neutropenia–ever), and the results are summarized in Supplementary Tables 1 and 2, available at Rheumatology online. The comparison of patient characteristics between lymphopenia–never vs lymphopenia–ever was very similar to what we described above between leucopenia–never and leucopenia–ever patients (Supplementary Table 1, available at Rheumatology online). In contrast, while the association with disease activity measures such as time adjusted SLEDAI, serological activity and elevated ESR was higher in patients who have ever experienced neutropenia, the number of patients treated with prednisolone and IS was not proportionately higher in the neutropenia–ever group (Supplementary Table 2, available at Rheumatology online).
Clinical associations of leucopenia
As the mechanisms of lymphopenia and neutropenia may be different in SLE, their associations with baseline and per-visit clinical parameters were examined separately (Supplementary Table 3, available at Rheumatology online). Since lymphopenia made up 85.4% of the visits that had leucopenia, the association patterns for leucopenia overall were similar to those observed with lymphopenia alone. Univariable associations of lymphopenia included age, serological activity, elevated ESR (≥25), medication use, PGA, SLEDAI score and category, flare, and LLDAS. There was no significant association between the presence of damage (SDI ≥1) and any of the leucopenia.
We next explored the associations of disease activity and medication use using multivariable analysis by a number of different models. These models were chosen due to the complexity of utilizing SLEDAI-2K alone as the only variable to reflect disease activity, as it contains an item (leucopenia, 1 point) that overlaps with the endpoints of interest (lymphopenia or neutropenia). Since LLDAS and SLEDAI-2K demonstrated perfect negative collinearity (tetrachoric rho = −1), independent associations of these variables were analysed separately. Moreover, SLEDAI-2K and serological activity were highly correlated; for instance, 93% of patient visits with SLEDAI-2K ≥6 were serology-positive.
After adjustment, increased ESR, prednisolone and IS/biologics use, as well as disease activity, remained independently associated with lymphopenia (Table 2). The association with active disease was shown positively with SLEDAI-2K score regardless of leucopenia domain (Table 2, models 2 and 3), serological activity (Table 2, model 1) and negatively with LLDAS (Table 2, model 5). The HR for lymphopenia with prednisolone use was 2.46 (95% CI: 1.83, 3.31) (P < 0.001), after accounting for potential confounding factors. SLEDAI ≥ 6 was associated with a significant increase in lymphopenia after controlling for high ESR and medications (Table 2, model 3). Patients who were in LLDAS at any given point during the study observation period were 43% less likely to have lymphopenia when compared with patients who were not in LLDAS [adjusted HR = 0.57 (95% CI: 0.50, 0.66); P < 0.001].
Table 2.
Multivariable Cox regression models examining the independent associations of lymphopenia
| HR (95% CI), P-value |
|||||
|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |
| Serology positive | 2.00 (1.67, 2.39), P < 0.001 | ||||
| ESR ≥ 25 | 1.65 (1.43, 1.90), P < 0.001 |
1.52 (1.32, 1.76), P < 0.001 |
1.52 (1.32, 1.76), P < 0.001 |
1.56 (1.35, 1.81), P < 0.001 |
1.61 (1.40, 1.87), P < 0.001 |
| PNL use | 2.53 (1.90, 3.37), P < 0.001 | 2.46 (1.83, 3.31), P < 0.001 | 2.47 (1.84, 3.32), P < 0.001 | 2.54 (1.89, 3.40), P < 0.001 | |
| IS or biologic use | 2.13 (1.78, 2.56), P < 0.001 |
2.06 (1.71, 2.48), P < 0.001 |
2.07 (1.72, 2.49), P < 0.001 |
2.13 (1.77, 2.56), P < 0.001 |
2.23 (1.86, 2.68), P < 0.001 |
| SLEDAI-2K score | 1.04 (1.02, 1.06), P < 0.001 | ||||
| SLEDAI-2K modifieda | 1.04 (1.02, 1.05), P < 0.001 | ||||
| SLEDAI-2K ≥ 6 | 1.18 (1.03, 1.37), P < 0.021 | ||||
| In LLDAS |
0.57 (0.50, 0.66), P < 0.001 |
||||
SLEDAI-2K modified excludes leucopenia. HR: hazard ratio; IS: immunosuppressant; LLDAS: lupus low disease activity state; PNL: prednisolone.
In relation to neutropenia, the HR for disease activity as measured by SLEDAI was attenuated after adjustment using the multivariable model. In contrast, elevated ESR, serological activity and AM use remained significant (Table 3).
Table 3.
Multivariable Cox regression models examining the independent associations of neutropenia
| HR (95% CI), P-value |
|||||
|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |
| Serology positive | 1.89 (1.26, 2.82), P = 0.001 | ||||
| ESR ≥ 25 | 1.71 (1.23, 2.35), P = 0.001 |
1.63 (1.17, 2.28), P = 0.004 |
1.68 (1.21, 2.35), P = 0.002 |
1.69 (1.22, 2.38), P = 0.002 |
1.62 (1.40, 1.87), P = 0.004 |
| AM use | 1.80 (1.28, 2.68), P = 0.005 | 1.89 (1.26, 2.82), P = 0.002 | 1.89 (1.27, 2.83), P = 0.002 | 1.89 (1.27, 2.82), P = 0.002 | 1.89 (1.26, 2.83), P = 0.002 |
| SLEDAI-2K score | 1.03 (0.99, 1.06), P = 0.138 | ||||
| SLEDAI-2K modifieda |
0.99 (0.96, 1.03), P = 0.8 |
||||
| SLEDAI-2K ≥ 6 |
0.87 (0.60, 1.24), P = 0.4 |
||||
| In LLDAS |
0.56 (0.42, 0.74), P < 0.001 |
||||
SLEDAI-2K modified excludes leucopenia. AM: anti-malarials; LLDAS: lupus low disease activity state.
Differential associations of medications with lymphopenia and neutropenia
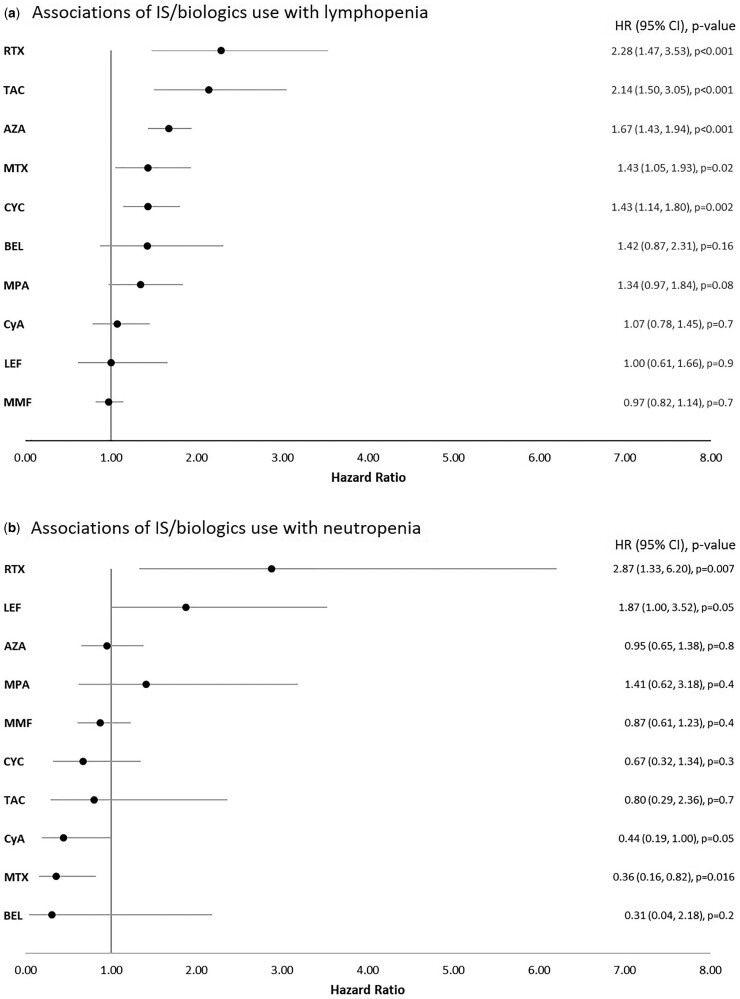
Different associations were observed between medication classes and lymphopenia and neutropenia. The use of prednisolone was strongly associated with lymphopenia but not with neutropenia. The use of several IS medications such as MMF, MPA, AZA, MTX, TAC and CYC was significantly associated with lymphopenia but not neutropenia in univariable models (Supplementary Table 3, available at Rheumatology online). Interestingly, AM use was associated with an increased risk of neutropenia, but not lymphopenia (Supplementary Table 3, available at Rheumatology online). In addition, LEF and RTX use were significantly associated with neutropenia occurring during the study period (Supplementary Table 3, available at Rheumatology online). We further examined these associations by controlling for potential confounding effects of disease activity (SLEDAI-2K), prednisolone use and AM use. The results are presented in Fig. 2. AZA, MTX, TAC, CYC and RTX remained independently associated with an increased hazard for lymphopenia, but the associations of MMF, MPA and belimumab were attenuated. TAC and RTX had the highest HR (Figure 2A). AM, LEF and RTX use remained significantly associated with neutropenia (Figure 2B). The use of CyA and MTX remained independently protective of neutropenia after adjustment (Figure 2B).
Fig. 2.
Forest plots depicting hazard ratio (HR) with corresponding 95% CI of immunosuppressant/biologic use with lymphopenia (A) and neutropenia (B)
HR was adjusted for prednisolone use, anti-malarial use and SLEDAI-2K score. BEL: belimumab; CyA: ciclosporin; MPA: mycophenolic acid; RTX: rituximab; TAC: tacrolimus.
We explored the association of AM use with neutropenia by categorizing visits to be either AM monotherapy or combination therapy. In 85% (9907/11 656) of visits where AM were used, they were used in combination with an IS or prednisolone. The HR for neutropenia was significantly elevated with AM in only combination therapy. Compared with patients who were not on medication, the HR for AM alone was 1.71 (95% CI: 0.85, 3.42) (P = 0.132), whereas for AM in combination with prednisolone and/or IS, HR was 2.25 (95% CI: 1.16, 4.35) (P = 0.016). (Supplementary Table 4, available at Rheumatology online).
Discussion
This study aimed to provide a comprehensive analysis of the prevalence and associations of leucopenia in SLE. We studied patients enrolled in the Asia Pacific Lupus Collaboration (APLC) cohort, one of the largest lupus observational cohorts in the world, in which detailed information on disease parameters and medication use as well as leucocyte counts is collected prospectively. Leucopoenia was observed at least once in 44% of patients, and 19.6% of all visits, figures comparable to previously reported studies [1]. While leucopenia was found in only 19.6% of all visits, over approximately two-thirds of these patients' episodes were recurrent. Most leucopenia episodes were driven by lymphopenia, which is recognized as the most common white blood cell abnormality among lupus patients [1]. Neutropenia was observed at least once in 12.5% of patients, lower than a recently reported European cohort [15], and occurred in 3.4% of visits. Severe neutropenia was rare (0.95%).
The examination of clinical associations of disease activity and medication use with lymphopenia and neutropenia can help clinicians evaluate the risk profile of these variables. Even though causality cannot be assumed in observational studies, we have shown that disease activity was a significant independent predictor of lymphopenia, consistent with the previously reported association of lymphopenia and disease activity [16].
Our study is the first per-visit analysis examining this association, taking into account the fluctuating nature of disease activity and medication use. The analysis showed a 30% increase in the risk of lymphopenia for visits with SLEDAI ≥6, or a 4% increase for each SLEDAI-2K point rise, whether we used the SLEDAI-2K or the modified version excluding the item of leucopenia. The relationship between disease activity and lymphopenia was further supported by the observation of an independently protective association with LLDAS, which is a composite state definition taking into account SLEDAI and PGA, and allowing the use of immunosuppression and low dose prednisolone. The protective effect of LLDAS on lymphopenia was consistent regardless of whether we omitted the item of leucopenia in the SLEDAI component of the LLDAS definition.
Neutropenia had a somewhat different profile of associations, and the relationship between neutropenia and overall disease activity is less clear. Like lymphopenia, after adjustment for confounders, neutropenia was significantly associated with elevated ESR, but not with SLEDAI-2K, IS use, or the modified LLDAS.
Our study was the first to comprehensively examine the different associations of medication use with lymphopenia and neutropenia in SLE. Mechanisms of leucopenia are complex, ranging from a direct or indirect effect on marrow production, ineffective maturation, increased immune-mediated cytotoxicity or apoptosis, or increased sequestration. Medications can affect haematological lineages differently, and they can interact with disease effects as well as with other medications.
Prednisolone use had the strongest association with lymphopenia, after adjustment. We also demonstrated that IS use was independently associated with lymphopenia. Of note, AZA, MTX, TAC, CYC and RTX were independently associated with lymphopenia, whereas MMF/MPA was not.
The associations of neutropenia with medication were somewhat surprising. Overall IS, when studied collectively, did not increase the risk of neutropenia. AM, on the other hand, was associated with increased risk of neutropenia, although we had shown that this was mostly when it was used in combination with another IS. These findings may demonstrate a degree of confounding by indication rather than a causal relationship, and we are observing other factors that may influence treatment decision-making of clinicians that may take into account prior experience and disease activity of patients. Our study has shown that among all IS, MTX and CyA use was associated with reduced risk of neutropenia.
These findings are highly relevant to clinicians. Among the IS, the myelosuppressive effect of some are better studied than others; for example, the relationship between deficiency of the enzyme thiopurine methyltransferase and AZA-induced leucopenia, which can be inherited or due to a functional deficiency, is well recognized [17, 18]. When SLE patients treated with drugs such as AZA present with neutropenia, the question of causation by drug vs disease activity is raised. Although caution is needed in ascribing causation based on observational data, our findings do not suggest a significant association between neutropenia and the IS drugs commonly used to treat SLE, suggesting that disease activity is a more likely cause, a finding supported by the association of neutropenia with ESR and negative association with LLDAS attainment.
MTX is also known for its myelosuppressive effect at high doses, but when used in low doses as in most SLE cases, leucopenia is not commonly observed [19]. In our study, we observed a protective association of MTX use with neutropenia. In the scenario in which patients with neutropenia require treatment with IS, this study would suggest MTX or CyA would make a reasonable choice based on the risk profile.
Finally, neutropenia is a well-described phenomenon after RTX treatment that can occur typically after 3–4 weeks. It can be protracted in some cases. In our study, the HR of lymphopenia and neutropenia was highest in association with RTX use among the IS and biologics. It was interesting to note a lack of association between CYC use and neutropenia, which could be due to our study design in which exposure to RTX or CYC was captured if the patient had received it in the preceding 6 months. It is well recognized that the myelosuppressive effect of intravenous CYC is often transient and best observed around day 7–10 following infusion [20].
This study has several important limitations. The study was done across multiple Asia Pacific lupus centres and is therefore a predominantly Asian cohort (89%). Genetic factors relating to ethnicity can certainly influence the severity and frequency of leucopenia; for example, we have already seen that our cohort had a lower frequency of neutropenia compared with another recent report of lupus patients in Europe [15]. Our findings, however, are still largely generalizable, as the overall prevalence of lymphopenia (which made up the majority of leucopenia), the patterns of disease activity and medication use were all similar to other cohorts. The clinical associations of disease activity and specific medications with leucopenia are valuable observations and can be generalized to other populations. Finally, we have used a combination of SLEDAI-2K (or SLEDAI-2K based endpoints) and ESR as best measures of disease activity. As SLEDAI-2K has components that are based on laboratory assessment of serological activity, the lack of homogeneity in laboratory assays, particularly for anti-dsDNA, is a limitation of this multicentre study. Nonetheless, the definition of SLEDAI-2K accepts anti-dsDNA values above the local laboratory reference, and the assessment of overall disease activity using this SLEDAI-2K definition is still considered the gold-standard.
Conclusion
Using a large, prospectively followed cohort, we have demonstrated independent, differential associations of lymphopenia and neutropenia in SLE. ESR, serological activity and, in the opposite direction, LLDAS were associated with both lymphopenia and neutropenia in multivariable analysis. While IS medications including AZA, MTX, TAC, CYC and RTX were independently associated with lymphopenia, only LEF and RTX use were associated with neutropenia; MTX and CyA were protective. AM use, particularly in combination with other medications, was associated with neutropenia. In SLE studies, lymphopenia and leucopenia are commonly grouped as leucopenia, including in disease activity measures such as SLEDAI-2K and hence treatment response measures used in clinical trials, such as SRI. Our findings suggest that lymphocyte and neutrophil counts should be recorded separately in the assessment of SLE.
Funding: The APLC received funding from AstraZeneca, Bristol-Myers Squibb, Eli Lilly, EMD Serono, GlaxoSmithKline, Janssen, and UCB Biopharma in support of its research activities. The funders had no role in study design, data analysis, results interpretation or writing of this manuscript.
Disclosure statement: The authors declare no conflict of interest.
Data availability statement
The data underlining this article cannot be publicly shared due to the strict protocols and procedures outlined in the Asia Pacific Lupus Collaboration (APLC) Data Access Policy to protect patients’ privacy and to maintain data security and ethical principles.
Supplementary data
Supplementary data are available at Rheumatology online.
Supplementary Material
References
- 1. Carli L, Tani C, Vagnani S, Signorini V, Mosca M.. Leukopenia, lymphopenia, and neutropenia in systemic lupus erythematosus: prevalence and clinical impact—a systematic literature review. Semin Arthritis Rheum 2015;45:190–4. [DOI] [PubMed] [Google Scholar]
- 2. Tan EM, Cohen AS, Fries JF. et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982;25:1271–7. [DOI] [PubMed] [Google Scholar]
- 3. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725. [DOI] [PubMed] [Google Scholar]
- 4. Petri M, Orbai AM, Alarcon GS. et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012;64:2677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aringer M, Costenbader K, Daikh D. et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis 2019;78:1151–9. [DOI] [PubMed] [Google Scholar]
- 6. Hepburn AL, Narat S, Mason JC.. The management of peripheral blood cytopenias in systemic lupus erythematosus. Rheumatology (Oxford) 2010;49:2243–54. [DOI] [PubMed] [Google Scholar]
- 7. Fayyaz A, Igoe A, Kurien BT. et al. Haematological manifestations of lupus. Lupus Sci Med 2015;2:e000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kandane-Rathnayake R, Golder V, Louthrenoo W. et al. Development of the Asia Pacific Lupus Collaboration cohort. Int J Rheum Dis 2019;22:425–33. [DOI] [PubMed] [Google Scholar]
- 9. Gladman DD, Ibanez D, Urowitz MB.. Systemic lupus erythematosus disease activity index 2000. J Rheumatol 2002;29:288–91. [PubMed] [Google Scholar]
- 10. Isenberg DA, Allen E, Farewell V. et al. An assessment of disease flare in patients with systemic lupus erythematosus: a comparison of BILAG 2004 and the flare version of SELENA. Ann Rheum Dis 2011;70:54–9. [DOI] [PubMed] [Google Scholar]
- 11. Petri M, Buyon J, Kim M.. Classification and definition of major flares in SLE clinical trials. Lupus 1999;8:685–91. [DOI] [PubMed] [Google Scholar]
- 12. Stoll T, Seifert B, Isenberg DA.. SLICC/ACR Damage Index is valid, and renal and pulmonary organ scores are predictors of severe outcome in patients with systemic lupus erythematosus. Br J Rheumatol 1996;35:248–54. [DOI] [PubMed] [Google Scholar]
- 13. Golder V, Kandane-Rathnayake R, Huq M. et al. Lupus low disease activity state as a treatment endpoint for systemic lupus erythematosus: a prospective validation study. Lancet Rheumatol 2019;1:e95–102. [DOI] [PubMed] [Google Scholar]
- 14. Amorim LD, Cai J.. Modelling recurrent events: a tutorial for analysis in epidemiology. Int J Epidemiol 2015;44:324–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meyer A, Guffroy A, Blaison G. et al. Systemic lupus erythematosus and neutropaenia: a hallmark of haematological manifestations. Lupus Sci Med 2020;7:e000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vila LM, Alarcon GS, McGwin G Jr, et al. ; Lumina Study Group. Systemic lupus erythematosus in a multi-ethnic US cohort, XXXVII: association of lymphopenia with clinical manifestations, serologic abnormalities, disease activity, and damage accrual. Arthritis Rheum 2006;55:799–806. [DOI] [PubMed] [Google Scholar]
- 17. Chen D, Lian F, Yuan S. et al. Association of thiopurine methyltransferase status with azathioprine side effects in Chinese patients with systemic lupus erythematosus. Clin Rheumatol 2014;33:499–503. [DOI] [PubMed] [Google Scholar]
- 18. Clunie GP, Lennard L.. Relevance of thiopurine methyltransferase status in rheumatology patients receiving azathioprine. Rheumatology (Oxford) 2004;43:13–8. [DOI] [PubMed] [Google Scholar]
- 19. Lim AY, Gaffney K, Scott DG.. Methotrexate-induced pancytopenia: serious and under-reported? Our experience of 25 cases in 5 years. Rheumatology (Oxford) 2005;44:1051–5. [DOI] [PubMed] [Google Scholar]
- 20. Poikonen P, Saarto T, Lundin J, Joensuu H, Blomqvist C.. Leucocyte nadir as a marker for chemotherapy efficacy in node-positive breast cancer treated with adjuvant CMF. Br J Cancer 1999;80:1763–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlining this article cannot be publicly shared due to the strict protocols and procedures outlined in the Asia Pacific Lupus Collaboration (APLC) Data Access Policy to protect patients’ privacy and to maintain data security and ethical principles.