Abstract
Objective
SSc reduces upper extremity function and performance of everyday activities; however, there are few evidence-based rehabilitation interventions. This study examined short and longer-term effects of two occupational therapy interventions on hand disability.
Methods
Participants with diffuse cutaneous SSc were randomized to one of two 18-week interventions: Intensive group, receiving eight weekly in-person occupational therapy sessions with App-delivered home exercises, or App Alone group. The primary outcome was QuickDASH hand disability; secondary outcomes were physical function (PROMIS scale), and total active hand motion. Linear mixed models were used to examine treatment effects.
Results
Most participants were female (72%); the mean age was 52 years (13.4) (n = 32). There were no significant between-group effects on QuickDASH (P = 1.0; mean change −6.4 on 0–100 scale in both groups at 18 weeks). Left lateral pinch, an exploratory outcome, improved in App Alone compared with Intensive from baseline to 18 weeks. Within groups, the Intensive group had the largest improvements after 8 weeks (−8.5 on QuickDASH; P = 0.03), but then lost gains from 8 to 18 weeks while the App Alone group had modest improvements from baseline to 8 weeks, but then continued to improve. Of completers, 50% had clinically meaningful improvement on QuickDASH in the Intensive group and 64% had improvement in App Alone.
Conclusion
Both interventions showed beneficial effects on hand disability. Participants in the App Alone group improved equally to the Intensive group at 18 weeks. Our findings provide support for further study into telehealth rehabilitation approaches.
Trial registration
Keywords: occupational therapy, rehabilitation, scleroderma and related disorders, hand, quality of life
Rheumatology key messages
This study provided a rigorous feasibility test of two replicable occupational therapy interventions.
The App Alone group had similar improvements to those in the Intensive intervention at 18 weeks.
Tailored rehabilitation delivered via App is a feasible method to reach and treat SSc.
Introduction
SSc or scleroderma is a rare, incurable and debilitating connective tissue disease. While drug therapies help control symptoms, patients with SSc face significant challenges managing a chronic condition that greatly impacts daily life. Upper extremity (UE) functional limitations from skin thickening and contractures develop early [1] and are associated with disability [2, 3] and reduced quality of life [4–6]. People with the diffuse subtype tend to have more severe disease within the first 5 years after diagnosis than people with the limited subtype, although both groups experience UE limitations.
Rehabilitation can mitigate SSc functional consequences, and occupational therapy typically addresses UE limitations. Despite the need for evidence-based treatments, there are only a few high-quality rehabilitation clinical trials [7, 8]. Most involve small samples, vary in dose or delivery, and are not UE-focused. The strongest evidence of effectiveness of SSc rehabilitation is from a trial in which 220 participants were randomized to individualized rehabilitation or usual care [9]. Intervention over 4 weeks included aerobic exercise, range of motion, functional adaptations, splinting, and mouth exercises. A written home exercise programme was provided. Self-reported disability and pain improved at 4 weeks and mobility at 6 months; however, most benefits dissipated by 12 months. This study demonstrated that intensive SSc rehabilitation treatment had short-term benefits. However, UE treatment did not include thermal modalities [10] or tissue mobilization [11, 12], which have supporting evidence and are clinically recommended, and included splinting, which has little evidence in SSc [13, 14]. Importantly, this study showed that home exercise impacted longer-term outcomes. Participants who adhered had better 12-month outcomes [9]; however, most participants had poor adherence, possibly due to passive administration of the programme.
The current trial, called Rehabilitation for Arm Coordination and Hand Movement in SSc (REACH), examined effects of an intensive occupational therapy intervention vs App-delivered home exercise alone. In a preliminary trial, we found that participants with diffuse cutaneous SSc had high adherence to 8-week occupational therapy intervention and demonstrated significant, clinically meaningful improvements in patient-reported and objective functional measures [15]. Participants were provided a written home exercise programme, but adherence was not tracked. Due to rarity of SSc and that these patients often travel long distances to receive care, home exercise engagement is critical to self-manage SSc functional consequences. An App to increase exercise engagement allows for objective tracking and standardized prescription processes. The objective of this study was to examine the short- and longer-term effects of two occupational therapy interventions on hand disability for people with diffuse cutaneous SSc. We hypothesized that compared with participants in the App Alone group, participants in the Intensive group would have statistically significant improvements in hand disability (measured by QuickDASH), physical function, and total active hand function (TAM) following eight weekly in-person sessions and after follow-up (18 weeks).
Methods
Design
We conducted a two-arm pilot randomized controlled trial in which participants were randomized in blocks of four into the Intensive group, which involved occupational therapy intervention plus App-delivered home exercise, or the App Alone group. We were primarily interested in examining effects of the interventions; however, as we were trying the App for the first time as an intervention strategy, we conducted a pilot trial [16]. Our target sample size was 50. Outcome assessments were at baseline, following in-person sessions in the Intensive group (8 weeks) and at follow-up (an additional 10 weeks in which all participants were asked to continue home exercises via the App). This project was approved by University of Michigan Medical School’s Human Subjects Review Board.
Sample
Recruitment took place at the Scleroderma Program at Michigan Medicine where around 1500 patients receive care from rheumatologists who specialize in scleroderma. Potentially eligible participants were approached in clinic after screening their medical records. Interested people could also contact the study team from fliers, website postings, targeted social media ads, or from an online university research participant portal. To be eligible, participants needed to be age ≥18 years; have a diagnosis of diffuse cutaneous SSc; have a contracture of the hand and joint in at least one arm (e.g. wrist or elbow) with ability to demonstrate active range of motion; English-speaking; have ‘early’ disease (≤5 years from first non-Raynaud symptom); have no concurrent medical issues; and willing to travel to Michigan Medicine for study visits. Participants needed to have an Android, iPhone, iPad or tablet to load the App.
Procedure
Individuals eligible from screening by research staff were scheduled for a baseline session. After obtaining written consent, the study occupational therapist conducted additional screening to determine the presence of contractures per inclusion criteria and if that criterion was met, she initiated the baseline assessments. The participant was then randomized into treatment arm. The therapist conducted all treatments and outcome assessments so was not able to be blinded. Participants were guided through downloading the App and prescribed a home exercise programme tailored by the therapist via clinical dashboard. The participant was then instructed on how to access and track exercises. The therapist checked on progress via dashboard, communicated with the participant as needed, and uploaded other information (such as digital ulcer management and home paraffin wax treatment) weekly on the App for all participants. Participants randomized into App Alone scheduled 8-week and 18-week assessments. Participants randomized into Intensive group received their first therapy session following baseline assessment and were scheduled for seven weekly therapy visits. All participants were asked to come in person for assessments. Participants were given a separate survey to ascertain treatment satisfaction.
Intensive group
The eight weekly in-person sessions, described in detail elsewhere [15], consisted of 60 min of treatment-preparatory thermal modalities, tissue mobilization using the Lymphatouch device (Lymphatouch LLC), and UE passive and active range of motion exercises. The in-person treatment was performed in this specific order to increase flexibility and prepare for functional movement. For example, each participant began with hot packs and/or paraffin wax treatment on the hands and ended with active range of motion exercises such as manipulating small foam cubes or rolling putty. Participants were also asked to participate in therapist-prescribed exercises using the App.
App-delivered home exercise in both groups
The Pt Pal App (Health Tech Pal Corp) was the UE exercise delivery method. There were 27 exercises possible involving passive or active UE stretching and included tendon gliding (see Supplementary Table 1, available at Rheumatology online). The therapist prescribed most of the exercises with a standard number of sets and repetitions, but tailored programmes based upon participant capabilities. Participants were asked to perform exercises daily at minimum. Short exercise videos were recorded with therapist narration so they could follow along (see Fig. 1). Time participating in each exercise was recorded. Estimated time needed to complete all exercises once daily differed by participant, but this sample averaged 45 min.
Fig. 1.
App screenshots for home exercise
Outcomes
Assessments occurred at baseline, 8 weeks, and 18 weeks. The primary outcome was QuickDASH, a reliable, validated self-reported UE function measure used in the SSc population [17]. Difficulty, interference and symptom severity in performing different UE tasks were rated and the item average was converted to a 0–100 scale in which worse function is indicated by higher scores. The minimal clinically important difference (MCID) has not been established in SSc; in related samples, MCIDs of 8 points [18] and 16 points have been reported [19].
Secondary outcomes included both self-reported and objective measures. PROMIS Physical Function v.2, 8-item short form was used to assess physical function. PROMIS scoring involves converting raw scores to a T-metric where the US population mean is 50 (s.d. of 10), and better function is indicated by a higher score [20]. Two-point improvement is considered clinically meaningful [21]. TAM was evaluated bilaterally, calculated by measuring degrees of movement in each digit via goniometer and summing total degrees [22]. A total score of 1175 degrees was possible for each hand. Exploratory measures included 9-hole peg test, an assessment of hand dexterity and coordination [23], and handgrip strength measured in kilograms of pressure via Jamar dynamometer (Lafayette Instruments, IL) [24]; six trials were performed, three on each arm and the maximum value was used. Other exploratory outcomes included pinch strength, average of three trials using a pinch gauge in the left and right hand [24], and skin thickness assessed by a rheumatologist not part of the team using modified Rodnan skin thickness score (mRSS) [25].
Home exercise adherence
Because of limited information about how people with SSc engage with Apps and their expected adherence, we examined utility of two methods: daily App engagement and adherence ‘quality’. Both methods provide different objective information, unlike participant logs. We selected daily App engagement as our primary method because it accommodated different ways that participants used the App. Some participants reported only opening the App to obtain the exercises and some reported they only viewed the more difficult exercises to perform. Daily App engagement was measured by whether participants opened the App each day (1 = yes, 0 = no), which was summed and divided by total days spent in the study from baseline to 18-week assessment. This adherence measure was calculated for two time periods (baseline to 8-week assessment; 8 weeks to 18 weeks). Adherence quality was based on time spent viewing exercise videos. Each participant’s prescribed exercise time was used as the denominator to calculate daily percent adherence. For example, if a person was prescribed 45 min of exercises, 100% daily adherence would be 45 min and 33% adherence would be 15 min. Percentage of time spent performing exercises was aggregated similar to daily App engagement (baseline to 8 weeks and 8–18 weeks).
Sample size determination
The study was powered taking into account the linear mixed-model analysis and utilized missing data estimates from our previous study [15]. With 50 participants, we estimated having 90% power to detect a difference in QuickDASH that would be at least half of the 14-point improvement achieved by our preliminary sample.
Data analysis
Descriptive statistics were used to summarize background variables for the sample and by treatment arm. Baseline variables were compared using t tests, Fisher’s exact and χ2 tests. The primary analysis included 32 participants allocated to treatment arms with 2–7 participants having missing data at various time points. Missing data in these outcome measures were imputed as recommended by established guidelines [26]. We used PROC MI and PROC MI ANALYZE in SAS to create five imputed datasets and analysed pooled estimates from these iterations. Linear mixed models were performed to measure primary and secondary outcome measures. In these models, change in measure was the outcome, and interaction terms of treatment arm X time point (8 or 18 weeks) were used to examine treatment effects by arm. Least square means (LSM) at each time point by treatment arm are presented. The same models were used to examine exploratory outcomes. Adherence was examined using descriptive statistics and plotting. Post hoc analyses included proportion of people who achieved a clinically meaningful change in outcome measures.
Results
Participants
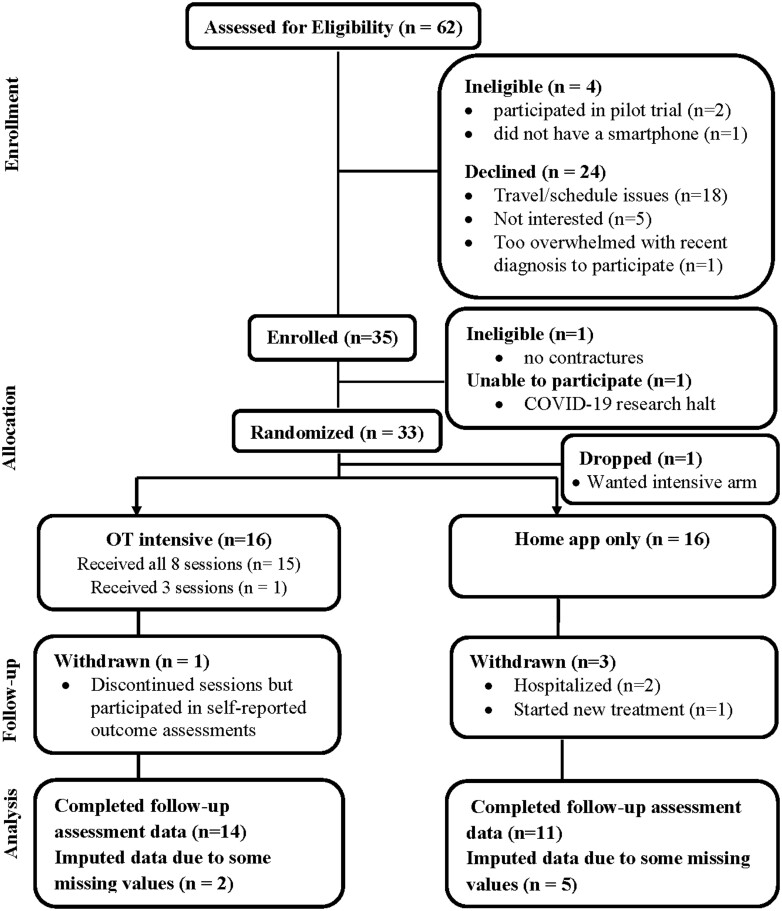
Participants were mainly approached in clinic; of 62 people approached, 57% enrolled (Fig. 2). The main reason for not enrolling was travel and scheduling issues (i.e. too far away or unable to meet on days dedicated to research in clinic); reported by 29% of people approached. Participants were initially enrolled in the study if they met eligibility by research staff (n = 35), but two of them were deemed ineligible because they had participated in our pilot trial or they had no UE contractures as determined by occupational therapist examination. Of 33 people randomized, 32 participated (16 in Intensive; 16 in App Alone). The study was prematurely discontinued due to Covid-19 as in-person research was temporarily suspended at the university, which prevented rehabilitation sessions in one treatment arm. Restarting the trial to achieve the final sample size was not feasible for a variety of reasons (cost, uncertainty, etc.); the decision was made to evaluate the feasibility on what had been completed.
Fig. 2.
REACH trial study flow diagram
In the Intensive group, 94% (15/16) received all eight sessions. One person who withdrew from treatment after three sessions completed self-reported measures remotely. In the App Alone, three people became ineligible due to being hospitalized or starting new treatment (two within the first 8 weeks, one after 8 weeks) and two others had incomplete 18-week QuickDASH data. The sample’s mean age was 52 years (range 20–73); 72% were female and 22% were African American (Table 1). They had a mean (s.d.) mRSS of 19.6 ( 9.4) indicative of moderate skin disease. Mean disease duration from diagnosis was 1.2 years (1.6).
Table 1.
Baseline characteristics of participants with diffuse SSc
| Characteristics | Sample (n = 32) |
Intensive Group (n = 16) |
App Alone Group (n = 16) |
|---|---|---|---|
| Age | 51.6 (13.4) | 48.5 (12.6) | 54.8 (13.7) |
| Female (%, n) | 71.9% (23) | 75% (12) | 68.8% (11) |
| African American (%, n) | 21.8% (7) | 18.8% (3) | 25.0% (4) |
| Hispanic/Latino (%, n) | 3.1% (1) | 6.3% (1) | 0 0 |
| Married (%, n) | 56.3% (18) | 50% (8) | 62.5%(10) |
| High school education (%, n) | 25% (8) | 25%(4) | 25% (4) |
| Modified Rodnan skin score (mean, s.d., possible range 0–51) | 19.6 (9.4) | 22.7 (11.1) | 16.5 (6.3) |
| Handgrip strength (maximum)(kg/pressure, mean, s.d.) | 21.1 (9.5) | 20.3 (9.9) | 21.8 (9.5) |
| Right 3 tip pinch strength (kg/pressure, mean, s.d.) | 5.6 (1.9) | 5.6 (2.0) | 5.5 (2.0) |
| Left 3 tip pinch strength (kg/pressure, mean, s.d.) | 5.4 (1.5) | 5.3 (1.5) | 5.5 (1.6) |
| Right 9-hole peg test (sec, mean, s.d.) | 26.6 (11.8) | 24.2 (12.1) | 29.0 (11.3) |
| Left 9-hole peg test (sec, mean, s.d.) | 24.8 (9.1) | 23.0 (5.6) | 26.6 (11.5) |
| QuickDASH disability score (mean, s.d., possible range 0–100) | 48.8 (21.5) | 53.5 (22.2) | 44.0 (20.4) |
| PROMIS physical function (t score, mean, s.d.) | 37.3 (6.2) | 37.3 (7.5) | 37.2 (4.9) |
| PROMIS satisfaction with participation (t score, mean, s.d.) | 38.0 (9.8) | 37.0 (10.6) | 39.0 (9.1) |
| Left hand total active motion (mean, s.d.) | 806.6 (176.0) | 789.8 (184.3) | 823.4 (171.6) |
| Right hand total active motion (mean, s.d.) | 754.8 (167.7) | 747.0 (149.1) | 762.6 (189.1) |
Note: For QuickDASH, a higher score denotes more disability. PROMIS measures are on a t metric in which the mean = 50 and s.d. = 10. A higher score denotes more physical function or satisfaction. For right and left total active motion the maximum possible is 1175 degrees.
Participants had moderate baseline disability on QuickDASH [mean score of 48.8 out of 100 (s.d. = 21.5)], and had low physical function (>1 s.d. below US population). Left and right hand TAM was 807 and 755 degrees, respectively, 64–69% of complete unrestricted active motion. Handgrip strength and pinch strength measures were well below age- and sex-matched norms. For example, the mean handgrip strength in this sample for women was 17.4 kg/pressure and for men was 30.1 kg/pressure, which are most similar to norms of healthy adults 75 and over (19.3 kg/pressure for women and 29.8 kg/pressure for men; right hand) [24].
Primary outcome
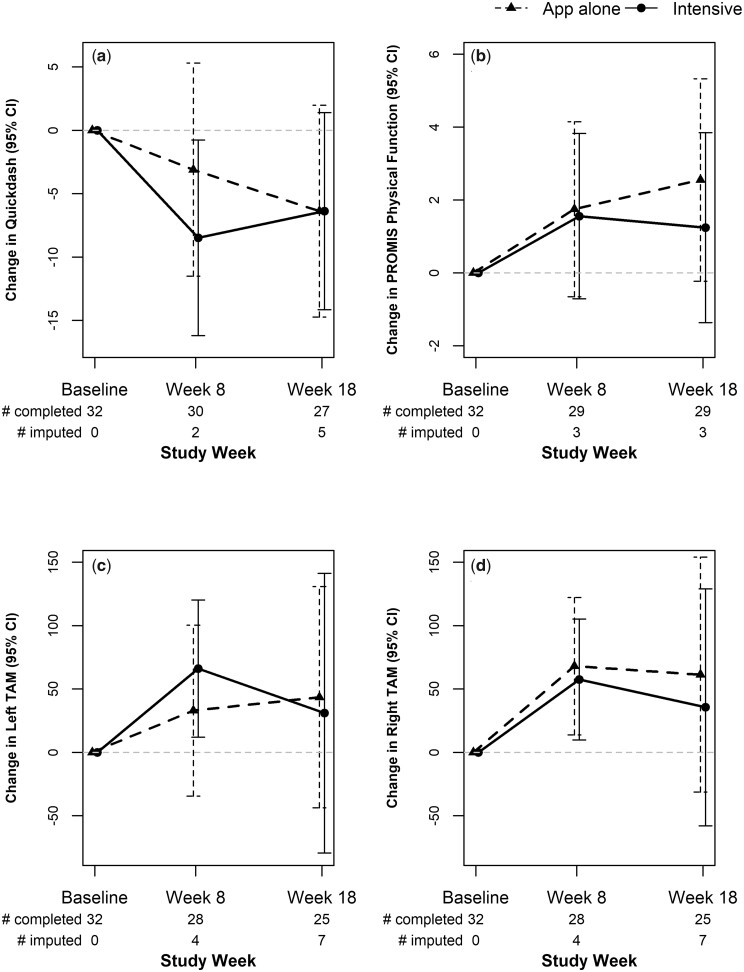
There were no significant between-group differences on QuickDASH at 8 and 18 weeks (LSM difference of treatment arm = −2.7; 95% CI: −12.9, 7.6). Fig. 3a depicts LSM for each group, which is the interaction of week by arm. Each group had mean improvements in QuickDASH scores. Improvements were significant and clinically meaningful in the Intensive group at 8 weeks [−8.5 (3.9); P = 0.03], but not significantly different from improvement shown in App Alone [−3.1 (4.3); P = 0.47]. At 8 weeks, 38% achieved a MCID of ≥8 points in the Intensive group (n = 16) vs 21% in App Alone (n = 14). While improvements were modest in App Alone at 8 weeks, participants continued to improve over 18 weeks, where the Intensive group lost some gains made from weekly sessions after they ended. At 18 weeks, both groups achieved equivalent improvement on QuickDASH (mean of −6.4 points). In total 50% of participants achieved an MCID of ≥8 points in the Intensive group (n = 16) and 64% in App Alone (n = 11).
Fig. 3.
Treatment group least square mean changes over the study period for primary and secondary outcomes
(a) QuickDASH, (b) PROMIS physical function, (c) Left total active range of motion, (d) Right total active range of motion. For QuickDASH (a), negative change denotes improvement. For all others, positive change denotes improvement.
Secondary outcomes
No significant between-group improvements were found for physical function, or TAM (Fig. 3b). Within-group improvements were shown for TAM; the Intensive group had significant gains at 8 weeks for the left hand [LSM 66 (28) degrees of added motion, P = 0.02] and for the right hand [LSM 57 (24) degrees of added motion, P = 0.02], but gains decreased at 18 weeks to means of 31 and 36 degrees of TAM for left and right hand, respectively. The App Alone group had significant improvement in TAM (right hand) at 8 weeks [68 (27) degrees of added motion], which worsened at 18 weeks to 61 degrees (Fig. 3c and d). Supplementary Table 2, available at Rheumatology online, shows the values at each time point that correspond to Fig. 3.
Exploratory analyses
Participants in App Alone had significantly improved lateral pinch in the left hand compared with participants in the Intensive group from baseline to 18 weeks [LSM difference 0.64 (0.27) kg/pressure]. There were no other between-group differences in exploratory outcomes. Participants within each treatment group had significant improvements in 9-hole peg test at one or both time points for the right hand and at 18 weeks for the left hand. Handgrip was improved in the App Alone group over time, but not in the Intensive group. Improvement in mRSS was seen within the Intensive group only after 8 weeks.
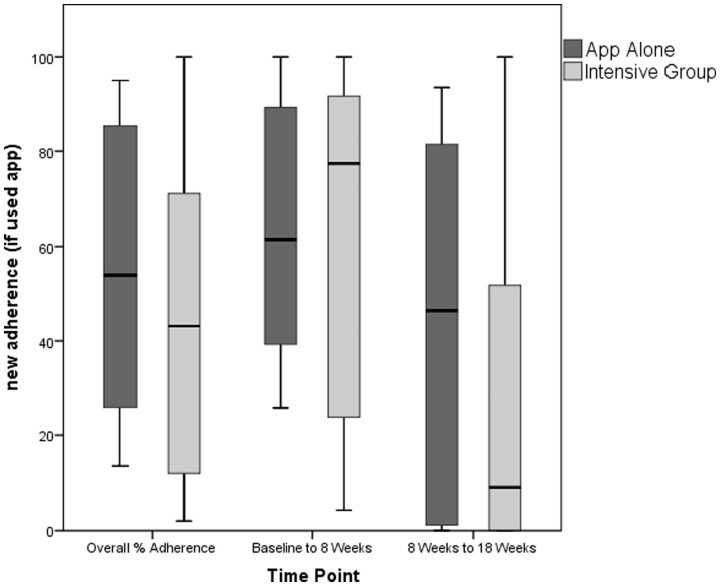
Adherence
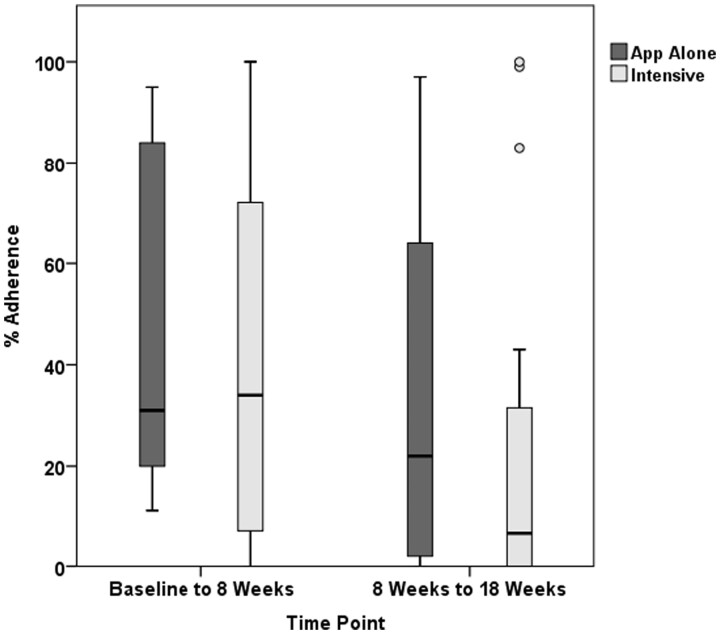
Overall median sample adherence was 49%, meaning participants engaged with the App almost half of all study days. For the Intensive group, the median was 43%; in the App Alone group it was 55% (Fig. 4). From baseline to 8 weeks, the Intensive group had higher App usage than App Alone (median of 77% to 62%, respectively), but this pattern reversed from 8–18 weeks, where the App Alone group had 46% adherence compared with 9% adherence in the Intensive group. With regard to adherence quality, median adherence was 25%, meaning that participants followed along to videos about one quarter of the time. Median adherence quality was higher in the Intensive group compared with App Alone in the first 8 weeks (34% vs 31%), but decreased over follow-up (Fig. 5). In App Alone, median adherence quality was 22% while the Intensive group had a median of 7%. When asked if they perform exercises without the App, 50% of 24 respondents at 18 weeks said that they regularly performed some or all exercises without the App, and eight people reported they performed all exercises without the App. Participants could retain the App and continue use after the study. From objective data collected, 69% of App Alone continued using the App, while only 29% of the Intensive group continued.
Fig. 4.
Adherence to app by participants assigned to App Alone or Intensive group
Fig. 5.
Adherence quality measured by time spent viewing guiding videos by group
Impression of change and satisfaction
Participants were sent a follow-up survey of which 21/32 participants (66%) responded (13 in Intensive; 8 in App Alone). When asked how they felt since they started the study, 77% of the Intensive group felt they improved at least minimally due to intensive treatment; while 100% in App Alone reported improvements. Most frequently reported improvements were in strength, coordination and pain, such as ‘I can now use a screwdriver, open jars, grip and hold onto something’, and ‘I can hold a hairbrush and toothbrush better’.
Discussion
We examined effects of two interventions to improve UE function in early diffuse cutaneous SSc. There were no significant between-group effects primary or secondary outcomes; however, each group demonstrated improvements with similar benefits by 18 weeks despite differences in treatment mode and dose. Some within-group improvements could be regression towards the mean although they were not specifically selected on the outcome measure per se.
There are few high-quality rehabilitation clinical trials [8]; of those, only one focused on UE rehabilitation [12]. Our Intensive intervention consisted of ≥1 components that have been supported in small studies including thermal treatments [27, 28], negative pressure treatment [15], stretching and range of motion [29, 30]. Participants in the Intensive group had statistically, clinically significant UE function improvements at 8 weeks, consistent with our preliminary study [15], and provides additional evidence supporting use of this protocol for patients with diffuse cutaneous SSc. Although larger studies are needed to examine between-group effects, this treatment protocol provides an evidence-based resource for therapists, which is important because SSc is not commonly seen in clinic settings. The in-person treatment protocol was designed with translation into clinical practice in mind, striking a balance between replicability and flexibility. However, more treatment tailoring may be needed. Research by Maddali-Bongi and colleagues supports treatment tailored to disease complications [12, 31]. They examined effects of manual lymph drainage on hand volume and function in patients with oedematous SSc and improvements were demonstrated and maintained at follow-up [12]. In our study, the therapist made minor adaptations to tailor treatment; however, all components were performed with all participants despite having heterogeneous complications. In future studies, it may increase treatment potency by developing different treatment protocols for specific complications such as oedema or tendon friction rubs.
Although short-term statistically significant effects were found after the intensive intervention, they were not as robust as effects estimated from our preliminary study. Further, improvements waned after in-person sessions ended. Rannou and colleagues also found that short-term benefits dissipated over follow-up [9]. The authors attributed this to lack of home exercise adherence. Despite having an App with exercise videos and instruction to continue, participants in our Intensive group markedly dropped App use after in-person sessions, from 77% median adherence during the first 8 weeks to 9% during follow-up. In contrast, the App Alone group had higher median adherence (62% during the first 8 weeks; 46% during follow-up). They also made steady gains in UE function, roughly equivalent to the Intensive group. Improvement in lateral pinch strength in the left hand for participants in the App Alone group was significantly greater than for participants in the Intensive group; however, additional studies are needed to better understand group differences in objective UE function. Participants in App Alone were more likely to continue using the App after the study. These findings support the use of a less intensive, but more sustainable approach to managing SSc disease manifestations. The main reason people did not enroll in this study was due to travel and scheduling constraints of in-person visits. Delivery of rehabilitation through telehealth offers several benefits for SSc clinical management, particularly increasing access to care—especially important for people with rare disease who lack access to specialty centres.
This study provides insights into how people with SSc interact with a home exercise App and the ability to track adherence. Our median adherence rate of 49% was lower than others [29, 31]; however, prior studies used self-reported adherence, which may have response bias. Further, our home exercises were more time-intensive (requiring ∼45 min); other studies estimated that daily exercises took 20–30 min [9, 31]. Measuring adherence quality using time spent watching videos was a rigorous measurement, but it did not allow for adaptation of how exercises were completed. Not all participants liked viewing videos; some reported doing all or some exercises without videos. Our adherence measure of opening the App did not provide feedback on exercise performance or time. In future studies, it may be better to have people self-track adherence on the App after each set to allow for flexibility without sacrificing rigour.
Limitations and strengths
Our RCT was underpowered as we had to stop recruitment due to the Covid-19 pandemic. With our planned 50 participants, the study would have achieved >80% power assuming 25 per arm for a mean difference of 5 (s.d. of 4.2). While any superiority study not showing significant differences is subject to type II errors, the longer-term adherence in the App Alone group presents an interesting finding along with the short-term effects of little difference. A larger study is needed to show between-group effects and further provide information on predictors of compliance and response. Second, the treating therapist performed the outcome assessments and was not blinded, but this might have been thought to bias away from the null hypothesis. Adherence was affected by issues with smartphones, especially early in the study, but they were resolved as they arose by the App developers. While it is a strength to have sampled early diffuse cutaneous SSc patients, findings can only be generalized to this group. Other strengths include making home exercise available and trackable via App, and insight about participant interaction with an App. A sustainable platform for use in multi-site studies was developed and has potential uptake by health systems.
In conclusion, we found no significant between-group effects between an intensive intervention and an App alone; however, there were positive effects on UE function for each group. Use of the App alone with remote therapist interaction was acceptable, feasible and perceived positively. Further study is needed using telehealth to deliver occupational therapy with an App in SSc.
Funding: This work was supported by an Established Investigator Research grant from the Scleroderma Foundation (grant# 006605, S.L.M./D.K., co-PIs). D.K.’s work was also supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases (K24-AR-063129).
Disclosure statement: S.L.M. received separate funding from Lymphatouch LLC. D.K. is consultant to Acceleron, Abbvie, Actelion, Amgen, Bayer, BMS, Boehringer Ingelheim, CSL Behring, Corbus, Galapagos, Genentech/Roche, GSK, Horizon, MitsubishiTanabe Pharma, Sanofi-Aventis, and United Therapeutics. He has stock options in Eicos Sciences, Inc.
The other authors have declared no conflicts of interest.
Data availability statement
The data underlying this article will be shared on reasonable request to the corresponding author.
Supplementary data
Supplementary data are available at Rheumatology online.
Supplementary Material
References
- 1. Bálint Z, Farkas H, Farkas N. et al. A three-year follow-up study of the development of joint contractures in 131 patients with systemic sclerosis. Clin Exp Rheumatol 2014;32(6 Suppl 86):S68–74. [PubMed] [Google Scholar]
- 2. Sandqvist G, Eklund M, Åkesson A, Nordenskiöld U.. Daily activities and hand function in women with scleroderma. Scand J Rheumatol 2004;33:102–7. [DOI] [PubMed] [Google Scholar]
- 3. Sandqvist G, Scheja A, Hesselstrand R.. Pain, fatigue and hand function closely correlated to work ability and employment status in systemic sclerosis. Rheumatology 2010;49:1739–46. [DOI] [PubMed] [Google Scholar]
- 4. Frantz C, Avouac J, Distler O. et al. Impaired quality of life in systemic sclerosis and patient perception of the disease: a large international survey. Semin Arthritis Rheum 2016;46:115–23. [DOI] [PubMed] [Google Scholar]
- 5. Khanna D, Yan X, Tashkin DP. et al. ; Scleroderma Lung Study Group. Impact of oral cyclophosphamide on health-related quality of life in patients with active scleroderma lung disease: results from the scleroderma lung study. Arthritis Rheumatol 2007;56:1676–84. [DOI] [PubMed] [Google Scholar]
- 6. Maddali-Bongi S, Rosso AD, Mikhaylova S. et al. Impact of hand and face disabilities on global disability and quality of life in systemic sclerosis patients. Clin Exp Rheumatol 2014;32(6 Suppl 86):S15–20. [PubMed] [Google Scholar]
- 7. Poole JL. Musculoskeletal rehabilitation in the person with scleroderma. Curr Opin Rheumatol 2010;22:205–12. [DOI] [PubMed] [Google Scholar]
- 8. Willems LM, Vriezekolk JE, Schouffoer AA. et al. Effectiveness of nonpharmacologic interventions in systemic sclerosis: a systematic review. Arthritis Care Res 2015;67:1426–39. [DOI] [PubMed] [Google Scholar]
- 9. Rannou F, Boutron I, Mouthon L. et al. Personalized physical therapy versus usual care for patients with systemic sclerosis: a randomized controlled trial. Arthritis Care Res 2017;69:1050–9. [DOI] [PubMed] [Google Scholar]
- 10. Mancuso T, Poole J.. The effect of paraffin and exercise on hand function in persons with scleroderma: a series of single case studies. J Hand Ther 2009;22:71–8. [DOI] [PubMed] [Google Scholar]
- 11. Maddali Bongi S, Del Rosso A, Galluccio F. et al. Efficacy of a tailored rehabilitation program for systemic sclerosis. Clin Exp Rheumatol 2009;27(3 Suppl 54):S44–50. [PubMed] [Google Scholar]
- 12. Maddali BS, Del Rosso A, Passalacqua M, Miccio S, Cerinic MM.. Manual lymph drainage improving upper extremity edema and hand function in patients with systemic sclerosis in edematous phase. Arthritis Care Res 2011;63:1134–41. [DOI] [PubMed] [Google Scholar]
- 13. Harvey LA, Katalinic OM, Herbert RD. et al. Stretch for the treatment and prevention of contractures. Cochrane Database Syst Rev 2017;1:1–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Seeger MW, Furst DE.. Effects of splinting in the treatment of hand contractures in progressive systemic sclerosis. Am J Occup Ther 1987;41:118–21. [DOI] [PubMed] [Google Scholar]
- 15. Murphy SL, Barber M, Homer K. et al. Occupational therapy treatment to improve upper extremity function in individuals with early systemic sclerosis: a pilot study. Arthritis Care Res 2018;70:1653–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thabane L, Ma J, Chu R. et al. A tutorial on pilot studies: the what, why, and how. BMC Med Res Methodol 2010;10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kennedy CA, Beaton DE, Smith P. et al. Measurement properties of the QuickDASH (Disabilities of the Arm, Shoulder and Hand) outcome measure and cross-cultural adaptations of the QuickDASH: a systematic review. Qual Life Res 2013;22:2509–47. [DOI] [PubMed] [Google Scholar]
- 18. Mintken PE, Glynn P, Cleland JA.. Psychometric properties of the shortened disabilities of the Arm, Shoulder, and Hand Questionnaire (QuickDASH) and Numeric Pain Rating Scale in patients with shoulder pain. J Shoulder Elbow Surg 2009;18:920–6. [DOI] [PubMed] [Google Scholar]
- 19. Franchignoni F, Vercelli S, Giordano A. et al. Minimal clinically important difference of the disabilities of the arm, shoulder and hand outcome measure (DASH) and its shortened version (QuickDASH). J Orthop Sports Phys Ther 2014;44:30–9. [DOI] [PubMed] [Google Scholar]
- 20. Cella D, Riley W, Stone A, PROMIS Cooperative Group et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005. J Clin Epidemiol 2010;63:1179–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hays RD, Spritzer KL, Fries JF, Krishnan E.. Responsiveness and minimally important difference for the Patient-Reported Outcomes Measurement Information System (PROMIS) 20-item physical functioning short form in a prospective observational study of rheumatoid arthritis. Ann Rheum Dis 2015;74:104–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Adams L, Greene L, Topoozian E.. Range of motion. In: American Society of Hand Therapists, ed. Clinical Assessment Recommendations, 2nd edn. Chicago: The Society, 1992: 55–70. [Google Scholar]
- 23. Grice KO, Vogel KA, Le V. et al. Adult norms for a commercially available nine hole peg test for finger dexterity. Am J Occup Ther 2003;57:570–3. [DOI] [PubMed] [Google Scholar]
- 24. Mathiowetz V, Weber K, Volland G, Kashman N.. Reliability and validity of grip and pinch strength evaluations. J Hand Surg 1984;9:222–6. [DOI] [PubMed] [Google Scholar]
- 25. Clements P, Lachenbruch P, Seibold J. et al. Skin thickness score in systemic sclerosis: an assessment of interobserver variability in 3 independent studies. J Rheumatol 1993;20:1892–6. [PubMed] [Google Scholar]
- 26. Moher D, Hopewell S, Schulz KF. et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pils K, Graninger W, Sadil F.. Paraffin hand bath for scleroderma. Euro J Phys Rehabil Med 1991;1:19–21. [Google Scholar]
- 28. Sandqvist G, Åkesson A, Eklund M.. Evaluation of paraffin bath treatment in patients with systemic sclerosis. Disabil Rehabil 2004;26:981–7. [DOI] [PubMed] [Google Scholar]
- 29. Landim SF, Bertolo MB, Marcatto de Abreu MF. et al. The evaluation of a home-based program for hands in patients with systemic sclerosis. J Hand Ther 2019;32:313–21. [DOI] [PubMed] [Google Scholar]
- 30. Mugii N, Hasegawa M, Matsushita T. et al. The efficacy of self-administered stretching for finger joint motion in Japanese patients with systemic sclerosis. J Rheumatol 2006;33:1586–92. [PubMed] [Google Scholar]
- 31. Maddali Bongi S, Del Rosso A, Galluccio F. et al. Efficacy of connective tissue massage and Mc Mennell joint manipulation in the rehabilitative treatment of the hands in systemic sclerosis. Clin Rheumatol 2009;28:1167–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.