Abstract
Dominance inhibition of shoot growth by fruit load is a major factor that regulates shoot architecture and limits yield in agriculture and horticulture crops. In annual plants, the inhibition of inflorescence growth by fruit load occurs at a late stage of inflorescence development termed the end of flowering transition. Physiological studies show this transition is mediated by production and export of auxin from developing fruits in close proximity to the inflorescence apex. In the meristem, cessation of inflorescence growth is controlled in part by the age-dependent pathway, which regulates the timing of arrest. Here, we show the end of flowering transition is a two-step process in Arabidopsis (Arabidopsis thaliana). The first stage is characterized by a cessation of inflorescence growth, while immature fruit continues to develop. At this stage, dominance inhibition of inflorescence growth by fruit load is associated with a selective dampening of auxin transport in the apical region of the stem. Subsequently, an increase in auxin response in the vascular tissues of the apical stem where developing fruits are attached marks the second stage for the end of flowering transition. Similar to the vegetative and floral transition, the end of flowering transition is associated with a change in sugar signaling and metabolism in the inflorescence apex. Taken together, our results suggest that during the end of flowering transition, dominance inhibition of inflorescence shoot growth by fruit load is mediated by auxin and sugar signaling.
Dominance inhibition of inflorescence shoot growth by fruit load involves auxin and sugar signaling during the end of flowering transition.
Introduction
Understanding how growing units in a shoot system are regulated, including apical and lateral buds, as well as fruits, is a key to developing elite breeding lines and management tools aimed at optimizing plant architecture and increasing yield in agriculture and horticulture crops (Teichmann and Muhr, 2015; Guo et al., 2020). The activity and development of apical and lateral buds, as well as fruits, are controlled by light, temperature, hormone, sugar, and nutrient signaling (Montgomery, 2008; Pfeiffer et al., 2017; Barbier et al., 2019). Moreover, these endogenous and environmental signaling pathways facilitate communication between the growing shoot apex and lateral sinks (meristems or fruits) to ensure plants adopt the appropriate architecture and reproductive capacity based on carbohydrate, nutrient, and water availability (Walker and Bennett, 2018; Barbier et al., 2019).
The correlative or dominance inhibition hypothesis predicts that sinks with a high growth potential inhibit the growth of younger or subordinate organs with a lower sink activity (Bangerth, 1989; Smith and Samach, 2013; Walker and Bennett, 2018). As a result, the dominant sink is able to direct water, assimilates, and nutrients required for growth and development. Dominance inhibition occurs among fruits within an inflorescence or between apical and axillary buds within a shoot. Interestingly, in perennial tree crops, dominance inhibition between shoots and fruits is highly plastic, as changes in the growth potential between these competing sinks can change over the course of season. For example, a high rate of immature fruit abscission in late spring/early summer is associated with the outgrowth of preformed vegetative shoots in avocado (Persea americana Mill; Salazar-García et al., 2013). Therefore, it has been hypothesized that dominance exerted by the vegetative shoot with a high growth potential results in the abscission of developing fruitlets, which have a low growth potential. However, as “retained” avocado fruitlets enter a phase of rapid growth and the sink potential increases, dominance between shoots and fruits switches and shoot growth is inhibited by the developing fruit (Salazar-García et al., 1998; Ziv et al., 2014). Dominance inhibition of shoot growth by fruit load is a major driver of biennial or alternate bearing in which the outgrowth of late season vegetative flushes is severely reduced in trees carrying a high crop load (Bangerth, 1989; Smith and Samach, 2013). As new inflorescences primarily develop from the late season vegetative flushes, a severe reduction in flowering and yield occurs the following year. Therefore, dynamic dominance interaction between developing fruits and shoots are of significant interest, as fruit abscission and the inhibition of shoot growth by fruit load significantly reduces yield in tree crops (Samach and Smith, 2013; Smith and Samach, 2013; Sawicki et al., 2015).
Auxin is a major regulator of dominance inhibition of lateral buds by the growing shoot apex, termed apical dominance (Barbier et al., 2019; Schneider et al., 2019), as well as among developing fruits within an inflorescence shoot (Bangerth et al., 2000; Smith and Samach, 2013; Walker and Bennett, 2018). In both cases, dominance inhibition is initiated by the biosynthesis and basipetal transport of auxin from the growing shoot apex or dominant fruit. For apical dominance, the polar auxin transport system (PATS) channels auxin basipetally in the stem in association with the vascular tissues (Galweiler et al., 1998). A local auxin transport system called the connective auxin transport system (CATS) also distributes this hormone in stem tissues (Bennett et al., 2016; van Rongen et al., 2019). Together, movement of auxin via the PATS and CATS indirectly inhibits bud outgrowth (Barbier et al., 2019). The canalization hypothesis predicts that a high stream of auxin channeled basipetally in the stem from the dominant shoot apex indirectly dampens auxin transport out of the lateral bud, which prevents release (Muller and Leyser, 2011). The second-messenger hypothesis reasons that high auxin concentration in the stem promotes strigolactone (SL) biosynthesis and this hormone moves into the bud to inhibit growth (Rameau et al., 2014; Barbier et al., 2019). In the bud, SL acts in part to dampen auxin transport to prevent bud outgrowth (Crawford et al., 2010; Shinohara et al., 2013). Furthermore, this mobile hormone is implicated in suppressing auxin biosynthesis and response genes in the bud (Wang et al., 2020). SL also functions to regulate key bud dormancy related transcription factors including DWARF53/SUPPRESSOR OF MAX2-LIKE 6, 7, and 8 (Jiang et al., 2013; Zhou et al., 2013; Soundappan et al., 2015; Wang et al., 2015, 2020), as well as BRANCHED1 (BRC1)/TEOSINTE BRANCHED1 (TB1; Aguilar-Martinez et al., 2007; Braun et al., 2012; Wang et al., 2020). Interestingly, studies in Cucumis sativus suggest that BRC1/TB1 prevents bud release in part by repressing transcription of a polar auxin transporter gene involved in branching (Shen et al., 2019). Finally, bud dormancy is maintained in part through the suppression of cell division and ribosome production (Gonzalez-Grandio et al., 2013), as well as the upregulation of abscisic acid (ABA) and jasmonic acid (JA; Gonzalez-Grandio et al., 2017; Dong et al., 2019).
An underlying factor in dominance interaction is the ability of a developing sink to maintain a high growth potential via uptake and metabolism of sugars, including sucrose (Eveland and Jackson, 2012; Barbier et al., 2015; Pfeiffer et al., 2017). For example, the growth potential of shoot and root apices, as well as developing fruits, are dependent upon invertase activity, which functions to metabolize sucrose to glucose and fructose (Ruan et al., 2012; Bihmidine et al., 2013). In addition, sugar catabolic pathways mediated by glycolysis/the tricarboxylic acid and oxidative pentose phosphate pathway also regulate shoot growth (Wang et al., 2021). The demand of growing sinks for carbohydrates is due to the fact that the sugars are key drivers of cell division and differentiation required for growth (Ruan et al., 2012; Sablowski and Carnier Dornelas, 2014). Indeed, sugar availability plays a major role in branching (Mason et al., 2014; Barbier et al., 2015), as well as meristem activity (Wu et al., 2005; Pfeiffer et al., 2016). Although sugars are essential for energy and cell wall biosynthesis, glucose and sucrose also function as signals that regulate plant developmental programs (Eveland and Jackson, 2012; Barbier et al., 2015), including the vegetative phase transition (Yang et al., 2013; Yu et al., 2013). In addition to sucrose and glucose, trehalose 6-phosphate (T6P) functions as a sugar signal that regulates growth in response to sucrose availability (Nunes et al., 2013; Lastdrager et al., 2014; Baena-Gonzalez and Lunn, 2020). For example, the T6P pathway regulates the vegetative and floral transition in response to the sugar availability to ensure sufficient carbohydrates are accessible to support reproductive development (Wahl et al., 2013; Ponnu et al., 2020). In addition, T6P plays a role in regulating branching and bud outgrowth in response to decapitation (Satoh-Nagasawa et al., 2006; Fichtner et al., 2017). Taken together, sugar signaling and metabolism are the key drivers of plant growth and developmental processes.
In annual plants, inflorescence growth and fruit development coexist for a definite period of time before inflorescence growth ceases (Bleecker and Patterson, 1997; Nooden and Penney, 2001; Gonzalez-Suarez et al., 2020). This developmental transition is referred to as the “end of flowering” phase transition (Gonzalez-Suarez et al., 2020), which is confined to the later stage of inflorescence development (Balanza et al., 2018; Gonzalez-Suarez et al., 2020; Ware et al., 2020). Inflorescence growth cessation is mediated by the fruit load, as removing these seed-bearing structures restores flower and fruit production. The inhibition of growth appears to be a separate step from senescence, which usually follows arrest (Bleecker and Patterson, 1997; Nooden and Penney, 2001; Wuest et al., 2016; Wang et al., 2020; Ware et al., 2020). A recent hypothesis predicts that inflorescence apices acquire a competency to undergo growth cessation late in inflorescence development (Ware et al., 2020). Once inflorescences acquire this competency, export of auxin from developing fruits induces growth cessation. Competency for inflorescence arrest involves the FRUITFUL (FUL)/APETALA2 (AP2) age dependent module, which indirectly regulates stem-cell homeostasis through the WUSCHEL (WUS) transcription factor (Balanza et al., 2018; Martinez-Fernandez et al., 2020). Interestingly, transcript levels for ABA signaling and response genes associated with lateral bud dormancy are higher in arrested inflorescence meristems at the end of flowering compared to active meristems during the growing phase of inflorescence development (Wuest et al., 2016). In addition, the FUL/AP1 module appears to directly regulate ABA response genes at the end of flowering transition (Martinez-Fernandez et al., 2020). Lastly, experimental studies indicate that JA may also play a role in the end of flowering phase transition (Kim et al., 2013).
Here, our results show that the end of flowering phase transition in Arabidopsis (Arabidopsis thaliana) is a two-stage process that involves auxin. The first stage is marked by the selective dampening of auxin transport in the apical region of the stem. Further, the transition from the first to the second stage is accompanied by an increase in auxin response in the vascular tissues where developing fruits are attached to the stem. Together, the dampening of auxin transport followed by an increase in auxin response in the apical region of the stem may function to prevent canalization required for flower production and development. Consistent with previous studies showing that sugar metabolism and signaling regulate the vegetative and flower transitions, the first stage of the end of flowering transition is associated with a significant reduction in sugar signaling and metabolism. We propose that inhibition of inflorescence growth by fruit load is regulated by auxin and sugar signaling for end of flowering transition in annual plants.
Results
Characterization of the end of flowering phase transition
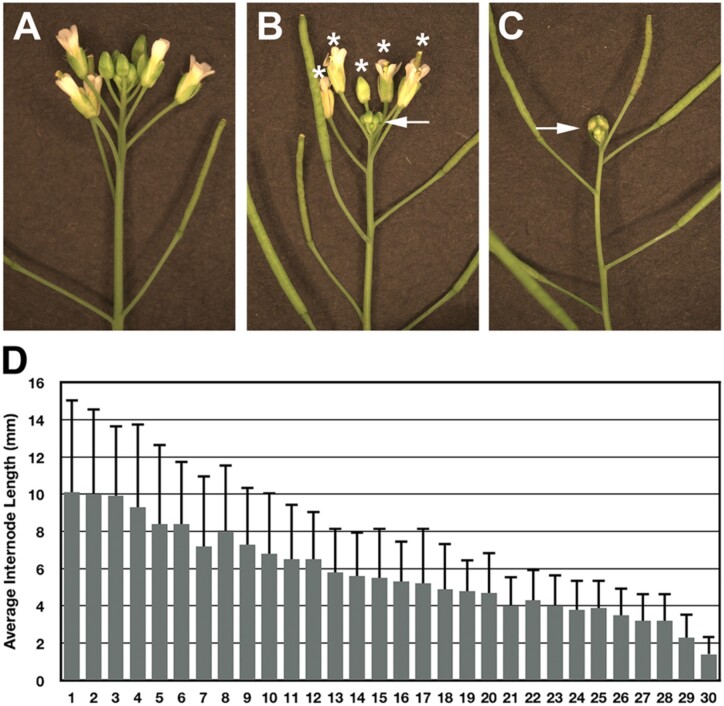
To better understand the end of flowering phase transition, the inflorescence arrest phenotype was characterized. During the growing phase of inflorescence development, the inflorescence meristem produces floral meristems, which give rise to flowers and floral organs (Figure 1A). As flowers develop into fruits, the subtending internodes elongate, which separates the siliques. Characterization of the inflorescence arrest phenotype indicated that the end of flowering phase can be divided into two stages. During the first stage, only the apical bud, which consists of the inflorescence meristem, young unopened flower primordia and the immediate subtending internodes, transitioned to a quiescent state (Figure 1B). In contrast, 4–6 mature flowers with developing fruits attached to elongated pedicels continued to develop (Figure 1B). For the purposes of this study, we defined the first stage of growth cessation stage as quiescent 1 (Q1). The end of flowering phase was completed at the quiescent 2 (Q2) stage, when growth at the inflorescence apex arrested, including the last set of fruits to develop (Figure 1C). As previously shown, removing fruits at this stage restores inflorescence growth, indicating that growth arrest is reversible (Bleecker and Patterson, 1997; Walker and Bennett, 2018; Gonzalez-Suarez et al., 2020).
Figure 1.
Characterization of the end of flowering transition. A, An active inflorescence shoot apex containing numerous flowers at different stages of development. B, Q1 inflorescence shoot apex with a white arrow pointing at the compact quiescent apex, which consists of young unopened flower buds. The asterisks mark mature flowers with developing fruit attached to elongated pedicels. C, Inflorescence shoot apex at the Q2 stage of development, in which growth at the apex, including the fruits, ceased. D, Average internode length was determined for the last 30 internodes produced at the Q2 stage of the end of flowering transition (n = 30 plants). Note: internode 30 is the last internode to elongate before Q1 stage arrest.
In actively growing Arabidopsis inflorescences, the shoot meristem allocates cells that give rise to flowers and internodes (Serrano-Mislata and Sablowski, 2018). The gradual decline in meristem size indicates that meristem activity decreases during inflorescence development (Balanza et al., 2018; Wang et al., 2020). To further support this hypothesis, the average length for the last 30 internodes produced on the primary stem was determined. Results showed that over the course of inflorescence development, internode length gradually declined (Figure 1D). The steady decline in internode development indicates that the meristem allocates fewer and fewer cells to support stem growth due to a gradual decrease in meristem activity.
Transition to the Q1 stage is associated with a cessation of meristem activity
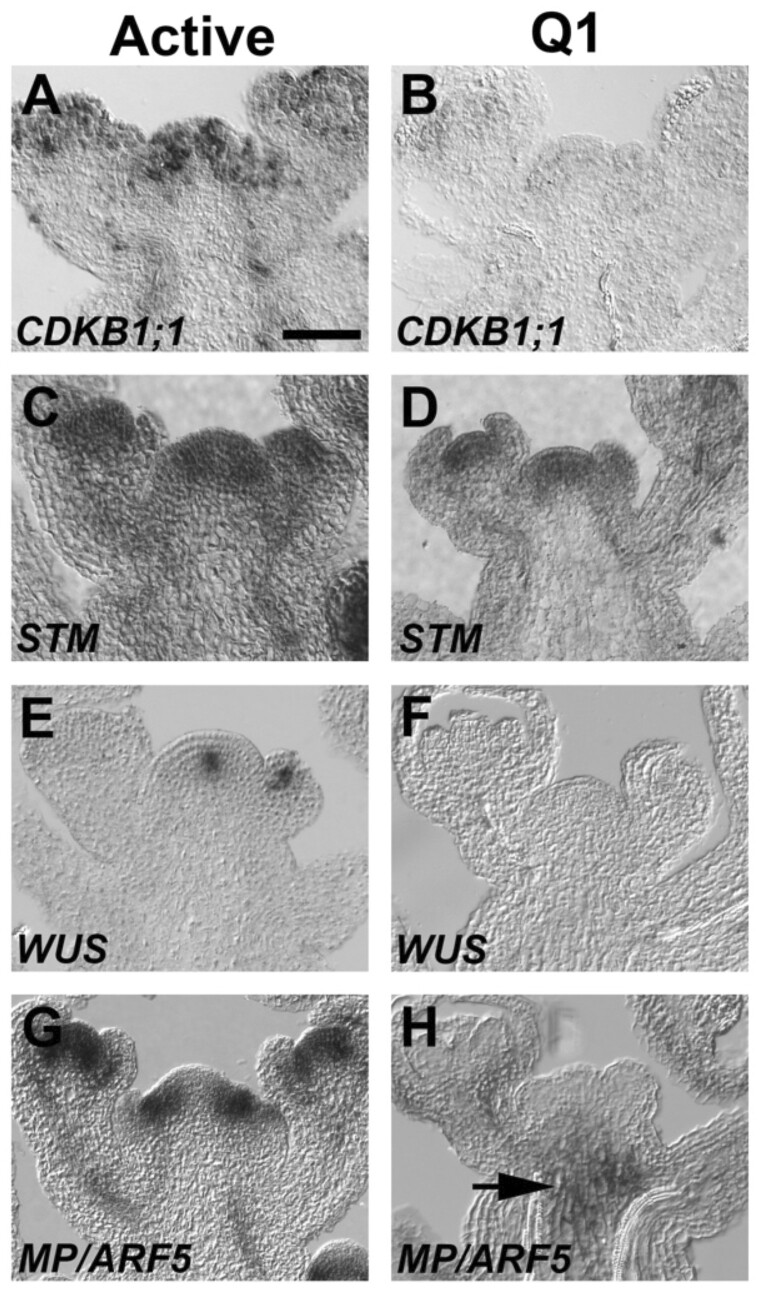
To evaluate the effect of fruit load on growth processes in the inflorescence apex, a series of mRNA in situ hybridizations were performed with genes that control meristem activity. To demonstrate the cessation of inflorescence growth occurred at the Q1 stage, the cell division marker, CYCLIN DEPENDENT KINASE B1;1 (CDKB1;1) was used as a marker to assess whether the shoot apex was active (Segers et al., 1996). Results showed that CDKB1;1 was expressed in inflorescence and flower meristems, as well as the vasculature of actively growing inflorescence apices (Figure 2A). In contrast, transcripts for CDKB1;1 were not readily detected in Q1 shoot apices (Figure 2B). SHOOTMERISTEMLESS (STM) is a regulator of shoot meristem identity (Long et al., 1996). Therefore, to determine if the fate of the inflorescence meristem cells had changed during Q1, the expression pattern of STM was examined. Results showed that STM was expressed in both active and the Q1 inflorescence and floral meristems (Figure 2, C and D).
Figure 2.
Expression patterns for key genes that control shoot growth. Gene expression patterns were shown for (A, C, E, and G) active and (B, D, F, and H) Q1 apices. A and B, CDKB1;1 is a mitotic regulator expressed in shoot meristem (Segers et al., 1996). C and D, STM is a shoot meristem identity gene (Long et al., 1996). E and F, WUS is key regulator of stem cell homeostasis (Laux et al., 1996). G and H, MP regulates flower formation and vascular development (Hardtke and Berleth, 1998). A, The length of the bar is 50 µm, which applies to all panels. H, Arrow points at the MP expression in the subapical region of the meristem and pith cells in the Q1 apex.
To investigate the impact of fruit load on stem cell homeostasis, the expression pattern for WUSCHEL (WUS) was evaluated in active and Q1 inflorescence apices. In active inflorescence apices, WUS was expressed in the central domain of the inflorescence meristem (Figure 2E; Laux et al., 1996; Clark et al., 1997). In contrast to active inflorescence apices, WUS expression was not detected in Q1 meristems (Figure 2F). MONOPTEROS (MP)/AUXIN RESPONSE FACTOR (ARF5) encodes an auxin response factor that is expressed in the periphery of the shoot meristem where it controls auxin mediated leaf and flower formation, as well as vascular development (Przemeck et al., 1996; Hardtke and Berleth, 1998; Schuetz et al., 2008). In active inflorescence apices, MP/ARF5 expression was detected in peripheral region of the shoot meristem and vascular tissues of the inflorescence apex (Figure 2G). Interestingly, the expression pattern of MP/ARF5 was altered in Q1 inflorescence apices, as the mRNA localized to the subapical region of the inflorescence meristem (Figure 2H). Further, MP/ARF5 expression was no longer detected in the periphery of the inflorescence meristem, as well as the quiescent floral meristems and vasculature tissues of the stem and pedicels (Figure 2H). Taken together, the expression studies show that key determinants of cell division, stem cell homeostasis, and auxin-mediated organogenesis are suppressed at the Q1 stage. However, meristem identity is maintained in Q1 meristems, as indicated by the expression of STM.
Selective inhibition of auxin transport in the apical inflorescence stem associates with arrest
We speculated that the end of flowering transition involved dominance inhibition of inflorescence growth by fruit load. Moreover, dominance inhibition was predicted to associate with the selective inhibition of auxin transport in the apical region of the stem below the inflorescence apex. To test this hypothesis, basipetal auxin transport was measured in two sets of stem segments during inflorescence development using 14C-indole-3-acetic acid (14C-IAA). First, auxin transport was determined in apical stem (AS) segments (Figure 3, A and B), from the stem region just below the inflorescence apex to the site of stem where developing fruits were attached. The region of the stem where developing fruits are attached was referred as the zone of fruit development (ZFD; Figure 3, A and B, white box). In basal stem (BS) segments, auxin transport was also measured below the ZFD (Figure 3, A and B).
Figure 3.
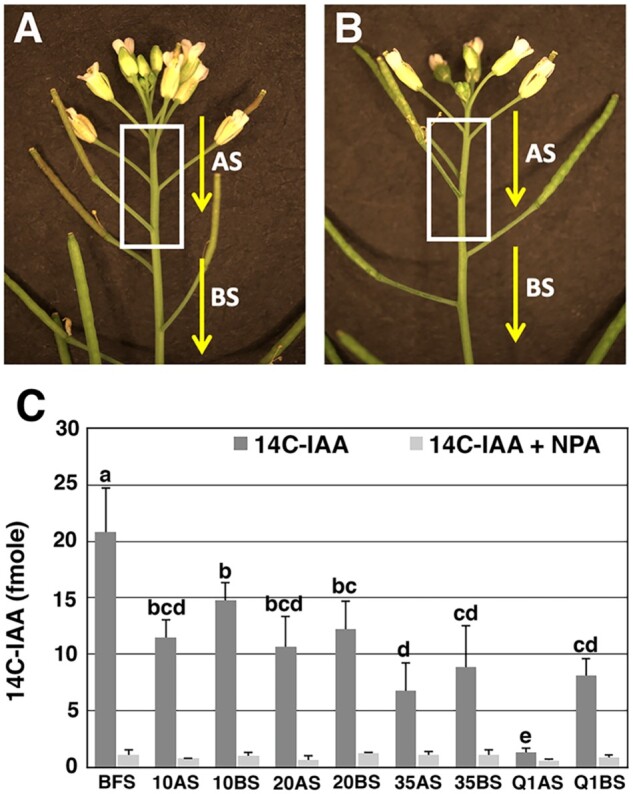
14C-IAA transport in stem segments during inflorescence development. Images of (A) active and (B) Q1 inflorescence shoots. The white box marks the region of the stem where developing fruits are attached. This region is referred as the ZFD. IAA transport was determined in AS segments and BS segments relative to the ZFD. C, Radiolabeled IAA transport was measured during inflorescence development starting at a time just before the first fruit set (BFS). After fruit set, 14C-IAA was determined in AS and BS segments when 10, 20, and 35 fruits were produced, as well as the Q1 stage. The light color boxes represent control stem segments in which 14C-IAA transport was measured in the presence of NPA, an inhibitor of polar auxin transport. The values are the mean ± sd (n = 5 biological samples). The letters above the bars determine whether differences in 14C-IAA transport were statistically significant using analysis of variance, Tukey’s honest significant difference (adjusted P-value < 0.05).
In this analysis, auxin transport was measured early in inflorescence development before fruit set (BFS) and results showed that these stem segments transported an average 20.8 fmoles 14C-IAA (Figure 3C). After 10 fruit set, a significant decline in radiolabeled IAA transport occurred in AS and BS segments (Figure 3C). The level of 14C-IAA transport was maintained in AS and BS segments up to the time in which 20 fruit set. At the 35-fruit set time point, an apparent further decline in radiolabeled IAA transport occurred (Figure 3C). At the Q1 stage, auxin transport was severely reduced in AS segments, as these segments transported an average 1.3 fmoles of 14C-IAA (Figure 3C). Interestingly, the average amount of 14C-IAA transported in Q1 BS segments was 8.1 fmoles, which was similar to the transport capacity of 35 BS segments. In addition, there was an apparent trend in which BS segments displayed higher 14C-IAA transport capacity than the AS segments when shoots produced 10, 20, and 35 fruits (Figure 3C). In summary, these results indicate that fruit load modulates auxin transport and supports the hypothesis that inflorescence growth arrest associates with a selective dampening of auxin transport in the AS segments at the Q1 stage.
Fruit removal restores shoot growth and auxin transport in the apical inflorescence stem
To further examine the effect of fruit load on IAA transport in the AS, fruits were removed from inflorescences at the Q2 stage and IAA transport was examined over a 72-h period. At the Q2 stage, the inflorescence apex arrested in a tight cluster of immature and unopened flowers (Figure 4A). Auxin transport in Q2 AS segments was markedly low, as these stem segments transported an average 1.1 fmoles of 14C-IAA (Figure 4D). However, 36- to 48-h after removing fruits the inflorescence apex expanded due to the production of new flowers (Figure 4B). Forty-eight hours after defruiting inflorescences, the average amount of 14C-IAA transported in the defruited (DF) AS segments increased to 3.95 fmoles (Figure 4D). Shoot expansion was apparent 72-h after defruiting inflorescences due to internode elongation, which separated the arrested floral buds (Figure 4C). A further increase in auxin transport was detected at this time point, as these DF AS segments transported an average 11.0 fmoles of 14C-IAA (Figure 4D), which was similar to the auxin transport capacities in AS segments early in inflorescence development (Figure 3C). Taken together, the increase in auxin transport in AS segments after defruiting Q2 inflorescences associates with flower production and shoot expansion.
Figure 4.
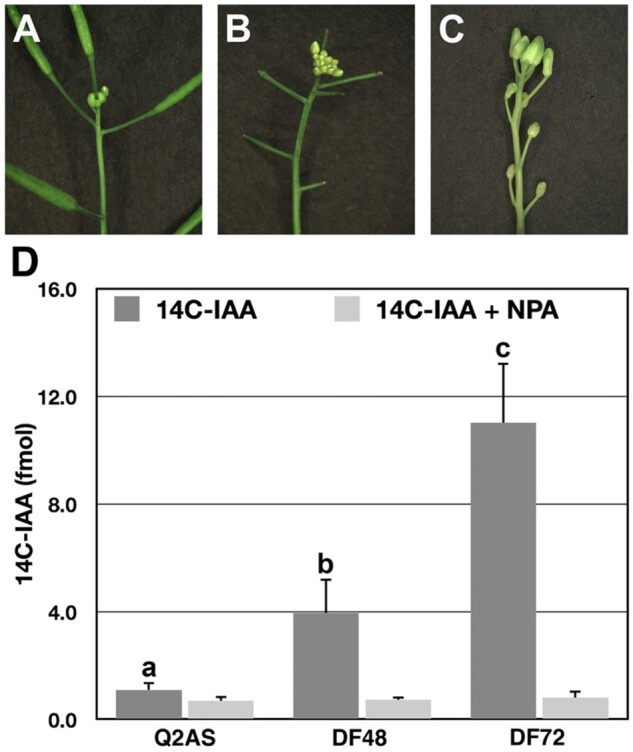
Defruiting Q2 inflorescences restored inflorescence growth and 14C-IAA transport in AS segments. A, An arrested inflorescence apex at the Q2 stage. B, As new flowers were produced, the shoot apex increased in size from 36- to 48-h after inflorescences were DF. C, At 72-h after defruiting inflorescences, shoot expansion was apparent. D, Radiolabeled IAA transport was measured in AS segments at the Q2 stage and 48- and 72-h after inflorescence shoots were DF. Note: the 48-h time point represented 14C-IAA transport at the later stage of floral bud production, while the 72-h time point measured radiolabeled IAA transport during shoot expansion. 14C-IAA + NPA served as a negative control and is indicated by the light shaded boxes. The values are the mean ± sd (n = 5 biological samples). The letters above the bars determine whether differences in 14C-IAA transport were statistically significant using analysis of variance, Tukey’s honest significant difference (adjusted P-value < 0.05).
Auxin response increase is primarily associated with Q2 stage of growth cessation
To further characterize the role of auxin in meristem arrest, auxin response was examined in active and arrested inflorescences at the Q1 and Q2 stages using the synthetic auxin responsive promoter termed DR5 fused to the reporter gene beta-glucuronidase (GUS; Ulmasov et al., 1997). In active inflorescence apices, DR5:GUS activity was detected primarily in young floral buds (Figure 5A). In addition, DR5:GUS expression was also detected in fruits (Figure 5A) and pedicels, particularly at the base of the developing fruits (Figure 5B). At the Q1 stage, DR5:GUS was detected in the dormant floral buds but to a lesser extent compared to active inflorescence apices (Figure 5C). In addition, DR5:GUS was detected in the last set of developing fruits (Figure 5C). Interestingly, in 37% of the Q1 shoots analyzed, DR5:GUS activity was apparent in the ZFD and throughout the pedicels of developing fruits (Figure 5, C and D). In the remaining 63% of Q1 DR5:GUS inflorescences, little or no DR5:GUS activity was displayed in the ZFD, similar to actively growing inflorescences (Supplemental Figure S1). At the Q2 stage, auxin response was detected primarily in the inflorescence stem and pedicels just below the inflorescence apex where the last set of fruit developed (Figure 5, E and F). The “stripe-like” pattern of DR5:GUS activity in the stem suggests that auxin response was induced in the vascular tissue of the main stem and pedicels (Figure 5F). To test this hypothesis, histological experiments were performed in active and Q2 inflorescence stems below the inflorescence apex where the last two to three fruits had set. Results showed that DR5:GUS was not detected in cross sections through the ZFD during active inflorescence growth (Figure 5G). In contrast, DR5:GUS activity was readily observed in vascular cells of the Q2 stems where the last set of fruit developed (Figure 5H). Taken together, results show that auxin response increases in the vascular tissues of the AS, which corresponds to the site where the last set of fruit completed their developmental program during the transition from Q1 to Q2.
Figure 5.
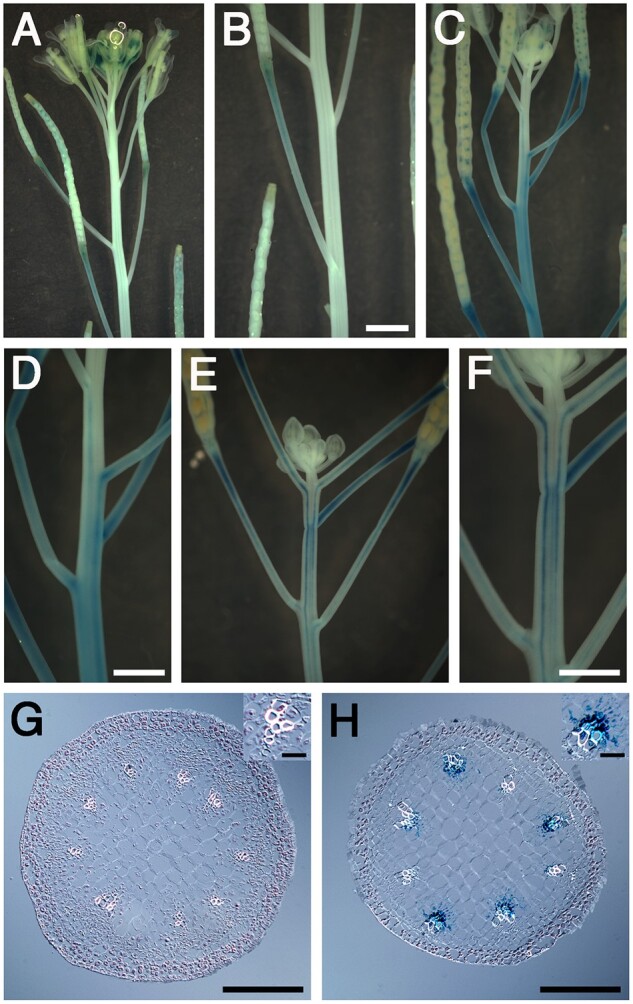
A change in auxin response in apical inflorescence stems at end of flowering transition. DR5:GUS staining patterns in (A) active, (C) Q1, and (E) Q2 shoot apices. Close up of stems where developing fruits are attached in (B) active, (D) Q1, and (F) Q2 shoot apices. Histological cross sections in the AS for an (G) active and (H) Q2 stage inflorescence. Note: inset of vascular bundle displayed in upper right corner of each image. In actively growing shoots, the section shown in (G) was through the region of the stem where the developing fruits were attached. The section displayed in (H) was in a region of the stem where the last fruits set. B, D, and F, Bar length is 2 mm. G and H, The length of the bar in bottom corner is 200 µM. The length of the bar in the inset images is 25 µM.
Carbohydrate status is reduced in Q1 inflorescence apices
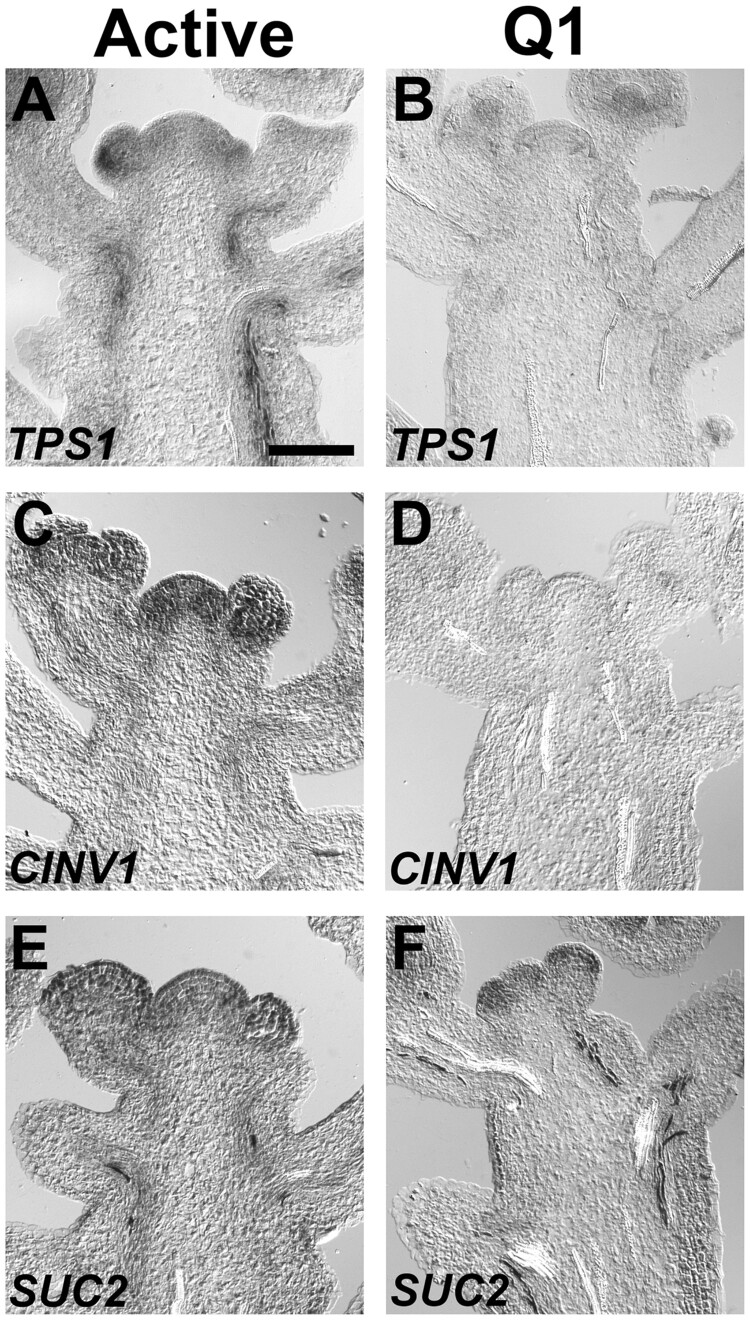
As sugar signaling and metabolism plays a critical role in developmental phase transitions (Poethig, 2013; Wang, 2014), and influences auxin signaling and transport (Le et al., 2010; Lilley et al., 2012; Sairanen et al., 2012; Barbier et al., 2015; Lauxmann et al., 2016), we examined the carbohydrate status of inflorescence apices after the transition to the Q1 stage. The expression patterns of key genes involved in sugar signaling, metabolism and transport were investigated. TREHALOSE 6-PHOSPHATE SYNTHASE 1 (TPS1) encodes an essential enzyme that catalyzes T6P from glucose-6-phophate and UDP-glucose (Lastdrager et al., 2014; Baena-Gonzalez and Lunn, 2020). As expression of TPS1 associates with T6P levels during inflorescence development (Wahl et al., 2013), this biosynthetic gene was used as a marker to assess the T6P pathway in Q1 shoot apices. Results showed that TPS1 was primarily expressed in the vascular system of active inflorescence apices, as well as the flanks of the inflorescence meristem (Figure 6A). In contrast to actively growing inflorescence apices, TPS1 was not detected in the vascular system or inflorescence meristems in Q1 apices (Figure 6B). Invertases are key enzymes involved in regulating sink activity in meristems and fruits (Ruan et al., 2012; Bihmidine et al., 2013). The CYTOSOLIC INVERTASE 1 (CINV1) and related genes in Oryza sativa (OsCYT-INV1), Lotus japonicas (LjINV1) and Solanum lycopersicum (NI6) are required for growth and carbon partitioning (Lou et al., 2007; Qi et al., 2007; Jia et al., 2008; Barratt et al., 2009; Welham et al., 2009; Barnes and Anderson, 2018; Leskow et al., 2020). In active inflorescence apices, CINV1 was expressed in inflorescence and flower meristems, vascular cells and young floral organ primordia (Figure 6C). Similar to the results obtained with TPS1, CINV1 expression was not detected in the Q1 apices (Figure 6D), indicating that sucrose metabolism and sink activity is highly reduced in arrested meristems. To investigate a possible effect of inflorescence growth arrest by fruit load on carbohydrate partitioning, the SUCROSE TRANSPORTER 2 (SUC2) was examined, as this transporter is expressed in the vasculature of inflorescence stems (Truernit and Sauer, 1995; Gottwald et al., 2000). SUC2 was expressed in the vasculature tissues of active and Q1 apices (Figure 6, E and F). Although SUC2 is expressed in Q1 apices, the decrease in CINV1 and TPS1 expression indicates that the carbohydrate status was reduced when shoot apices transition to the Q1 stage.
Figure 6.
Expression patterns for sugar signaling, metabolism, and transport genes. Gene expression patterns were determined in (A, C, and E) active and (B, D, and F) Q1 apices. A and B, TPS1, which is expressed in the vasculature of the inflorescence stem, was used as a marker to assess the T6P pathway (Wahl et al., 2013; Ponnu et al., 2020). C and D, CINV1 is an invertase, which is required for growth (Barratt et al., 2009). E and F, SUC2 is a sucrose transporter expressed in the vascular tissues of inflorescences (Truernit and Sauer, 1995; Gottwald et al., 2000). The length of the bar is 50 µm, which applies to all panels.
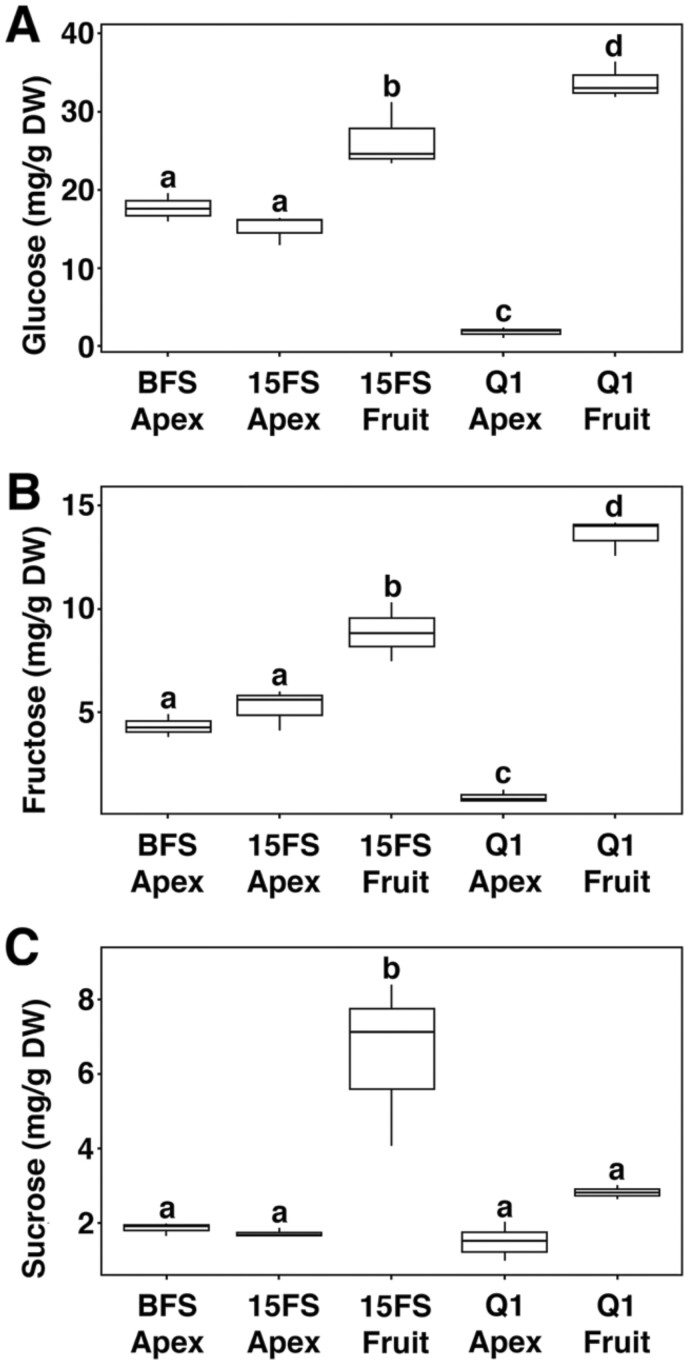
To further investigate a role for sugar metabolism and signaling in the end of flowering phase transition, glucose, fructose and sucrose were measured in inflorescence apices before and after 15 fruits set, as well as the Q1 stage. These sugars were also measured in developing fruits after 15 fruits set and at the Q1 stage. Levels of glucose and fructose were similar in active inflorescence apices before and after 15 fruits set (Figure 7, A and B). In contrast, the levels of these monosaccharides were significantly reduced in Q1 inflorescence apices compared to active inflorescence apices (Figure 7, A and B). In active and Q1 inflorescence apices, the level of sucrose was similar, indicating that sucrose transport was not affected at the Q1 stage (Figure 7C), which is consistent with the expression of SUC2 in arrested inflorescences. Together, these results indicate that sucrose metabolism but not transport is significantly reduced when inflorescence apices transition from an active to a quiescent state. In developing fruits, the levels of glucose, fructose, and sucrose were significantly higher compared with the active inflorescence apices (Figure 7, A and B). These results indicate that developing fruits have a higher sink potential than active inflorescence apices during inflorescence development. Interestingly, developing fruits at Q1 had the highest levels of glucose and fructose indicating that sucrose metabolism is increased in fruits at the end of flowering phase (Figure 7, A and B). Taken together, results from above indicate that a change in sugar signaling and metabolism in arrested inflorescence apices and developing fruits is associated when active inflorescence apices transition to the Q1 stage during the end of flowering transition.
Figure 7.
Sugar content in shoot apices and developing fruits. Sugar levels determined in shoot apices BFS (Apex), after 15 fruit set (15FS Apex) and at the Q1 stage (Q1 Apex). Sugar levels were also determined in developing fruits when 15 fruits set (15FS Fruit) and at the Q1 stage (Q1 Fruit). The levels of (A) Glucose, (B) fructose, and (C) sucrose were measured from DW tissue. n = 3 biological samples. The sugar profiles determined for each biological measurement were derived from extracting carbohydrates from at least 40 shoot apices or 30 developing fruits. The box plot displays the interquartile range with whiskers representing maximum and minimum values. The horizontal line within the box denotes the median. The letters above the bars determine whether differences in the means for 14C-IAA transport were statistically significant using analysis of variance, Tukey’s honest significant difference (adjusted P-value < 0.05).
Discussion
Dominance interaction between fruits and shoot apices is a major factor that influences shoot architecture and yield (Bangerth, 1989; Smith and Samach, 2013; Walker and Bennett, 2018). During inflorescence development, the decline in meristem size and activity is associated with a decrease in stem cell renewal based on WUS expression (Balanza et al., 2018; Wang et al., 2020). As the growth potential of the inflorescence apex declines, and the meristem becomes competent for arrest, the end of flowering phase is initiated (Ware et al., 2020). To further extend these studies, our results showed that the end of flowering phase transition is a two-step process. At the Q1 stage, a cessation of growth selectively occurs in the inflorescence apex, while the remaining immature fruits continue to develop. This is supported by expression studies indicating that stem cell renewal and auxin mediated organogenesis in the inflorescence meristem, as well as cell division, are significantly reduced in the Q1 shoot apex. The end of flowering phase is completed at the Q2 stage when fruit growth and development is completed.
Results from a recent study show that production and export of auxin from developing fruits at a late stage of inflorescence development promotes inflorescence arrest (Ware et al., 2020). The end of flowering model proposed by Ware et al. (2020) predicts that auxin export from developing fruits induces inflorescence arrest by disrupting polar auxin transport in the inflorescence stem. Results from our study show that growing fruits impact auxin transport from the inflorescence apex. First, auxin transport in the AS was the highest BFS. However, after 10 fruits were produced, a significant decline in auxin transport occurred in AS and BS segments. Second, after 20 fruits were produced, an apparent gradual decrease in auxin transport primarily occurred in AS segments. At the Q1 stage, the inhibition of inflorescence growth is associated with a selective dampening of auxin transport in the apical region of the stem below the shoot apex, while transport below the ZFD is functional. Furthermore, an increase in auxin response in the vascular tissues in the ZFD is initiated at Q1 and reaches a maximum at Q2. Finally, removal of fruits at the Q2 stage restores growth and auxin transport in the apical region of the stem. Taken together, we propose that the transition to the Q1 stage involves the dampening of auxin transport, which prevents auxin canalization in the apical region of the inflorescence stem. In addition, growth arrest is maintained as the inflorescence apices advance from the Q1 to the Q2 stage by an increase in auxin response. During this advancement, it is tempting to speculate that the increase in auxin response acts to repress auxin transport in the apical region of the stem until seeds fully mature.
Sugar signaling plays an essential role in plant developmental phase transitions (Bolouri Moghaddam and Van den Ende, 2013; Poethig, 2013). Flowering is a major phase transition that is regulated by florigen, FLOWERING LOCUS T (FT), and the age-dependent pathway mediated by the microRNA156 (miR156)/SQUAMOSA PROMOTER BINDING PROTEIN-LIKE (SPL) module (Turck et al., 2008; Srikanth and Schmid, 2011; Wang, 2014). Experimental studies show that the T6P pathway regulates FT and the miR156/SPL module in leaves and shoot apices, respectively (Wahl et al., 2013). In addition, the suppression of miR156 during the vegetative phase transition is mediated by sugars, including glucose and sucrose (Yang et al., 2013; Yu et al., 2013), as well as the T6P pathway (Ponnu et al., 2020). In our study, we show that glucose and fructose levels, as well as TSP1 expression, are significantly reduced in Q1 apices compared to actively growing inflorescence apices. As the biosynthesis of T6P is dependent upon glucose and TSP1 expression, our results indicate that the T6P pathway is highly reduced in Q1 apices. Therefore, we propose that the suppression of sugar signaling mediated by glucose and the T6P pathway is involved in the end of flowering phase transition.
The reduction in glucose and fructose but not sucrose in Q1 apices at the end of flowering indicates that sucrose metabolism is selectively inhibited in inflorescence apices but not developing fruits. Consistent with this view, CINV1 expression is suppressed when inflorescence apices transition to the Q1 stage. In contrast, developing fruits at Q1 stage display an increase in sucrose metabolism compared to growing fruits at an earlier stage of inflorescence development. This is supported by the fact that the levels of glucose and fructose are higher, while sucrose is lower in developing fruits at the Q1 stage. Therefore, it is tempting to speculate that end of flowering phase transition not only functions to repress inflorescence growth, but also acts to further increase the growth potential of fruits. The increase in the growth potential of developing fruit at the Q1 stage may be mediated by the end of flowering-competence factors.
Expanding on the models proposed by Ware et al. (2020) and Balanza et al. (2018), we propose that inflorescence growth is maintained by: (1) the basipetal auxin transport system in the apical domain of the inflorescence stem, (2) sugar signaling and metabolism, and (3) AP2 function in the inflorescence apex (Figure 8). During the active stage of inflorescence development, the shoot system can support both shoot and fruit growth by the maintenance of a positive feedback loop between polar auxin transport and sugar signaling and metabolism in the shoot apex and subtending region of the stem. However, with the continuous decline in meristem activity, auxin transport out of the inflorescence apex and AP2 function, a competence juncture is reached in which the apical bud, including the inflorescence and floral meristems, immature flowers and subtending internodes, can no longer maintain growth, as fruits continue to develop. At this competence juncture, the switch from active inflorescence growth to the Q1 stage of arrest is mediated by: (1) the age-dependent FUL/AP2 module and (2) the export of auxin from developing fruits, which selectively impairs auxin transport in the AS below the inflorescence apex (Figure 8). We propose that impairment of auxin transport in the apical inflorescence stem results in a significant decrease in sugar signaling and metabolism in the inflorescence apex, which negatively impacts stem-cell renewal and organogenesis. This is supported by the fact that auxin-mediated organogenesis and stem cell renewal is dependent upon sugar availability and signaling (Lauxmann et al., 2016; Pfeiffer et al., 2016). Further, the increase in auxin response in the vascular tissues of the AS from the Q1 to the Q2 stage acts to maintain growth arrest by dampening auxin export from the inflorescence meristem and immature floral buds at the Q2 stage (Figure 8).
Figure 8.
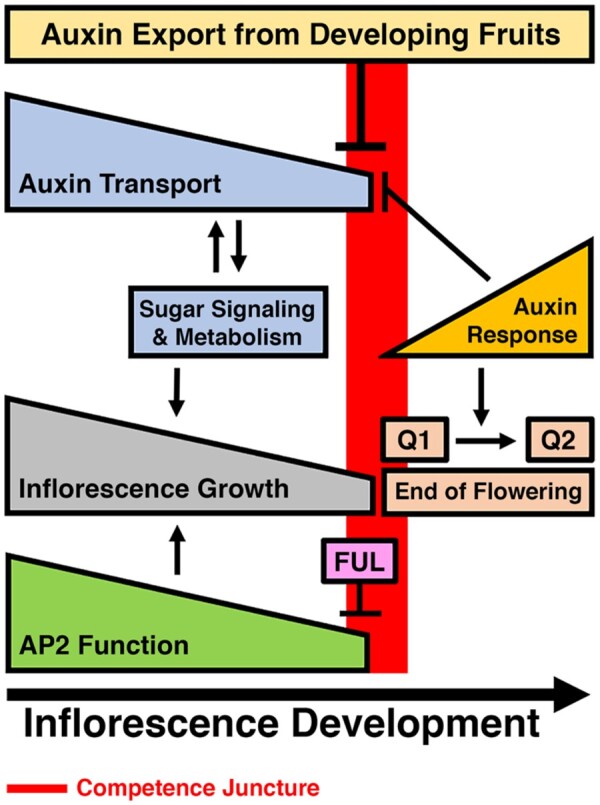
Model for inflorescence arrest in response to fruit load in the AS and shoot apex at the end of flowering phase transition. In this model, inflorescence growth is dependent upon auxin transport in the AS, as well as sugar signaling and metabolism and AP2 function in the inflorescence apex. Feedback between auxin transport and sugar signaling and metabolism is critical for inflorescence growth. During inflorescence development, the decline in auxin transport and AP2 function reduces the growth potential of the inflorescence. Late in inflorescence development, the inflorescence acquires competency to undergo arrest. At this competence juncture (red rectangle), the transition to the Q1 stage is induced by: (1) the selective dampening of auxin transport in the AS via export of auxin from developing fruits, which decrease sugar signaling and metabolism in the shoot apex and (2) the repression of AP2 by FUL, which reduces meristem activity. During the advancement to Q2, auxin response increases, and functions to maintain growth arrest by suppressing auxin transport in the AS until seed maturity is completed in the last set of developing fruits. In this model, the end of flower phase transition is induced when active inflorescences transition to the Q1 stage. This phase transition is completed at Q2 stage.
In fruit tree crops, inhibition of shoot growth by fruit load is a major driver of biennial or alternate bearing, which is a major challenge for fruit tree crop industries worldwide (Samach and Smith, 2013; Smith and Samach, 2013). Because of the significant challenges and barriers associated with the usage of genetic and molecular manipulations in fruit tree crops, Arabidopsis may serve as a model system to understand the physiological basis of shoot growth arrest in response to fruit load. Translational research from Arabidopsis to fruit tree crops can be utilized to develop new innovative tools to limit the impact of fruits on shoot growth in order to maximize yield and reduce seasonal variation.
Materials and methods
Plant materials and growth conditions
The Arabidopsis (Arabidopsis thaliana) Columbia-0 (Col-0) accession was used to characterize inflorescence arrest in response to fruit load. Auxin response was evaluated during inflorescence development using the DR5:GUS system (Ulmasov et al., 1997). Plants were grown at 22°C under long day growth conditions, 16-h light/8-h dark.
Internode measurements
The length of the last 30 internodes was measured in the primary inflorescence after the end of flowering transition was completed in 30 plants. The average length in millimeter and standard deviation (sd) for each internode were determined.
Gene expression analyses
To examine the expression pattern of key genes that regulate meristem activity and sugar signaling, in situ hybridization was performed using the standard method of fixation, sectioning, and mRNA hybridization as previously described (Jackson, 2001; Chuck et al., 2002). Active inflorescence apices were harvested after 5–10 fruits were produced. Quiescent apices were harvested at the Q1 stage of the end of flowering transition. Synthesis of UTP-digoxigenin anti-sense probes were previously described for STM (Long et al., 1996), WUS (Yadav et al., 2009), MP (Zhao et al., 2010), and TPS1 (Zhao et al., 2010). Primer sequences were used to PCR amplify CDKB1;1, CINV1, and SUC2 DNA fragments for the synthesis of UTP-digoxigenin antisense probes. The sequences for CDKB1;1, CINV1, and SUC2 primers were CDKB1;1-F (CGAGATGGACGAAGAAGGTATTCCACC), CDKB1;1-R (GAAATAATACGACTCACTATAGGGACTCGTGAGAAGATCAACTCCTTGAGGTG), CINV1-F (CCGATGGAGATGGCAGAGAGG), CINV1-R (GAAATAATACGACTCACTATAGGGACTGGCCAAGACGCAGATCGCTTGATGAC), SUC2-F (CTGAGTCATGCGATCTCTACTGCG) and SUC2-R (GAAATAATACGACTCACTATAGGGACTCTTACCGCTGCCGCAATCGCTCC). The method to visualize GUS activity in DR5:GUS inflorescence shoots was described previously (Sundaresan et al., 1995; Springer et al., 2000). DR5:GUS was evaluated in inflorescences during the active period of inflorescence development when apices produced 10–20 fruits, as well as the Q1 and Q2 stages.
14C-IAA transport assay
Basipetal auxin transport was measured in inflorescence stems using a 14C-IAA protocol previously described (Lewis and Muday, 2009). Briefly, 20-mm stem segments were harvested from inflorescences BFS. After the shoot apex produced 10, 20, and 35 fruits and at the Q1 and Q2 stages of development, AS and BS segments were isolated from each inflorescence as described in the results section. To measure auxin transport in each stem segment, the apical end of the stem was placed in 20 µL of auxin transport buffer (100 nM 14C-IAA, 0.05% w/v MES, pH 5.7). To measure movement mediated by diffusion, a separate set of stem segments were isolated and the apical end of each stem was placed in auxin transport buffer containing naphthylphthalamic acid (NPA) to a final concentration of 10 µM. After 10 h of auxin transport at 22°C, each segment was removed from the auxin transport buffer (±NPA) and a 5-mm section at the apical end was cleaved and discarded. Next, each stem segment was transferred to an Eppendorf tube and ground in scintillation fluid using a plastic pestle. For each sample, the extract was transferred to a single scintillation vial containing 20 mL of scintillation fluid and 14C disintegrations per minute (d.p.m.) was determined before conversion to fmoles. Five biological replicates were used to calculate the mean and sd. Analysis of variance and Tukey’s honest significant difference analysis was performed using standard statistical packages in R.
Sugar measurements
For sugar extraction, ∼100 mg of inflorescence apices and developing fruit were collected during inflorescence development in triplicate. After collection, the material was freeze-dried for 12 h and the dry weight (DW) for each sample was determined. Each sample was ground with a mortar and pestle in 1.0 mL of 80% ethanol. Next, samples were incubated at 80°C for 30 min to extract soluble sugars. After the insoluble material was pelleted at 10,000g and the supernatant decanted, the tissues were re-extracted two more times with 80% ethanol. After combining and mixing the three separate 80% ethanol extracts, 650 mL of the soluble extract was placed in an Eppendorf tube and dried in a Gene miVac Quattro (SP Industries, Warminster, PA, USA) for 1.5 h at 55°C. Each dried sample was resuspended in 20-µL sterile H2O. Glucose, fructose, and sucrose were separated by High-Performance Liquid Chromatography using the Sugar-Pak cation-exchange column (Waters, Rydalmere, NSW, Australia). The Aglient Technologies 1200 G1362A infinity refractive index detector (Santa Clara, CA, USA) was used to identify and quantify separated sugars in each of the samples by comparison to the glucose, fructose, and sucrose standards. Analysis of variance and Tukey’s honest significant difference analysis was performed using standard statistical packages in R.
Accession numbers
The accession numbers for the genes studied in this manuscript are: AT3G54180 (CDKB1;1), AT1G35580 (CINV1), AT1G19850 (MP/ARF5), AT1G62360 (STM), AT1G22710 (SUC2), AT1G78580 (TPS1), and AT2G17950 (WUS).
Supplementary Material
Acknowledgments
We thank Tom Bennett (University of Leeds, UK) for discussions and reviewing the manuscript. We also thank Kate Tepper and Rhys Webber for the maintenance of Arabidopsis plants and Dr Tom Guilfoyle for providing DR5:GUS seed.
Funding
This research was funded by CSIRO Agriculture & Food—Strategic Future Funds (grant no. R-04816-01).
Conflict of interest statement. None declared.
H.M.S. conceived the project, supervised the experiments, performed the in situ hybridization experiments, wrote the manuscript, and agrees to serve as the author responsible for contact and communication; M.G. quantified soluble sugar in active and quiescent apices and fruits; M.R. performed DR5:GUS analyses in active and quiescent apices.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys/pages/general-instructions) is: Harley M. Smith (harley.smith@csiro.au)
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1. Auxin response patterns in the ZFD during active and quiescent stages of inflorescence development.
References
- Aguilar-Martinez JA, Poza-Carrion C, Cubas P (2007) Arabidopsis BRANCHED1 acts as an integrator of branching signals within axillary buds. Plant Cell 19: 458–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baena-Gonzalez E, Lunn JE (2020) SnRK1 and trehalose 6-phosphate—two ancient pathways converge to regulate plant metabolism and growth. Curr Opin Plant Biol 55: 52–59 [DOI] [PubMed] [Google Scholar]
- Balanza V, Martinez-Fernandez I, Sato S, Yanofsky MF, Kaufmann K, Angenent GC, Bemer M, Ferrandiz C (2018) Genetic control of meristem arrest and life span in Arabidopsis by a FRUITFULL-APETALA2 pathway. Nat Commun 9: 565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangerth F (1989) Dominance among fruits/sinks and the search for a correlative signal. Physiol Plant 76: 608–614 [Google Scholar]
- Bangerth F, Li CJ, Gruber J (2000) Mutual interaction of auxin and cytokinin in regulating correlative dominance. Plant Growth Regul 32: 205–217 [Google Scholar]
- Barbier F, Peron T, Lecerf M, Perez-Garcia MD, Barriere Q, Rolcik J, Boutet-Mercey S, Citerne S, Lemoine R, Porcheron B, et al. (2015) Sucrose is an early modulator of the key hormonal mechanisms controlling bud outgrowth in Rosa hybrida. J Exp Bot 66: 2569–2582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbier FF, Dun EA, Kerr SC, Chabikwa TG, Beveridge CA (2019) An update on the signals controlling shoot branching. Trends Plant Sci 24: 220–236 [DOI] [PubMed] [Google Scholar]
- Barbier FF, Lunn JE, Beveridge CA (2015) Ready, steady, go! A sugar hit starts the race to shoot branching. Curr Opin Plant Biol 25: 39–45 [DOI] [PubMed] [Google Scholar]
- Barnes WJ, Anderson CT (2018) Cytosolic invertases contribute to cellulose biosynthesis and influence carbon partitioning in seedlings of Arabidopsis thaliana. Plant J 94: 956–974 [DOI] [PubMed] [Google Scholar]
- Barratt DH, Derbyshire P, Findlay K, Pike M, Wellner N, Lunn J, Feil R, Simpson C, Maule AJ, Smith AM (2009) Normal growth of Arabidopsis requires cytosolic invertase but not sucrose synthase. Proc Natl Acad Sci USA 106: 13124–13129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett T, Hines G, van Rongen M, Waldie T, Sawchuk MG, Scarpella E, Ljung K, Leyser O (2016) Connective auxin transport in the shoot facilitates communication between shoot apices. PLoS Biol 14: e1002446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bihmidine S, Hunter CT 3rd, Johns CE, Koch KE, Braun DM (2013) Regulation of assimilate import into sink organs: update on molecular drivers of sink strength. Front Plant Sci 4: 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleecker AB, Patterson SE (1997) Last exit: senescence, abscission, and meristem arrest in Arabidopsis. Plant Cell 9: 1169–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolouri Moghaddam MR, Van den Ende W (2013) Sugars, the clock and transition to flowering. Front Plant Sci 4: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun N, de Saint Germain A, Pillot JP, Boutet-Mercey S, Dalmais M, Antoniadi I, Li X, Maia-Grondard A, Le Signor C, Bouteiller N, et al. (2012) The pea TCP transcription factor PsBRC1 acts downstream of Strigolactones to control shoot branching. Plant Physiol 158: 225–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuck G, Muszynski M, Kellogg E, Hake S, Schmidt RJ (2002) The control of spikelet meristem identity by the branched silkless1 gene in maize. Science 298: 1238–1241 [DOI] [PubMed] [Google Scholar]
- Clark SE, Williams RW, Meyerowitz EM (1997) The CLAVATA1 gene encodes a putative receptor kinase that controls shoot and floral meristem size in Arabidopsis. Cell 89: 575–585 [DOI] [PubMed] [Google Scholar]
- Crawford S, Shinohara N, Sieberer T, Williamson L, George G, Hepworth J, Muller D, Domagalska MA, Leyser O (2010) Strigolactones enhance competition between shoot branches by dampening auxin transport. Development 137: 2905–2913 [DOI] [PubMed] [Google Scholar]
- Dong Z, Xiao Y, Govindarajulu R, Feil R, Siddoway ML, Nielsen T, Lunn JE, Hawkins J, Whipple C, Chuck G (2019) The regulatory landscape of a core maize domestication module controlling bud dormancy and growth repression. Nat Commun 10: 3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eveland AL, Jackson DP (2012) Sugars, signalling, and plant development. J Exp Bot 63: 3367–3377 [DOI] [PubMed] [Google Scholar]
- Fichtner F, Barbier FF, Feil R, Watanabe M, Annunziata MG, Chabikwa TG, Hofgen R, Stitt M, Beveridge CA, Lunn JE (2017) Trehalose 6-phosphate is involved in triggering axillary bud outgrowth in garden pea (Pisum sativum L.). Plant J 92: 611–623 [DOI] [PubMed] [Google Scholar]
- Galweiler L, Guan C, Muller A, Wisman E, Mendgen K, Yephremov A, Palme K (1998) Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 282: 2226–2230 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Grandio E, Pajoro A, Franco-Zorrilla JM, Tarancon C, Immink RG, Cubas P (2017) Abscisic acid signaling is controlled by a BRANCHED1/HD-ZIP I cascade in Arabidopsis axillary buds. Proc Natl Acad Sci USA 114: E245–E254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Grandio E, Poza-Carrion C, Sorzano CO, Cubas P (2013) BRANCHED1 promotes axillary bud dormancy in response to shade in Arabidopsis. Plant Cell 25: 834–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Suarez P, Walker CH, Bennett T (2020) Bloom and bust: understanding the nature and regulation of the end of flowering. Curr Opin Plant Biol 57: 24–30 [DOI] [PubMed] [Google Scholar]
- Gottwald JR, Krysan PJ, Young JC, Evert RF, Sussman MR (2000) Genetic evidence for the in planta role of phloem-specific plasma membrane sucrose transporters. Proc Natl Acad Sci USA 97: 13979–13984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Chen L, Herrera-Estrella L, Cao D, Tran LP (2020) Altering plant architecture to improve performance and resistance. Trends Plant Sci 25: 1154–1170 [DOI] [PubMed] [Google Scholar]
- Hardtke CS, Berleth T (1998) The Arabidopsis gene MONOPTEROS encodes a transcription factor mediating embryo axis formation and vascular development. EMBO J 17: 1405–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D (2001) In-situ hybridization in plants. In Gurr SJ, McPherson MJ, Bowles DJ, eds, Molecular Plant Pathology: A Practical Approach, Vol 1. Oxford University Press, Oxford, pp 163–174 [Google Scholar]
- Jia L, Zhang B, Mao C, Li J, Wu Y, Wu P, Wu Z (2008) OsCYT-INV1 for alkaline/neutral invertase is involved in root cell development and reproductivity in rice (Oryza sativa L.). Planta 228: 51–59 [DOI] [PubMed] [Google Scholar]
- Jiang L, Liu X, Xiong G, Liu H, Chen F, Wang L, Meng X, Liu G, Yu H, Yuan Y. et al. (2013) DWARF 53 acts as a repressor of strigolactone signalling in rice. Nature 504: 401–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Dotson B, Rey C, Lindsey J, Bleecker AB, Binder BM, Patterson SE (2013) New clothes for the jasmonic acid receptor COI1: delayed abscission, meristem arrest and apical dominance. PLoS One 8: e60505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lastdrager J, Hanson J, Smeekens S (2014) Sugar signals and the control of plant growth and development. J Exp Bot 65: 799–807 [DOI] [PubMed] [Google Scholar]
- Laux T, Mayer KF, Berger J, Jurgens G (1996) The WUSCHEL gene is required for shoot and floral meristem integrity in Arabidopsis. Development 122: 87–96 [DOI] [PubMed] [Google Scholar]
- Lauxmann MA, Annunziata MG, Brunoud G, Wahl V, Koczut A, Burgos A, Olas JJ, Maximova E, Abel C, Schlereth A,. et al. (2016) Reproductive failure in Arabidopsis thaliana under transient carbohydrate limitation: flowers and very young siliques are jettisoned and the meristem is maintained to allow successful resumption of reproductive growth. Plant Cell Environ 39: 745–767 [DOI] [PubMed] [Google Scholar]
- Le CS, Schmelz EA, Chourey PS (2010) Sugar levels regulate tryptophan-dependent auxin biosynthesis in developing maize kernels. Plant Physiol 153: 306–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leskow CC, Conte M, del Pozo T, Bermúdez L, Lira BS, Gramegna G, Baroli I, Burgos E, Zavallo D, Kamenetzky L,. et al. (2020) The cytosolic invertase NI6 affects vegetative growth, flowering, fruit set and yield in tomato. J Exp Bot 72: 2525–2543 [DOI] [PubMed] [Google Scholar]
- Lewis DR, Muday GK (2009) Measurement of auxin transport in Arabidopsis thaliana. Nat Protoc 4: 437–451 [DOI] [PubMed] [Google Scholar]
- Lilley JL, Gee CW, Sairanen I, Ljung K, Nemhauser JL (2012) An endogenous carbon-sensing pathway triggers increased auxin flux and hypocotyl elongation. Plant Physiol 160: 2261–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JA, Moan EI, Medford JI, Barton MK (1996) A member of the KNOTTED class of homeodomain proteins encoded by the STM gene of Arabidopsis. Nature 379: 66–69 [DOI] [PubMed] [Google Scholar]
- Lou Y, Gou JY, Xue HW (2007) PIP5K9, an Arabidopsis phosphatidylinositol monophosphate kinase, interacts with a cytosolic invertase to negatively regulate sugar-mediated root growth. Plant Cell 19: 163–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Fernandez I, Menezes de Moura S, Alves-Ferreira M, Ferrandiz C, Balanza V (2020) Identification of Players Controlling Meristem Arrest downstream of the FRUITFULL-APETALA2 pathway. Plant Physiol 184: 945–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MG, Ross JJ, Babst BA, Wienclaw BN, Beveridge CA (2014) Sugar demand, not auxin, is the initial regulator of apical dominance. Proc Natl Acad Sci USA 111: 6092–6097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery BL (2008) Right place, right time: spatiotemporal light regulation of plant growth and development. Plant Signal Behav 3: 1053–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller D, Leyser O (2011) Auxin, cytokinin and the control of shoot branching. Ann Bot 107: 1203–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nooden LD, Penney JP (2001) Correlative controls of senescence and plant death in Arabidopsis thaliana (Brassicaceae). J Exp Bot 52: 2151–2159 [DOI] [PubMed] [Google Scholar]
- Nunes C, O'Hara LE, Primavesi LF, Delatte TL, Schluepmann H, Somsen GW, Silva AB, Fevereiro PS, Wingler A, Paul MJ (2013) The trehalose 6-phosphate/SnRK1 signaling pathway primes growth recovery following relief of sink limitation. Plant Physiol 162: 1720–1732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer A, Janocha D, Dong Y, Medzihradszky A, Schone S, Daum G, Suzaki T, Forner J, Langenecker T, Rempel E,. et al. (2016) Integration of light and metabolic signals for stem cell activation at the shoot apical meristem. Elife 5: e17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer A, Wenzl C, Lohmann JU (2017) Beyond flexibility: controlling stem cells in an ever changing environment. Curr Opin Plant Biol 35: 117–123 [DOI] [PubMed] [Google Scholar]
- Poethig RS (2013) Vegetative phase change and shoot maturation in plants. Curr Top Dev Biol 105: 125–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnu J, Schlereth A, Zacharaki V, Dzialo MA, Abel C, Feil R, Schmid M, Wahl V (2020) The trehalose 6-phosphate pathway impacts vegetative phase change in Arabidopsis thaliana. Plant J 104: 768–780 [DOI] [PubMed] [Google Scholar]
- Przemeck GK, Mattsson J, Hardtke CS, Sung ZR, Berleth T (1996) Studies on the role of the Arabidopsis gene MONOPTEROS in vascular development and plant cell axialization. Planta 200: 229–237 [DOI] [PubMed] [Google Scholar]
- Qi X, Wu Z, Li J, Mo X, Wu S, Chu J, Wu P (2007) AtCYT-INV1, a neutral invertase, is involved in osmotic stress-induced inhibition on lateral root growth in Arabidopsis. Plant Mol Biol 64: 575–587 [DOI] [PubMed] [Google Scholar]
- Rameau C, Bertheloot J, Leduc N, Andrieu B, Foucher F, Sakr S (2014) Multiple pathways regulate shoot branching. Front Plant Sci 5: 741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan YL, Patrick JW, Bouzayen M, Osorio S, Fernie AR (2012) Molecular regulation of seed and fruit set. Trends Plant Sci 17: 656–665 [DOI] [PubMed] [Google Scholar]
- Sablowski R, Carnier Dornelas M (2014) Interplay between cell growth and cell cycle in plants. J Exp Bot 65: 2703–2714 [DOI] [PubMed] [Google Scholar]
- Sairanen I, Novak O, Pencik A, Ikeda Y, Jones B, Sandberg G, Ljung K (2012) Soluble carbohydrates regulate auxin biosynthesis via PIF proteins in Arabidopsis. Plant Cell 24: 4907–4916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar-García S, Garner LC, Lovatt CJ (2013) Reproductive biology.InSchaffer B, Wolstenholme BN, Whiley AW, eds, The Avocado, Ed 2. CABI, Wallingford, Oxfordshire OX10 8DE, UK, pp 118–167 [Google Scholar]
- Salazar-García S, Lord EM, Lovatt CJ (1998) Inflorescence and flower development of the ‘Hass’ avocado (Persea americana Mill.) during ‘on’ an d’off’ crop years. J Am Soc Horticult Sci 123: 537–544 [Google Scholar]
- Samach A, Smith HM (2013) Constraints to obtaining consistent annual yields in perennials. II: environment and fruit load affect induction of flowering. Plant Sci 207: 168–176 [DOI] [PubMed] [Google Scholar]
- Satoh-Nagasawa N, Nagasawa N, Malcomber S, Sakai H, Jackson D (2006) A trehalose metabolic enzyme controls inflorescence architecture in maize. Nature 441: 227–230 [DOI] [PubMed] [Google Scholar]
- Sawicki M, Ait Barka E, Clement C, Vaillant-Gaveau N, Jacquard C (2015) Cross-talk between environmental stresses and plant metabolism during reproductive organ abscission. J Exp Bot 66: 1707–1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Godin C, Boudon F, Demotes-Mainard S, Sakr S, Bertheloot J (2019) Light regulation of axillary bud outgrowth along plant axes: an overview of the roles of sugars and hormones. Front Plant Sci 10: 1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetz M, Berleth T, Mattsson J (2008) Multiple MONOPTEROS-dependent pathways are involved in leaf initiation. Plant Physiol 148: 870–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segers G, Gadisseur I, Bergounioux C, de Almeida Engler J, Jacqmard A, Van Montagu M, Inze D (1996) The Arabidopsis cyclin-dependent kinase gene cdc2bAt is preferentially expressed during S and G2 phases of the cell cycle. Plant J 10: 601–612 [DOI] [PubMed] [Google Scholar]
- Serrano-Mislata A, Sablowski R (2018) The pillars of land plants: new insights into stem development. Curr Opin Plant Biol 45: 11–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Zhang Y, Ge D, Wang Z, Song W, Gu R, Che G, Cheng Z, Liu R, Zhang X (2019) CsBRC1 inhibits axillary bud outgrowth by directly repressing the auxin efflux carrier CsPIN3 in cucumber. Proc Natl Acad Sci USA 116: 17105–17114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara N, Taylor C, Leyser O (2013) Strigolactone can promote or inhibit shoot branching by triggering rapid depletion of the auxin efflux protein PIN1 from the plasma membrane. PLoS Biol 11: e1001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith HM, Samach A (2013) Constraints to obtaining consistent annual yields in perennial tree crops. I: heavy fruit load dominates over vegetative growth. Plant Sci 207: 158–167 [DOI] [PubMed] [Google Scholar]
- Soundappan I, Bennett T, Morffy N, Liang Y, Stanga JP, Abbas A, Leyser O, Nelson DC (2015) SMAX1-LIKE/D53 family members enable distinct MAX2-dependent responses to strigolactones and karrikins in Arabidopsis. Plant Cell 27: 3143–3159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer PS, Holding DR, Groover A, Yordan C, Martienssen RA (2000) The essential Mcm7 protein PROLIFERA is localized to the nucleus of dividing cells during the G(1) phase and is required maternally for early Arabidopsis development. Development 127: 1815–1822 [DOI] [PubMed] [Google Scholar]
- Srikanth A, Schmid M (2011) Regulation of flowering time: all roads lead to Rome. Cell Mol Life Sci 68: 2013–2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan V, Springer P, Volpe T, Haward S, Jones JD, Dean C, Ma H, Martienssen R (1995) Patterns of gene action in plant development revealed by enhancer trap and gene trap transposable elements. Genes Dev 9: 1797–1810 [DOI] [PubMed] [Google Scholar]
- Teichmann T, Muhr M (2015) Shaping plant architecture. Front Plant Sci 6: 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truernit E, Sauer N (1995) The promoter of the Arabidopsis thaliana SUC2 sucrose-H+ symporter gene directs expression of beta-glucuronidase to the phloem: evidence for phloem loading and unloading by SUC2. Planta 196: 564–570 [DOI] [PubMed] [Google Scholar]
- Turck F, Fornara F, Coupland G (2008) Regulation and identity of florigen: FLOWERING LOCUS T moves center stage. Annu Rev Plant Biol 59: 573–594 [DOI] [PubMed] [Google Scholar]
- Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ (1997) Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9: 1963–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rongen M, Bennett T, Ticchiarelli F, Leyser O (2019) Connective auxin transport contributes to strigolactone-mediated shoot branching control independent of the transcription factor BRC1. PLoS Genet 15: e1008023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl V, Ponnu J, Schlereth A, Arrivault S, Langenecker T, Franke A, Feil R, Lunn JE, Stitt M, Schmid M (2013) Regulation of flowering by trehalose-6-phosphate signaling in Arabidopsis thaliana. Science 339: 704–707 [DOI] [PubMed] [Google Scholar]
- Walker CH, Bennett T (2018) Forbidden fruit: dominance relationships and the control of shoot architecture. Annual Plant Reviews 1: 217–254 [Google Scholar]
- Wang JW (2014) Regulation of flowering time by the miR156-mediated age pathway. J Exp Bot 65: 4723–4730 [DOI] [PubMed] [Google Scholar]
- Wang L, Wang B, Jiang L, Liu X, Li X, Lu Z, Meng X, Wang Y, Smith SM, Li J (2015) Strigolactone signaling in Arabidopsis regulates shoot development by targeting D53-Like SMXL repressor proteins for ubiquitination and degradation. Plant Cell 27: 3128–3142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Wang B, Yu H, Guo H, Lin T, Kou L, Wang A, Shao N, Ma H, Xiong G,. et al. (2020) Transcriptional regulation of strigolactone signalling in Arabidopsis. Nature 583: 277–281 [DOI] [PubMed] [Google Scholar]
- Wang M, Perez-Garcia MD, Daviere JM, Barbier F, Oge L, Gentilhomme J, Voisine L, Péron T, Launay-Avon A, Clément G,. et al. (2021) Axillary bud outgrowth in rose is controlled by sugar metabolic and signalling pathways. J Exp Bot 72: 3044–3060 [DOI] [PubMed] [Google Scholar]
- Wang Y, Kumaishi K, Suzuki T, Ichihashi Y, Yamaguchi N, Shirakawa M, Ito T (2020) Morphological and physiological framework underlying plant longevity in Arabidopsis thaliana. Front Plant Sci 11: 600726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware A, Walker CH, Simura J, Gonzalez-Suarez P, Ljung K, Bishopp A, Wilson ZA, Bennett T (2020) Auxin export from proximal fruits drives arrest in temporally competent inflorescences. Nat Plants 6: 699–707 [DOI] [PubMed] [Google Scholar]
- Welham T, Pike J, Horst I, Flemetakis E, Katinakis P, Kaneko T, Sato S, Tabata S, Perry J, Parniske M,. et al. (2009) A cytosolic invertase is required for normal growth and cell development in the model legume, Lotus japonicus. J Exp Bot 60: 3353–3365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Dabi T, Weigel D (2005) Requirement of homeobox gene STIMPY/WOX9 for Arabidopsis meristem growth and maintenance. Curr Biol 15: 436–440 [DOI] [PubMed] [Google Scholar]
- Wuest SE, Philipp MA, Guthorl D, Schmid B, Grossniklaus U (2016) Seed production affects maternal growth and senescence in Arabidopsis. Plant Physiol 171: 392–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav RK, Girke T, Pasala S, Xie M, Reddy GV (2009) Gene expression map of the Arabidopsis shoot apical meristem stem cell niche. Proc Natl Acad Sci U S A 106: 4941–4946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Xu M, Koo Y, He J, Poethig RS (2013) Sugar promotes vegetative phase change in Arabidopsis thaliana by repressing the expression of MIR156A and MIR156C. Elife 2: e00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Cao L, Zhou CM, Zhang TQ, Lian H, Sun Y, Wu J, Huang J, Wang G, Wang JW (2013) Sugar is an endogenous cue for juvenile-to-adult phase transition in plants. Elife 2: e00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Andersen SU, Ljung K, Dolezal K, Miotk A, Schultheiss SJ, Lohmann JU (2010) Hormonal control of the shoot stem-cell niche. Nature 465: 1089–1092 [DOI] [PubMed] [Google Scholar]
- Zhou F, Lin Q, Zhu L, Ren Y, Zhou K, Shabek N, Wu F, Mao H, Dong W, Gan L,. et al. (2013) D14-SCF(D3)-dependent degradation of D53 regulates strigolactone signalling. Nature 504: 406–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv D, Zviran T, Zezak O, Samach A, Irihimovitch V (2014) Expression profiling of FLOWERING LOCUS T-like gene in alternate bearing ‘Hass’ avocado trees suggests a role for PaFT in avocado flower induction. PLoS One 9: e110613. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.